Abstract
Neospora caninum, the causative agent of abortion in cattle, has a major economic impact worldwide. This review aims to provide an overview of key advances over the last 10 years in understanding host−pathogen interactions, molecular mechanisms, and emerging control strategies and puts them into a context with previously published important findings. More recently, novel diagnostic tools with improved sensitivity and specificity have been developed. These have supplemented the already existing methods to detect infection in clinical cases and are essential for investigations on parasite distribution, disease incidence and prevalence, and transmission of N. caninum. Epidemiological studies have revealed the influence of environmental, genetic, and ecological factors on parasite transmission dynamics, and emphasized the importance of integrated “One Health” strategies. Characteristics of different Neospora strains have been elucidated through animal models and molecular tools such as clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9 (CRISPR/Cas9)-based gene editing, high-throughput sequencing, and advanced proteomics, aiming to shed light on stage-specific gene regulation and virulence factors, contributing to the development of interventions against neosporosis. Insights into immune modulation, immune evasion, and parasite persistence contributed to the efforts towards vaccine development. In terms of therapeutics, both repurposed drugs and more targeted inhibitors have shown promising efficacy in reducing parasite burden and mitigating vertical transmission in laboratory models. Here, more recent innovations in nanoparticle-based drug delivery systems and immunomodulatory strategies are prone to enhancing therapeutic outcomes. However, a significant challenge remains the integration of molecular and immunological insights into practical applications.
1. Neospora caninum and Neosporosis
Neospora caninum is an apicomplexan parasite that was isolated from dogs with encephalomyelitis and myositis [1]. After its discovery, it has emerged as a significant pathogen causing abortion and stillbirth in cattle and neurological disorders in dogs. Canids such as dogs, coyotes, gray wolves, and dingoes act as definitive hosts [2,3], while with respect to intermediate hosts N. caninum infects a diverse range of other farm animals and wildlife species [4,5,6]. This broad host range contributes to parasite persistence in various ecosystems and complicates control efforts.
The life cycle of N. caninum includes three distinct invasive stages. The rapidly proliferating tachyzoite stage causes acute disease and can invade and replicate in many tissues and cell types [7]. In vitro culture allows the visualization of tachyzoites, their interactions with the host cells, and their intracellular development by different microscopical techniques. Surface antigens (SAGs) mediate the first contact between host cell surface and parasite membrane, closely followed by the secretion of three types of secretory organelles crucially involved in host cell invasion. Initially, micronemes and rhoptries sequentially secrete MIC and ROP proteins, respectively, through the apical tip of the parasites. MIC and ROP proteins interact with host cell surface receptors and are involved in the formation of a gliding junction, which allows the parasite to invade by pushing the host cell surface membrane into the cytoplasm, until the parasite is located intracellularly and proliferates within a parasitophorous vacuole (PV), delineated by a parasitophorous vacuole membrane (PVM) [2,3].
Host cell invasion is followed by the secretion of dense granule (GRA) proteins into the lumen of the PV, some of which form the intravacuolar tubular network, modify the PVM or cross the PVM and enter the host cell nucleus to act as transcription factors. Several MIC, ROP, and GRA proteins constitute virulence factors and have been exploited as vaccine candidates [7,8]. In an immunocompetent host, tachyzoites will differentiate into slowly replicating bradyzoites that form tissue cysts and cause chronic infection. Tissue cysts are found mainly in the central nervous system (CNS) and in muscle tissue without causing symptoms. They are orally infectious for a carnivorous host. The third invasive stage is the sporozoites that are formed in the environment following the sexual development and oocyst formation that takes place in the intestinal tissues of the definitive host.
The life cycle of N. caninum starts by accidental uptake of infective oocysts containing sporozoites through contaminated food or water or ingestion of meat containing N. caninum tissue cysts formed by bradyzoites. These stages lead to the infection of enterocytes and proliferation within intestinal epithelial cells. Parasites multiply and undergo egress, followed by invasion of cells of the reticulo-endothelial system, including antigen-presenting dendritic cells, in which they further disseminate into other locations and tissues in the body as tachyzoites [9]. Infection goes largely unnoticed, but the inflammatory immune response eliminates a large part of the parasite population. The associated physiological stress triggers tachyzoite-to-bradyzoite differentiation in a subset of these parasites, resulting in the formation of tissue cysts, which finally persists mainly in the CNS and in skeletal muscle [10]. Ingestion of tissues containing bradyzoites by a definitive host can then lead to sexual development and oocyst formation in the intestine [6].
In pregnant animals, tachyzoites can be vertically transmitted and infect the fetus. Vertical transmission is highly relevant in cattle but has been reported also in several other animal species [6,8]. Transplacental vertical transmission can occur through the exogenous route following a primary infection during pregnancy or endogenously in chronically infected animals upon recrudescence of bradyzoites and conversion into tachyzoites during pregnancy. In both cases, parasites exploit the pregnancy-associated impairment of inflammatory immunity normally serving to protect the developing fetus, while at the same time increasing the risk of fetal infection. Vertical transmission rates vary significantly between host species [6,7], with fetal infection often resulting in abortion [11]. In intermediate hosts, particularly cattle, endogenous transplacental transmission can occur repeatedly through successive pregnancies. This process of interconversion between developmental stages is crucial for both parasite persistence and transmission, involving complex immunological interactions during both acute and chronic phases of infection [12]. The molecular mechanisms controlling the tachyzoite-bradyzoite-stage conversion in N. caninum are not well understood, as reliable in vitro models to study bradyzoite development have not been set up. In vitro stage conversion has been initiated by specific stress conditions such as pH changes and nitric oxide exposure [13,14,15], but the efficiency of these methods is limited [16].
The clinical manifestations of neosporosis vary, depending on the host species. In cattle, the primary impact is on reproductive health. Abortion mostly occurs in the second trimester of pregnancy, but neosporosis can also lead to birth of weak offspring or persistently infected calves devoid of any clinical signs. However, in subsequent pregnancies, these healthy offspring can transmit the parasite to the next generation, which represents a severe complication for the introduction of control measures [17]. Thus, early and accurate diagnosis is crucial for the effective management. In dogs, N. caninum infection can cause severe neuromuscular disease [1]. Neurological manifestations are often multifocal with predominantly cerebello-vestibular signs. The prognosis is generally poor, with complete clinical improvement in only about 6% of cases and a relapse rate of >25% [18]. Clinical presentations vary with age. In dogs under six months, manifestations primarily involve the peripheral nervous system with more generalized neuromuscular disorders including decreased spinal reflexes, tetraparesis, progressive weakness, muscle loss, and myalgia. In dogs over one-year old, the central nervous system appears affected to a higher degree, with cerebellar ataxia, head tremors, vestibular syndrome, and facial nerve deficits. Additional manifestations can include mega-esophagus, hyperthermia, and hepatomegaly [19]. In puppies, particularly those infected transplacentally, the infection can be particularly severe and often leads to death [20]. N. caninum imposes substantial economic burden most notably on the global cattle industry. Thus, vaccine development remains a primary research focus. Overall, traditional vaccine approaches have shown limited efficacy, but significant advances in reverse vaccinology, incorporating modern in silico methods and advanced genomic analyses, offer new opportunities for identifying promising vaccine candidates [7,21]. The complex nature of N. caninum transmission necessitates a One Health approach, recognizing the interconnections between livestock, wildlife, and human activities. This approach requires sustained international collaboration and coordinated surveillance, while considering ecological contexts and environmental factors [22]. To visualize the interconnected research domains and translational pathways discussed in this review, a schematic framework integrating diagnosis, epidemiology, molecular and immunological mechanisms, intervention strategies, and future directions is presented in Figure 1.
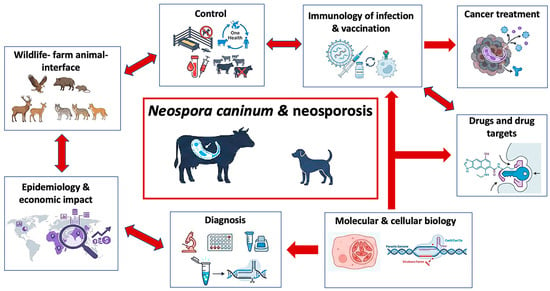
Figure 1.
Integrated research framework for Neospora caninum and neosporosis organized into eight thematic sections, partially interlinked as indicated by the red arrows. These include diagnosis (traditional and more recently developed methods), epidemiology and economic impact, the interface of wildlife and farm animals, control measures to limit infection and transmission of the disease, most notably in cattle, molecular and cell biological studies that identify and characterize virulence factors and potential vaccine and drug targets, and exploiting N. caninum as a cancer immunotherapy platform. Arrows indicate which research areas have impacted each other. Images were generated through NotebookLM.
In this review, we summarize information on key advances in the field over the last 10 years and relate them to important findings published earlier.
2. Diagnosis of N. caninum Infection
The diagnosis of N. caninum infection relies on indirect serological detection of infection and direct demonstration of the parasite via molecular tools and immuno-histopathology. Serological techniques such as enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent antibody test (IFAT) have been widely used in different animal species [6,23]. ELISA, using crude antigens or subcellular fractions, offers high throughput for large-scale screening, while IFAT on intact tachyzoites provides high specificity. However, ELISA may present cross-reactivity issues, and IFAT is labor-intensive. In addition, a plethora of different PCR-based techniques were established. A brief overview of the diagnostic laboratory methods that have been developed since the discovery of N. caninum is shown in Table 1.

Table 1.
General overview of the traditional laboratory methods used for the diagnosis of N. caninum infection.
More recently, improvements in ELISA have been achieved through the development of chimeric antigens, such as a recombinant antigen based on the two surface antigens NcSRS2 and NcSAG1 and the dense granule antigen NcGRA7 [34], achieving high sensitivity (86.7%) and specificity (96.1%) in detecting N. caninum antibodies in cattle. Udonsom et al. [35] evaluated the immunodiagnostic performances of specific recombinant proteins including NcPrx2, NcMIC4, and NcSAG1, with NcSAG1 and NcMIC4 demonstrating particular promise as reliable diagnostic markers. Fereig et al. [36] validated a NcSAG1-based immunochromatographic test (ICT) for the detection of N. caninum infection in cattle, which demonstrated high sensitivity (84.2%) and specificity (93.5%). This ICT is a rapid tool for on-site diagnosis, representing a significant advancement in field-applicable diagnostics for neosporosis control. To complement traditional diagnostic approaches and to identify active infections, quantification of pro-inflammatory cytokines such as IL-12 and IFN-γ can aid in distinguishing chronic and active infections [37], which is relevant given that N. caninum can establish persistent infections with varying levels of immune response at different stages.
Histopathological examination, particularly immunohistochemistry (IHC), remains the gold standard for definitive diagnosis. In cases of abortion, examination of both placental and fetal tissues is crucial. Advances in immunodiagnostics have improved the ability to differentiate between N. caninum and T. gondii in tissue samples. Lepore et al. [38] developed species-specific polyclonal antibodies targeting recombinant proteins (rNcSRS2 and rTgSRS2) that achieved 90.1% agreement between IHC and PCR testing. This advancement addresses a critical challenge in diagnostic specificity, particularly in abortion cases. While IHC is highly useful and informative, the quality and reliability of results depend significantly on several factors including infection stage, tissue preservation methods, and timing of sample collection. The characterization of stage-specific immunodominant antigens has further enhanced our understanding of diagnostic targets. For instance, evaluation of monoclonal and polyclonal antibodies against different parasitic stages demonstrated that antibodies directed against NcSAG1 specifically recognized N. caninum tachyzoites, while antibodies directed against the bradyzoite antigen NcSAG4 displayed broader cross-reactivity among parasite stages [39]. These findings have important implications for the development of stage-specific diagnostic tools and highlight the need to consider developmental stages when designing diagnostic tests.
N. caninum diagnosis has significantly benefited from advances in molecular techniques, providing unparalleled sensitivity and specificity. Quantitative PCR (qPCR) targeting the Nc5 gene enables detection of as few as 5 copies/µL in complex samples [40]. Building on qPCR technology, a high-resolution melting (HRM) assay developed by Gouvias et al. [41] enabled simultaneous detection of ten abortifacient pathogens including N. caninum in one sample, providing a cost-effective diagnostic solution for multiple pathogen screening. Optimized multiplexed qPCR assays allow simultaneous detection of N. caninum and T. gondii with a detection limit of less than a single tachyzoite [42]. Droplet digital PCR (dPCR) could be a further improvement to enhance diagnostic precision. In the case of T. gondii, dPCR achieved absolute quantification, with strong concordance (96.7%) with qPCR results and detection limits as low as 0.07 copies/µL [43], highlighting the potential application of dPCR also for N. caninum diagnostics. Emerging platforms, such as Loop-Mediated Isothermal Amplification (LAMP) and CRISPR-based systems, continue to push the boundaries of diagnostic innovation. LAMP demonstrated field applicability with sensitivity and specificity exceeding 90% [44,45]. Further validation of LAMP technology comes from Liu et al. [46], who developed a colorimetric detection method that enables reliable visual identification of N. caninum DNA without specialized equipment. In addition, CRISPR-based detection platforms have shown increasing promise, such as a recombinase polymerase amplification (RPA)-CRISPR/Cas12a-based detection using fluorescent readouts [47].
Numerous commercial diagnostic kits for serological and molecular detection of N. caninum infection are available on the market. However, bridging the gap between the laboratory- and field-based diagnosis by the integration of CRISPR and especially LAMP into portable kits could enormously advance sensitive and timely diagnosis. Importantly, each diagnostic technique exhibits its distinct advantages for specific applications. PCR-based molecular methods, while technically demanding, provide highly sensitive and specific detection of N. caninum [48], with advanced multiplexed qPCR systems achieving detection limits of less than one tachyzoite [42]. IFAT serves as a reference method, though its labor-intensive nature limits large-scale applications and subjective interpretation can be a problem [35]. ELISA provides a practical tool for herd-level screening despite varied kit agreement [49]. Emerging platforms such as RPA-CRISPR-based detection show high sensitivity with detection limits of one parasite/mL using fluorescent systems [47], while LAMP, not requiring extensive infrastructure, offers field applicability [45].
3. Distribution and Transmission of Neosporosis in Farm Animals
Earlier studies had shown that N. caninum infection in cattle is distributed worldwide with prevalence rates ranging from 10% to 60% (reviewed in [6]). Intensive farming systems generally showed a higher risk of infections with prevalence rates appearing to be influenced by management practices and animal husbandry.
In Europe, varying levels of N. caninum prevalence have been recorded in the last 5 years. In Switzerland, an overall low seroprevalence of 4.2% was observed in female cattle, with 16.2% of farms having at least one seropositive animal [50]. In Portugal, the animal-level seroprevalence was 17.2% and the herd-level seroprevalence was 68.6%, with notable differences between production systems—dairy farms showed a higher prevalence of 26.8%, compared to beef farms (14.4%) [51]. A large-scale study carried out on dairy cows in Northern Italy discovered significant regional variations in N. caninum prevalence. Analysis of bulk tank milk from 586 dairy herds showed an overall seroprevalence of 30.7%, with a higher prevalence in small−medium farms and older animals. Environmental factors such as temperature and altitude, as well as land use patterns, significantly influenced prevalence rates in different areas [52]. Studies on Neospora seroprevalences in bulk milk were also carried out in Canada, where a seasonal study in Alberta showed fluctuating prevalence rates throughout the year, ranging from 7.4% to 18.2% [53]. In Colombia, an overall seroprevalence of 20.6% was reported, with notably higher rates in beef herds compared to dairy herds [54]. In China, comprehensive meta-analyses were carried out. For instance, a nationwide review found an overall seroprevalence of 12.2% (20.9% in Southern China, 9.4% in Northwest China) [55]. Another meta-analysis examining the results obtained from 33,945 cattle from 51 studies across mainland China found an overall prevalence of 13.69% [56]. At the provincial level, significant variations were observed, with particularly high rates in Hebei Province where 37.34% of dairy cows tested positive [57], while lower rates were found in Hunan Province with 2.1% seroprevalence in beef cattle [58]. In Central India, a study of 576 cattle revealed a seroprevalence of 24.8%, with risk factors including age, movement of dogs on farms, drinking pond water, and history of abortion [59]. The first molecular study carried out in Bangladesh [60] identified N. caninum in aborted fetuses by nested PCR, with infection rates of 16.0% in cattle, 14.8% in sheep, and 11.8% in goats. Contrasting patterns between regions were also found on the African continent. In Egypt, two studies showed consistently high seroprevalence rates: 28.9% in Northern governorates [61] and 24.6% in the Beheira region [62]. In contrast, South Africa demonstrated much lower rates with an overall seroprevalence of 2.3%, although significant regional variations ranging from 7.5% in KwaZulu-Natal to 0.1% in Western Cape were observed [63]. In Somalia, a comprehensive survey revealed a seroprevalence of 3.6% in ruminants, with cattle showing the highest prevalence (6.2%), followed by goats and sheep (both 2.2%) [64]. An unpublished serological survey was conducted in the Biskra and Aures regions of Algeria. Of 64 serum samples obtained from female cattle, 8 were found positive (Debache et al., unpublished findings). Although serological screening can be informative, the antibody titers in persistently infected animals can fluctuate considerably. A recent study from De Oliveira et al. [65] demonstrated significant alterations in N. caninum antibody titres throughout pregnancy in naturally infected crossbred cows, with notable increases in serological titration per trimester. These findings suggest that pregnancy influences antibody levels and that serological testing from the sixth month of gestation onwards may reduce false negative results. In South America, bovine neosporosis represents a serious veterinary and economic problem. Neosporosis accounts for approximately 9% of bovine abortions in Buenos Aires Province, and epidemiological studies across Argentina revealed a high distribution of Neospora infections not only in dairy but also in beef cattle, with seroprevalence rates of 16.6–88.8% and 0–73%, respectively [66]. One study carried out in Brazil identified N. caninum as the primary cause of bovine abortion in 53.8% of cases, with PCR confirmation in 71.4% of positive samples [67]. Further epidemiological studies in Brazil’s southern region have revealed important environmental risk factors, with the presence of dogs being the most important one. In Santa Catarina State, Remor-Sebolt et al. [68] reported a 4.2% seroprevalence in canine populations, with housing conditions and outdoor exposure significantly influencing infection rates. A study of free-roaming dogs in Ecuador found a seroprevalence of 6.8%, with no significant differences between urban and rural areas, highlighting the widespread distribution of N. caninum in canine populations across diverse environments [69]. In contrast, lower seroprevalences in dogs and cats were measured in urban settings in Poland, with rates of 1.0% in dogs and 3.3% in cats [70]. This highlights the importance of environmental factors in N. caninum transmission, particularly in areas where dogs have unrestricted access to outdoor spaces and can thus influence the transmission dynamics.
N. caninum is transmitted either vertically from the dam to the fetus or horizontally through the ingestion of oocysts containing sporozoites or infected meat containing tissue cysts and bradyzoites. Vertical transmission represents the predominant route in both domestic and wild hosts and remains the most critical barrier to effective control. In one study on Iranian dairy cattle, vertical transmission resulted in 13.6% infection rates among offspring of seropositive dams, with 94.1% of aborted fetuses being N. caninum-positive [11]. Lagomarsino et al. [71] reported horizontal transmission rates of N. caninum reaching 22.7% in dairy cattle in an endemic region in Argentina. In South American deer, transmission rates of >80% were recorded [72], and in goats, rates reached up to 100% across successive generations [65]. Studies with wild ungulates in Northern Italy demonstrated an efficiency of congenital transmission reaching 87.5% [73], highlighting the significance of this pathway in maintaining parasite populations.
Horizontal transmission through ingestion of sporulated oocysts or the oral uptake of meat infected with N. caninum tissue cysts is influenced by environmental factors and host diversity. In Brazilian cattle, the access of dogs to pastures and improper disposal of fetal remains were key risk factors [74], and environmental factors such as altitude, precipitation, and temperature significantly affected N. caninum seropositivity rates [75]. Management practices focusing on preventing definitive host access to cattle feed and water sources remain critical for controlling horizontal transmission. The complexity of transmission dynamics, involving both domestic and wildlife cycles, necessitates integrated approaches to disease control. In this respect, a 2023 meta-analysis revealed a global seroprevalence of 5% in rodents, confirming their potential role as reservoir hosts [76].
The worldwide prevalence of neosporosis has a considerable economic impact. An earlier systematic review by Reichel et al. [77] estimated global losses exceeding US $1.298 billion per annum, with nearly two-thirds of the losses (US $842.9 million) incurred by the dairy industry. Ribeiro et al. [78] reported that N. caninum-infected cattle were 2.66 times more likely to experience abortion, highlighting the direct link between infection rates and economic losses. A more recent report from Turkey [79] estimated costs associated with Neospora infection of approximately 710 US$ per dairy cow. In Australia, studies have consistently estimated annual losses at AU$110 million [80,81], while in Argentina the annual economic impact amounts up to US$33 million in the dairy cattle and 12 million in the beef cattle industry [66]. Financial losses through neosporosis are particularly pronounced in regions with intensive cattle production systems, and respective assessments are not straightforward, due to the differences in surveillance capabilities across regions. Despite the importance of livestock production for food security and livelihoods in many communities, comprehensive surveillance data for abortifacient pathogens including N. caninum remain limited in many parts of Africa and Asia [82]. This gap in surveillance suggests that the true global economic impact may be substantially underestimated, highlighting the need for improved monitoring and control strategies.
4. The Farm Animal−Wildlife Animal Interface and Control
Due to its implications for livestock health, the role of wildlife as reservoirs for N. caninum has garnered increasing attention. The presence of N. caninum was reported in migratory birds, underscoring their potential as long-distance vectors contributing to the geographic spread of the parasite [73]. Other studies identified elevated transmission risks in regions where domestic animals interact closely with wildlife [83]. In addition, wild ungulates acting as intermediate hosts were suggested to contribute to the presence of N. caninum in livestock [84]. Additional wildlife reservoirs were identified by Haydett et al. [85] and Zanet et al. [73] in wild pigs and birds of prey. In a long-term study lasting 18 years, the seroprevalence of N. caninum in wild rabbits was shown to be >6%, with significant seasonal variations and peak rates during spring, most likely related to prolonged oocyst survival [86]. Huaman et al. [81] detected a 3.7% seroprevalence in wild deer across Southeastern Australia, and Baldini et al. [72] demonstrated an 81.25% vertical transmission rate of N. caninum in South American deer. Thus, control approaches for N. caninum should also take into account the contribution of the sylvatic cycle.
Spatial analyses in endemic regions of the Amazonas in Brazil have revealed significant heterogeneity in parasite distribution, with seroprevalences ranging from 2.2% to 69.2% [87]. Similarly, epidemiological studies in Argentina revealed seroprevalence rates ranging from 16.6% to 88.8% in dairy cattle and 0% to 73% in beef cattle, demonstrating dramatic regional differences. Thus, it is important to integrate genetic, environmental, and management factors into tailored intervention strategies to mitigate the diverse impacts of N. caninum [66]. Key risk factors for neosporosis were identified, including dairy farming systems, mixed land use patterns, water source management, and the presence of domestic dogs, with the latter as a continuous source of environmental contamination with oocysts [78,87]. Another factor that influences the epidemiology of neosporosis is genetic variability. A study on N. caninum isolates from aborted bovine fetuses carried out in Northern Italy showed that spatial distance between sampling sites can be linked to genetic variation [88]. Thus, localized adaptation can potentially lead to genetic divergence. In the Amazonian region of Brazil, environmental factors such as high humidity, extensive floodplains, and dense livestock−wildlife interfaces significantly impact parasite transmission [87].
Host−pathogen interactions further complicate integrated control strategies. Holstein cattle show increased susceptibility to N. caninum-induced abortion compared to beef breeds [89], while embryo transfer studies demonstrate that donor seropositivity can affect embryo quality without direct transmission [90]. In Argentina, selective breeding strategies and embryo transfer have demonstrated efficacy in reducing vertical transmission and seroprevalence in dairy herds, providing a promising avenue for mitigating reproductive losses [66]. Experimental infections of Texel sheep with N. caninum at different stages of gestation demonstrated vertical transmission of the parasite to the fetus, but absence of abortions and other clinical signs suggested that Texel sheep may potentially have resistance to N. caninum-induced abortion [91]. These findings suggest that genetic and reproductive management strategies must be considered alongside broader epidemiological interventions.
5. Molecular and Cellular Biology of N. caninum
Significant progress in molecular techniques has revolutionized our understanding of N. caninum through multiple technological breakthroughs. High-throughput sequencing analysis has already earlier revealed novel insights into the genome of N. caninum, such as the presence of an expanded repertoire of SAG1-related proteins compared to T. gondii [92]. More recent studies have employed high-throughput RNA sequencing to study microRNAs (miRNAs) involved in the regulation of gene expression in parasites and host cells. For instance, Liu et al. [93] identified 300 miRNAs in N. caninum tachyzoites, and bioinformatics analyses showed that 10 were conserved among metazoan miRNA families, while 290 were novel miRNAs. In another bioinformatics study, Das et al. [94] performed a homology search on 336 non-redundant Expressed Sequence Tags (ESTs) of the N. caninum genome to identify conserved miRNAs. A total of 1041 mature miRNAs of reference organisms were employed; one putative miRNA “nca-miR-9388-5p” of 19 nucleotides was identified, and 16 potential target genes associated with different protozoal physiological functions were identified. This work has been complemented on the host cell side by temporal transcriptomic analyses of miRNA expression in caprine endometrial epithelial cells infected with N. caninum at 24 h and 48 h post-infection, identifying numerous up- and downregulated miRNAs at both time points. Bioinformatically predicted targets are genes involved in host immune responses, metabolism, and multiple signaling pathways. One upregulated chi-miR-146a was found to promote tachyzoite proliferation [95].
A pivotal breakthrough in genetic manipulation tools came with the implementation of CRISPR technology. Arranz-Solís et al. [96] established an efficient gene disruption system using CRISPR/Cas9 plasmids earlier developed for T. gondii, successfully targeting GFP in a reporter strain and disrupting NcGRA7 through pyrimethamine-resistance cassette (mdhfr-ts) insertion. However, it was recognized that CRISPR/Cas9 mutagenesis is not free of off-target effects that can lead to integration of multiple mdhfr-ts copies into other sites of the genome. Thus, TaqMan-quantitative PCR assays were developed that allowed determination of the copy numbers of the integrated selectable markers in CRISPR/Cas9-generated N. caninum KO strains [97]. To prevent multiple insertions, another highly efficient genetic manipulation system was established by Mineo et al. [98] through disrupting the ku80 gene in the N. caninum-Liverpool reference strain. This modification led to improved homologous recombination efficiency and enabled precise gene targeting with shorter homology regions, bringing the manipulation capabilities closer to those available for T. gondii.
The work by Arranz-Solis et al. [96] was closely followed by Nishikawa et al. [99] who reported that NcGRA7 KO strains exhibit a reduced virulence and that this protein is involved in modulation of the immune response [99]. The same was shown in the pregnant neosporosis mouse model [100]. In addition, NcGRA7 was shown to interact with cellular immune factors in mice, such as mediating the aggregation of IRGa6—an interferon-inducible GTPase—at the PVM, which affects the pathogenicity of N. caninum [101]. However, cross-species translation of results such as from mice to farm animals should be done with caution. The basic features of murine and bovine immunity, including transplacental passage of immunoglobulins, Th1- and Th2-immunity, the composition of the T cell repertoire, and the functionality of interferon-induced GTPases, show clear differences [7].
Other GRA proteins were characterized by generating CRISPR KO strains such as NcGRA17 [102], NcGRA6 [103], and NcGRA2 [104], demonstrating that these proteins were important for parasite virulence. Biotinylation revealed interaction partners with NcGRA17, named NcGRA23 and NcGRA11, but the corresponding CRISPR KO strains did not show any impairment. Three novel GRA proteins, NcGRA27, NcGRA61, and NcGRA85, were identified by the proximity-dependent biotin identification (BioID) technique [105]. Deletion of the NcGRA27 gene reduced the in vitro replication and the pathogenicity of N. caninum tachyzoites in mice, while deletion of NcGRA61 and NcGRA85 had no impact on virulence [105]. CRISPR/Cas9 was also employed to clarify the roles of two rhoptry proteins as virulence factors, namely NcROP40 and NcROP2 in N. caninum development and host−parasite interactions. NcROP40 KO strains exhibited a modestly reduced virulence compared to wild-type parasites when assessed in the pregnant mouse model [100], while NcROP2 KO tachyzoites exhibited more impaired virulence in the pregnant neosporosis mouse model, were less susceptible to IFN-γ-mediated inhibition and were more readily converted to the semi-dormant bradyzoite stage compared to the wild type [106]. The transcription factor NcAP2XII-4, whose expression was previously shown to be altered upon deletion of the gene coding for NcROP5, was also functionally characterized by employing CRISPR/Cas9 [107]. CRISPR/Cas9-mediated deletion of NcAP2XII-4 resulted in parasites that were not able to undergo egress from the parasitophorous vacuole and could not form plaques, and subsequent investigations confirmed that NcAP2XX-4 regulated ROP5 transcription by binding to its promoter. Other proteins whose roles as virulence factors were confirmed are the major surface antigen NcSAG1 as a major determinant mediating pathology in pregnant and non-pregnant mice [108], the virulence factors NcPuf [109], and the c-myc-proto-oncogene regulatory protein NcMyr1 [110].
Besides these molecular advances, classical and modern microscopy approaches were applied to characterize endodyogeny in N. caninum tachyzoites [111]. Expansion microscopy and regular confocal microscopy were used to study the dynamics of the cell assembly and nuclear division, describing centrosome, centriole, and apicoplast dynamics, during the cell cycle. In addition, the implementation of three-dimensional culture systems has opened new avenues for studying host−pathogen interactions, especially in apicomplexan parasites related to N. caninum such as Plasmodium, Cryptosporidium, Eimeria, and Toxoplasma [112,113]. Such cultures, including organoids and microfluidic devices, provide more physiologically relevant contexts for studying parasite life cycles and the implementation of advanced molecular techniques.
6. Immunological Control of Infection and Vaccination
The host immune response against N. caninum infection has a profound influence on the outcome of pregnancy and fetal survival. In general, as for many other intracellular pathogens, a Th1-biased immune response characterized by pro-inflammatory cytokine expression mediates protection against N. caninum infection. However, it has been shown in murine models that pregnancy outcome can be impaired due to excessive inflammatory responses [12,114], and the same could be true for bovine hosts [115]. A further factor complicating the situation is the fact that different N. caninum isolates were shown to differ considerably in virulence. Differences in terms of innate and adaptive immune responses were observed in a study on placentomes of cattle experimentally infected with a highly virulent (Nc-Spain7) isolate and a low-virulence (Nc-Spain 1H) isolate [116]. Infection with the low-virulence isolate resulted in the upregulated expression of pathogen recognition receptors, chemokines, and pro-inflammatory cytokines that mediate control, as well as other mechanisms implicated in the maintenance of extracellular matrix integrity, resulting in ensuring fetal survival. In contrast, these responses were impaired in placentomes of cattle infected with the more virulent Nc-Spain7 isolate during the first 10 days post-infection (10 dpi). Subsequently (20 dpi), a predominantly pro-inflammatory Th1-based response and increased leucocyte infiltration were observed, and fetal death was associated with higher expression levels of IL-8, TNF-α, iNOS, and SERP-1 genes, and lower expression of the metalloproteases and their inhibitors, compared to placentomes from animals carrying viable fetuses [116]. Understanding these immunological mechanisms is essential for developing effective control strategies based on vaccines, not only in cattle but also in sheep and goats [117].
Early efforts in vaccine development, carried out in murine models and to a lesser extent also in small and large ruminant models, included vaccines composed of N. caninum tachyzoite antigen extracts and/or subcellular fractions and those composed of recombinant antigens as monovalent and chimeric formulations, as well as polyvalent recombinant vaccine candidates expressed in different expression systems and formulated with different adjuvants (reviewed in [7]). Table 2 summarizes a range of studies which were done in the actual target hosts, namely one study in sheep [118] and others in cattle, employing either parasite extracts/subunit vaccines or live vaccines (attenuated N. caninum isolates). With one exception [118], attenuated live vaccines were those that provided meaningful protection against abortion and/or vertical transmission, while subunit vaccines showed lower efficacy [7].

Table 2.
Selected studies on subunit and live vaccines for prevention of N. caninum infection in cattle models. One study was carried out in ewes [118]. Vaccines contained either recombinant antigens or killed tachyzoite extracts, emulsified in different adjuvants [118,119,120,121,122,123,124,125], or were composed of attenuated live vaccines [126,127,128,129,130,131,132].
Most vaccine candidates studied to date are immunogenic surface, microneme, and rhoptry of dense granule proteins functionally involved in host cell adhesion/invasion. Parasite proteins that elicit inflammatory responses and are responsible for the immunopathology that leads to abortion have also been considered as vaccine candidates. Of note, a dual-antigen vaccine combining recombinant N. caninum cyclophilin (NcCyP) and profilin (NcPro), both important mediators of inflammatory responses, has shown significant protection in sheep, with 69.2% of vaccinated ewes giving birth to viable lambs compared to complete abortion in control animals [118]. More recently, Mendoza-Morales et al. [133] introduced a recombinant subunit vaccine with a dual Differentiation of Infected from Vaccinated Animals (DIVA)-like capability, which comprises recombinant NcSAG1 and the carrier/adjuvant heat shock protein 81.2 from Arabidopsis thaliana (rAtHsp81.2, a known B-cell mitogen). The vaccine formulation has been demonstrated earlier to be safe and efficacious in the murine neosporosis model and allowed differentiation between vaccinated and infected animals [134]. In cattle, this formulation stimulated a broad and potent humoral and cellular immune response, characterized by an IgG1/IgG2 isotype profile and IFN-γ secretion, and also allowed differentiation between vaccinated and infected heifers by two different DIVA compliant test approaches [133]. Studies on the efficacy of this vaccine approach are still pending, but using this strategy will allow more precise disease monitoring, making it a critical step forward in controlling N. caninum in cattle.
Although in earlier studies the most promising vaccine results were obtained with live-attenuated parasites in both murine models [135,136,137,138] and cattle [127,128,129], safety concerns, production costs, and the anticipated non-stability of live vaccines blocked their further development into commercial products [7]. As an alternative, an approach that utilizes the attenuated Listeria monocytogenes vaccine vector Lm3Dx devoid of three important virulence genes but expresses one or more N. caninum antigens has been introduced. The safety and outstanding efficacy of this Lm3Dx-based Neospora vaccine vector has been demonstrated in the pregnant neosporosis mouse model [139,140,141].
Additionally, immunoinformatic approaches have been followed for the virtual to design and evaluation of vaccine candidates, predicting a number of important features such as antigenicity, solubility, potential allergenic domains, posttranslational modifications, transmembrane domains and signal peptides, secondary and tertiary structures, and linear and conformational B-cell epitopes, and potential MHC class I and II presentation and T cell cytotoxicity. Using this approach, Shams et al. [142] predicted a multiepitope vaccine candidate incorporating epitopes from six key proteins (SRS2, MIC3, MIC6, GRA1, IMP-1, and profilin). In addition, bioinformatic evaluation of the NcSRS2 protein identified immunogenic epitopes capable of inducing dual immune responses, with high antigenicity and no allergenicity, positioning it as a strong candidate for next-generation vaccines [143].
Progress has also been made in the development of delivery methods and formulations. Yao et al. [144] highlighted the importance of adjuvant combinations for preventing high-incidence diseases in bovines, such as the addition of TLR2 and TLR9 as adjuvants for recombinant NcPro to achieve higher levels of IFN-γ and to ensure a prolonged recall B-cell response in cattle compared to other adjuvants [124]. Others have used di-palmitoyl phosphatidyl glycerol-loaded nanoparticles (DGNP) loaded with N. caninum extract and N. caninum glycosylphosphatidylinositol (GPI) and found an adjuvant effect in murine bone marrow-derived dendritic cells with higher levels of interleukin (IL)-1β, IL-6, IL-12p40, and IL-10, and decreased expression of major histocompatibility complex (MHC) molecules. GPI also modulated the responses of bovine peripheral blood mononuclear cells (PBMCs) by increasing the production of IFN-γ and by decreasing the expression of MHC molecules [145], suggesting that the GPI adjuvant effect should be exploited for vaccination. In addition, recent developments of more advanced DNA vaccine platforms [146], self-assembling protein nanoparticles (SAPNs), and virus-like particles (VLPs) with demonstrated abilities to enhance antigen uptake, B-cell activation, and lymph node trafficking [147] await further exploitation in the Neospora vaccine field.
Subunit vaccine candidates could also be incorporated into mRNA vaccines. As reviewed in [7], mRNA can overcome mis-folding during in vitro expression of recombinant proteins, and mRNAs, as opposed to DNA vaccines, are considered to have very little safety issues as they retain a cytoplasmic localization, thus minimizing the risk of gene recombination and conversion into malignant cells. Messenger RNA vaccines are designed relatively easily, thus incurring little costs of production and time requirements. The major caveat is their short intracellular half-life and the rapid degradation during storage [7]. However, variability in host immune responses remains a significant barrier, necessitating further standardization and optimization.
7. Recent Advances in Therapeutic Strategies
Traditional therapeutic approaches against N. caninum have relied on folate biosynthesis inhibitors, primarily sulfadiazine and pyrimethamine, or alternatively a combination of trimethoprim, another folic acid antagonist, and sulfamethoxazole. These compounds, while useful for managing clinical neosporosis in dogs, show limited efficacy in cattle, particularly due to their potential adverse effects in pregnancy and their minimal impact on tissue cysts [148,149]. Other repurposed drugs have been investigated but in many cases have not been applied clinically in cattle such as the protein synthesis inhibitor clindamycin, the ionophore antibiotic monensin, the triazinetrione derivative toltrazuril, the cytochrome bc1 inhibitors decoquinate, buparvaquone and endochin-like quinolones (ELQs), a number of anti-malarials such as artemisinin and its derivatives and mefloquine, and the alkyl phospholipid miltefosine (for review see [7,150]). Systematic screening efforts of open-source libraries such the Medicines for Malaria Venture (MMV) Malaria Box and the MMV Pathogen Box employing transgenic parasites that express bacterial beta-galactosidase have significantly expanded the arsenal or repurposed drugs [151,152]. More recently, CRISPR/Cas9 was used to generate transgenic tachyzoites that allow high-throughput in vitro drug screening [153], leading to the discovery of TAK-632, a selective pan-rapidly accelerated fibrosarcoma (pan-RAF) kinase inhibitor employed in cancer. In experimentally infected mice, TAK-632 attenuated the virulence of N. caninum and significantly reduced the parasite burden in the brain.
Additionally, Harada et al. [154] developed a novel high-standard chemiluminescent assay targeting nucleoside triphosphate hydrolase (NTPase), leading to the identification of 19 synthetic compounds and six marine bacterial extracts with inhibitory activity, providing potential candidates for anti-parasitic drug development. This screening platform was further refined by Kurata et al. [155], who implemented robotic automation to enhance throughput and precision, establishing a more efficient drug discovery pipeline for both N. caninum and T. gondii. A comprehensive evaluation of antimalarial compounds against N. caninum revealed distinct efficacy profiles [156]. Atovaquone emerged as the most potent compound (IC50 = 8 nM), comparable to its activity against T. gondii and Plasmodium. Tetracycline showed significant efficacy with an IC50 of 19.6 μM, while primaquine and quinine demonstrated very low activity (IC50 = 44.4 μM and 56.6 μM, respectively). Notably, chloroquine required concentrations above 100 μM for inhibitory effects. The study also revealed important differences in drug susceptibility patterns among apicomplexan parasites, suggesting species-specific therapeutic targets [156].
Another example of a repurposed drug is niclosamide, a compound that was developed for clinical use in tapeworm infections and blocks glucose uptake. Niclosamide was shown to act against N. caninum through a novel mechanism involving the NLRP3 inflammasome activation in mice. Niclosamide enhanced macrophage-mediated parasite clearance but also exhibited direct antiparasitic activity, significantly reducing the parasite burden in tissues and improving survival rates in murine models. Notably, niclosamide treatment affected the mitochondrial membrane potential and ATP production in N. caninum tachyzoites, suggesting multiple mechanisms of action [46].
The efficacy of natural products such as a cyclic natural compound isolated from Tetragonisca angustula honey was demonstrated, exerting a 40–56% reduction in tachyzoite numbers and a dose-dependent inhibition of parasite proliferation up to 50%, all without toxicity to host cells [157]. Another natural compound, Inonotus obliquus polysaccharide (IOP) was identified by Tang et al. [158]. IOP treatment at 2 mg/10 g provided protection against N. caninum infection in mice, reducing parasite burden in multiple organs and modulating immune responses through regulation of immunoglobulin levels (IgG1, IgG2a) and cytokine production (IL-12, TNF-α). Additionally, IOP treatment showed no toxicity in vitro or in vivo, while effectively balancing hormonal responses, suggesting its potential as a safe therapeutic option [158]. Cordycepin, a nucleoside antibiotic derived from Chinese medicine Cordyceps militaries, was shown to exhibit profound activity against N. caninum in vitro and in vivo. Upon experimental infection, mice treated with cordycepin showed reduced clinical symptoms, increased food intake and significantly increased body weight [159].
More targeted therapies have been achieved with Bumped Kinase Inhibitors (BKIs). These compounds target two apicomplexan kinases, namely calcium-dependent protein kinase 1 (CDPK1) that is involved in signaling events that regulate host cell invasion and egress [160], as well as a specialized mitogen-activated protein kinase (MAPKL1) that localizes to the centrosome where it prevents overduplication and is crucial for proper centrosome function during asexual replication and cell division [161]. The latest generation BKIs, namely BKI-1748 and BKI-1708, efficiently impaired N. caninum tachyzoite proliferation (IC50 < 500 nM) and effectively reduced vertical transmission in experimentally infected mice [162,163]. Interestingly, BKIs do not kill the parasites in vitro but induce the formation of multinucleated complexes consisting of newly formed zoites that are blocked in the final stages of cell division and remain stuck within the host cell cytoplasm. This affects the humoral and cellular immune response as shown in experimentally infected mice treated with another BKI-family member, BKI-1294 [138], as well as with BKI-1748 and BKI-1708 [162,163]. By applying affinity chromatography, BKI-1748 was shown to bind not only to NcCDPK1 but also to N. caninum proteins and enzymes that are crucially involved in RNA- and DNA-binding and -modification processes, most notably splicing factors, suggesting that other targets besides the activity against these two kinases are involved [164].
The field has progressed through combination approaches and drug delivery innovations. Anghel et al. [165] studied the efficacy of ELQs that were originally developed for the treatment of toxoplasmosis, with one derivative, ELQ-334, achieving a 50% reduction of vertical transmission in pregnant mouse models. A treatment with a combined ELQ-334 plus BKI-1748 treatment achieved a synergistic effect in vitro and resulted in complete inhibition of vertical transmission in the mouse model [166]. Efforts on drug delivery options are underway to improve bioavailability, pharmacokinetic properties, and targeted action of compounds. This could be achieved by formulating compounds in solid dispersions, microparticles, polymeric micelles, nanosuspensions, lipid-based nanocarriers or liposomes, and other suitable agents.
Modulating the host immune response to combat N. caninum infection offers another promising therapeutic pathway. High and low virulence isolates of N. caninum induce virulence-dependent pro-inflammatory responses in bovine macrophages via the NF-κB signaling pathway [167]. These differences provide a foundation for therapeutic interventions aimed at enhancing protective immunity while reducing parasite evasion mechanisms. Targeting host signaling pathways offers further opportunities. Bhandage et al. [168] revealed that N. caninum activates GABAergic signaling in mononuclear phagocytes, enhancing their motility and facilitating parasite dissemination. Therapeutic strategies that disrupt GABA receptor or calcium signaling could limit parasite spread. Similarly, Mota et al. [169] identified the p38 MAPK pathway as a critical mechanism of immune evasion, with its inhibition leading to increased cytokine production and improved host survival. Additional research has focused on host-based strategies.
8. N. caninum as a Therapeutic Agent for Cancer Treatments
Most interestingly, within the last few years, N. caninum has switched its role from being a therapeutic problem to becoming a therapeutic agent, most notably in cancer treatment. Lantier et al. [170] exploited the following facts: (i) tumor growth induces an immune-modulated environment; and (ii) infection with an intracellular pathogen can potentially twist this modulated, or partially downregulated, immunity into eliciting an inflammatory response that could also affect tumor cells. Injection of N. caninum tachyzoites into mice inoculated with thymoma EG7 or human Merkel cell carcinoma led to severe impairment or even eradication of the tumor mass. This effect was based not only on direct lysis of tumor cells, but also on the reactivation of immunosuppressed cells and the development of a protective anti-tumor response dependent on natural killer (NK) cells, CD8-T cells, and interferon (IFN)-γ secretion into the tumor microenvironment. A transgenic N. caninum strain expressing and secreting human interleukin (IL)-15 induced both proliferation of human peripheral blood mononuclear cells (PBMCs) and IFN-γ secretion, and was used to successfully treat murine lung metastases by intranasal administration, leading to increased numbers of NK and cytotoxic T cells and macrophages, with the latter showing a polarization towards the antitumoral M1 phenotype [171]. Another transgenic N. caninum strain expressed and secreted a single-chain variable fragment fused to an Fc domain (scFv-Fc) binding to human programmed cell death ligand 1 (PD-L1). The scFv-Fc bound to PD-L1 on mouse and human tumor cells, blocked the programmed cell death protein 1 (PD-1)/PD-L1 pathway and induced T cell activity, antibody-dependent cellular phagocytosis, and cellular cytotoxicity [172]. Thus, N. caninum behaves as a live biotherapeutic capable of modulating both tumor growth and host immunity.
These findings place N. caninum within a broader emerging field where parasites are actively explored as immunostimulatory tools and sources for the development of anti-neoplastic strategies [173,174]. In parallel, engineered bacteria and other living microorganisms are being developed as programmable cancer immunotherapies and live biotherapeutic products. They incorporate genetic circuits that control tumor colonization, payload delivery, and safety profiles [175,176,177]. In this conceptual framework, N. caninum can be viewed as a potential Trojan horse-like programmable immuno-oncology platform. The parasite chassis can enter and perturb tumors while carrying defined therapeutic cargos and attenuation modules. Such a view bridges classical neosporosis control with the wider field of engineered live biotherapeutics.
9. Conclusions and Perspectives
Integrated control strategies remain paramount in mitigating the impact of N. caninum across diverse ecosystems. As shown in Argentina [66,71], selective breeding programs and embryo transfer have resulted in reduced vertical transmission and seroprevalence in dairy herds, providing a promising avenue for limiting reproductive losses in livestock. However, these programs require sustained commitment and consistent monitoring to ensure long-term success. Control strategies must also address the role of definitive hosts in environmental transmission, as well as the interface between farm and wildlife animals. The integration of “One Health” principles, linking human, animal, and environmental health, is crucial for the sustained success of control programs, particularly in regions with high human–livestock interactions.
The development of novel vaccines to be applied in cattle as the economically most important target host is important. These include live-attenuated and subunit vaccines, vaccines based on secretory components or exosomes, carbohydrate-based vaccines, and DNA or—based on the proven success against viral infections—mRNA vaccines [7,178]. Relevant vaccine targets in apicomplexan parasites are now being discovered by employing high-throughput approaches based on genomics, transcriptomics, and proteomics, together with techniques to genetically manipulate N. caninum. This will further improve our understanding of the biology of N. caninum and its interaction with the host, and will lead to the identification of determinants of virulence and triggers of immunity, thus contributing to the identification of novel targets for intervention [179]. Bridging the gap between immunological variability and vaccine development is essential for advancing effective intervention. In any case, a successful vaccine must elicit not only B cell responses, but also T-helper cell and cytotoxic T cell responses [65].
The chronic bradyzoite stage has a key role in transmission of N. caninum and should be more thoroughly investigated. The persistence of asymptomatic carriers represents a major challenge for control, and chronically infected animals maintain parasite populations within herds without exhibiting clinical signs, complicating detection and management [72]. In addition, bradyzoites pose significant barriers to treatment and immunological intervention, being largely resistant to chemotherapeutic agents and immune responses. Investigations on transcription factors that govern stage conversion in N. caninum could enhance our understanding of its chronic persistence, metabolic adaptation, and asymptomatic nature, which still remain critical knowledge gaps.
Overall, to overcome persistent obstacles in neosporosis control, a multidisciplinary approach is essential. Integrating genomic, proteomic, and transcriptomic data will provide a comprehensive understanding of host−parasite interactions, while international repositories of well-characterized N. caninum strains will enhance research consistency. The combined efforts of molecular research, therapeutic innovation, and collaborative surveillance frameworks will be pivotal in bridging the gap between laboratory discoveries and practical solutions.
Author Contributions
Conceptualization, K.D. and A.H.; investigation, K.D. and A.H.; resources, K.D. and A.H.; writing—original draft preparation, K.D.; writing—review and editing, K.D. and A.H.; visualization, A.H.; funding acquisition, A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swiss National Science Foundation (grant number: 310030_214897). The APC was funded by the Swiss National Science Foundation (grant number: 310030_214897).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable in this article.
Acknowledgments
The authors thank the Institute of Parasitology, Vetsuisse Faculty, University of Bern, for access to facilities and infrastructure that supported the underlying work in previous years and contributed to this review.
Conflicts of Interest
Author, Karim Debache, was employed by the company, Debache Switzerland. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Dubey, J.P.; Hattel, A.L.; Lindsay, D.S.; Topper, M.J. Neonatal Neospora caninum Infection in Dogs: Isolation of the Causative Agent and Experimental Transmission. J. Am. Vet. Med. Assoc. 1988, 193, 1259–1263. [Google Scholar] [CrossRef]
- Gondim, L.F.P.; McAllister, M.M.; Pitt, W.C.; Zemlicka, D.E. Coyotes (Canis latrans) Are Definitive Hosts of Neospora caninum. Int. J. Parasitol. 2004, 34, 159–161. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.M.; Dubey, J.P.; Lindsay, D.S.; Jolley, W.R.; Wills, R.A.; McGuire, A.M. Dogs Are Definitive Hosts of Neospora caninum. Int. J. Parasitol. 1998, 28, 1473–1478. [Google Scholar] [CrossRef]
- Hemphill, A. The Host-Parasite Relationship in Neosporosis. Adv. Parasitol. 1999, 43, 47–104. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Vonlaufen, N.; Naguleswaran, A. Cellular and Immunological Basis of the Host-Parasite Relationship during Infection with Neospora caninum. Parasitology 2006, 133, 261–278. [Google Scholar] [CrossRef]
- Dubey, J.P.; Hemphill, A.; Calero-Bernal, R.; Schares, G. Neosporosis in Animals, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Oxfordshire, UK, 2017. [Google Scholar]
- Imhof, D.; Hänggeli, K.P.A.; De Sousa, M.C.F.; Vigneswaran, A.; Hofmann, L.; Amdouni, Y.; Boubaker, G.; Müller, J.; Hemphill, A. Working towards the Development of Vaccines and Chemotherapeutics against Neosporosis-with All of Its Ups and Downs-Looking Ahead. Adv. Parasitol. 2024, 124, 91–154. [Google Scholar] [CrossRef]
- Pastor-Fernández, I.; Collantes-Fernández, E.; Jiménez-Pelayo, L.; Ortega-Mora, L.M.; Horcajo, P. Modeling the Ruminant Placenta-Pathogen Interactions in Apicomplexan Parasites: Current and Future Perspectives. Front. Vet. Sci. 2021, 7, 634458. [Google Scholar] [CrossRef]
- Collantes-Fernandez, E.; Arrighi, R.B.G.; Alvarez-García, G.; Weidner, J.M.; Regidor-Cerrillo, J.; Boothroyd, J.C.; Ortega-Mora, L.M.; Barragan, A. Infected Dendritic Cells Facilitate Systemic Dissemination and Transplacental Passage of the Obligate Intracellular Parasite Neospora caninum in Mice. PLoS ONE 2012, 7, e32123. [Google Scholar] [CrossRef]
- Peters, M.; Lütkefels, E.; Heckeroth, A.R.; Schares, G. Immunohistochemical and Ultrastructural Evidence for Neospora caninum Tissue Cysts in Skeletal Muscles of Naturally Infected Dogs and Cattle. Int. J. Parasitol. 2001, 31, 1144–1148. [Google Scholar] [CrossRef]
- Gharekhani, J.; Yakhchali, M. Vertical Transmission of Neospora Caninum in Iranian Dairy Cattle. Ann. Parasitol. 2020, 66, 495–500. [Google Scholar] [CrossRef]
- Aguado-Martínez, A.; Basto, A.P.; Leitão, A.; Hemphill, A. Neospora caninum in Non-Pregnant and Pregnant Mouse Models: Cross-Talk between Infection and Immunity. Int. J. Parasitol. 2017, 47, 723–735. [Google Scholar] [CrossRef]
- Weiss, L.M.; Ma, Y.F.; Halonen, S.; McAllister, M.M.; Zhang, Y.W. The In Vitro Development of Neospora caninum Bradyzoites. Int. J. Parasitol. 1999, 29, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, N.; Müller, N.; Keller, N.; Naguleswaran, A.; Bohne, W.; McAllister, M.M.; Björkman, C.; Müller, E.; Caldelari, R.; Hemphill, A. Exogenous Nitric Oxide Triggers Neospora caninum Tachyzoite-to-Bradyzoite Stage Conversion in Murine Epidermal Keratinocyte Cell Cultures. Int. J. Parasitol. 2002, 32, 1253–1265. [Google Scholar] [CrossRef]
- Vonlaufen, N.; Guetg, N.; Naguleswaran, A.; Müller, N.; Björkman, C.; Schares, G.; von Blumroeder, D.; Ellis, J.; Hemphill, A. In Vitro Induction of Neospora caninum Bradyzoites in Vero Cells Reveals Differential Antigen Expression, Localization, and Host-Cell Recognition of Tachyzoites and Bradyzoites. Infect. Immun. 2004, 72, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Sokol-Borrelli, S.L.; Coombs, R.S.; Boyle, J.P. A Comparison of Stage Conversion in the Coccidian Apicomplexans Toxoplasma gondii, Hammondia hammondi, and Neospora caninum. Front. Cell. Infect. Microbiol. 2020, 10, 608283. [Google Scholar] [CrossRef]
- Horcajo, P.; Regidor-Cerrillo, J.; Aguado-Martínez, A.; Hemphill, A.; Ortega-Mora, L.M. Vaccines for Bovine Neosporosis: Current Status and Key Aspects for Development. Parasite Immunol. 2016, 38, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Seferidis, N.; Zilli, J.; Roberts, T.; Harcourt-Brown, T. Insights into the Clinical Presentation, Diagnostics and Outcome in Dogs Presenting with Neurological Signs Secondary to Infection with Neospora caninum: 41 Cases (2014–2023). J. Small Anim. Pract. 2024, 65, 582–588. [Google Scholar] [CrossRef]
- Alf, V.; Tirrito, F.; Fischer, A.; Cappello, R.; Kiviranta, A.-M.; Steinberg, T.A.; Poli, F.; Stotz, F.; Del Vecchio, O.V.; Dörfelt, S.; et al. A Multimodal Approach to Diagnosis of Neuromuscular Neosporosis in Dogs. J. Vet. Intern. Med. 2024, 38, 2561–2570. [Google Scholar] [CrossRef]
- Morganti, G.; Rigamonti, G.; Brustenga, L.; Calgaro, V.; Angeli, G.; Moretta, I.; Diaferia, M.; Veronesi, F. Exploring Similarities and Differences between Toxoplasma gondii and Neospora caninum Infections in Dogs. Vet. Res. Commun. 2024, 48, 3563–3577. [Google Scholar] [CrossRef]
- Goodswen, S.J.; Kennedy, P.J.; Ellis, J.T. A Guide to Current Methodology and Usage of Reverse Vaccinology towards in Silico Vaccine Discovery. FEMS Microbiol. Rev. 2023, 47, fuad004. [Google Scholar] [CrossRef]
- Minicucci, L.A.; Carstensen, M.; Cornicelli, L.; Elmore, S.A.; Dubey, J.P.; Wolf, P.; Hildebrand, E.; Tunseth, D. Risk Perception and Transmission Potential of Neospora caninum at the Wildlife and Livestock Interface in Minnesota. Front. Vet. Sci. 2025, 12, 1552390. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S. A Review of Neospora caninum and Neosporosis. Vet. Parasitol. 1996, 67, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Baszler, T.V.; Adams, S.; Vander-Schalie, J.; Mathison, B.A.; Kostovic, M. Validation of a Commercially Available Monoclonal Antibody-Based Competitive-Inhibition Enzyme-Linked Immunosorbent Assay for Detection of Serum Antibodies to Neospora caninum in Cattle. J. Clin. Microbiol. 2001, 39, 3851–3857. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.P.; Alvarez-García, G.; Chiapparrone, M.L.; Regidor-Cerrillo, J.; Lischinsky, L.H.; de Yaniz, M.G.; Odeón, A.C.; Ortega-Mora, L.M.; Campero, C.M. Neospora caninum Tachyzoites Inoculated by the Conjunctival Route Are Not Vertically Transmitted in Pregnant Cattle: A Descriptive Study. Vet. Parasitol. 2014, 199, 1–7. [Google Scholar] [CrossRef]
- van Maanen, C.; Wouda, W.; Schares, G.; von Blumröder, D.; Conraths, F.J.; Norton, R.; Williams, D.J.L.; Esteban-Redondo, I.; Innes, E.A.; Mattsson, J.G.; et al. An Interlaboratory Comparison of Immunohistochemistry and PCR Methods for Detection of Neospora caninum in Bovine Foetal Tissues. Vet. Parasitol. 2004, 126, 351–364. [Google Scholar] [CrossRef]
- Baszler, T.V.; Gay, L.J.; Long, M.T.; Mathison, B.A. Detection by PCR of Neospora caninum in Fetal Tissues from Spontaneous Bovine Abortions. J. Clin. Microbiol. 1999, 37, 4059–4064. [Google Scholar] [CrossRef] [PubMed]
- Collantes-Fernández, E.; Zaballos, A.; Alvarez-García, G.; Ortega-Mora, L.M. Quantitative Detection of Neospora caninum in Bovine Aborted Fetuses and Experimentally Infected Mice by Real-Time PCR. J. Clin. Microbiol. 2002, 40, 1194–1198. [Google Scholar] [CrossRef]
- Wapenaar, W.; Barkema, H.W.; Vanleeuwen, J.A.; McClure, J.T.; O’Handley, R.M.; Kwok, O.C.H.; Thulliez, P.; Dubey, J.P.; Jenkins, M.C. Comparison of Serological Methods for the Diagnosis of Neospora caninum Infection in Cattle. Vet. Parasitol. 2007, 143, 166–173. [Google Scholar] [CrossRef]
- Dong, J.; Otsuki, T.; Kato, T.; Park, E.Y. Development of a Diagnostic Method for Neosporosis in Cattle Using Recombinant Neospora caninum Proteins. BMC Biotechnol. 2012, 12, 19. [Google Scholar] [CrossRef]
- Ghalmi, F.; China, B.; Jenkins, M.; Azzag, N.; Losson, B. Comparison of Different Serological Methods to Detect Antibodies Specific to Neospora caninum in Bovine and Canine Sera. J. Vet. Diagn. Investig. 2014, 26, 136–140. [Google Scholar] [CrossRef]
- Regidor-Cerrillo, J.; Gómez-Bautista, M.; Pereira-Bueno, J.; Aduriz, G.; Navarro-Lozano, V.; Risco-Castillo, V.; Férnandez-García, A.; Pedraza-Díaz, S.; Ortega-Mora, L.M. Isolation and Genetic Characterization of Neospora caninum from Asymptomatic Calves in Spain. Parasitology 2008, 135, 1651–1659. [Google Scholar] [CrossRef]
- Canada, N.; Meireles, C.S.; Mezo, M.; González-Warleta, M.; Correia da Costa, J.M.; Sreekumar, C.; Hill, D.E.; Miska, K.B.; Dubey, J.P. First Isolation of Neospora caninum from an Aborted Bovine Fetus in Spain. J. Parasitol. 2004, 90, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Yang, C.-Y.; Ayanniyi, O.-O.; Chen, Y.-Q.; Lu, Z.-X.; Zhang, J.-Y.; Liu, L.-Y.; Hong, Y.-H.; Cheng, R.-R.; Zhang, X.; et al. Development and Application of an Indirect ELISA to Detect Antibodies to Neospora caninum in Cattle Based on a Chimeric Protein rSRS2-SAG1-GRA7. Front. Vet. Sci. 2022, 9, 1028677. [Google Scholar] [CrossRef]
- Udonsom, R.; Adisakwattana, P.; Popruk, S.; Reamtong, O.; Jirapattharasate, C.; Thiangtrongjit, T.; Rerkyusuke, S.; Chanlun, A.; Hasan, T.; Kotepui, M.; et al. Evaluation of Immunodiagnostic Performances of Neospora caninum Peroxiredoxin 2 (NcPrx2), Microneme 4 (NcMIC4), and Surface Antigen 1 (NcSAG1) Recombinant Proteins for Bovine Neosporosis. Animals 2024, 14, 531. [Google Scholar] [CrossRef]
- Fereig, R.M.; Abdelbaky, H.H.; Nishikawa, Y. Comparative Evaluation of Four Potent Neospora caninum Diagnostic Antigens Using Immunochromatographic Assay for Detection of Specific Antibody in Cattle. Microorganisms 2021, 9, 2133. [Google Scholar] [CrossRef] [PubMed]
- Fereig, R.M.; Nishikawa, Y. From Signaling Pathways to Distinct Immune Responses: Key Factors for Establishing or Combating Neospora caninum Infection in Different Susceptible Hosts. Pathogens 2020, 9, 384. [Google Scholar] [CrossRef]
- Lepore, T.; Macrae, A.I.; Cantón, G.J.; Cantile, C.; Martineau, H.M.; Palarea-Albaladejo, J.; Cahalan, S.; Underwood, C.; Katzer, F.; Chianini, F. Evaluation of Species-Specific Polyclonal Antibodies to Detect and Differentiate between Neospora caninum and Toxoplasma gondii. J. Vet. Diagn. Investig. 2024, 36, 418–427. [Google Scholar] [CrossRef]
- Dellarupe, A.; Moré, G.; Unzaga, J.M.; Pardini, L.; Venturini, M.C. Study of Specific Immunodominant Antigens in Different Stages of Neospora caninum, Toxoplasma gondii, Sarcocystis spp. and Hammondia spp. Exp. Parasitol. 2024, 262, 108772. [Google Scholar] [CrossRef] [PubMed]
- Bandelj, P.; Kušar, D.; Šimenc, L.; Jamnikar-Ciglenečki, U.; Vengušt, G.; Vengušt, D.Ž. First Molecular Detection of Neospora caninum in Feces of Grey Wolf (Canis lupus) and Golden Jackal (Canis aureus) Populations in Slovenia. Animals 2023, 13, 3089. [Google Scholar] [CrossRef]
- Gouvias, I.; Lysitsas, M.; Batsidis, A.; Malefaki, S.; Bitchava, D.; Tsara, A.; Nickovic, E.; Bouzalas, I.; Malissiova, E.; Guatteo, R.; et al. Molecular Investigation of Small Ruminant Abortions Using a 10-Plex HRM-qPCR Technique: A Novel Approach in Routine Diagnostics. Microorganisms 2024, 12, 1675. [Google Scholar] [CrossRef]
- Truong, M.; Šlapeta, J. Analytical Sensitivity of a Multiplex Quantitative PCR for Toxoplasma gondii and Neospora caninum. Parasitol. Res. 2023, 122, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Nabet, C.; Brossas, J.-Y.; Poignon, C.; Bouzidi, A.; Paris, L.; Touafek, F.; Varlet-Marie, E.; Sterkers, Y.; Passebosc-Faure, K.; Dardé, M.-L.; et al. Assessment of Droplet Digital PCR for the Detection and Absolute Quantification of Toxoplasma gondii: A Comparative Retrospective Study. J. Mol. Diagn. 2023, 25, 467–476. [Google Scholar] [CrossRef]
- Ramos, A.E.; Muñoz, M.; Cortés-Vecino, J.A.; Barato, P.; Patarroyo, M.A. A Novel Loop-Mediated Isothermal Amplification-Based Test for Detecting Neospora caninum DNA. Parasites Vectors 2017, 10, 590. [Google Scholar] [CrossRef]
- Erber, A.C.; Sandler, P.J.; De Avelar, D.M.; Swoboda, I.; Cota, G.; Walochnik, J. Diagnosis of Visceral and Cutaneous Leishmaniasis Using Loop-Mediated Isothermal Amplification (LAMP) Protocols: A Systematic Review and Meta-Analysis. Parasites Vectors 2022, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, K.; Chen, M.; Hong, H.; Jiang, X.; Huang, R.; Wang, Y.; Huang, J.; Yu, X.; Liu, Q.; et al. A Colorimetric Assay for Neospora caninum Utilizing the Loop-Mediated Isothermal Amplification Technique. Res. Vet. Sci. 2024, 179, 105395. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Li, L.; Cao, L.; Zhao, Z.; Huang, T.; Li, J.; Zhang, X.; Cao, S.; Zhang, N.; et al. Establishment of an Ultrasensitive and Visual Detection Platform for Neospora caninum Based-on the RPA-CRISPR/Cas12a System. Talanta 2024, 269, 125413, Erratum in Talanta 2024, 271, 125722. https://doi.org/10.1016/j.talanta.2024.125722. [Google Scholar] [CrossRef]
- Müller, N.; Zimmermann, V.; Hentrich, B.; Gottstein, B. Diagnosis of Neospora caninum and Toxoplasma gondii Infection by PCR and DNA Hybridization Immunoassay. J. Clin. Microbiol. 1996, 34, 2850–2852. [Google Scholar] [CrossRef] [PubMed]
- da Silva Silveira, C.; Armendano, J.I.; Moore, D.P.; Cantón, G.J.; Macías-Rioseco, M.; Riet-Correa, F.; Giannitti, F. A comparative study of commercial ELISAs for antibody detection in the diagnostic investigation of Neospora caninum -associated abortion in dairy cattle herds in Uruguay. Rev. Argent. Microbiol. 2020, 52, 107–114. [Google Scholar] [CrossRef]
- Gliga, D.S.; Basso, W.; Ardüser, F.; Moore-Jones, G.; Schares, G.; Zanolari, P.; Frey, C.F. Switzerland-Wide Neospora caninum Seroprevalence in Female Cattle and Identification of Risk Factors for Infection. Front. Vet. Sci. 2022, 9, 1059697. [Google Scholar] [CrossRef]
- Waap, H.; Bärwald, A.; Nunes, T.; Schares, G. Seroprevalence and Risk Factors for Toxoplasma gondii and Neospora caninum in Cattle in Portugal. Animals 2022, 12, 2080. [Google Scholar] [CrossRef]
- Villa, L.; Allievi, C.; Di Cerbo, A.R.; Zanzani, S.A.; Sommariva, F.; Zanini, L.; Mortarino, M.; Manfredi, M.T. Neospora caninum Antibodies in Bulk Tank Milk from Dairy Cattle Herds in Italy in Relation to Reproductive and Productive Parameters and Spatial Analysis. Acta Trop. 2024, 254, 107194. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, W.; de Jong, E.; McCubbin, K.D.; Biesheuvel, M.M.; van der Meer, F.J.U.M.; De Buck, J.; Lhermie, G.; Hall, D.C.; Kalbfleisch, K.N.; Kastelic, J.P.; et al. Herd-Level Prevalence of Bovine Leukemia Virus, Salmonella Dublin, and Neospora caninum in Alberta, Canada, Dairy Herds Using ELISA on Bulk Tank Milk Samples. J. Dairy Sci. 2024, 107, 8313–8328. [Google Scholar] [CrossRef]
- Idarraga-Bedoya, S.E.; Álvarez-Chica, J.; Bonilla-Aldana, D.K.; Moore, D.P.; Rodríguez-Morales, A.J. Seroprevalence of Neospora caninum Infection in Cattle from Pereira, Colombia. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100469. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-Y.; An, Q.; Xue, N.-Y.; Chen, Y.; Chen, Y.-Y.; Zhang, Y.; Zhao, Q.; Wang, C.-R. Seroprevalence and Risk Factors of Neospora caninum Infection in Cattle in China from 2011 to 2020: A Systematic Review and Meta-Analysis. Prev. Vet. Med. 2022, 203, 105620. [Google Scholar] [CrossRef]
- Ying, Z.; Zhu, Z.-F.; Yang, X.; Liu, J.; Liu, Q. Prevalence and Associated Risk Factors of Neospora caninum Infection among Cattle in Mainland China: A Systematic Review and Meta-Analysis. Prev. Vet. Med. 2022, 201, 105593. [Google Scholar] [CrossRef]
- Ma, L.; Li, S.; Zhang, Y.; Wen, Z. Seroprevalence of Toxoplasma gondii and Neospora caninum in Dairy Cows in Hebei Province, China. Anim. Biotechnol. 2021, 32, 451–453. [Google Scholar] [CrossRef]
- Yi, X.-L.; Yang, W.-H.; Zheng, H.-L.; Cao, M.-L.; Xiong, J.; Chen, W.-C.; Zhou, Y.-J.; Li, F.; Zhu, X.-Q.; Liu, G.-H. Seroprevalence and Molecular Detection of Toxoplasma gondii and Neospora caninum in Beef Cattle and Goats in Hunan Province, China. Parasites Vectors 2024, 17, 195. [Google Scholar] [CrossRef]
- Hebbar, B.K.; Mitra, P.; Khan, W.; Chaudhari, S.; Shinde, S.; Deshmukh, A.S. Seroprevalence and Associated Risk Factors of Toxoplasma gondii and Neospora caninum Infections in Cattle in Central India. Parasitol. Int. 2022, 87, 102514. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Biswas, P.; Kabir, A.; Beni Amin, A.R.M.; Parijat, S.S.; Ahmed, N.; Hossain, M.Z.; Wakid, M.H. First Report of Neospora caninum from Aborted Fetuses of Cattle, Sheep, and Goats in Bangladesh. J. Adv. Vet. Anim. Res. 2024, 11, 618–626. [Google Scholar] [CrossRef]
- Selim, A.; Alshammari, A.; Gattan, H.S.; Marzok, M.; Salem, M.; Al-Jabr, O.A. Neospora caninum Infection in Dairy Cattle in Egypt: A Serosurvey and Associated Risk Factors. Sci. Rep. 2023, 13, 15489. [Google Scholar] [CrossRef] [PubMed]
- Metwally, S.; Hamada, R.; Sobhy, K.; Frey, C.F.; Fereig, R.M. Seroprevalence and Risk Factors Analysis of Neospora caninum and Toxoplasma gondii in Cattle of Beheira, Egypt. Front. Vet. Sci. 2023, 10, 1122092. [Google Scholar] [CrossRef]
- Tagwireyi, W.M.; Thompson, P.N.; Garcia, G.A.; Morar-Leather, D.; Neves, L. Seroprevalence and Associated Risk Factors for Neospora caninum Infection in Dairy Cattle in South Africa. Parasitol. Res. 2024, 123, 298. [Google Scholar] [CrossRef]
- Kakimori, M.T.A.; Osman, A.M.; Silva, A.C.S.; Ibrahim, A.M.; Shair, M.A.; Cavallieri, A.C.; Barros, L.D.; Garcia, J.L.; Vieira, T.S.W.J.; Hassan-Kadle, A.A.; et al. Serological and Molecular Detection of Toxoplasma gondii and Neospora caninum in Ruminants from Somalia. Parasitol. Res. 2024, 123, 376. [Google Scholar] [CrossRef]
- De Oliveira Junior, I.M.; Mesquita, L.E.D.S.; Miranda, D.N.P.; Gomes, T.A.; Vasconcelos, B.K.S.; Penha, L.C.; Silveira, L.C.S.; Redondo, A.R.R.; Costa, R.C.; Bruhn, F.R.P.; et al. Endogenous Transplacental Transmission of Neospora caninum in Successive Generations of Congenitally Infected Goats. Vet. Parasitol. 2020, 284, 109191. [Google Scholar] [CrossRef]
- Campero, L.M.; Basso, W.; Moré, G.; Fiorani, F.; Hecker, Y.P.; Echaide, I.; Cantón, G.J.; Cirone, K.M.; Campero, C.M.; Venturini, M.C.; et al. Neosporosis in Argentina: Past, Present and Future Perspectives. Vet. Parasitol. Reg. Stud. Rep. 2023, 41, 100882. [Google Scholar] [CrossRef] [PubMed]
- da Costa, L.S.; Withoeft, J.A.; Bilicki, J.V.; Melo, I.C.; Snak, A.; das Neves, G.B.; Miletti, L.C.; de Moura, A.B.; Casagrande, R.A. Neospora caninum -Associated Abortions in Cattle from Southern Brazil: Anatomopathological and Molecular Characterization. Vet. Parasitol. Reg. Stud. Rep. 2022, 36, 100802. [Google Scholar] [CrossRef] [PubMed]
- Remor-Sebolt, A.P.; de Lima, F.R.; Américo, L.; Padilha, M.A.C.; Chryssafidis, A.L.; de Moura, A.B. Occurrence of Antibodies and Epidemiological Significance of Toxoplasma gondii and Neospora caninum Infections in Canine Populations of Laguna, State of Santa Catarina. Vet. Res. Commun. 2024, 48, 3349–3354. [Google Scholar] [CrossRef]
- Rodriguez-Pazmiño, A.S.; Brito, C.M.; Salas-Rueda, M.; Orlando, S.A.; Garcia-Bereguiain, M.A. A First Insight into Seropositivity of Neospora caninum and Associated Risk Factors in Free-Roaming Dogs from Ecuador. Acta Trop. 2024, 256, 107245. [Google Scholar] [CrossRef] [PubMed]
- Turlewicz-Podbielska, H.; Ruszkowski, J.J.; Wojciechowski, J.; Pomorska-Mól, M. Seroprevalence of Neospora caninum in Pet Cats, Dogs and Rabbits from Urban Areas of Poland. BMC Vet. Res. 2024, 20, 37. [Google Scholar] [CrossRef]
- Lagomarsino, H.; Scioli, A.; Rodríguez, A.; Armendano, J.; Fiorani, F.; Bence, Á.; García, J.; Hecker, Y.; Gual, I.; Cantón, G.; et al. Controlling Endemic Neospora caninum-Related Abortions in a Dairy Herd From Argentina. Front. Vet. Sci. 2019, 6, 446. [Google Scholar] [CrossRef]
- Baldini, M.H.M.; Sandoval, E.D.P.; Duarte, J.M.B. Assessment of Transplacental Transmission of Neospora caninum and Toxoplasma gondii in Neotropical Deer: An Estimative Based on Serology. Vet. Parasitol. 2022, 303, 109677. [Google Scholar] [CrossRef] [PubMed]
- Zanet, S.; Poncina, M.; Ferroglio, E. Congenital Transmission of Neospora caninum in Wild Ungulates and Foxes. Front. Vet. Sci. 2023, 10, 1109986. [Google Scholar] [CrossRef]
- Marian, L.; Withoeft, J.A.; Costa, L.d.S.; Ribeiro, L.R.; Melo, I.C.; Alves, R.S.; Baumbach, L.F.; Pinto, M.G.L.; Snak, A.; Miletti, L.C.; et al. Causes of Fetal Death in the Flemish Cattle Herd in Brazil. Vet. World 2023, 16, 766–772. [Google Scholar] [CrossRef]
- Montiel-González, G.; Franco-Robles, E.; García-Munguía, C.A.; Valencia-Posadas, M.; Martínez-Jaime, O.A.; López-Briones, S.; Gutiérrez-Chávez, A.J. Co-Infection by Toxoplasma gondii and Neospora caninum in Goats Reared in Extensive System of Mexico. Vector Borne Zoonotic Dis. 2024, 24, 591–596. [Google Scholar] [CrossRef]
- Hamzavi, Y.; Salimi, Y.; Ahmadi, M.; Adimi, P.; Falahi, S.; Bozorgomid, A. Global Prevalence of Neospora caninum in Rodents: A Systematic Review and Meta-Analysis. Vet. Med. Sci. 2023, 9, 2192–2200. [Google Scholar] [CrossRef]
- Reichel, M.P.; Alejandra Ayanegui-Alcérreca, M.; Gondim, L.F.P.; Ellis, J.T. What Is the Global Economic Impact of Neospora caninum in Cattle—the Billion Dollar Question. Int. J. Parasitol. 2013, 43, 133–142. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Soares, I.R.; Mendes, R.G.; De Santis Bastos, P.A.; Katagiri, S.; Zavilenski, R.B.; De Abreu, H.F.P.; Afreixo, V. Meta-Analysis of the Prevalence and Risk Factors Associated with Bovine Neosporosis. Trop. Anim. Health Prod. 2019, 51, 1783–1800. [Google Scholar] [CrossRef] [PubMed]
- Demir, P.A.; Eşki, F.; Ütük, A.E. Estimating the Total Economic Costs of Neospora caninum Infections in Dairy Cows in Turkey. Trop. Anim. Health Prod. 2020, 52, 3251–3258. [Google Scholar] [CrossRef]
- Davidson, M.J.; Huaman, J.L.; Pacioni, C.; Stephens, D.; Hitchen, Y.; Carvalho, T.G. Active Shedding of Neospora caninum Detected in Australian Wild Canids in a Nonexperimental Context. Transbound. Emerg. Dis. 2022, 69, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Huaman, J.L.; Pacioni, C.; Doyle, M.; Forsyth, D.M.; Helbig, K.J.; Carvalho, T.G. Evidence of Australian Wild Deer Exposure to N. caninum Infection and Potential Implications for the Maintenance of N. caninum Sylvatic Cycle. BMC Vet. Res. 2023, 19, 153. [Google Scholar] [CrossRef]
- Semango, G.P.; Buza, J. Review of the Current Status on Ruminant Abortigenic Pathogen Surveillance in Africa and Asia. Vet. Sci. 2024, 11, 425. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Millán, J.; Chirife, A.D.; Ortega-Mora, L.M.; Calero-Bernal, R. Molecular Survey for Cyst-Forming Coccidia (Toxoplasma gondii, Neospora caninum, Sarcocystis spp.) in Mediterranean Periurban Micromammals. Parasitol. Res. 2020, 119, 2679–2686. [Google Scholar] [CrossRef]
- Moreno-Torres, K.I.; Pomeroy, L.W.; Moritz, M.; Saville, W.; Wolfe, B.; Garabed, R. Host Species Heterogeneity in the Epidemiology of Neospora caninum. PLoS ONE 2017, 12, e0183900. [Google Scholar] [CrossRef] [PubMed]
- Haydett, K.M.; Peper, S.T.; Reinoso Webb, C.; Tiffin, H.S.; Wilson-Fallon, A.N.; Jones-Hall, Y.L.; Webb, S.L.; Presley, S.M. Prevalence of Neospora caninum Exposure in Wild Pigs (Sus scrofa) from Oklahoma with Implications of Testing Method on Detection. Animals 2021, 11, 2487. [Google Scholar] [CrossRef]
- McKenny, L.; O’Handley, R.; Kovaliski, J.; Mutze, G.; Peacock, D.; Lanyon, S. Evidence of Infection with Toxoplasma gondii and Neospora caninum in South Australia: Using Wild Rabbits as a Sentinel Species. Aust. Vet. J. 2020, 98, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Azevedo Filho, P.C.G.d.; Ribeiro-Andrade, M.; Santos, J.F.D.; Reis, A.C.D.; Pinheiro Júnior, J.W.; Valença, S.R.F.d.A.; Samico-Fernandes, E.F.T.; Mota, R.A. Neospora caninum Infection in Cattle in the State of Amazonas, Brazil: Seroprevalence, Spatial Distribution and Risk Factors. Rev. Bras. Parasitol. Vet. 2021, 30, e020820. [Google Scholar] [CrossRef]
- Villa, L.; Maksimov, P.; Luttermann, C.; Tuschy, M.; Gazzonis, A.L.; Zanzani, S.A.; Mortarino, M.; Conraths, F.J.; Manfredi, M.T.; Schares, G. Spatial Distance between Sites of Sampling Associated with Genetic Variation among Neospora caninum in Aborted Bovine Foetuses from Northern Italy. Parasites Vectors 2021, 14, 47. [Google Scholar] [CrossRef]
- Dinon, A.; Fiorani, F.; Campero, L.M.; Moore, D.P.; Corva, P.M. The Role of Genetic Variability of the Host on the Resistance to Neospora caninum Infection in Cattle. Anim. Genet. 2024, 55, 304–318. [Google Scholar] [CrossRef]
- Grillo, G.F.; Couto, S.R.B.; Guerson, Y.B.; Ferreira, J.E.; Teixeira, E.F.; Silva, A.F.; Palhano, H.B.; Mello, M.R.B. Neospora caninum Is Not Transmissible via Embryo Transfer, but Affects the Quality of Embryos in Dairy Cows. Vet. Parasitol. 2024, 331, 110287. [Google Scholar] [CrossRef]
- Pivatto, R.A.; Reiter, J.C.; Rodrigues, R.B.; Miletti, L.C.; Palácios, R.; Snak, A.; Chryssafidis, A.L.; Moura, A.B. de Experimental Infection with Neospora caninum in Texel Ewes at Different Stages of Gestation. Vet. Parasitol. Reg. Stud. Rep. 2023, 37, 100817. [Google Scholar] [CrossRef]
- Reid, A.J.; Vermont, S.J.; Cotton, J.A.; Harris, D.; Hill-Cawthorne, G.A.; Könen-Waisman, S.; Latham, S.M.; Mourier, T.; Norton, R.; Quail, M.A.; et al. Comparative Genomics of the Apicomplexan Parasites Toxoplasma gondii and Neospora caninum: Coccidia Differing in Host Range and Transmission Strategy. PLoS Pathog. 2012, 8, e1002567. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jia, L.; Shao, Q.; Lu, H.; Zhao, J.; Yin, J. MicroRNA Profiling of Neospora caninum Tachyzoites (NC-1) Using a High-Throughput Approach. Parasitol. Res. 2021, 120, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Hasan, M.; Akter, S.; Roy, S.; Sharma, B.; Chowdhury, M.S.R.; Ahsan, M.I.; Akhand, R.N.; Uddin, M.B.; Ahmed, S.S.U. In Silico Investigation of Conserved miRNAs and Their Targets From the Expressed Sequence Tags in Neospora caninum Genome. Bioinform. Biol. Insights 2021, 15, 11779322211046729. [Google Scholar] [CrossRef]
- Chen, J.-M.; Zhao, S.-S.; Tao, D.-L.; Li, J.-Y.; Yang, X.; Fan, Y.-Y.; Song, J.-K.; Liu, Q.; Zhao, G.-H. Temporal Transcriptomic Changes in microRNAs Involved in the Host Immune Response and Metabolism during Neospora caninum Infection. Parasites Vectors 2023, 16, 28. [Google Scholar] [CrossRef]
- Arranz-Solís, D.; Regidor-Cerrillo, J.; Lourido, S.; Ortega-Mora, L.M.; Saeij, J.P.J. Toxoplasma CRISPR/Cas9 Constructs Are Functional for Gene Disruption in Neospora caninum. Int. J. Parasitol. 2018, 48, 597–600. [Google Scholar] [CrossRef]
- Rico-San Román, L.; Hänggeli, K.P.A.; Hemphill, A.; Horcajo, P.; Collantes-Fernández, E.; Ortega-Mora, L.M.; Boubaker, G. TaqMan-Quantitative PCR Assays Applied in Neospora caninum Knock-Outs Generated through CRISPR-Cas9 Allow to Determine the Copy Numbers of Integrated Dihydrofolate Reductase-Thymidylate Synthase Drug Selectable Markers. Front. Cell. Infect. Microbiol. 2024, 14, 1419209. [Google Scholar] [CrossRef]
- Mineo, T.W.P.; Chern, J.H.; Thind, A.C.; Mota, C.M.; Nadipuram, S.M.; Torres, J.A.; Bradley, P.J. Efficient Gene Knockout and Knockdown Systems in Neospora caninum Enable Rapid Discovery and Functional Assessment of Novel Proteins. mSphere 2022, 7, e0089621. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Shimoda, N.; Fereig, R.M.; Moritaka, T.; Umeda, K.; Nishimura, M.; Ihara, F.; Kobayashi, K.; Himori, Y.; Suzuki, Y.; et al. Neospora caninum Dense Granule Protein 7 Regulates the Pathogenesis of Neosporosis by Modulating Host Immune Response. Appl. Environ. Microbiol. 2018, 84, e01350-18. [Google Scholar] [CrossRef]
- Rico-San Román, L.; Amieva, R.; Regidor-Cerrillo, J.; García-Sánchez, M.; Collantes-Fernández, E.; Pastor-Fernández, I.; Saeij, J.P.J.; Ortega-Mora, L.M.; Horcajo, P. NcGRA7 and NcROP40 Play a Role in the Virulence of Neospora caninum in a Pregnant Mouse Model. Pathogens 2022, 11, 998. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Song, X.; Ma, L.; Yang, J.; Liu, Q.; Liu, J. Function of Neospora caninum Dense Granule Protein 7 in Innate Immunity in Mice. Parasitol. Res. 2021, 120, 197–207. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Ma, L.; Zhang, X.; Zhang, X.; Zhou, B.; Zhu, X.; Liu, Q. NcGRA17 Is an Important Regulator of Parasitophorous Vacuole Morphology and Pathogenicity of Neospora caninum. Vet. Parasitol. 2018, 264, 26–34. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, N.; Dong, J.; Li, J.; Wang, X.; Li, X.; Li, X.; Yang, J.; Gong, P.; Zhang, X. Effects of Dense Granular Protein 6 (GRA6) Disruption on Neospora caninum Virulence. Front. Vet. Sci. 2020, 7, 562730. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, N.; Zhao, P.; Li, J.; Cao, L.; Wang, X.; Li, X.; Yang, J.; Zhang, X.; Gong, P. Disruption of Dense Granular Protein 2 (GRA2) Decreases the Virulence of Neospora caninum. Front. Vet. Sci. 2021, 8, 634612. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, J.; Zhou, Y.; Zhang, H.; Ayanniyi, O.O.; Luo, S.; Zhang, Y.; Xu, Q.; Wang, C.; Yang, C. Functional Characterization of Three Novel Dense Granule Proteins in Neospora caninum Using the CRISPR-Cas9 System. Acta Trop. 2024, 256, 107250. [Google Scholar] [CrossRef] [PubMed]
- Amieva, R.; Coronado, M.; Powell, J.; Arranz-Solís, D.; Hassan, M.A.; Collantes-Fernández, E.; Ortega-Mora, L.M.; Horcajo, P. NcROP2 Deletion Reduces Neospora caninum Virulence by Altering Parasite Stage Differentiation and Hijacking Host Immune Response. Front. Immunol. 2025, 16, 1617570. [Google Scholar] [CrossRef]
- Nan, H.; Lu, X.; Zhang, C.; Yang, X.; Ying, Z.; Ma, L. Identification and Function Characterization of NcAP2XII-4 in Neospora caninum. Parasites Vectors 2024, 17, 392. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaky, H.H.; Rahman, M.M.; Shimoda, N.; Chen, Y.; Hasan, T.; Ushio, N.; Nishikawa, Y. Neospora caninum Surface Antigen 1 Is a Major Determinant of the Pathogenesis of Neosporosis in Nonpregnant and Pregnant Mice. Front. Microbiol. 2023, 14, 1334447. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Liu, J.; Liu, Q. NcPuf1 Is a Key Virulence Factor in Neospora caninum. Pathogens 2020, 9, 1019. [Google Scholar] [CrossRef]
- Du, B.; Chen, M.; Chang, L.; Zhang, X.; Zhang, X.; Wang, X.; Gong, P.; Zhang, N.; Zhang, X.; Li, X.; et al. Immunization with the NcMYR1 Gene Knockout Strain Effectively Protected C57BL/6 Mice and Their Pups against the Neospora caninum Challenge. Virulence 2024, 15, 2427844. [Google Scholar] [CrossRef]
- Tomasina, R.; González, F.C.; Echeverría, S.; Cabrera, A.; Robello, C. Insights into the Cell Division of Neospora caninum. Microorganisms 2023, 12, 61. [Google Scholar] [CrossRef]
- Ramírez-Flores, C.J.; Tibabuzo Perdomo, A.M.; Gallego-López, G.M.; Knoll, L.J. Transcending Dimensions in Apicomplexan Research: From Two-Dimensional to Three-Dimensional In Vitro Cultures. Microbiol. Mol. Biol. Rev. 2022, 86, e0002522. [Google Scholar] [CrossRef]
- Feix, A.S.; Cruz-Bustos, T.; Ruttkowski, B.; Joachim, A. In Vitro Cultivation Methods for Coccidian Parasite Research. Int. J. Parasitol. 2023, 53, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Martínez, A.; Basto, A.P.; Tanaka, S.; Ryser, L.T.; Nunes, T.P.; Ortega-Mora, L.-M.; Arranz-Solís, D.; Leitão, A.; Hemphill, A. Immunization with a Cocktail of Antigens Fused with OprI Reduces Neospora caninum Vertical Transmission and Postnatal Mortality in Mice. Vaccine 2019, 37, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.S.; Hecker, Y.P.; Quintana, S.; Pérez, S.E.; Leunda, M.R.; Cantón, G.J.; Cobo, E.R.; Moore, D.P.; Odeón, A.C. Immunization with Inactivated Antigens of Neospora caninum Induces Toll-like Receptors 3, 7, 8 and 9 in Maternal-Fetal Interface of Infected Pregnant Heifers. Vet. Parasitol. 2017, 243, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pelayo, L.; García-Sánchez, M.; Collantes-Fernández, E.; Regidor-Cerrillo, J.; Horcajo, P.; Gutiérrez-Expósito, D.; Espinosa, J.; Benavides, J.; Osoro, K.; Pfarrer, C.; et al. Crosstalk between Neospora caninum and the Bovine Host at the Maternal-Foetal Interface Determines the Outcome of Infection. Vet. Res. 2020, 51, 83. [Google Scholar] [CrossRef]
- Hasan, T.; Nishikawa, Y. Advances in Vaccine Development and the Immune Response against Toxoplasmosis in Sheep and Goats. Front. Vet. Sci. 2022, 9, 951584. [Google Scholar] [CrossRef]
- Tuo, W.; Feng, X.; Cao, L.; Vinyard, B.; Dubey, J.P.; Fetterer, R.; Jenkins, M. Vaccination with Neospora caninum-Cyclophilin and -Profilin Confers Partial Protection against Experimental Neosporosis-Induced Abortion in Sheep. Vaccine 2021, 39, 4534–4544. [Google Scholar] [CrossRef]
- Baszler, T.V.; Shkap, V.; Mwangi, W.; Davies, C.J.; Mathison, B.A.; Mazuz, M.; Resnikov, D.; Fish, L.; Leibovitch, B.; Staska, L.M.; et al. Bovine Immune Response to Inoculation with Neospora caninum Surface Antigen SRS2 Lipopeptides Mimics Immune Response to Infection with Live Parasites. Clin. Vaccine Immunol. 2008, 15, 659–667. [Google Scholar] [CrossRef]
- Staska, L.M.; Davies, C.J.; Brown, W.C.; McGuire, T.C.; Suarez, C.E.; Park, J.Y.; Mathison, B.A.; Abbott, J.R.; Baszler, T.V. Identification of Vaccine Candidate Peptides in the NcSRS2 Surface Protein of Neospora caninum by Using CD4+ Cytotoxic T Lymphocytes and Gamma Interferon-Secreting T Lymphocytes of Infected Holstein Cattle. Infect. Immun. 2005, 73, 1321–1329. [Google Scholar] [CrossRef]
- Nishimura, M.; Kohara, J.; Kuroda, Y.; Hiasa, J.; Tanaka, S.; Muroi, Y.; Kojima, N.; Furuoka, H.; Nishikawa, Y. Oligomannose-Coated Liposome-Entrapped Dense Granule Protein 7 Induces Protective Immune Response to Neospora caninum in Cattle. Vaccine 2013, 31, 3528–3535. [Google Scholar] [CrossRef]
- Hecker, Y.P.; Cóceres, V.; Wilkowsky, S.E.; Jaramillo Ortiz, J.M.; Morrell, E.L.; Verna, A.E.; Ganuza, A.; Cano, D.B.; Lischinsky, L.; Angel, S.O.; et al. A Neospora caninum Vaccine Using Recombinant Proteins Fails to Prevent Foetal Infection in Pregnant Cattle after Experimental Intravenous Challenge. Vet. Immunol. Immunopathol. 2014, 162, 142–153. [Google Scholar] [CrossRef]
- Mansilla, F.C.; Moore, D.P.; Quintana, M.E.; Cardoso, N.; Hecker, Y.P.; Gual, I.; Czepluch, W.; Odeón, A.C.; Capozzo, A.V. Safety and Immunogenicity of a Soluble Native Neospora caninum Tachyzoite-Extract Vaccine Formulated with a Soy Lecithin/β-Glucan Adjuvant in Pregnant Cattle. Vet. Immunol. Immunopathol. 2015, 165, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, F.C.; Quintana, M.E.; Cardoso, N.P.; Capozzo, A.V. Fusion of Foreign T-Cell Epitopes and Addition of TLR Agonists Enhance Immunity against Neospora caninum Profilin in Cattle. Parasite Immunol. 2016, 38, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Novoa, M.B.; Sarli, M.; Reidel, I.G.; Veaute, C.; Valentini, B.; Primo, M.E. Neospora caninum Truncated Recombinant Proteins Formulated with Liposomes and CpG-ODNs Triggered a Humoral Immune Response in Cattle after Immunisation and Challenge. Vet. Immunol. Immunopathol. 2021, 238, 110285. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.L.; Guy, C.S.; Smith, R.F.; Ellis, J.; Björkman, C.; Reichel, M.P.; Trees, A.J. Immunization of Cattle with Live Tachyzoites of Neospora caninum Confers Protection against Fetal Death. Infect. Immun. 2007, 75, 1343–1348. [Google Scholar] [CrossRef]
- Weber, F.H.; Jackson, J.A.; Sobecki, B.; Choromanski, L.; Olsen, M.; Meinert, T.; Frank, R.; Reichel, M.P.; Ellis, J.T. On the Efficacy and Safety of Vaccination with Live Tachyzoites of Neospora caninum for Prevention of Neospora-Associated Fetal Loss in Cattle. Clin. Vaccine Immunol. 2013, 20, 99–105. [Google Scholar] [CrossRef]
- Rojo-Montejo, S.; Collantes-Fernández, E.; Pérez-Zaballos, F.; Rodríguez-Marcos, S.; Blanco-Murcia, J.; Rodríguez-Bertos, A.; Prenafeta, A.; Ortega-Mora, L.M. Effect of Vaccination of Cattle with the Low Virulence Nc-Spain 1H Isolate of Neospora caninum against a Heterologous Challenge in Early and Mid-Gestation. Vet. Res. 2013, 44, 106. [Google Scholar] [CrossRef]
- Hecker, Y.P.; Moore, D.P.; Quattrocchi, V.; Regidor-Cerrillo, J.; Verna, A.; Leunda, M.R.; Morrell, E.; Ortega-Mora, L.M.; Zamorano, P.; Venturini, M.C.; et al. Immune Response and Protection Provided by Live Tachyzoites and Native Antigens from the NC-6 Argentina Strain of Neospora caninum in Pregnant Heifers. Vet. Parasitol. 2013, 197, 436–446. [Google Scholar] [CrossRef]
- Mazuz, M.L.; Fish, L.; Wolkomirsky, R.; Leibovich, B.; Reznikov, D.; Savitsky, I.; Golenser, J.; Shkap, V. The Effect of a Live Neospora caninum Tachyzoite Vaccine in Naturally Infected Pregnant Dairy Cows. Prev. Vet. Med. 2015, 120, 232–235. [Google Scholar] [CrossRef]
- Mazuz, M.L.; Leibovitz, B.; Savitsky, I.; Blinder, E.; Yasur-Landau, D.; Lavon, Y.; Sharir, B.; Tirosh-Levy, S. The Effect of Vaccination with Neospora caninum Live-Frozen Tachyzoites on Abortion Rates of Naturally Infected Pregnant Cows. Vaccines 2021, 9, 401. [Google Scholar] [CrossRef]
- Hecker, Y.P.; Burucúa, M.M.; Fiorani, F.; Maldonado Rivera, J.E.; Cirone, K.M.; Dorsch, M.A.; Cheuquepán, F.A.; Campero, L.M.; Cantón, G.J.; Marín, M.S.; et al. Reactivation and Foetal Infection in Pregnant Heifers Infected with Neospora caninum Live Tachyzoites at Prepubertal Age. Vaccines 2022, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Morales, L.F.; Fiorani, F.; Morán, K.D.; Hecker, Y.P.; Cirone, K.M.; Sánchez-López, E.F.; Ramos-Duarte, V.A.; Corigliano, M.G.; Bilbao, M.G.; Clemente, M.; et al. Immunogenicity, Safety and Dual DIVA-like Character of a Recombinant Candidate Vaccine against Neosporosis in Cattle. Acta Trop. 2024, 257, 107293. [Google Scholar] [CrossRef]
- Bengoa-Luoni, S.A.; Corigliano, M.G.; Sánchez-López, E.; Albarracín, R.M.; Legarralde, A.; Ganuza, A.; Clemente, M.; Sander, V.A. The Potential of a DIVA-like Recombinant Vaccine Composed by rNcSAG1 and rAtHsp81.2 against Vertical Transmission in a Mouse Model of Congenital Neosporosis. Acta Trop. 2019, 198, 105094. [Google Scholar] [CrossRef]
- Marugán-Hernández, V.; Ortega-Mora, L.M.; Aguado-Martínez, A.; Jiménez-Ruíz, E.; Álvarez-García, G. Transgenic Neospora caninum Strains Constitutively Expressing the Bradyzoite NcSAG4 Protein Proved to Be Safe and Conferred Significant Levels of Protection against Vertical Transmission When Used as Live Vaccines in Mice. Vaccine 2011, 29, 7867–7874. [Google Scholar] [CrossRef]
- Miller, C.; Quinn, H.; Ryce, C.; Reichel, M.P.; Ellis, J.T. Reduction in Transplacental Transmission of Neospora caninum in Outbred Mice by Vaccination. Int. J. Parasitol. 2005, 35, 821–828. [Google Scholar] [CrossRef]
- Rojo-Montejo, S.; Collantes-Fernández, E.; López-Pérez, I.; Risco-Castillo, V.; Prenafeta, A.; Ortega-Mora, L.M. Evaluation of the Protection Conferred by a Naturally Attenuated Neospora caninum Isolate against Congenital and Cerebral Neosporosis in Mice. Vet. Res. 2012, 43, 62. [Google Scholar] [CrossRef]
- Winzer, P.; Imhof, D.; Anghel, N.; Ritler, D.; Müller, J.; Boubaker, G.; Aguado-Martinez, A.; Ortega-Mora, L.-M.; Ojo, K.K.; VanVoorhis, W.C.; et al. The Impact of BKI-1294 Therapy in Mice Infected With the Apicomplexan Parasite Neospora caninum and Re-Infected During Pregnancy. Front. Vet. Sci. 2020, 7, 587570. [Google Scholar] [CrossRef] [PubMed]
- Imhof, D.; Pownall, W.R.; Monney, C.; Oevermann, A.; Hemphill, A. A Listeria Monocytogenes-Based Vaccine Formulation Reduces Vertical Transmission and Leads to Enhanced Pup Survival in a Pregnant Neosporosis Mouse Model. Vaccines 2021, 9, 1400. [Google Scholar] [CrossRef] [PubMed]
- Imhof, D.; Pownall, W.; Hänggeli, K.P.A.; Monney, C.; Román, L.R.-S.; Ortega-Mora, L.-M.; Forterre, F.; Oevermann, A.; Hemphill, A. Immunization with a Multivalent Listeria Monocytogenes Vaccine Leads to a Strong Reduction in Vertical Transmission and Cerebral Parasite Burden in Pregnant and Non-Pregnant Mice Infected with Neospora caninum. Vaccines 2023, 11, 156. [Google Scholar] [CrossRef]
- Pownall, W.R.; Imhof, D.; Trigo, N.F.; Ganal-Vonarburg, S.C.; Plattet, P.; Monney, C.; Forterre, F.; Hemphill, A.; Oevermann, A. Safety of a Novel Listeria Monocytogenes-Based Vaccine Vector Expressing NcSAG1 (Neospora caninum Surface Antigen 1). Front. Cell. Infect. Microbiol. 2021, 11, 675219. [Google Scholar] [CrossRef]
- Shams, M.; Maleki, B.; Kordi, B.; Majidiani, H.; Nazari, N.; Irannejad, H.; Asghari, A. Towards the First Multiepitope Vaccine Candidate against Neospora caninum in Mouse Model: Immunoinformatic Standpoint. Biomed. Res. Int. 2022, 2022, 2644667. [Google Scholar] [CrossRef] [PubMed]
- Asghari, A.; Kordi, B.; Maleki, B.; Majidiani, H.; Shams, M.; Naserifar, R. Neospora caninum SRS2 Protein: Essential Vaccination Targets and Biochemical Features for Next-Generation Vaccine Design. Biomed. Res. Int. 2022, 2022, 7070144. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Z.; Yang, Z. The Combination of Vaccines and Adjuvants to Prevent the Occurrence of High Incidence of Infectious Diseases in Bovine. Front. Vet. Sci. 2023, 10, 1243835. [Google Scholar] [CrossRef] [PubMed]
- Débare, H.; Moiré, N.; Ducournau, C.; Schmidt, J.; Laakmann, J.-D.; Schwarz, R.T.; Dimier-Poisson, I.; Debierre-Grockiego, F. Neospora caninum Glycosylphosphatidylinositols Used as Adjuvants Modulate Cellular Immune Responses Induced in Vitro by a Nanoparticle-Based Vaccine. Cytokine 2021, 144, 155575. [Google Scholar] [CrossRef]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; K, P.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The Next-Generation DNA Vaccine Platforms and Delivery Systems: Advances, Challenges and Prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef]
- Sun, M.; Pratama, A.C.; Qiu, H.; Liu, Z.; He, F. Toward Innovative Veterinary Nanoparticle Vaccines. Anim. Dis. 2024, 4, 14. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Effects of Sulfadiazine and Amprolium on Neospora caninum (Protozoa: Apicomplexa) Infections in Mice. J. Parasitol. 1990, 76, 177–179. [Google Scholar] [CrossRef]
- Dubey, J.P.; Schares, G. Neosporosis in Animals--the Last Five Years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Vázquez, P.; Ferre, I.; Ortega-Mora, L.M. Treatment of Toxoplasmosis and Neosporosis in Farm Ruminants: State of Knowledge and Future Trends. CTMC 2018, 18, 1304–1323. [Google Scholar] [CrossRef]
- Müller, J.; Aguado, A.; Laleu, B.; Balmer, V.; Ritler, D.; Hemphill, A. In Vitro Screening of the Open Source Pathogen Box Identifies Novel Compounds with Profound Activities against Neospora caninum. Int. J. Parasitol. 2017, 47, 801–809. [Google Scholar] [CrossRef]
- Müller, J.; Winzer, P.A.; Samby, K.; Hemphill, A. In Vitro Activities of MMV Malaria Box Compounds against the Apicomplexan Parasite Neospora caninum, the Causative Agent of Neosporosis in Animals. Molecules 2020, 25, 1460. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xue, Y.; Pei, Y.; Yin, M.; Sun, Z.; Zhou, Z.; Liu, J.; Liu, Q. Construction of Luciferase-Expressing Neospora caninum and Drug Screening. Parasites Vectors 2024, 17, 118. [Google Scholar] [CrossRef]
- Harada, M.; Nagai, J.; Kurata, R.; Shimizu, K.; Cui, X.; Isagawa, T.; Semba, H.; Ishihara, J.; Yoshida, Y.; Takeda, N.; et al. Establishment of Novel High-Standard Chemiluminescent Assay for NTPase in Two Protozoans and Its High-Throughput Screening. Mar. Drugs 2020, 18, 161. [Google Scholar] [CrossRef]
- Kurata, R.; Harada, M.; Nagai, J.; Cui, X.; Isagawa, T.; Semba, H.; Yoshida, Y.; Takeda, N.; Maemura, K.; Yonezawa, T. Nucleoside Triphosphate Hydrolases Assay in Toxoplasma gondii and Neospora caninum for High-Throughput Screening Using a Robot Arm. J. Vis. Exp. 2022, 185. [Google Scholar] [CrossRef]
- Pereira, L.M.; De Luca, G.; Abichabki, N.D.L.M.; Brochi, J.C.V.; Baroni, L.; Abreu-Filho, P.G.; Yatsuda, A.P. Atovaquone, Chloroquine, Primaquine, Quinine and Tetracycline: Antiproliferative Effects of Relevant Antimalarials on Neospora caninum. Rev. Bras. Parasitol. Vet. 2021, 30, e022120. [Google Scholar] [CrossRef]
- de Oliveira Lima, Â.C.; Nolasco, M.; Freitas, L.D.S.; Pinheiro, A.M.; de Carvalho, C.A.L.; de Freitas, H.F.; Pita, S.S.d.R.; Vieira, I.J.C.; Braz Filho, R.; Branco, A. A New Cyclodipeptide from Tetragonisca Angustula Honey Active against Neospora caninum and in Silico Study. Nat. Prod. Res. 2024, 38, 2909–2914. [Google Scholar] [CrossRef]
- Tang, Z.; Xie, S.; Min, P.; Li, H.; Zhao, F.; Liu, M.; Jin, W.; Wang, L.; Zhao, J.; Jia, L. Protective Effect of Inonotus Obliquus Polysaccharide on Mice Infected with Neospora caninum. Int. J. Biol. Macromol. 2024, 261, 129906. [Google Scholar] [CrossRef]
- Huang, R.; Jiang, X.; Jiang, Y.; Qian, Y.; Huang, J.; Liu, T.; Wang, Y.; Hu, K.; Yang, Z.; Wei, Z. Efficacy of Cordycepin against Neospora caninum Infection in Vitro and in Vivo. Vet. Parasitol. 2024, 331, 110284. [Google Scholar] [CrossRef] [PubMed]
- Ojo, K.K.; Reid, M.C.; Kallur Siddaramaiah, L.; Müller, J.; Winzer, P.; Zhang, Z.; Keyloun, K.R.; Vidadala, R.S.R.; Merritt, E.A.; Hol, W.G.J.; et al. Neospora caninum Calcium-Dependent Protein Kinase 1 Is an Effective Drug Target for Neosporosis Therapy. PLoS ONE 2014, 9, e92929. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Alday, P.H.; Choi, R.; Khim, M.; Staker, B.L.; Hulverson, M.A.; Ojo, K.K.; Fan, E.; Van Voorhis, W.C.; Doggett, J.S. Bumped Kinase Inhibitors Inhibit Both Toxoplasma Gondii MAPKL1 and CDPK1. ACS Infect. Dis. 2025, 11, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Imhof, D.; Anghel, N.; Winzer, P.; Balmer, V.; Ramseier, J.; Hänggeli, K.; Choi, R.; Hulverson, M.A.; Whitman, G.R.; Arnold, S.L.M.; et al. In Vitro Activity, Safety and in Vivo Efficacy of the Novel Bumped Kinase Inhibitor BKI-1748 in Non-Pregnant and Pregnant Mice Experimentally Infected with Neospora caninum Tachyzoites and Toxoplasma Gondii Oocysts. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 90–101. [Google Scholar] [CrossRef]
- de Sousa, M.C.F.; Imhof, D.; Hänggeli, K.P.A.; Choi, R.; Hulverson, M.A.; Arnold, S.L.M.; Van Voorhis, W.C.; Fan, E.; Roberto, S.-S.; Ortega-Mora, L.M.; et al. Efficacy of the Bumped Kinase Inhibitor BKI-1708 against the Cyst-Forming Apicomplexan Parasites Toxoplasma gondii and Neospora caninum in Vitro and in Experimentally Infected Mice. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100553. [Google Scholar] [CrossRef]
- Müller, J.; Anghel, N.; Imhof, D.; Hänggeli, K.; Uldry, A.-C.; Braga-Lagache, S.; Heller, M.; Ojo, K.K.; Ortega-Mora, L.-M.; Van Voorhis, W.C.; et al. Common Molecular Targets of a Quinolone Based Bumped Kinase Inhibitor in Neospora caninum and Danio Rerio. Int. J. Mol. Sci. 2022, 23, 2381. [Google Scholar] [CrossRef] [PubMed]
- Anghel, N.; Balmer, V.; Müller, J.; Winzer, P.; Aguado-Martinez, A.; Roozbehani, M.; Pou, S.; Nilsen, A.; Riscoe, M.; Doggett, J.S.; et al. Endochin-Like Quinolones Exhibit Promising Efficacy Against Neospora caninum in Vitro and in Experimentally Infected Pregnant Mice. Front. Vet. Sci. 2018, 5, 285. [Google Scholar] [CrossRef]
- Anghel, N.; Imhof, D.; Winzer, P.; Balmer, V.; Ramseier, J.; Haenggeli, K.; Choi, R.; Hulverson, M.A.; Whitman, G.R.; Arnold, S.L.M.; et al. Endochin-like Quinolones (ELQs) and Bumped Kinase Inhibitors (BKIs): Synergistic and Additive Effects of Combined Treatments against Neospora caninum Infection in Vitro and in Vivo. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 92–106. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, M.; Jiménez-Pelayo, L.; Horcajo, P.; Collantes-Fernández, E.; Ortega-Mora, L.M.; Regidor-Cerrillo, J. Neospora caninum Infection Induces an Isolate Virulence-Dependent pro-Inflammatory Gene Expression Profile in Bovine Monocyte-Derived Macrophages. Parasites Vectors 2020, 13, 374. [Google Scholar] [CrossRef]
- Bhandage, A.K.; Olivera, G.C.; Kanatani, S.; Thompson, E.; Loré, K.; Varas-Godoy, M.; Barragan, A. A Motogenic GABAergic System of Mononuclear Phagocytes Facilitates Dissemination of Coccidian Parasites. Elife 2020, 9, e60528. [Google Scholar] [CrossRef]
- Mota, C.M.; Oliveira, A.C.M.; Davoli-Ferreira, M.; Silva, M.V.; Santiago, F.M.; Nadipuram, S.M.; Vashisht, A.A.; Wohlschlegel, J.A.; Bradley, P.J.; Silva, J.S.; et al. Neospora caninum Activates P38 MAPK as an Evasion Mechanism against Innate Immunity. Front. Microbiol. 2016, 7, 1456, Erratum in Front. Microbiol. 2019, 10, 548. https://doi.org/10.3389/fmicb.2019.00548. [Google Scholar] [CrossRef] [PubMed]
- Lantier, L.; Poupée-Beaugé, A.; di Tommaso, A.; Ducournau, C.; Epardaud, M.; Lakhrif, Z.; Germon, S.; Debierre-Grockiego, F.; Mévélec, M.-N.; Battistoni, A.; et al. Neospora caninum: A New Class of Biopharmaceuticals in the Therapeutic Arsenal against Cancer. J. Immunother. Cancer 2020, 8, e001242. [Google Scholar] [CrossRef]
- Battistoni, A.; Lantier, L.; di Tommaso, A.; Ducournau, C.; Lajoie, L.; Samimi, M.; Coënon, L.; Rivière, C.; Epardaud, M.; Hertereau, L.; et al. Nasal Administration of Recombinant Neospora caninum Secreting IL-15/IL-15Rα Inhibits Metastatic Melanoma Development in Lung. J. Immunother. Cancer 2023, 11, e006683. [Google Scholar] [CrossRef]
- Riviere, C.; Aljieli, M.; Mévélec, M.-N.; Lantier, L.; Boursin, F.; Lajoie, L.; Ducournau, C.; Germon, S.; Moiré, N.; Dimier-Poisson, I.; et al. Neospora caninum as Delivery Vehicle for Anti-PD-L1 scFv-Fc: A Novel Approach for Cancer Immunotherapy. Mol. Ther. Oncol. 2025, 33, 200968. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.M.; Salem, A.E.; El Skhawy, N. Parasites Revive Hope for Cancer Therapy. Eur. J. Med. Res. 2024, 29, 489. [Google Scholar] [CrossRef]
- Bruno, P.S.; Biggers, P.; Nuru, N.; Versaci, N.; Chirila, M.I.; Darie, C.C.; Neagu, A.-N. Small Biological Fighters Against Cancer: Viruses, Bacteria, Archaea, Fungi, Protozoa, and Microalgae. Biomedicines 2025, 13, 665. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.-H.; Chong, A.; Hong, Y.; Min, J.-J. Bioengineering of Bacteria for Cancer Immunotherapy. Nat. Commun. 2023, 14, 3553. [Google Scholar] [CrossRef]
- Rutter, J.W.; Dekker, L.; Owen, K.A.; Barnes, C.P. Microbiome Engineering: Engineered Live Biotherapeutic Products for Treating Human Disease. Front. Bioeng. Biotechnol. 2022, 10, 1000873. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, Y.; Xu, W.; Hao, X.; Li, Y.; Huang, S.; Xiang, D.; Wu, J. Bacteria-Based Immunotherapy for Cancer: A Systematic Review of Preclinical Studies. Front. Immunol. 2023, 14, 1140463. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent Advances in mRNA Vaccine Technology. Curr. Opin. Immunol. 2020, 65, 14–20. [Google Scholar] [CrossRef]
- Tomazic, M.L.; Marugan-Hernandez, V.; Rodriguez, A.E. Next-Generation Technologies and Systems Biology for the Design of Novel Vaccines Against Apicomplexan Parasites. Front. Vet. Sci. 2022, 8, 800361. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.