Abstract
Migratory beekeeping is a widely extended practice aimed at increasing the yield of products and pollination services of honey bee colonies. However, it represents a stress factor, as it facilitates the dissemination of diseases and may compromise the genetic identity of the colonies involved. To analyze the extent of these effects, pathogens infestation rate and genetic composition were monitored in a field experiment comparing stationary and migratory colonies sharing the same environmental conditions but differing in management (stationary vs. migratory) and genetic background. We studied the pathogens infestation rate (Varroa destructor, Nosema spp., and Deformed Wing Virus (DWV)) at four different times: before migratory operation, two weeks later, at the end of the migratory period, and two weeks after the return of the migratory hives. An increased incidence of V. destructor and Nosema ceranae and a lower DWV viral load were found in migratory colonies. Temporary changes in genetic diversity were detected regardless of colony type, suggesting that stressors other than management affect the genetic diversity of the colonies. Our study demonstrates that migratory practices have variable effects on the health and genetic diversity of honey bee colonies, which should be taken into account for the development of sustainable beekeeping.
1. Introduction
The importance of the honey bee (Apis mellifera) is related to its crucial role in the pollination of wild plant species and in agroecosystems [1,2,3]. The economic benefits of beekeeping are derived both from the products generated, including honey, wax, pollen, and royal jelly, and the pollination that honey bees provide to many crops [4]. This pollination activity is even more important in the case of migratory beekeeping, as crops and wild plants from different regions and different flowering seasons benefit from honey bee foraging behavior [5].
There has been a demise of honey bee colonies in North America and Europe over the last few decades, with beekeepers reporting unexpected high winter losses of about 30% among managed honey bees in the last decade [6,7,8,9]. To date, large-scale surveys of managed honey bee populations in the US and Europe have been unable to identify a single factor responsible for this colony loss. This has led researchers to hypothesize that a combination of factors is acting in synergy to compromise honey bee survival [10,11,12,13,14]. Among the most important factors that have a negative impact on the longevity of honey bee colonies are parasites, primarily the mite V. destructor [15,16] and its associated viruses [17,18,19] as the Deformed Wing Virus (DWV) [20], and pathogens such as the microsporidia from the genus Nosema, specifically Nosema ceranae [21,22,23,24] and several other bacterial and fungal brood pathogens [25,26,27]. Other factors believed to enhance the detrimental effects of these parasites and pathogens are pesticide exposure [28], poor nutrition [29], reduced genetic diversity [30], and queen failure [31], as well as beekeeper expertise [32] and current honey bee management practices such as migratory beekeeping [33].
To date, the potentially harmful effects of migratory beekeeping have seldom been investigated [34,35,36,37]. Honey bee confinement, vibration and noise, and marked changes in colony temperature are among the negative influences on colonies associated with such practices. Moreover, the installation of colonies in new pollination locations might increase their exposure to pesticides [34,36,38] and pathogens [39,40]. These colonies must also adapt to new environmental conditions and potential stressors, including orientation cues, daily oscillations in temperature, humidity, and wind regimes. A study of the impact of migratory management on honey bee health and their stress response showed a significant decrease in lifespan and an increase in oxidative stress in migratory as opposed to stationary worker honey bees in the USA [38]. Migratory practices also lead to higher drifting rates [41], which increases the horizontal transmission of pathogens and parasites [42]. In fact, transportation and pollination services were recently proposed to increase the infestation rate and abundance of Nosema ceranae [39] and some viruses [33,43] in A. mellifera worker bees.
In Spain, migratory beekeeping is a common practice due to the marked seasonality of the Mediterranean climate. About 80% of the 2.8 million of colonies in Spain are moved in an annual cycle (data from 2017 [44]) that involves transportation over distances of at least 400 km in summer, from the southern and warmer regions of the Iberian peninsula to the northern regions with a milder climate and later flowering season. The extended practice of migratory beekeeping in Spain represents an evident risk for disease spread and a higher infestation rate of pathogens. However, this practice favors the gene flow between migratory and stationary colonies over a large geographic scale in the Iberian Peninsula [45,46]. At the intra-colony level, the environmental conditions of the different settlements of apiaries and the proliferation of pathogens may also affect the genetic diversity in colonies, as both are important selective factors [12,47,48]. Furthermore, there is accumulating evidence that genetic variation can influence host susceptibility to pathogens [49,50,51]. As a result, we set out to determine the effects of migratory beekeeping on the infestation rate of important pathogens (V. destructor, Nosema spp. and DWV) in honey bee colonies in Spain. The hypothesis tested was that stress factors associated with migratory management affect colony health and disease transmission, resulting in a higher infestation rate of pathogens and viruses in the migratory colonies. We also aimed to determine whether migratory beekeeping has an effect on the genetic diversity in the individual colonies, comparing the changes in genetic diversity between migratory and stationary colonies from the same apiary. It is hypothesized that factors associated with migratory beekeeping favor particular patrilines within the colonies, depending on their selective value against external stressors prevailing in the migratory areas. Additionally, changes in the genetic composition of colonies due to queen replacement and its subsequent mating in the region of migration are also expected, which is a practice that represents a significant mechanism of gene flow among honey bee colonies over large geographic distances in Spain.
2. Materials and Methods
2.1. Ethical Statement
The study was conducted under the supervision of researchers of the University of Murcia and the Regional Institute for Food and Forestry Research and Development, Spain. Beekeeping practices were performed in compliance with the Spanish Ministry of Agriculture, Fisheries and Food regulations.
No permits were required to conduct the study.
2.2. Experimental Design
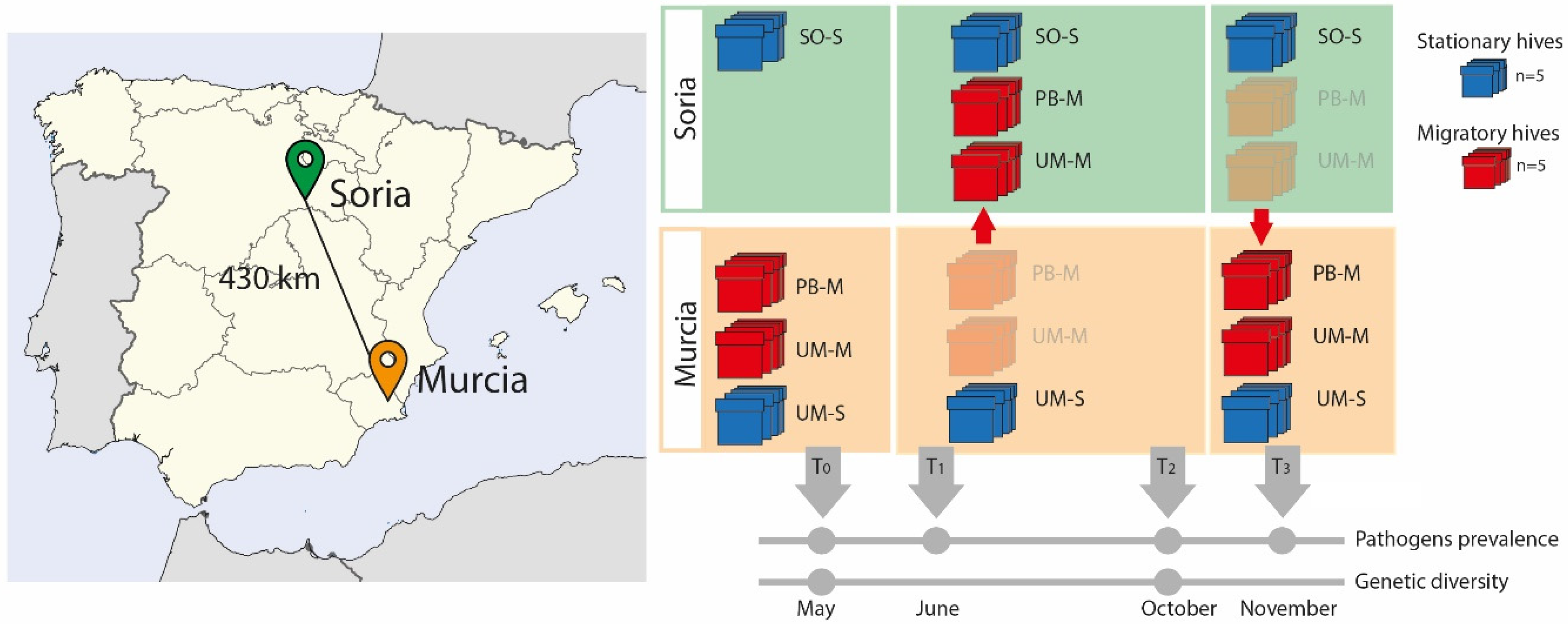
Following a similar research study [35] in this field experiment, ten sentinel hives from the apiary located at the Campus of the University of Murcia (Southeast Spain) were divided into two groups of five hives each. While one group remained stationary (UM-S) during the experiment, the second was moved (UM-M) 430 km to the region of Soria (Northern–Central Spain) during the summer (June–October 2015) (Figure 1). This latter group was moved together with another five hives belonging to a professional beekeeper from Murcia (PB-M) who usually carries out migratory beekeeping. The transported colonies were settled in Soria around 13 km from a stationary apiary of a hobbyist beekeeper (SO-S), thereby sharing the same environmental conditions. Five of these stationary colonies were also sampled. At the beginning of October, the UM-M and PB-M colonies were transported back from Soria to Murcia in the same truck. Thus, samples investigated in the experiment came from four groups of colonies, namely, two from the university campus of Murcia (hives that remained in Murcia, UM-S and those transported to Soria, UM-M), one from the professional beekeeper (hives transported to Soria, PB-M), and that of stationary colonies from Soria (SO-S).
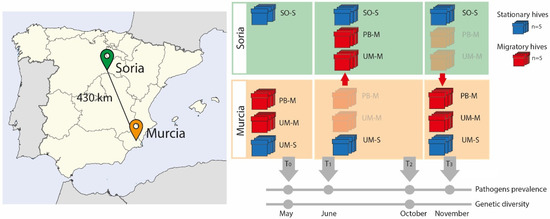
Figure 1.
Graphic representation of the sample design. The map on the left shows the location of the two localities from where the hives were moved (Murcia) to the new location (Soria). On the right are representations of the two types of hives sampled (red = migratory, blue = stationary) and the timeline indicating the months in which samples were taken for the different analyses. (UM means Murcia, PB is professional beekeeper, SO is Soria, M = migratory, S = stationary).
This experimental design was established to assess the effects of migratory movements in a single year, as environmental factors are not homogeneous from one year to the following. In this design, we included groups of migratory (UM-M and PB-M) and stationary (UM-S and SO-S) hives. Comparisons were made between (i) groups sharing similar genetic background but subjected to different management strategies (UM-M vs. UM-S); (ii) groups with different genetic backgrounds but subjected to the same management (UM-M vs. PB-M); and (iii) groups settled in the same location (Soria) sharing the same environmental conditions but with different genetic background (UM-M vs. PB-M), and different management strategies (SO-S) (Figure 1). See Table S1 for colony codes.
2.3. Sampling Dates and Collection
The 20 colonies included in the experiment were sampled on four different months in 2015: in May before moving the hives from Murcia to Soria (T0); in June, two weeks after transportation and settling the migratory hives in Soria (T1); in October, at the end of the migratory season (T2); and in November, two weeks after transportation of hives back to Murcia (T3). The stationary colonies from Soria (SO-S) were only sampled during the migratory season (T1 and T2), when the UM-M and PB-M hives were in their proximity (Figure 1). Samples of adult honey bee workers were taken from combs located between those with sealed brood and those with fresh honey in order to sample both nursing and foraging workers [52]. Samples were kept either in absolute ethanol at −20 °C or frozen directly at −80 °C. Due to colony die-off, we were unable to sample honey bees from all 20 colonies at each time point, as explained later.
2.4. Varroa Destructor Detection
The infestation rate of V. destructor was estimated from about 200 worker honey bees per colony at each sampling time. A total of 70 samples were evaluated: 15 colonies (5 UM-M, 5 PB-M and 5 UM-S) and 4 sampling times, plus 5 colonies of the SO-S apiary sampled at T1 and T2. Varroa mites were dislodged from honey bee workers preserved in ethanol after being shaken, and the proportion of infested individuals per colony was calculated by dividing the number of mites counted by the number of honey bees in each sample [53].
2.5. Extraction and Purification of DNA and RNA
Simultaneous RNA and DNA extraction was carried out to detect Nosema spp. and to quantify DWV. For this, the abdomen of 22 honey bees from each colony and sampling time were macerated in AL buffer [54]. The abdomen of each single honey bee was removed and placed in one well of a 96-well plate containing glass beads (2 mm; Sigma®, St. Louis, MO, USA) and then macerated in 180 µL of AL buffer (Qiagen® 19075, Hilden, Germany) and shaken in a Tissuelyser (Qiagen®) for 6 min at 30 Hz. Afterward, the homogenate was transferred to another plate (Deepwell, Eppendorf®, Hamburg, Germany) and incubated for 10 min with 20 µL of proteinase K (10 mg/mL) and 200 µL of the AL buffer at 70 °C, shaking at 300 rpm.
DNA and RNA purification was performed simultaneously in a Biosprint 96 workstation (Quiagen®, Hilden, Germany) following the BS96 DNA Tissue extraction protocol. Total nucleic acids (RNA and DNA) were eluted in 100 µL of elution buffer, and aliquots of 75 µL of this eluted product were frozen until further processing to detect Nosema spp. The remaining 25 µL obtained from each honey bee from the same colony and sampling time were pooled (hereafter referred to as pooled cDNA) [55], and any genomic DNA was completely removed by digestion with DNase I (Quiagen® kit 79254). Ten µL of this DNA-free pooled RNA were used to generate cDNA using the iScriptTM cDNA Synthesis Kit (Biorad®, Hercules, CA, USA). Negative and positive controls were run in parallel for each step (maceration, nucleic acid extraction, and reverse transcription).
2.6. Nosema spp. Detection
Aliquots of 2.5 µL of the total nucleic acids eluted from the abdomen of each individual honey bee were used to detect N. apis and N. ceranae. PCR reactions were performed as described previously [56], using an internal PCR control to determine the reliability of the analysis. The infestation rate per colony was estimated from the number of nosema positive honey bee individuals divided by the total number (22 per colony) of honey bees analyzed [57].
2.7. Quantification of DWV
Quantification of the viral load of each colony and time was performed by qPCR in a 384-well plate using the LightCycler® 480 Real Time PCR System (Roche®, Basel, Switzerland). The qPCR was carried out in a total volume of 10 µL containing 2× LightCycler® 480 Probes Master, 0.3 µM of the primers DWV958F and DWV9711R, 0.1 µM of the FAM marked probe DWV9627T [58], and 2.5 µL of the pooled cDNA. The qPCR conditions were 95 °C for 10 min, and 45 cycles of 95 °C 10 s and 60 °C 40 s (annealing temperature). Each sample was run in duplicate, and the average Cq values were calculated. Positive individuals were only considered when the average Cq value was <40 [59]. A known amount of DWV PCR product was run in parallel to obtain a standard curve that was used to convert the Cq data to the initial concentration of DWV RNA in the reaction tube. Positive and negative PCR controls were also included. The online tool “dsDNA copy number calculator” (hosted in http://cels.uri.edu/gsc/cndna.html) was utilized to calculate the equivalence between amount (ng) of DWV RNA and DWV copy number in each qPCR reaction. Viral load was calculated for each colony and sampling time by averaging the amount of RNA extracted from 80.5 mg of honey bee abdomens. To calculate the viral load per individual honey bee, we determined the average amount of RNA in a single honey bee by taking into account the elution volume and the average weight of one honey bee [55]. Prior to data analysis, virus copy number was log10 transformed to account for the exponential distribution of the data [60]. Since zero values cannot be log-transformed, negative samples were assigned a hypothetical Cq value of 40 and later converted to the theoretical virus copy number as described above.
2.8. Number of Combs
The number of combs with a capped brood per colony was annotated for every colony at each of the four sampling times, as a qualitative measure of their strength and general health status [61]. This also served to assess the extent to which this characteristic, often evaluated by beekeepers when checking health and vitality status of the colonies, is related to pathogen infestation rate.
2.9. Statistical Analysis
Calculation and graphical representation of the different descriptive measures were implemented with the R v. 3.2.2 software [62]. The normal distribution of the data obtained of each group of colonies and the equality of variance between the groups of data were tested with the Kolmogorov–Smirnov normality test and the F-test of equality of variances, respectively. Thereafter, T-tests or Mann–Whitney-U tests if required were used to compare means between groups. All these analyses were carried out in PAST v.3.14 [62]. In collapsing colonies, the number of brood combs was considered as zero, and the pathogen infestation rate and viral load were considered as not available (NA) at the sampling times subsequent to colony collapse. Possible correlations between the different variables were assessed with the Pearson’s correlation test using PAST v.3.14.
A Principal Component Analysis (PCA) was also performed with PAST v.3.14 software to compare the relative weight of the different variables (brood combs, pathogens infestation rate, and virus load), as well as to assess the explanatory power of these variables on the dispersion of the colonies, according to the type of beekeeping management.
2.10. Sampling for Genetic Diversity and Patrilineal Composition of the Colonies
Honey bee workers were sampled at T0 (May) prior to the movement of the five migratory UM-M hives to Soria, and at T2 (October) after the migratory season (Figure 1). The analysis of the genetic diversity of 48 workers per colony that results from the queen and the multiple droned she mated with, allows the assessment of the impact of transhumance due to queen replacement and subsequent mating with local drones. Samples of 48 worker honey bees per colony and sampling time were preserved in absolute ethanol at −20 °C until processed.
2.11. Microsatellite Amplification and Detection
The determination of the number of patrilines and the genetic diversity were inferred from the analysis of five microsatellite loci [63]. The total DNA from each worker honey bee (48 per colony) was extracted using the Chelex® method [64], and 2 µL of this DNA was used for multiplex microsatellite amplification in a total volume of 10 µL containing 1× reaction buffer, 1.2 mM MgCl2, 0.3 mM of each dNTP, 0.4 µM of each primer (one of each pair fluorescence labeled), and 1.5 units of Taq polymerase (Biotools®, Madrid, Spain). The annealing temperature was set at 54 °C, and the PCR products were visualized by capillary electrophoresis (ABI-3730, Applied Biosystems®, Foster City, CA, USA), scoring individual alleles using GeneMapper v3.7 software (Applied Biosystems®).
2.12. Microsatellite Data Analysis
The following population genetic parameters were calculated for each group of colonies (migratory and stationary), and at each sampling time (T0 and T2) based on allele frequency and using the GenAlex v. 6.41 software [65]: expected heterozygosity (He), number of different alleles (Na), number of effective alleles (Ne), and number of private alleles (Pa). Fisher’s exact test was applied to detect genetic differentiation [66] in colonies between T0 and T2 in Genepop On the Web (http://genepop.curtin.edu.au/genepop_op3.html). Honey bee worker genotypes were analyzed with the Colony 2.0.5 software [67] to unravel the patrilineal composition of each colony at each sampling time. Events of queen replacement and honey bee drifting were also extrapolated with this software.
3. Results
3.1. Parasite and Pathogen Assays
3.1.1. Varroa Destructor
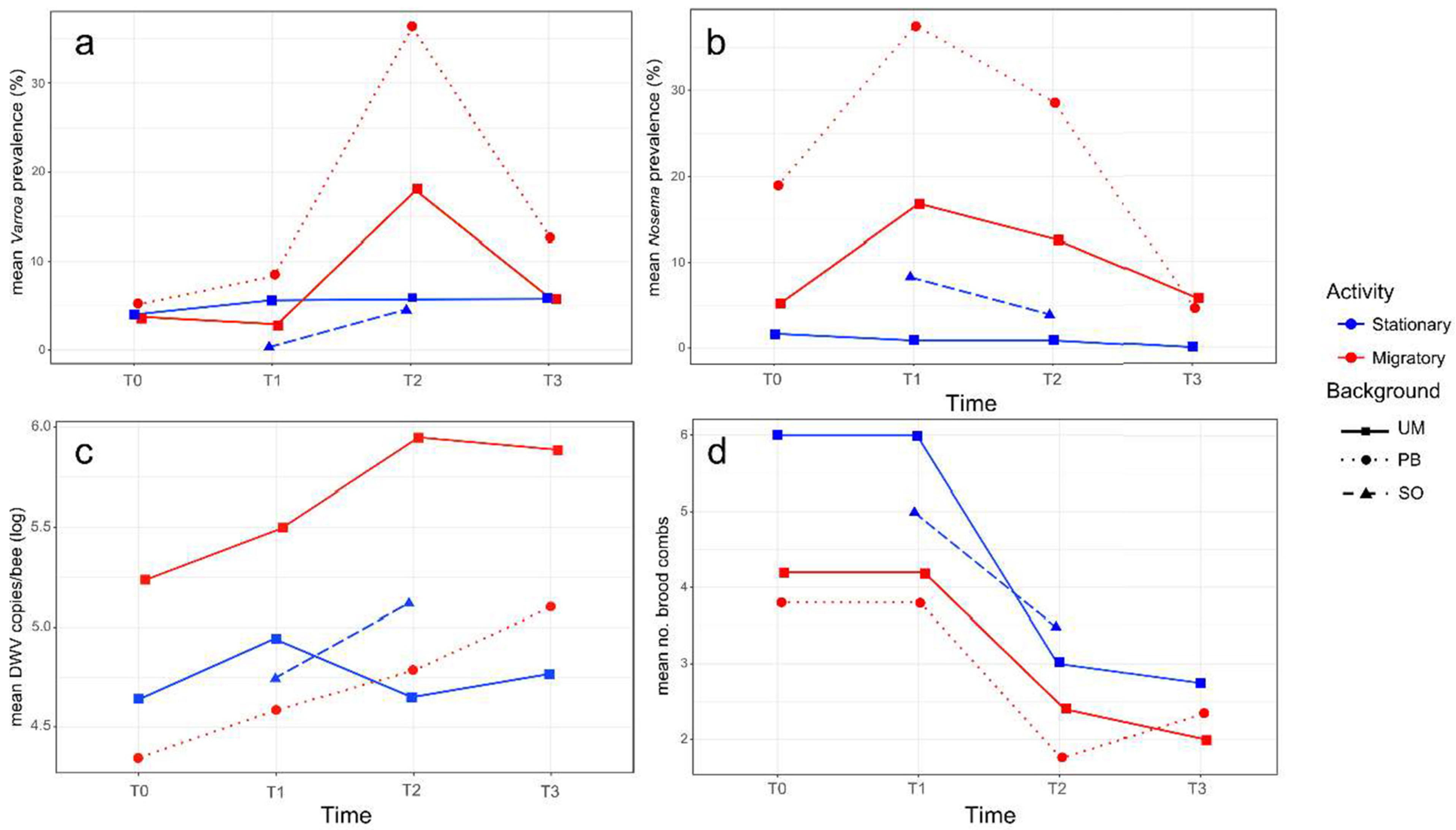
A low infestation rate of V. destructor was detected in all the colonies at the beginning of the study (T0: mean values 4.5 ± 6.2% in the migratory that is UM-M plus PB-M groups, and 4.0 ± 2.3% in the stationary colonies, that is UM-S plus SO-S). No significant changes (N = 35, p > 0.05) were observed after transportation to Soria in the migratory and stationary colonies respectively (T1: 5.7 ± 11.1% and 3.0 ± 4.0%), although a significant increase (N = 39, z = −2.165, p = 0.030) in the infestation rate of varroa was detected in migratory colonies after the summer period (T2: 26.1 ± 29.2%); the infestation rate in stationary colonies remained low (T2: 5.2 ± 6.8%). Indeed, the former displayed a significantly higher mite infestation rate than the stationary colonies at T2 (N = 19, t = 2.205, p = 0.042) (see Figure 2a for mean values per group), with colonies 1M (UM-M), 7M (PB-M), and 10M (PB-M) registering the highest infestation rate at T2 (42%, 83%, and 61% respectively, Table S1).
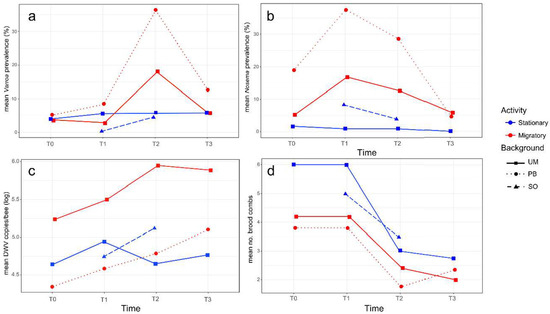
Figure 2.
Average data per colony group (stationary in blue and migratory in red), genetic background (squares for the University of Murcia (UM), circles for the professional beekeeper (PB) and triangles for Soria (SO)) and sampling time (T0, T1, T2 and T3) of the variables: (a) infestation rate of V. destructor; (b) infestation rate of N. ceranae; (c) log10 DWV copy number/bee; (d) number of brood combs.
When comparing migratory and stationary colonies from the University of Murcia (same genetic background), a trend toward a higher infestation rate of varroa mites (N = 10, t = 1.503, p = 0.078) was observed in the migratory colonies at T2 (UM-M: 18.0 ± 14.2% vs. UM-S: 8.0 ± 9.1%). This difference was not significant (N = 8, p > 0.05) after the colonies returned to Murcia at T3, due to a decrease in V. destructor infestation rate in these migratory group of colonies (UM-M: 8.56 ± 11.04%), while the infestation rate remained stable in the stationary colonies (UM-S: 5.8 ± 3.9%). Migratory colonies with different genetic backgrounds, UM-M and PB-M, displayed a distinct response to V. destructor, as the PB-M colonies suffered a stronger increase in varroa infestation rate (from T0: 5.12 ± 8.57% to T2: 36.28 ± 41.98%) than the UM-M colonies (from T0: 3.78 ± 2.28% to T2: 17.96 ± 9.06%) in the migratory period. The standard deviation was also higher in the PB-M hives, showing large differences in varroa infestation rate among individual colonies within the group (Table S1). The stationary colonies in Soria (SO-S) that shared the location and environmental conditions with UM-M and PB-M colonies from June to October (T1 and T2) showed the lowest infestation rate of V. destructor in June (T1: 0.28 ± 0.63%). This rate increased slightly in October (T2: 4.58 ± 4.59%), showing a similar value to that recorded in the UM-S group at that time (T2: 5.76 ± 9.06%).
3.1.2. Nosema spp.
The total number of worker honey bee individuals analyzed by PCR was 1413, as four colonies died off throughout the experiment (Table S1). Nosema spp. were detected in 10.9% of them; concretely, N. ceranae was detected in 84% of the infected workers, N. apis was detected in 8% and co-infection with both species was detected in the remaining 8%. Co-infection increased from T0 to T1 (Table S1), but since the N. apis infestation and co-infection rates were low and unsuitable for proper statistical analysis, the following data mainly refer to N. ceranae infection.
The infection rate of the microsporidium was distinct among the colonies at the beginning of the experiment possibly due to the random selection of the hives analyzed (Figure 2b), as at T0, the infection rate of N. ceranae in migratory colonies was 9.9 ± 10.03% and 1.7 ± 1.7% in the stationary colonies. At T1, the infection rate of N. ceranae increased significantly (N = 35, z = −2.387, p = 0.017) in migratory (T1: 26.8 ± 15.74%) but not in the stationary hives, as UM-S colonies maintained a lower infection rate (T1: 0.9 ± 2.0%). Therefore, a higher N. ceranae infection rate was detected in migratory as opposed to stationary colonies at T1 (N = 20, z = −3.184, p = 0.002). After the summer period (T2), the infection rate of this microsporidium tended to decrease in the two groups of colonies, yet the migratory colonies (T2: 18.1 ± 15.54%) still maintained a higher infection rate of N. ceranae (N = 19, z = −2.892, p = 0.004) than the stationary colonies (T2: 2.1 ± 3.15%). Two weeks after their transportation back to Murcia (T3), the infection rate of N. ceranae kept the decreasing trend in the migratory colonies.
Between the two groups of migratory colonies (with different genetic background), PB-M colonies had a greater infection rate of N. ceranae (T1: 36.7 ± 14.57%) than UM-M colonies after they were transported to Soria (T1: 16.8 ± 9.9%; N = 10, z = −2.089, p = 0.037).
3.1.3. DWV Loads
From the 65 pools of RNA extracted from 22 worker honey bees per colony and at the distinct sampling times, DWV was detected in 35 pools (53.9%) with mean viral loads ranging from 2.22 × 104 to 6.89 × 106 DWV copies/bee (Table S1). Due to the random selection of the hives, the initial viral load at T0 differed widely among the colonies irrespective of their management. However, at T1, the average DWV load tended to increase for the migratory and stationary colonies, albeit not significantly (N = 35, p > 0.05). After the migratory period, at T2, there were no clear trends in DWV load between the stationary and migratory colonies (Figure 2c). In general, the largest differences were detected among the individual colonies over the entire study period (T0 to T3), although a temporal fluctuation at the colony level was also observed, irrespective of the management group.
The UM-M and UM-S colonies registered a distinct initial situation, with a tendency (although not significant, N = 10, t = 1.80, p = 0.31) toward higher DWV loads in UM-M (T0: 8.13 × 105 ± 1.38 × 106 DWV copies/bee) than in UM-S (T0: 6.58 × 104 ± 7.14 × 104 DWV copies/bee). In June (T1), both groups of colonies showed a slight increase in DWV copies/bee, while at T2 (at the end of the migration period), the DWV load had increased in UM-M (T2: 1.4 × 106 ± 1.5 × 106 copies/bee), whereas it had decreased in UM-S (T2: 4.4 × 104 ± 6.0 × 106 copies/bee). Nevertheless, no significant differences (N = 10, t = 2.03, p = 0.07)) were found in the DWV load between the two groups at T2 due to the high standard deviation registered. Likewise, similar viral loads were found in the two groups of colonies at T3 (Figure 2c).
For migratory colonies that differed in their genetic background (UM-M and PB-M), the initial situation was also distinct. The DWV viral load was below the detection threshold in all the PB-M colonies at T0, while the UM-M colonies registered an average viral load of 4.45 × 104 ± 2.47 × 104 DWV copies/bee. However, despite the initial differences, both groups showed a tendency toward a higher DWV load at T1 (UM-M: 6.7 × 105 ± 9.6 × 105 and PB-M: 2.4 × 104 ± 1.5 × 104 copies/bee) and T2 (UM-M: 1.4 × 106 ± 1.5 × 106 and PB-M: 1.8 × 105 ± 3.4 × 105 copies/bee), while at T3, the DWV load tended to decrease slightly in UM-M (8.1 × 105 ± 6.3 × 105 copies/bee) and tended to increase (although not significantly, N = 9, t = 0.36, p = 0.73) in PB-M (7.6 × 105 ± 1.3 × 106 copies/bee). In the case of the SO-S colonies established close to the migratory area, a tendency toward an increased DWV load was observed from T1 (8.2 × 104 ± 1.4 × 105 copies/bee) to T2 (7.9 × 105 ± 1.7 × 106 copies/bee, p > 0.05), while in the colonies settled in Murcia (UM-S), a tendency toward a decrease in DWV load was registered in the same period (T1: 1.1 × 105 ± 1.36 × 105 to T2: 4.4 × 104 ± 6.0 × 106 copies/bee, p > 0.05).
3.1.4. Brood Combs
There was a slightly higher mean number of brood combs in stationary as opposed to migratory colonies at the beginning of the experiment (T0: 5.5 ± 2.1 and 4.0 ± 3.0, respectively). The same number of brood combs was also found at T1, two weeks after the transportation of the migratory colonies to Soria. At T2, the colony 6M (from the PB-M group) collapsed, and fewer brood combs were detected after the summer period in both groups of colonies (T2: 3.2 ± 1.6 and 2.1 ± 1.0 respectively, Figure 2d). This decrease was significant in the stationary group (N = 10, t = 2.680, p = 0.016), although at T3, after transporting the migratory hives back to Murcia, a similar low mean number of brood combs was observed in the migratory and stationary colonies (2.14 ± 0.7 and 2.8 ± 1.9, respectively). Colonies 1M (UM-M), 4S (UM-S), 6M, and 7M (PB-M) had collapsed by that time (T3).
3.1.5. Correlation Analysis
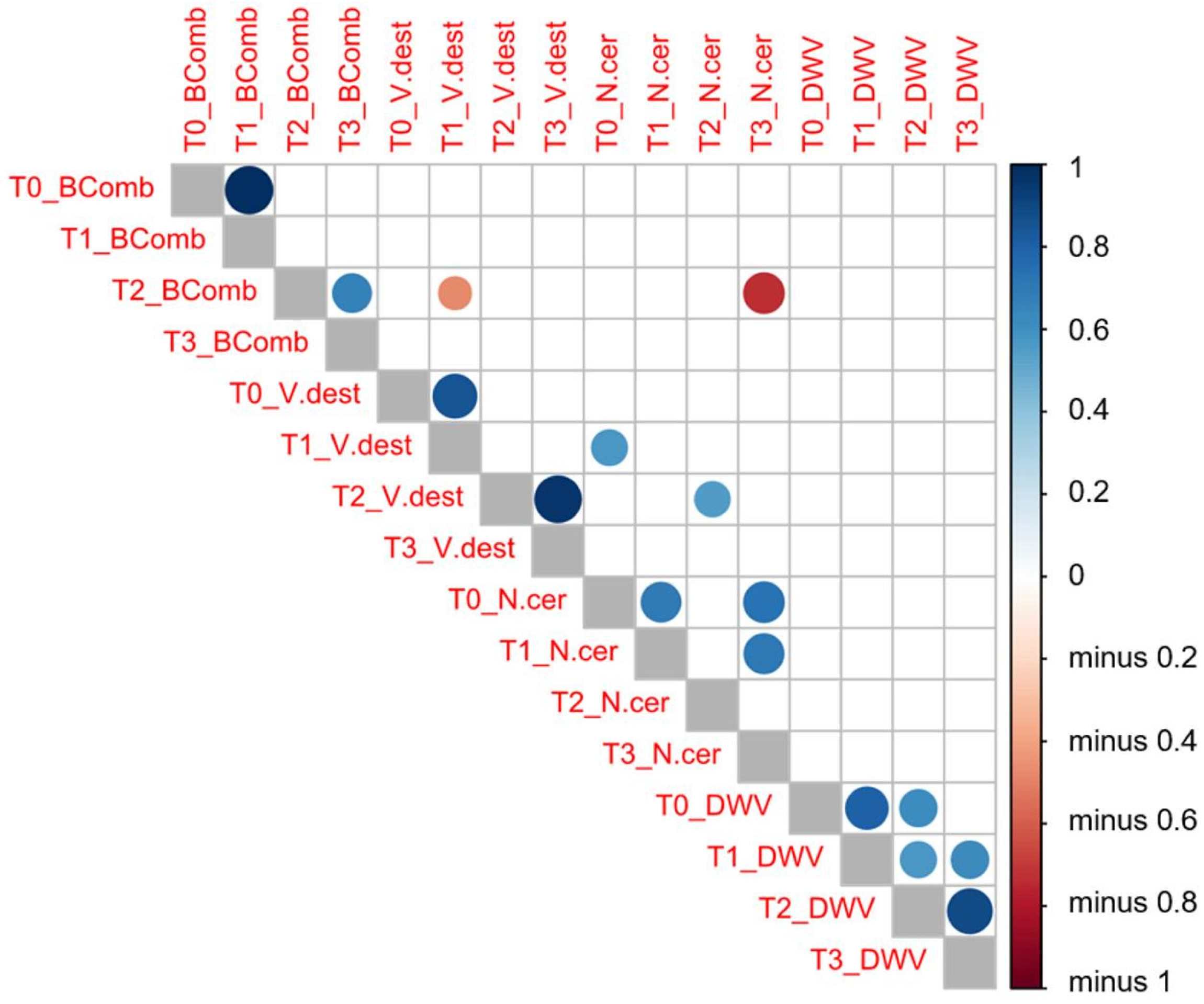
A Pearson’s correlation test (Figure 3) between the variables analyzed and the sampling times highlighted a positive correlation in the number of brood combs between T0 and T1 (same number of brood combs, r = 1, p = 0.000) and also between T2 and T3 (r = 0.662, p = 0.001). However, no correlation was found between the number of brood combs recorded in T1 and that observed in T2 (p > 0.05).
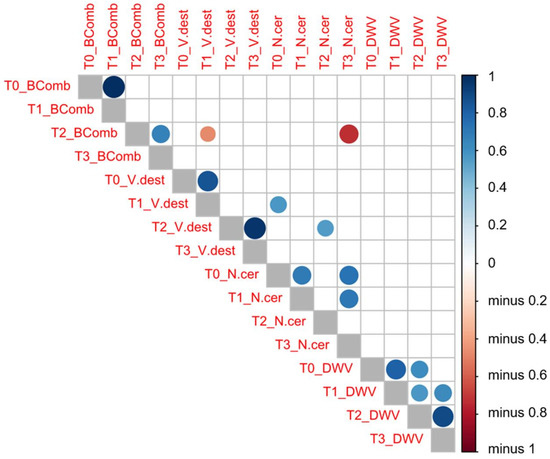
Figure 3.
Pearson’s correlation tests between the variables analyzed (V. destructor (V.dest) and N. ceranae (N.cer) infestation rate values, DWV load (DWV) and number of brood combs (BComb)) at each sampling time (T0, T1, T2, and T3). Dark blue means a positive correlation between these two variables, whereas dark red means a negative correlation as indicated in the vertical bar on the right side of the figure.
An increased infestation rate of V. destructor at T1 was negatively correlated (r = −0.471, p = 0.042) with the number of brood combs at the next sampling time (T2). This negative correlation was stronger in migratory (r = −0.672, p = 0.033) than in stationary colonies (r = −0.472, p = 0.042). Similarly, this negative correlation was also found in migratory colonies (r = −0.691, p = 0.039), whereby the infestation rate of V. destructor at T2 was negatively related to the mean number of brood combs at T3. Likewise, the lower number of brood combs at T2 appeared to be strongly correlated to the higher infection rate of N. ceranae a few weeks later (T3: r = −0.731, p = 0.011), although there was no correlation between the number of brood combs and the DWV loads. The infection rate of N. ceranae at T0 appeared to be positively correlated to the V. destructor infestation rate at T1 (r = 0.571, p = 0.026) and T2 (r = 0.551, p = 0.014), yet there was no correlation between varroa or nosema infestation rate and DWV load.
3.1.6. PCA Analysis
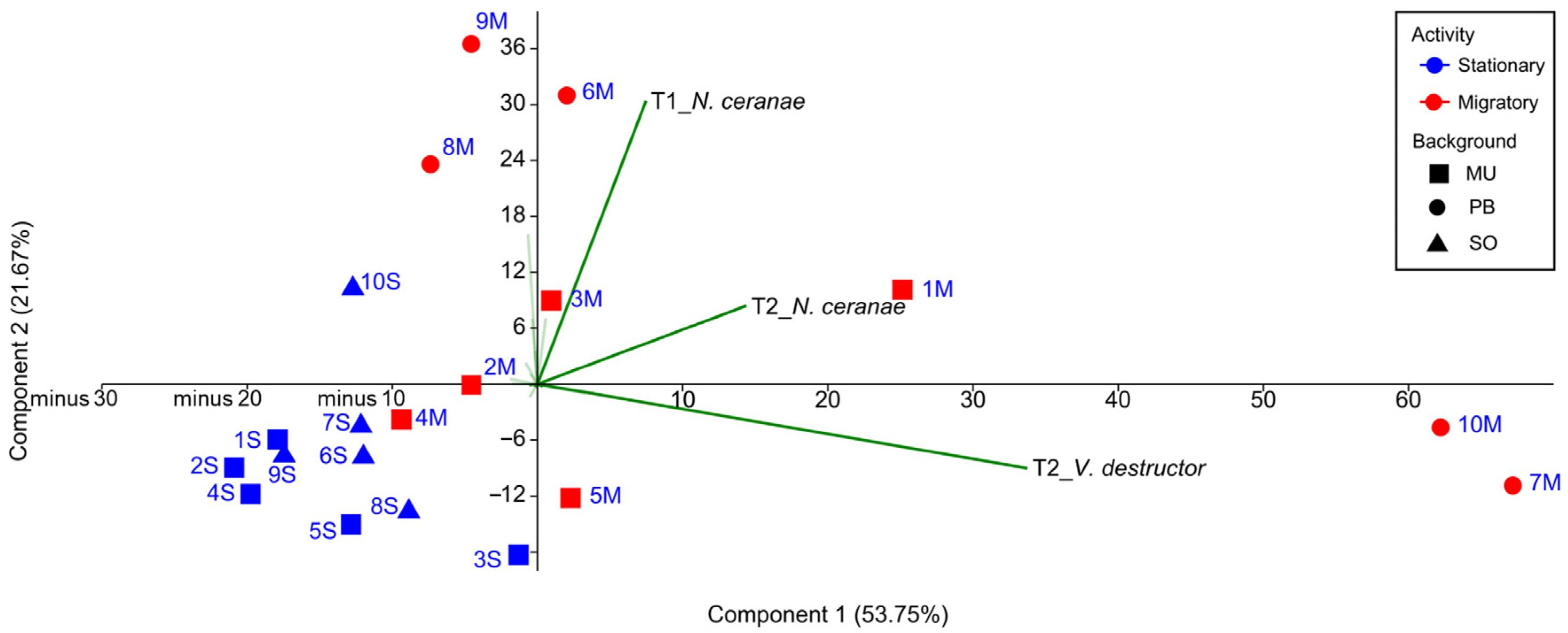
A first PCA analysis, including all the colonies and taking as variables the number of brood combs, varroa and nosema infestation rate, and DWV viral load at the four sampling times (Table S1), allowed us to assess the relative weight of each variable in the clustering of the colonies throughout the study (T0–T3), without making any prior assumption regarding beekeeping management. Eigenvalues of the two principal components explained 75.42% of the overall variability. The infestation rate of V. destructor and N. ceranae at T2 (PC 1) and the infection rate of N. ceranae at T1 (PC 2) were the variables with the strongest influence on the dispersion of the colonies in the PCA (Figure 4). The dispersion of the migratory colonies was based on the most prevalent pathogens (UM-M-3, PB-M-8, PB-M-6, and PB-M-9 along the T1_N. ceranae vector, UM-M-1 along with T2_N. ceranae, and UM-M-5, PB-M-7, and PB-M-10 along with T2_V. destructor vector), while the dispersion of the stationary colonies was not affected by these vectors. A second PCA with only the data from UM-M and UM-S colonies (the same genetic background) showed a similar result with same variables influencing the dispersion, i.e., V. destructor and N. ceranae at T2, and the infection rate of N. ceranae at T1.
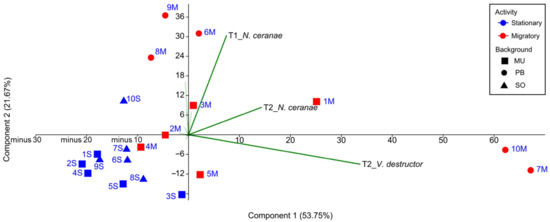
Figure 4.
Scatterplot of the colonies showing dissimilar dispersion of stationary and migratory colonies according to the rates of parasitism. The code of the colonies has been abbreviated for better visualization (stationary in blue and migratory in red, squares for the University of Murcia (UM), circles for the professional beekeeper (PB) and triangles for Soria (SO)).
3.2. Genetic Diversity and Patrilineal Composition of the Colonies
3.2.1. Genetic Diversity
In order to assess the impact of beekeeping management on the genetic variation of the colonies, population genetic parameters were inferred from the genotypes of 48 worker honey bees per colony before (T0) and after migration (T2). Colonies in Soria (SO-S) were sampled only in T1 and T2, and both datasets were also included in this analysis. In total, 1728 worker honey bees were genotyped, since one colony died off in T2. Temporal variation of the population parameters was not statistically significant in the migratory or stationary colonies, although differences were observed in the mean number of alleles, in the number of private alleles, and in the genetic diversity in some individual colonies (Table 1). In general, the greatest differences in genetic diversity were found among individual colonies (e.g., UM-M-5 vs. PB-M-7 or UM-S-2 vs. SO-S-7), irrespective of their management (migratory and stationary) or sampling time (T0 and T2). To further analyze the temporal differences between T0 and T2, Fisher’s exact test was performed for each colony. Significant genetic differences (p < 0.001) were detected in seven colonies (UM-M-1, UM-M-3, UM-M-4, PB-M-7, PB-M-8, PB-M-9, and PB-M-10) within the group of migratory colonies, whereas in the stationary colonies, significant differences (p < 0.001) were only found in the UM-S-4 and SO-S-8 colonies.

Table 1.
Population genetic parameters of migratory (M) and stationary colonies (S) at two sampling times: at the beginning of the experiment (T0, except the stationary colonies from Soria (SO-S) that were sampled at T1) and at the end of the migratory period (T2). Colony PB-M-6 † already died off at T2, therefore, data is not available (N.A.).
3.2.2. Patriline Analysis, Queen Replacement, and Drifting Workers
Paternal lines within the colonies and the relationships among the worker offspring were studied at T0 and T2. The number of patrilines ranged from 7 to 15 in the migratory and from 5 to 18 in the stationary colonies. Temporal variation in the number and frequency of patrilines was observed in individual colonies from both groups (Table 2). Despite the equivalent number of patrilines in colonies UM-M-1, UM-M-2, UM-S-1, UM-S-2, and UM-S-4, a replacement of some patrilines was detected between T0 and T2, with some low frequency patrilines no longer detected and new patrilines recorded. At the colony level, some patrilines were more frequently found at one particular sampling time. Despite the trends observed in favor of some patrilines rather than others in both individual migratory and stationary colonies, no selective patterns could be detected related to the management.

Table 2.
Number of patrilines, queen replacement events and drifting workers per colony and sampling times: at the beginning of the experiment (T0, except the stationary colonies from Soria (SO-S) that were sampled at T1) and at the end of the migratory period (T2). Colony PB-M-6 † already died off at T2; therefore, data are not available (N.A.).
No replacement of the honey bee queen was detected in the stationary colonies but in six of the migratory colonies: UM-M-1, UM-M-3, UM-M-4, PB-M-7, PB-M-8, and PB-M-10. This replacement was seemingly related to the genetic differences at the beginning of the experiment and those observed between T0 and T2. In the colonies UM-M-1, UM-M-3, PB-M-8, and PB-M-10, worker offspring from the new queen were only detected at T2, suggesting that the replacement of the queen occurred in Soria during the migratory period. For colony UM-M-4, worker offspring from the new queen were already present at T0, which indicates that the replacement and mating of the new queen took place in Murcia prior to transportation of the colonies to Soria. For colony PB-M-7, worker offspring from three different queens were detected, which indicates that the replacement and mating occurred in both periods (T0 and T2). Drifting workers (Table 2) were detected in 16 colonies at T0 (nine migratory and seven stationary) and in 10 colonies (five migratory and five stationary) at T2, although these values were not significantly different.
4. Discussion
This study provides further insights into the impact of migratory beekeeping practices on honey bee health [35,36] and on the infestation rate of pathogens and diseases in the colonies [39,40,68]. Our major finding is that migratory beekeeping and its associated stress lead to higher V. destructor and N. ceranae infestation rates in the colonies, which partially confirms our initial hypothesis.
In the case of V. destructor, the type of colony management (migratory vs. stationary) seems to influence the proliferation of the mite during the summer months. Although the two groups of migratory and stationary colonies display similar low varroa infestation rates in May (T0) and June (T1), a significantly higher infestation rate of V. destructor was recorded in migratory colonies compared to the stationary colonies after the summer period (T2). Varroa destructor population dynamics are known to be highly influenced by the host population dynamics [16]. Indeed, the increase of mites in migratory colonies may be partially explained by the existence of sufficient brood combs [69,70], thanks to favorable weather conditions in Soria and the availability of ample resources. However, colonies that are permanently located in Soria did not show such a large increase in varroa mite, despite sharing the same environmental conditions. Thus, the stress associated with migratory management can also contribute to a high infestation rate of the pathogen. For example, phoretic infestation by mites from highly infected colonies to healthy ones during migratory operations have been noted [41]: a clear case of horizontal pathogen transmission [42]. Genetic background is another factor to be considered in terms of the susceptibility of colonies to pathogens [46,71], which may explain the higher increase in V. destructor infestation rate after summer (T2) in PB colonies (owned by the professional beekeeper) compared to displaced UM colonies. It should be noted that both PB and UM colonies were transported together, placed in the same site in Soria, and showed similar varroa infestation rates (after being submitted to the same varroa treatment) at the beginning of the experiment.
Nosema ceranae was more prevalent than N. apis, which is in consonance with previous reports in Spain [46,56,72] and elsewhere [21,22,73]. Despite the low rates of N. apis infection, the infection rate of combined infections of the two microsporidia species was higher in migratory colonies. This result is worth noting, as it has been demonstrated that combined nosema infection significantly decreases honey bee survival when compared to single species infection [74]. Nosema ceranae was significantly more prevalent in migratory than in stationary colonies in June (T1), just two weeks after the hives were transported to Soria. Its infection rate was also high in October (T2). Hence, management would be related to the infection rate of N. ceranae in A. mellifera workers [39]. It has been hypothesized that transportation to ensure pollination weakens the immune system of honey bees, making them more susceptible to infection. Stressful factors involved in this management that affect the honey bee immune system and that thereby favor pathogen proliferation, including truck vibration, noises, marked temperature changes during transport of the hives, and the release of nosema spores from infected honey bees that die during transportation [21,75,76,77], are events that inevitably occurred while moving the hives in this experiment. In addition to individual immunity, transhumance can affect social immunity. The resilience of a honey bee colony to a chronic stress factor, such as N. ceranae, is also based on its ability to maintain a homogeneous population, with the right balance between nurse and foragers workers. When this balance is altered, the compensation mechanisms of the honey bee colony can fail, thus weakening the colony, and yielding to a final collapse [78]. Transhumance can lead to a further loss of foragers honey bees, which unbalances the population and favors the spread of nosema.
The significant increase in Nosema spp. from T0 to T2 was not found at T3, after the migratory colonies had returned to Murcia (beginning of November). Factors that may have counterbalanced the negative effects of transportation are perhaps related to the better weather encountered by the honey bees on their return to their original location. During their stay in Soria, the temperatures were often close to 10 °C and rainfall was frequent (e.g., in mid-October, in the two weeks before their return, there was 40% of rainy days and a mean temperature of 11.2 °C). Cold temperatures and honey bee confinement are thought to enhance the infestation rate and density of Nosema spp. inside colonies through trophallaxis and oral–fecal routes [42,79,80]. When hives returned to Murcia, honey bees encountered only 10% of rainy days and a mean temperature of 18.5 °C, which are weather conditions that favor a lower infestation rate of microsporidia. Another explanation is the “natural dilution” that occurs in the number of infected honey bees due to the natural growth cycle of the colonies [23]. If the number of “new” honey bees increases faster than the transmission of the parasite within the colony, the relative percentage of infected honey bees decreases over time. This occurs most effectively in colonies with young queens and in periods of climatic and resource bonanza.
In terms of DWV load, the virus was detected in 53% of the samples analyzed, which is a similar result as that reported in France for adult A. m. mellifera bees from March to November [17]. No clear trends were found between stationary and migratory colonies during our survey, and the largest differences were found among individual colonies rather than between colony groups. The viral loads detected were comparable to those reported in asymptomatic colonies in other studies employing a similar methodology [55]. Despite the evident differences in DWV load among colonies in May (T0), a tendency toward a greater viral load (although not significant) was detected in the two groups of colonies (migratory and stationary) in June (T1). The seasonal load variation of DWV is known to closely follow that of the varroa mite due to the role of this mite as a vector of DWV [19,81]. Thus, DWV load usually increases as the honey bee season progresses [16] and V. destructor parasitism augments, mainly in spring and early summer [15].
We found a significant reduction in the number of brood combs present in the colonies in October (T2), probably reflecting the increase of varroa infestation. Higher infestation rates of V. destructor at T1 were negatively correlated with the strength of the colony at T2 (inferred from the number of brood combs), both in migratory and stationary colonies. This result agrees with a number of studies where V. destructor is identified as the main factor affecting colony strength [59]. Indeed, we found that migratory colonies that collapsed before the end of the study had a high infestation rate of varroa mites. In addition, colonies with fewer brood combs at T2 also had higher levels of nosema a few weeks later (T3) after their return to Murcia. This observation may be related to the above mentioned: the colonies having less brood, had less non-infected young honey bees and therefore, there is a relative increase of parasitized honey bees. That is, the population of the parasite grows faster than the honey bee colony and thus increases the relative percentage of infected honey bees.
A positive correlation was detected between V. destructor and N. ceranae infestation rates, which is consistent with previous studies showing that weak colonies (less than five brood combs) are more frequently associated with higher numbers of pathogens in adult honey bees [81,82]. Unexpectedly, no correlation was found between V. destructor infestation rate and DWV load, in contrast to many studies that point to varroa as the most important vector for DWV, positively affecting the DWV copy number in honey bees [83]. However, some studies have shown a lag in the correlation [19] between the mite and the virus [84], suggesting that the epidemic’s dynamics are unique to individual colonies or even to different times during the season [85]. As summarized by the PCA analyses, the infection rate of N. ceranae in June (T1) and the infestation rate of V. destructor and N. ceranae in October (T2), appeared to be the variables that most strongly influence the status of migratory and stationary colonies.
In relation to the genetic diversity, migratory beekeeping had only a weak impact on the colonies. Short migration periods may be insufficient to produce changes on the overall genetic diversity, although trends were evident within the colonies in relation to allele frequency. This is exemplified by the fact that the strongest differences in genetic parameters were found between individual colonies irrespective of the management group or the sampling time (T0 or T2). Temporal changes in allele frequencies and the patriline composition of each colony were also greater within each management group than between groups, suggesting the influence of other non-management stressors affecting the colonies individually. Stressors such as the pathogen infestation rate and climatic condition are important selective forces that shape the genetic composition of colonies [12,47,48]. Furthermore, there is accumulating evidence that genetic variation can influence host susceptibility to pathogens [49,50,51]. Previous studies found different incidence of N. ceranae [86] and bacterial pathogens [87] among patrilines inside the colonies, as well as changes in allele frequencies and the signal of selection determined by varroa and nosema parasitism [46,88,89]. As climatic conditions were shared by all migratory colonies in this study, temporal genetic variation may rather be influenced by the infestation rate and the synergic effects of the particular combination of pathogens affecting each colony [90,91]. These stressors, along with the queen replacement events detected in some migratory colonies, may be the causes of the significant genetic differences detected. Queen replacement, as well as brood diseases, high varroa infestation rate, and DWV load have been proposed as the main factors leading to colony collapse in migratory beekeeping operations [31]. Moreover, queen replacement during migration and the subsequent mating of the queen in migratory areas, as observed in this study, have been also highlighted as an important factor for genetic homogenization and the loss of local adaptation in the Iberian honey bee populations [45,46]. However, the true impact of each individual stressor could not be weighed here due to the difficulty in controlling all the variables affecting the colonies in field conditions.
This study is a first attempt to identify the effects of migratory operations on honey bee colonies under field conditions, where the combined interactions of stress factors may have a significant effect on the colonies. Together, these findings indicate that genetic diversity is not notably affected by the type of beekeeping management. Thus, further studies are necessary to fully untangle these interactions between management, genetic background, environment, and other factors related to the status of the colony itself, weighing the effect of each factor on the honey bee colonies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/9/1/22/s1, Table S1: Data obtained for each colony in the four sampling times (T0–T3).
Author Contributions
L.J., C.R., J.S. and P.D.l.R. were involved in the study design and fieldwork. L.J., C.R., P.D.l.R. and R.M.-H. conceived the experimental laboratory design. L.J. and I.M. performed the data collection and together with C.R. the data analysis. M.H. contributed with laboratory facilities and costs. All authors were involved in the discussion of the results and manuscript writing and all authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Institute for Agricultural and Food Research and Technology (INIA) and FEDER funds (grant numbers: RTA2013-00042-C10-05 and 06) and the Seneca Foundation (Science and Technology Agency of the Murcia Region, grant number 19908/GERM/2015). Irene Muñoz was supported by Saavedra Fajardo fellowship from the Seneca Foundation (20036/SF/16) and a MINECO Spanish postdoctoral grant “Juan de la Cierva-Incorporación” (JCI2018-036614-I).
Acknowledgments
The authors would like to thank the help of the beekeepers Antonio Franco and Jesús Andalúz Romanillas for their help during the transport of the hives.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B 2018, 285, 2017–2140. [Google Scholar] [CrossRef] [PubMed]
- FAO Food and Agriculture Organization of the United Nations. Faostat. 2009. Available online: http://faostat.fao.org (accessed on 20 October 2020).
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and Temporal Trends of Global Pollination Benefit. PLoS ONE 2012, 7, e35954. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Underwood, R.; Caron, D.; Hayes, J., Jr. An estimate of managed colony losses in the winter of 2006–2007: A report commissioned by the Apiary Inspectors of America. Am. Bee J. 2007, 147, 599–603. [Google Scholar]
- VanEngelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Pettis, J. A Survey of Honey Bee Colony Losses in the U.S., Fall 2007 to Spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar] [CrossRef]
- Lee, K.V.; Steinhauer, N.; Rennich, K.; Wilson, M.E.; Tarpy, D.R.; Caron, D.M.; Rose, R.; Delaplane, K.S.; Baylis, K.; Lengerich, E.J.; et al. A national survey of managed honey bee 2013 2014 annual colony losses in the USA. Apidologie 2015, 46, 292–305. [Google Scholar] [CrossRef]
- COLOSS. Welcome, organisational matters, update on colony losses during the last winter, general information on findings and applications. In Proceedings of the 4th COLOSS Conference, Zagreb, Croatia, 3–4 March 2009; Available online: http://www.coloss.org (accessed on 20 October 2020).
- Oldroyd, B.P. What’s killing American honey bees? PLoS Biol. 2007, 5, e168. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Martínez-Salvador, A.; Garrido-Bailón, E.; González-Porto, A.V.; Meana, A.; Bernal, J.L.; Noza, M.J.; Bernal, J. A preliminary study of the epidemiological factors related to honey bee colony loss in Spain. Environ. Microbiol. 2010, 2, 243–250. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, 80–95. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.L.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Sammataro, D.; Gerson, U.; Needham, G. Parasitic mites of honey bees: Life history, implications, and impact. Annu. Rev. Entomol. 2000, 45, 519–548. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Tentcheva, D.; Gauthier, L.; Zappulla, N.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 2004, 70, 7185–7191. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Siede, R. Honey bee viruses. Adv. Virus Res. 2007, 70, 33–80. [Google Scholar] [CrossRef]
- Mondet, F.; de Miranda, J.R.; Kretzschmar, A.; Le Conte, Y.; Mercer, A.R. On the Front Line: Quantitative Virus Dynamics in Honeybee (Apis mellifera L.) Colonies along a New Expansion Front of the Parasite Varroa destructor. PLoS Pathog. 2014, 10, e1004323. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the Microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef]
- Paxton, R.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Huang, Z.Y. Nosema ceranae, a newly identified pathogen of Apis mellifera in the USA and Asia. Apidologie 2010, 41, 364–374. [Google Scholar] [CrossRef]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef]
- Aronstein, K.A.; Murray, K.D. Chalkbrood disease in honey bees. J. Invertebr. Pathol. 2010, 103, S20–S29. [Google Scholar] [CrossRef]
- Genersch, E. Paenibacillus larvae and American Foulbrood-long since known and still surprising. J. Verbrauch. Lebensm. 2008, 3, 429–434. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Mattila, H.R.; Seeley, T.D. Genetic diversity in honey bee colonies enhances productivity and fitness. Science 2007, 317, 362–364. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Tarpy, D.R.; Lengerich, E.J.; Pettis, J.S. Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev. Vet. Med. 2013, 108, 225–233. [Google Scholar] [CrossRef]
- Jacques, A.; Laurent, M.; EPILOBEE Consortium; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.P. A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE 2017, 12, e0172591. [Google Scholar] [CrossRef]
- Alger, S.A.; Burnham, P.A.; Lamas, Z.S.; Brody, A.K.; Richardson, L.L. Home sick: Impacts of migratory beekeeping on honey bee (Apis mellifera) pests, pathogens, and colony size. PeerJ 2018, 6, e5812. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.; Pettis, J.; Tarpy, D.; Mullin, C.A.; Frazier, J.L.; Frazier, M.; van Engelsdorp, D. In-hive pesticide exposome: Assessing risks to migratory honey bees from in-hive pesticide contamination in the Eastern United States. Sci. Rep. 2016, 6, 33207. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Li-Byarlay, H.; Huang, M.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 2016, 6, 32023. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Xie, X.; Riddle, J.; Pettis, J.; Huang, Z.Y. Effects of Long Distance Transportation on Honey Bee Physiology. Psyche 2012, 2012, 193029. [Google Scholar] [CrossRef]
- Melicher, D.; Wilson, E.S.; Bowsher, J.H.; Peterson, S.S.; Yocum, G.D.; Rinehart, J.P. Long-Distance Transportation Causes Temperature Stress in the Honey Bee, Apis mellifera (Hymenoptera: Apidae). Environ. Entomol. 2019, 48, 691–701. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; van Engelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, S.; Huang, Z.Y. Transportation and pollination service increase abundance and prevalence of Nosema ceranae in honey bees (Apis mellifera). J. Apic. Res. 2014, 53, 469–471. [Google Scholar] [CrossRef]
- Jara, L.; Martínez-López, D.; Muñoz, I.; De la Rúa, P. Epidemiological Survey of Ascosphaera apis in Small-Scale Migratory Apis mellifera iberiensis Colonies. Sociobiology 2018, 65, 285–290. [Google Scholar] [CrossRef]
- Nelson, D.L.; Jay, S.C. The effect of colony relocation on loss and disorientation of honeybees. Apidologie 1989, 20, 245–250. [Google Scholar] [CrossRef]
- Fries, I.; Camazine, S. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 2001, 32, 199–214. [Google Scholar] [CrossRef]
- Welch, A.; Drummond, F.; Tewari, S.; Averill, A.; Burand, J.P. Presence and prevalence of viruses in local and migratory honeybees (Apis mellifera) in Massachusetts. Appl. Environ. Microbiol. 2009, 75, 7862–7865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- REGA. 2017. Available online: https://www.mapa.gob.es/es/ganaderia/estadisticas/indicadoreseconomicossectordelamiel2017_pub_tcm30-419675.pdf (accessed on 20 October 2020).
- Cánovas, F.; De la Rúa, P.; Serrano, J.; Galián, J. Microsatellite variability reveals beekeeping influences on Iberian honeybee populations. Apidologie 2011, 42, 235–251. [Google Scholar] [CrossRef]
- Jara, L.; Muñoz, I.; Cepero, A.; Martín-Hernández, R.; Serrano, J.; Higes, M.; De la Rúa, P. Stable genetic diversity despite parasite and pathogen spread in honey bee colonies. Sci. Nat. 2015, 102, 9–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spivak, M.; Reuter, G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef]
- Chávez-Galarza, J.; Henriques, D.; Johnston, J.S.; Azevedo, J.C.; Patton, J.C.; Muñoz, I.; De la Rúa, P.; Pinto, M.A. Signatures of selection in the Iberian honey bee (Apis mellifera iberiensis) revealed by a genome scan analysis of single nucleotide polymorphisms. Mol. Ecol. 2013, 22, 5890–5907. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation between Fitness and Genetic Diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Spielman, D.; Brook, B.W.; Briscoe, D.A.; Frankham, R. Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv. Genet. 2004, 5, 439–448. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; Tinsley, M.C.; Brown, M.J.F.; Darvill, B.; Goulson, D. Genetic diversity, parasite prevalence and immunity in wild bumblebees. Proc. R. Soc. Lond. B 2011, 278, 1195–1202. [Google Scholar] [CrossRef]
- Jack, C.J.; Lucas, H.M.; Webster, T.C.; Sagili, R.R. Colony Level Prevalence and Intensity of Nosema ceranae in Honey Bees (Apis mellifera L.). PLoS ONE. 2016, 11, e0163522. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Tannahill, C.; Frey, E.; Ziegelmann, B.; Rosenkranz, P.; et al. Standard methods for varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Jara, L.; Rogerio, C.; De la Rúa, P.; Higes, M.; Martín-Hernández, R. Improving the DNA extraction method for pathogen (Nosema spp.) detection in honeybees. In Poster Communication, Proceedings of the Seventh European Conference of Apidology; Eurbee: Cluj-Napoca, Romania, 2016; pp. 7–9. [Google Scholar]
- Highfield, A.C.; El Nagar, A.; Mackinder, L.C.; Noël, L.M.; Hall, M.J.; Martin, S.J.; Schroeder, D.C. Deformed wing virus implicated in overwintering honey bee colony losses. Appl. Environ. Microbiol. 2009, 75, 7212–7220. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Botías, C.; Bailón, E.G.; Martínez-Salvador, A.; Prieto, L.; Meana, A.; Higes, M. Microsporidia infecting Apis mellifera: Coexistence or competition. Is Nosema ceranae replacing Nosema apis? Environ. Microbiol. 2012, 14, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Chantawannakul, P.; Ward, L.; Boonham, N.; Brown, M. A scientific note on detection of honeybee viruses using real-time PCR (TaqMan) in Varroa mites collected from Thai honeybee (Apis mellifera) apiary. J. Invertebr. Pathol. 2006, 91, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Budge, G.E.; Pietravalle, S.; Brown, M.; Laurenson, L.; Jones, B.; Tomkies, V.; Delaplane, K.S. Pathogens as Predictors of Honey Bee Colony Strength in England and Wales. PLoS ONE 2015, 10, e0133228. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Bailey, L.; Ball, B.V.; Blanchard, P.; Budge, P.E.; Chejanovsky, N.; Chen, Y.-P.; Gauthier, L.; Genersch, E.; de Graaf, D.C.; et al. Standard methods for virus research in Apis mellifera. J. Apic. Res. 2013, 52, 1–56. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 20 October 2017).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 20 October 2017).
- Hernández-García, R.; De la Rúa, P.; Serrano, J. Mating frequency of Apis mellifera iberiensis queens. J. Apic. Res. Bee World. 2009, 48, 121–125. [Google Scholar] [CrossRef]
- Evans, J.D.; Schwarz, R.S.; Chen, Y.P.; Budge, G.; Cornman, R.S.; De la Rúa, P.; de Miranda, J.R.; Foret, S.; Foster, L.; Gauthier, L.; et al. Standard methodologies for molecular research in Apis mellifera. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Rousset, F. Genepop’007: A complete reimplementation of the Genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.; Wang, J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Resour. 2010, 10, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Cavigli, I.; Daughenbaugh, K.F.; Martin, M.; Lerch, M.; Banner, K.; García, E.; Brutscher, L.M.; Flenniken, M.L. Pathogen prevalence and abundance in honey bee colonies involved in almond pollination. Apidologie 2016, 47, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Calis, J.N.M.; Fries, I.; Ryrie, S.C. Population modelling of Varroa jacobsoni Oud. Apidologie 1999, 30, 111–124. [Google Scholar] [CrossRef]
- Martin, S.J. The role of varroa and viral pathogens in the collapse of honey bee colonies: A modeling approach. J. Appl. Ecol. 2001, 38, 1082–1093. [Google Scholar] [CrossRef]
- Behrens, D.; Huang, Q.; Geßner, C.; Rosenkranz, P.; Frey, E.; Locke, B.; Moritz, R.F.A.; Kraus, F.B. Three QTL in the honey bee Apis mellifera L. suppress reproduction of the parasitic mite Varroa destructor. Ecol. Evol. 2011, 1, 451–458. [Google Scholar] [CrossRef]
- Jara, L.; Cepero, A.; Garrido-Bailón, E.; Martín-Hernández, R.; Higes, M.; De la Rúa, P. Linking evolutionary lineage with parasite and pathogen prevalence in the Iberian honey bee. J. Invertebr. Pathol. 2012, 110, 8–13. [Google Scholar] [CrossRef]
- Huang, Q.; Lattorff, H.M.G.; Kryger, P.; Le Conte, Y.; Moritz, R.F.A. A selective sweep in a microsporidian parasite Nosema-tolerant honey bee population, Apis mellifera. Anim. Genet. 2013, 45, 267–273. [Google Scholar] [CrossRef]
- Milbrath, M.O.; van Tran, T.; Huang, W.F.; Solter, L.F.; Tarpy, D.R.; Lawrence, F.; Huang, Z.Y. Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera). J. Invertebr. Pathol. 2015, 125, 9–15. [Google Scholar] [CrossRef]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef]
- Webster, T.C. Nosema apis spore transmission among honey bees. Am. Bee J. 1993, 133, 869–870. [Google Scholar]
- Malone, L.A.; Gatehouse, H.S.; Tregidga, E.L. Effects of Time, Temperature, and Honey on Nosema apis (Microsporidia: Nosematidae), a Parasite of the honeybee, Apis mellifera (Hymenoptera: Apidae). J. Invertebr. Pathol. 2001, 77, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R. Nosema ceranae in Apis mellifera: A 12 years post-detection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; García-Palencia, P.; Marín, P.; Meana, A. Horizontal transmission of Nosema ceranae (Microsporidia) from worker honeybees to queens (Apis mellifera). Environ. Microbiol. Rep. 2009, 1, 495–498. [Google Scholar] [CrossRef]
- Smith, M.L. The honey bee parasite Nosema ceranae: Transmissible via food exchange? PLoS ONE 2012, 7, e43319. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Cornman, R.S.; Tarpy, D.R.; Chen, Y.; Jeffreys, L.; Lopez, D.; Pettis, J.S.; van Engelsdorp, D.; Evans, J.D. Pathogen webs in collapsing honey bee colonies. PLoS ONE 2012, 7, e43562. [Google Scholar] [CrossRef]
- Tentcheva, D.; Gauthier, L.; Bagny, L.; Fievet, J.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Comparative analysis of deformed wing virus (DWV) RNA in Apis mellifera and Varroa destructor. Apidologie 2006, 37, 41–50. [Google Scholar] [CrossRef]
- Meixner, M.D.; Francis, R.M.; Gajda, A.; Kryger, P.; Andonov, S.; Uzunov, A.; Topolska, G.; Costa, C.; Amiri, E.; Berg, S.; et al. Occurrence of parasites and pathogens in honey bee colonies used in a European genotype-environment-interactions experiment. J. Apic. Res. 2014, 53, 215–229. [Google Scholar] [CrossRef]
- Prisco, G.D.; Zhang, X.; Pennacchio, F.; Caprio, E.; Li, J.; Evans, J.D.; Degrandi-Hoffman, G.; Hamilton, M.; Chen, Y.P. Dynamics of persistent and acute deformed wing virus infections in honey bees, Apis mellifera. Viruses 2011, 3, 2425–2441. [Google Scholar] [CrossRef]
- Bourgeois, A.L.; Rinderer, T.E.; Sylvester, H.A.; Holloway, B.; Oldroyd, B.P. Patterns of Apis mellifera infestation by Nosema ceranae support the parasite hypothesis for the evolution of extreme polyandry in eusocial insects. Apidologie 2012, 43, 539–548. [Google Scholar] [CrossRef]
- Palmer, K.A.; Oldroyd, B.P. Evidence for Intra-Colonial Genetic Variance in Resistance to American Foulbrood of Honey Bees (Apis Mellifera): Further Support for the Parasite/Pathogen Hypothesis for the Evolution of Polyandry. Naturwissenschaften 2003, 90, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Tanner, G.; Lodesani, M.; Maistrello, L.; Neumann, P. Negative correlation between Nosema ceranae spore loads and deformed wing virus infection levels in adult honey bee workers. J. Invertebr. Pathol. 2011, 108, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Runckel, C.; Flenniken, M.L.; Engel, J.C.; Ruby, J.G.; Ganem, D.; Andino, R.; DeRisi, J.L. Temporal Analysis of the Honey Bee Microbiome Reveals Four Novel Viruses and Seasonal Prevalence of Known Viruses, Nosema, and Crithidia. PLoS ONE 2011, 6, e20656. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- Martin, S.J.; Hardy, J.; Villalobos, E.; Martín-Hernández, R.; Nikaido, S.; Higes, M. Do the honeybee pathogens Nosema ceranae and deformed wing virus act synergistically? Environ. Microbiol. Rep. 2013, 5, 506–510. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).