Simple Summary
Disease is a frequently encountered problem in aquaculture, which always causes global economic losses. White spot syndrome virus (WSSV) and Vibrio parahaemolyticus are two of the most destructive pathogens causing severe loss of shrimp aquaculture. Understanding the host immune responses against different pathogens is vital for developing effective disease control technologies. The lymphoid organ is a vital part of the shrimp immune system and exhibits important immune functions including cellular and humoral immunity. However, the immune function of the lymphoid organ and its responses against different pathogens are still largely unclear. In the present study, transcriptomic analysis was applied to compare the differentially expressed genes (DEGs) in the lymphoid organ of shrimp after Vibrio or WSSV challenge. Data showed that Vibrio challenge induced broad immune responses in the lymphoid organ including activation of several pattern recognition receptors, the proPO activating system, phagocytosis related genes, and immune effectors. In contrast, the immune responses seemed to be inhibited after WSSV infection. The present study suggests that the shrimp lymphoid organ plays different functions in response to the infection of distinct pathogens at early stage, which provides new insights into the immune functions of lymphoid organ in shrimp.
Abstract
The lymphoid organ is an essential part of the immune system involved in cellular and humoral immune responses in shrimp. However, its roles in the immune responses against different pathogens are still largely unclear. In the present study, transcriptomic analysis was applied to compare the differentially expressed genes (DEGs) in the lymphoid organ of shrimp after Vibrio or WSSV challenge. In total, 2127 DEGs were screened in the lymphoid organ of shrimp at 6 h post Vibrio parahaemolyticus injection, and 1569 DEGs were obtained at the same time after WSSV challenge. KEGG pathway enrichment analysis of these DEGs revealed that two significantly enriched pathways including “neuroactive ligand–receptor interaction” and “protein digestion and absorption” were responsive to both pathogens. In contrast, “lysosome” was the significantly enriched pathway only in Vibrio challenge whereas carbohydrate metabolism related pathways were the significantly enriched pathways only in WSSV challenge. Further analysis on immune-related DEGs showed that Vibrio challenge induced broad immune responses in the lymphoid organ including activation of several pattern recognition receptors, the proPO activating system, phagocytosis related genes, and immune effectors. In contrast, the immune responses seemed to be inhibited after WSSV infection. The data suggest that the shrimp lymphoid organ plays different functions in response to the infection of distinct pathogens at the early stage, which provides new insights into the immune functions of lymphoid organ in shrimp.
1. Introduction
The Pacific whiteleg shrimp Litopenaeus vannamei is one of the most important crustaceans in global aquaculture [1]. During the development of the shrimp aquaculture industry, disease is a frequently encountered problem, which always causes global economic losses. Viruses, bacteria, and even parasites are the main pathogens of shrimp in aquaculture [2]. Vibrio parahaemolyticus and white spot syndrome virus (WSSV) are two of the most destructive pathogens causing severe loss of shrimp aquaculture [3,4]. Similar to other invertebrates, shrimp usually relies on its innate immune system including humoral and cellular immunity to fight against the invasive pathogens [5]. Circulating hemocytes play a crucial role both in cellular and humoral immune responses [6,7]. Besides the hemocytes, the lymphoid organ is also an important target providing immune defense against invasive pathogens.
The lymphoid organ, also called Oka, was first identified in Penaeus orientalis (now called Fenneropenaeus chinensis) [8] and then found in other penaeid shrimp including Penaeus monodon [9] and L. vannamei [10]. Therefore, the lymphoid organ was once thought to be unique to penaeid shrimps [11]. However, recent studies showed that the lymphoid organ also existed in Macrobrachium rosenbergii [12] and Procambarus clarkii [13]. The normal lymphoid organ in shrimp is composed of two similar lobes situated ventral-anteriorly to the hepatopancreas, and its size is affected by many factors including developmental stage, animal size, species and health status, and connects directly to the heart through the subgastric artery [11]. The organ filtrates and removes foreign materials from the hemolymph [11].
The lymphoid organ is a vital part of the shrimp immune system and exhibits important immune functions. The lymphoid organ is important for phagocytosis [14], which plays a major role both in bacteriostasis [15] and viral degradation [16] and functions in humoral immunity. In P. clarkii and M. rosenbergii, some pattern recognition receptors and immune signaling pathways were differentially expressed at the late stages of WSSV infection [12,13]. In F. chinensis, one anti-lipopolysaccharide factor (ALF) specifically expressed in the lymphoid organ showed strong antibacterial and antiviral activities, which further suggested the importance of the lymphoid organ in shrimp humoral immunity [17]. However, the immune function of the lymphoid organ is still largely unclear. How does the lymphoid organ response to the infection of pathogens? What are the differences of the lymphoid organ responsive to different pathogens? These questions are still less investigated.
To understand the early immune responses of the lymphoid organ of shrimp against different pathogens, cDNA libraries from the lymphoid organ of the whiteleg shrimp L. vannamei challenged by V. parahaemolyticus or WSSV were sequenced using the Illumina Hiseq 4000 platform in the present study. A total of 2127 and 1569 DEGs were identified in the lymphoid organ of shrimp at 6 h post Vibrio or WSSV injection, respectively. KEGG signal pathway enrichment analysis displayed the similarities and differences between antibacterial and antiviral immune responses in the organ. The data will enrich our understanding of the molecular mechanisms of the lymphoid organ against early infection of different pathogens.
2. Materials and Methods
2.1. Immune Challenge and Shrimp Tissues Preparation
Healthy shrimp about 9–10 g were obtained from our laboratory (Qingdao, China) and cultured in tanks filled with aerated seawater. Shrimp were fed thrice a day with artificial food pellets and acclimatized for three days before challenge experiments.
V. parahaemolyticus was isolated from diseased shrimp. Bacteria were cultured in trypticase soybroth (TSB) media (17.0 g/L tryptone, 3.0 g/L phytone, 5.0 g/L NaCl, 2.5 g/L KH2PO4, 2.5 g/L glucose) at 30 °C with shaking at 200 rpm. When the cultured bacteria reached the logarithmic growth phase, formaldehyde solution was added to cultures at a final concentration of 0.5% (vol/vol) and set for 24 h at 4 °C. Excess formaldehyde was removed by three washes with PBS. The formalinized V. parahaemolyticus cell suspension was stored at 4 °C before injection. In addition, live WSSV particles were extracted from pathologically infected shrimp by differential centrifugation and density gradient centrifugation as previously described [18]. These particles were stored at −80 °C before use.
Forty-five shrimps were randomly grouped into three groups including two experimental groups and a control group. For one experimental group, each shrimp was injected with 107 CFU formalinized bacterial cells. For another group, 1000 copies of WSSV particles suspended in 10 μL sterile PBS were injected in the shrimp abdominal segments III and IV. In the control group, equal volume of PBS was injected. At 6 hpi, the lymphoid organ was collected from each shrimp and frozen in liquid nitrogen for RNA extraction.
2.2. RNA Extraction and Illumina Sequencing
The total RNA was extracted with RNAiso Plus (TaKaRa, Kyoto, Japan) according to the manufacturer’s instructions. Equal amounts of the total RNA from five lymphoid organs in each group were pooled as a biological replicate. Each group was set with three biological replicates. The RNA samples from the control group were designated as POka-1~3, while the RNA samples from the experimental groups were designated as VOka-1~3 and WOka-1~3 for the Vibrio and WSSV challenges, respectively. All nine pools were used for sequencing at Gene Denovo (Guangzhou, China) following the manufacturer’s protocol (Illumina Inc., San Diego, CA, USA). Briefly, the mRNA with poly (A) was enriched from the total RNA using oligo (dT) magnetic beads and fragmented with ultra-sonication. Then, the first strand cDNA was reverse-transcribed using reverse transcriptase and random primers. The second-strand cDNA was synthesized under DNA polymerase I. Subsequently, sequencing adapters were added to short fragments, and the libraries were sent for sequencing using an Illumina HiSeq™ 4000.
2.3. De Novo Assembly and Transcriptome Analysis
High-quality clean reads were obtained after raw reads filtration. Next, all clean reads from three sets of samples were assembled into transcripts using Trinity software [19] with default parameters. The redundant transcripts were removed by clustering in TGICL [20] with default parameters. After de novo assembly was completed, the unigenes were aligned against four protein databases including Nr, KEGG, Swiss-Prot, and KOG using BLASTX at the E-value less than 10−5. The best alignment results were used to predict the sequence direction and the coding sequence of the unigenes.
2.4. Differential Expression Analysis and Functional Characterization
To calculate gene abundances, clean reads from each sample were mapped to the assembled transcripts using the RSEM software [21]. Gene expression levels were calculated with the fragments per kilobase of exon model per million mapped reads (FPKM) value and DEGs were identified using the DESeq R package. Besides, false discovery rate (FDR) was used to correct for E-value. Genes were described as DEGs based on the RPKM value in POka and VOka groups or POka and WOka groups, followed by a multiple hypothesis testing: FDR < 0.05 and the absolute value of log2 fold change (FC) > 1. For functional analysis of DEGs, the DEGs were enriched in GO functions and KEGG pathways by the Blast2GO program [22] and KAAS (KEGG Automatic Annotation Server) [23], respectively. The genes related to the immune function were identified based on the functional gene profiling analysis.
2.5. Data Reliability Confirmed by qRT-PCR Assay
Four DEGs including Unigene0055376 (alpha 2 macroglobulin), Unigene0018885 (solute carrier organic anion transporter), Unigene0024101 (neuroparsin 1), and Unigene0028929 (C-type lectin) were randomly selected for real-time qPCR (qRT-PCR) in three replicates from the POka, VOka, and WOka groups, respectively. Briefly, 1 μg of total RNA was reverse-transcribed using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Japan) following the manufacturer’s protocol. The qRT-PCR reactions were carried out using SYBR (TOYOBO, Osaka, Japan) with gene-specific primers designed by PRIMER 5.00 (Premier Biosoft, San Francisco, CA, USA). The information of primers used in this study is shown in Table S1. The 18S rRNA gene was used as the internal reference gene and the relative gene expression levels were calculated by the comparative Ct method with the formula 2-ΔΔCt. All the expression data were obtained from at least three parallel tests. Then, the qRT-PCR results were compared with the transcriptome data.
3. Results and Discussion
3.1. The Basic Information of the Transcriptome
The detailed information of the transcriptome sequencing is shown in Table 1. A total of 152,386,028 reads were obtained from the POka group, 156,938,950 reads were obtained from the VOka group, and 143,090,744 reads were obtained from the WOka group, respectively. After data filtration, both the clean reads percentage and Q20 (percentage of bases the quality of which was greater than 20 in clean reads) in each sample were higher than 95%. Using the Trinity program, the transcriptome assembly yielded a total of 59,583 unigenes, with a N50 of 2103 bp.

Table 1.
Summary of the transcriptome data.
3.2. Identification and Verification of DEGs
To identify DEGs in the lymphoid organ of shrimp after different treatments, the RPKM value was used to compare the expression differences of each gene. According to the definition of DEGs, 2127 DEGs were identified in the VOka group including 809 upregulated DEGs and 1318 downregulated DEGs (Figure 1A,C, Table S2), while 1569 DEGs were identified in the WOka group including 138 upregulated DEGs and 1431 downregulated DEGs (Figure 1B,C, Table S2).
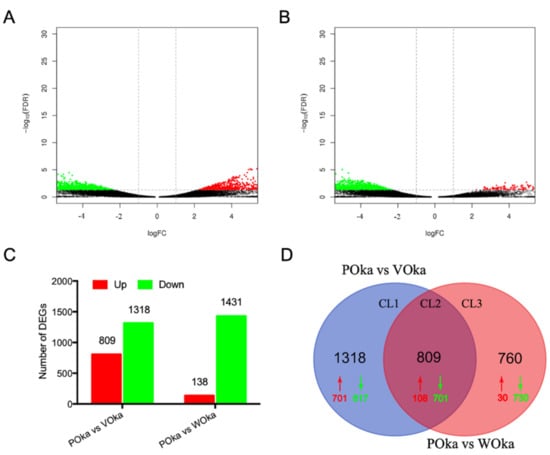
Figure 1.
Analysis of the DEGs upon Vp and WSSV challenges. (A) Volcano plot displaying DEGs between the VOka group and the POka group. (B) Volcano plot displaying DEGs between the WOka group and the POka group. The X axis displays the log of the FC (VOka or WOka group relative to POka group) and the Y axis represents the negative log of the FDR (base 10). Black dots represent genes whose expression levels did not reach statistical significance. (C) Statistical results of DEGs between the control group and experimental groups. (D) A two-set Venn diagram showing the number of DEGs in different treatments. Upregulated genes are marked with red and downregulated genes are shown in green.
To better understand the immune responses in the lymphoid organ of shrimp caused by V. parahaemolyticus and WSSV challenges, a Venn diagram was used to show the DEGs between the VOka group and WOka group. A total of 1318 DEGs in cluster 1 (CL1) and 760 DEGs in cluster 3 (CL3) were specific for the V. parahaemolyticus and WSSV challenges, respectively (Figure 1D, Table S2), and 809 DEGs in cluster 2 (CL2) were in the overlapping region (Figure 1D, Table S2), which indicated that these genes might be involved in immune responses against different pathogens.
To verify the accuracy of the transcriptome data, four DEGs were randomly selected for qRT-PCR from 809 DEGs located at the intersection of Figure 1D. The expression profiles of them in the transcriptome data were in good agreement with the qPCR results (Figure 2). Unigene0055376 (Figure 2A) and Unigene0018885 (Figure 2B) were significantly downregulated in the lymphoid organ of shrimp under both the Vp and WSSV stimulation, while Unigene0024101 (Figure 2C) and Unigene0028929 (Figure 2D) were obviously upregulated.
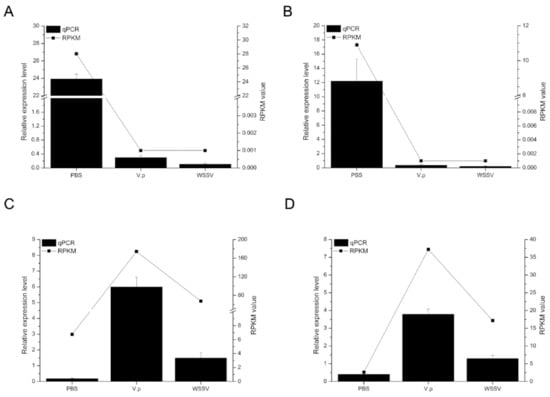
Figure 2.
Expression profiles of four unigenes. (A) Unigene0055376 (alpha 2 macroglobulin); (B) Unigene0018885 (solute carrier organic anion transporter family member 5A1-like); (C) Unigene0024101 (neuroparsin 1); (D) Unigene0028929 (C-type lectin). The X axis represents the different treatments. Columns and bars represent the means and standard error of relative expression levels from the qPCR results (Y axis at left). Lines represent the RPKM value from the transcriptome results (Y axis at right).
3.3. Functional Characterization of DEGs
To understand the function of DEGs during V. parahaemolyticus and WSSV challenges in shrimp, all genes were mapped to terms in the GO and KEGG database. A total of 532 GO terms were successfully enriched in VOka vs. POka (Table S3), and 307 GO terms were enriched in WOka vs. POka (Table S4). The functional distribution of all DEGs is shown in Figure 3. Compared to WOka vs. POka, the level 2 GO terms of VOka vs. POka had some specific terms in each category including biological adhesion in the biological process; antioxidant activity in the molecular function; and virion and virion part in the cellular component (Figure 3). This result provided more information for exploring the differences between bacterial and viral challenges in the lymphoid organ.

Figure 3.
GO term (level 2) distribution for the DEGs from the present transcriptome analysis of lymphoid organ after V. parahaemolyticus (A) and WSSV (B) challenges. The horizontal axis displays the name of GO subcategories. The vertical axis represents the number of genes. Red indicated upregulated DEGs and green showed downregulated DEGs.
Signaling pathways were defined using the KEGG database (Tables S5 and S6) and the top 20 pathways were shown in Figure 4. Among them, the top five pathways in the lymphoid organ challenged by two pathogens had significant differences (p-value < 0.01 and q-value < 0.05) (Figure 4). Importantly, “neuroactive ligand–receptor interaction” and “protein digestion and absorption” were the significantly enriched pathways in both the VOka and WOka groups (Figure 4) and might play important roles in response to pathogen challenge in the lymphoid organ. “Lysosome” was the significantly enriched pathway only in the VOka group (Figure 4, Table S5) and probably served as an important organelle involved in the clearance of invasive Vibrio. Carbohydrate metabolism related pathways including “inositol phosphate metabolism”, “propanoate metabolism” and “glycolysis/gluconeogenesis” were the significantly enriched pathways only in the WOka group (Figure 4, Table S6). It had been reported that WSSV could induce changes in carbohydrate metabolism in multiple tissues of shrimp including hemocytes, hepatopancreas, and muscle [24,25]. In shrimp hemocytes, WSSV induces the invertebrate Warburg effect (or aerobic glycolysis) in the early infection stage and significantly activates several metabolic pathways, which is essential for successful viral replication [26]. Our results showed that carbohydrate metabolism in shrimp lymphoid organ was very likely to be altered by WSSV infection.
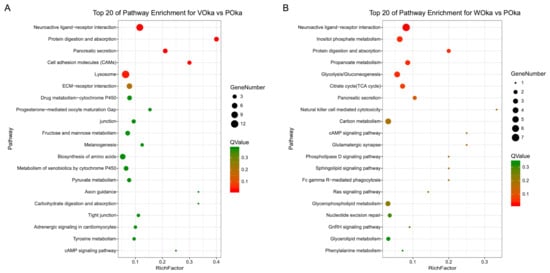
Figure 4.
The top 20 KEGG pathways enriched in shrimp lymphoid organ after V. parahaemolyticus (A) and WSSV (B) challenges. Enrichment analysis of KEGG pathways was performed using the KEGG Automatic Annotation Server [23]. “Rich factor” represents the ratio of the DEG number to the number of all genes annotated in this pathway. The Rich factor is proportional to the degree of enrichment.
3.4. In-Depth Analysis of Immune-Related DEGs
To further understand the host immune responses in the lymphoid organ against Vibrio and WSSV challenges, we identified immune-related DEGs based on their functional annotation. DEGs responsive to Vibrio and WSSV challenges were mainly divided into several categories including pattern recognition receptors (PRRs), proPO activating system, signaling pathway, phagocytosis, chitin-binding protein (CBP), antimicrobial peptides (AMPs), etc.
3.4.1. PRRs
In the transcriptome data, 30 transcripts were identified as PRRs. These transcripts were annotated as beta-1,3-glucan-binding protein (BGBP), lipopolysaccharide and beta-1,3-glucan binding protein (LGBP), lectins (mainly C-type lectins, CTLs), NOD-like receptors (NLRs), fibrinogen-related proteins (FREPs), and leucine rich repeat only protein (Table 2). All identified BGBP and LGBP transcripts in DEGs were increased significantly at the early challenge stage of Vp while no transcript was changed after WSSV infection (Table 2), indicating the key role of the lymphoid organ in bacteria removal. All CTL transcripts in DEGs were induced obviously by Vp challenge, whereas only two of them were responsive to WSSV infection (Table 2). All the other PRRs were only upregulated after Vp challenge while no change after WSSV infection, except one transcript encoding FREP2, which was downregulated after WSSV infection while not responsive to Vp (Table 2).

Table 2.
Differentially expressed PRRs in the lymphoid organ after Vibrio or WSSV challenge.
3.4.2. ProPO Activating System
In the Vp-challenged lymphoid organ, 20 SP transcripts changed significantly, of which 16 were upregulated, whereas in the WSSV-infected lymphoid organs, only eight transcripts changed apparently, of which five were upregulated (Table 3). Like SP, more SPIs were responsive to Vp challenge than to WSSV infection (Table 3). Notably, all 12 proPO transcripts including two laccase transcripts and ten hemocyanin transcripts were upregulated by Vp challenge, but no transcript changed after WSSV infection (Table 3).

Table 3.
DEGs related to the proPO system in the lymphoid organ after Vibrio or WSSV challenge.
3.4.3. Signaling Pathways
In invertebrates, signaling pathways such as Toll, IMD, JAK/STAT, and RNA interference pathways play important roles in the innate immunity [27,28]. However, only six genes related to immune signaling pathways were differentially expressed after Vibrio or WSSV challenge (Table S7). Four of them encoding TRAF6, SOCS2, and AGO4 were responsive to WSSV infection. AGO4 and TRAF6 were downregulated significantly after WSSV infection, whereas SOCS2 was upregulated (Table S7). AGO is an important member of the RNA-induced silencing complex (RISC) in the RNAi pathway [29]. Here, we found that AGO4 was also expressed highly (RPKM = 309.54) in the lymphoid organ in L. vannamei and its expression was decreased rapidly to a very low level (RPKM = 0.08) after WSSV infection. TRAF6 is a central signaling adapter protein that activates nuclear factor κB (NF-κB), mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K) and interferon regulatory factor pathways via its TRAF domain and a RING finger domain [30]. The role of TRAF6 in the antiviral response has been shown in two kinds of shrimp [31,32]. SOCS2 is an inhibitor of the JAK/STAT pathway that mediates antiviral immune response, and knockdown of SOCS2 could enhance resistance against pathogens in both Marsupenaeus japonicus and L. vannamei [33,34]. Therefore, downregulation of AGO4 and TRAF6 and upregulation of SOCS2 in the lymphoid organ might inhibit antiviral function and increase the shrimp body’s susceptibility to WSSV.
3.4.4. Phagocytosis
In the present transcriptome, 14 protease transcripts changed obviously after Vp challenge, 12 of which increased, whereas no transcript changed after WSSV infection (Table 4). Seven cathepsin transcripts that belong to three categories (C, D, and L) were significantly induced after Vp challenge (Table 4).

Table 4.
DEGs related to lysosome in the lymphoid organ after Vibrio or WSSV challenge.
3.4.5. Chitin-Binding Proteins (CBPs)
CBPs, which include chitinases, peritrophic matrix proteins, and several other proteins with unknown function, play important roles in immune responses in many peripheral tissues of shrimp such as stomach, epidermis, and gill [35,36,37,38]. In the transcriptome data, CBPs exhibited differential expression under the treatment of two pathogens in the lymphoid organ. Up to 34 transcripts encoding CBPs were responsive to Vp or WSSV challenge. Among them, 25 transcripts specifically responded to Vibrio challenge, eight transcripts responded to both pathogens, while only one transcript was specific to WSSV challenge (Table S7). Furthermore, all the 14 peritrophin encoding transcripts were upregulated after Vibrio challenge and four of them were upregulated after WSSV challenge (Table S7, bolded). However, most of the other CBPs were downregulated after Vibrio or WSSV challenge. The data showed that CBPs were also abundantly expressed in the lymphoid organ and played important roles immediately after pathogen infection.
3.4.6. Immune Effectors
Immune effectors are essential factors of the host immune system, which could directly kill invasive pathogens and induce amplified host immune responses. Antimicrobial peptides are one of the important immune effectors in shrimp, which mainly include penaeidins, anti-lipopolysaccharide factors (ALFs), crustins, and stylins [39]. The expression of two ALFs and three crustins was apparently changed after Vibrio or WSSV challenge. The two ALFs were only induced by Vibrio challenge, while one crustin was induced after challenge with both Vibrio and WSSV (Table 5). However, the other two crustins were downregulated after Vibrio or WSSV challenge. The data indicated that different antimicrobial peptides in the lymphoid organ were responsive to distinct pathogens in different ways.

Table 5.
DEGs related to immune effectors in the lymphoid organ after Vibrio or WSSV challenge.
It was noted that eight transcripts encoding vitelline membrane outer layer protein I (VMO-I) displayed the same expression pattern that was upregulated in lymphoid organ after Vibrio challenge while not responsive to WSSV challenge (Table 5). VMO-I was initially found in the outer layer of the vitelline membrane (VM) from hen yolk [40]. The VM in arthropods also possess a similar structure and function [41,42]. In the lobster, VMO-I from hepatopancreatic tissue was responsible for humoral immune responses against parasitic infection [43]. In the crayfish, VMO-I is a component of the granules in the granular hemocytes [44]. These findings indicated that VMO-I might be a kind of immune effectors in crustacean. Although the mechanism of VMO-I function remains largely unknown, our findings still provide information about the potential function of VMO-I during Vibrio challenge in shrimp lymphoid organ.
4. Discussion
The present study compared the transcriptome differences of the shrimp lymphoid organ against challenges of different pathogens. We focused on the early immune responses (6 hpi) of the host lymphoid organ against different pathogens, and in vivo WSSV propagation needs a much longer time than 6 h post injection [45]. Therefore, we used formalinized bacteria and live viral particles for immune challenges. The data showed that the host early responses, especially the immune responses, differed significantly against Vibrio and WSSV challenges. PRRs play a crucial role in the recognition of the pathogen-associated molecular patterns (PAMPs) derived from bacteria, fungi, virus, and parasites, and initiate downstream signaling events. So far, there are 11 types of PRRs reported in shrimp [46]. Some PRRs including BGBP, LGBP, CTLs, and FREPs could be activated by Vibrio challenge at the early infection stage. The complex formed by BGBP or LGBP and its corresponding pathogenic ingredient induces degranulation and the activation of prophenoloxidase in shrimp [47]. As a large family of lectins in crustaceans, CTLs show a ubiquitous spectrum of microbe-associated molecular patterns (MAMPs) binding and induce the subsequent immune responses including phagocytosis, activation of prophenoloxidase and respiratory burst [48]. A few shrimp CTLs are involved in the anti-viral response through interaction with viral structural proteins [49,50]. According to their functions, some lectins are regarded as important PRRs and others are thought to act as immune effectors. Activation of these PRRs after Vibrio challenge indicated that a rapid immune response occurred during bacterial infection.
Melanization of pathogens is one major and rapid immune response in invertebrates, which depends on the proPO activating system [51]. The recognition of PRRs to PAMPs on the pathogen activates the proPO system [52]. A serine proteinase (SP) cascade is then triggered and converts the inactive proPO into the active PO [52]. SPs constitute one of the families of proteolytic enzymes including clip-domain serine proteinase designated as a proPO-activating enzyme (PPAE) and large numbers of serine proteinase homologues without precise functions in the SP cascade [52]. The active PO produces melanin and toxic reactive intermediates to fight against invasive pathogens [53]. Due to the high toxicity of intermediates in the melanization process, the process is tightly controlled by SPI via inhibiting the SP cascade [52]. Laccase is a member of POs (copper-containing enzymes) and oxidizes o-diphenols, p-diphenol, and p-diamines [54]. In insects, laccase has a variety of biological functions such as degradation of secondary phenolic compounds, metal ion metabolism, cuticle sclerotization, pigment formation, and immune defense [55]. A laccase from L. vannamei was reported to be involved in anti-bacterial host defense [56]. Hemocyanin (HMC) acts as an oxygen carrier, a precursor of some AMPs and PO [57]. Until now, HMC-derived PO activity has been characterized in more than 40 species of invertebrates [57]. The data showed that many genes in the proPO activating system including SP, SPI, and proPO were mainly responsive to Vibrio challenge. The results were in accordance with the responses of PRRs, which play important roles in triggering the proPO system.
As an evolutionarily conserved process, phagocytosis plays a vital role in both host defense and tissue homeostasis in a wide diversity of organisms. Phagocytosis is a very complex process including particle recognition and internalization, phagosome maturation, and particle destruction [58]. The phagosome matures via fusion with a lysosome that is known as an “enzyme warehouse” and a cellular “digestive system” due to many intrinsic hydrolytic enzymes [59]. Among them, cathepsins are proteases present in all animals as well as other organisms and they could be divided into three subgroups based on the active amino acid residue: cysteine proteases (including types B, C, F, H, K, L, O, S, V, W, and X), aspartic proteases (including types D and E), and serine proteases (including types A and G) [60,61]. Cathepsin C, D, and L were reported to play important roles during immune response against pathogen infection in shrimp or crayfish [62,63,64,65,66]. The present data showed that the lysosome mediated digestion of invasive pathogens was immediately activated in the lymphoid organ during Vibrio challenge, whereas this system was not responsive to viral challenge.
5. Conclusions
This is the first report on the comparative transcriptome analysis of the shrimp lymphoid organ in response to the early challenge of two different pathogens, V. parahaemolyticus and WSSV. Vibrio challenge activated broad immune responses including the humoral and cellular immune responses in the lymphoid organ. However, the immune responses seemed to be inhibited in the lymphoid organ at the early infection stage of WSSV. Instead, the carbohydrate metabolism related pathways were induced after WSSV infection. The data suggest that the shrimp lymphoid organ plays different functions in response to the early infection of distinct pathogens, which provides new insights into the immune functions of the lymphoid organ in shrimp.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11082160/s1, Table S1: Primers used for qPCR validation, Table S2: Expression and annotation of DEGs identified from VOka group and WOka group, Table S3: GO enrichment analysis of all DEGs from VOka group, Table S4: GO enrichment analysis of all DEGs from WOka group, Table S5: KEGG pathway analysis of all DEGs from VOka group, Table S6: KEGG pathway analysis of all DEGs from WOka group, Table S7: Differentially expressed genes related to signaling pathways, chitin-binding protein, ECM–receptor interaction, neuroactive ligand–receptor interaction, and cellular stress response in Oka after Vp.
Author Contributions
S.L. and F.L. conceived and designed the project. F.W. and S.L. performed all experiments and prepared the figures. F.W., S.L., and F.L. wrote and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Key Program of the National Natural Science Foundation of China (31830100); the General Program of National Natural Science Foundation of China (31972829, 41776158); the China Agriculture Research System of MOF and MARA; and the Distinguished Young Scientists Research Fund of Key Laboratory of Experimental Marine Biology, Chinese Academy of Sciences (KLEMB-DYS01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are included in the Supplementary Materials or available in the NCBI Sequence Read Archive (SRA, https://www.ncbi.nlm.nih.gov/bioproject/713898, accessed on 11 March 2021) with the accession number PRJNA713898.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| ALFs | Anti-Lipopolysaccharide Factors |
| CBPs | Chitin-Binding Proteins |
| DEGs | Differentially Expressed Genes |
| HMC | Hemocyanin |
| PRRs | Pattern Recognition Receptors |
| RPKM | Reads per Kilobase per Million Reads |
| VMO-I | Vitelline Membrane Outer Layer Protein I |
References
- Ghaffari, N.; Sánchez-Flores, A.; Doan, R.; Garcia-Orozco, K.D.; Chen, P.L.; Ochoa-Leyva, A.; López-Zavala, A.A.; Carrasco, J.S.; Hong, C.; Brieba, L.G.; et al. Novel transcriptome assembly and improved annotation of the whiteleg shrimp (Litopenaeus vannamei), a dominant crustacean in global seafood mariculture. Sci. Rep. 2014, 4, 7081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Flegel, T.W.; Itsathitphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- Citarasu, T.; Sivaram, V.; Immanuel, G.; Rout, N.; Murugan, V. Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeus monodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol. 2006, 21, 372–384. [Google Scholar] [CrossRef]
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar] [CrossRef]
- Li, F.; Xiang, J. Recent advances in researches on the innate immunity of shrimp in China. Dev. Comp. Immunol. 2013, 39, 11–26. [Google Scholar] [CrossRef]
- Söderhäll, I. Crustacean hematopoiesis. Dev. Comp. Immunol. 2016, 58, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zheng, S.-C.; Li, Y.-L.; Li, J.; Liu, H.-P. Hemocyte-Mediated Phagocytosis in Crustaceans. Front. Immunol. 2020, 11, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, M. Studies on Penaeus orientalis Kishinouye-VIII structure of the newly found lymphoid organ. Bull. Jpn. Soc. Sci. Fish. 1969, 35, 245–250. [Google Scholar] [CrossRef]
- Lightner, D.V.; Hedrick, R.P.; Fryer, J.L.; Chen, S.N.; Liao, I.C.; Kou, G.H. A survey of cultured penaeid shrimp in Taiwan for viral and other important diseases. Fish. Pathol. 1987, 22, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Bonami, J.R.; Lightner, D.V.; Redman, R.M.; Poulos, B.T. Partial Characterization of a Togavirus (Lovv) Associated with Histopathological Changes of the Lymphoid Organ of Penaeid Shrimps. Dis. Aquat. Organ. 1992, 14, 145–152. [Google Scholar] [CrossRef]
- Rusaini; Owens, L. Insight into the lymphoid organ of penaeid prawns: A review. Fish Shellfish Immunol. 2010, 29, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wu, L.; Jin, M.; Li, T.; Hui, K.; Ren, Q. Transcriptome profiling of the Macrobrachium rosenbergii lymphoid organ under the white spot syndrome virus challenge. Fish Shellfish Immunol. 2017, 67, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Q. Comparative transcriptome analysis reveals three potential antiviral signaling pathways in lymph organ tissue of the red swamp crayfish, Procambarus clarkii. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Van de Braak, C.; Botterblom, M.; Taverne, N.; Van Muiswinkel, W.; Rombout, J.; Van der Knaap, W. The roles of haemocytes and the lymphoid organ in the clearance of injected Vibrio bacteria in Penaeus monodon shrimp. Fish Shellfish Immunol. 2002, 13, 293–309. [Google Scholar] [CrossRef]
- Burgents, J.E.; Burnett, L.E.; Stabb, E.V.; Burnett, K.G. Localization and bacteriostasis of Vibrio introduced into the Pacific white shrimp, Litopenaeus vannamei. Dev. Comp. Immunol. 2005, 29, 681–691. [Google Scholar] [CrossRef]
- Anggraeni, M.S.; Owens, L. The haemocytic origin of lymphoid organ spheroid cells in the penaeid prawn Penaeus monodon. Dis. Aquat. Org. 2000, 40, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Guo, S.; Li, F.; Xiang, J. Characterization and function analysis of an anti-lipopolysaccharide factor (ALF) from the Chinese shrimp Fenneropenaeus chinensis. Dev. Comp. Immunol. 2014, 46, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, F.; Chi, Y.; Xiang, J. Enhanced resistance of marine shrimp Exopalamon carincauda Holthuis to WSSV by injecting live VP28-recombinant bacteria. Acta Oceanol. Sin. 2013, 32, 52–58. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef] [Green Version]
- Pertea, G.; Huang, X.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B.; et al. TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conesa, A.; Götz, S.; Garcia-Gomez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.-T.; Aoki, T.; Huang, Y.-T.; Hirono, I.; Chen, T.-C.; Huang, J.-Y.; Chang, G.-D.; Lo, C.-F.; Wang, H.-C. White Spot Syndrome Virus Induces Metabolic Changes Resembling the Warburg Effect in Shrimp Hemocytes in the Early Stage of Infection. J. Virol. 2011, 85, 12919–12928. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Nair, A.K.K.; Anandan, R.; Gopalan, P.; Nair, N.V.; Devadasan, K. Biochemical studies on changes associated with enzymes of glucose metabolism in white spot syndrome virus (WSSV) infected with Penaeus monodon (Fabricius). Afr. J. Biotechnol. 2007, 6. [Google Scholar] [CrossRef]
- Su, M.-A.; Huang, Y.-T.; Chen, I.-T.; Lee, D.-Y.; Hsieh, Y.-C.; Li, C.-Y.; Ng, T.H.; Liang, S.-Y.; Lin, S.-Y.; Huang, S.-W. An invertebrate Warburg effect: A shrimp virus achieves successful replication by altering the host metabolome via the PI3K-Akt-mTOR pathway. PLOS Pathog. 2014, 10, e1004196. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Zhang, X. Host defense against DNA virus infection in shrimp is mediated by the siRNA pathway. Eur. J. Immunol. 2013, 43, 137–146. [Google Scholar] [CrossRef]
- Li, F.; Xiang, J. Signaling pathways regulating innate immune responses in shrimp. Fish Shellfish Immunol. 2013, 34, 973–980. [Google Scholar] [CrossRef]
- Labreuche, Y.; Warr, G.W. Insights into the antiviral functions of the RNAi machinery in penaeid shrimp. Fish Shellfish Immunol. 2013, 34, 1002–1010. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.C.; Lee, J.; Choi, Y. Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 2015, 266, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Wan, D.H.; Gu, Z.H.; Deng, X.X.; Weng, S.P.; Yu, X.Q.; He, J.G. Litopenaeus vannamei tumor necrosis factor receptor-associated factor 6 (TRAF6) responds to Vibrio alginolyticus and white spot syndrome virus (WSSV) infection and activates antimicrobial peptide genes. Dev. Comp. Immunol. 2011, 35, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Huang, Y.; Wang, B.; Jian, J.; Xu, Y. Tumor necrosis factor receptor-associated factor 6 (TRAF6) participates in peroxinectin gene expression in Fenneropenaeus penicillatus. Fish Shellfish Immunol. 2017, 64, 193–201. [Google Scholar] [CrossRef]
- Wang, S.; Song, X.; Zhang, Z.; Li, H.; Lǚ, K.; Yin, B.; He, J.; Li, C. Shrimp with knockdown of LvSOCS2, a negative feedback loop regulator of JAK/STAT pathway in Litopenaeus vannamei, exhibit enhanced resistance against WSSV. Dev. Comp. Immunol. 2016, 65, 289–298. [Google Scholar] [CrossRef]
- Sun, J.-J.; Lan, J.-F.; Xu, J.-D.; Niu, G.-J.; Wang, J.-X. Suppressor of cytokine signaling 2 (SOCS2) negatively regulates the expression of antimicrobial peptides by affecting the Stat transcriptional activity in shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2016, 56, 473–482. [Google Scholar] [CrossRef]
- Shen, Z.; Jacobs-Lorena, M. Evolution of Chitin-Binding Proteins in Invertebrates. J. Mol. Evol. 1999, 48, 341–347. [Google Scholar] [CrossRef]
- Yang, F.; Li, S.; Li, F.; Xiang, J. A cuticle protein from the Pacific white shrimp Litopenaeus vannamei involved in WSSV infection. Dev. Comp. Immunol. 2018, 81, 303–311. [Google Scholar] [CrossRef]
- Wang, L.; Li, F.; Wang, B.; Xiang, J. A new shrimp peritrophin-like gene from Exopalaemon carinicauda involved in white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2013, 35, 840–846. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.; Li, S.; Xiang, J.; Li, F. A novel cuticle protein involved in WSSV infection to the Pacific white shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 2020, 102, 103491. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.D.; Barracco, M.A. Antimicrobial peptides in crustaceans. Invertebr. Surviv. 2010, 7, 262–284. [Google Scholar]
- Kido, S.; Morimoto, A.; Kim, F.; Doi, Y. Isolation of a novel protein from the outer layer of the vitelline membrane. Biochem. J. 1992, 286, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngernsoungnern, P.; Ngernsoungnern, A.; Chaiseha, Y.; Sretarugsa, P. Role of vitelline envelope during fertilization in the black tiger shrimp, Penaeus monodon. Acta Histochem. 2012, 114, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, T.; Perrino, J.; Mahowald, A.; Waring, G. Eggshell Assembly inDrosophila:Processing and Localization of Vitelline Membrane and Chorion Proteins. Dev. Biol. 1996, 177, 590–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, K.F.; Acorn, A.R.; Greenwood, S.J. A transcriptomic analysis of American lobster (Homarus americanus) immune response during infection with the bumper car parasite Anophryoides haemophila. Dev. Comp. Immunol. 2013, 40, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Back, J.F.; Bain, J.M.; Vadehra, D.; Burley, R. Proteins of the outer layer of the vitelline membrane of hen’s eggs. Biochim. et Biophys. Acta (BBA)—Protein Struct. Mol. Enzym. 1982, 705, 12–19. [Google Scholar] [CrossRef]
- Sun, Y.; Li, F.; Xiang, J. Analysis on the dynamic changes of the amount of WSSV in Chinese shrimp Fenneropenaeus chinensis during infection. Aquaculture 2013, 376-379, 124–132. [Google Scholar] [CrossRef]
- Wang, X.-W.; Wang, J.-X. Pattern recognition receptors acting in innate immune system of shrimp against pathogen infections. Fish Shellfish Immunol. 2013, 34, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Albores, F.; Yepiz-Plascencia, G. Beta glucan binding protein and its role in shrimp immune response. Aquaculture 2000, 191, 13–21. [Google Scholar] [CrossRef]
- Wang, X.-W.; Wang, J.-X. Diversity and multiple functions of lectins in shrimp immunity. Dev. Comp. Immunol. 2013, 39, 27–38. [Google Scholar] [CrossRef]
- Junkunlo, K.; Prachumwat, A.; Tangprasittipap, A.; Senapin, S.; Borwornpinyo, S.; Flegel, T.W.; Sritunyalucksana, K. A novel lectin domain-containing protein (LvCTLD) associated with response of the whiteleg shrimp Penaeus (Litopenaeus) vannamei to yellow head virus (YHV). Dev. Comp. Immunol. 2012, 37, 334–341. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Yin, Z.-X.; Xu, X.-P.; Weng, S.-P.; Rao, X.-Y.; Dai, Z.-X.; Luo, Y.-W.; Yang, G.; Li, Z.-S.; Guan, H.-J.; et al. A Novel C-Type Lectin from the Shrimp Litopenaeus vannamei Possesses Anti-White Spot Syndrome Virus Activity. J. Virol. 2009, 83, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Lage, C.; Bok Luel, L.; Kenneth, S.D.L. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends. Immunol. 2008, 29, 263–271. [Google Scholar]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Le Clec’h, W.; Anderson, T.J.C.; Chevalier, F.D. Characterization of hemolymph phenoloxidase activity in two Biomphalaria snail species and impact of Schistosoma mansoni infection. Parasites Vectors 2016, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittmer, N.T.; Kanost, M.R. Insect multicopper oxidases: Diversity, properties, and physiological roles. Insect Biochem. Mol. Biol. 2010, 40, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chan, S.; Li, C.; Zhang, S. Identification and characterization of a laccase from Litopenaeus vannamei involved in anti-bacterial host defense. Fish Shellfish Immunol. 2017, 66, 1–10. [Google Scholar] [CrossRef]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012, 7, 61–98. [Google Scholar] [CrossRef]
- Li, W.-W.; Jin, X.-K.; He, L.; Jiang, H.; Gong, Y.-N.; Xie, Y.-N.; Wang, Q. Molecular cloning, characterization, expression and activity analysis of cathepsin L in Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2010, 29, 1010–1018. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Sloane, B.F. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer. 2006, 6, 764–775. [Google Scholar] [CrossRef]
- Liaudet-Coopman, E.; Beaujouin, M.; Derocq, D.; Garcia, M.; Glondu-Lassis, M.; Laurent-Matha, V.; Prébois, C.; Rochefort, H.; Vignon, F. Cathepsin D: Newly discovered functions of a long-standing aspartic protease in cancer and apoptosis. Cancer Lett. 2006, 237, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Q.; Zhang, X.-W.; Sun, Y.-D.; Sun, S.-S.; Zhou, J.; Wang, Z.-H.; Zhao, X.-F.; Wang, J.-X. Two cysteine proteinases respond to bacterial and WSSV challenge in Chinese white shrimp Fenneropenaeus chinensis. Fish Shellfish Immunol. 2010, 29, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, L.; Zhang, F.; Wu, P.; Li, E.; Qin, J. Molecular characterization of cathepsin L cDNA and its expression during oogenesis and embryogenesis in the oriental river prawn Macrobrachium nipponense (Palaemonidae). Genet. Mol. Res 2013, 12, 5215–5225. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Jiang, S.; Huang, J.; Wang, W.; Zhang, D.; Wu, Q.; Yang, K. Molecular cloning and mRNA expression of cathepsin C gene in black tiger shrimp (Penaeus monodon). Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2008, 150, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.S.; Lin, S.T.; Wu, Z.H.; Jian, J.C. Molecular cloning and mRNA expression of cathepsin C in white shrimp, Litopenaeus vannamei. Aquac. Res. 2011, 42, 1569–1576. [Google Scholar]
- Yu, X.-M.; Chen, J.-L.; Abbas, M.N.; Gul, I.; Kausar, S.; Dai, L.-S. Characterization of the cathepsin D in Procambarus clarkii and its biological role in innate immune responses. Dev. Comp. Immunol. 2020, 111, 103766. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).