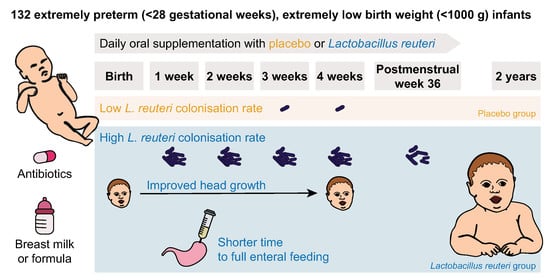
Lactobacillus reuteri Colonisation of Extremely Preterm Infants in a Randomised Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Clinical Outcomes
2.3. Lactobacillus reuteri Cultures
2.4. DNA Extraction
2.5. Quantitative PCR
2.6. Human Milk Oligosaccharide Analysis
2.7. Statistical Analyses
3. Results
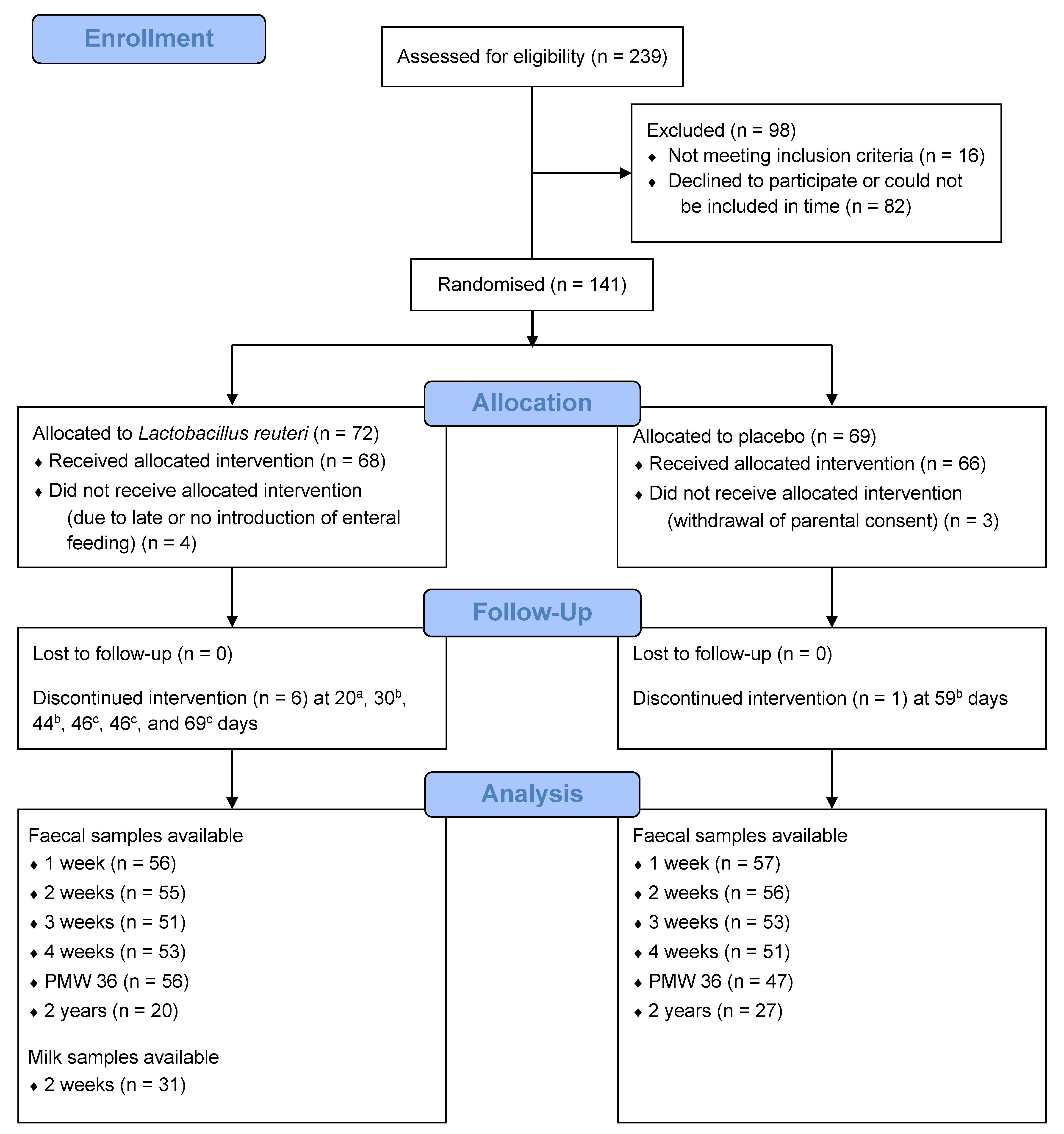
3.1. Study Participants and Samples
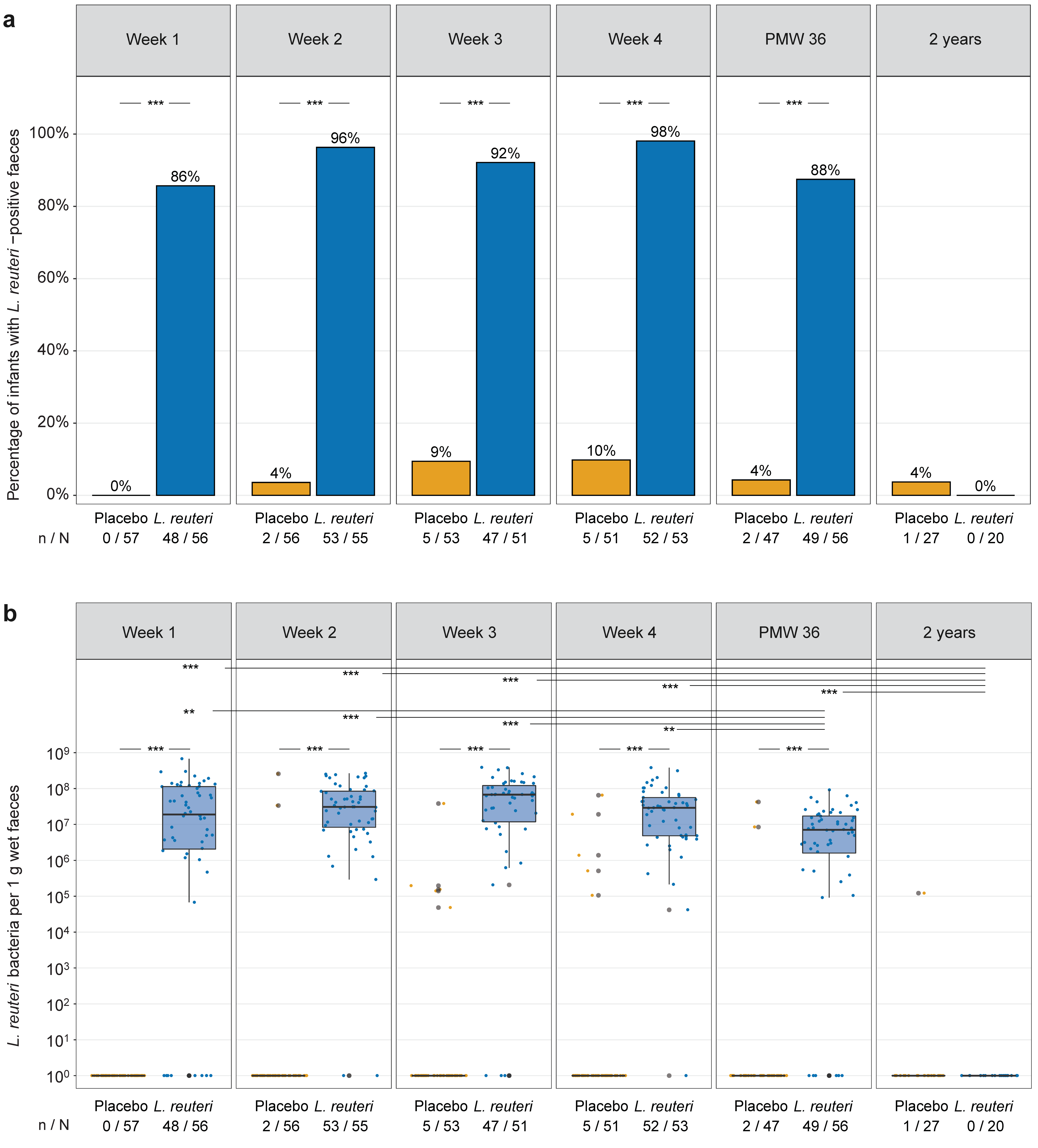
3.2. Lactobacillus reuteri in Infant Faeces
3.3. Maternal and Infant Characteristics and Lactobacillus reuteri Colonisation
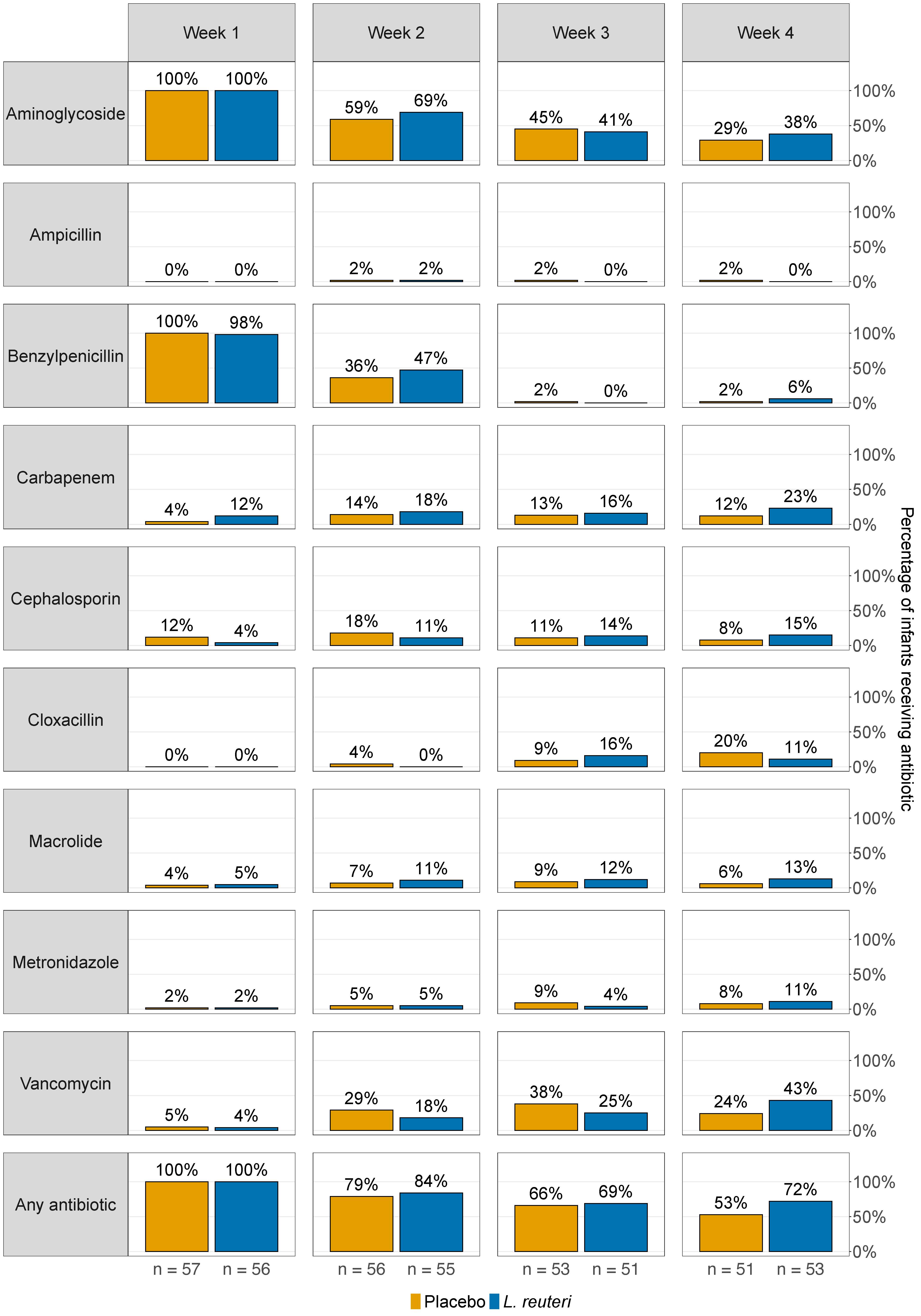
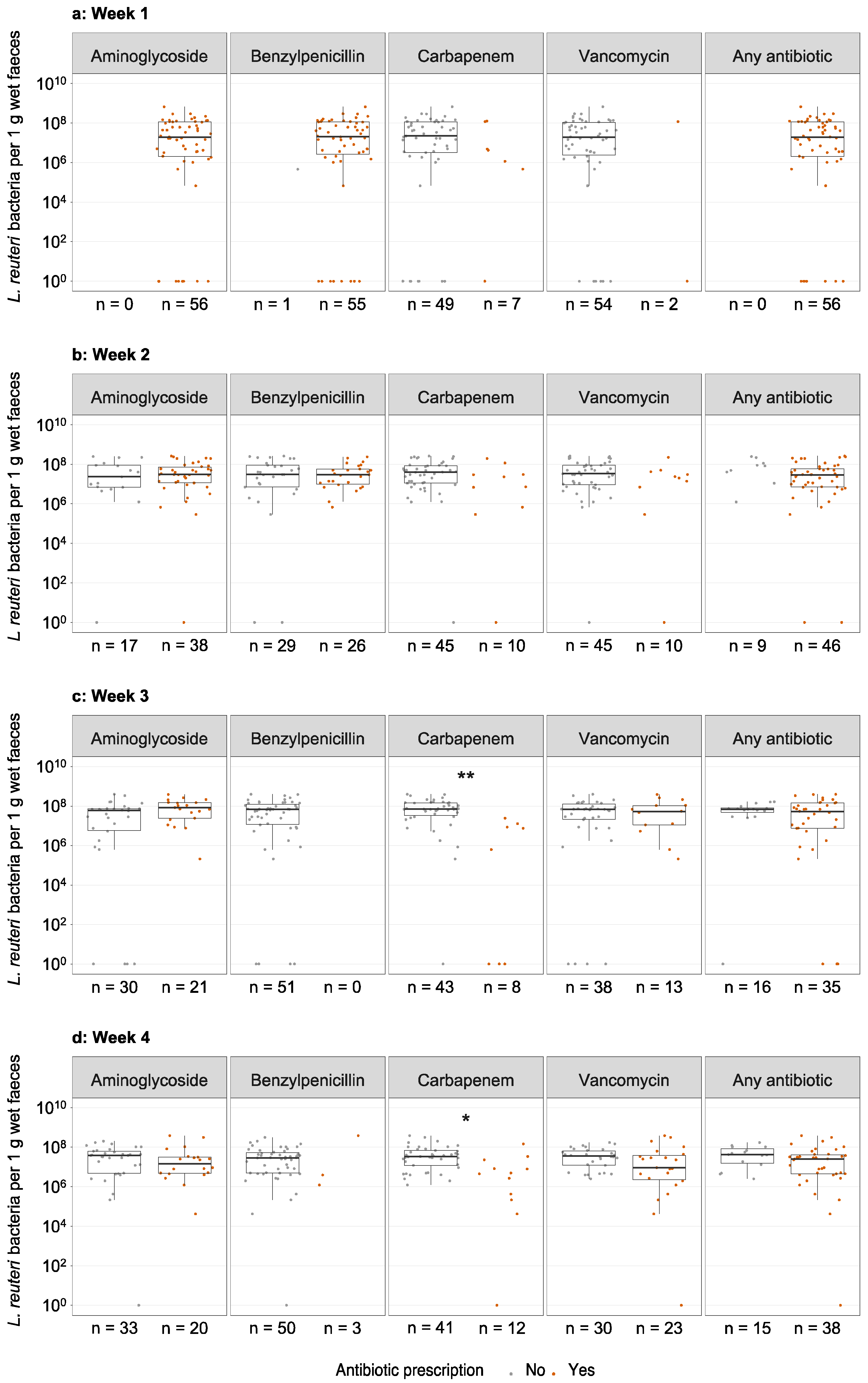
3.4. Antibiotic Treatment and Lactobacillus reuteri Colonisation
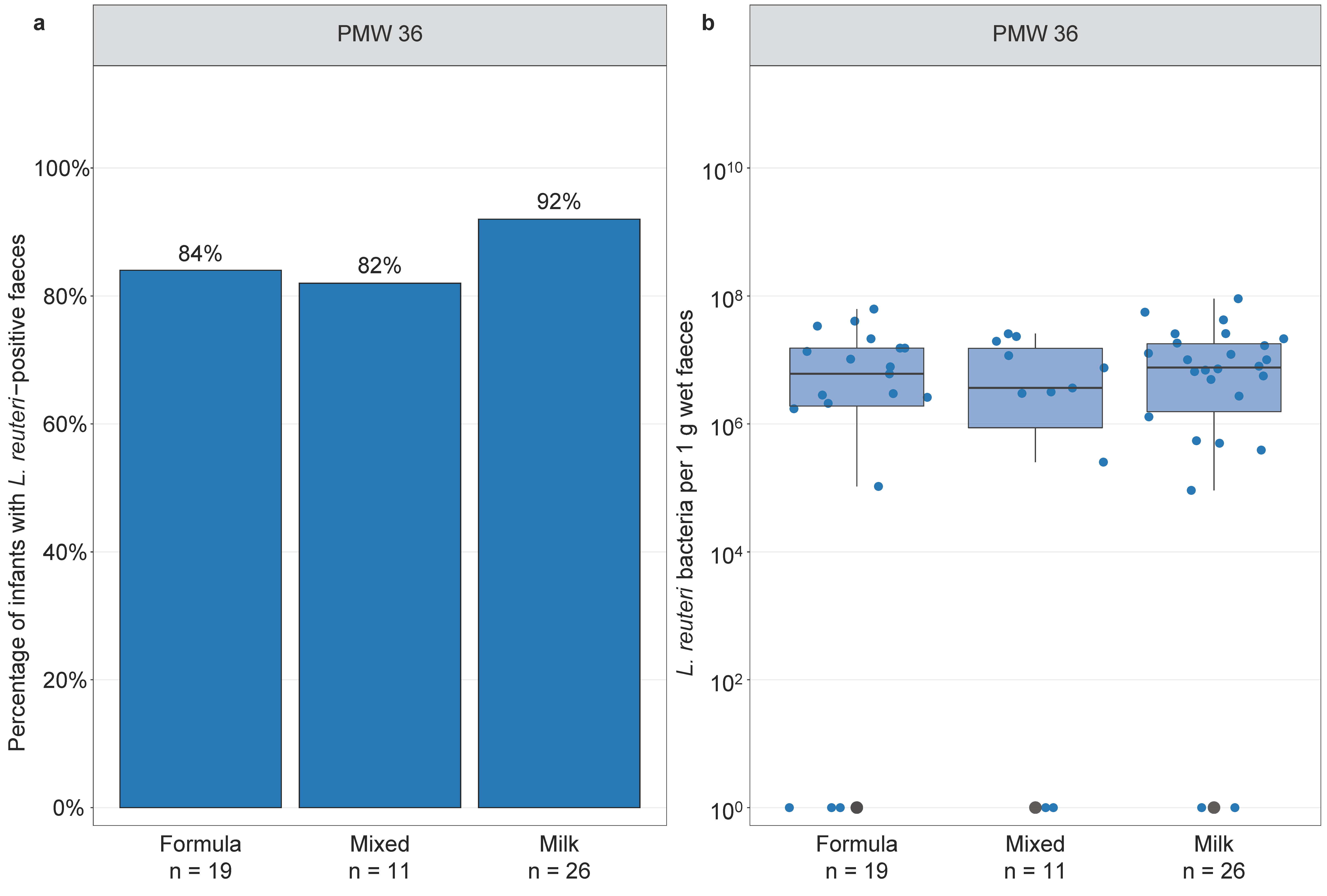
3.5. Infant Feeding and Lactobacillus reuteri Colonisation
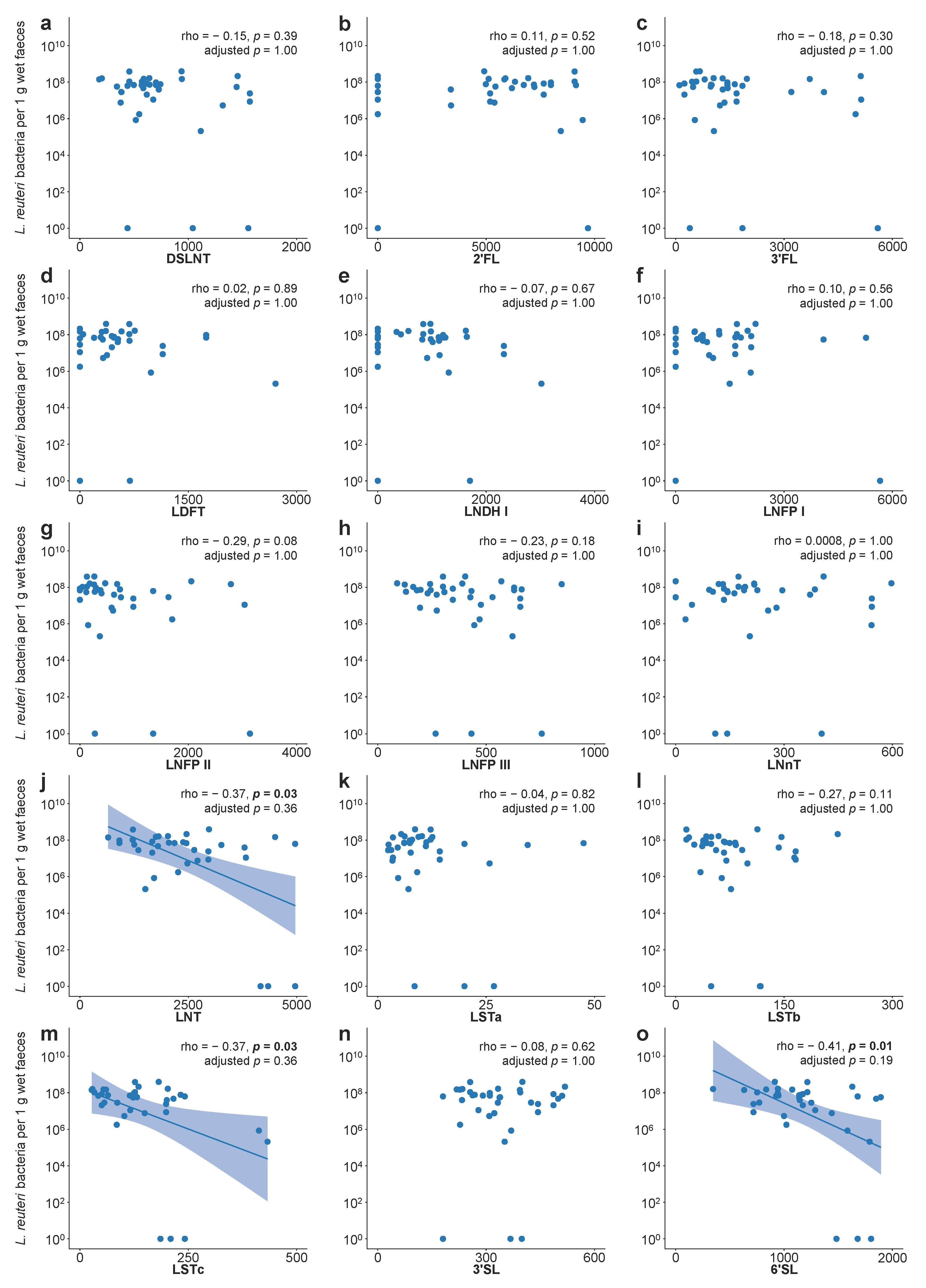
3.6. Human Milk Oligosaccharides and Lactobacillus reuteri Colonisation
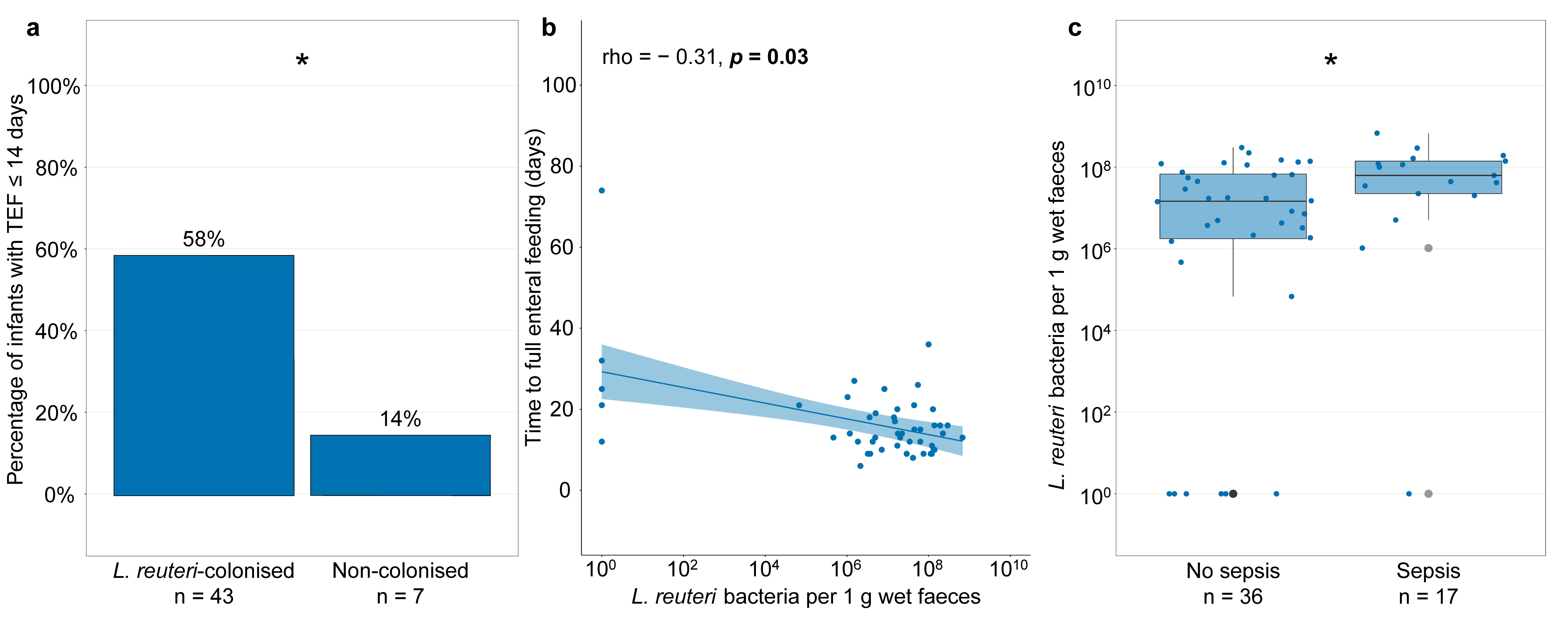
3.7. Lactobacillus reuteri Colonisation and Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norman, M.; Hallberg, B.; Abrahamsson, T.; Björklund, L.J.; Domellöf, M.; Farooqi, A.; Foyn Bruun, C.; Gadsbøll, C.; Hellström-Westas, L.; Ingemansson, F.; et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA 2019, 321, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- AlFaleh, K.; Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.P.; Raine, T.; Reddy, S.; Belteki, G. Probiotics for the prevention of necrotising enterocolitis in very low-birth-weight infants: A meta-analysis and systematic review. Acta Paediatr. 2017, 106, 1729–1741. [Google Scholar] [CrossRef]
- Van den Akker, C.H.P.; van Goudoever, J.B.; Szajewska, H.; Embleton, N.D.; Hojsak, I.; Reid, D.; Shamir, R. Probiotics for preterm infants: A strain specific systematic review and network meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Oncel, M.Y.; Sari, F.N.; Arayici, S.; Guzoglu, N.; Erdeve, O.; Uras, N.; Oguz, S.S.; Dilmen, U. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, 110–116. [Google Scholar] [CrossRef]
- Cui, X.; Shi, Y.; Gao, S.; Xue, X.; Fu, J. Effects of Lactobacillus reuteri DSM 17938 in preterm infants: A double-blinded randomized controlled study. Ital. J. Pediatr. 2019, 45, 1–7. [Google Scholar] [CrossRef]
- Kaban, R.K.; Wardhana, B.H.; Rohsiswatmo, R.; Handryastuti, S.; Amelia, N.; Muktiarti, D.; Indrio, F.; Vandenplas, Y. Lactobacillus reuteri DSM 17938 improves feeding intolerance in preterm infants. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 545–553. [Google Scholar] [CrossRef]
- Wejryd, E.; Marchini, G.; Frimmel, V.; Jonsson, B.; Abrahamsson, T. Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta Paediatr. 2019, 108, 62–69. [Google Scholar] [CrossRef]
- Eaton, S.; Rees, C.M.; Hall, N.J. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology 2017, 111, 423–430. [Google Scholar] [CrossRef]
- Rougé, C.; Piloquet, H.; Butel, M.-J.; Berger, B.; Rochat, F.; Ferraris, L.; Des Robert, C.; Legrand, A.; de la Cochetiere, M.-F.; N’Guyen, J.-M.; et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2009, 89, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016, 387, 649–660. [Google Scholar] [CrossRef]
- Valeur, N.; Engel, P.; Carbajal, N.; Connolly, E.; Ladefoged, K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl. Environ. Microbiol. 2004, 70, 1176–1181. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Sinkiewicz, G.; Jakobsson, T.; Fredrikson, M.; Björkstén, B. Probiotic Lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Spreckels, J.E.; Ranasinghe, P.D.; Wejryd, E.; Marchini, G.; Sverremark-Ekström, E.; Jenmalm, M.C.; Abrahamsson, T. Effects of Lactobacillus reuteri supplementation on the gut microbiota in extremely preterm infants in a randomized placebo-controlled trial. Cell Reports Med. 2021, 2. [Google Scholar] [CrossRef]
- Rosander, A.; Connolly, E.; Roos, S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl. Environ. Microbiol. 2008, 74, 6032–6040. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Kesavelu, D.; Veligandla, K.C.; Muni, S.K.; Mehta, S.C. Lactobacillus reuteri DSM 17938: Review of evidence in functional gastrointestinal disorders. Pediatr. Ther. 2018, 8. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- De Vries, L.S.; Eken, P.; Dubowitz, L.M. The spectrum of leukomalacia using cranial ultrasound. Behav. Brain Res. 1992, 49, 1–6. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Papile, L.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Niklasson, A.; Albertsson-Wikland, K. Continous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Romani Vestman, N.; Hasslöf, P.; Keller, M.K.; Granström, E.; Roos, S.; Twetman, S.; Stecksén-Blicks, C. Lactobacillus reuteri influences regrowth of mutans Streptococci after full-mouth disinfection: A double-blind, randomised controlled trial. Caries Res. 2013, 47, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Wejryd, E.; Martí, M.; Marchini, G.; Werme, A.; Jonsson, B.; Landberg, E.; Abrahamsson, T.R. Low diversity of human milk oligosaccharides is associated with necrotising enterocolitis in extremely low birth weight infants. Nutrients 2018, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Egervärn, M. Antibiotic Resistance in Lactobacillus Reuteri and Lactobacillus Plantarum. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2009. [Google Scholar]
- La Rosa, P.S.; Warner, B.B.; Zhou, Y.; Weinstock, G.M.; Sodergren, E.; Hall-Moore, C.M.; Stevens, H.J.; Bennett, W.E.; Shaikh, N.; Linneman, L.A.; et al. Patterned progression of bacterial populations in the premature infant gut. Proc. Natl. Acad. Sci. USA 2014, 111, 12522–12527. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Tran, D.Q.; Roos, S.; Rhoads, J.M.; Liu, Y. Human breast milk promotes the secretion of potentially beneficial metabolites by probiotic Lactobacillus reuteri DSM 17938. Nutrients 2019, 11, 1548. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Thongaram, T.; Hoeflinger, J.L.; Chow, J.; Miller, M.J. Human milk oligosaccharide consumption by probiotic and human-associated Bifidobacteria and Lactobacilli. J. Dairy Sci. 2017, 100, 7825–7833. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Gänzle, M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides. FEMS Microbiol. Lett. 2011, 315, 141–148. [Google Scholar] [CrossRef]
- Lin, H.-C.; Hsu, C.-H.; Chen, H.-L.; Chung, M.-Y.; Hsu, J.-F.; Lien, R.-I.; Tsao, L.-Y.; Chen, C.-H.; Su, B.-H. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: A Multicenter, Randomized, Controlled trial. Pediatrics 2008, 122, 693–700. [Google Scholar] [CrossRef]
- Bienenstock, J.; Kunze, W.; Forsythe, P. Microbiota and the gut-brain axis. Nutr. Rev. 2015, 73, 28–31. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Chen, R.Y.; Mostafa, I.; Hibberd, M.C.; Das, S.; Mahfuz, M.; Naila, N.N.; Islam, M.; Huq, S.; Alam, M.A.; Zaman, M.U.; et al. A microbiota-directed food intervention for undernourished children. N. Engl. J. Med. 2021, 384, 1517–1528. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef]
- Serenius, F.; Ewald, U.; Farooqi, A.; Fellman, V.; Hafström, M.; Hellgren, K.; Maršál, K.; Ohlin, A.; Olhager, E.; Stjernqvist, K.; et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr. 2016, 170, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Romeo, M.G.; Romeo, D.M.; Trovato, L.; Oliveri, S.; Palermo, F.; Cota, F.; Betta, P. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: Incidence of late-onset sepsis and neurological outcome. J. Perinatol. 2011, 31, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Akar, M.; Eras, Z.; Oncel, M.Y.; Arayici, S.; Guzoglu, N.; Canpolat, F.E.; Uras, N.; Oguz, S.S. Impact of oral probiotics on neurodevelopmental outcomes in preterm infants. J. Matern. Neonatal Med. 2017, 30, 411–415. [Google Scholar] [CrossRef] [PubMed]

| a: Week 1 | |||||
| L. reuteri-Colonised (n = 48) | Non-Colonised (n = 8) | p Value | |||
| Gestational age, weeks, mean (SD) | 25.4 | (1.3) | 25.8 | (1.1) | 0.43 1 |
| Gestational weeks 23–25, n (%) | 31 | (65%) | 4 | (50%) | 0.46 2 |
| Birth weight, g, mean (SD) | 728 | (130) | 702 | (142) | 0.65 1 |
| Birth weight, z-score, mean (SD) | −1.2 | (1.2) | −1.8 | (1.1) | 0.17 1 |
| Small for gestational age (weight < 2 SD), n (%) | 13 | (27%) | 4 | (50%) | 0.23 2 |
| Apgar score at 5 min, median (IQR) | 6.5 | (4.0–8.0) | 8 | (4.0–8.2) | 0.65 3 |
| Male/Female, n/n (%/%) | 22/26 | (46%/54%) | 2/6 | (25%/75%) | 0.44 2 |
| Infant from multiple pregnancy, n (%) | 16 | (33%) | 3 | (38%) | 1.00 2 |
| Caesarean section, n (%) | 36 | (75%) | 6 | (75%) | 1.00 2 |
| Chorioamnionitis, n (%) | 14 | (29%) | 2 | (25%) | 1.00 2 |
| Preeclampsia, n (%) | 3 | (6%) | 2 | (25%) | 0.14 2 |
| Preterm premature rupture of membranes, n (%) | 19 | (40%) | 2 | (25%) | 0.70 2 |
| Maternal smoking, n (%) | 4 | (8%) | 0 | (0%) | 1.00 2 |
| Maternal antibiotics, n (%) | 32 | (67%) | 3 | (38%) | 0.14 2 |
| Antenatal corticosteroids, n (%) | 47 | (98%) | 8 | (100%) | 1.00 2 |
| Full course of antenatal corticosteroids, n (%) | 31 | (65%) | 8 | (100%) | 0.09 2 |
| Inclusion site—Linköping/Stockholm, n/n (%/%) | 19/29 | (40%/60%) | 0/8 | (0%/100%) | 0.04 2 |
| Start of supplementation within 72 h, n (%) a | 41 | (85%) | 7 | (88%) | 1.00 2 |
| Age when receiving first dose, h, mean (SD) | 47 | (20) | 56 | (21) | 0.29 1 |
| Total time with probiotic product, h, mean (SD) b,c | 117 | (39) | 64 | (30) | 0.005 1 |
| b: Postmenstrual Week 36 | |||||
| L. reuteri-Colonised (n = 49) | Non-Colonised (n = 7) | pValue | |||
| Total time without probiotic product, days, median (IQR) | 1 | (0–3) | 27 | (13–39) | 0.02 3 |
| Total time with acid inhibitors, days, median (IQR) | 0 | (0–5) | 5 | (0–10) | 0.35 3 |
| Total time with antibiotics, days, median (IQR) | 26 | (20–37) | 19 | (17–52) | 0.86 3 |
| Total time with insulin, days, median (IQR) | 0 | (0–1) | 0 | (0–1) | 0.86 3 |
| Total time with continuous opioids, days, median (IQR) | 5 | (0–16) | 3 | (0–10) | 0.79 3 |
| Total time with opioid antagonists, days, median (IQR) | 0 | (0–16) | 0 | (0–4) | 0.36 3 |
| Total time with postnatal steroids, days, median (IQR) | 0 | (0–3) | 0 | (0–0) | 0.48 3 |
| Total time with postnatal inhaled steroids, days, median (IQR) d | 24 | (5–39) | 20 | (20–38) | 0.76 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spreckels, J.E.; Wejryd, E.; Marchini, G.; Jonsson, B.; de Vries, D.H.; Jenmalm, M.C.; Landberg, E.; Sverremark-Ekström, E.; Martí, M.; Abrahamsson, T. Lactobacillus reuteri Colonisation of Extremely Preterm Infants in a Randomised Placebo-Controlled Trial. Microorganisms 2021, 9, 915. https://doi.org/10.3390/microorganisms9050915
Spreckels JE, Wejryd E, Marchini G, Jonsson B, de Vries DH, Jenmalm MC, Landberg E, Sverremark-Ekström E, Martí M, Abrahamsson T. Lactobacillus reuteri Colonisation of Extremely Preterm Infants in a Randomised Placebo-Controlled Trial. Microorganisms. 2021; 9(5):915. https://doi.org/10.3390/microorganisms9050915
Chicago/Turabian StyleSpreckels, Johanne E., Erik Wejryd, Giovanna Marchini, Baldvin Jonsson, Dylan H. de Vries, Maria C. Jenmalm, Eva Landberg, Eva Sverremark-Ekström, Magalí Martí, and Thomas Abrahamsson. 2021. "Lactobacillus reuteri Colonisation of Extremely Preterm Infants in a Randomised Placebo-Controlled Trial" Microorganisms 9, no. 5: 915. https://doi.org/10.3390/microorganisms9050915
APA StyleSpreckels, J. E., Wejryd, E., Marchini, G., Jonsson, B., de Vries, D. H., Jenmalm, M. C., Landberg, E., Sverremark-Ekström, E., Martí, M., & Abrahamsson, T. (2021). Lactobacillus reuteri Colonisation of Extremely Preterm Infants in a Randomised Placebo-Controlled Trial. Microorganisms, 9(5), 915. https://doi.org/10.3390/microorganisms9050915