Postweaning Grouping as a Strategy to Reduce Singly Housed Male Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Grouping Experiment
2.2.1. Experimental Groups
2.2.2. Experimental procedure
2.3. Testosterone Determination
2.3.1. Experimental Groups
2.3.2. Experimental procedure
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
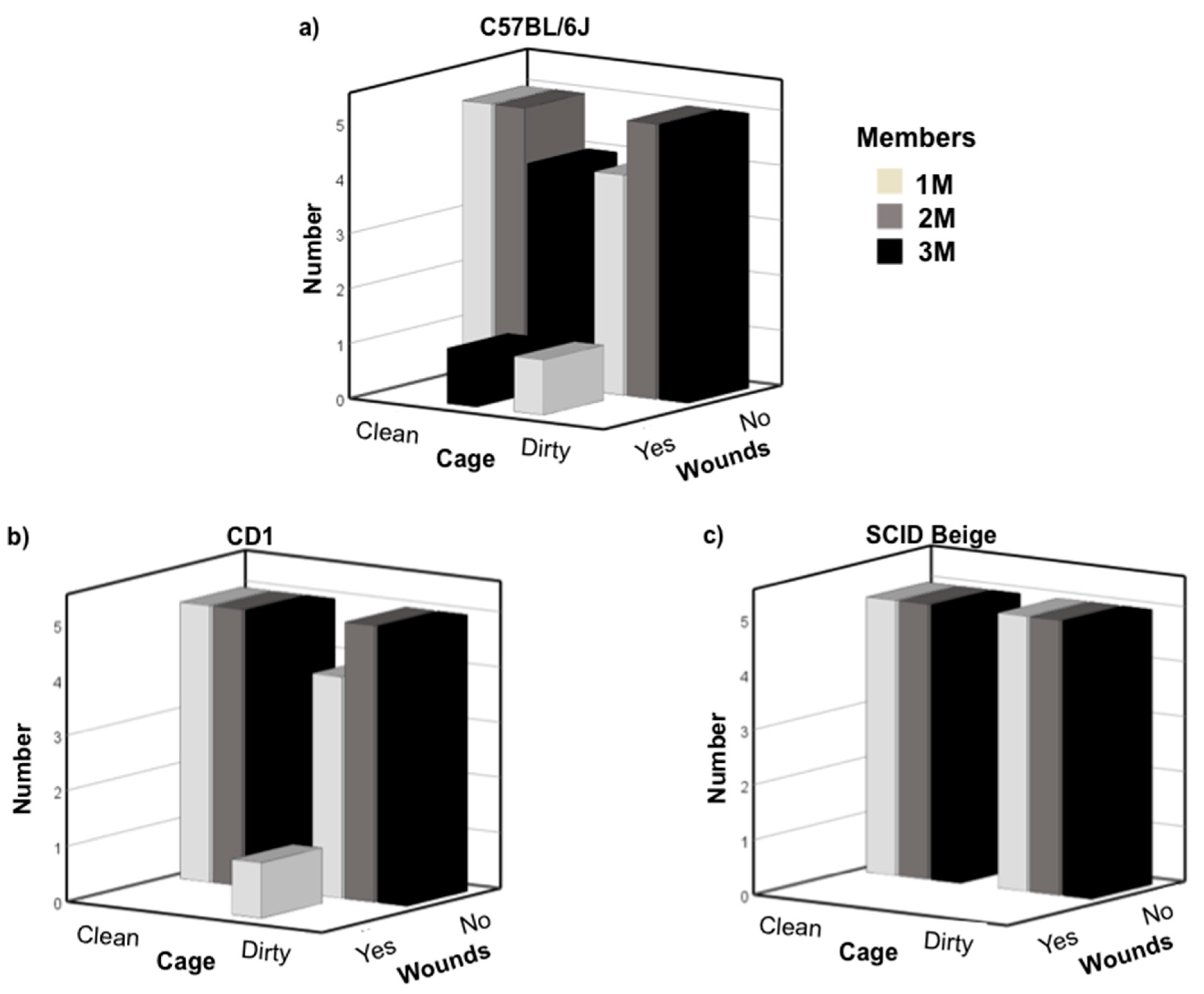
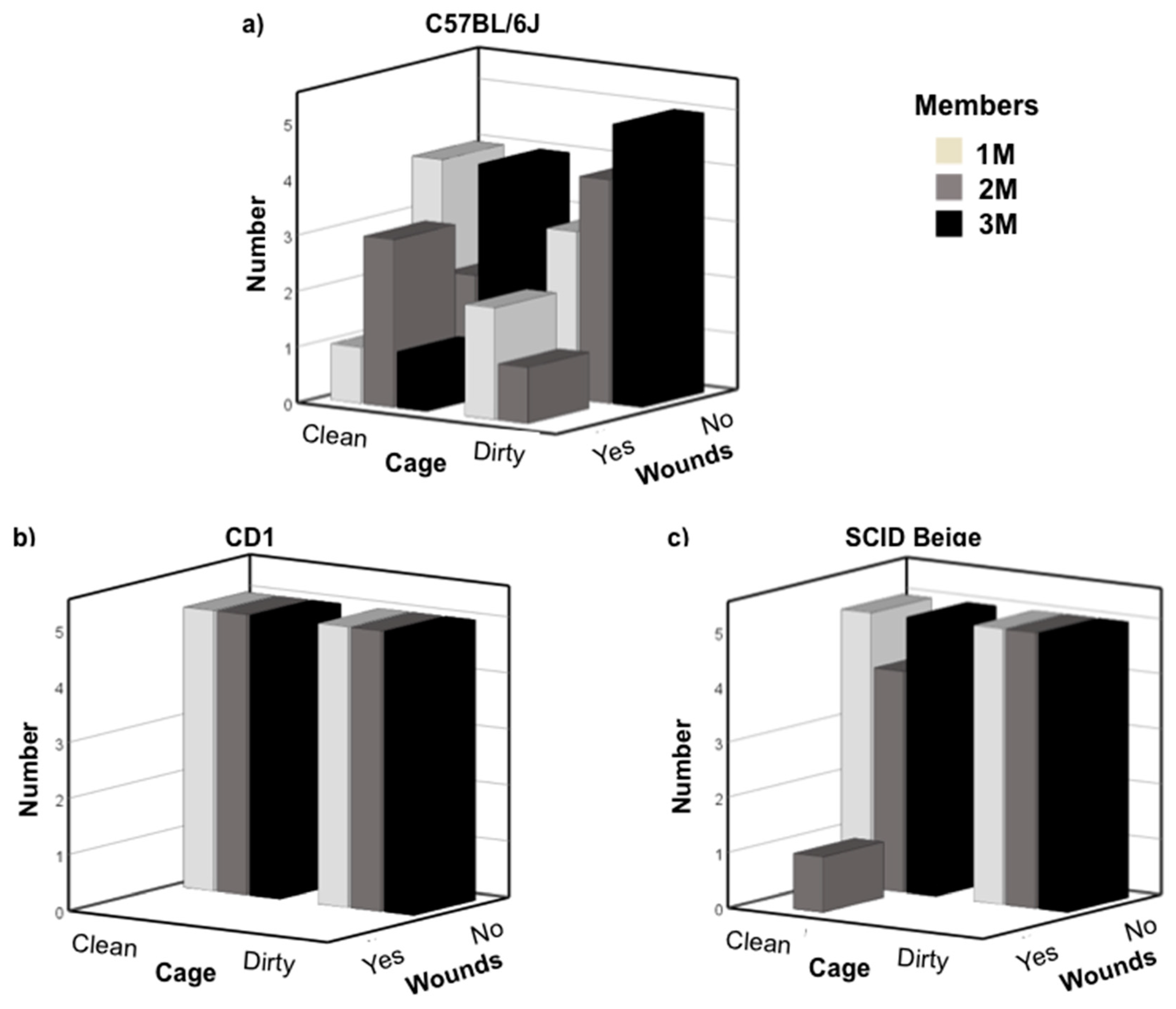
3.1. Newly Weaned Mice Can Be Safely Grouped with Late Juvenile Males
3.2. C57BL/6J Early Pubescent Males Showed More Agonistic Behaviour towards Newly Weaned Mice
3.3. C57BL/6J Late Pubescent Males Showed More Agonistic Behaviour towards Newly Weaned Mice
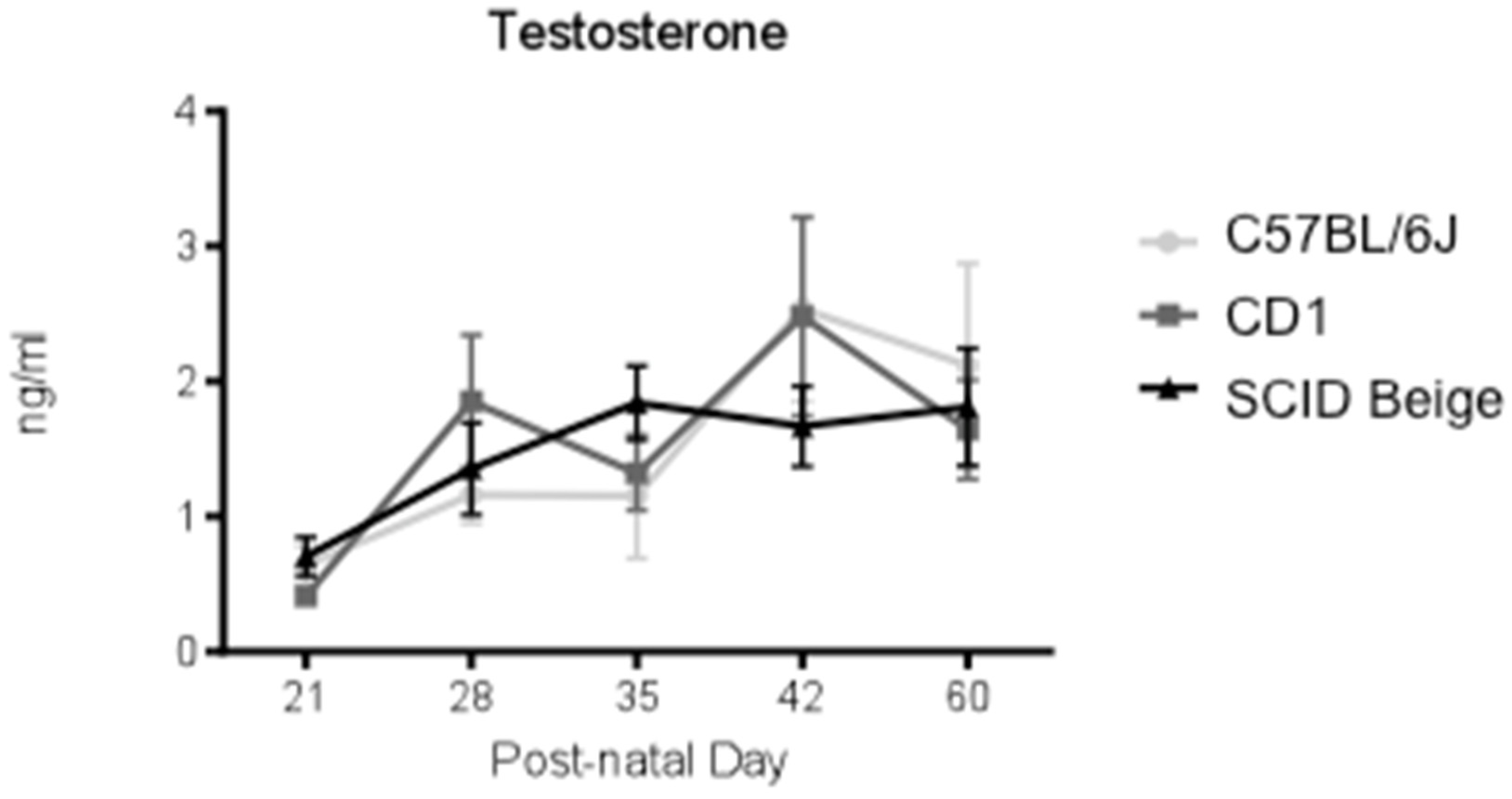
3.4. No Differences in Testosterone Plasma Levels among Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Zegeren, K. Variation in Aggressiveness and the Regulation of Numbers in House Mouse Populations. Neth. J. Zool. 1980, 30, 635. [Google Scholar] [CrossRef]
- Crowcroft, P. Mice All over; G.T. Foulis Company, 7 Milford Lane: London, UK, 1966; p. 123. [Google Scholar]
- Hurst, J.L. Behavioural variation in wild house mice Mus domesticus Rutty: A quantitative assessment of female social organization. Anim. Behav. 1987, 35, 1846–1857. [Google Scholar] [CrossRef]
- Cohn, J.; MacPhail, R.C. Ethological and experimental approaches to behavior analysis: Implications for ecotoxicology. Environ. Health Perspect. 1996, 104 (Suppl. 2), 299–305. [Google Scholar] [PubMed]
- Blanchard, R.J.; Hebert, M.; Sakai, R.R.; McKittrick, C.; Henrie, A.; Yudko, E.; McEwen, B.S.; Blanchard, D.C. Chronic social stress: Changes in behavioral and physiological indices of emotion. Aggress. Behav. 1998, 24, 307–324. [Google Scholar] [CrossRef]
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci Methods 2014, 234, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H. Ethological approach to social isolation effects in behavioral studies of laboratory rodents. Behav. Brain Res. 2018, 341, 98–108. [Google Scholar] [CrossRef]
- Haj-Mirzaian, A.; Amiri, S.; Kordjazy, N.; Rahimi-Balaei, M.; Marzban, H.; Aminzadeh, A.; Dehpour, A.R.; Mehr, S.E. Blockade of NMDA receptors reverses the depressant, but not anxiogenic effect of adolescence social isolation in mice. Eur. J. Pharmacol. 2015, 750, 160–166. [Google Scholar] [CrossRef]
- Okada, R.; Fujiwara, H.; Mizuki, D.; Araki, R.; Yabe, T.; Matsumoto, K. Involvement of dopaminergic and cholinergic systems in social isolation-induced deficits in social affiliation and conditional fear memory in mice. Neuroscience 2015, 299, 134–145. [Google Scholar] [CrossRef]
- Medendorp, W.E.; Petersen, E.D.; Pal, A.; Wagner, L.M.; Myers, A.R.; Hochgeschwender, U.; Jenrow, K.A. Altered Behavior in Mice Socially Isolated During Adolescence Corresponds With Immature Dendritic Spine Morphology and Impaired Plasticity in the Prefrontal Cortex. Front. Behav. Neurosci. 2018, 12, 87. [Google Scholar] [CrossRef]
- Ago, Y.; Takahashi, K.; Nakamura, S.; Hashimoto, H.; Baba, A.; Matsuda, T. Anxiety-like and exploratory behaviors of isolation-reared mice in the staircase test. J. Pharmacol. Sci. 2007, 104, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Ibi, D.; Mizoguchi, H.; Nagai, T.; Nitta, A.; Takuma, K.; Nabeshima, T.; Yoneda, Y.; Yamada, K. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav. Brain Res. 2009, 202, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Haj-Mirzaian, A.; Rahimi-Balaei, M.; Razmi, A.; Kordjazy, N.; Shirzadian, A.; Ejtemaei Mehr, S.; Sianati, H.; Dehpour, A.R. Co-occurrence of anxiety and depressive-like behaviors following adolescent social isolation in male mice; possible role of nitrergic system. Physiol. Behav. 2015, 145, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.J.; Dewsbury, D.A.; Wakeland, E.K.; Potts, W.K. Communal nesting and communal nursing in house mice, Mus musculus domesticus. Anim. Behav. 1995, 50, 741–751. [Google Scholar] [CrossRef]
- Terranova, M.L.; Laviola, G.; de Acetis, L.; Alleva, E. A description of the ontogeny of mouse agonistic behavior. J. Comp. Psychol. 1998, 112, 3–12. [Google Scholar] [CrossRef]
- Makinodan, M.; Rosen, K.M.; Ito, S.; Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012, 337, 1357–1360. [Google Scholar] [CrossRef]
- Himmler, B.T.; Pellis, S.M.; Kolb, B. Juvenile play experience primes neurons in the medial prefrontal cortex to be more responsive to later experiences. Neurosci. Lett. 2013, 556, 42–45. [Google Scholar] [CrossRef]
- Sisk, C.L.; Zehr, J.L. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005, 26, 163–174. [Google Scholar] [CrossRef]
- Luciano, D.; Lore, R. Aggression and social experience in domesticated rats. J. Comp. Physiol. Psychol. 1975, 88, 917–923. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Chirieleison, A.; Gioiosa, L.; Ceresini, G.; Parmigiani, S.; Palanza, P. Age at group formation alters behavior and physiology in male but not female CD-1 mice. Physiol. Behav. 2004, 82, 425–434. [Google Scholar] [CrossRef]
- Jirkof, P.; Bratcher, N.; Medina, L.; Strasburg, D.; Ebert, P.; Gaskill, B.N. The effect of group size, age and handling frequency on inter-male aggression in CD 1 mice. Sci. Rep. 2020, 10, 2253. [Google Scholar] [CrossRef] [PubMed]
- Annas, A.; Bengtsson, C.; Törnqvist, E. Group housing of male CD1 mice: Reflections from toxicity studies. Laboratory. Animals 2013, 47, 127–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Loo, P.L.; Van Zutphen, L.F.; Baumans, V. Male management: Coping with aggression problems in male laboratory mice. Lab. Anim. 2003, 37, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Azkona, G.; Caballero, J.M. Implementing strategies to reduce singly housed male mice. Lab. Anim. 2019, 53, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Mähler Convenor, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab. Anim. 2014, 48, 178–192. [Google Scholar]
- Hutchinson, E.; Avery, A.; Vandewoude, S. Environmental enrichment for laboratory rodents. ILAR. J. 2005, 46, 148–161. [Google Scholar] [CrossRef]
- Weber, E.M.; Dallaire, J.A.; Gaskill, B.N.; Pritchett-Corning, K.R.; Garner, J.P. Aggression in group-housed laboratory mice: Why can’t we solve the problem? Lab. Anim. 2017, 46, 157–161. [Google Scholar] [CrossRef]
- Ortega-Saez, I.; Azkona, G. Estudio comparativo de tres materiales utilizados como lecho para ratones de laboratorio. XIV Congreso SECAL. 2017. Available online: https://www.researchgate.net/publication/344225781_ESTUDIO_COMPARATIVO_DE_TRES_MATERIALES_UTILIZADOS_COMO_LECHO_PARA_RATONES_DE_LABORATORIO (accessed on 15 June 2017). [CrossRef]
- Lockworth, C.R.; Kim, S.J.; Liu, J.; Palla, S.L.; Craig, S.L. Effect of Enrichment Devices on Aggression in Manipulated Nude Mice. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 731–736. [Google Scholar]
- Thrusfield, M.; Ortega, C.; de Blas, I.; Noordhuizen, J.P.; Frankena, K. WIN EPISCOPE 2.0: Improved epidemiological software for veterinary medicine. Vet. Rec. 2001, 148, 567–572. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani, S.; Palanza, P.; Rogers, J.; Ferrari, P.F. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci. Biobehav. Rev. 1999, 23, 957–969. [Google Scholar] [CrossRef]
- Bryant, C.D. The blessings and curses of C57BL/6 substrains in mouse genetic studies. Ann. N. Y. Acad. Sci. 2011, 1245, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Mosier, D.E.; Stell, K.L.; Gulizia, R.J.; Torbett, B.E.; Gilmore, G.L. Homozygous scid/scid;beige/beige mice have low levels of spontaneous or neonatal T cell-induced B cell generation. J. Exp Med. 1993, 177, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.M.; Pothuizen, H.H.; Spruijt, B.M. Ethological concepts enhance the translational value of animal models. Eur. J. Pharmacol. 2015, 759, 42–50. [Google Scholar] [CrossRef]
- Gaskill, B.N.; Stottler, A.M.; Garner, J.P.; Winnicker, C.W.; Mulder, G.B.; Pritchett-Corning, K.R. The effect of early life experience, environment, and genetic factors on spontaneous home-cage aggression-related wounding in male C57BL/6 mice. Lab. Anim. 2017, 46, 176–184. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Perez, A.; Holloway, L.P.; Barbaro, R.P.; Barbaro, J.R.; Wilson, L.M.; Threadgill, D.W.; Lauder, J.M.; et al. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav. Brain Res. 2007, 176, 4–20. [Google Scholar] [CrossRef]
- Sankoorikal, G.M.; Kaercher, K.A.; Boon, C.J.; Lee, J.K.; Brodkin, E.S. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol. Psychiatry 2006, 59, 415–423. [Google Scholar] [CrossRef]
- Curley, J.P.; Jordan, E.R.; Swaney, W.T.; Izraelit, A.; Kammel, S.; Champagne, F.A. The meaning of weaning: Influence of the weaning period on behavioral development in mice. Dev. Neurosci. 2009, 31, 318–331. [Google Scholar] [CrossRef]
- Bisazza, A. Social organization and territorial behaviour in three strains of mice. Boll. Zool. 1981, 48, 157–167. [Google Scholar] [CrossRef]
- Haemisch, A.; Voss, T.; Gärtner, K. Effects of environmental enrichment on aggressive behavior, dominance hierarchies, and endocrine states in male DBA/2J mice. Physiol. Behav. 1994, 56, 1041–1048. [Google Scholar] [CrossRef]
- Lidster, K.; Owen, K.; Browne, W.J.; Prescott, M.J. Cage aggression in group-housed laboratory male mice: An international data crowdsourcing project. Sci. Rep. 2019, 9, 15211. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, P.L.; Van der Meer, E.; Kruitwagen, C.L.J.J.; Koolhaas, J.M.; Van Zutphen, L.F.M.; Baumans, V. Strain-specific aggressive behavior of male mice submitted to different husbandry procedures. Aggress. Behav. 2003, 29, 69–80. [Google Scholar] [CrossRef]
- Golden, S.A.; Aleyasin, H.; Heins, R.; Flanigan, M.; Heshmati, M.; Takahashi, A.; Russo, S.J.; Shaham, Y. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain. Behav. 2017, 16, 44–55. [Google Scholar] [CrossRef]
- Van Loo, P.L.; Mol, J.A.; Koolhaas, J.M.; Van Zutphen, B.F.; Baumans, V. Modulation of aggression in male mice: Influence of group size and cage size. Physiol. Behav. 2001, 72, 675–683. [Google Scholar] [CrossRef]
- Poole, T.B.; Morgan, H.D. Differences in aggressive behaviour between male mice (Mus musculus L.) in colonies of different sizes. Anim. Behav. 1973, 21, 788–795. [Google Scholar] [CrossRef]
- Gray, S.; Hurt, J. The effects of cage cleaning on aggression within groups of male laboratory mice. Anim. Behav. 1995, 49, 821–826. [Google Scholar] [CrossRef]
- Van Loo, P.L.P.; Kruitwagen, C.L.J.J.; Van Zutphen, L.F.M.; Koolhaas, J.M.; Baumans, V. Modulation of Aggression in Male Mice: Influence of Cage Cleaning Regime and Scent Marks. Anim. Welf. 2000, 9, 281–295. [Google Scholar]
- Tsuda, M.C.; Yamaguchi, N.; Ogawa, S. Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport 2011, 22, 259–263. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Jiang, X.; Sun, J.; Tian, L.; Jiao, R.; Tang, Y.; Bai, W. Cyanidin-3-O-glucoside protects against cadmium-induced dysfunction of sex hormone secretion via the regulation of hypothalamus-pituitary-gonadal axis in male pubertal mice. Food Chem. Toxicol. 2019, 129, 13–21. [Google Scholar] [CrossRef]

| Age | Juvenil | Early Pubertal | Late Pubertal | |||
|---|---|---|---|---|---|---|
| Cage Members | Clean | Dirty | Clean | Dirty | Clean | Dirty |
| 1M | 5 | 5 | 5 | 5 | 5 | 5 |
| 2M | 5 | 5 | 5 | 5 | 5 | 5 |
| 3M | 5 | 5 | 5 | 5 | 5 | 5 |
| Grade | Number of Puncture Wounds | Severity Classification |
|---|---|---|
| 1 | 1–10 | Mild |
| 2 | 11–20 | Moderate |
| 3 | ≥21 | Severe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grífols, R.; Zamora, C.; Ortega-Saez, I.; Azkona, G. Postweaning Grouping as a Strategy to Reduce Singly Housed Male Mice. Animals 2020, 10, 2135. https://doi.org/10.3390/ani10112135
Grífols R, Zamora C, Ortega-Saez I, Azkona G. Postweaning Grouping as a Strategy to Reduce Singly Housed Male Mice. Animals. 2020; 10(11):2135. https://doi.org/10.3390/ani10112135
Chicago/Turabian StyleGrífols, Roger, Carolina Zamora, Iván Ortega-Saez, and Garikoitz Azkona. 2020. "Postweaning Grouping as a Strategy to Reduce Singly Housed Male Mice" Animals 10, no. 11: 2135. https://doi.org/10.3390/ani10112135
APA StyleGrífols, R., Zamora, C., Ortega-Saez, I., & Azkona, G. (2020). Postweaning Grouping as a Strategy to Reduce Singly Housed Male Mice. Animals, 10(11), 2135. https://doi.org/10.3390/ani10112135






