Simple Summary
In response to the conservation of threatened livestock species, different strategies to improve semen quality have been developed. However, spermatozoa remain sensitive to cryopreservation damages especially that of avian species, thus limiting the use of reproductive biotechnologies such as artificial insemination in the conservation programs. Improving semen quality through dietary inclusion of long-chain polyunsaturated fatty acids sources mainly omega n-3 has received research interest. This review explains the role of flaxseed oil as a source of omega n-3 fatty acids to improve semen quality. Comprehensive information elaborated in this review is believed to promote the use of flaxseed oil as an alternative source of omega n-3 fatty acids to fish oil. This is because fisheries are over-exploited and could collapse.
Abstract
The demand to conserve indigenous species through the cryo-gene bank is increasing. Spermatozoa remain sensitive to cryopreservation damages especially that of avian species thus limiting the use of reproductive biotechnologies such as artificial insemination in the conservation programs. Long-chain polyunsaturated fatty acid (LCPUFAs), specifically omega n-3, expanded a research interest to improve animal reproductive efficiency through improving spermatozoa quality. This is driven by the fact that mammals cannot synthesize omega-3 de-novo because they lack delta-12 and delta-15 desaturase enzymes thus supplemented in the diet is mandatory. Delta-12 and delta-15 add a double bond at the 12th and 15th carbon-carbon bond from the methyl end of fatty acids, lengthening the chain to 22 carbon molecules. Fish oil is a pioneer source of omega n-3 and n-6 fatty acids. However, there is a report that numerous fisheries are over-exploited and could collapse. Furthermore, processing techniques used for processing by-products could complement alterations of the amino acid profile and reduce protein retrieval. Alternatively, flaxseed oil contains ±52–58% of total fatty acids and lignans in the form of α-linolenic and linoleic acid. Alpha-linolenic acid (ALA,18:3n-3) is enzymatically broken-down de-novo by delta-6 desaturase and lengthened into a long-chain carbon molecule such as eicosapentaenoic acid (C20:5n-3). Nevertheless, controversial findings following the enrichment of diet with flaxseed oil have been reported. Therefore, this paper is aimed to postulate the role of flaxseed oil as an alternative source of omega n-3 and n-6 fatty acids to improve semen quality and quantity from livestock animals. These include the interaction between docosahexaenoic acid (DHA) and spermatogenesis, the interaction between docosahexaenoic acid (DHA) and testicular cells, and the effect of flaxseed oil on semen quality. It additionally assesses the antioxidants to balance the level of PUFAs in the semen.
1. Introduction
The demand to conserve indigenous species through the cryo-gene bank is increasing [1]. This is driven by the fact that livestock production could be sustained through genetic improvement of vital species together with the control of the diseases affecting their production [2]. Smallholder farmers own sheep, goats, chickens, and cattle among other species to address poverty in developing countries [3]. Through these species, rural farmers produce meat for consumption, maintain household income, produce manure, and graze marginal land not conducive for crop farming [3,4,5], thus playing a huge role in food security [6]. However, there are impediments hampering their reproduction efficiencies. Among various impediments, ±20% of males are incompatible for mating during the breeding season [7]. Moreover, another ±10 to 15% of males show a decreased reproduction efficiency or questionable fertility and semen quality [7].
Artificial insemination (AI) is an alternative tool to improve livestock genetic material and conservation [8]. This, however, requires good quality semen (+70% total motility) before freezing to survive the damaging effects imposed by the cryopreservation processes [9]. Frozen-thawed spermatozoa survival is still challenging in various species [10] making it difficult to perform AI [9,11]. Cryopreservation damages target the sperm plasma membrane making it more permeable. Thereafter, reactive oxygen species (ROS) take advantage and cause harm to the acrosome integrity and sperm motility [12,13]. Sperm cell plasma membrane is made of lipids in the form of polyunsaturated fatty acids (PUFAs) and attached by ROS because of their unconjugated double bonds separated by methylene groups [14]. This occurs due to the lower concentration of sifting enzymes and body defenses confined within the sperm cytoplasm [14]. For that reason, it is pivotal to enhance a balance between lipids, ROS, and antioxidants [15].
Molecular studies have shown the loss of proteins, lipids, and ions during cryopreservation to be a primary cause of poor post-thawed sperm quality [13]. Enriching diets with fatty acids such as omega n-3 has been shown to improve sperm motility [16], sperm membrane fluidity, and reproductive performance [17] in chickens [18], bulls [19,20,21,22], rams [23], and even in buffalos [17]. Ahmad et al. [16], reported that the dietary inclusion of PUFA’s plays a vital role in improving fertilization rate through improving sperm membrane integrity. Khoshniat et al. [22], associated the spermatozoa’s ability to resist cold shock with sperm membrane lipid composition. Hence, there has been a great interest in modifying sperm quality through long-chain polyunsaturated fatty acids (LCPUFA’s) enriched diets indifferent livestock species [10,24,25]. Nevertheless, other authors reported some controversial results following the enrichment of diets with flaxseed oil [22,26]. In their studies, some find no positive effect of feeding flaxseed oil-rich feed on semen quality and fertility. Therefore, this study is aimed to review the role of flaxseed oil as a source of polyunsaturated fatty acids to improve semen quality from livestock animals. Among various livestock animals available, this review is limited to avian, pigs, cattle, buffalo, sheep, and goats.
2. Flaxseed Oil Composition
The botanic name for flax is Linum usitatissimum, belonging to the Linaceae family [27]. It is known that alsi or linseed or flaxseed is a good source of α-linolenic and improves the tissue concentration of both α-linolenic acid and eicosapentaenoic acid, which synthesizes pivotal for reproductive hormones [28]. Flaxseed is a source of omega n-3 fatty acids containing about 52–58% of total fatty acids and lignans [29]. Moreover, flaxseed oil contains 58% α-linolenic acid, which is a vital antioxidant source that improves animal health not only reproductively [20,30] but also through improving inflammation and brain development [27]. Flaxseed oil is further comprised of stearic, oleic, linoleic, and palmitic acids, which contain a high content of vitamins including vitamin E [30]. Vitamins such as vitamin E and C (ascorbic acid) play a pivotal role as an antibiotic in the cell.
Flaxseed oil contains precursors (α-linolenic acid) of eicosapentaenoic (EPA) and docosahexaenoic (DHA), which are broken down de novo to form long-chain PUFAs [30] and later converted to docosahexaenoic acid which is vital for testes’ functioning [18,31] (Figure 1). In human beings, flaxseed oil protects the body against cardiovascular disease, inhibits pro-inflammatory mediators, reduces low-density lipoprotein (LDL) cholesterol, plays a significant role in bone health, and reduces hormone-related cancers [32]. Furthermore, flaxseed oil increases the level of α-linolenic and eicosapentaenoic acid that are important in the synthesis of reproductive hormones [27]. Essential α-linolenic acid (ALA, 18:3n-3) is enzymatically broken down de novo by delta-6-desaturase and lengthened into long-chains such as eicosapentaenoic acid (C20:5n-3) [30], stearidonic acid (SDA) eicosapentaenoic acid (EPA, 20:5n-3), and converted into docosapentaenoic acid then to docosahexaenoic acid (DHA, 22:6n-3) [31]. In the n-6 family, essential linolenic acid (LA, 18:2n-6) is altered into long-chain arachidonic acid (AA, 20:4n-6) [31].
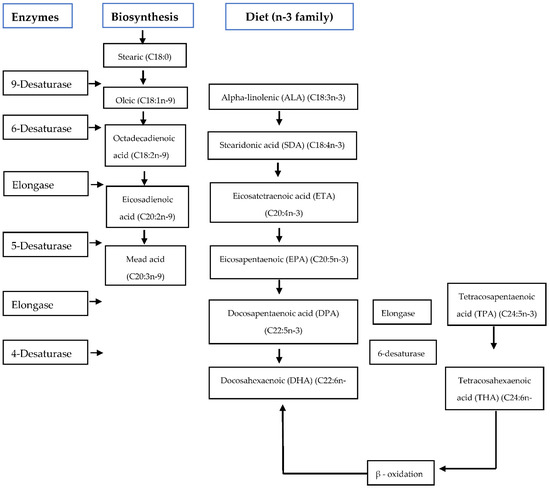
Figure 1.
De-novo biosynthesis and the transformation of the omega n-3 family PUFA’s by desaturation and elongation [33,34].
3. Classifications and General Functions of Long-Chain Polyunsaturated Fatty Acids
Long-chain fatty acids can be distinguished into three groups: n-3, n-6, and n-9, depending on the location of the first double bond [30]. Most n-9 fatty acids (FA) are mono-unsaturated fatty acids including oleic acid. Polyunsaturated fatty acids involve n-3 and n-6, which are distinguished with more than one double bond between their carbon atoms [35,36]. In cooperation, n-3 and n-6 are classified as essential fatty acids because mammals cannot synthesize them de-novo.
Generally, PUFA’s have several benefits in the body and in the reproductive cells. These benefits range from maintaining the structure and functions of the cell membrane, enhancing immune function, promoting growth and development, regulating lipid metabolism and related gene expression, and in the reduction of thrombosis [17]. Omega-3 fatty acids play a significant role in human health through immune system responses [37,38,39], controlling chronic diseases such as cardiovascular diseases [40] and cancer [41], act as bioactive cellular components of membrane phospholipids [35,40,42]. Therefore, supplementing PUFAs to animals’ diet would also be beneficial to human health because omega-3 FA fed to the animal might later be found in their meat or milk.
PUFAs can be retrieved from all-natural foods but vary in quantities, with fatty foods and some seafood containing extra. Vegetable oils such as sunflower oil, safflower oil, corn oil, flax oil, sesame seed oil, pumpkin seed oil, and rapeseed oil are also sources of PUFAs. Moreover, they are also found in marine fish such as mackerel, salmon, sardine, herring, and smelt. However, there are alarming studies in some countries like Indonesia where serious fish over-exploitation is reported [43]. Furthermore, processing techniques used for processing by-products could complement alterations of the amino acid profile reduce protein recovery [44]. Therefore, diverting to plant-based sources will be advantageous should a similar quality be maintained.
4. Interaction between Docosahexaenoic Acid (DHA) and Spermatogenesis
Docosahexaenoic acid (DHA, C22: 6n-3) is a long-chain omega n-3 and widespread unsaturated fatty acid found in mammalian cells [45]. It is synthesized from its precursor’s alpha-linolenic and linoleic acid with the aid of delta-5 and delta-6-desaturase enzymes [46]. Docosahexaenoic acid accounts for 10% of the brain phospholipids and has a high content in the central nervous system, distributed in acetylcholine, amino phospholipids, and serine glycerol [47]. If DHA concentration in the spermatozoa is lower than that of normal level is associated with infertility [30,48,49]. Docosahexaenoic acids are major elements for human and ruminants’ spermatozoa phospholipids [50]. DHA makes up 30% of esterified fatty acids in phospholipids and 73% of all PUFAs [7]. Therefore, neurons, photoreceptors, and spermatozoa are the cells richest in DHA content [47].
Docosahexaenoic acid has numerous functions such as the role in brain cell function to improve communication between the brain cells. Thus, the lack of n-3 PUFAs in the body can cause a brain communication breakdown [51]. It also appears that DHA influences the membrane structure of the sperm cells by providing essential sperm membrane fluidity and participates in cell response mediation in protein [47]. The DHA concentration in the testicles supports the role of astrocytes, retinal pigment epithelial cells, and Sertoli cells [52]. This could in turn affect the production of lipid-mediated conductors, cell signal transduction, and gene expression. Furthermore, DHA is known to be immunomodulatory, which can affect both innate and adaptive immunity and be used in developing treatments for inflammatory diseases such as rheumatoid arthritis, psoriasis, and ulcerative colitis [41]. Testicular cells especially the Sertoli cells and spermatozoa, are rich in polyunsaturated fatty acids and a fatty acid-enriched diet can control fatty acids profiles in the reproductive tissue [50]. Spermatogenesis involves a sequence of proliferative phases, differentiation, cell divisions to mitotic, meiotic, and spermatogenic stages [53].
Different stages of spermatogenesis include spermatogonia, spermatocytes, and spermatids. Sertoli cells regulate spermatogenesis through playing a vital role in the endocrine and paracrine and providing seminiferous tubules (that manufacture spermatozoon) with nutrition, transporting mature spermatids to the seminiferous tubules’ lumen, discharging androgen-binding protein, and cooperate with Leydig cells for sperm production [53]. During these spermatogenesis stages, lipid droplets occur throughout the process [54], suggesting a very close link between lipid metabolism and fertility during spermatogenesis [53].
Docosahexaenoic acid influences spermatogenesis and sperm quality (Table 1). It is believed that 99% of DHA is found in the tail of the sperm cell [47]. This suggests that DHA in the sperm tail is associated with fluidity and flexibility as well as movements or sperm motility. However, other authors of studies in bulls and humans suggested that a high concentration of DHA is rather found in the sperm head than the tail [55,56]. This suggests the role of DHA in nuclear transfer and fusion with zona-pellucida.

Table 1.
Influence of dietary supplementation with PUFAs sourced from flaxseed oil in livestock animals’ reproduction.
5. Interaction between Docosahexaenoic Acid (DHA) and Testicular Cells
Testicular cells, particularly Sertoli cells, are active in the conversion of 18 and 20 carbon omega n-3 into 22 carbon omega n-3 PUFAs [68] because testes have high levels of desaturase mRNA and elongate enzyme. However, higher PUFAs have been reported in germ cells [69]. Germ cells are higher in PUFAs than Sertoli cells but Sertoli cells can convert essential fatty acids (LA and ALA) to derivatives DPA and DHA more than germ cells, hence there are higher 5 and 6 desaturase enzymes in Sertoli cells than germ cells [52]. Testicles have high capacities for the conversion of C20:5n-3 to long C22:6n-3 due to an active elongation and desaturase enzymes in the Sertoli cells [68]. These make testicles an extraordinary organ for PUFA (LA and ALA to derivatives ARA, EPA, DPA, and DHA) metabolism like that of the liver organ [52]. However, when spermatozoa transit to the epididymis for storage, caudal PUFAs are continuously drained from the testicles. Moreover, unsaturated fatty acids sometimes are altered by hormones such as luteinizing hormones (LH) and or adrenocorticotropic hormone (ACTH) in the testis by altering the functions of the enzymes [52].
6. Comparison between the Flaxseed and the Fish Oil to Improve Fresh Semen Quality of Livestock Animals
Fish oil is a common source of omega n-3, specifically DHA and EPA [50,68]. Omega n-3 sourced from fish oil has been found to improve semen from rams [10,69], roosters [70,71], and bucks [72,73] through improving total motility [72,74,75,76]. Nevertheless, numerous problems associated with feeding fish by-products have been articulated [77], leading to an interest in replacing fish by-products with plant-based by-products [78]. Therefore, plant base by-products such as flaxseed oil is essential as an alternative to fish by-products in providing necessary omega n-3 fatty acid.
The effects of flaxseed oil on semen quality have been investigated (Table 1) in rabbits [33], avian [18,58], and cattle [22,79,80]. Furthermore, flaxseed oil modulated semen production, quality, quantity, freezability, testicular biometrics, and endocrinological profiles in Mithun bulls [20]. However, the suitable quantity of flaxseed oil necessary for each species is still controversial [18,58,79,80,81]. Noteworthy, depending on the species, sperm motility decreased with the increase of flaxseed oil percentage in the diet (Table 2).

Table 2.
Comparison between the flaxseed and the fish oil to improve fresh semen quality of livestock animals.
7. Flaxseed Oil Alteration between Monogastric and Ruminant Livestock
Ruminants have low fatty acids intake ranging between 2.5–3.5% [Table 2]. Based on the information above (Table 2), avian needs only 2% of flaxseed oil which is the same as that of fish oil to improve semen quality. This was evident when 2 and 4% inclusion of flaxseed was used and was concluded that semen was improved in the 2% group than in the 4% treatment group [71]. This supports a hypothesis that high flaxseed oil is detrimental or has no effect on semen quality especially when no natural antioxidants are used to cause an imbalance, suggesting a necessity to add vitamin E or C on the diet [10]. In pigs, low (3%) flaxseed oil is necessary to improve semen quality and fertility [81]. However, there is still a lack of information with regards to pigs when flaxseed oil is reduced to below or higher than 3% and when no antioxidants are used. In large ruminants (cattle, buffalo), flaxseed oil seems to bypass rumen when dosed [83].
8. Role of Dietary Inclusion of Omega n-3 on the Post-Thawed Sperm Quality
Cryopreserving semen is essential, particularly because it facilitates the global trade of semen and enables the long-term storage of sperm from superior sires [84,85]. Furthermore, it subsidizes the enlargement of assisted reproductive techniques such as AI and in-vitro fertilization [86] and is useful in difficulties such as extinction, infertility, and injury [87]. However, sperm cells succumb easily to cryopreservation, with subsequent irreversible motility loss [88]. Irreversible motility loss caused by the lipid peroxidation and deoxyribonucleic acid (DNA) damage leading to the less fertilizing ability of the sperm cells [87].
Benefits to supplement dietary fish oil on semen quality of livestock animals through improving sperm plasma membrane of post cryopreservation has been reported [10,80,88]. There was improvement on post-thawed sperm motility in chicken [89], bulls [90], and bucks [87] following supplementation with flaxseed oil. Nevertheless, flaxseed oil-treated bulls produced high total and progressive motility in frozen-thawed semen despite the high content of DHA in fish oil [30]. Moreover, flaxseed supplementation led to 47.8% total post-thawed goat sperm motility [66].
9. Role of Antioxidant against PUFAs’ Vulnerability to Oxidation
Testicles contain reproductive tissues and cells that are involved in spermatogenesis with a clear role in long-chain polyunsaturated fatty acids (LCPUFAs) production [91]. Nevertheless, testicles are considered oxidation-sensitive organs due to higher PUFAs [92]. It is well known that PUFAs are susceptible to oxidation via free radicals [92] due to their double bonds between the carboxyl chain [93]. Although seminal plasma contains reactive oxygen species (ROS) scavengers, dietary inclusion of PUFAs cause an imbalance of the antioxidant. Therefore, the inclusion of antioxidants is necessary [94]. See Figure 2 which illustrates the process of PUFA supplementation. There is still not enough information on the effect of antioxidants to defend spermatozoa from oxidative stress when flaxseed oil is used as a source of PUFAs. However, on the fresh, the effectiveness of antioxidants seems to be species-specific when supplemented with flaxseed oil (Table 3). In short, when flaxseed oil is increased with no antioxidants, semen quality decreases in avian [18]. Nevertheless, no effect of increasing flaxseed oil concentration in Buffalo bulls [79] sheep [84], and cattle [78] even without an antioxidant. As previously mentioned, flaxseed has some antioxidants in itself scavenging ROS [30]. Nevertheless, more studies evaluating the role of antioxidants in sperm quality when supplemented with flaxseed oil in livestock are still needed.
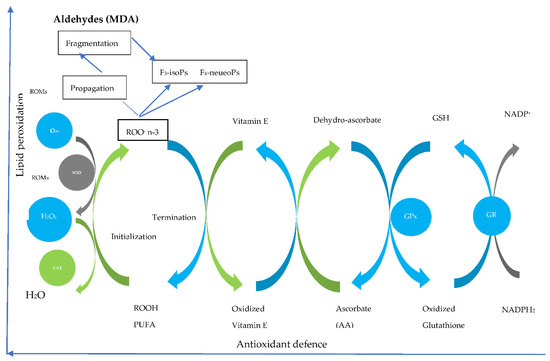
Figure 2.
Steps of the omega n-3 oxidative chain and antioxidant interactions [92]. MDA-malondialdehyde, ROMs-reactive oxygen species, GSH-glutathione, GPX-glutathione peroxidase, GR-glutathione reductase, F3isoPs-Isoprostanes, F4-NeuroPs-F4-Neuroprostanes, SOD-superoxide dismutase, CAT-catalase.

Table 3.
The role of antioxidant against PUFAs’ vulnerability to oxidation.
Antioxidants are in the form of enzymes that absorb hypothetically lethal ROS such as hydrogen peroxide (H2O2), superoxide anion, and small molecular mass scavengers that can terminate free-radical-mediated chain reactions [95]. The enzyme antioxidants involved in H2O2 metabolism are the glutathione peroxidase system and/or catalase. Those involved in superoxide anion include superoxide dismutase and/or sometimes indoleamine dioxygenase. Moreover, vitamin C, E, and a variety of polyphenols are classified as small molecular mass scavengers that aid in terminating free radical-mediated chain reactions [95]. Their function is to protect ejaculated spermatozoa from the notorious effects of ROS [96].
10. Conclusions
Long-chain polyunsaturated fatty acids (LCPUFAs) are of current prodigious interest in livestock species to improve semen quality. This review described that dietary inclusion of flaxseed oil improves semen quality in livestock animals. Flaxseed oil provides adequate alpha-linolenic as a precursor to omega n-3 through desaturase enzymes, to form long-chain polyunsaturated fatty acids, and hence can be a good alternative to fish oil. This may further address the issues related to fish sustainability and the contamination with meat and bone products during the manufacturing processes. Nevertheless, most of the data has been tested using in-vitro spermatozoa quality, hence more work is needed to test in-vivo fertility e.g., lambing, calving, and the farrowing rate following feeding with flaxseed oil. Conversely, the flaxseed oil quantity necessary for superior results differs in each species. Therefore, it is suggested that more studies are conducted to evaluate the different concentrations of flaxseed oil and the role of antioxidants in each species. This will make a comprehensive conclusion on whether LCPUFAs improve livestock production or not which may be helpful particularly for conservation purposes and disseminating semen from superior sires globally through cryopreservation and artificial insemination.
Author Contributions
Conceptualization, J.N.N., F.V.R. and T.L.N.; methodology, not applicable software, not applicable; writing—original draft preparation, J.N.N., writing—review and editing, J.N.N., F.V.R., K.A.N., T.J.M., T.C.C. and T.L.N.; supervision, F.V.R. and T.L.N. All authors have read and agreed to the published version of the manuscript.
Funding
Department of Agriculture, Land Reform and Rural Development (DALRRD)-Farm Animal Genetic Resource (FAnGR) project number 21.1.1/18/GR–02/API and Agricultural Research Council project number P02000172.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were collected or analyzed in this study.
Acknowledgments
Department of Agriculture, Land Reform and Rural Development (DALRRD)-Farm Animal Genetic Resource (FAnGR), Tshwane University of Technology (TUT), Agricultural Research Council (ARC) and Agriculture Sector Education Training Authority (AgriSETA) are acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Department of Agriculture, Forestry and Fisheries, South Africa. National Plan for Conservation and Sustainable Use of Farm Animal Genetic Resources; Department of Agriculture, Forestry and Fisheries, South Africa: Pretoria, South Africa, 2016.
- Ngcobo, J.N.; Nephawe, K.A.; Maqhashu, A.; Nedambale, T.L. Seasonal variation in semen parameters of Zulu rams preserved at 10 °C for 72 h during breeding and non-breeding season. Am. J. Anim. Vet. Sci. 2020, 15, 226–239. [Google Scholar] [CrossRef]
- Mavule, B.S.; Muchenje, V.; Kunene, N.W. Characterization of Zulu sheep production system: Implications for conservation and improvement. Acad. J. 2011, 8, 1226–1238. [Google Scholar]
- Hasani, N.; Ebrahim, M.; Ghasemi-Panahi, B.; Khani, A.H. Evaluating reproductive performance of three estrus synchronization protocols in Ghezel ewes. Theriogenology 2018, 122, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Molotsi, A.H.; Dube, B.; Cloete, S.W.P. The current status of indigenous ovine genetic resources in Southern Africa and future sustainable utilization to improve livelihoods. Divers 2020, 12, 1–16. [Google Scholar]
- Tsioumani, E. The state of the world’s biodiversity for food and agriculture: A call to action? Environ. Policy Law 2019, 49, 2–3. [Google Scholar] [CrossRef]
- Aké-Villanueva, J.R.; Aké-López, J.R.; Magaña-Monforte, J.G.; Segura-Correa, J.C. Reproductive behaviour in hair sheep rams under tropical conditions. Trop. Anim. Health Pro. 2019, 51, 1627–1635. [Google Scholar] [CrossRef]
- Palacín, I.; Yániz, J.L.; Fantova, E.; Blasco, M.E.; Quintín-Casorrán, F.J.; Sevilla-Mur, E.; Santolaria, P. Factors affecting fertility after cervical insemination with cooled semen in meat sheep. Anim. Reprod. Sci. 2012, 132, 139–144. [Google Scholar] [CrossRef]
- Salamon, S.; Maxwell, W.M.C. Storage of ram semen. Anim. Reprod. Sci. 2000, 62, 77–111. [Google Scholar] [CrossRef]
- Díaz, R.; Torres, M.A.; Paz, E.; Quiñones, J.; Bravo, S.; Farías, J.G.; Sepúlveda, N. Dietary inclusion of fish oil changes the semen lipid composition but does not improve the post-thaw semen quality of ram. Anim. Reprod. Sci. 2017, 183, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm cryodamage in ruminants: Understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Nur, Z.; Zik, B.; Ustuner, B.; Sagirkaya, H.; Ozguden, C.G. Effects of different cryoprotective agents on ram sperm morphology and DNA integrity. Theriogenology 2010, 73, 1267–1275. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Cesari, A.; Garde, J.J.; Villar, M.; Soler, A.J. Cryopreservation of ram sperm alters the dynamic changes associated with in vitro capacitation. Theriogenology 2020, 145, 100–108. [Google Scholar] [CrossRef]
- Solomon, M.C.; Cho, C.-L.; Henkel, R.R. Basic Aspects of Oxidative Stress in Male Reproductive Health; Academic Press: Amsterdam, The Netherlands, 2019; pp. 27–36. [Google Scholar]
- Fair, S.; Doyle, D.N.; Diskin, M.G.; Hennessy, A.A.; Kenny, D.A. The effect of dietary n-3 polyunsaturated fatty acids supplementation of rams on semen quality and subsequent quality of liquid stored semen. Theriogenology 2014, 81, 210–219. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, S.; Abbas, A.; Abbas, R.Z.; Zubair, M.; Raza, M.A.; Bano, N.; Shafi, M.U.; Badar, S.N.; Hussain, K.; et al. Effects of dietary supplementation of linseed oil (Omega-3) on quality parameters of Nili Ravi bull spermatozoa. Livest. Sci. 2019, 224, 57–59. [Google Scholar] [CrossRef]
- Tran, L.; Malla, B.A.; Kumar, S.; Tyagi, A.K. Polyunsaturated fatty acids in male ruminant reproduction—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Zanussi, H.P.; Shariatmadari, F.; Sharafi, M.; Ahmadi, H. Dietary supplementation with flaxseed oil as source of omega-3 fatty acids improves seminal quality and reproduction performance in aged broiler breeder roosters. Theriogenology 2019, 130, 41–48. [Google Scholar] [CrossRef]
- Gürler, H.; Calisici, O.; Calisici, D.; Ballwein, H. Effect of feeding omega-3 fatty acids on fatty acid composition and quality of bovine sperm and on antioxidant capacity of bovine seminal plasma. Anim. Reprod. Sci. 2015, 160, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.; Chang, S.; Khate, K.; Vupru, K.; Bag, S. Flaxseed oil modulates semen production and its quality profiles, freezability, testicular biometrics and endocrinology profiles in mithun. Theriogenology 2019, 136, 47–59. [Google Scholar] [CrossRef]
- Khoshvaght, A.; Townhidi, A.; Zare-Shahneh, A.; Noruozi, M.; Zhandi, M.; Davachi, N.D.; Karimi, R. Dietary n-3 PUFAs improve fresh and post-thawed semen quality in Holstein bulls via alteration of sperm fatty acid composition. Theriogenology 2016, 85, 807–812. [Google Scholar] [CrossRef]
- Khoshniat, M.T.; Towhidi, A.; Rezayazdi, K.; Zhandi, M.; Rostan, F.; Davachi, N.D.; Khalooee, F.; Kastelic, J. Dietary omega-3 acids from linseed oil improve quality of post-thaw but not fresh sperm in Holstein bulls. Cryobiology 2020, 93, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, R.; Sharafi, M.; Shahneh, A.Z.; Towhidi, A.; Kohram, H.; Zhandi, M.; Esmaeili, V.; Shahverdi, A. Effect of dietary fish oil supplementation on ram semen freeze ability and fertility using soybean lecithin—And egg yolk-based extenders. Theriogenology 2016, 86, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaifah, H.; Al-Nasser, A.; Givens, D.I.; Ryner, C.; Yaqoob, P. Comparison of different dietary sources of n-3 polyunsaturated fatty acids on immune response in broiler chickens. Heliyon 2020, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Mattioli, S.; Signorini, C.; Cotozzolo, E.; Noto, D.; Moretti, E.; Brecchia, G.; Dal Bosco, A.; Belmonte, G.; Durand, T.; et al. Effect of dietary n-3 source on rabbit male reproduction. Oxid. Med. Cell. Longev. 2020, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.; Holden, S.A.; Lyons, A.; Souza, J.C.; Fair, S. In-vitro addition of docosahexaenoic acid improves the quality of cooled but not frozen-thawed stallion semen. Reprod. Fertil. Dev. 2017, 29, 2021–2027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, H.K.; Qureshi, M.S.; Khan, I.; Rehman, S.; Mehsud, T.; Ullah, I.; Aftab, M.; Rehman, F. Dietary flaxseed oil supplementation effect on bovine semen quality parameters. Vet. J. 2015, 3, 9–13. [Google Scholar]
- Petit, H.V.; Germiquet, C.; Lebel, D. Effect of feeding whole, unprocessed sunflower seeds and flaxseed on milk production, milk composition and prostaglandins secretion in dairy cows. J. Dairy Sci. 2004, 87, 3889–3898. [Google Scholar] [CrossRef]
- Oomar, D.B. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar]
- Moallen, U. Roles of dietary n-3 fatty acids in performance, milk fat composition, and immune system in dairy cattle. J. Dairy Sci. 2018, 101, 8641–8661. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine omega-3 (N-3) fatty acids for cardiovascular health: And update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef]
- Nelson, J.R.; Raskin, S. The eicosapentaenoic acid: Arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgraduate Med. 2019, 4, 268–277. [Google Scholar] [CrossRef]
- Mourvaki, E.; Cardinali, R.; Bosco, A.D.; Corazzi, L.; Castellini, C. Effects of flaxseed dietary supplementation on sperm quality and on lipids composition of sperm subfractions and prostatic granules in rabbit. Theriogenology 2010, 73, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ration in cardiovascular disease and other chronic diseases. SEBM 2009, 233, 674–688. [Google Scholar]
- Raes, K.; De Smet, S.; Demeyer, D. Effect of dietary fatty acids on incorporation of long chain polyunsaturated fatty acids and conjugated linoleic acid in lamb, beef and pork meat: A review. Anim. Feed Sci. Technol. 2004, 113, 199–221. [Google Scholar] [CrossRef]
- Lee, H.; Park, W.J. Unsaturated fatty acids, desaturase, and human health. J. Med. Food 2014, 17, 189–197. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Hogervorst, J.H.C.; Garssen, J.; Wichers, H.J.; Willemsen, E.M. Long Chain Polyunsaturated Fatty Acids (LCPUFAs) in the prevention of food allergy. Front Immunol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Ruiz-López, N.; Sayanova, O.; Napier, J.A.; Haslam, R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthesis pathway into transgenic plants. J. Exp. Bot. 2012, 63, 2397–2410. [Google Scholar] [CrossRef]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Superko, H.R.; Superko, A.R.; Lundberg, G.P.; Margolis, B.; Carrett, B.C.; Nasir, K.; Agatston, A.S. Omega-3 fatty acid blood levels clinical significance update. Curr. Cardiovasc. Risk Rep. 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Fasano, E.; Serini, S.; Cittadini, A.; Calviello, G. Long-chain n-3 PUFA against breast and prostate cancer: Which are the appropriate doses for intervention studies in animals and humans? Crit Rev. Food Sci. Nutr. 2017, 57, 2245–2262. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, S.D.; Brenna, J.T. Desaturase and elongase limiting endogenous long chain polyunsaturated fatty acids biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103–110. [Google Scholar] [CrossRef]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef]
- Warren, C.; Steenbergen, D.J. Fisheries decline, local livelihoods and conflicted governance: An Indonesian case. Ocean Coast Manag. 2021, 202, 1–13. [Google Scholar] [CrossRef]
- Gasco, L.; Acuti, G.; Bani, P.; Zotte, A.D.; Danieli, P.P.; Angelis, A.; Fortina, R.; Marino, R.; Oarisi, G.; Piccolo, G.; et al. Insect and fish by-product as sustainable alternatives to conventional animal protein in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef]
- Zhu, G.; Jiang, X.; Qu, Q.; Zhang, T.; Wang, M.; Sun, G.; Wang, Z.; Sun, J.; Ge, T. Enhanced production of docosahexanoic acid in mammalian cells. PLoS ONE 2014, 9, e96503. [Google Scholar]
- Nandi, A.; Wadhwani, N.; Joshi, S.R. Vitamin D deficiency influences fatty Acid metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2019, 140, 57–63. [Google Scholar] [CrossRef]
- Sarkadi-Nagy, E.; Wijendran, V.; Diau, G.Y.; Chao, A.C.; Hsieh, A.T.; Turpeinen, A.; Lawrence, P.; Nathanielsz, P.W.; Brenna, J.T. Formula feeding potentiates docosahexaenoic and arachidonic acid biosynthesis in term and preterm baboon neonates. J. Lipid Res. 2004, 45, 71–80. [Google Scholar] [CrossRef]
- Aksoy, Y.; Aksoy, H.; Altinkaynak, K.; Aydin, H.R.; Özkan, A. Sperm fatty acid composition in subfertile men. Prostaglandins Leukot. Essent. 2006, 75, 75–79. [Google Scholar] [CrossRef]
- Martínez-Soto, J.C.; Domingo, J.C.; Cordobilla, B.; Nicolás, M.; Fernández, L.; Albero, P.; Gadea, J.; Landeras, J. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidants status and decreases sperm DNA fragmentation. Syst. Biol. Reprod. Med. 2016, 62, 387–395. [Google Scholar] [CrossRef]
- Alizadeh, A.; Esmaeili, V.; Shahverdi, A.; Rashidi, L. Dietary fish oil can change parameters and fatty acids profiles of ram sperm during oil consumption period and after removal of oil source. Cell J. 2014, 16, 289–298. [Google Scholar] [PubMed]
- Saether, T.; Tran, T.N.; Rootwelt, H.; Grav, H.J.; Christophersen, B.O.; Haugen, T.B. Essential fatty acid deficiency induces fatty acid desaturase expression in rat epididymis, but not in testis. Reproduction 2007, 133, 467–477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Collodel, G.; Castellini, C.; Lee, J.C.; Signorini, C. Relevance of fatty acids to sperm maturation and quality. Oxid. Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Gadella, B.M. Sperm surface changes and physiological consequences induced by sperm handling and storage. Soc. Reprod Fertil. 2011, 142, 759–778. [Google Scholar] [CrossRef]
- Argov-Argman, N.; Mahgrefthe, K.; Zeron, Y.; Roth, Z. Season-induced variation in lipid composition is associated with semen quality in Holstein bulls. Reproduction 2013, 145, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Argov-Argman, N.; Mahgrefthe, K.; Zeron, Y.; Roth, Z. Variation in lipids profiles within semen compartment-the bovine model of aging. Theriogenology 2013, 80, 712–721. [Google Scholar] [CrossRef]
- Rotterstøl, K.; Haugen, T.B.; Tran, T.N.; Christophersen, O. Studies on the metabolism of essential fatty acids in isolated human testicular cells. Reproduction 2001, 121, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, B.; Sharifi, S.D.; Ghazanfari, S.; Mohammadi-Sangcheshmeh, A.; Honaebakhsh, S. Effect of dietary supplementation of whole flaxseed on sperm traits and sperm fatty acid profile in aged broiler breeder. Reprod. Domest. Anim. 2020, 55, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; El-Sayed, R.; Khater, S.I.; Said, E.N.; El-Mandrawy, S.A.M. Changing dietary n-6: N-3 ratio using different oil sources affects performance, behaviour, cytokines mRNA expression and meat fatty acid profile of broiler chickens. Anim. Nutr. 2018, 4, 44–51. [Google Scholar] [CrossRef]
- Shahid, M.S.; Wu, Y.; Xiao, Z.; Raza, T.; Dong, X.; Yuan, J. Duration of the flaxseed diet promotes deposition of n-3 fatty acids in the meat and skin of Peking ducks. J. Food Nutr. Res. 2019, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, K.E.; Hofmo, P.O.; Tverdal, A.; Miller, R.R. Within and between breed differences in freezing tolerance plasma membrane fatty acids composition of boar sperm. Soc. Reprod. Fertil. 2006, 131, 887–894. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, X.; Mao, J.; Wu, D.; Ren, B.; Xu, S.; Fang, Z.; Che, L.; Wu, C.; Li, J. Effects of different dietary n-6/n-3 polyunsaturated fatty acids ratios on boar reproduction. Lipids Health Dis. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Nazir, G.; Ghuman, S.P.S.; Singh, J.; Honparkhe, M.; Ahuja, C.S.; Dhaliwal, G.S.; Sangha, M.K.; Saijpaul, S.; Agarwal, S.K. Improvement of conception rate in postpartum flaxseed supplemented buffalo with Ovsynch + CIDR protocol. Anim. Reprod. 2013, 137, 15–22. [Google Scholar] [CrossRef]
- Gholami, H.; Chamani, M.; Towhidi, A.; Fazeli, M.H. Effect of feeding a docosahexaenoic acid-enriched nutriceutical on the quality of fresh and frozen-thawed semen in Holstein bulls. Theriogenology 2010, 74, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Moallem, U.; Neta, N.; Zeron, Y.; Zachut, M.; Roth, Z. Dietary α-linolenic acid from flaxseed oil or eicosapentaenoic and docosahexaenoic acids from fish oil differentially alter fatty acid composition and characteritics of fresh and frozen-thawed bull semen. Theriogenology 2015, 83, 1110–1120. [Google Scholar] [CrossRef]
- Kargar, R.; Forouzanfar, M.; Ghalamkari, G.; Esfahani, M.H.N. Dietary flaxseed oil and/or vitamin E improve sperm parameters of cloned goats following freezing-thawing. Cryobiology 2017, 74, 110–114. [Google Scholar] [CrossRef]
- Souza, R.S.; Barbosa, L.P.; Aguiar, C.S.; Vieira, R.L.A.; Ribeiro, M.O.; Araújo, R.C.S.A.; Silva, M.A.A.; Santana, A.L.A. Cryopreservation of semen from goats fed diet supplemented with flaxseed. Rev. Bras. Saude Prod. Anim. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Samadian, F.; Towhidi, A.; Rezayadi, K.; Bahreini, M. Effects of dietary n-3 fatty acids on characteristics and lipid composition of ovine sperm. Animals 2010, 4, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Zamir, M.J.; Akhlaghi, A.; Shahvedi, A.H.; Alizadeh, A.R.; Jaafarzedah, M.R. Effect of dietary fish oil with or without vitamin E supplementation on fresh and cryopreserved ovine sperm. Anim. Prod. Sci. 2017, 57, 441–447. [Google Scholar] [CrossRef]
- Asl, R.S.; Shariatmadari, F.; Sharafi, M.; Torshizi, M.A.K.; Shahverdi, A. Dietary fish oil supplemented with vitamin E improves quality indicators of rooster cold-stored semen through reducing lipid peroxidation. Cryobiology 2018, 84, 15–19. [Google Scholar]
- Qi, X.; Shang, M.; Chen, C.; Chen, Y.; Hua, J.; Sheng, X.; Wang, X.; Xing, K.; Ni, H.; Guo, Y. Dietary supplementation with linseed oil improves semen quality, reproductive hormone, gene and protein expression related to testosterone synthesis in aging layer breeder rooster. Theriogenology 2019, 131, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mahla, A.S.; Chaudhari, R.K.; Verma, A.K.; Singh, A.K.; Singh, S.K.; Singh, G.; Sarkar, M.; Dutta, N.; Kumar, H.; Krishnaswamy, N. Effect od dietary supplementation of omega-3 polyunsaturated fatty acid (PUFA) rich fish oil on reproductive perfomance of goat (Capra hircus). Theriogenology 2017, 99, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Dolatpanah, M.B.; Towhidi, A.; Farshad, A.; Rashidi, A.; Rezayadzi, A. Effects of dietary fish oil on semen quality of goats. Asian-Aust. J. Anim. Sci. 2008, 21, 29–34. [Google Scholar]
- Ran, Z.; Xu, J.; Liao, K.; Monroig, Ó.; Navarro, J.C.; Oboh, A.; Jin, M.; Zhou, Q.; Zhou, C.; Tocher, D.R.; et al. Biosynthesis of long-chain polyunsaturated fatty acids in the razor clam Sinonocula constricta: Characterization of four fatty acyl elongases and a novel desaturase capacity. BBA-Mol Cell Biol. L. 2019, 1864, 1083–1090. [Google Scholar]
- Kumar, D.; Bhatt, R.S.; Balagnur, K.; De, K.; Mahla, A.S.; Sahoo, A. Milk replacer and linseed supplementation promotes puberty and semen quality in growing male lambs. Small Rumin. Res. 2021, 202, 1–6. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Nhiem, D.V.; Berg, J.; Kjos, N.P. Effects of replacing fish meal with soy cake in a diet based on urea-treated rice straws on performance of growing Laisind beef cattle. Trop. Anim. Prod. 2013, 45, 907–909. [Google Scholar]
- EI-Hamd, M.A.A.; Metwally, A.S.M.; Hegazy, M.M.; Ghallab, Z.R.; Elateeqy, O.A. Effect of supplementation of omega-3 fatty acids on blood parameters and semen quality of Friesian bulls. Slov. Vet. Res. 2019, 56, 765–772. [Google Scholar]
- Shah, S.M.H.; Ali, S.; Zubair, M.; Jamil, H.; Ahmad, N. Effect of supplementation of feed with flaxseed (Linumusitatisimum) oil on libido and semen quality of Nilli-Ravi buffalo bulls. J. Anim. Sci. Technol. 2016, 58, 1–6. [Google Scholar] [CrossRef]
- Gandeshmini, A.P.; Sharafi, M.; Alizadeh, A. Enhancement of rooster semen freezing ability with the use of dietary sources of omega-3 and omega-6 fatty acids. Anim. Feed Sci. Technol. 2020, 268, 1–9. [Google Scholar] [CrossRef]
- Singh, M.; Mollier, R.T.; Sharma, P.R.; Kadirvel, G.; Doley, S.; Sanjukta, R.K.; Rajkhowa, D.J.; Kandpal, B.K.; Kumar, D.; Khan, M.H.; et al. Dietary flaxseed oil improve boar semen quality, antioxidant status and in-vitro fertility in humid sub-tropical region of North East India. Theriogenology 2021, 159, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Weng, X.X.; Yuan, L.F.; Li, F.; Yue, X.P.; Li, F.D. Effect of feeding linseed diet on testis development, antioxidant capacity, and epididymal cauda sperm concentration in Chinese Hu lamb. Theriogenology 2021, 159, 69. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.L. Omega-3 fatty acids in metabolism, health, and nutrition and for modified animal product foods. Prof. Anim. Sci. 2009, 25, 207–249. [Google Scholar] [CrossRef]
- Peña, F.J.; García, B.M.; Samper, J.C.; Aparicio, I.M.; Tapia, J.A.; Ferrulosa, C.O. Dissecting the molecular damage to stallion spermatozoa: The way to improve current cryopreservation protocols? Theriogenology 2011, 76, 1177–1186. [Google Scholar] [CrossRef]
- Medeiros, C.M.O.; Forrell, F.; Oliveira, A.T.D.; Rodrigues, J.L. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- Andrabi, S.M.H.; Maxwell, W.M.C. A review on reproductive biotechnologies for conservation of endangered mammalian species. Anim. Reprod. Sci. 2007, 99, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.; Chamuah, J.K.; Rajkhowa, C. Effect of catalase on the liquid storage of mithun (Bos frontalis) semen. Asian Pac. J. Reprod. 2013, 2, 209–214. [Google Scholar] [CrossRef]
- Gangwar, C.; Kharche, S.D.; Ranjan, R.; Kumar, S.; Goel, A.K.; Jindal, S.K.; Agarwal, S.K. Effect of vitamin C supplementation of freezability of Barbari buck semen. Small Ruminant Res. 2015, 129, 104–107. [Google Scholar] [CrossRef]
- Amini, R.M.; Kohram, H.; Shahaneh, A.Z.; Zhandi, M.; Sharideh, H.; Nabi, M.M. The effects of different levels of vitamin E and vitamin C in modified Beltville extender on rooster post-thawed sperm quality. Cell Tissue Bank 2015, 16, 587–592. [Google Scholar] [CrossRef]
- Asadpour, R.; Jafari, R.; Tayefi-Nasrabadi, H. Influence of added vitamin C and vitamin E on frozen-thawed bovine sperm cryopreserved in citrate and tris-based extenders. Vet. Res. Forum. 2011, 1, 37–44. [Google Scholar]
- Mattiolli, S.; Bosco, A.D.; Maranesi, M.; Petrucci, L.; Rebollar, P.G.; Castellini, C. Dietary fish oil and flaxseed for rabbit does: Fatty acids distribution and ∆6-desaturase enzymes expression of different tissues. Animals 2019, 13, 1934–1942. [Google Scholar]
- Mattiolli, S.; Collodel, G.; Signorini, C.; Cotozzolo, E.; Noto, D.; Cerretani, D.; Micheli, L.; Fiaschi, A.I.; Brecchia, G.; Menchetti, L.; et al. Tissue antioxidant status and lipid peroxidation are related to dietary intake on n-3 polyunsaturated acids: A rabbit model. Antioxidants 2021, 10, 681. [Google Scholar] [CrossRef]
- Moallen, U.; Lehrer, H.; Livshits, L.; Zachut, M. The effects of omega-3 α-linolenic acid from flaxseed oil supplemented to high-yielding dairy cows on production, health, and fertility. Livest. Sci. 2020, 242, 1–8. [Google Scholar]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2003, 66, 21–32. [Google Scholar] [CrossRef]
- Anane, R.; Creppy, E.E. Lipid peroxidation as pathway of aluminium cytotoxicity in human skin fibroblast culture: Prevention by superoxide dismutase + catalase and vitamins E and C. Hum. Exp. Toxicol. 2001, 20, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Burnaugh, L.; Sabeur, K.; Ball, B.A. Generation of superoxide anion by equine spermatozoa as detected by dihydroethidium. Theriogenology 2007, 67, 580–589. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).