Evaluation of the Safety and Feasibility of Apheresis in Dogs: For Application in Metastatic Cancer Research
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
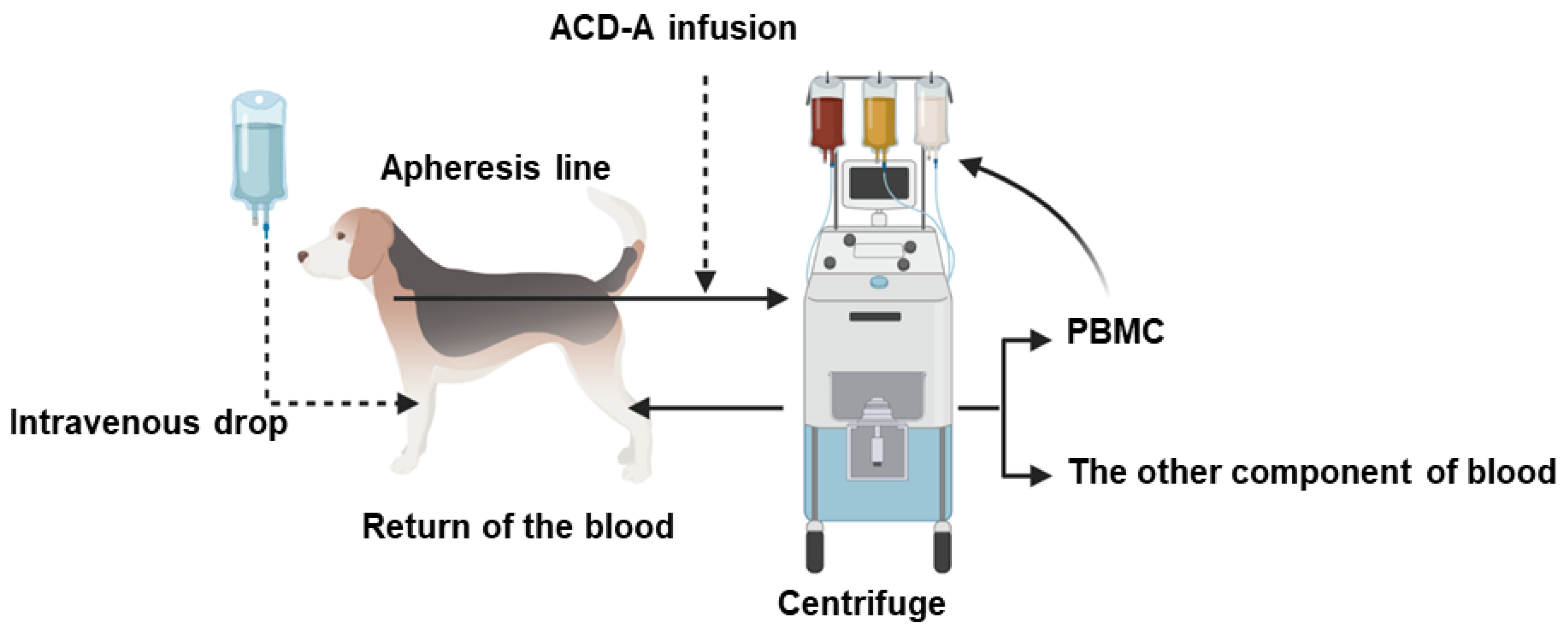
2.1. The Spectra Optia Apheresis System
2.2. MCF7 Cells and Antibodies
2.3. Animals
2.4. Preparation of Dogs for Apheresis
2.5. Procedure of Apheresis
2.6. Management after Apheresis
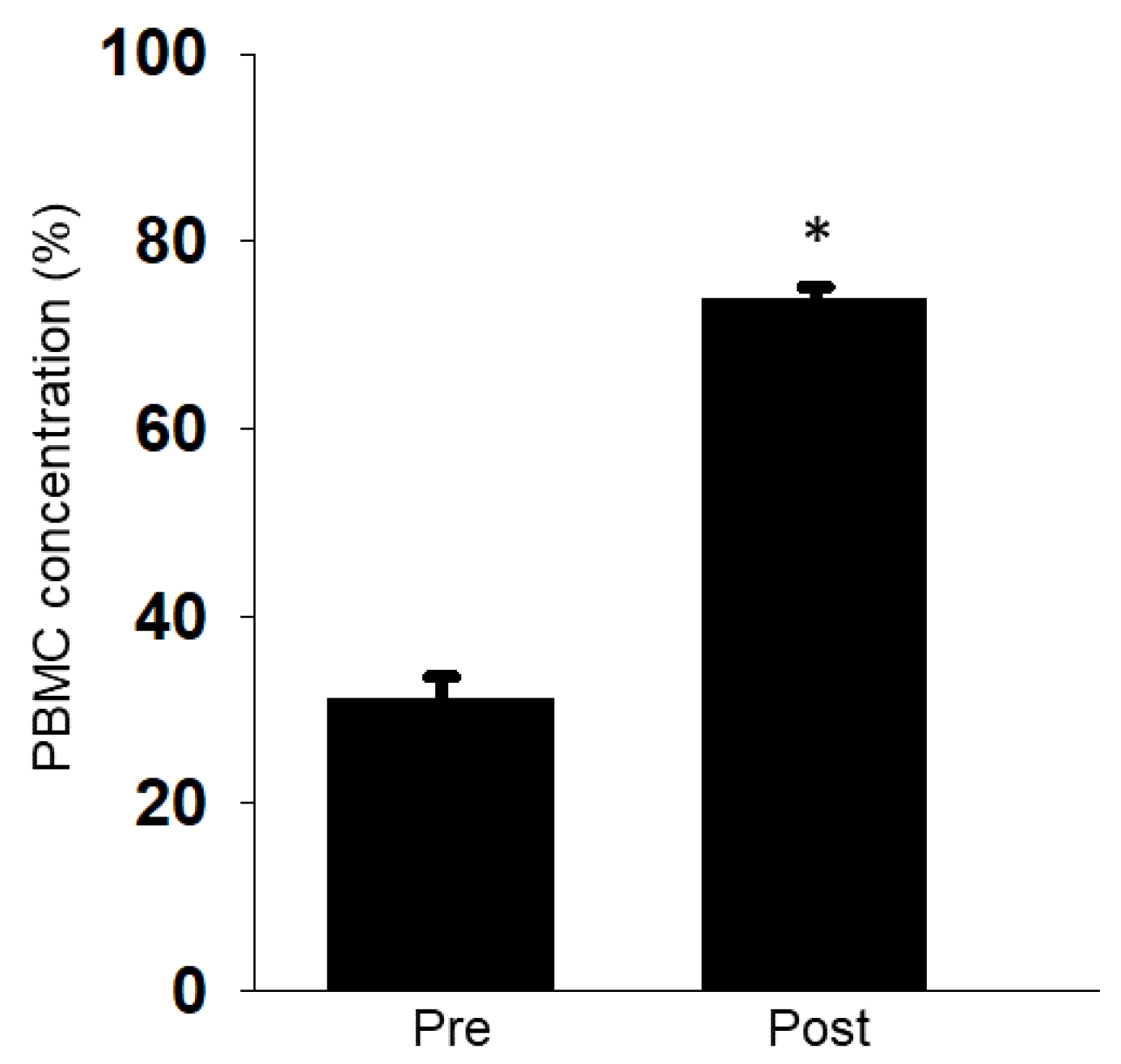
2.7. Comparison of PBMC Concentration Rate
2.8. Cell Culture and Characterization after Apheresis
2.9. Immunofluorescence Staining
2.10. Data Analysis
3. Results
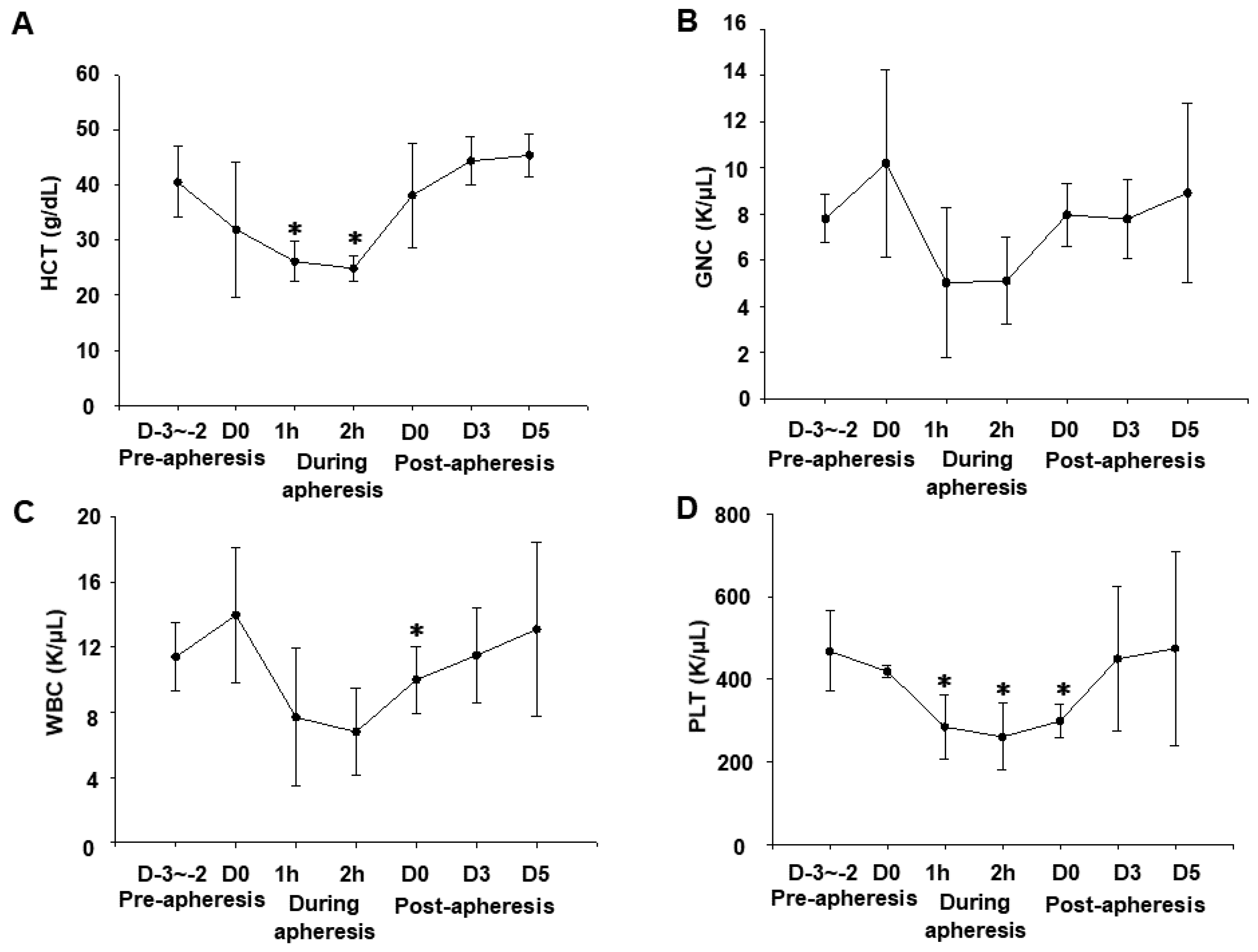
3.1. Effects of Apheresis on Blood Parameters
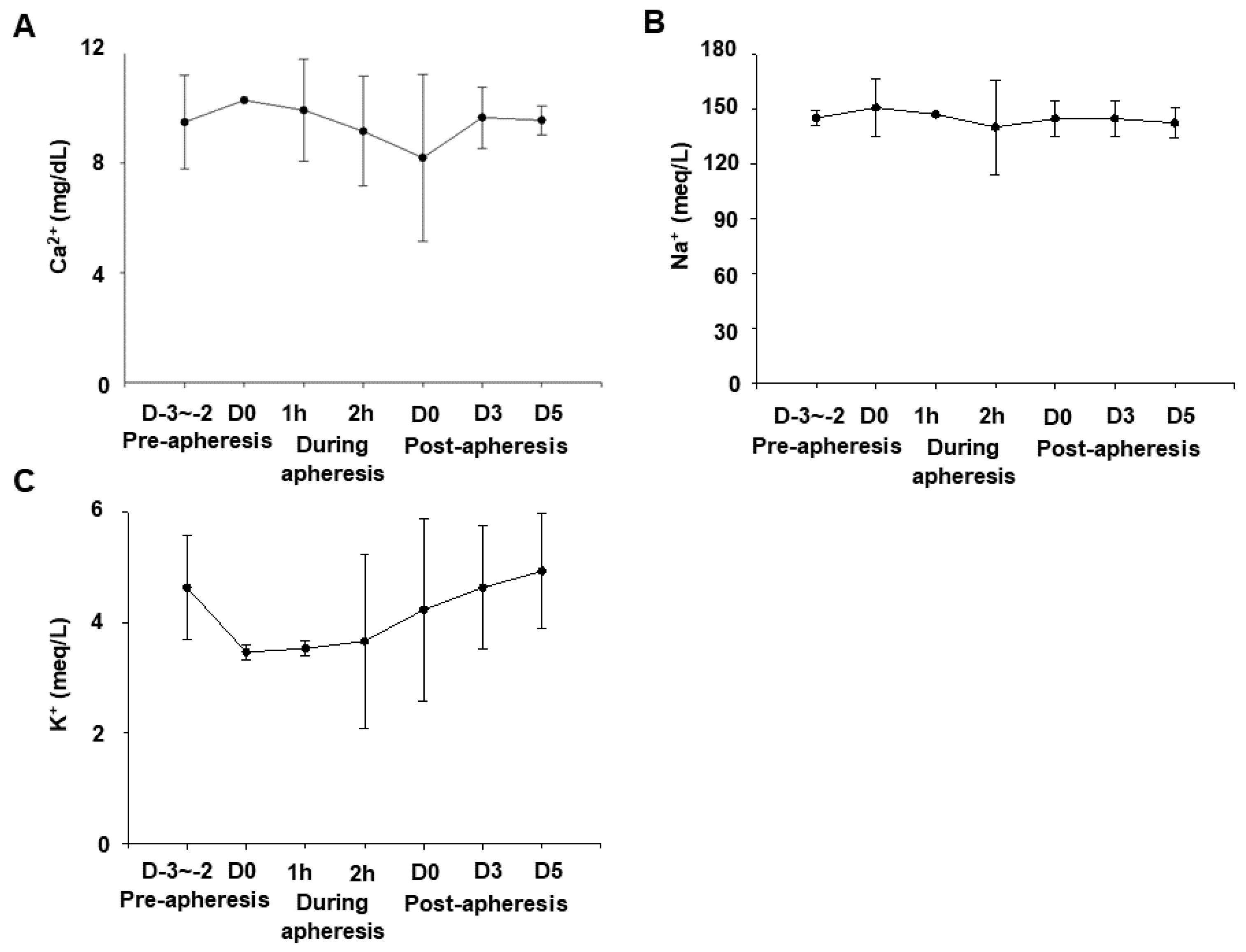
3.2. Effects of Apheresis on Serum Calcium, Sodium, and Potassium Levels
3.3. Efficacy of Mononuclear Cell Collection by Apheresis
3.4. Occurrence of a Thrombus during Apheresis
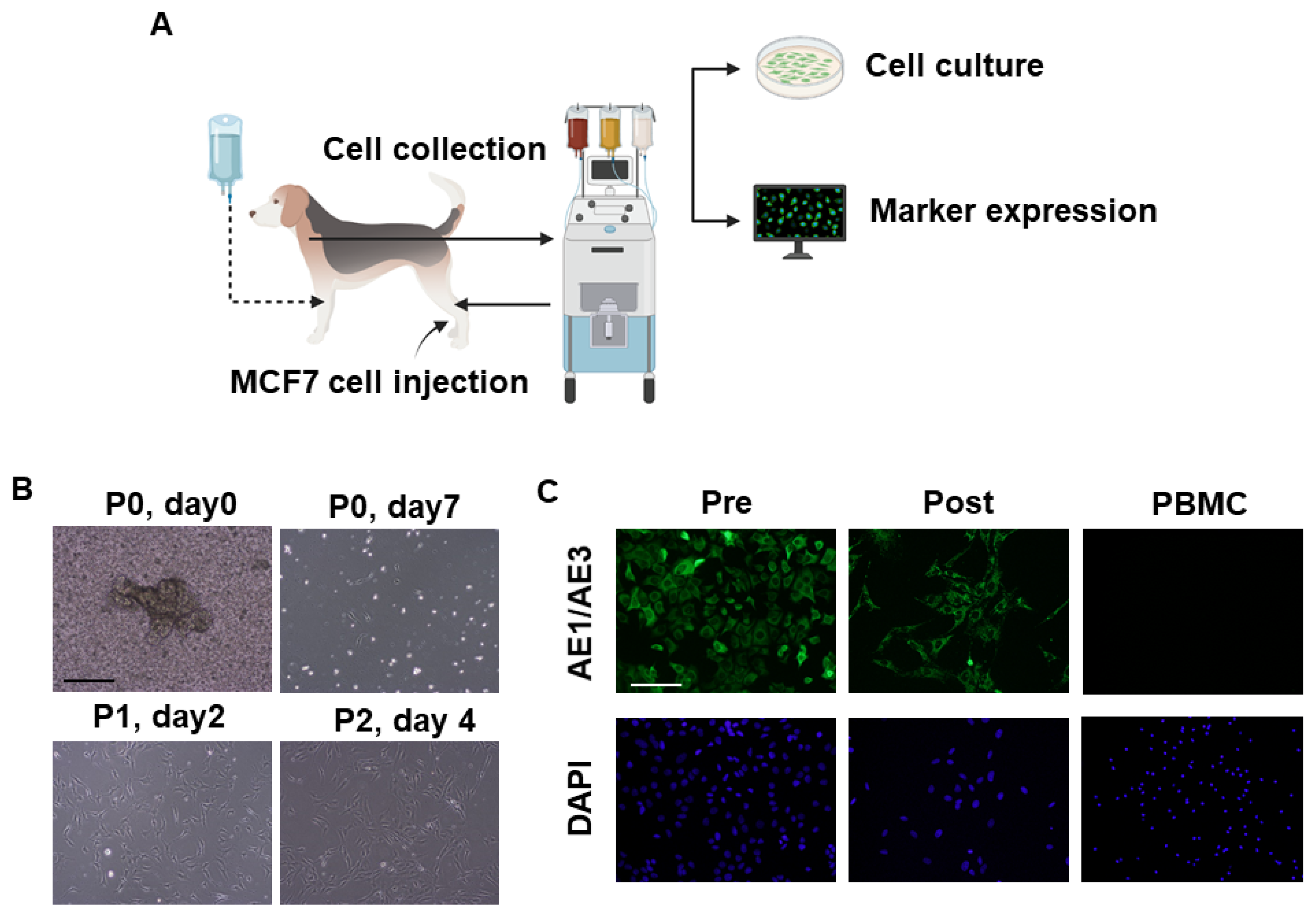
3.5. Collection of Intravenously Infused Cancer Cells by Apheresis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alix-Panabieres, C.; Pantel, K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Peeters, D.J.; Brouwer, A.; Van den Eynden, G.G.; Rutten, A.; Onstenk, W.; Sieuwerts, A.M.; Van Laere, S.J.; Huget, P.; Pauwels, P.; Peeters, M.; et al. Circulating tumour cells and lung microvascular tumour cell retention in patients with metastatic breast and cervical cancer. Cancer Lett. 2015, 356, 872–879. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Urtishak, S.; Alpaugh, R.K.; Weiner, L.M.; Swaby, R.F. Clinical utility of circulating tumor cells: A role for monitoring response to therapy and drug development. Biomark. Med. 2008, 2, 137–145. [Google Scholar] [CrossRef]
- Smerage, J.B.; Barlow, W.E.; Hortobagyi, G.N.; Winer, E.P.; Leyland-Jones, B.; Srkalovic, G.; Tejwani, S.; Schott, A.F.; O’Rourke, M.A.; Lew, D.L.; et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 3483–3489. [Google Scholar] [CrossRef]
- Li, X.C.; Li, Y.; Shao, W.Q.; Li, Z.; Zhao, R.; Ye, Z.L. Strategies for enrichment of circulating tumor cells. Transl. Cancer Res. 2020, 9, 2012–2025. [Google Scholar] [CrossRef]
- Fischer, J.C.; Niederacher, D.; Topp, S.A.; Honisch, E.; Schumacher, S.; Schmitz, N.; Zacarias Fohrding, L.; Vay, C.; Hoffmann, I.; Kasprowicz, N.S.; et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc. Natl. Acad. Sci. USA 2013, 110, 16580–16585. [Google Scholar] [CrossRef] [Green Version]
- Padmanabhan, A.; Connelly-Smith, L.; Aqui, N.; Balogun, R.A.; Klingel, R.; Meyer, E.; Pham, H.P.; Schneiderman, J.; Witt, V.; Wu, Y.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J. Clin. Apher. 2019, 34, 171–354. [Google Scholar] [CrossRef] [PubMed]
- Connelly-Smith, L.; Dunbar, N.M. The 2019 guidelines from the American Society for Apheresis: What’s new? Curr. Opin. Hematol. 2019, 26, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Bouhairie, V.E.; Goldberg, A.C. Familial hypercholesterolemia. Cardiol. Clin. 2015, 33, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Lambros, M.B.; Seed, G.; Sumanasuriya, S.; Gil, V.; Crespo, M.; Fontes, M.; Chandler, R.; Mehra, N.; Fowler, G.; Ebbs, B.; et al. Single-Cell Analyses of Prostate Cancer Liquid Biopsies Acquired by Apheresis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5635–5644. [Google Scholar] [CrossRef] [Green Version]
- Klingele, M.; Allmendinger, C.; Thieme, S.; Baerens, L.; Fliser, D.; Jan, B. Therapeutic apheresis within immune-mediated neurological disorders: Dosing and its effectiveness. Sci. Rep. 2020, 10, 7925. [Google Scholar] [CrossRef]
- Aguirre-Valencia, D.; Naranjo-Escobar, J.; Posso-Osorio, I.; Macia-Mejia, M.C.; Nieto-Aristizabal, I.; Barrera, T.; Obando, M.A.; Tobon, G.J. Therapeutic Plasma Exchange as Management of Complicated Systemic Lupus Erythematosus and Other Autoimmune Diseases. Autoimmune Dis. 2019, 2019, 5350960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.J.; Kozminsky, M.; Nagrath, S. Emerging role of nanomaterials in circulating tumor cell isolation and analysis. ACS Nano 2014, 8, 1995–2017. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, C.; Hayes, D.F. Circulating Tumor Cells. Adv. Exp. Med. Biol. 2016, 882, 235–258. [Google Scholar] [CrossRef]
- Tibbe, A.G.; Miller, M.C.; Terstappen, L.W. Statistical considerations for enumeration of circulating tumor cells. Cytom. Part A J. Int. Soc. Anal. Cytol. 2007, 71, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eifler, R.L.; Lind, J.; Falkenhagen, D.; Weber, V.; Fischer, M.B.; Zeillinger, R. Enrichment of circulating tumor cells from a large blood volume using leukapheresis and elutriation: Proof of concept. Cytom. Part B Clin. Cytom. 2011, 80, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Gardner, H.L.; Fenger, J.M.; London, C.A. Dogs as a Model for Cancer. Annu. Rev. Anim. Biosci. 2016, 4, 199–222. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.K.; Mazcko, C.N. Improving human cancer therapy through the evaluation of pet dogs. Nat. Rev. Cancer 2020, 20, 727–742. [Google Scholar] [CrossRef]
- Elbadawy, M.; Usui, T.; Mori, T.; Tsunedomi, R.; Hazama, S.; Nabeta, R.; Uchide, T.; Fukushima, R.; Yoshida, T.; Shibutani, M.; et al. Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture. Cancer Sci. 2019, 110, 2806–2821. [Google Scholar] [CrossRef]
- Abugomaa, A.; Elbadawy, M.; Yamanaka, M.; Goto, Y.; Hayashi, K.; Mori, T.; Uchide, T.; Azakami, D.; Fukushima, R.; Yoshida, T.; et al. Establishment of 2.5D organoid culture model using 3D bladder cancer organoid culture. Sci. Rep. 2020, 10, 9393. [Google Scholar] [CrossRef]
- Kim, T.H.; Wang, Y.; Oliver, C.R.; Thamm, D.H.; Cooling, L.; Paoletti, C.; Smith, K.J.; Nagrath, S.; Hayes, D.F. A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nat. Commun. 2019, 10, 1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, T.; Brisson, B.A.; Wood, G.A.; Oblak, M.; Mutsaers, A.J.; Sabine, V.; Skowronski, K.; Belanger, C.; Tiessen, A.; Bienzle, D. Flow Cytometric Detection of Circulating Osteosarcoma Cells in Dogs. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2019, 95, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.N.; Meier-Stiegen, F.; Driemel, C.; Jager, B.; Reinhardt, F.; Naskou, J.; Franken, A.; Neubauer, H.; Neves, R.P.L.; van Dalum, G.; et al. Diagnostic leukapheresis for CTC analysis in breast cancer patients: CTC frequency, clinical experiences and recommendations for standardized reporting. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2018, 93, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Lupu, M.; Gooley, T.; Zellmer, E.; Graves, S.S.; Storb, R. Principles of peripheral blood mononuclear cell apheresis in a preclinical canine model of hematopoietic cell transplantation. J. Vet. Intern. Med. 2008, 22, 74–82. [Google Scholar] [CrossRef]
- Posner, L.P.; Willcox, J.L.; Suter, S.E. Apheresis in three dogs weighing <14 kg. Vet. Anaesth. Analg. 2013, 40, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, T.; Vigani, A.; Ripoll, A.Z.; Taylor, S.; Culler, C.; Suter, S.E. Clinical Application of Apheresis in Very Small Dogs Weighing <8 kg to Pediatric Patients. Ther. Apher. Dial. 2020, 24, 333–342. [Google Scholar] [CrossRef]
- Even-Or, E.; Eden-Walker, A.; Di Mola, M.; McDougall, E.; Schechter, T.; Ali, M.; Svajger, G.; Gassas, A.; Licht, C.; Krueger, J. Comparison of two apheresis systems for autologous stem cell collections in pediatric oncology patients. Transfusion 2017, 57, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.; Cottler-Fox, M. Optia(R) continuous mononuclear collection (CMNC) system is a safe and efficient system for hematopoietic progenitor cells-apheresis (HPC-a) collection and yields a lower product hematocrit (HCT%) than the COBE(R) spectra system: A retrospective study. J. Clin. Apher. 2018, 33, 505–513. [Google Scholar] [CrossRef]
- Horwitz, K.B.; Costlow, M.E.; McGuire, W.L. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids 1975, 26, 785–795. [Google Scholar] [CrossRef]
- Nakasone, H.; Kanda, Y.; Ueda, T.; Matsumoto, K.; Shimizu, N.; Minami, J.; Sakai, R.; Hagihara, M.; Yokota, A.; Oshima, K.; et al. Retrospective comparison of mobilization methods for autologous stem cell transplantation in multiple myeloma. Am. J. Hematol. 2009, 84, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Storb, R.; Little, M.T.; Joslyn, A.; Spector, M.; Kuhr, C.S. Percutaneous central dual-lumen catheter for apheresis in the canine. J. Investig. Surg. Off. J. Acad. Surg. Res. 2002, 15, 337–341. [Google Scholar] [CrossRef]
- Kim, S.; Hosoya, K.; Kobayashi, A.; Okumura, M. Comparison of three mobilization protocols for peripheral blood stem cell apheresis with Spectra Optia continuous mononuclear cell protocol in healthy dogs. Vet. Comp. Oncol. 2019, 17, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Abugomaa, A.; Elbadawy, M. Patient-derived organoid analysis of drug resistance in precision medicine: Is there a value? Expert Rev. Precis. Med. Drug Dev. 2020, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Perdue, J.J.; Chandler, L.K.; Vesely, S.K.; Duvall, D.S.; Gilcher, R.O.; Smith, J.W.; George, J.N. Unintentional platelet removal by plasmapheresis. J. Clin. Apher. 2001, 16, 55–60. [Google Scholar] [CrossRef]
- Lee, G.; Arepally, G.M. Anticoagulation techniques in apheresis: From heparin to citrate and beyond. J. Clin. Apher. 2012, 27, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Lydon, H.; Brooks, R.; McCaskie, A.; Henson, F. Peripheral mononuclear blood cell apheresis in a preclinical ovine model. BMC Vet. Res. 2018, 14, 47. [Google Scholar] [CrossRef]
| BW (kg) | Sex | TBV (mL) | Total Length (min) | AC Flow (mL/min) | AC Volume (mL) | Inlet Flow (mL/min) | Inlet Volume (mL) | Plasma Flow (mL/min) | Collection Flow (mL/min) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dog 1 | 11 | female | 990 | 154.0 | 0.9 | 138.6 | 10.8 | 1660.1 | 5.7 | 0.7 |
| Dog 2 | 9 | female | 810 | 133.0 | 0.9 | 119.7 | 11.0 | 1463.0 | 5.4 | 0.5 |
| Dog 3 | 10 | female | 900 | 153.0 | 0.9 | 131.1 | 10.3 | 1569.3 | 5.4 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, H.; Elbadawy, M.; Fujisaka, K.; Sato, Y.; Ohmori, T.; Shinohara, Y.; Hatano, Y.; Kobayashi, D.; Gomyo, A.; Sudo, Y.; et al. Evaluation of the Safety and Feasibility of Apheresis in Dogs: For Application in Metastatic Cancer Research. Animals 2021, 11, 2770. https://doi.org/10.3390/ani11102770
Yamamoto H, Elbadawy M, Fujisaka K, Sato Y, Ohmori T, Shinohara Y, Hatano Y, Kobayashi D, Gomyo A, Sudo Y, et al. Evaluation of the Safety and Feasibility of Apheresis in Dogs: For Application in Metastatic Cancer Research. Animals. 2021; 11(10):2770. https://doi.org/10.3390/ani11102770
Chicago/Turabian StyleYamamoto, Haru, Mohamed Elbadawy, Koudai Fujisaka, Yomogi Sato, Takahiro Ohmori, Yuta Shinohara, Yui Hatano, Daichi Kobayashi, Ayana Gomyo, Yuji Sudo, and et al. 2021. "Evaluation of the Safety and Feasibility of Apheresis in Dogs: For Application in Metastatic Cancer Research" Animals 11, no. 10: 2770. https://doi.org/10.3390/ani11102770
APA StyleYamamoto, H., Elbadawy, M., Fujisaka, K., Sato, Y., Ohmori, T., Shinohara, Y., Hatano, Y., Kobayashi, D., Gomyo, A., Sudo, Y., Azakami, D., Uchide, T., Fukushima, R., Morita, S., Abugomaa, A., Yamawaki, H., Kaneda, M., Usui, T., & Sasaki, K. (2021). Evaluation of the Safety and Feasibility of Apheresis in Dogs: For Application in Metastatic Cancer Research. Animals, 11(10), 2770. https://doi.org/10.3390/ani11102770








