On the Doorstep, Rodents in Homesteads and Kitchen Gardens
Simple Summary
Abstract
1. Introduction
2. Material and Methods
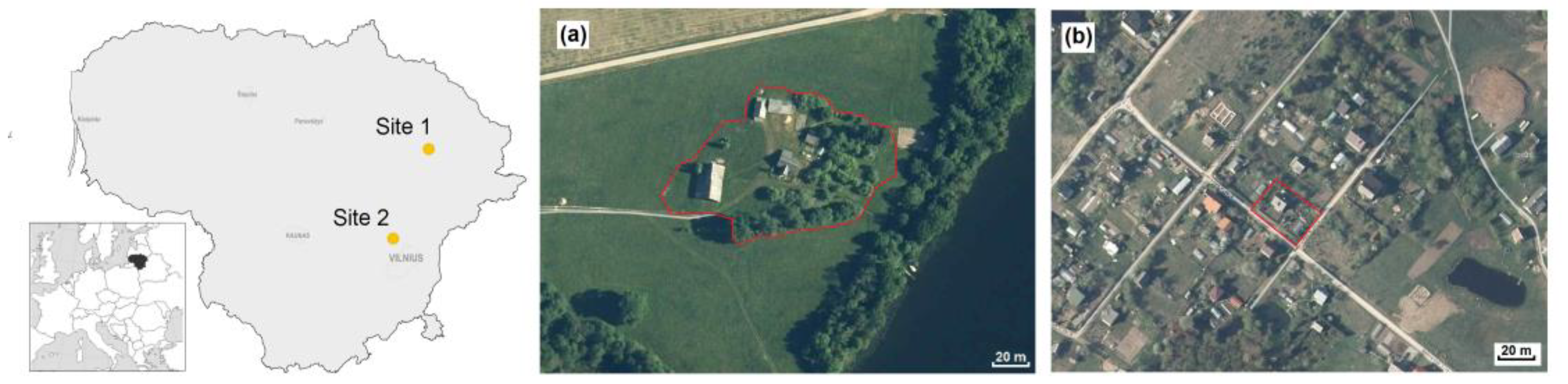
2.1. Study Sites
2.2. Small Mammal Trapping and Evaluation
2.3. Data Analysis
3. Results
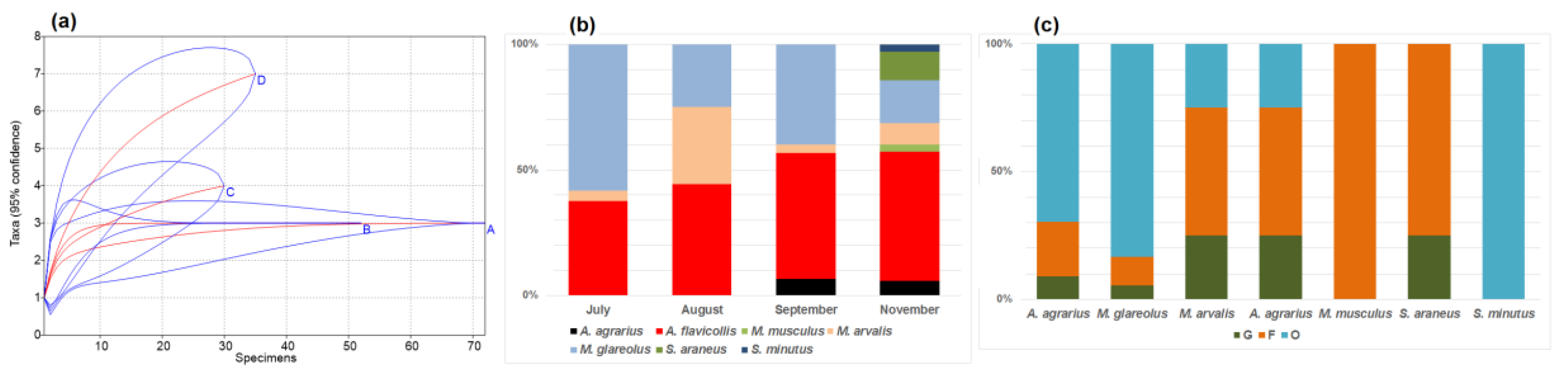
3.1. Diversity and Dominance in Relation to Food Sources (Habitat)
3.2. Seasonal Changes in the Small Mammal Community
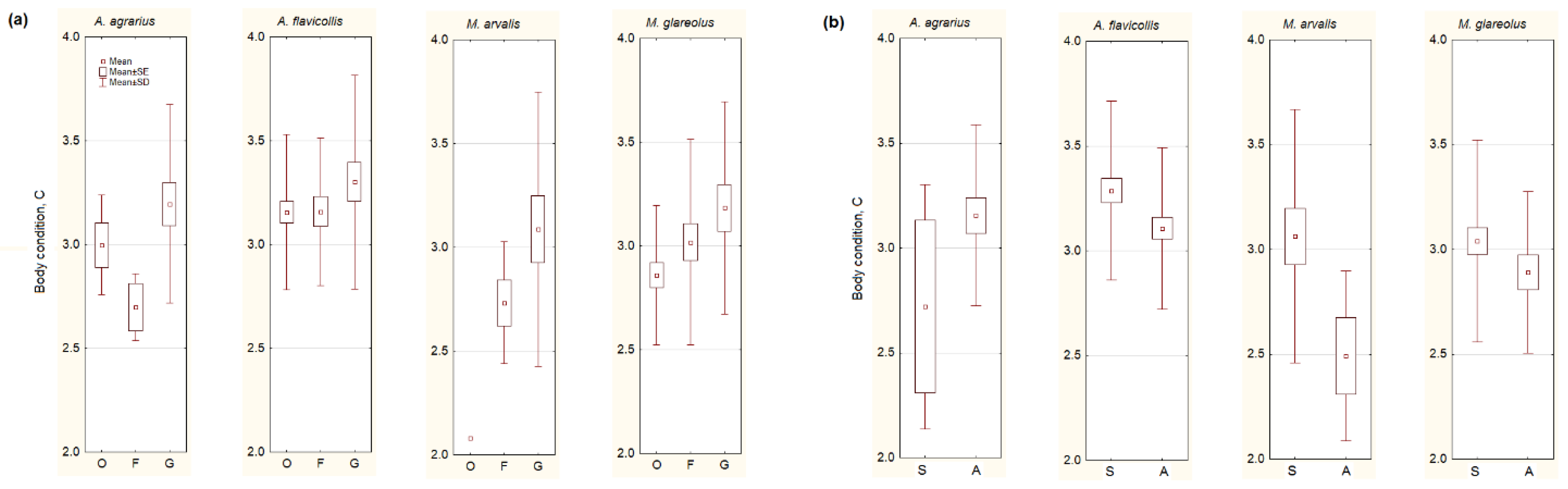
3.3. Body Condition of Small Mammals
3.4. Breeding of Small Mammals in the Commensal Habitats
4. Discussion
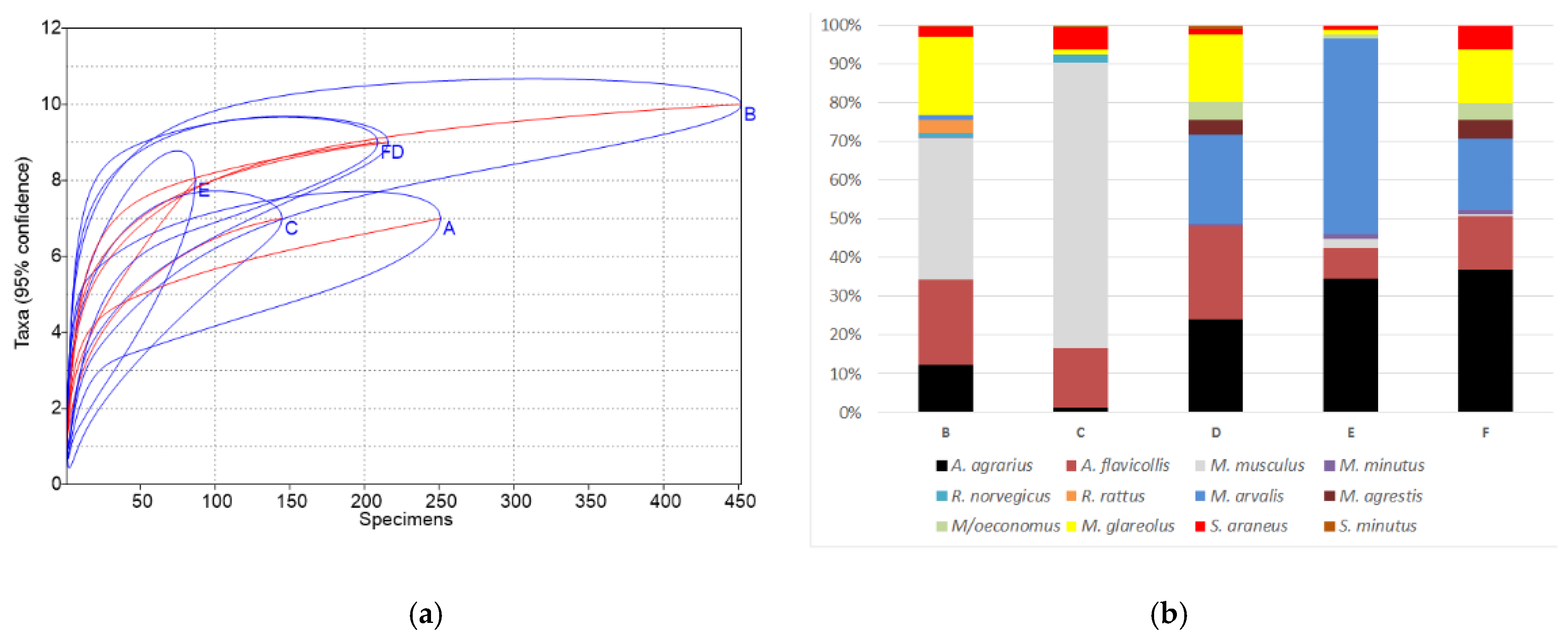
4.1. Species Composition and Diversity of Small Mammal Communities in the Other Homesteads and Commercial Orchards
4.2. Body Condition of Small Mammals in Commensal Habitats, Seasonal Changes and Importance
4.3. Breeding Failures in Rodents under Anthropogenic Impact
4.4. Significance of Small Mammal Studies in Commensal Habitats
5. Conclusions
- Out of seven small mammal species recorded in the commensal habitats, A. agrarius, A. flavicollis, M. arvalis, and M. glareolus may be referred as anthropophilic.
- Gardens and outbuildings were, by numbers and relative abundance, dominated by A. flavicollis, while buildings with food available by M. glareolus.
- The number of recorded species and diversity significantly increased in the autumn months.
- Body condition was the highest in rodents trapped in the homestead gardens, and it decreased in the autumn (with the exception of A. agrarius).
- Breeding failures were registered in all of the most numerous species of rodents from homesteads.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hulme-Beaman, A.; Dobney, K.; Cucchi, T.; Searle, J.B. An ecological and evolutionary framework for commensalism in anthropogenic environments. Trends Ecol. Evol. 2016, 31, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Adams, R.I.; Bateman, A.; Bik, H.M.; Hawks, J.; Hird, S.M.; Hughes, D.; Kembel, S.W.; Kinney, K.; Kolokotronis, S.-O.; et al. Evolution of the indoor biome. Trends Ecol. Evol. 2015, 30, 223–232. [Google Scholar] [CrossRef]
- Cavia, R.; Muschetto, E.; Cueto, G.R.; Suárez, O.V. Commensal rodents in the City of Buenos Aires: A temporal, spatial, and environmental analysis at the whole city level. EcoHealth 2015, 12, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J.; Ansell, R.J.; Dodds, P.A.A.; Webber, C.E.; Harris, S. Factors affecting the distribution of small mammals in an urban area. Mammal Rev. 2003, 33, 95–100. [Google Scholar] [CrossRef]
- Baker, S.E.; Maw, S.A.; Johnson, P.J.; Macdonald, D.W. Not in My Backyard: Public Perceptions of Wildlife and ‘Pest Control’ in and around UK Homes, and Local Authority ‘Pest Control’. Animals 2020, 10, 222. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Leirs, H.; Skonhoft, A.; Davis, S.A.; Pech, R.P.; Andreassen, H.P.; Singleton, G.R.; Lima, M.; Machang’u, R.S.; Makundi, R.H.; et al. Mice, rats, and people: The bio-economics of agricultural rodent pests. Front. Ecol. Environ. 2003, 1, 367–375. [Google Scholar] [CrossRef]
- Tikhonova, G.N.; Tikhonov, I.A.; Bogomolov, P.L.; Davydova, L.V. Certain aspects of interactions between cohabiting sibling species Microtus arvalis and M. rossiaemeridionalis. Biol. Bull. 2006, 33, 59–64. [Google Scholar] [CrossRef]
- Gortat, T.; Barkowska, M.; Gryczyńska-Siemią Tkowska, A.; Pieniążek, A.; Kozakiewicz, A.; Kozakiewicz, M. The Effects of Urbanization—Small Mammal Communities in a Gradient of Human Pressure in Warsaw City, Poland. Pol. J. Ecol. 2014, 62, 163–172. [Google Scholar] [CrossRef]
- Kays, R.; Parsons, A.W. Mammals in and around suburban yards, and the attraction of chicken coops. Urban Ecosyst. 2014, 17, 691–705. [Google Scholar] [CrossRef]
- Lambert, M.; Vial, F.; Pietravalle, S.; Cowan, D. Results of a 15-year systematic survey of commensal rodents in English dwellings. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Langton, S.D.; Cowan, D.P.; Meyer, A.N. The occurrence of commensal rodents in dwellings as revealed by the 1996 English House Condition Survey. J. Appl. Ecol. 2001, 38, 699–709. [Google Scholar] [CrossRef]
- Pocock, M.J.; Searle, J.B.; White, P.C. Adaptations of animals to commensal habitats: Population dynamics of house mice Mus musculus domesticus on farms. J. Anim. Ecol. 2004, 73, 878–888. [Google Scholar] [CrossRef]
- Khlyap, L.A.; Warshavsky, A.A. Synanthropic and agrophilic rodents as invasive alien mammals. Russ. J. Biol. Invasions 2010, 1, 301–312. [Google Scholar] [CrossRef]
- Atkočaitis, O. Small mammals trapped inside farmstead buildings. [Sodybos pastatuose sugauti smulkieji žinduoliai]. Theriologia Lituanica 2003, 3, 57–61. [Google Scholar]
- Balčiauskienė, L.; Balčiauskas, L.; Vitkauskas, V.; Podėnas, S. Indoor small mammals in Lithuania: Some morphometrical, body condition, and reproductive characteristics. Zool. Ecol. 2015, 25, 305–313. [Google Scholar] [CrossRef]
- Zorenko, T.; Leontyeva, T. Species Diversity and Distribution of Mammals in Riga. Acta Zool. Litu. 2003, 13, 78–86. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Mažeikytė, R.; Baranauskas, K. Diversity of mammals in the Vilnius city. Acta Biol. Univ. Daugavp. 2005, 5, 55–66. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L.; Stirkė, V. Mow the Grass at the Mouse’s Peril: Diversity of Small Mammals in Commercial Fruit Farms. Animals 2019, 9, 334. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Pieniążek, A.; Sokół, M.; Kozakiewicz, M. Ecological characteristics of two closely related rodent species in urban environment—Permanent inhabitant vs. newcomer. Nat. Resour. 2017, 8, 69–80. [Google Scholar] [CrossRef]
- Łopucki, R.; Kitowski, I. How small cities affect the biodiversity of ground-dwelling mammals and the relevance of this knowledge in planning urban land expansion in terms of urban wildlife. Urban Ecosyst. 2017, 20, 933–943. [Google Scholar] [CrossRef]
- Klimant, P.; Klimantová, A.; Baláž, I.; Jakab, I.; Tulis, F.; Rybanský, Ľ.; Vadel, Ľ.; Krumpálová, Z. Small mammals in an urban area: Habitat preferences and urban-rural gradient in Nitra city, Slovakia. Pol. J. Ecol. 2017, 65, 144–157. [Google Scholar] [CrossRef]
- Cavia, R.; Guidobono, J.S.; Fraschina, J.; Busch, M. Effects of physical barriers and eradication on recolonization of rodents in poultry farms. Int. J. Pest Manag. 2019, 65, 370–380. [Google Scholar] [CrossRef]
- Krijger, I.M.; Belmain, S.R.; Singleton, G.R.; Groot Koerkamp, P.W.; Meerburg, B.G. The need to implement the landscape of fear within rodent pest management strategies. Pest Manag. Sci. 2017, 73, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.S.; Whitetail-Eagle, J.; Corneli, A.L.; Person, B.; Ettestad, P.J.; Dimenna, M.; Norstog, J.; Creswell, J.; Khan, A.S.; Olson, J.G.; et al. Experimental evaluation of rodent exclusion methods to reduce hantavirus transmission to residents in a Native American community in New Mexico. Vector-Borne Zoonot. 2002, 2, 61–68. [Google Scholar] [CrossRef]
- Wahl-Jensen, V.; Radoshitzky, S.R.; de Kok-Mercado, F.; Taylor, S.L.; Bavari, S.; Jahrling, P.B.; Kuhn, J.H. Role of rodents and bats in human viral hemorrhagic fevers. In Viral Hemorrhagic Fevers; Singh, S.K., Ruzek, D., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 99–127. [Google Scholar]
- Hornok, S.; Földvári, G.; Rigó, K.; Meli, M.L.; Gönczi, E.; Répási, A.; Farkas, R.; Papp, I.; Kontschán, J.; Hofmann-Lehmann, R. Synanthropic rodents and their ectoparasites as carriers of a novel haemoplasma and vector-borne, zoonotic pathogens indoors. Parasites Vectors 2015, 8, 27. [Google Scholar] [CrossRef]
- Bastien, M.; Vaniscotte, A.; Combes, B.; Umhang, G.; Germain, E.; Gouley, V.; Pierlet, A.; Quintaine, T.; Forin-Wiart, M.-A.; Villena, I.; et al. Identifying drivers of fox and cat faecal deposits in kitchen gardens in order to evaluate measures for reducing contamination of fresh fruit and vegetables. Food Waterborne Parasitol. 2019, 14, e00034. [Google Scholar] [CrossRef]
- Nau, L.H.; Emirhar, D.; Obiegala, A.; Mylius, M.; Runge, M.; Jacob, J.; Bier, N.; Nöckler, K.; Imholt, C.; Below, D.; et al. Leptospirosis in Germany: Current knowledge on pathogen species, reservoir hosts, and disease in humans and animals. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2019, 62, 1510–1521. [Google Scholar] [CrossRef]
- Vanwambeke, S.O.; Zeimes, C.B.; Drewes, S.; Ulrich, R.G.; Reil, D.; Jacob, J. Spatial dynamics of a zoonotic orthohantavirus disease through heterogenous data on rodents, rodent infections, and human disease. Sci. Rep. 2019, 9, 2329. [Google Scholar] [CrossRef]
- Krawczyk, A.I.; Van Duijvendijk, G.L.; Swart, A.; Heylen, D.; Jaarsma, R.I.; Jacobs, F.H.; Fonville, M.; Sprong, H.; Takken, W. Effect of rodent density on tick and tick-borne pathogen populations: Consequences for infectious disease risk. Parasites Vectors 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Fischer, S.; Franke, A.; Imholt, C.; Gethmann, J.; Spierling, N.G.; Jacob, J.; Beer, M.; Hoffmann, D.; Ulrich, R.G. Patchy Occurrence of Cowpox Virus in Voles from Germany. Vector-Borne Zoonotic Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.M.; Williamson, D.H.Z.; Pearson, M.; Saikawa, E.; Gribble, M.O.; Kegler, M. Safe community gardening practices: Focus groups with garden leaders in Atlanta, Georgia. Local Environ. 2020, 25, 18–35. [Google Scholar] [CrossRef]
- Kingsley, J.; Foenander, E.; Bailey, A. “It’s about community”: Exploring social capital in community gardens across Melbourne, Australia. Urban For. Urban Green. 2020, 49, 126640. [Google Scholar] [CrossRef]
- Kimber, C.T. Gardens and dwelling: People in vernacular gardens. Geogr. Rev. 2004, 94, 263–283. [Google Scholar] [CrossRef]
- Guitart, D.; Pickering, C.; Byrne, J. Past results and future directions in urban community gardens research. Urban For. Urban Green. 2012, 11, 364–373. [Google Scholar] [CrossRef]
- Ciftcioglu, G.C. Social preference-based valuation of the links between home gardens, ecosystem services, and human well-being in Lefke Region of North Cyprus. Ecosyst. Serv. 2017, 25, 227–236. [Google Scholar] [CrossRef]
- Matteson, K.C.; Langellotto, G. Evaluating Community Gardens as Habitat for an Urban Butterfly. Cities Environ. 2012, 5, 10. [Google Scholar] [CrossRef]
- Santini, L.; González-Suárez, M.; Russo, D.; Gonzalez-Voyer, A.; von Hardenberg, A.; Ancillotto, L. One strategy does not fit all: Determinants of urban adaptation in mammals. Ecol. Lett. 2019, 22, 365–376. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Janonytė, A. Reproduction of the root vole (Microtus oeconomus) at the edge of its distribution range. Turk. J. Zool. 2012, 36, 668–675. [Google Scholar] [CrossRef]
- Moors, P.J. Norway rats (Rattus norvegicus) on the Noises and Motukawao islands, Hauraki Gulf, New Zealand. N. Z. J. Ecol. 1985, 8, 37–54. Available online: https://www.jstor.org/stable/24052744 (accessed on 15 April 2010).
- Balčiauskienė, L.; Balčiauskas, L.; Čepukienė, A. Demographic and morphometric parameters of the yellow-necked mouse (Apodemus flavicollis) in late autumn-early spring in Lithuania. Acta Biol. Univ. Daugavp. 2009, 9, 25–34. [Google Scholar]
- Balčiauskienė, L.; Balčiauskas, L.; Čepukienė, A. Growth of the bank vole Myodes glareolus in the non-vegetative period in NE Lithuania. Est. J. Ecol. 2009, 58, 86–93. [Google Scholar] [CrossRef]
- Balčiauskienė, L.; Balčiauskas, L.; Čepukienė, A. Winter growth depression of common vole (Microtus arvalis). Acta Zool. Litu. 2009, 19, 85–92. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Addison-Wesley Educational Publishers, Inc.: Menlo Park, CA, USA, 1999; 620p. [Google Scholar]
- Past 3.x—The Past of the Future. Available online: http://folk.uio.no/ohammer/past (accessed on 10 October 2018).
- TIBCO Software Inc. Data Science Textbook. 2020. Available online: https://docs.tibco.com/data-science/textbook (accessed on 15 January 2020).
- Martineau, J.; Pothier, D.; Fortin, D. Processes driving short-term temporal dynamics of small mammal distribution in human-disturbed environments. Oecologia 2016, 181, 831–840. [Google Scholar] [CrossRef]
- Meillère, A.; Brischoux, F.; Parenteau, C.; Angelier, F. Influence of Urbanization on Body Size, Condition, and Physiology in an Urban Exploiter: A Multi-Component Approach. PLoS ONE 2015, 10, e0135685. [Google Scholar] [CrossRef]
- Shonfield, J.; Bayne, E.M. Effects of industrial disturbance on small mammal abundance and activity of small mammals. Can. J. Zool. 2019, 97, 1013–1020. [Google Scholar] [CrossRef]
- Foley, J.A. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Arets, E.J.M.M.; Andeweg, K.; Conijn, J.G.; Hassink, J.; Hiemstra, S.J.; Klapwijk, C.J.; Lahr, J.; Van Rooijen, N.; Wosten, H.; Zijlstra, J. Food Systems and Biodiversity: Progress and Output 2019; Wageningen Environmental Research: Wageningen, The Netherlands, 2019; 21p. [Google Scholar]
- Baker, P.J.; Harris, S. Urban mammals: What does the future hold? An analysis of the factors affecting patterns of use of residential gardens in Great Britain. Mammal Rev. 2007, 37, 297–315. [Google Scholar] [CrossRef]
- Bókony, V.; Seress, G.; Nagy, S.; Lendvai, Á.Z.; Liker, A. Multiple indices of body condition reveal no negative effect of urbanization in adult house sparrows. Landsc. Urban Plan. 2012, 104, 75–84. [Google Scholar] [CrossRef]
- Brakes, C.R.; Smith, R.H. Exposure of non-target small mammals to rodenticides: Short-term effects, recovery and implications for secondary poisoning. J. Appl. Ecol. 2005, 42, 118–128. [Google Scholar] [CrossRef]
- Dickman, C.R. Habitat fragmentation and vertebrate species richness in an urban environment. J. Appl. Ecol. 2008, 24, 337–351. [Google Scholar] [CrossRef]
- Lundholm, J.T.; Richardson, P.J. Habitat analogues for reconciliation ecology in urban and industrial environments. J. Appl. Ecol. 2010, 47, 966–975. [Google Scholar] [CrossRef]
- Büchner, S.; Trout, R.; Adamík, P. Conflicts with Glis glis and Eliomys quercinus in households: A practical guideline for sufferers (Rodentia: Gliridae). Lynx 2018, 49, 19–26. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Woods, W.A., Jr. Condition indices for conservation: New uses for evolving tools. Integr. Comp. Biol. 2006, 46, 1169–1190. [Google Scholar] [CrossRef] [PubMed]
- Peig, J.; Green, A.J. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Funct. Ecol. 2010, 24, 1323–1332. [Google Scholar] [CrossRef]
- Jánová, E.; Heroldová, M.; Bryja, J. Conspicuous Demographic and Individual Changes in a Population of the Common Vole in a Set-Aside Alfalfa Field. Ann. Zool. Fenn. 2008, 45, 39–54. [Google Scholar] [CrossRef]
- Keane, B.; Bryant, L.; Goyal, U.; Williams, S.; Kortering, S.L.; Lucia, K.E.; Richmond, A.R.; Solomon, N.G. No effect of body condition at weaning on survival and reproduction in prairie voles. Can. J. Zool. 2007, 85, 718–727. [Google Scholar] [CrossRef]
- Taylor, C.H.; Wanelik, K.M.; Friberg, I.M.; Lowe, A.; Hall, A.J.; Ralli, C.; Birtles, R.J.; Begon, M.; Paterson, S.; Jackson, J.A.; et al. Physiological, but not fitness, effects of two interacting haemoparasitic infections in a wild rodent. Int. J. Parasitol. 2018, 48, 463–471. [Google Scholar] [CrossRef]
- Beldomenico, P.M.; Telfer, S.; Lukomski, L.; Gebert, S.; Bennett, M.; Begon, M. Host condition and individual risk of cowpox virus infection in natural animal populations: Cause or effect? Epidemiol. Infect. 2009, 137, 1295. [Google Scholar] [CrossRef]
- Zejda, J. Differential growth of three cohorts of the bank vole, Clethrionomys glareolus Schreb. 1780. Zool. Listy. Folia Zool. 1971, 20, 229–245. [Google Scholar]
- Aars, J.; Ims, R.A. Intrinsic and climatic determinants of population demography: The winter dynamics of tundra voles. Ecology 2002, 83, 3449–3456. [Google Scholar] [CrossRef]
- Ebensperger, L.A.; Tapia, D.; Ramírez-Estrada, J.; León, C.; Soto-Gamboa, M.; Hayes, L.D. Fecal cortisol levels predict breeding but not survival of females in the short-lived rodent, Octodon degus. Gen. Comp. Endocr. 2013, 186, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Vekhnik, V.A. Effect of food availability on the reproduction in edible dormice (Glis glis L., 1766) on the eastern periphery of the range. Mammal Res. 2019, 64, 423–434. [Google Scholar] [CrossRef]
- Mappes, T.; Ylonen, H. Reproductive effort of female bank voles in a risky environment. Evol. Ecol. 1997, 11, 591–598. [Google Scholar] [CrossRef]
- Stanko, M. Notes on the litter size of Microtus arvalis (Arvicolidae). Biologia 1996, 51, 607–611. [Google Scholar]
- Innes, D.G.L.; Millar, J.S. Life histories of Clethrionomys and Microtus (Microtinae). Mammal Rev. 1994, 24, 179–207. [Google Scholar] [CrossRef]
- Martin, A.R.; Richardson, M.G. Rodent eradication scaled up: Clearing rats and mice from South Georgia. Oryx 2019, 53, 27–35. [Google Scholar] [CrossRef]
- Danforth, M.E.; Messenger, S.; Buttke, D.; Weinburke, M.; Carroll, G.; Hacker, G.; Niemela, M.; Andrews, E.S.; Jackson, B.T.; Kramer, V.; et al. Long-Term Rodent Surveillance after Outbreak of Hantavirus Infection, Yosemite National Park, California, USA, 2012. Emerg. Infect. Dis. 2020, 26, 560–567. [Google Scholar] [CrossRef]
- Strakova, P.; Jagdmann, S.; Balčiauskas, L.; Balčiauskienė, L.; Drewes, S.; Ulrich, R.G. Puumala Virus in Bank Voles, Lithuania. Emerg. Infect. Dis. 2017, 23, 158–160. [Google Scholar] [CrossRef]
| Species | Habitats | Total | |||||
|---|---|---|---|---|---|---|---|
| G | F | O | N (%) | ♂♂:♀♀ | Ad:Sub:Juv | RA ± SE | |
| Apodemus agrarius | 21 | 2 | 5 | 28 (11.2) | 22:6 | 7:7:14 | 4.9 ± 1.8 |
| Apodemus flavicollis | 30 | 24 | 55 | 109 (43.4) | 63:46 | 48:48:13 | 13.4 ± 2.0 |
| Mus musculus | 0 | 1 | 0 | 1 (0.4) | 0:1 | 0:1:0 | 0.2 ± 0.2 |
| Microtus arvalis | 17 | 7 | 1 | 25 (10.0) | 11:14 | 6:1:18 | 2.9 ± 1.2 |
| Myodes glareolus | 21 | 31 | 31 | 83 (33.1) | 51:32 | 19:19:45 | 9.3 ± 3.6 |
| Sorex araneus | 1 | 3 | 0 | 4 (1.6) | 3:1 | 1.0 ± 0.8 | |
| Sorex minutus | 0 | 0 | 1 | 1 (0.4) | 0:1 | 0.3 ± 0.3 | |
| Total, N | 90 | 68 | 93 | 251 | |||
| Species number, S | 5 a | 6 a | 5 a | 7 | |||
| Diversity, H | 2.02 a | 1.82 a,b | 1.34 c | 1.89 | |||
| Dominance, D | 0.26 a | 0.35 b | 0.46 c | 0.32 | |||
| Species | July | August | September | November |
|---|---|---|---|---|
| Apodemus agrarius | 2 | 2 | ||
| Apodemus flavicollis | 27 | 23 | 15 | 18 |
| Mus musculus | 1 | |||
| Microtus arvalis | 3 | 16 | 1 | 3 |
| Myodes glareolus | 42 | 13 | 12 | 6 |
| Sorex araneus | 4 | |||
| Sorex minutus | 1 | |||
| Total, N | 72 | 52 | 30 | 35 |
| Species number, S | 3 a | 3 a | 4 a | 7 b |
| Diversity, H | 1.18 a | 1.54 b | 1.45 b | 2.12 c |
| Dominance, D | 0.48 a | 0.35 b | 0.42 b | 0.32 b |
| Species | N | C | Gender | Age | |||
|---|---|---|---|---|---|---|---|
| Male | Female | Ad | Sub | Juv | |||
| Apodemus agrarius | 28 | 3.12 ± 0.08 | 3.09 ± 0.10 a | 3.24 ± 0.13 a | 3.13 ± 0.19 a | 3.32 ± 0.23 a | 3.03 ± 0.09 a |
| Apodemus flavicollis | 109 | 3.20 ± 0.04 | 3.15 ± 0.05 a | 3.26 ± 0.07 a | 3.11 ±0.05 a | 3.21 ± 0.06 a | 3.48 ± 0.16 b |
| Mus musculus | 1 | 4.44 | 4.44 | 4.44 | |||
| Microtus arvalis | 25 | 2.95 ± 0.12 | 2.95 ± 0.19 a | 2.95 ± 0.16 a | 2.57 ± 0.12 a | 3.16 | 3.06 ± 0.16 a |
| Myodes glareolus | 83 | 3.00 ± 0.05 | 2.92 ± 0.07 a | 3.12 ± 0.07 a | 2.74 ± 0.07 a | 2.80 ± 0.09 a | 3.20 ± 0.07 b |
| Sorex araneus | 4 | 2.80 ± 0.24 | 2.98 ± 0.24 | 2.29 | |||
| Sorex minutus | 1 | 3.82 | 3.82 | ||||
| Species | N | Males | Females | Litter Size, Young ± SE | Disturbances, % | |
|---|---|---|---|---|---|---|
| Potential | Observed | |||||
| Apodemus agrarius | 3 | 2 | 1 | 7.0 | 5.0 | 100 |
| Apodemus flavicollis | 25 | 14 | 11 | 7.5 ± 1.5 | 6.7 ± 1.1 | 50.0 |
| Microtus arvalis | 5 | 5 | 6.0 ± 0.4 | 5.6 ± 0.7 | 20.0 | |
| Myodes glareolus | 17 | 11 | 6 | 5.7 ± 0.8 | 4.5 ± 0.9 | 16.7% |
| HM 1 | FO 2 | BP 2 | NM 2 | ||||
|---|---|---|---|---|---|---|---|
| Species | A–W | S | A | S | A | S | A |
| Apodemus agrarius | 2.95 ± 0.42 | 3.26 ± 0.07 | 3.94 | 3.47 ± 0.10 # | 3.75 ± 0.25 | 3.30 ± 0.06 # | |
| A. flavicollis | 3.17 ± 0.10 | 3.24 ± 0.06 | 3.48 ± 0.21 | 3.55 ± 0.12 | 3.43 ± 0.13 | 3.33 ± 0.07 | |
| Mus musculus | 3.39 ± 0.05 | - | - | 4.02 | - | - | 4.00 |
| Microtus arvalis | - | 3.28 ± 0.17 | 3.38 ± 0.08 | 3.73 ±0.05 | 3.21 ± 0.08 * | 3.80 ± 0.17 | 3.18 ± 0.11 * |
| Myodes glareolus | 2.99 ± 0.35 | 3.48 ± 0.14 | 3.23 ± 0.09 | - | 2.98 | 3.09 ± 0.20 | 3.02 ± 0.11 |
| Species | Litter Size, Young ± SE | Disturbances, % | |
|---|---|---|---|
| Potential | Observed | ||
| Apodemus agrarius | 6.57 ± 0.53 | 6.29 ± 0.52 | 28.6 |
| Apodemus flavicollis | 6.50 ± 0.57 | 6.33 ± 0.57 | 14.3 |
| Microtus arvalis | 5.51 ± 0.22 | 5.02 ± 0.22 | 43.9 |
| Myodes glareolus | 5.43 ± 0.53 | 5.14 ± 0.52 | 28.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčiauskas, L.; Balčiauskienė, L. On the Doorstep, Rodents in Homesteads and Kitchen Gardens. Animals 2020, 10, 856. https://doi.org/10.3390/ani10050856
Balčiauskas L, Balčiauskienė L. On the Doorstep, Rodents in Homesteads and Kitchen Gardens. Animals. 2020; 10(5):856. https://doi.org/10.3390/ani10050856
Chicago/Turabian StyleBalčiauskas, Linas, and Laima Balčiauskienė. 2020. "On the Doorstep, Rodents in Homesteads and Kitchen Gardens" Animals 10, no. 5: 856. https://doi.org/10.3390/ani10050856
APA StyleBalčiauskas, L., & Balčiauskienė, L. (2020). On the Doorstep, Rodents in Homesteads and Kitchen Gardens. Animals, 10(5), 856. https://doi.org/10.3390/ani10050856






