Genome-Wide Identification and Expression Analysis of the bZIP Transcription Factors in the Mycoparasite Coniothyrium minitans
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Cultural Media
2.2. Identification of the bZIP Transcription Factors in C. minitans
2.3. Multiple Sequence Alignment and Phylogenetic Analysis
2.4. Sequence Analysis and Gene Structural Characterization
2.5. Culture Conditions, Biological Samples Collection, and Total RNA Extractions
2.6. Reverse Transcription and Fluorescence Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. RNA-Sequencing (RNA-seq) Data Analysis
2.8. Disruption of Four CmbZIP Genes
2.9. Phenotypic Characterization
3. Results
3.1. Identification of bZIP Genes in C. minitans
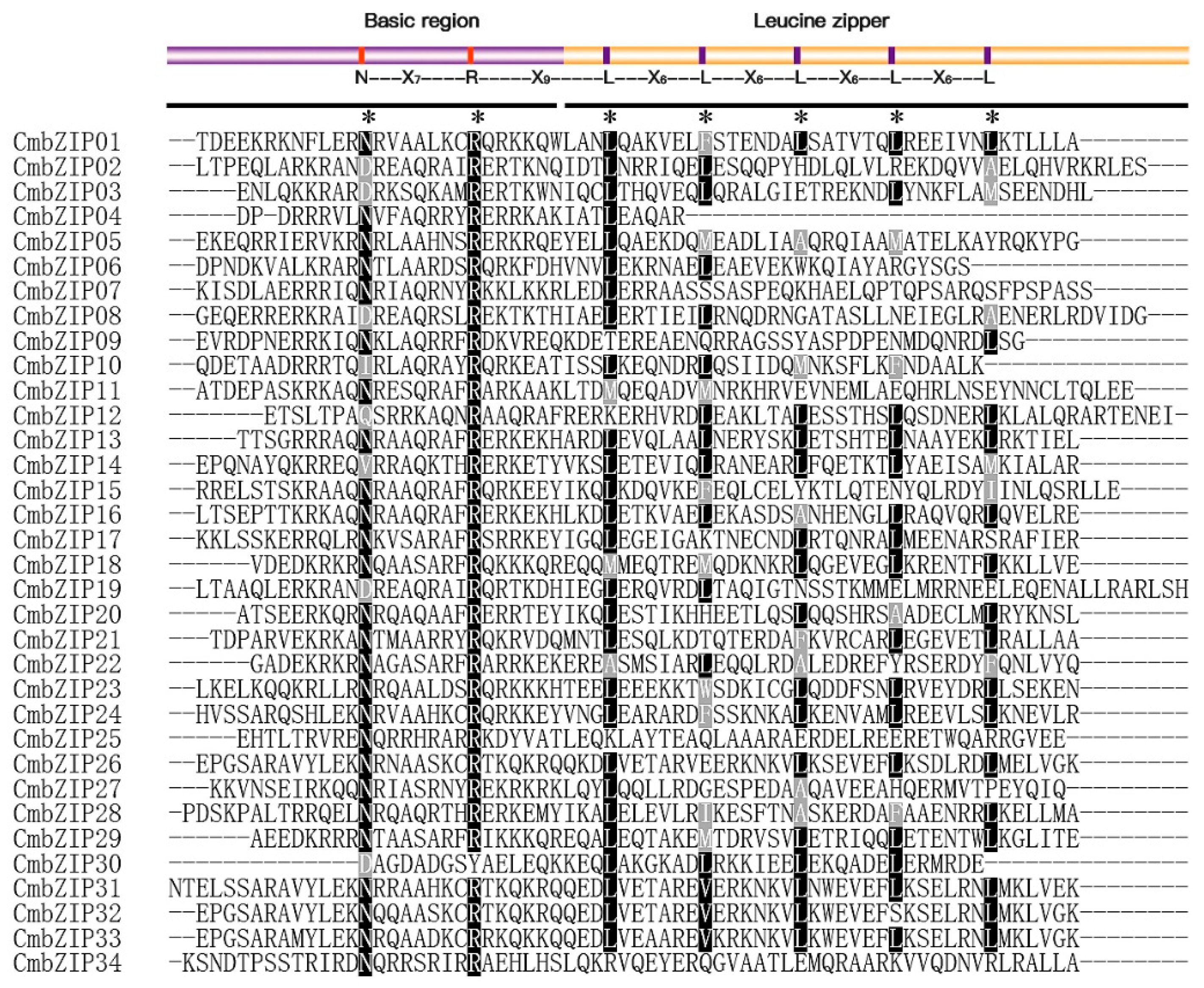
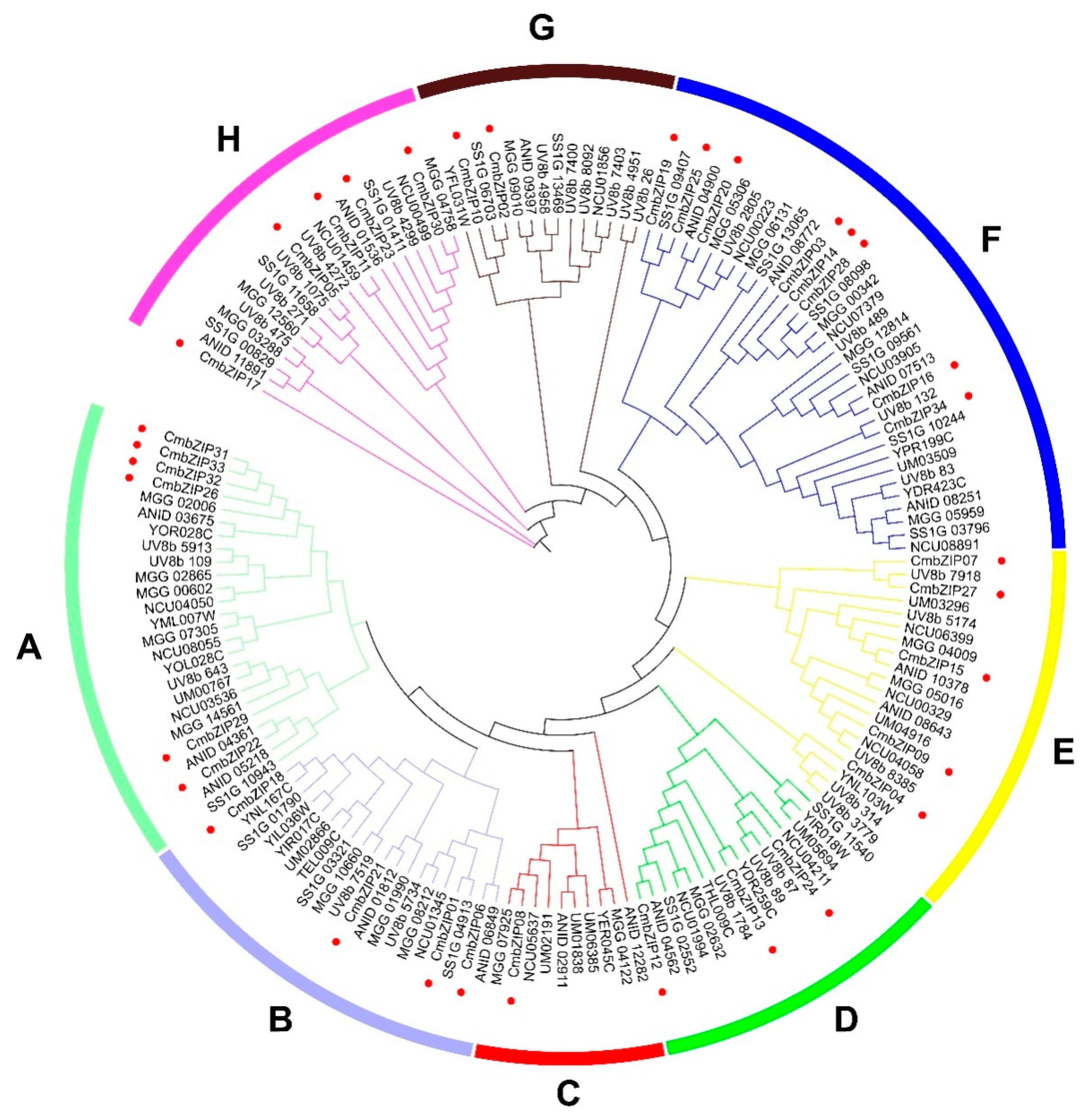
3.2. Multiple Sequence Alignment and Phylogenetic Analysis of bZIP Gene Family
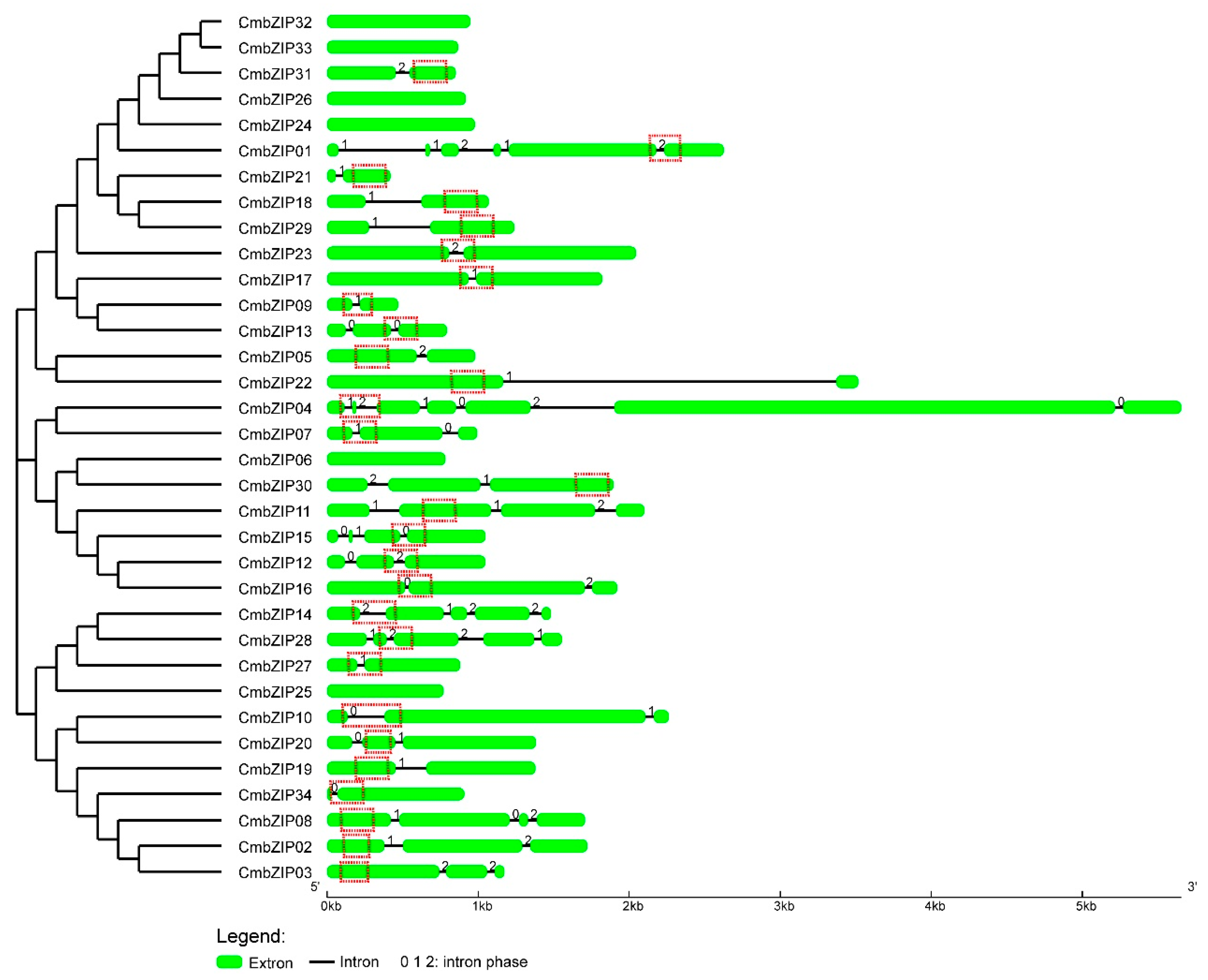
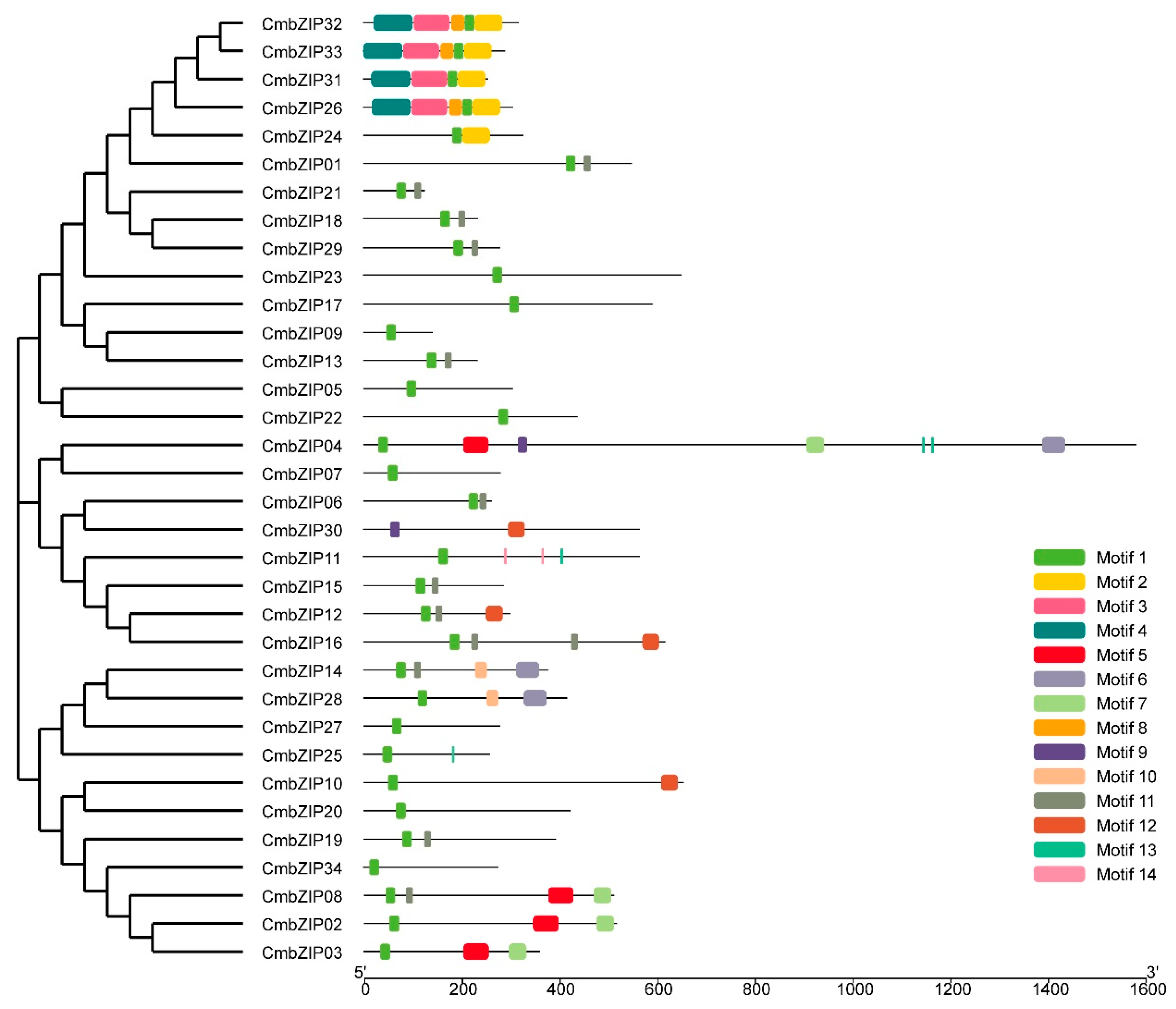
3.3. Intron Distribution Patterns and Insertion Sites in CmbZIP Genes
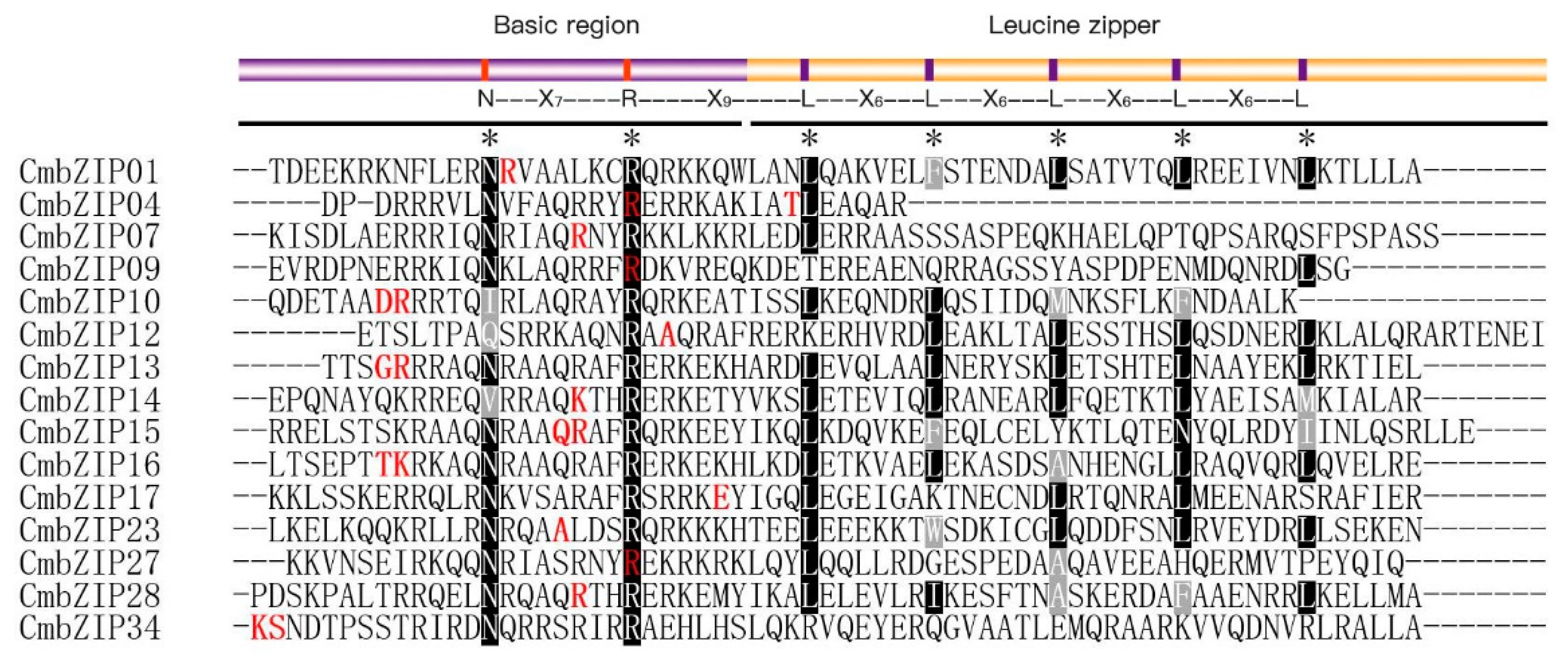
3.4. Verification of Additional Structural Features in the C. minitans bZIP Genes
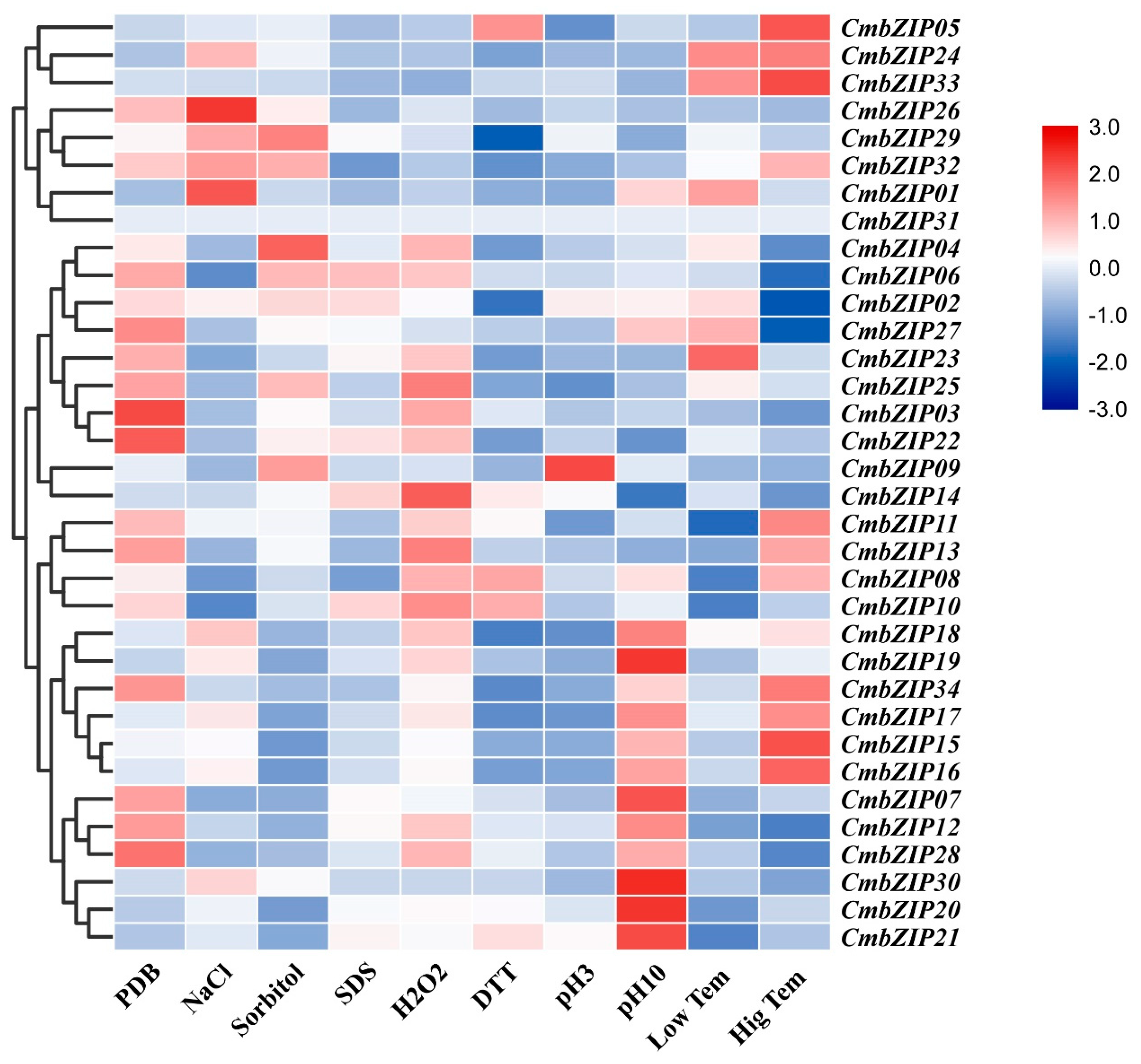
3.5. Expression Patterns of bZIP Genes Response to Abiotic Stress
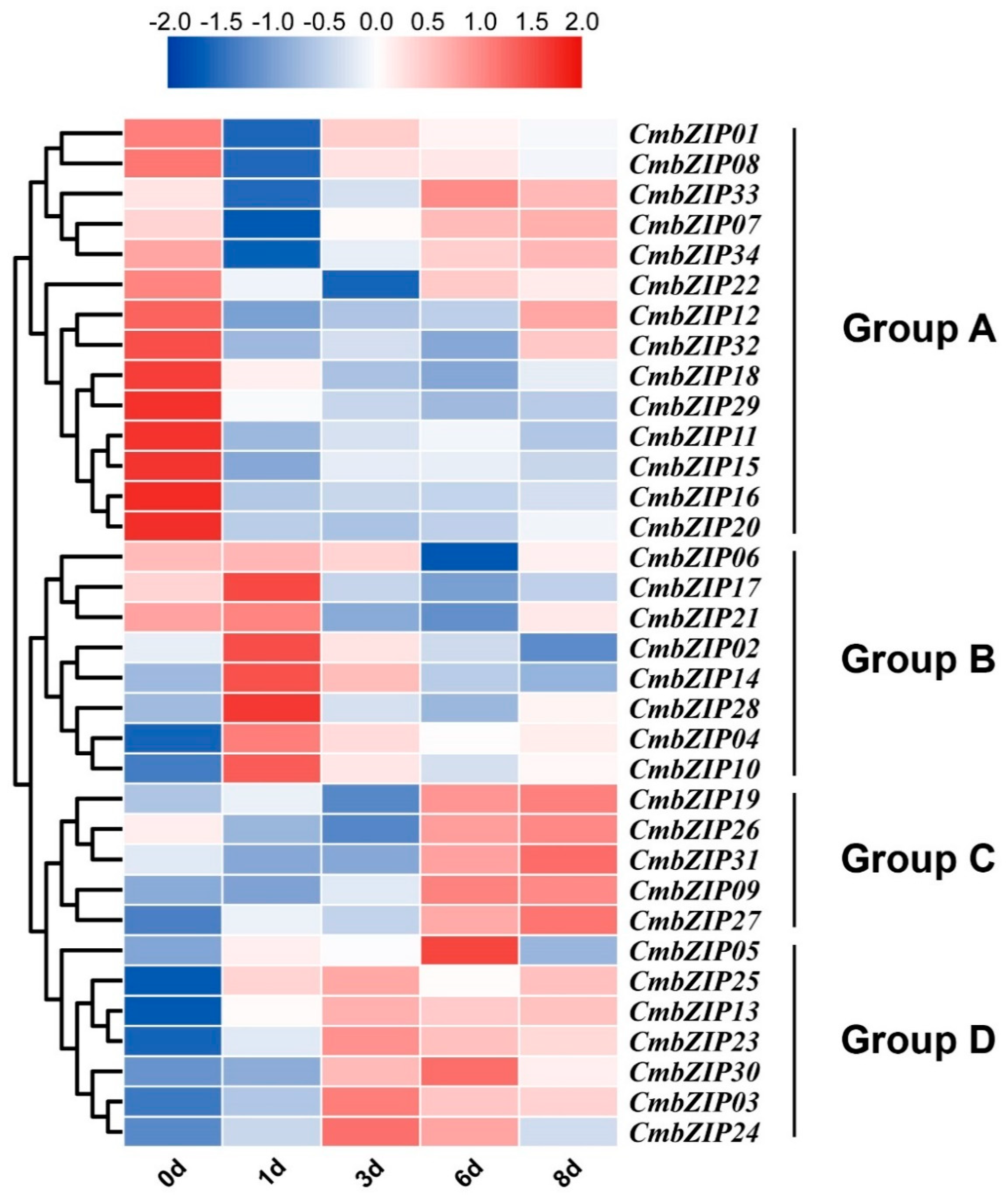
3.6. Expression Patterns of bZIP Genes in Different Stages of Conidial Development
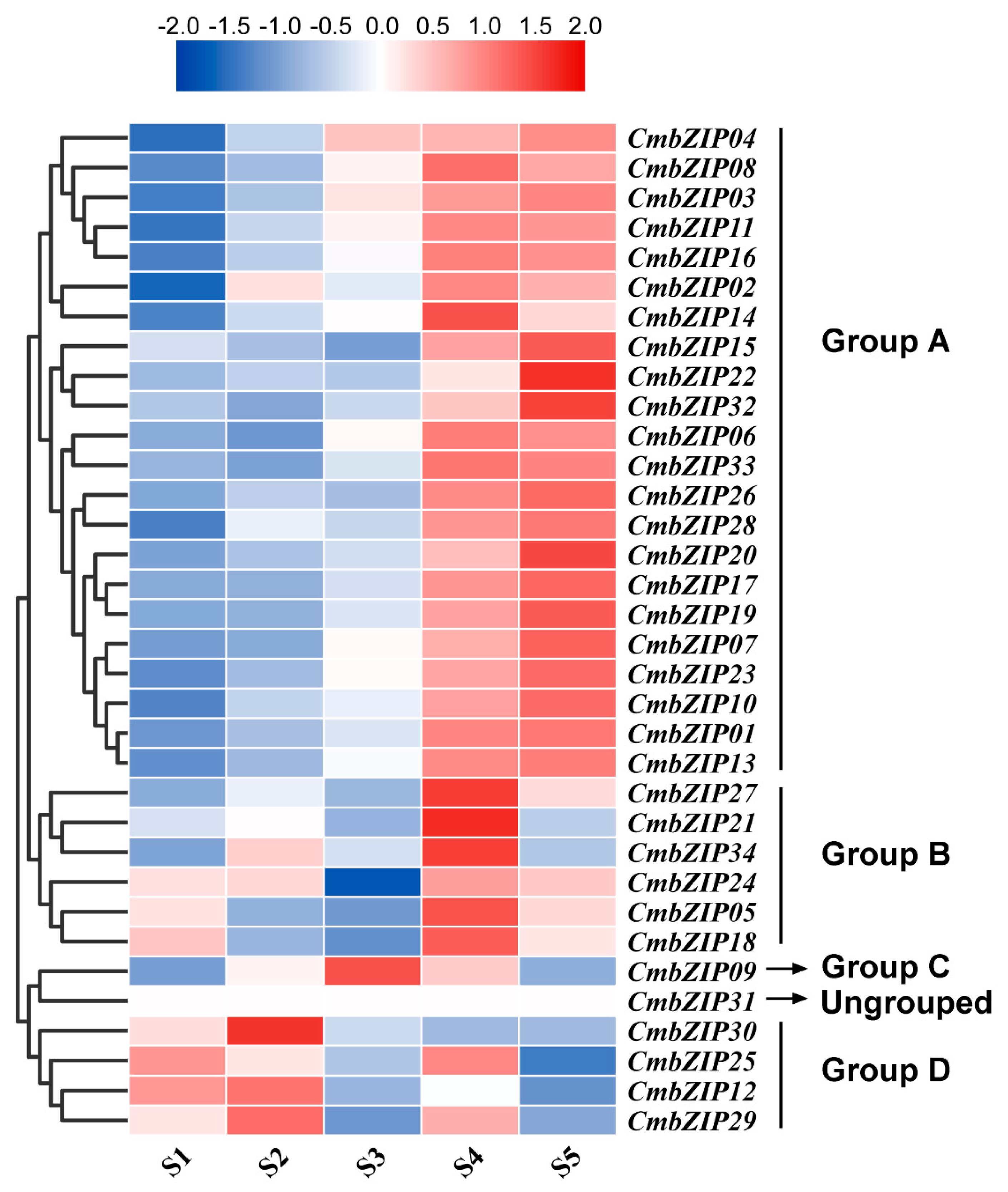
3.7. Transcriptome Analyses of bZIP Genes in the Process of Mycoparasitism
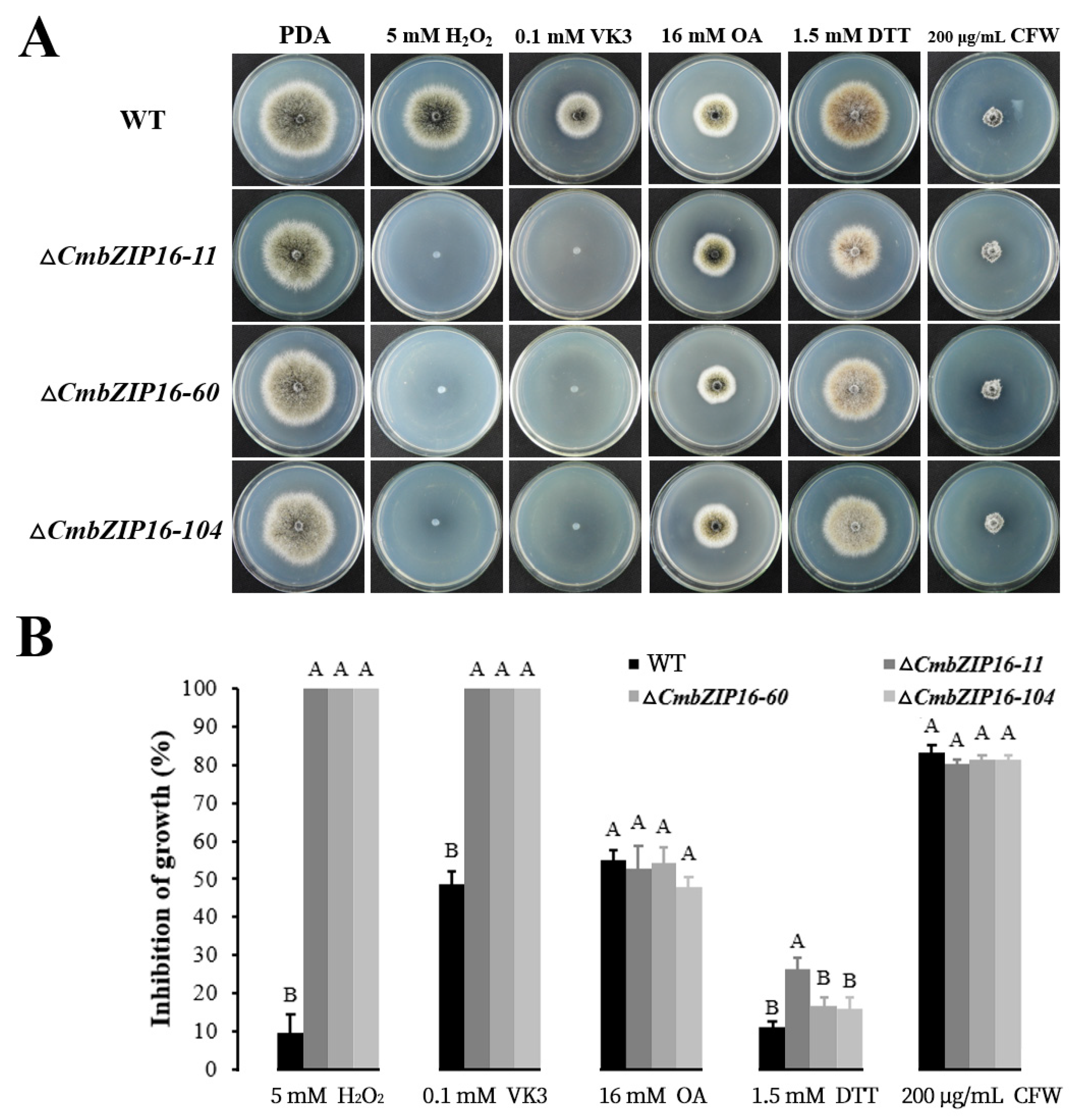
3.8. Function Analysis of Individual CmbZIP Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Campbell, W.A. A new species of Coniothyrium parasitic on sclerotia. Mycologia 1947, 39, 190–195. [Google Scholar] [CrossRef]
- de Vrije, T.; Antoine, N.; Buitelaar, R.M.; Bruckner, S.; Dissevelt, M.; Durand, A.; Gerlagh, M.; Jones, E.E.; Lüth, P.; Oostra, J.; et al. The fungal biocontrol agent Coniothyrium minitans: Production by solid-state fermentation, application and marketing. Appl. Microbiol. Biotechnol. 2001, 56, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.J.; Tribe, H.T. Preliminary field plot trails on biological control of Sclerotinia trifoliorum by Coniothyrium minitans. Plant Pathol. 1975, 24, 109–113. [Google Scholar] [CrossRef]
- Whipps, J.M.; Gerlagh, M. Biology of Coniothyrium minitans and its potential in disease control. Mycol. Res. 1992, 96, 897–907. [Google Scholar] [CrossRef]
- Gerlagh, M.; Goossen-van de Geijn, H.M.; Fokkema, N.J.; Vereijken, P.F.G. Long-term biosanitation by application of Coniothyrium minitans on Sclerotinia sclerotiorum-infected crops. Phytopathology 1999, 89, 141–147. [Google Scholar] [CrossRef]
- Partridge, D.E.; Sutton, T.B.; Jordan, D.L.; Curtis, V.L.; Bailey, J.E. Management of sclerotinia blight of peanut with the biological control agent Coniothyrium minitans. Plant Dis. 2006, 90, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Giczey, G.; Kerényi, Z.; Fülöp, L.; Hornok, L. Expression of cmg1, an exo-β-1, 3-glucanase gene from Coniothyrium minitans, increases during sclerotial parasitism. Appl. Environ. Microbiol. 2001, 67, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, G.Q.; Han, Y.C.; Jiang, D.H.; Huang, H.C. Degradation of oxalic acid by Coniothyrium minitans and its effects on production and activity of β-1, 3-glucanase of this mycoparasite. Biol. Control 2007, 43, 1–11. [Google Scholar] [CrossRef]
- Zeng, L.M.; Zhang, J.; Han, Y.C.; Yang, L.; Wu, M.D.; Jiang, D.H.; Chen, W.D.; Li, G.Q. Degradation of oxalic acid by the mycoparasite Coniothyrium minitans plays an important role in interacting with Sclerotinia sclerotiorum. Environ. Microbiol. 2014, 16, 2591–2610. [Google Scholar] [CrossRef]
- Lou, Y.; Han, Y.C.; Yang, L.; Wu, M.D.; Zhang, J.; Cheng, J.S.; Wang, M.Y.; Jiang, D.H.; Chen, W.D.; Li, G.Q. CmpacC regulates mycoparasitism, oxalate degradation and antifungal activity in the mycoparasitic fungus Coniothyrium minitans. Environ. Microbiol. 2015, 17, 4711–4729. [Google Scholar] [CrossRef] [PubMed]
- Hurst, H.C. Transcription factors. 1: bZIP proteins. Protein Profile 1994, 1, 123–168. [Google Scholar] [PubMed]
- Ellenberger, T.E.; Brandl, C.J.; Struhl, K.; Harrison, S.C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted helices: Crystal structure of the protein-DNA complex. Cell 1992, 71, 1223–1237. [Google Scholar] [CrossRef]
- Landschulz, W.H.; Johnson, P.F.; McKnight, S.L. The leucine zipper: A hypothetical structure common to a new class of DNA binding proteins. Science 1988, 240, 1759–1764. [Google Scholar] [CrossRef]
- Vinson, C.R.; Sigler, P.B.; McKnight, S.L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science 1989, 246, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Vinson, C.; Myakishev, M.; Acharya, A.; Mir, A.A.; Moll, J.R.; Bonovich, M. Classification of human B-ZIP proteins based on dimerization properties. Mol. Cell. Biol. 2002, 22, 6321–6335. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, X.H.; Gao, W.J.; Li, S.Y. The oncogenic and tumor suppressor activities of ATF-2. Life Sci. Res. 2013, 17, 73–77. [Google Scholar]
- Lopez-Bergami, P.; Lau, E.; Ronai, Z. Emerging roles of ATF-2 and the dynamic AP1 network in cancer. Nat. Rev. Cancer 2010, 10, 65–76. [Google Scholar] [CrossRef]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Nijhawan, A.; Jain, M.; Tyagi, A.K.; Khurana, J.P. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol. 2008, 146, 333–350. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhou, J.X.; Zhang, B.L.; Vanitha, J.; Ramachandran, S.; Jiang, S.Y. Genome-wide expansion and expression divergence of the basic leucine zipper transcription factors in higher plants with an emphasis on sorghum. J. Integr. Plant Biol. 2011, 53, 212–231. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chen, N.N.; Chen, F.; Cai, B.; Santo, S.D.; Tornielli, G.B. Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera). BMC Genom. 2014, 15, 281. [Google Scholar] [CrossRef]
- Li, Y.Y.; Meng, D.; Li, M.J.; Cheng, L.L. Genome-wide identification and expression analysis of the bZIP gene family in apple (Malus domestica). Tree Genet. Genomes 2016, 12, 82. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.H.; Shi, H.; Guo, M.L.; Chai, M.N.; He, Q.; Yan, M.K.; Cao, D.; Zhao, L.H.; Cai, H.Y.; et al. Evolutionary and expression analyses of soybean basic Leucine zipper transcription factor family. BMC Genom. 2018, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Ohmiya, K.; Hattori, T. A rice bZIP protein, designated OSBZ8, is rapidly induced by abscisic acid. Plant J. 1996, 9, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Hobo, T.; Kowyama, Y.; Hattori, T. A bZIP factor, TRAB1, interacts with VP1 and mediates abscisic acid-induced transcription. Proc. Natl. Acad. Sci. USA 1999, 96, 15348–15353. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zou, H.F.; Wei, W.; Hao, Y.J.; Tian, A.G.; Huang, J.; Liu, Y.F.; Zhang, J.S.; Chen, S.Y. Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of ABA signaling and confer salt and freezing tolerance in transgenic Arabidopsis. Planta 2008, 228, 225–240. [Google Scholar] [CrossRef]
- Hossain, M.A.; Cho, J.I.; Han, M.; Ahn, C.H.; Jeon, J.S.; An, G.; Park, P.B. The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice. J. Plant Physiol. 2010, 167, 1512–1520. [Google Scholar] [CrossRef]
- Fukazawa, J.; Sakai, T.; Ishida, S.; Yamaguchi, I.; Kamiya, Y.; Takahashi, Y. Repression of shoot growth, a bZIP transcriptional activator, regulates cell elongation by controlling the level of gibberellins. Plant Cell 2000, 12, 901–915. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Harada, M.; Suzuki, N.; Sugawara, K. A maize DNA-binding factor with a bZIP motif is induced by low temperature. Mol. Gen. Genet. 1995, 248, 507–517. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Li, C.W.; Su, R.C.; Cheng, C.P.; Sanjaya; Tsai, Y.C.; Chan, M.T. A tomato bZIP transcription factor, SlAREB, is involved in water deficit and salt stress response. Planta 2010, 231, 1459–1473. [Google Scholar] [CrossRef]
- Chen, H.; Chen, W.; Zhou, J.L.; He, H.; Chen, L.B.; Chen, H.D.; Deng, X.W. Basic leucine zipper transcription factor OsbZIP16 positively regulates drought resistance in rice. Plant Sci. 2012, 193–194, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tak, H.; Mhatre, M. Cloning and molecular characterization of a putative bZIP transcription factor VvbZIP23 from Vitis vinifera. Protoplasma 2013, 250, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.; Ma, L.G.; Qu, L.J.; Deng, X.W. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 2002, 16, 1247–1259. [Google Scholar] [CrossRef]
- Huang, X.; Ouyang, X.; Yang, P.; Lau, O.S.; Li, G.; Li, J.; Chen, H.; Deng, X.W. Arabidopsis FHY3 and HY5 positively mediate induction of COP1 transcription in response to photomorphogenic UV-B light. Plant Cell 2012, 24, 4590–4606. [Google Scholar] [CrossRef]
- Hanson, J.; Hanssen, M.; Wiese, A.; Hendriks, M.M.W.B.; Smeekens, S. The sucrose regulated transcription factor bZIP11 affects amino acid metabolism by regulating the expression of ASPARAGINE SYNTHETASE1 and PROLINE DEHYDROGENASE2. Plant J. 2008, 53, 935–949. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.Y.; Cho, D.I.; Park, J.H.; Kim, S.Y. ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J. 2004, 40, 75–87. [Google Scholar] [CrossRef]
- Kang, S.G.; Price, J.; Lin, P.C.; Hong, J.C.; Jang, J.C. The Arabidopsis bZIP1 transcription factor is involved in sugar signaling, protein networking, and DNA binding. Mol. Plant 2010, 3, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Thurow, C.; Schiermeyer, A.; Krawczyk, S.; Butterbrodt, T.; Nickolov, K.; Gatz, C. Tobacco bZIP transcription factor TGA2.2 and related factor TGA2.1 have distinct roles in plant defense responses and plant development. Plant J. 2005, 44, 100–113. [Google Scholar] [CrossRef]
- Lee, S.C.; Choi, H.W.; Hwang, I.S.; Choi, D.S.; Hwang, B.K. Functional roles of the pepper pathogen-induced bZIP transcription factor, CAbZIP1, in enhanced resistance to pathogen infection and environmental stresses. Planta 2006, 224, 1209–1225. [Google Scholar] [CrossRef]
- Kuge, S.; Arita, M.; Murayama, A.; Maeta, K.; Izawa, S.; Inoue, Y. Regulation of the yeast Yap1p nuclear export signal is mediated by redox signal-induced reversible disulfide bond formation. Mol. Cell. Biol. 2001, 21, 6139–6150. [Google Scholar] [CrossRef]
- Lessing, F.; Kniemeyer, O.; Wozniok, I.; Loeffler, J.; Kurzai, O.; Haertl, A.; Brakhage, A.A. The Aspergillus fumigatus transcriptional regulator AfYap1 represents the major regulator for defense against reactive oxygen intermediates but is dispensable for pathogenicity in an intranasal mouse infection model. Eukaryot. Cell 2007, 6, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Yang, S.L.; Chung, K.R. The YAP1 homolog-mediated oxidative stress tolerance is crucial for pathogenicity of the necrotrophic fungus Alternaria alternata in citrus. Mol. Plant-Microbe Interact. 2009, 22, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Chen, Y.; Du, Y.; Dong, Y.H.; Guo, W.; Zhai, S.; Zhang, H.F.; Dong, S.M.; Zhang, Z.G.; Wang, Y.C.; et al. The bZIP transcription factor MoAP1 mediates the oxidative stress response and is critical for pathogenicity of the rice blast fungus Magnaporthe oryzae. PLoS Pathog. 2011, 7, e1001302. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Davidson, M.K.; Wahls, W.P. Distinct regions of ATF/CREB proteins Atf1 and Pcr1 control recombination hotspot ade6-M26 and the osmotic stress response. Nucleic Acids Res. 2008, 36, 2838–2851. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, D.; Asano, Y.; Yamashino, T.; Mizuno, T. Characterization of bZip-type transcription factor AtfA with reference to stress responses of conidia of Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2008, 72, 2756–2760. [Google Scholar] [CrossRef]
- Guo, M.; Guo, W.; Chen, Y.; Dong, S.M.; Zhang, X.; Zhang, H.F.; Song, W.W.; Wang, W.; Wang, Q.; Lv, R.L.; et al. The basic leucine zipper transcription factor Moatf1 mediates oxidative stress responses and is necessary for full virulence of the rice blast fungus Magnaporthe oryzae. Mol. Plant Microbe. Interact. 2010, 23, 1053–1068. [Google Scholar] [CrossRef]
- Balázs, A.; Pócsi, I.; Hamari, Z.; Leiter, É.; Emri, T.; Miskei, M.; Oláh, J.; Tóth, V.; Hegedüs, N.; Prade, R.A.; et al. AtfA bZIP-type transcription factor regulates oxidative and osmotic stress responses in Aspergillus nidulans. Mol. Genet. Genom. 2010, 283, 289–303. [Google Scholar] [CrossRef]
- Etxebeste, O.; Ni, M.; Garzia, A.; Kwon, N.J.; Fischer, R.; Yu, J.H.; Espeso, E.A.; Ugalde, U. Basic-zipper-type transcription factor FlbB controls asexual development in Aspergillus nidulans. Eukaryot. Cell 2008, 7, 38–48. [Google Scholar] [CrossRef]
- Xiao, P.; Shin, K.S.; Wang, T.H.; Yu, J.H. Aspergillus fumigatus flbB encodes two basic leucine zipper domain (bZIP) proteins required for proper asexual development and gliotoxin production. Eukaryot. Cell 2010, 9, 1711–1723. [Google Scholar] [CrossRef]
- Mulder, H.J.; Saloheimo, M.; Penttilä, M.; Madrid, S.M. The transcription factor HACA mediates the unfolded protein response in Aspergillus niger, and upregulates its own transcription. Mol. Genet. Genom. 2004, 271, 130–140. [Google Scholar] [CrossRef]
- Richie, D.L.; Hartl, L.; Aimanianda, V.; Winters, M.S.; Fuller, K.K.; Miley, M.D.; White, S.; McCarthy, J.W.; Latgé, J.P.; Feldmesser, M. A role for the unfolded protein response (UPR) in virulence and antifungal susceptibility in Aspergillus fumigatus. PLoS Pathog. 2009, 5, e1000258. [Google Scholar] [CrossRef]
- Kong, S.Y.; Park, S.Y.; Lee, Y.H. Systematic characterization of the bZIP transcription factor gene family in the rice blast fungus, Magnaporthe oryzae. Environ. Microbiol. 2015, 17, 1425–1443. [Google Scholar] [CrossRef]
- Tang, W.; Ru, Y.Y.; Hong, L.; Zhu, Q.; Zuo, R.F.; Guo, X.X.; Wang, J.Z.; Zhang, H.F.; Zheng, X.B.; Wang, P. System-wide characterization of bZIP transcription factor proteins involved in infection-related morphogenesis of Magnaporthe oryzae. Environ. Microbiol. 2015, 17, 1377–1396. [Google Scholar] [CrossRef]
- Wong, K.H.; Hynes, M.J.; Todd, R.B.; Davis, M.A. Transcriptional control of nmrA by the bZIP transcription factor MeaB reveals a new level of nitrogen regulation in Aspergillus nidulans. Mol. Microbiol. 2007, 66, 534–551. [Google Scholar] [CrossRef]
- López-Berges, M.S.; Rispail, N.; Prados-Rosales, R.C.; Di Pietro, A. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. Plant Cell 2010, 22, 2459–2475. [Google Scholar] [CrossRef]
- Polley, S.D.; Caddick, M.X. Molecular characterisation of meaB, a novel gene affecting nitrogen metabolite repression in Aspergillus nidulans. FEBS Lett. 1996, 388, 200–205. [Google Scholar] [CrossRef]
- Yin, W.X.; Cui, P.; Wei, W.; Lin, Y.; Luo, C.X. Genome-wide identification and analysis of the basic leucine zipper (bZIP) transcription factor gene family in Ustilaginoidea virens. Genome 2017, 60, 1051–1059. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Zhou, T.; Xie, J.T.; Cheng, J.S.; Chen, T.; Jiang, D.H.; Fu, Y.P. Mycoparasitism illuminated by genome and transcriptome sequencing of Coniothyrium minitans, an important biocontrol fungus of the plant pathogen Sclerotinia sclerotiorum. Microbial Genom. 2020, 6, e000345. [Google Scholar] [CrossRef]
- Ye, W.W.; Wang, Y.; Dong, S.M.; Tyler, B.M.; Wang, Y.C. Phylogenetic and transcriptional analysis of an expanded bZIP transcription factor family in Phytophthora sojae. BMC Genom. 2013, 14, 839. [Google Scholar] [CrossRef]
- Kershaw, M.J.; Talbot, N.J. Genome-wide functional analysis reveals that infection-associated fungal autophagy is necessary for rice blast disease. Proc. Natl. Acad. Sci. USA 2009, 106, 15967–15972. [Google Scholar] [CrossRef]
- Luo, C.W.; Zhao, H.Z.; Yang, X.X.; Qiang, C.C.; Cheng, J.S.; Xie, J.T.; Chen, T.; Jiang, D.H.; Fu, Y.P. Functional analysis of the melanin-associated gene CmMR1 in Coniothyrium minitans. Front. Microbiol. 2018, 9, 2658. [Google Scholar] [CrossRef]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 2013, 147, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Y.; Fu, Y.P.; Jiang, D.H.; Li, G.Q.; Yi, X.H.; Peng, Y.L. L-arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet. Biol. 2007, 44, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fu, Y.P.; Jiang, D.H.; Xie, J.T.; Cheng, J.S.; Li, G.Q.; Hamid, M.I.; Yi, X.H. Cyclic GMP as a second messenger in the nitric oxide-mediated conidiation of the mycoparasite Coniothyrium minitans. Appl. Environ. Microbiol. 2010, 76, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Gao, S.Q.; Tang, Y.M.; Li, L.; Zhang, F.J.; Feng, B.N.; Fang, Z.F.; Ma, L.J.; Zhao, C.P. Genome-wide identification and evolutionary analyses of bZIP transcription factors in wheat and its relatives and expression profiles of anther development related TabZIP genes. BMC Genom. 2015, 16, 976. [Google Scholar] [CrossRef]
- Jindrich, K.; Degnan, B.M. The diversification of the basic leucine zipper family in eukaryotes correlates with the evolution of multicellularity. BMC Evol. Biol. 2016, 16, 28. [Google Scholar] [CrossRef]
- Callis, J.; Fromm, M.; Walbot, V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987, 1, 1183–1200. [Google Scholar] [CrossRef]
- Boudet, N.; Aubourg, S.; Toffano-Nioche, C.; Kreis, M.; Lecharny, A. Evolution of intron/exon structure of DEAD helicase family genes in Arabidopsis, Caenorhabditis, and Drosophila. Genome Res. 2001, 11, 2101–2114. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Manimekalai, R.; Sharoni, A.M.; Satoh, K.; Kondoh, H.; Ooka, H.; Kikuchi, S. Genome-wide analysis of NAC transcription factor family in rice. Gene 2010, 465, 30–44. [Google Scholar] [CrossRef]
- Deppmann, C.D.; Alvania, R.S.; Taparowsky, E.J. Cross-species annotation of basic leucine zipper factor interactions: Insight into the evolution of closed interaction networks. Mol. Biol. Evol. 2006, 23, 1480–1492. [Google Scholar] [CrossRef]
- Tan, K.; Feizi, H.; Luo, C.; Fan, S.H.; Ravasi, T.; Ideker, T.G. A systems approach to delineate functions of paralogous transcription factors: Role of the Yap family in the DNA damage response. Proc. Natl. Acad. Sci. USA 2008, 105, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, Y.; Zhao, H.; Wu, M.; Zhang, J.; Chen, W.; Li, G.; Yang, L. Genome-Wide Identification and Expression Analysis of the bZIP Transcription Factors in the Mycoparasite Coniothyrium minitans. Microorganisms 2020, 8, 1045. https://doi.org/10.3390/microorganisms8071045
Xu Y, Wang Y, Zhao H, Wu M, Zhang J, Chen W, Li G, Yang L. Genome-Wide Identification and Expression Analysis of the bZIP Transcription Factors in the Mycoparasite Coniothyrium minitans. Microorganisms. 2020; 8(7):1045. https://doi.org/10.3390/microorganisms8071045
Chicago/Turabian StyleXu, Yuping, Yongchun Wang, Huizhang Zhao, Mingde Wu, Jing Zhang, Weidong Chen, Guoqing Li, and Long Yang. 2020. "Genome-Wide Identification and Expression Analysis of the bZIP Transcription Factors in the Mycoparasite Coniothyrium minitans" Microorganisms 8, no. 7: 1045. https://doi.org/10.3390/microorganisms8071045
APA StyleXu, Y., Wang, Y., Zhao, H., Wu, M., Zhang, J., Chen, W., Li, G., & Yang, L. (2020). Genome-Wide Identification and Expression Analysis of the bZIP Transcription Factors in the Mycoparasite Coniothyrium minitans. Microorganisms, 8(7), 1045. https://doi.org/10.3390/microorganisms8071045




