Abstract
The growth of a large number of poisonous plants is an indicator of grassland degradation. Releasing allelochemicals through root exudates is one of the strategies with which poisonous plants affect neighboring plants in nature. Arbuscular mycorrhizal fungi (AMF) can form a mutualistic symbiosis with most of the higher plants. However, the manner of interaction between root exudates of poisonous plants and AMF on neighboring herbage in grasslands remains poorly understood. Stellera chamaejasme L., a common poisonous plant with approved allelopathy, is widely distributed with the dominant grass of Leymus chinensis in the degradeds of Northern China. In this study, we investigated the addition of S. chamaejasme root exudates (SRE), the inoculation of AMF, and their interaction on the growth and tissue nitrogen contents of L. chinensis, the characteristics of rhizosphere AMF, and soil physicochemical properties. Results showed that SRE had significant effects on ramet number, aboveground biomass, and total nitrogen of L. chinensis in a concentration dependent manner. Additionally, SRE had a significant negative effect on the rate of mycorrhiza infection and spore density of the AMF. Meanwhile, the addition of SRE significantly affected soil pH, electrical conductivity, available nitrogen (AN), available phosphorus (AP), total nitrogen (TN), and total carbon (TC) contents; while neither inoculation of AMF itself nor the interaction of AMF with SRE significantly affected the growth of L. chinensis. The interaction between AMF and SRE dramatically changed the pH, AP, and TC of rhizosphere soil. Therefore, we suggested SRE of S. chamaejasme affected the growth of L. chinensis by altering soil pH and nutrient availability. AMF could change the effect of SRE on soil nutrients and have the potential to regulate the allelopathic effects of S. chamaejasme and the interspecific interaction between the two plant species. We have provided new evidence for the allelopathic mechanism of S. chamaejasme and the regulation effects of AMF on the interspecific relationship between poisonous plants and neighboring plants. Our findings reveal the complex interplay between the root exudates of poisonous plants and rhizosphere AMF in regulating population growth and dynamics of neighboring plants in degraded grassland ecosystems.
1. Introduction
Grassland occupies approximately one third of the land area of China, and provides great ecological and economic value to the country [1,2]. However, grassland degradation has exacerbated during the past 50 years due to intense human disturbance, especially overgrazing [3]. One important indicator of grassland degradation is increasing abundance of poisonous plants. These plants commonly accumulate and exude secondary metabolites that are toxic to livestock or humans [4,5,6]. Moreover, poisonous plants directly compete with palatable forage plants for resources such as water and mineral nutrition [7,8].
Stellera chamaejasme L. is a common poisonous plant species in the degraded grasslands of northern China [9], which even becomes the dominant species in some extremely degraded grasslands [10,11]. A growing number of studies have shown that S. chamaejasme can exert allelopathic substance, and inhibit the germination and seedling growth of other plants [12,13,14]. The fresh roots, stems, and leaves of S. chamaejasme, as well as the comminuted substance from different plant parts, have been found to have negative effects on many important forage plant species, including Medicago sativa L., Astragalus adsurgens Pall., Elymus dahuricus Turcz., and Elymus sibiricus L. [12,13]. The root has stronger allelopathic influence than the stem and leaf. For example, the water and alcohol extracts of S. chamaejasme roots can inhibit germination of Arthraxon hispidus (Thunb.) Makino by 5%–15% and seedling growth of Galium verum L. by more than 50% [14]. In parallel, many secondary metabolites such as coumarins, flavonoids, lignans, and terpenoids have been isolated and identified from S. chamaejasme [15,16,17,18]. Mechanistically, allelochemicals could affect photosynthesis, respiration, and the metabolism of certain groups of proteins and nucleic acid of other plants, which in turn regulate their growth [17,19]. Some studies have shown that allelopathic substance can alter soil pH and nutrient availability, e.g., terpenoids may play an important role in the inhibition of nitrification [20]. In S. chamaejasme, the flavonoids secreted by the root could indirectly increase the edaphic available phosphorus (AP) [21]. However, despite these significant advances, little is known about whether and how allelopathic substance of S. chamaejasme affects soil characteristics and the growth of neighboring grasses.
The interspecific competition is a classical topic in ecology [22]. Previous studies on interspecific competition in plants have usually focused on competition for nutrients [23,24], or the niche overlap [25]. In addition to poisonous plants, the infection of arbuscular mycorrhizal fungi (AMF) is another important biotic factor in structuring plant communities and regulating the interspecific relationship [26]. A growing body of studies has shown that AMF exist widely in terrestrial ecosystems and form symbionts with plants to promote nutrients uptake and increase their utilization rate [27,28,29,30]. AMF could mediate the interaction between plant species and significantly affect the distribution of individual size in plant population and plant community structure [31,32,33,34,35]. Therefore, AMF may play an essential role in regulating the relationship between plants, including intra- and inter-specific competitions [36,37]. However, whether AMF also play such a role in regulating the interactive relationships between S. chamaejasme and neighboring plants has been rarely investigated.
Leymus chinensis L. is a rhizomatous perennial grass, with a high AMF infection rate on its root system [38]. This species is a primary forage grass for livestock and often coexists with S. chamaejasme in the degraded grasslands of northern China [39]. With grassland degradation, S. chamaejasme can become a dominant species in the community and compete with neighboring plants for space and resources [7,8]. However, the interspecific relationship between this poisonous plant and L. chinensis and the mechanisms underlying interactions are remain poorly understood. Interestingly, a field study carried out in the grasslands of Inner Mongolia, China, found that there was no AMF infection in the root of S. chamaejasme [40]. Studies have found that the rhizosphere soils of S. chamaejasme had lower relative abundance of AMF [41]. These findings indicated that the root exudates of S. chamaejasme may be able to inhibit AMF in the rhizosphere. However, little is known about whether poisonous plants can interact with AMF infection to regulate the growth of neighboring plants.
In this study, we performed a two-factor interactive experiment to test how root exudates of S. chamaejasme and AMF inoculation, as well as their interaction, could affect the growth of L. chinensis, the characteristics of rhizosphere AMF, and soil physicochemical properties. We hypothesized that (i) the root exudates of S. chamaejasme would have negatively allelopathic effects on the growth of L. chinensis by altering soil physicochemical properties; (ii) there would be an interactive effect of AMF and root exudates on the growth of L. chinensis; and (iii) AMF could mediate the allelopathic effects of S. chamaejasme on L. chinensis via changing soil pH and nutrient availability.
2. Material and Methods
2.1. Material Preparation
L. chinensis is naturally highly colonized by Glomus spp. of AMF in the fields [38,42], so Glomus tortuosum Schenck and Smith were selected as the AMF inoculation agent in our study. The stain was purchased from the Germplasm Repository of AMF of Beijing Academy of Agricultural and Forestry Sciences. M. sativa L. was used for fungus propagation. The expanding propagation of AM fungal spores was completed from June to August 2016 in a greenhouse, which was located in the College of Life Sciences, Northeast Normal University, Changchun City, China (43°51′38″ N, 125°19′26″ E).
The seeds of L. chinensis were collected from natural grasslands in Changling County, Jilin Province in the fall of 2016. After disinfection with 0.5% potassium permanganate solution, L. chinensis seeds were sown in the nursery with sterilized soil and germinated in a greenhouse (illuminated for 12 h, temperature is 26 °C in the day and 22 °C in the night) in early May 2017. After the seedlings grew to the trefoil stage, they were transplanted into the pots containing sterilized soil and sand (volume ratio of soil and sand, 4:1). Three plant individuals were planted in each pot. The soil was collected from the native grasslands of L. chinensis. The soil and sand were sterilized at 121 °C for 30 min before combining together. The weight of soil substrates in each pot was identical, and the pot size was 18 cm in diameter and 20 cm in height.
S. Chamaejasme, a poisonous perennial plant, widely distributes in the overgrazed steppe of Inner Mongolia of China [9]. Root exudates from S. chamaejasme were collected by in vivo culture as described previously [43,44]. Briefly, entire living S. chamaejasme plants were taken without hurting their roots from the degraded grassland which is located in Tianshan town, Chifeng city, eastern Inner Mongolia. The roots were washed by water to remove debris like soil and gravel, followed by sterilization by 70% alcohol. We used 20 S. chamaejasme plants aged eight-year-old for collecting root exduates. Each plant was placed in a special tin barrel containing 6 L Hoagland’s nutrient solution, with stems and leaves exposed to normal light. Seven days were allowed for the secretion of root exudates into the culturing solution and then mixed all of the culturing solution to be recovered and condensed by a reverse osmosis membrane (Model: TW30-1812-50, Dow, IA, USA) suction filter. The roots of all S. chamaejasm were dried at 105 °C for 48 h to measure the root biomass. Finally, the culturing solution was condensed to a specified level of 0.1 g/mL (i.e.,0.1 g is the root biomass of S. chamaejasme). The prepared root exudates were stored in a refrigerator at 4 °C.
2.2. Experimental Design and Treatments
The experimental design was a completely randomized two-factor interaction. AMF treatment included two levels: inoculation with and without AMF. The treatment of S. chamaejasme root exudates (SRE) had three concentration levels: the original solution (SRE0, mass concentration 0.1 g/mL), five-fold dilution (SRE5, mass concentration 0.02 g/mL), and twenty-fold dilution (SRE20, mass concentration 0.005 g/mL). The Hoagland’s nutrient solution was used as a control (CK). The dilutions (SRE5 and SRE20) were obtained by diluting the original solution with the Hoagland’s nutrient solution. These concentrations of root exudates (SRE0, SRE5 and SRE20) were designed basing on the methods and results of the previous studies on S. chamaejasme [45,46]. Combining the two factors, there were eight treatments in total (including CK), and each treatment had eight replicates (pots). The addition of root exudates and the inoculation with the AMF were carried out in July, 2017. The seedlings of L. chinensis had grown for two months after seed germination, whose height was about 12 cm. The spores collected by a 100 g expansion matrix of G. tortuosum were suspended to 100 mL in distilled water, and then were poured into all the pots simultaneously. For the negative control, the same amount of distilled water was poured into the pots without the AMF inoculation. There were approximately 400 AM fungal spores in each pot. The root exudate with the above three concentration gradients was added with 100 mL per pot, and the same amount of nutrient solution was poured in CK. The addition of root exudate treatment was performed every ten days for a total of four times.
Moreover, to mimic the natural composition of the microbial communities, all the pots were treated with 120 mL filtrate from non-sterile grassland soils. The filtrate was obtained by passing a 50:60 suspension of the soil inoculum through a filter paper (the pore size < 10 μm) to remove AM propagules [47]. The pots were placed in the greenhouse and arranged randomly. During the experiment, an equal amount of distilled water (100 mL) was added to each pot twice a week to keep soil moisturized.
2.3. Measurements of the Characteristics of Plant, AMF, and Soil
Ramet number, total rhizome length, above-ground and underground biomass of L. chinensis were measured on 19 August 2017. More specifically, ramet number of L. chinensis in each pot was recorded, and then the aboveground and underground parts were separated. Subsequentially, the underground part was washed with water, and then total rhizome length was measured as described previously [48]. The aboveground and underground parts were dried at 105 °C for 15 min (keeping the chemical composition of plant samples unchanged), then at 65 ºC for 72 h for measuring biomass, respectively. The dried plant samples were ground using a grinding miller to make the particles less than 1 mm. Total aboveground nitrogen (shoot TN) and total underground nitrogen (root TN) of L. chinensis were measured on a Kjeldahl apparatus (Kjeltec 8400, FOSS, Hillerød, Denmark) after digesting the plant samples with sulphuric acid (ω ≈ 98%).
The rhizosphere soil of L. chinensis was collected by the buffeting method to detect the AM fungal spore density and soil chemical properties. A small amount of fresh fibrous roots were selected and stored in a 4-degree refrigerator for detecting infection rate of AMF in the root systems of L. chinensis. Finally, the biomass of the fibrous roots was incorporated into underground biomass. Mycorrhizal infection was determined by a mycorrhizal staining method [49,50]. Spore density of AMF in the rhizosphere soil was determined by an improved wet sieve-sucrose gradient centrifugation method [51].
A portion of the soil samples was air-dried, ground and then sieved through a 0.15 mm mesh to measure soil pH, electrical conductivity (EC), the contents of available nitrogen (AN), total nitrogen (TN), available phosphorus (AP), total phosphorus (TP), and total carbon (TC). Soil pH and EC were measured in water suspension (water/soil = 2.5:1) using a pH meter (pH S-3C, INESA, Shanghai, China). EC was determined using an electrical conductivity instrument (DDS-307, INESA, Shanghai, China). AN was measured using a flow analyzer (Futura, AMS, Frépillon, France), and the pretreatment was completed using the alkaline hydrolysis and diffusion extraction method [52]. TN was measured on the Kjeldahl apparatus (Kjeltec 8400, FOSS, Hillerød, Denmark). AP was extracted with 0.5 mol·L1− NaHCO3 solution (pH 8.5) and was determined using Molybdenum antimony and the scandium colorimetry method on a spectrophotometer (UV5−500, METASH, Shanghai, China) [52]. TP was measured using an automatic discontinuous chemical analyzer (Smartchem 450, AMS, Rome, Italy) after digesting the soil samples with sulfuric acid-perchloric acid. TC was determined using the total organic carbon analyzer (Vario TOC, Elementar, Langenselbold, Germany).
2.4. Statistical Analysis
Before the ANOVA was carried out, all the data were tested for variance homogeneity (Levence-test) and normal distribution (Kolmogorov–Smirnov test). A two-way analysis of variance (ANOVA) (95% CI) was used to determine the differences of the interactive effects of the two factors (i.e., the addition of root exudates and inoculation with and without AMF on the characteristics of L. chinensis and soil properties). One-way ANOVA was also used to determine the significant effect of different concentration levels of S. chamaejasme root exudates. T-test was used to determine the significant difference in the growth characteristics of L. chinensis, shoot TN, root TN, and soil characteristics between with and without AMF inoculation, respectively. The multivariable stepwise regression analysis was used to identify the factor that could effectively explain the correlation among the characteristics of soils, AMF and L. chinensis. The statistical analysis was performed using SPSS software (SPSS Statistics 20.0, Chicago, IL, USA). All figures were produced using SigmaPlot software (Sigmaplot 12.5, Systat Software Inc., San Jose, CA, USA).
3. Results
3.1. The Growth of L. chinensis
3.1.1. Ramet Number and Rhizome Length
Within no AMF inoculation (AM−), SRE20 and SRE5 significantly increased ramet number of L. chinensis (p < 0.05), while there was no significant difference between SRE0 and the CK. With AMF inoculation (AM+), SRE20 significantly increased, and SRE0 significantly decreased ramet number (p < 0.05) (Figure 1a). Therefore, the treatments with lower root exudate concentrations showed positive effects on ramet number, whether L. chinensis was inoculated with AMF or not. However, high concentration of root exudates had an inhibitory effect on ramet number under inoculation of AMF.
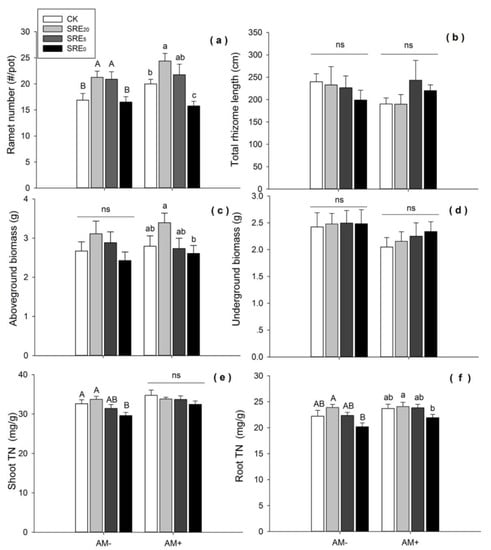
Figure 1.
Effects of the addition of S. chamaejasme root exudate (SRE) and inoculation without (AM−) and with (AM+) arbuscular mycorrhizal fungi (AMF) on (a) ramet number, (b) total rhizome length, (c) aboveground biomass, (d) underground biomass, (e) the content of shoot total nitrogen (TN), (f) the contene of root TN of L. chinensis. Values were means ± SE from eight repeat samples. Different capital letters, or lowercase letters indicate significant differences (p < 0.05, LSD) among the three SRE concentrations and CK within AM− or AM+ treatment, respectively. ns = no significance. SRE0, SRE5, and SRE20 represented the original solution, five-fold dilution, and twenty-fold dilution of S. chamaejasme root exudates. The mass concentration within these three SRE treatments was 0.1 g/mL, 0.02 g/mL, and 0.005 g/mL, respectively.
There was no significant difference in total rhizome length of L. chinensis among all concentrations of SRE within the treatment groups of AM− and AM+, respectively (Figure 1b).
3.1.2. Aboveground and Underground Biomass
SRE20 tended to increase the biomass of L. chinensis within non-inoculated AM−, but there was no significant difference among the three levels and CK. When AMF was inoculated, aboveground biomass of L. chinensis decreased gradually with the increasing levels of root exudates, and SRE0 significantly reduced aboveground biomass compared with SRE20 (p < 0.05) (Figure 1c). However, SRE0 did not significantly reduce this growth trait compared with CK. There were no significant differences in the underground biomass of L. chinensis between SRE and AMF treatments (Figure 1d). It seemed that underground biomass was insensitive to the addition of SRE and AMF inoculation.
Moreover, the results of the two-way ANOVA showed that SRE had significant effects on ramet number (p < 0.001) and aboveground biomass (p < 0.05) of L. chinensis, while AMF inoculation and their interaction of SRE × AMF had no significant effects on any growth parameters of L. chinensis (Table 1).

Table 1.
The ANOVA of the addition of S. chamaejasme root exudates (SRE) and inoculation with and without AM fungi (AMF) on the growth characteristics (ramet number, RN; rhizome length, RL; aboveground biomass, AB; underground biomass, UB) and nitrogen (N) contents of L.chinensis, infection rates and spore densities of AMF.
3.2. Total Nitrogen Content of Shoots and Roots
When AMF were not inoculated, SRE0 significantly reduced shoot TN of L. chinensis (p < 0.05). When AMF were inoculated, there was no difference in shoot TN among the three levels of root exudates and CK (Figure 1e). In general, higher concentration of root exudates had a negative effect on shoot TN in L. chinensis.
SRE0 significantly reduced root TN compared with the SRE20, while there were no significant differences between the three concentrations of root exudates and CK (Figure 1f). With or without AMF inoculation, there was no significant difference in root TN between all concentrations of root exudates with CK. In addition, T-test results showed that AMF inoculation significantly increased shoot TN (df = 62, p = 0.01) and root TN (df = 62, p = 0.042) compared with the condition without AMF inoculation.
The variance analysis showed the significant effects of SRE on shoot TN (p < 0.05) and root TN (p < 0.01) (Table 1). However, AMF treatment had a significant effect only on shoot TN (p < 0.01). Shoot TN of L. chinensis was significantly higher in the condition of AM+ than AM− at the concentration of SRE0 (df = 14, p = 0.029). This indicated that AMF inoculation had a positive effect on the nitrogen accumulation in the shoots of L. chinensis.
3.3. AM Fungal Characteristics
3.3.1. Infection Rate
The root exudate treatment had no significant effects on the infection rate in the non-inoculated (AM−) L. chinensis root system. SRE20 and SRE5 tended to increase infection rate compared with the CK treatment under the condition of G. tortuosum inoculation (AM+), while the increase was not significant at p < 0.05 statistical level (Figure 2a).
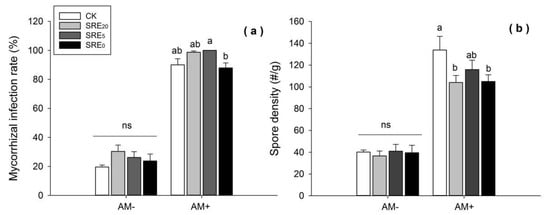
Figure 2.
Effects of the addition of S. chamaejasme root exudate (SRE) and inoculation without (AM−) or with (AM+) AMF on the (a) mycorrhizal infection rate and (b) spore density of AMF. Values were means ± SE from eight repeat samples. Different lowercase letters above bars indicate significant differences (p < 0.05, LSD) among the treatments within AM− or AM+ treatment. ns = no significance. SRE0, SRE5, and SRE20 represented the original solution, five-fold dilution, and twenty-fold dilution of S. chamaejasme root exudates. The mass concentration within these three SRE treatments was 0.1 g/mL, 0.02 g/mL, and 0.005 g/mL, respectively.
3.3.2. Spore Density
Under the AM− condition, spore density was not significantly affected by different concentration levels of root exudates (Figure 2b). However, spore density within SRE0 and SRE20 was significantly lower than that of CK under AM+ (p < 0.05). T-test results showed that AM+ significantly increased the mycorrhizal infection rate of L. chinensis (df = 62, p = 0.000) and AM spore density (df = 34, p = 0.000) compared with AM−.
The analysis of variance showed that SRE had a significant effect on the infection rate and spore density of AMF (p < 0.01). Similarly, AMF also had a significant effect on the above two parameters (p < 0.001). Yet, no obvious influence of their interaction between the two treatments (SRE × AMF) on the parameters of AMF was found (Table 1).
3.4. Soil Characteristics
SRE0 significantly increased soil pH compared with CK (p < 0.05) under the condition of AM−, while no significant effects of SRE20, and SRE5 on the pH were found. Under the AMF inoculation condition, SRE0 significantly increased but SRE5 decreased soil pH, respectively (p < 0.05) (Figure 3a).
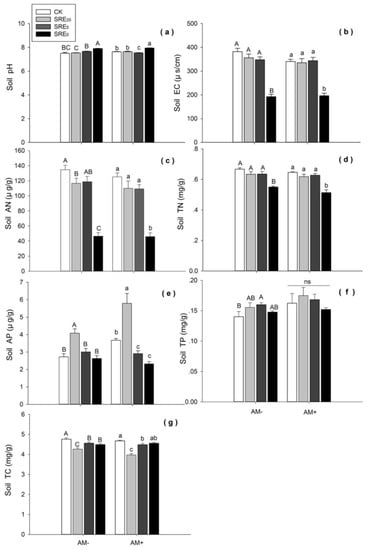
Figure 3.
Effects of the addition of S. chamaejasme root exudate (SRE) and inoculation without (AM−) or with (AM+) AMF on (a) soil pH, (b) soil electrical conductivity (EC), (c) soil available nitrogen (AN), (d) soil total nitrogen (TN), (e) soil available phosphorus (AP), (f) soil total phosphorus (TP), (g) soil total carbon (TC). Values were means ± SE from eight repeat samples. Different capital letters, or lowercase letters indicate significant differences (p < 0.05, LSD) among the three SRE concentrations and CK within AM− or AM+ treatment, respectively. SRE0, SRE5, and SRE20 represented the original solution, five-fold dilution, and twenty-fold dilution of S. chamaejasme root exudates. The mass concentration within these three SRE treatments was 0.1 g/mL, 0.02 g/mL, and 0.005 g/mL, respectively.
Whether L. chinensis was inoculated by AMF or not, the high concentration of SRE0 significantly decreased soil EC (p < 0.05), AN (p < 0.01), and TN (p < 0.05) (Figure 3b–d). However, there was no significant difference in EC and TN among SRE20, SRE5 and CK levels (Figure 3b,d).
SRE20 showed a remarkable increasing effect on soil AP compared with CK (p < 0.05) in both AM− and AM+ groups (Figure 3e). However, SRE5 and SRE0 significantly decreased soil AP compared with CK under the condition of AMF inoculation (p < 0.05), respectively. Lower concentrations of root exudates (SRE20) had a significantly positive effect on soil AP, while only negative effects of higher concentration were found in AM+ group.
Compared with CK, SRE5 significantly increased soil TP (p < 0.05) in AM− group, while SRE0 and SRE20 had no significant effects (Figure 3f). Under the condition of AMF inoculation, there was no significant difference among the three SRE concentrations and CK (p < 0.05). In addition, T-test results of AM+ and AM− groups showed that AMF inoculation significantly increased soil TP (df = 62, p = 0.040).
SRE20, SRE5, and SRE0 treatments significantly decreased soil TC compare with CK (p < 0.05) when L. chinensis was not inoculated with AMF. SRE20 and SRE5 significantly decreased soil TC compared in AM+ group (p < 0.05), while there was no significant difference between SRE0 and CK (Figure 3g). These results showed SRE20 had a stronger inhibitory effect on soil TC than the other two levels of SRE.
According to the two-way ANOVA, there were significant effects of SRE on soil pH, EC, AN, TN, AP and TC (p < 0.001). Additionally, AMF inoculation had significant effects on soil TN (p < 0.05), AP (p < 0.01), TP (p < 0.05) and TC (p < 0.01) (Table 2). Therefore, the addition of root exudates affected not only soil acidity and alkalinity, but also electrical conductivity and soil nutrients (including carbon, nitrogen and phosphorus). Their interaction between the two factors (SRE × AMF) had significant effects on soil pH (p < 0.01), AP (p < 0.001) and TC (p < 0.05) (Table 2).

Table 2.
The ANOVA of the addition of S. chamaejasme root exudates (SRE) and inoculation with and without AM fungi (AMF) on soil characteristics (electrical conductivity, EC; available phosphorous, AP; available nitrogen, AN; total nitrogen, TN; total phosphorous, TP; total carbon, TC).
3.5. The Correlation Between the Characteristics of L. chinensis and the Soil
The results of the multivariable stepwise regression analysis showed that ramet number of L. chinensis positively correlated with soil AP and with EC, but negatively related to soil pH (Table 3). There was a positive correlation between aboveground biomass of L. chinensis with soil AP. Underground biomass was negatively related to spore density (SD) of AMF. Shoot and root TN showed significant correlations with both soil AN and mycorrhizal infection rate (IR).

Table 3.
Multivariable stepwise regression analysis of the main soil and AMF parameters influencing the characteristics of L. chinensis. EC: electrical conductivity, AP: available phosphorous, AN: available nitrogen, IR: infection rate.SD: spore density.
4. Discussion
4.1. Effects of Root Exudates on Soil Properties and AMF
It is suggested that the magnitude of allelopathic substance on soil properties obviously highly depended on their concentration [53]. We had found that the higher concentrations of root exudates significantly reduced soil available nitrogen (AN), available phosphorous (AP), total nitrogen (TN) (Figure 3c–e). These results were similar to the findings of the previous studies, which found that the contents of soil total nitrogen and phosphorus decreased significantly in rhizosphere soil of S. chamaejasme [41,54]. Besides, the improvement of low-concentration root exudates on soil AP was consistent with the results of Cesco et al. (2012) [53]. The flavonoids secreted by the roots of S. chamaejasme could indirectly increase the soil AP through affecting the colonization of the AMF [21]. Shen et al. (2002) reported that in common bean organic acids might be secreted and could mobilize P from Al- and Fe-bound phosphates, and hence, increase soil AP [55]. Generally, the mechanism how S. chamaejasme root exudates affect soil nutrients was quite complicated, which might closely relate to the composition and concentration of the root exudates, soil microorganisms, enzyme activity, and litter decomposition [53,56,57,58].
Previous study reported that allelopathic substance (i.e., terpenes and flavonoids) of a poisonous plant, Solidago canadensis L., could increase soil pH [59]. We also found that high concentration of S. chamaejasme root exudates significantly increased soil pH. S. chamaejasme roots contain flavonoids, lignans, and terpenes and alkaloids [15,16,17]. S. chamaejasme root exudates collected from in vivo culture in our experiments were alkalescent (pH = 9.28) which could partially explain the increase of soil pH. Soil pH can directly or indirectly affect the availability of soil nutrient elements, soil microbial composition, enzyme activity, and the metabolism of allelochemicals [50,60,61,62]. Therefore, the alkalescent solution of root exudates may potentially explain why the root exudates had altered the soil carbon, nitrogen, and phosphorus contents.
It has been reported that plant allelochemicals or root exudates also can affect the composition and structure of microbial communities in the rhizosphere soil [44,63,64], but the conclusions are not coincident. The secondary metabolites of the invasive plant S. canadensis might promote its competitiveness by enhancing its own AMF symbionts [65]. Root exudates from Allium sativum L. could inhibit the growth of the mycelium and germination of zoospores of Phytophthora capsici [66]. Our results showed a particular inhibitory effect of S. chamaejasme root exudates on the AMF infection rate and spore density (Table 1, Figure 2). Many secondary metabolites have been isolated from the root of S. chamaejasme by organic solvent extraction such as coumarins, flavonoids, lignans, and diterpenes [15,16]. The bioactivity assay showed that some of these daphneolone analogues synthesized by S. chamaejasme were potentially active against plant pathogenic fungi, such as Rhizoctonia solani, Gibberella zeae, Bipolaris maydis, Sclerotia sclerotium, and Botrytis cirerea [67]. Therefore, we speculated that the components of S. chamaejasme root exudates might inhibit AMF infection. No AMF infection rate in the root system of S. chamaejasme in the field also supported such a speculation [40].
4.2. Effects of Root Exudates on L. chinensis
S. chamaejasme root exudates could affect the growth and development of the surrounding plants through allelopathy. The lower concentration had shown a tendency of positive effects on L. chinensis growth, whereas the higher concentrations had negative effects (Figure 1; Table 1). These results reinforced the previous findings of the effect of S. chamaejasme on the seedling growth of adjacent plants [13]. In our study, the influence of root exudates on the growth of L. chinensis can be better explained by the changes in rhizosphere soil nutrients. Low-concentration promotion and high-concentration inhibition were the general rules of the effects of root exudates on the growth of L. chinensis (Figure 1) and nutrient availability (Figure 2). Soil nutrient availability, especially nitrogen and phosphorus, is often a limiting factor for plant growth in grassland ecosystems [68,69], and is closely related to the nutrient content of the plant [70]. Our stepwise regression analysis also further proved that AN and AP were one of the critical factors affecting the ramet numbers and the above-ground biomass of L. chinensis (Table 3). Therefore, in consistent with our hypothesis, S. chamaejasme root exudates appeared to affect the growth and nitrogen accumulation of L. chinensis mainly by changing the soil pH and soil nutrient availability. In summary, this supported our first hypothesis.
4.3. Inoculation Effects of AMF
The mycorrhizal infection rate is an important parameter to evaluate the symbiotic relationship between AMF and host plants [71]. The successful colonization of AMF in many clonal plants, such as Prunella vulgaris L. and Potentilla reptans L. has been reported [72,73,74]. The high AMF colonization rate in L. chinensis (87.91~100%) indicated that AMF could establish a strong symbiotic relationship with the root system of L. chinensis. AM symbiosis could increase photosynthetic and water use efficiency, and further improve host plant growth [75]. A case study showed AMF inoculation promoted the formation of new ramets in Hedernepalensis var. sinensis. However, AMF failed to affect the biomass of some stoloniferous clonal species, such as Trifolium repens L. and Gnetum montanum Markgr. [47]. Our results also did not find a positive effect of AMF inoculation on the growth of L. chinensis (Table 1). These inconsistent conclusions determined that AMF inoculation effects on plant ecological performance might be species-specific. The relatively slow process of AMF from inoculation to the colonization in a host plant might lead to a time-lag effect of AMF in promoting plant growth.
Although no significant effects on population performance (i.e., ramet number and biomass) of L. chinensis, AMF inoculation had a significant effect on physiological indexes, i.e., shoot nitrogen content (Figure 1; Table 1). A number of studies had also shown that AMF could increase plant absorption of nitrogen [27,28,29,30]. After exogenous application of 15N, the nitrogen content in the shoot and root of mycorrhizal plants was higher than that of the control [76]. AMF inoculation could improve the formation of a large number of extracellular mycelia to increase the root area of plants. Meanwhile, because of ammonium transporters of AMF, extracellular mycelium could facilitate the absorption of NH4+ and NO3− from the soil, which was transferred to intracellular hyphae hydrolysis into the plants [77,78].
In addition, AMF inoculation significantly affected the TN, AP, TP, and TC (Table 2) in this study. AMF are usually known as obligate biotrophs that cannot utilize organic phosphorus, but they can release C-rich compounds into the soil, thereby promoting phosphorus-solubilizing bacteria to mineralize phosphorus [79]. Additionally, AMF might increase soil AP by improving the soil bacterial diversity and phosphorus-solubilizing bacteria (Acidobacteria) abundance [80]. Therefore, we suggested that AMF may indirectly affect plant growth by regulating soil chemical characteristics, especially the availability of phosphorus. According to the increasing effects on the mycorrhizal infection rate, spore density, and the shoot nitrogen content, our results suggested that AMF may have potentially facilitate the growth of L. chinensis by increasing nitrogen uptake and utilization rate of its root system.
4.4. Interaction Effects
The interaction between of S. chamaejasme root exudates and AMF significantly changed pH, AP, and TC of rhizosphere soil (Table 2). AMF altered the effects of the additions of the S. chamaejasme root exudates on soil properties (Table 1). These results verified the second hypothesis of our study. Soil microbes play an essential role in altering the activities of allelochemicals and can degrade some allelopathic active substance into non-allelopathic active [81]. Studies have shown that the connectivity of mycorrhizal fungi hyphae may play a key role in regulating the movement of allelochemicals in soil and affecting their bioactive zones [82]. The mycelium of AMF could mediate allelochemical transport, such as juglone [83].
Furthermore, the change of soil pH is closely related to the availability and supply capacity of soil mineral elements. Therefore, the inoculation of the AMF might have changed the effects of the S. chamaejasme allelochemicals through some direct or indirect approaches. Additionally, AMF infection rate (IR) and rhizosphere spore density (SD) were important factors in affecting the underground biomass and nitrogen content of the plant (Table 3). Hence, these results provided reliable evidence for the regulatory ability of AMF in the growth of L. chinensis. Therefore, in consistent with our third hypothesis, our results suggested that AMF have the potential to regulate the allelopathic effects of S. chamaejasme and the relationship between S. chamaejasme and L. chinensis.
Root exudates of poisonous plants may comprise a diverse of chemical compounds, which can differentially affect growth of neighboring plants [84]. However, there was no report about the chemical compounds of S. chamaejasme root exudates that were collected in vivo. In previous studies, many secondary metabolites have been isolated from the roots and rhizosphere soil of S. chamaejasme by organic solvent extraction, including coumarins, flavonoids, lignans, and diterpenes [15,16,17]. We speculated that the main active components of root exudates in this study might be the same as the results that have been identified above. As we know releasing root exudates is the primary way in which poisonous plants interact with neighboring plants in natural grasslands. In our study, the root exudates instead of the organic solvent extracts of S. chamaejasme could better simulate a natural process that exists in the grassland ecosystem. The chemical composition of S. chamaejasme root exudates and their differential effects still need further research in the future.
5. Conclusions
Our study highlighted the regulatory roles of AMF in mediating the interspecific relationship between poisonous and neighboring plants. In this study, we investigated the influence of S. chamaejasme root exudates, AMF inoculation, their interaction on the growth of L. chinensis and soil physicochemical properties in the rhizosphere. Firstly, we concluded that the S. chamaejasme root exudates had allelopathic effects on the growth of L. chinensis, and the effects were closely related to the concentration of the root exudates. Low concentration of S. chamaejasme root exudates positively improved the growth of L. chinensis, whereas high concentrations showed the negatively inhibiting effects. High concentration of root exudates also significantly reduced soil nutrients. The critical factors in affecting the growth of L. chinensis. Secondly, AMF could mediate the allelopathic effects of S. chamaejasme via changing nutrient availability. These results indicated that AMF could interact with the root exudates to change some soil chemical characteristics and then potentially regulate the interspecific relationship between poisonous plants and neighboring forage grasses. Taken together, our study provided empirical evidence revealing the allelopathic mechanism of S. chamaejasme and the regulatory effects of AMF on the interspecific relationship between poisonous plants and neighboring plants. The combination of the allelopathy of poisonous plants and the regulation of AMF will be meaningful to recover the dominance of palatable grasses in persistent degraded grassland ecosystems.
Author Contributions
Conceptualization, X.Z. and F.X.; data curation, X.Z. and X.L.; formal analysis, X.Z. and X.L.; funding acquisition, F.X. and Y.G.; investigation, X.Z.; methodology, X.Z., F.X. and G.H.; project administration, F.X.; software, C.C.; supervision, X.Z., X.L., F.X., C.C., G.H. and Y.G.; writing—original draft, X.Z.; writing—review and editing, X.Z., X.L., F.X., C.C. and Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (31670524, 31570452), the Program for Introducing Talents to Universities (B16011), the Bureau of Science & Technology, Jilin Province (20180101347JC), and the Bureau of Education of Jilin Province (JJKH20190263KJ).
Acknowledgments
We greatly appreciate Zhang Xiao and Jiang Xue for helping complete part of the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meng, B.P.; Ge, J.; Liang, T.G.; Yang, S.X.; Gao, J.L.; Feng, Q.S.; Cui, X.; Huang, X.D.; Xie, H.J. Evaluation of remote sensing inversion error for the above-ground biomass of alpine meadow grassland based on multi-source satellite data. Remote. Sens. 2017, 9, 372. [Google Scholar] [CrossRef]
- Feng, X.M.; Zhao, Y.S. Grazing intensity monitoring in Northern China steppe: Integrating CENTURY model and MODIS data. Ecol. Indic. 2011, 11, 175–182. [Google Scholar] [CrossRef]
- Akiyama, T.; Kawamura, K. Grassland degradation in China: methods of monitoring, management and restoration. Grass Sci. 2007, 53, 1–17. [Google Scholar] [CrossRef]
- Khan, R.U.; Mehmood, S.; Khan, S.U. Toxic effect of common poisonous plants of district bannu, khyber pakhtunkhwa, pakistan. Pak. J. Pharm. Sci. 2018, 31, 57–67. [Google Scholar] [PubMed]
- Holechec, J. Do most livestock losses to poisonous plants result from “poor” range management? J. Range Manage. 2002, 55, 270–276. [Google Scholar] [CrossRef]
- Tokarnia, C.H.; Döbereineb, J.; Peixoto, P.V. Poisonous plants affecting livestock in Brazik. Toxicon 2002, 40, 1635–1660. [Google Scholar] [CrossRef]
- Jandová, K.; Klinerová, T.; Müllerová, J.; Pysěk, P.; Pergl, J.; Cajthaml, T.; Dostál, P. Long-term impact of Heracleum mantegazzianum invasion on soil chemical and biological characteristics. Soil Biol. Biochem. 2014, 68, 270–278. [Google Scholar] [CrossRef]
- Li, Y.Y.; Dong, S.K.; Liu, S.L.; Wang, X.X.; Wen, L.; Wu, Y. The interaction between poisonous plants and soil quality in response to grassland degradation in the alpine region of the Qinghai-Tibetan Plateau. Plant Ecol. 2014, 215, 809–819. [Google Scholar] [CrossRef]
- Lu, H.; Wang, S.S.; Zhou, Q.W.; Zhao, Y.N.; Zhao, B.Y. Damage and control of major poisonous plants in the western grasslands of China—A review. Rangel. J. 2012, 34, 329. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yue, J.P.; Sun, H. Identification of twelve novel polymorphic microsatellite loci in the severe weed, Stellera chamaejasme L. (Thymelaeaceae). J. Genet. 2015, 94 (Suppl. 2), 24–26. [Google Scholar] [CrossRef]
- Xing, F. Ecological Study on Poisonous Plants in Grassland; Academy of Sciences Press: Beijing, China, 2016. [Google Scholar]
- Zhou, S.Q.; Wang, H. Allelopathy of S. chamaejasme on Elymus dahuricus. Grassl. Turf. 2010, 30, 63–65. (In Chinese) [Google Scholar]
- Guo, H.R.; Cui, H.Y.; Jin, H.; Yan, Z.Q.; Ding, L.; Qin, B. Potential allelochemicals in root zone soils of Stellera chamaejasme L. and variations at different geographical growing sites. Plant Growth Regul. 2015, 77, 335–342. [Google Scholar] [CrossRef]
- Cao, C.Y.; Fu, Y.; Wang, W.X.; Gao, F.F. Inhibition influence of extraction liquids from Stellera chamaejasme root on seed germination. J. Northeast. Univ. 2007, 28, 29–32. (In Chinese) [Google Scholar]
- Tatematsu, H.; Kurokawa, M.; Aiwa, M.; Hirata, Y. Piscicidal constituents of Stellera chamaejasme L. Chem. Pharm. Bull. 1984, 32, 1612–1613. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Tanaka, T.; Sakamoto, T. Biflavanones, diterpenes and coumarins from the roots of Stellera chamaejasme L. Chem. Pharm. Bull. 2002, 50, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Q.; Guo, H.G.; Yang, J.Y.; Liu, Q.; Jin, H.; Xu, R.; Cui, H.Y.; Qin, B. Phytotoxic flavonoids from roots of Stellera chamaejasme L. (Thymelaeaceae). Phytochemistry 2014, 106, 61–68. [Google Scholar] [CrossRef]
- Harborne, J.B. Introduction to Ecological Biochemistry, 3rd ed.; Academic Press: London, UK, 1988. [Google Scholar]
- Leu, E. Polyphenolic allelochemicals from the aquatic angiosperm myriophyllum spicatum inhibit photosystem Ⅱ. Plant Physiol. 2002, 130, 2011–2018. [Google Scholar] [CrossRef]
- White, C.S. Monoterpenes: Their effects on ecosystem nutrient cycling. J. Chem. Ecol. 1994, 20, 1381–1406. [Google Scholar] [CrossRef]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 12–15. [Google Scholar] [CrossRef]
- Vandermeer, J. Interspecific competition: a new approach to the classical theory. Science 1975, 188, 2532–2555. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Barton, A.M. Patterns and consequences of interspecific competition in natural communities a review of field experiments with plants. Am. Nat. 1992, 139, 771–801. [Google Scholar] [CrossRef]
- Mckane, R.B.; Johnson, L.C.; Shaver, G.R.; Nadelhoffer, K.J.; Rastetter, E.B.; Fry, B. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 2002, 415, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Klironomos, J.; Zoble, M.; Tibbett, M.; Stock, W.D.; Rillig, M.C.; Parrent, J.L.; Moora, M.; Koch, A.M.; Facellli, J.M.; Dickie, I.A.; et al. Forces that structure plant communities: quantifying the importance of the mycorrhizal symbiosis. New. Phytol. 2011, 189, 3663–3670. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; George, E.; Marschner, H. Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 1991, 136, 41–48. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Zhang, F.S.; Shen, J.B.; Feng, G. Rhizosphere Ecology: Processes & Management; China Agricultural University Press: Beijing, China, 2009. [Google Scholar]
- van der Heijden, M.G.A.; Martin, F.M.; Sanders, I.R. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Thomas, B.; Wiemken, A.; Sander, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Facelli, E.; Facelli, J.M.; Smith, S.E.; Mclaughlin, M.J. Interactive effects of arbuscular mycorrhizal symbiosis, intraspecific competition and resource availability on Trifolium subterraneum cv. Mt. Barker. New Phytol. 1999, 141, 535–547. [Google Scholar] [CrossRef]
- Scheublin, T.R.; van Logtestijn, R.S.P.; van der Heijden, M.G.A. Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J. Ecol. 2007, 95, 631–638. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Horton, T.R. Socialism in soil? The importance mycorrhizal fungal networks for facilitation in natural ecosystems. J. Ecol. 2009, 97, 1139–1150. [Google Scholar] [CrossRef]
- Ayres, R.L.; Gange, A.C.; Aplin, D.M. Interactions between arbuscular mycorrhizal fungi and intraspecific competition affect size, and size inequality, of Plantago lanceolata L. J. Ecol. 2010, 94, 285–294. [Google Scholar] [CrossRef]
- Wagg, C.; Jansa, J.; Stadler, M. Mycorrhizal fungal identity and diversity relaxes plant-plant competition. Ecology 2011, 92, 1303–1313. [Google Scholar] [CrossRef]
- Mariotte, P.; Meugnier, C.; Johnson, D.; Thébault, A.; Spiegelberger, T.; Buttler, A. Arbuscular mycorrhizal fungi reduce the differences in competitiveness between dominant and subordinate plant species. Mycorrhiza 2013, 23, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.M.; Caçador, I.; Martins-Loução, M.A. Temporal and spatial variation of arbuscular mycorrhizas in salt marsh plants of the Tagus estuary (Portugal). Mycorrhiza 2001, 11, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.L.; Du, J.; Zhang, B.T.; Ba, L.; Hodgkinson, K.C. Grazing intensity and phenotypic plasticity in the clonal grass Leymus chinensis. Rangel. Ecol. Manag. 2017, 70, 740–747. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Yan, W. Arbuscular mycorrhizae and their structural types on common plants in grasslands of mid-western Inner Mongolia. Biodivers. Sci. 2004, 12, 501–508. (In Chinese) [Google Scholar]
- He, W.; Detheridge, A.; Liu, Y.M.; Wang, L.; Wei, H.C.; Griffith, G.W.; Scullion, J.; Wei, Y.H. Variation in soil fungal composition associated with the invasion of Stellera chamaejasme L. in Qinghai–Tibet plateau grassland. Microorganisms 2019, 7, 587. [Google Scholar] [CrossRef]
- Sengupta, A.; Chaudhuri, S. Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India. Mycorrhiza 2002, 12, 169–174. [Google Scholar] [CrossRef]
- Jandová, K.; Dostá, P.; Cajthaml, T.; Kameník, Z. Intraspecific variability in allelopathy of Heracleum mantegazzianum is linked to the metabolic profile of root exudates. Ann. Bot. 2015, 115, 821–831. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal comunity composition and diversity. Appl. Environ. Microbiol. 2008, 73, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Meng, Z.J.; Dang, X.H.; Song, W.J.; Zhai, B. Allelopathic effects of Stellera chamaejasme on seed germination and seedling growth of alfalfa and two forage grasses. Acta Prataculturae Sinica 2019, 28, 130–138. (In Chinese) [Google Scholar]
- Bi, L.X.; Liao, R.S. Study on Allelopathic Effects of Aqueous Extracts from Stellera chamaejasme L. and Cynanchum komarovii. J. Anhui Agri. Sci. 2010, 38, 3294–3297. (In Chinese) [Google Scholar]
- Sudová, R. Different growth response of five co-existing stoloniferous plant species to inoculation with native arbuscular mycorrhizal fungi. Plant Ecol. 2009, 204, 135–143. [Google Scholar] [CrossRef]
- Gao, Y.; Xing, F.; Jin, Y.J.; Nie, D.D.; Wang, Y. Foraging responses of clonal plants to multi-patch environmental heterogeneity: spatial preference and temporal reversibility. Plant Soil 2012, 359, 137–147. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 5, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianiazzi-Pearson, V. Mesure du taux de mycorhization VA d’unsystème radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae, Gianiazzi-Pearson Vand Gianiazzi S; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Wang, Y.S.; Zhang, S.B.; Zhang, M.Q. Resources and Germplasm Resources of Arbuscular Mycorrhizal Fungi in China; China Agricultural Publishing House: Beijing, China, 2012. [Google Scholar]
- Shi, Y.; Sheng, L.; Wang, Z.; Zhang, X.; He, N.; Yu, Q. Responses of soil enzyme activity and microbial community compositions to nitrogen addition in bulk and microaggregate soil in the temperate steppe of Inner Mongolia. Eurasian Soil Sci. 2016, 49, 1149–1160. [Google Scholar] [CrossRef]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. a review. Biol. Fertil. Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Sun, G.; Luo, P.; Wu, N.; Qiu, P.F.; Gao, Y.H.; Chen, H.; Shi, F.S. Stellera chamaejasme L. increases soil N availability, turnover rates and microbial biomass in an alpine meadow ecosystem on the eastern Tibetan Plateau of China. Soil Biol. Biochem. 2009, 41, 86–91. [Google Scholar] [CrossRef]
- Shen, H.; Yan, X.L.; Zhao, M. Exudation of organic acids in common bean as related to mobilization of aluminum and ironbound phosphates. Environ. Exp. Bot. 2002, 48, 1–9. [Google Scholar] [CrossRef]
- Salam, A.K.; Helmke, P.A. The dependence of free ionic activities and total dissolved concentration of copper and cadmium in soil solution. Geoderma 1998, 83, 281–291. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Zobeck, T.M.; Gill, T.E.; Kennedy, A.C. Enzyme activities and microbial community structure in semiarid agricultural soils. Biol. Fertil. Soils 2003, 38, 216–227. [Google Scholar] [CrossRef]
- Zhan, S.X.; Wang, Y.; Zhu, Z.C.; Li, W.H.; Bai, Y.F. Nitrogen enrichment alters plant n: p stoichiometry and intensifies phosphorus limitation in a steppe ecosystem. Environ. Exp. Bot. 2016, 134, 21–32. [Google Scholar] [CrossRef]
- Zhang, C.B.; Wang, J.; Qian, B.Y.; Li, W.H. Effects of the invader Solidago canadensis on soil properties. Appl Soil Ecol. 2009, 43, 163–169. [Google Scholar] [CrossRef]
- Staddon, W.J.; Trevors, J.T.; Duchesne, L.C. Soil microbial diversity and community structure across a climatic gradient in western Canada. Biodivers Conserv. 1998, 7, 1081–1092. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Turner, B.L.; Haygarth, P.M. Phosphatase activity in temperate pasture soils: Potential regulation of labile organic phosphorus turnover by phosphodiesterase activity. Sci. Total Environ. 2005, 344, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gadkar, V.; David-Schwartz, R.; Nagahashi, G.; David, D.; Wininger, S.; Kapulnik, Y. Root exudate of pmi tomato mutant M161 reduces AM fungal proliferation in vitro. FEMS Microbiol. Lett. 2003, 223, 193–198. [Google Scholar] [CrossRef][Green Version]
- Haichar, F.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1211–1230. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Tang, J.J.; Leng, D.; Hu, S.J.; Yong Jean, W.H.; Chen, X. An invasive plant promotes its arbuscular mycorrhizal symbioses and competitiveness through its secondary metabolites: indirect evidence from activated carbon. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Muhammad, A.K.; Cheng, Z.H.; Xiao, X.M.; Khan, A.R.; Ahmed, S.S. Ultrastructural studies of the inhibition effect against phytophthora capsici of root exudates collected from two garlic cultivars along with their qualitative analysis. Crop Prot. 2011, 30, 1149–1155. [Google Scholar]
- Jin, H.; Geng, Y.; Yu, Z.; Tao, K.; Hou, T. Lead optimization and anti-plant pathogenic fungi activities of daphneolone analogues from Stellera chamaejasme L. Pestic. Biochem. Physiol. 2009, 93, 131–137. [Google Scholar] [CrossRef]
- Zhang, N.Y.; Guo, R.; Song, P.; Guo, J.X.; Gao, Y.Z. Effects of warming and nitrogen deposition on the coupling mechanism between soil nitrogen and phosphorus in Songnen Meadow Steppe, northeastern China. Soil Biol. Biochem. 2013, 65, 96–104. [Google Scholar] [CrossRef]
- Sun, S.N.; Xing, F.; Zhao, H.; Gao, Y.; Bai, Z.; Dong, Y. Response of bacterial community to simulated nitrogen deposition in soils and a unique relationship between plant species and soil bacteria in the Songnen grassland in Northeastern China J. Soil. Sci. Plant. Nut. 2014, 14, 565–580. [Google Scholar] [CrossRef][Green Version]
- Ordoñez, J.C.; van Bodegom, P.M.; Witte, J.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Cesco, S.; Tomasi, N.; Mimmo, T. Influence of different trap solutions on the determination of root exudates in Lupinus albus L. Biol. Fertil. Soils. 2015, 51, 757–765. [Google Scholar] [CrossRef]
- Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Clonal growth traits of two Prunella species are determined by cooccurring arbuscular mycorrhizal fungi from a calcareous grassland. J. Ecol. 1997, 85, 181–191. [Google Scholar] [CrossRef]
- Streiywolf-Engel, R.; van der Heijden, M.G.A.; Wiemken, A.; Sanders, I.R. The ecological significance of arbuscular mycorrhizal fungal effects on clonal reproduction in plants. Ecology 2001, 82, 2846–2859. [Google Scholar] [CrossRef]
- Sudová, R.; Vosátka, M. Effects of inoculation with native arbuscular mycorrhizal fungi on clonal growth of Potentilla reptans and Fragaria moschata (Rosaceae). Plant Soil 2008, 308, 55–67. [Google Scholar] [CrossRef]
- Hameed, A.; Dilfuza, E.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Ahmad, P. Salinity stress and arbuscular mycorrhizal symbiosis in Plants. In Use of Microbes for the Alleviation of Soil Stresses; Springer Science & Business Media: New York, NY, USA, 2014; Volume 1, pp. 139–159. [Google Scholar]
- Ames, R.N.; Reid, C.P.P.; Porter, L.K.; Cambardella, C. Hyphal uptake and transport of nitrogen from two 15N-labelled sources by Glomus mosseae, a vesicular-arbuscular mycorrhizal fungus. New Phytol. 1983, 95, 381–396. [Google Scholar] [CrossRef]
- Tian, C.; Kasiborski, B.; Koul, R.; Lammers, P.J.; Bücking, H.; Shachar-Hill, Y. Regulation of the nitrogen transfer pathway in the arbuscular mycorrhizal symbiosis: gene characterization and the coordination of expression with nitrogen flux. Plant Physiol. 2010, 153, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tienda, J.; Testillano, P.S.; Balestrini, R.; Fiorilli, V.; Azcón-Aguilar, C.; Ferrol, N. GintAMT2, a new member of the ammonium transporter family in the arbuscular mycorrhizal fungus Glomus intraradices. Fungal Genet. Biol. 2011, 48, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate- solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, S.D.; Palmer, A.S.; Winsley, T.; Lamb, E.; Bissett, A.; Brown, M.V.; Van Dors, J.; Ji, M.; Ferrari, C.; Grogan, P.; et al. Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with communities. Soil Biol. Biochem. 2014, 78, 10–20. [Google Scholar] [CrossRef]
- Inderjit. Soil microorganisms: an important determinant of allelopathic activity. Plant Soil 2005, 274, 227–236. [Google Scholar] [CrossRef]
- Barto, E.K.; Weidenhamer, J.D.; Cipollini, D.; Rillig, M.C. Fungal superhighways: do common mycorrhizal networks enhance below ground communication? Trends Plant Sci. 2012, 17, 633–637. [Google Scholar] [CrossRef]
- Achatz, M.; Rillig, M.C. Arbuscular mycorrhizal fungal hyphae enhance transport of the allelochemical juglone in the field. Soil Biol. Biochem. 2014, 78, 76–82. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.H.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–78. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).