Abstract
Oral mucosa healing is a complex process that involves the innate wound healing system, including the coagulation cascade, extracellular matrix remodeling, immune cell responses, and fibroblast and epithelial responses, within the context of a dynamic resident microbiome. Unlike cutaneous wounds, oral wounds heal rapidly with minimal scarring despite constant exposure to diverse microbial communities, saliva, and mechanical stress. Emerging evidence highlights the critical interplay between microbiome-mediated signaling and macrophage plasticity in shaping wound outcomes, suggesting that similar mechanisms operate within the oral cavity. Inflammation is an essential component of wound repair, and its resolution is necessary to promote tissue remodeling and functional regeneration. Macrophages play a central role in this transition through phenotype switching from a pro-inflammatory (M1) to a pro-resolving, anti-inflammatory (M2) state. This review synthesizes current understanding of the oral microbiome’s influence on macrophage polarization across distinct stages of oral wound healing and examines microbial-based strategies that modulate the immune response to enhance repair. Significant knowledge gaps remain, including limited clinical translation, inter-individual variability in microbiome composition, and complete mechanistic insight into host–microbe immune interaction. Addressing these challenges enables the development of precision microbiome-based therapeutics that restore microbial balance, direct macrophage-driven regeneration, and improve outcomes in oral wounds and chronic inflammatory conditions.
1. Introduction
Wound healing is a coordinated physiological process involving four overlapping phases—hemostasis, inflammation, proliferation, and remodeling—regulated through interactions among extracellular matrix components, immune cells, cytokines, chemokines, and growth factors [1]. Wounds are typically classified into acute and chronic. Acute wounds heal within 14 days and mostly occur as a result of burns, surgical sites and trauma, and are affected by factors including moisture, temperature and nutrients [2]. Chronic wounds are those that are delayed or fail to heal on their own within 14 days, and occur in conditions like venous ulcers, pressure ulcers, and diabetic ulcers, typically due to unresolved inflammation or impaired tissue regeneration [3]. Beyond host factors, the microbiome is now recognized as a critical regulator of the wound microenvironment. Eubiosis, defined as a balanced microbial community, is associated with efficient healing, whereas dysbiosis promotes sustained inflammation and chronic wound formation [4].
This microbial influence is particularly relevant in the oral cavity, where constant exposure to saliva, mechanical forces from mastication, and a highly diverse microbiota creates a uniquely dynamic healing environment [5]. The oral microbiome, the second largest microbial ecosystem after the gut, comprises approximately 700 bacterial species [6]. In health, commensal organisms maintain immune homeostasis, whereas dysbiosis characterized by the overrepresentation of pathobionts such as Streptococcus mutans, Porphyromonas gingivalis, Enterococcus faecalis, and Fusobacterium nucleatum is associated with inflammation, tissue breakdown, and impaired wound healing, as exemplified in periodontitis [7,8]. Immune regulation is central to this host–microbe interplay. Following injury, innate immune cells are recruited to the wound in response to cytokines and chemokines released during the hemostatic and inflammatory phases [9]. Macrophages orchestrate the transition from inflammation to tissue repair through a shift from a pro-inflammatory (M1) to a pro-regenerative (M2) phenotype [10]. Emerging evidence indicates that the oral microbiome is a key modulator of this phenotypic transition: eubiotic communities promote timely resolution of inflammation, enhance angiogenesis, and reduce scarring, whereas dysbiosis sustains M1 polarization and prevents progression into the regenerative phase [11,12].
Despite these insights, therapeutic strategies specifically targeting oral wound healing remain limited, and post-surgical complications related to microbial imbalance are common [13]. While cutaneous wound care has begun to incorporate microbiome-based approaches, including probiotic therapies and microbial metabolite delivery to modulate immune responses and enhance macrophage-driven repair, similar strategies for oral wounds are underdeveloped [14,15]. This review uniquely integrates current understanding of oral mucosal structure, immune regulation, and microbiome–macrophage interactions to define how microbial communities shape each phase of oral wound healing and to highlight emerging microbiome-based therapeutic strategies aimed at promoting regenerative repair.
2. The Oral Microenvironment: Structure, Immunity and Microbial Exposure
Antony van Leeuwenhoek, often hailed as the father of microbiology, was the first to visualize and identify oral bacteria [16]. The term oral microbiome collectively denotes the microorganisms inhabiting the human oral cavity. The oral microbiome, encompassing the collective of microorganisms within the oral cavity, constitutes the second largest microbial community in humans, following the gut microbiome. Using a microscope of his own construction, he made groundbreaking observations, discovering both protists and bacteria. In a noteworthy instance in 1674, he experimented with his own dental plaque and documented the presence of “little living animalcules which are prettily moving” [17]. The oral microbiome is a dynamic ecosystem where the core microbiome is present in all individuals, while the variable microbiome is affected by lifestyle and physiological differences, leading to a wide range of protein functions [18]. Within the oral cavity, microorganisms find distinct niches on both hard and soft surfaces which change over time, such as teeth, tongue, cheeks, gingival sulcus, tonsils, and palate [19]. These surfaces, coated with a complex bacterial biofilm, provide a rich environment for microbes to flourish. The oral cavity, along with the associated nasopharyngeal regions, offers an ideal environment for microbial growth. With a stable average temperature of 37 °C, and a consistent pH of 6.5–7 in saliva, which are favorable conditions for many bacterial species, the oral environment supports microbial survival, hydration, and nutrient transportation. In this intricate ecosystem, the oral microbiome plays a crucial role, contributing to both oral and systemic health [20].
The oral microbiome is made up of a core group of microorganisms that are shaped by factors such as diet, hygiene, immunity, medications, and overall health, which are common among individuals but can also vary. [20]. Disruption in the microbial balance that leads to diseases is termed dysbiosis. Pathogens such as Porphyromonas ginigivalis, Fusobacterium nucleatum and Candida albicans have been implicated in periodontitis, endodontic infections and failed root canal treatments [21].
Beyond bacteria, the microbiome includes a viral community (the virome) that actively shapes host immunity. Predominantly composed of bacteriophages, the virome regulates microbial ecology through lytic and lysogenic cycles, horizontal gene transfer, and modulation of bacterial virulence and biofilm dynamics. Virome dysbiosis can directly influence inflammatory signaling and immune activation, indicating that dysbiosis-driven inflammation cannot be fully explained by bacterial communities alone. This perspective expands the mechanistic framework beyond bacteriocentric models [22].
Viruses like human cytomegalovirus [23], herpes simplex virus [24], Epstein–Barr virus [25], human papilloma virus and human herpesvirus [26] are associated with oral pathology. Structurally the oral mucosa comprises stratified squamous epithelium, basal lamina, lamina propria and sometimes submucosa [27]. It has a unique environment unlike the skin, both anatomically and immunologically, that lacks appendages such as hair follicles and sebaceous glands, limiting stem cell reservoirs [28]. The gingiva and hard palate are keratinized due to mechanical stress, whereas other regions remain non-keratinized [29].
Skin-resident immune cells, comprising both myeloid and lymphoid lineages, are essential for maintaining tissue homeostasis and orchestrating immune responses [22]. Langerhans cells in the epidermis and dermal dendritic cells within the papillary dermis serve as pivotal antigen-presenting cells [23]. Macrophages and monocyte-derived macrophages are part of the innate immune system in the dermis that mediate phagocytosis and secrete cytokines and growth factors critical for tissue repair and remodeling [24]. Mast cells and eosinophils contribute to allergic inflammation and parasitic defense, whereas neutrophils rapidly infiltrate sites of infection or injury to eliminate pathogens and facilitate angiogenesis [25]. Humoral immunity, including T lymphocyte subsets such as CD4+, CD8+, and γδ T cells, modulates cutaneous immune responses and promotes wound healing [26]. B cells primarily mediate antibody production and regulate immune homeostasis within the skin microenvironment [27]. Differences in reduced neutrophil and macrophage infiltration in oral wounds, compared to cutaneous wounds, are associated with reduced expression of pro-fibrotic cytokines like TGF-1, which, in part, explains the lack of scarring after oral injury [24]. Immune homeostasis and tissue integrity within the oral cavity are maintained by commensal organisms like Streptococcus salivarius and Actinomyces naeslundii; however, pathogenic microbes may disrupt this balance through the secretion of virulence factors like lipopolysaccharide (LPS), outer membrane vesicles (OMV) and peptidoglycans (PG) that influence macrophage polarization [28].
Macrophages are differentiated from monocytes during the inflammatory phase of wound healing based on the signals from antigen-presenting cells and cytokines such as IL-1β and TNF-α produced from neutrophils and tissue-resident macrophages [30]. Macrophages can polarize into pro-inflammatory M1 or anti-inflammatory M2 phenotypes based on the environmental stimuli and will secrete pro-inflammatory or pro-regenerative cytokines, respectively [31]. The oral microbiome can modulate the polarization of macrophages to an inflammatory M1 phenotype in a dysbiotic environment and a pro-regenerative M2 phenotype in a eubiotic state, thereby impacting the wound outcomes, either by promoting resolution of inflammation or contributing to chronic inflammation [32].
3. The Impact of Oral Microbiome on Wound Healing Phases
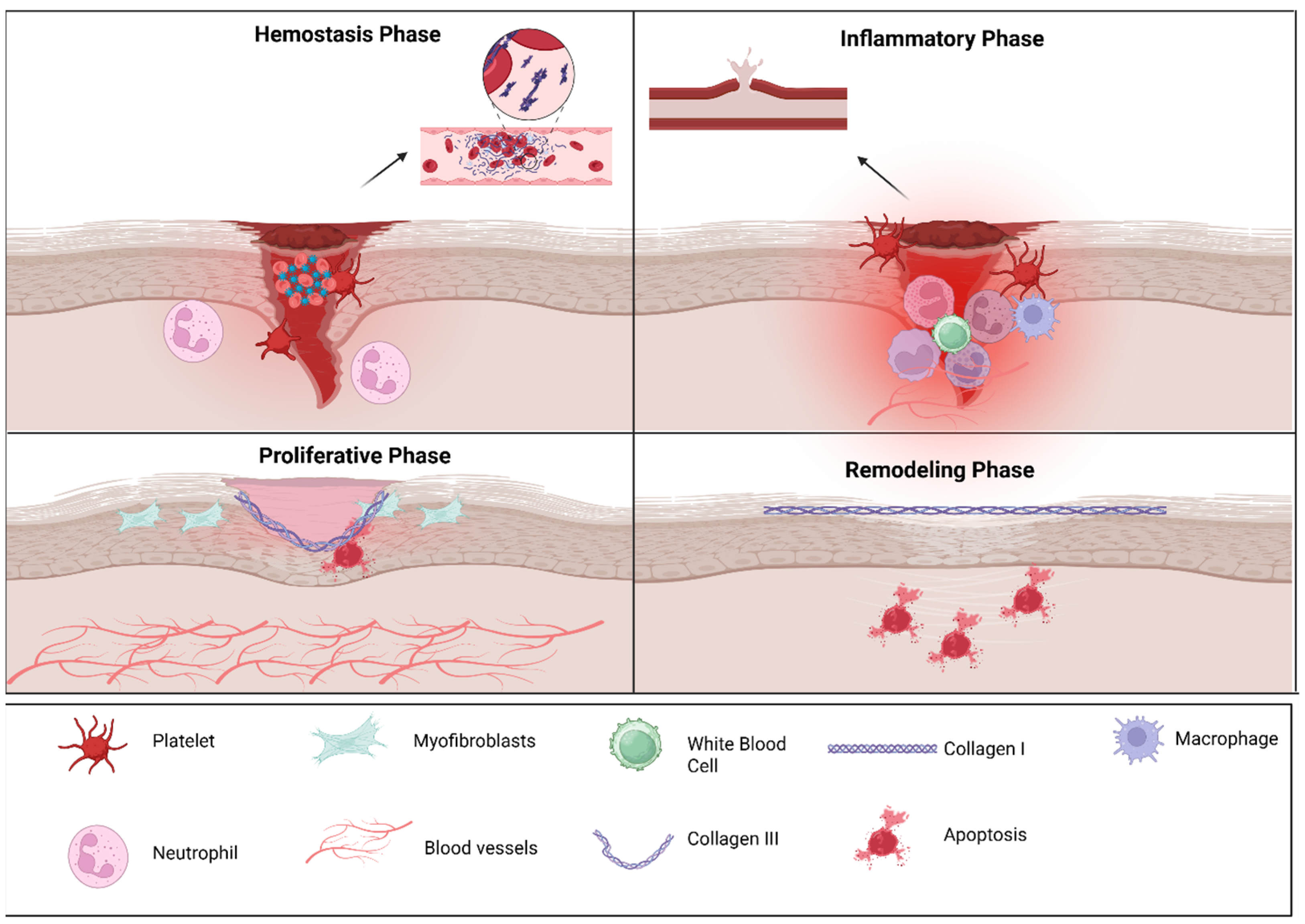
Healing of the oral mucosa occurs in four distinct stages—hemostasis, inflammation, proliferation and remodeling—driven by coordinated actions of immune cells, particularly macrophages, which help in the transition between pro-inflammatory and pro-repair phenotypes, and the molecular signals shaped by the microbiome (Figure 1) [33].
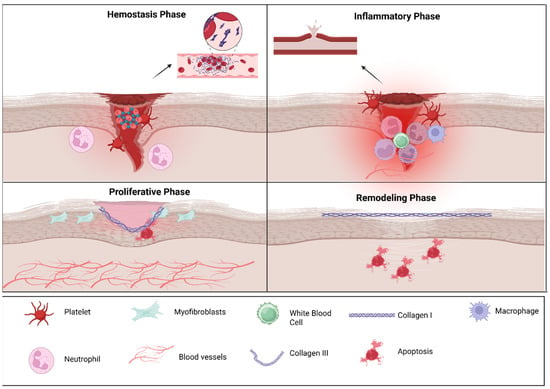
Figure 1.
Cellular players of oral wound healing: The figure illustrates the four principal phases of wound repair. During hemostasis, platelet aggregation and clot formation occur to rapidly control bleeding. In the inflammatory phase, infiltrating neutrophils and macrophages clear debris and pathogens while releasing cytokines that guide subsequent repair. The proliferative phase is characterized by myofibroblast activity, extracellular matrix deposition, angiogenesis, and re-epithelialization to restore tissue continuity. In the remodeling phase, collagen III is gradually replaced by collagen I, and extracellular matrix reorganization enhances tensile strength as excess cells undergo apoptosis. Key cellular and structural components involved in each phase are indicated in the legend. The above figure was created with BioRender.com. Goudy, S. (2026) https://BioRender.com/hgymzt8.
3.1. Hemostatic Phase
Immediately following tissue injury, vascular disruption initiates the hemostatic clotting cascade to prevent blood loss and create a matrix for cellular infiltration. Platelet activation leads to fibrin clot formation and release of vasoactive agents, cytokines such as IL-1β and TNF-α, and growth factors such as PDGF and TGFβ that recruit immune cells and contribute to ECM remodeling (Figure 1, Table 1) [5]. The release of these cytokines from platelets following wounding incites a local and systemic inflammatory response that induces inflammatory cell migration of neutrophils and macrophages into the wound bed. The fibrin clot acts as a scaffold for the neutrophils and macrophages to migrate into the wound and initiate wound repair. The fibrin matrix is rapidly stabilized by interaction with salivary components like proline-rich proteins, statherin, and histatins that facilitate cell adhesion and early epithelial migration [34]. Saliva also contributes antimicrobial peptides like defensins, cathelicidins, and histatins and enzymes that control bacterial colonization [35]. Ultimately the release of anti-inflammatory mediators such as lipoxins, resolvins, and protectins helps resolve inflammation and prevent excessive clotting and tissue damage [36]. Saliva contains tissue factor and histatins that quickly promote platelet aggregation and the formation of fibrin, operating faster than the coagulation process in the skin. Chewing increases saliva production to help clear away debris while the biofilm helps secure the clot against early microbial attacks [1].

Table 1.
Pro-inflammatory and anti-inflammatory mediators in oral wound healing.
Table 1.
Pro-inflammatory and anti-inflammatory mediators in oral wound healing.
| Wound Healing Phase | Description |
Role of Inflammatory
Markers | Role of Anti-Inflammatory Markers | References |
|---|---|---|---|---|
| Hemostasis phase | Initial response to injury involving blood clot formation. | Platelets release cytokines (e.g., IL-1β, TNF-α) and growth factors (e.g., PDGF, TGF-β) to initiate inflammation and recruit immune cells to the wound site. | Anti-inflammatory mediators such as lipoxins, resolvins, and protectins help resolve inflammation and prevent excessive clotting and tissue damage. | [37,38,39] |
| Inflammatory phase | Characterized by inflammation and removal of debris. | Neutrophils and macrophages release pro-inflammatory cytokines (e.g., IL-6, IL-8, TNF-α) to eliminate pathogens and cellular debris and stimulate angiogenesis and fibroblast proliferation. | Interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) help suppress inflammation, regulate immune responses, and promote tissue repair. | [40,41,42] |
| Proliferative phase | Formation of new tissue by fibroblasts and endothelial cells. | Fibroblasts produce collagen and extracellular matrix components, while endothelial cells promote angiogenesis and blood vessel formation. | Anti-inflammatory cytokines like IL-4 and IL-13 help modulate the inflammatory response and facilitate tissue remodeling and wound closure. | [43,44,45] |
| Remodeling phase | Maturation and remodeling of the newly formed tissue. | Matrix metalloproteinases (MMPs) produced by fibroblasts and macrophages degrade excess collagen and promote tissue remodeling. | Tissue inhibitors of metalloproteinases (TIMPs) control MMP activity, prevent excessive tissue degradation, and promote tissue maturation and strength. | [46,47,48] |
3.2. Inflammatory Phase
The inflammatory phase begins within hours post-injury in response to the cytokines released during the hemostatic phase (IL-1β, TNF-α, etc.) and peaks between 24 and 72 h. Neutrophils are the first response, tasked with clearing microbial and cellular debris through the release of reactive oxygen species (ROS), proteolytic enzymes, and neutrophil extracellular traps (NET) [49]. Neutrophils produce CCL2, a chemokine responsible for monocyte recruitment to inflamed tissue [50], and macrophages are also indirectly attracted by cytokines such as IL-1β and TNF-α [51]. GM-CSF [52] also plays a role to attract systemic monocytes, which gradually replace the neutrophils and differentiate into macrophages at the wound site [53]. The impact of the microbiome on neutrophil response is that eubiosis supports a balanced neutrophil response while dysbiosis can lead to an overactive or dysfunctional neutrophil response, hindering healing and prolonging the inflammatory phase [54].
Macrophages are the principal orchestrators of the inflammatory phase of wound healing, initially polarizing monocytes towards a classically activated M1 phenotype in response to pathogen-associated molecular patterns (PAMPs) that are found in bacteria (LPS) and by pro-inflammatory cytokines like IFN-γ and TNF-α. M1 macrophages release IL-1β, IL-6, TNF-α and inducible nitric oxide (iNOS) [55]. The secretion of these pro-inflammatory cytokines is crucial for microbial clearance and activation of downstream immune responses. Pro-inflammatory cytokines can also enhance vascular permeability and recruit neutrophils, monocytes and Th17 cells. The acute phase response of inflammatory wound healing is promoted by B cell differentiation and by the production of IL-6. Persistent inflammation leads to the excessive production of inflammatory cytokines, increasing the production of ROS, which leads to tissue damage and is perpetuated by the continuous recruitment and activation of neutrophils and M1 macrophage cells. Moreover, chronic stimulation of fibroblasts and endothelial cells under the influence of M1-derived cytokines results in the formation of persistent granulation tissue by neovascularization and collagen deposition, which lacks the maturation of reparative tissue. The inhibition of the shift to reparative M2 from M1 impairs wound healing, resulting in fibrosis and the formation of a chronic, non-healing wound [56]. The lack of interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) secretion by M2 macrophages allows unchecked inflammation, an ongoing immune response, and failure of tissue repair.
The beneficial microbes, such as Streptococcus salivarius and Actinomyces naeslundii, in a state of eubiosis help suppress excessive inflammation. The activity of these two bacterial strains during oral wound healing enhances epithelial barrier integrity and stimulates signaling pathways that favor M2 polarization. Together, these bacterial strains contribute to the resolution of the inflammatory phase of wound healing and begin the proliferative phase with rapid wound closure and minimal fibrosis [57]. However, dysbiosis marked by pathogenic species such as Porphyromonas gingivalis and Fusobacterium nucleatum sustains M1 activation through persistent stimulation by virulence factors including LPS and outer membrane vesicles [58]. The presence of LPS and OMV in the wound healing environment engages toll-like receptors (TLRs) on immune cells, which prevents transition to the M2 macrophage phenotype and prolongs the M1 macrophage phenotype, leading to unresolved inflammation and contributing to impaired healing or chronic wounds [59]. The persistent M1 phase of macrophages, associated with dysbiosis and inflammatory response to LPS and OMVs, leads to chronic inflammation, which is demonstrated by the continued production of inflammatory cytokines IFN-γ, TNF-α, IL-1β, IL-6, TNF-α and inducible nitric oxide (iNOS), all of which contribute to persistent inflammation [60].
The state of oral eubiosis, characterized by the presence of beneficial microbes such as Streptococcus salivarius and Actinomyces naeslundii, plays a crucial role in resolving inflammation and promoting tissue repair [57,61]. These commensals help suppress excessive immune responses, enhance epithelial barrier integrity, and activate signaling pathways that favor M2 macrophage polarization. This shift from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype facilitates the resolution of the inflammatory phase and the initiation of the proliferative phase of wound healing, resulting in rapid wound closure with minimal fibrosis [57]. Additionally, microbial metabolites such as short-chain fatty acids (SCFAs) and polysaccharide A from Bacteroides fragilis contribute to this effect by dampening TLR signaling, reducing NF-κB activation, and promoting regulatory immune responses conducive to M2 polarization [62].
In contrast, dysbiosis, marked by an overrepresentation of pathogenic species like Porphyromonas gingivalis, Fusobacterium nucleatum, and Escherichia coli, sustains M1 macrophage activation through persistent exposure to virulence factors such as LPS and OMV [58]. These microbial products stimulate toll-like receptors (especially TLR4) on immune cells, reinforcing NF-κB and STAT1 signaling [63]. This prolongs the M1 phase, characterized by the continued release of pro-inflammatory cytokines like IL-1β, TNF-α, and inducible nitric oxide synthase (iNOS)-derived nitric oxide [59]. As a result, the inflammatory phase is abnormally extended, impairing the transition to the M2 phenotype and leading to chronic inflammation, excessive tissue damage, and a failure to progress to the proliferative phase. This sustained M1 activity disrupts granulation tissue remodeling, promoting fibrosis and the development of chronic, non-healing wounds [64]. Salivary antimicrobial agents such as defensins and IL-10 cytokines rapidly manage neutrophil infiltration, in contrast to the prolonged pro-inflammatory period seen in skin. The act of chewing physically primes the immune system for prompt elimination, as biofilm encourages the modulation of commensals rather than fostering dysbiosis [2].
3.3. Proliferative Phase
The proliferative phase of wound healing is indicated by formation of granulation tissue, re-epithelialization, and neovascularization [1]. A key component of the proliferative phase of wound healing occurs post-injury, under the influence of IL-4, IL-10 and IL-13, by alternatively activated M2 phenotype macrophages [65]. Anti-inflammatory cytokines and growth factors produced by M2 macrophages, including TGF-β, VEGF- and IGF-1, promote angiogenesis, fibroblast activation and ECM deposition [66]. During the proliferative phase of wound healing, fibroblasts infiltrate the wound bed and initiate granulation tissue formation through the secretion of type 1 collagen and fibronectin [67]. Endothelial cells form new capillaries, restoring tissue perfusion and supporting epithelial regeneration accelerated by salivary histatins and growth factors [13]. Anti-inflammatory cytokines like IL-4 and IL-13 help modulate the inflammatory response and facilitate tissue remodeling and wound closure [66]. EGF and salivary growth factors promote the rapid migration of keratinocytes and the formation of new blood vessels, allowing oral wounds to heal in days compared to the weeks required for skin wounds. Regular chewing stimulates fibroblast mechanotransduction, helping to control contraction, while biofilm signaling enhances the optimal deposition of the extracellular matrix [2].
The oral environment is colonized by a complex and diverse microbiota that can significantly impact wound healing dynamics along with a constant presence of saliva that contains antimicrobial peptides (defensins, histatins, etc.) [68], enzymes and growth factors (EGF), vascular endothelial growth factor (VEGF), transforming growth factor alpha (TFG-α), transforming growth factor beta (TFG-β), nerve growth factor (NGF), fibroblast growth factor (FGF), and insulin-like growth factor (IGF) [69], which facilitate re-epithelization and angiogenesis, modulating the wound healing dynamics. The proliferative phase is disrupted by dysbiosis, prolonged inflammation, impaired fibroblast function and degrading ECM components [70]. Dysbiosis also modulates macrophage polarization, promoting pro-inflammatory M1 phenotypes, thereby delaying progression to granulation tissue formation and re-epithelization [12].
3.4. Remodeling Phase
Remodeling of oral wounds begins as early as one-week post-injury in soft tissue (e.g., oral mucosal wound) and may extend over several weeks or months in hard tissue (e.g., alveolar bone followed dental extraction) depending on the wound type and size. During this remodeling phase, fibroblasts differentiate into myofibroblasts, primarily driven by transforming growth factor (TGF-β1) signaling through the SMAD pathway and supported by platelet-derived growth factor (PGDF) and cytokines like interlukein-6 (IL-6), enabling wound contraction. Matrix metalloproteinases (MMP) produced by macrophages and fibroblasts degrade excess ECM proteins, which allows tissue architecture to be restored and prevents excessive fibrosis [71,72,73]. Meanwhile the tissue inhibitors of metalloproteinase (TIMPs) prevent excessive degradation of the ECM to maintain tissue homeostasis [74]. Pro-resolving features of a eubiotic oral microbiome reduce inflammation and fibrosis and promote the restoration of mucosal architecture by promoting the activity of M2 macrophages [75]. Saliva balances MMPs and TGF-β to create structured collagen alignment without fibrosis, unlike scars in the skin. Masticatory pressure enhances tensile strength adaptively, while consistent biofilms maintain vascular stability [2].
In oral tissues, remodeling is typically faster and results in less scarring than in cutaneous skin due to multiple factors including a lower fibrotic response and reduced production of TGF-β1 [76]. However, in the presence of dysbiosis and chronic inflammation, the proliferative phase may be disrupted by sustained pro-inflammatory signals, impaired macrophage polarization, and dysregulated TGF-β1 [77]. The repeated tissue injury from chronic inflammation and impaired barrier function leads to delayed healing, chronic inflammation or tissue breakdown, as observed in periodontitis and oral mucosal ulcers, and ultimately a fibrotic unhealed oral wound [78].
The following table summarizes the four major phases of wound healing hemostasis, inflammation, proliferation, and remodeling. It highlights the biological processes occurring in each phase and the contributions of key inflammatory and anti-inflammatory mediators involved in regulating tissue repair and restoration.
4. Eubiosis and Dysbiosis vs. Probiotics in Skin, Gut and Oral Habitat
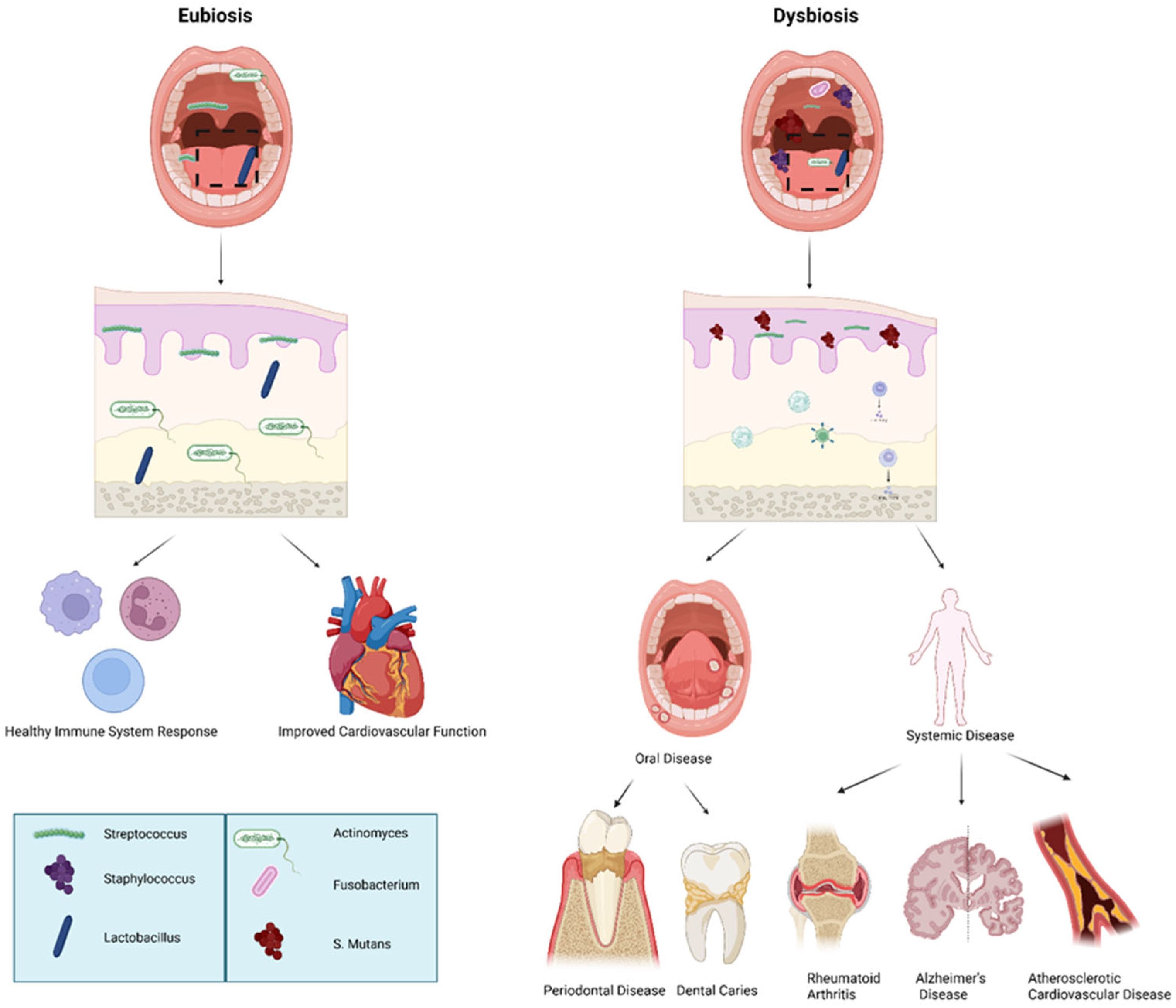
Eubiosis is the condition in which the host and its microbiota maintain a healthy equilibrium, characterized by microbial diversity and interactions that promote tissue homeostasis and immune regulation [79]. A dysregulation or misbalance in the host’s healthy microbiota is termed as dysbiosis, which often occurs in a range of conditions beginning in the oral cavity, including oral diseases such as periodontitis and dental caries; gastrointestinal disorders such as Clostridium difficile colitis, antibiotic-associated diarrhea, irritable bowel syndrome (IBS), and inflammatory bowel diseases (e.g., Crohn’s disease and ulcerative colitis); and systemic diseases including metabolic disorders (e.g., obesity and type 2 diabetes), allergies, and atopic dermatitis [80]. Probiotics are the beneficial bacteria that are used to maintain and/or restore eubiosis (Figure 2, Table 2) [81].
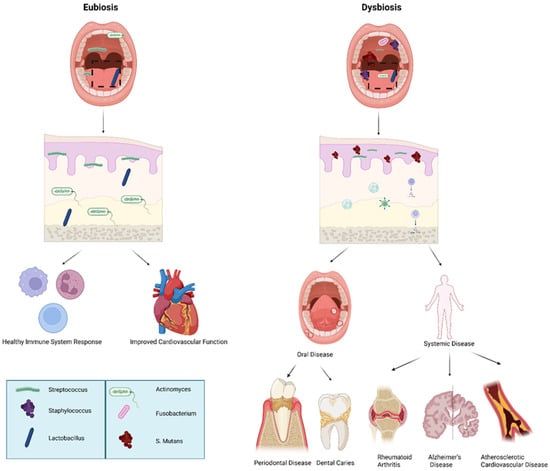
Figure 2.
Eubiotic vs. dysbiotic microbiome in human health and oral disease. Overview of oral microbiome balance (eubiosis) versus imbalance (dysbiosis) and associated systemic outcomes: Eubiosis is characterized by a predominance of beneficial commensal bacteria such as Streptococcus, Staphylococcus, and Lactobacillus, supporting mucosal integrity, balanced immune signaling, and improved cardiovascular health. In contrast, dysbiosis features increased pathogenic microorganisms including Fusobacterium, Actinomyces, and Streptococcus mutans, leading to oral disease (periodontal disease, dental caries) and contributing to systemic conditions such as rheumatoid arthritis, Alzheimer’s disease, and atherosclerotic cardiovascular disease. The above figure was created with BioRender.com. Goudy, S. (2026) https://BioRender.com/1o6wsje.

Table 2.
Comparison of microbiota in distinct ecology in eubiosis and dysbiosis: Comparison of microbiota composition under eubiosis versus dysbiosis across major body sites. Representative probiotic or beneficial taxa associated with eubiosis are listed alongside pathogenic or dysbiosis-associated microorganisms. Reference numbers correspond to supporting studies in the text.
Table 2.
Comparison of microbiota in distinct ecology in eubiosis and dysbiosis: Comparison of microbiota composition under eubiosis versus dysbiosis across major body sites. Representative probiotic or beneficial taxa associated with eubiosis are listed alongside pathogenic or dysbiosis-associated microorganisms. Reference numbers correspond to supporting studies in the text.
| Conditions | Eubiosis vs. Probiotic | Dysbiosis vs. Pathogenic |
|---|---|---|
| Gut/intestinal microbiota | Bacteroides fragilis [82] Faecalibacterium prausnitzii [83] Bifidobacterium breve [84] Lactobacillus rhamnosus GG [85], Bifidobacterium longum [86,87] | Clostridium difficile [88], Escherichia coli [89], Enterococcus faecalis [90] |
| Oral microbiota | Streptococcus salivarius [91], Actinomyces naeslundii, Streptococcus salivarius K12 [92], Lactobacillus reuteri [93] | Streptococcus mutans [94], Porphyromonas gingivalis [95], Fusobacterium nucleatum [96] |
| Skin microbiota | Staphylococcus epidermidis [97], Cutibacterium acnes [98], Corynebacterium accolens [99], Lactobacillus plantarum [100], Lactobacillus rhamnosus GG [101] | Staphylococcus aureus [102], Malassezia spp. [103] |
| Vaginal microbiota | Lactobacillus crispatus [104], Lactobacillus iners [105], Lactobacillus gasseri [106], Lactobacillus rhamnosus GR-1 [107], Lactobacillus reuteri RC-14 [108] | Gardnerella vaginalis (BV) [109], Candida albicans (yeast infection) [110], Atopobium [110] |
5. Macrophage Polarization in Oral Wound Healing
Macrophages are highly plastic innate immune cells capable of shifting their phenotypic and functional profiles in response to environmental cues. This dynamic transition, known as macrophage polarization, is guided by a variety of cytokines, surface markers and receptors [111]. In the context of oral wound healing, macrophages exhibit phase-specific roles—initially contributing to pathogen clearance and inflammation via the M1 phenotype and later facilitating tissue regeneration and resolution through the M2 phenotype [112].
5.1. M1 and M2 Phenotypes and Function
During the inflammatory phase of wound healing, macrophages adopt a classically activated M1 phenotype in response to microbial ligands (e.g., LPS, flagellin) and pro-inflammatory cytokines (e.g., IFN-γ) [113]. M1 macrophages are characterized by CD86, MHC class II, CCR7, CX3CR1 and inducible nitric oxide synthase (iNOS) [114]. M1 macrophages secrete high levels of TNF-α, IL-β, IL-6, IL-12 and ROS, which collectively contribute to debris clearance and pathogen elimination. However, sustained M1 activation, especially in dysbiotic oral environments, can contribute to chronic inflammation and impaired healing [115].
As wound healing transitions into the proliferative phase, macrophages begin to polarize from M1 to M2 phenotypes. This is stimulated by cytokines such as IL-4, IL-10, IL-13 and TGF-β. M2 macrophages are marked by expression of CD68, CD163, CD206, PD-L1 and PD-L2, with constitutive CSFIR expression playing a supportive role [116]. These cells promote an anti-inflammatory microenvironment by secreting cytokines and growth factors like IL-10, TGF-β, VEGF and IGF-1, which drives fibroblast activation, angiogenesis, extracellular matrix deposition and tissue regeneration [117].
In the remodeling phase, the high expression of CD206 and CD163 supports proper tissue structure restoration. PD-L1 and PD-L2 further help maintain tissue homeostasis [118]. M2 macrophages thus play an essential role in scar resolution and epithelial regeneration [119].
Importantly, macrophage polarization exists on a functional spectrum rather than as a binary switch. Mixed M1/M2 profiles are commonly observed in chronic oral wounds and biofilm-associated infections, illustrating the complexity of immune–microbe interactions in the oral microenvironment [120].
Oral wound healing involves a finely tuned immunological response orchestrated by macrophage plasticity and phenotypic shifts in response to dynamic cues such as cytokines, microbial factors and mechanical stress seen in oral wound healing [121]. Disruption in this balance, particularly prolonged M1 activation or insufficient M2 transition, can lead to chronic inflammation and delayed wound healing [64].
5.2. Microbial Influence on Macrophage Polarization
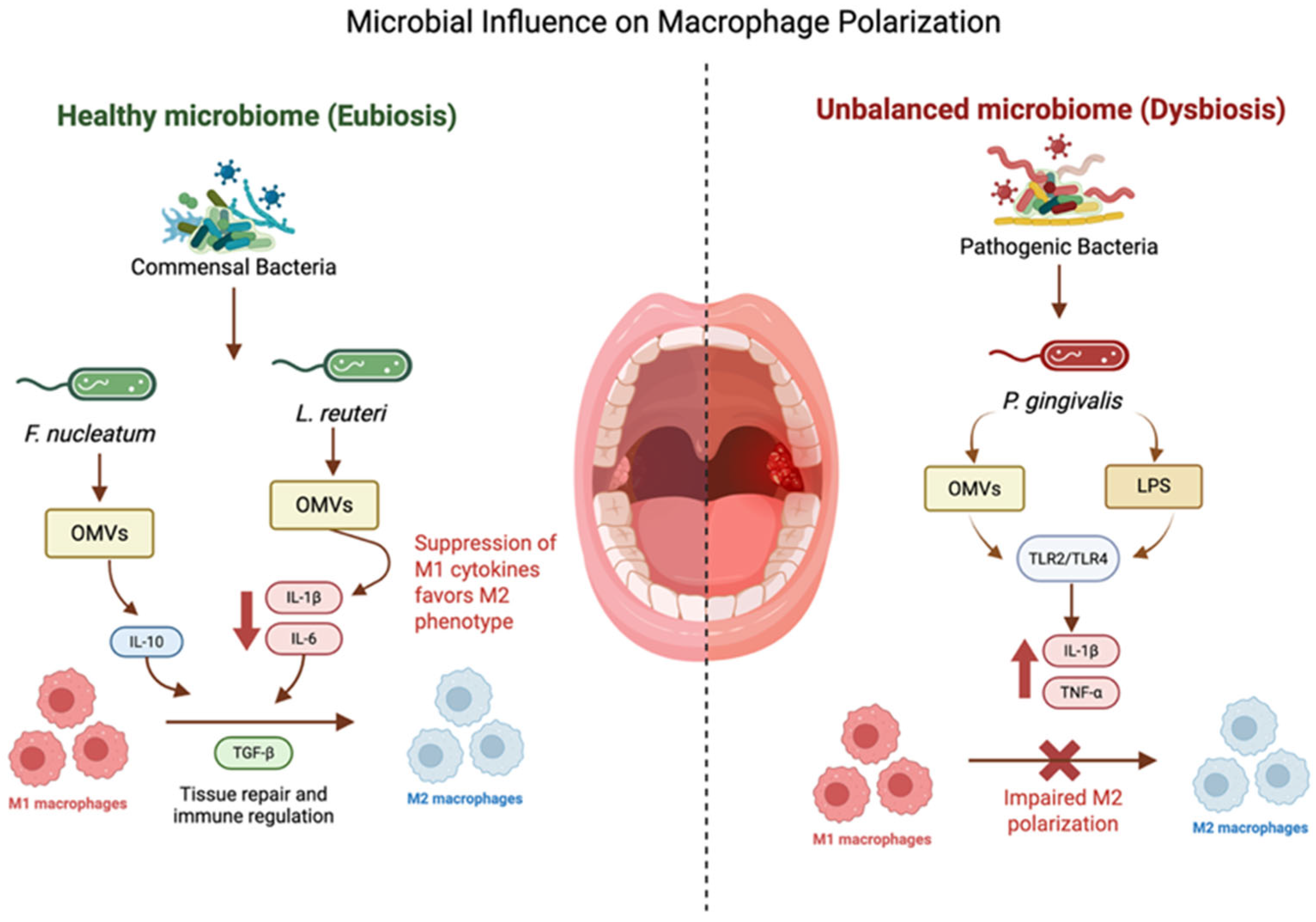
Microbial products are key regulators of macrophage function in oral tissues. Commensal and probiotic bacteria can promote M2 polarization and wound resolution, whereas pathogenic species often sustain or exacerbate M1-driven inflammation. Examples of the bacterial impact on macrophage phenotypes are well described [122] (Figure 3). These interactions suggest that targeted modulation of the microbiome may shape macrophage responses and redirect wound trajectories towards resolution rather than chronicity Table 5.
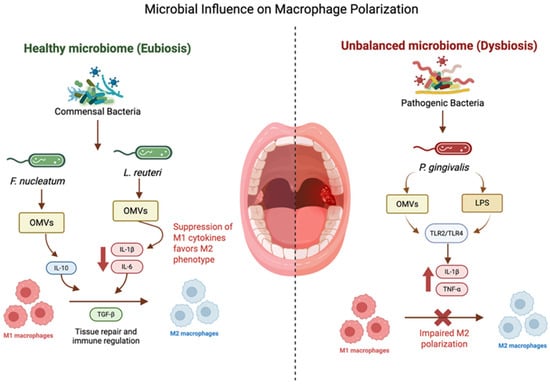
Figure 3.
Microbial influence on macrophage polarization: bacterial OMVs modulate macrophage polarization: F. nucleatum promotes M2-like traits, P. gingivalis induces inflammatory M1 responses and pyroptosis, and L. reuteri shifts macrophages toward M2 and enhances phagocytosis. The above figure was created with BioRender.com. Goudy, S. (2026) https://BioRender.com/ulcvvbs.
6. Microbiome-Based Therapeutics for Modulation of Wound Healing
The microbiome plays a dual role in oral wound healing, capable of supporting immune homeostasis or promoting chronic inflammation depending on its composition [123]. Increasing evidence suggests that therapeutic modulation of microbiomes, particularly through the delivery of probiotics or microbial metabolites, can influence macrophage polarization and improve wound healing outcomes [4]. Insights from immune-targeted microbiome therapies in chronic inflammatory diseases further support the feasibility of translating these approaches to wound healing contexts, where similar immunoregulatory mechanisms are operative [124]. Here, we review preclinical and clinical strategies that leverage microbial interventions across oral and systemic wound models.
Although preclinical results are encouraging, there are notable challenges in translating microbiome therapeutics into clinical practice. The stability of strains is threatened by factors such as salivary flow, fluctuations in pH, and mechanical disruption caused by chewing, which affect the viability of probiotics. The ability of these external strains to persist in colonization is generally low, as they face strong competition from established oral biofilms. There are safety issues to consider, including the risk of opportunistic infections and the transfer of antibiotic resistance genes in susceptible patients, while regulatory hurdles require thorough strain characterization and clinically relevant endpoints that are not yet established for applications related to oral wounds [56].
6.1. Probiotic-Based Therapeutics in Oral and Cutaneous Wounds
Probiotics like Lactobacillus reuteri, Bifidobacterium breve and Streptococcus salivarius have been explored in various wound contexts, with promising immunomodulatory effects [124]. These bacteria modulate local inflammation, influence cytokine profiles and promote a shift towards reparative M2 macrophage phenotypes [64] Table 4.
An advanced delivery system is needed to improve localization, preserve viability and ensure controlled therapeutic activity in vivo, particularly for mammalian cells, genetically engineered microbes, viruses, etc. [125]. Depending on the target tissue and desired therapeutic outcome, these agents can be administered through oral, topical or local application, intravenous and intratumoral injection, and surgical implantation using biomaterial-based scaffolds; among other delivery platforms, hydrogels typically composed of natural polymers such as alginate, collagen and chitosan or synthetic polymers such as polyethylene glycol (PEG) are the most extensively explored carriers. These carriers enable encapsulation, prolonged local retention, protection from antagonistic environments, and sustained release of living therapeutics [126]. In addition, direct delivery of living cells and bacteria including stem cells, CAR-T cells, dendritic cells and engineered microbes into target tissues with enhanced spatial control and retention is performed by minimally invasive biomaterial-based microneedles [127]. Supplemental approaches such as conjugation of aptamers, antibodies and other affinity ligands to the surface of bacteria and mammalian cells have been developed to improve precision and minimize off-target effects [128].
6.2. Mechanisms of Microbial Modulation: Cytokine Signaling and Receptor Engagement
Beneficial bacteria modulate macrophage function through a variety of secretory products:
- Outer membrane vesicles (OMVs): OMVs from F. nucleatum and P. gingivalis influence TLR2/TLR4 signaling and activation of inflammation, with downstream effects on IL-1β, IL-6, TNF-α, and IFN-β production [129].
- Lipoteichoic acids and peptidoglycans: structural components of Gram-positive bacteria such as S. mutans and A. naeslundii stimulate release of pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6 in macrophages [130].
- Lantibiotics (e.g., salivaricins): produced by S. salivarius K12, these peptides inhibit NF-κB activation and reduce IL-8 and IL-6 signaling, promoting a homeostatic environment [131].
- Metabolites (e.g., reuterin): produced by L. reuteri, reuterin exhibits anti-inflammatory properties by neutralizing LPS and suppressing macrophage polarization toward the M1 state [132].
6.3. Probiotic-Mediated M2 Polarization—A Therapeutic Opportunity
In vivo and in vitro, studies consistently demonstrate that select probiotic strains enhance the M2 polarization of macrophages, marked by increased CD163/CD206 expression and IL-10/VEGF/TGF-β production [75]. These immunological shifts are accompanied by improved re-epithelization, enhanced vascularization, reduced inflammatory infiltrate, and suppressed M1-associated cytokines [12]. These findings have important implications for translational wound care, particularly in managing oral wounds with chronic inflammation or microbial dysbiosis, including diabetic oral ulcers, periodontitis and postsurgical effects [13].
The active process whereby macrophages change the phenotype guided by various cytokines, cell surface markers and receptors in a plastic manner is referred to as polarization. M1 macrophages are characterized by the expression of CD86 [133], MHC class II [133], CCR7 [134], and CX3CR1 [135]. In the initial inflammatory phase, markers like TNF-α, IL-1β, IL-6, and IL-12 [136] signal to M1 macrophages, which are involved in the process of clearing debris and pathogens. In the proliferative phase, markers like PD-L1 [137], PD-L2 [138], and constant CSFIR expression promote an anti-inflammatory and repairing mechanism with M2 macrophage polarization.
Reduced inflammation and enhanced fibroblast activity are involved in extracellular matrix deposition and promote angiogenesis, facilitated by M2 macrophages that are characterized mainly by surface markers such as CD68 [139], CD163 [140], and CD206 [141]. Cytokines like IL-10 and growth factors like TGF-β and VEGF are produced by M2 macrophages and are involved in tissue regeneration, angiogenesis, and fibroblast activation in the proliferative stage [142]. High expression of CD206 and CD163 for ensuring proper tissue structure (especially in the remodeling phase [143] and additionally PD-L1 and PD-L2 are involved in homeostasis during this phase [144].
Oral wound healing is orchestrated by specific macrophage phenotypes and cytokines, and the phases involved are inflammatory, proliferative and remodeling. Prolonged or delayed wound healing is termed as chronic phase or chronic inflammation phase [145,146] Table 3.

Table 3.
Role of macrophages, cytokines, and phases of healing in various oral wound conditions.
Table 3.
Role of macrophages, cytokines, and phases of healing in various oral wound conditions.
| Phenotype | Cytokines/ Chemokines/Growth Factor | Role in Oral Wound Healing | Phases | Oral Wound Conditions | References |
|---|---|---|---|---|---|
| M1 | IL-1β, IL-6, TNF-α, IFN-γ, ROS | Initiate the inflammatory response, clear pathogens, and recruit immune cells to the injury site. | Inflammatory Phase | Acute Oral Wounds (e.g., dental extraction, minor trauma) | [117,147] |
| M2 | IL-10, TGF-β, VEGF, IGF-1 | Suppress inflammation, promote angiogenesis, and stimulate fibroblast activity and extracellular matrix deposition. | Proliferative Phase | Chronic Periodontal Wounds (e.g., periodontitis) | [148] |
| M2 | M2b-like (Immunoregulatory) IL-10, IL-1RA, TGF-β | Modulate immune responses, balancing pro- and anti-inflammatory signals. | Transitional Phase | Diabetic Oral Wounds (delayed healing) | [149] |
| M2 | TGF-β, IL-10, PDGF, VEGF | Facilitate tissue remodeling, collagen deposition, and scar formation. | Remodeling Phase | Oral Mucosal Wounds (e.g., ulcers, post-surgery) | [150] |
| M1 | TNF-α, IL-1β, IL-6 (low IL-10) | Persistent inflammation, chronic wounds, and impaired healing. | Chronic Wound Phase | Chronic Periodontitis | [151] |
| Mixed M1/M2 Phenotypes | IL-6, IL-10, VEGF, TGF-β, IFN-γ | Adapt to saliva, oral microbiota, and mechanical stress for tissue-specific repair. | Oral Wound Healing | Oral Wounds in Immunocompromised Patients | [152] |
| M1- | IL-1β, TNF-α, IL-6, IL-17 | Delayed resolution of inflammation, impairing wound healing. | Chronic Inflammation | Oral Wounds in Smoking (e.g., impaired healing) | [153] |
| Mixed M1/M2 | IL-1β, TNF-α, IL-6, IL-10 | Biofilm-associated wounds trigger prolonged inflammation and microbial persistence. | Inflammatory/Chronic | Dental Implants and Peri-implantitis | [154] |
| Tumor-associated Macrophages (TAMs) | TGF-β, IL-10, VEGF, IL-6 | Promote tumor invasion and immunosuppression but can support tissue healing in surgical resection wounds. | Variable | Oral Squamous Cell Carcinoma (OSCC) Wounds | [155] |
This table highlights the different types of macrophages (M1, M2) and their associated cytokines, specific functions, and roles across different phases of wound healing in various oral conditions, including acute wounds, chronic periodontal wounds, diabetic oral wounds, and biofilm-related infections.

Table 4.
Microbiome-based interventions in wound healing: use of microbiome interventions for wound healing, detailing their application methods, wound models, impact on macrophages, and overall effects on healing outcomes. N/A—Not applicable.
Table 4.
Microbiome-based interventions in wound healing: use of microbiome interventions for wound healing, detailing their application methods, wound models, impact on macrophages, and overall effects on healing outcomes. N/A—Not applicable.
| Source | Microbiome Intervention | Application | Wound Healing Model | Impact on Macrophages | Effect |
|---|---|---|---|---|---|
| Wälivaara D.Å 2019 (Clinical Trial) [156] | Lactobacillus reuteri-containing lozenges | Oral | Surgical extraction of impacted mandibular third molars | N/A | No significant influence of probiotics on wound healing mechanism. Did observe reduced swelling and pain |
| Li 2023 [157] | Bifidobacterium breve | Oral | C57BL/6N mice hard palate mucosal defect model | N/A | Therapeutic potential of Bifidobacterium breve on oral mucosal wound healing after impairment caused by Sunitinib. Promoted wound healing by intestinal dendritic cell-derived IL-10 |
| Han 2020 [158] | L. reuteri | Inoculated in the wound | Palate mucosal model on C57BL/6 mice | N/A | Reuterin in L. reuteri neutralized the LPS in L. gingivalis and inhibiting inflammation while promoting wound healing |
| Xu 2024 [159] | Lactobacillus paracasei TYM202 | Injection | Full-thickness skin injury on Sprague Dawley rats | Found higher levels of M2 macrophages | The probiotic hydrogel encapsulated with L. paracasei TYM202 (HAEPS@L.sei gel) promotes wound healing by reducing inflammation and promoting angiogenesis and increasing collagen deposition |
| Li 2024 [160] | Gluconacetobacter (formerly Acetobacter), Agrobacterium, and Aerobacter | Skin wounds on the back of Sprague Dawley rats | N/A | Bacterial cellulose hydrogel (Ag-BCN) and carbon nanodots with resveratrol reduced wound healing time and ROS and promoted wound healing | |
| Mohtashami 2021 [161] | Lactobacillus bulgaricus and Lactobacillus plantarum | Topical | Diabetic cutaneous wounds in Wistar rats | Promoted the proliferation of macrophages | The bacteria modulated the immune response and accelerated the wound healing process |
| Evelina Vågesjö 2018 [162] | Lactobacilli with a plasmid encoding CXCL12 | Topical | Full-thickness wound on the hind limb of C57BL/6 mice | Promoted proliferation of TGF-β+ macrophages | Accelerated wound closure |
| Emelie Öhnstedt 2023 [163] (human clinical trial) | Engineered Limosilactobacillus reuteri R2LC to produce CXCL12-a (ILP100-Topical) | Topical | Full-thickness wound punching on the arm | N/A | Overall safe. Multiple doses resulted in larger amount of healed wounds |

Table 5.
Bacterial influence on macrophage phenotype and inflammatory responses: The effects of various bacterial species and their secretory products on macrophage phenotype and immune signaling. This includes details on cytokine modulation, inflammasome activation, and macrophage polarization (M1/M2).
Table 5.
Bacterial influence on macrophage phenotype and inflammatory responses: The effects of various bacterial species and their secretory products on macrophage phenotype and immune signaling. This includes details on cytokine modulation, inflammasome activation, and macrophage polarization (M1/M2).
| Oral Microbiota Bacteria | Product/Secretory Factors | Expected Effects with Reference Links |
|---|---|---|
| Streptococcus salivarius | --- | Inhibits NF-KB activation and IL-8 secretion (both use HT-29 primary tumor epithelial cells) |
| --- | Increases IL-6 and TNF-α expression (mentions “infected oral epithelial cells also expressed relatively high levels of the chemo attractive cytokine IL-8”) | |
| Actinomyces naeslundii | Peptidoglycan purified | Increases IL-1β, IL-6, and TNF-α gene expression in mouse peritoneal macrophages (and stimulates osteoclast genesis in alveolar bone resorption) |
| Streptococcus mutans | --- | Induces IL-1β production via inflammasome activation in THP1 macrophages [164] |
| lipoteichoic acid purified | Induces TNF-α and nitric oxide (NO) production in RAW264.7 murine macrophages [165] | |
| Outer membrane vesicles (OMVs) purified | S. mutans OMVs increased production of IL-1β, IL-6, TNF-α and IL-8 (especially IL-1β) in THP1 cells; also reduces macrophage phagocytosis [166] | |
| Porphyromonas gingivalis | Outer membrane vesicles (OMVs) | OMV-stimulated macrophages produce a large amount of TNF-α, IL-12p70, IL-6, IL-10, IFNβ, and nitric oxide; OMVs can also induce NK-KB activation 9 [167] |
| --- | Activates NLRP3 and AIM2 inflammasomes in THP1 cells via TLR2 and TLR4 signaling, leading to IL-1β secretion and pyroptotic cell death (pro-inflammatory cell death; apoptosis = non-inflammatory) via caspase-1 activation [168] | |
| Outer membrane vesicles (OMVs) purified | Macrophages require a second signal (e.g., exogenous ATP), whereas monocytes (and THP-1 cells) only require one signal for inflammasome activation. This distinction may neatly explain why P. gingivalis activation of the inflammasome and IL-1β production has been demonstrated in THP-1 and Mono-Mac-6 cell lines (Bostanci et al., 2009; Hamedi et al., 2009), as well as in human monocytes (Huang et al., 2009; Jung et al., 2015) [169]. In comparison, studies in mature macrophage populations found that P. gingivalis fails to activate the inflammasome (Taxman et al., 2012; Slocum et al., 2014) unless stimulated with a secondary signal. Used murine bone-marrow-der. macrophage (BMM); human monocyte-der. macrophage (MDM)
| |
| Fusobacterium nucleatum | --- | Fn-challenged Mφ (unpolarized mac, THP1) had significantly lower mRNA level of M1 markers iNOS and TNF-α but significantly higher mRNA level of M2 markers IL-10 and CD206 = suggests Fn may be involved in M2-like polarization [170] |
| Outer membrane vesicles (OMVs) purified | Secreted OMVs can activate TLR4 and downstream targets ERK, CREB, and NF-κB, which promotes pro-inflammatory cytokine production. These effects were observed in colonic HT29 cells, as well as in human colonoid (organoid) monolayers [129]. | |
| --- | Activates both TLR2 and TLR4 in bone-marrow-derived macrophages to stimulate IL-6 production (and TNF-α) [171] | |
| Fn-cell wall; Lipopolysaccharides |
| |
Two more promising papers:
| ||
| Streptococcus salivarius K12 | Salivaricin A2 and B (lantibiotics = antimicrobial peptides) | Salivaricins inhibit other pathogens; K12 inhibits NF-KB pathway/inflammation and stimulates type I and II interferon responses (type 2 = INF-Gamma!) “Our analyses…indicated that K12 didn’t initiate synthesis of proinflammatory cytokines/chemokines, nor did it regulate genes involved in responses to such molecules.” [131] |
| Lactobacillus reuteri | Membrane vesicles | MVs inhibit LPS-induced macrophage polarization towards pro-inflammatory phenotype, promote their polarization towards anti-inflammatory phenotype, and reduce inflammation levels in vitro 9 [175] |
| “L. reuteri and inactivated L. reuteri treatment enhance macrophage phagocytic activity to kill intracellular pathogens (Figure 3A,B). Previous studies have also confirmed that Lactobacillus can activate macrophages, enhance phagocytosis ability and inhibit intracellular pathogen survival, thus playing a role in resisting pathogens [24,25].” | ||
| Streptococcus oralis | Hydrogen peroxide (H2O2) | Cytotoxic, kills monocytes and epithelial cells [176] |
7. Future Directions for Therapeutic Innovation
Oral wound healing represents a highly coordinated process involving immune signaling, microbial ecology and tissue regeneration [177]. Traditionally research efforts have emphasized the role of growth factors, scaffolds and re-epithelization mechanisms. However, emerging studies now highlight the critical importance of the immune–microbiome axis, particularly the plasticity of macrophages and the influence of microbial communities on inflammation and healing outcomes.
A central finding from both experimental and clinical studies is the necessity for timely macrophage polarization from a pro-inflammatory M1 phenotype to an anti-inflammatory, tissue-repairing M2 phenotype [10]. Successful transition between these macrophage states is vital for resolving the initial inflammatory response and facilitating the proliferative and remodeling phases essential for tissue integrity. Dysregulation of this polarization, often triggered by persistent microbial imbalance or chronic pro-inflammatory signaling, is a key mechanism underlying delayed healing in oral pathologies such as periodontitis [178] and diabetic ulcers [179].
During the inflammatory phase of wound healing, M1 macrophages, characterized by surface markers such as CD86, MHC class II and CCR7, release pro-inflammatory cytokines IL-1β, IL-6, TNF-α and inducible nitric oxide (iNOS) to eliminate pathogens and recruit immune cells for debris clearance. However sustained M1 activation contributes to chronic inflammation and delayed healing [12]. In contrast, during the proliferative phase, polarization towards M2 macrophages, marked by CD163, CD206, PD-L1 and PD-L2, supports tissue repair, angiogenesis, fibroblast activation, and extracellular matrix deposition, particularly important for minimizing scarring and promoting vascularization [180]. In the remodeling phase, M2 macrophages contribute to collagen deposition and tissue homeostasis [181]. Impaired or dysregulated macrophage activity, therefore, represents a significant barrier to efficient wound healing.
Recent studies have demonstrated that the oral microbiome is a key player in oral wound repair [21]. In a state of eubiosis, balanced microbial communities support immune homeostasis, reduce inflammation, and inhibit pathogenic overgrowth, thereby promoting efficient healing [158]. In contrast, dysbiosis, characterized by microbial imbalance, leads to persistent inflammation, increased susceptibility to infection, and impaired tissue repair [182]. Probiotic interventions aimed at restoring microbial balance have demonstrated potential in enhancing angiogenesis, promoting structural integrity, and supporting tissue regeneration [81].
Beneficial microbes such as Lactobacillus reuteri and Bifidobacterium breve have been shown to promote M2 macrophage polarization, reduce oxidative stress and increase the expression of anti-inflammatory cytokines like IL-10 and TGF-β [124]. These immunomodulatory effects are mediated through microbial metabolites, outer membrane vesicles and peptides that engage host pattern recognition receptors (PRRs) [183]. Nevertheless, not all probiotic therapies yield consistent clinical outcomes. For instance, while some clinical trials report modest reductions in postoperative inflammation following probiotic lozenge use, improvements in healing kinetics have been limited [156]. These findings highlight the complexity of host–microbe interactions and the need for optimized delivery strategies tailored to the wound environment.
Conversely, pathogenic bacteria such as Porphyromonas gingivalis and Fusobacterium nucleatum exacerbate wound pathology by sustaining M1-type inflammation through TLR-dependent pathways and activation of inflammation [184]. These pathogens effectively disrupt wound homeostasis, prolonging the inflammatory phase, and hinder progression to repair stages.
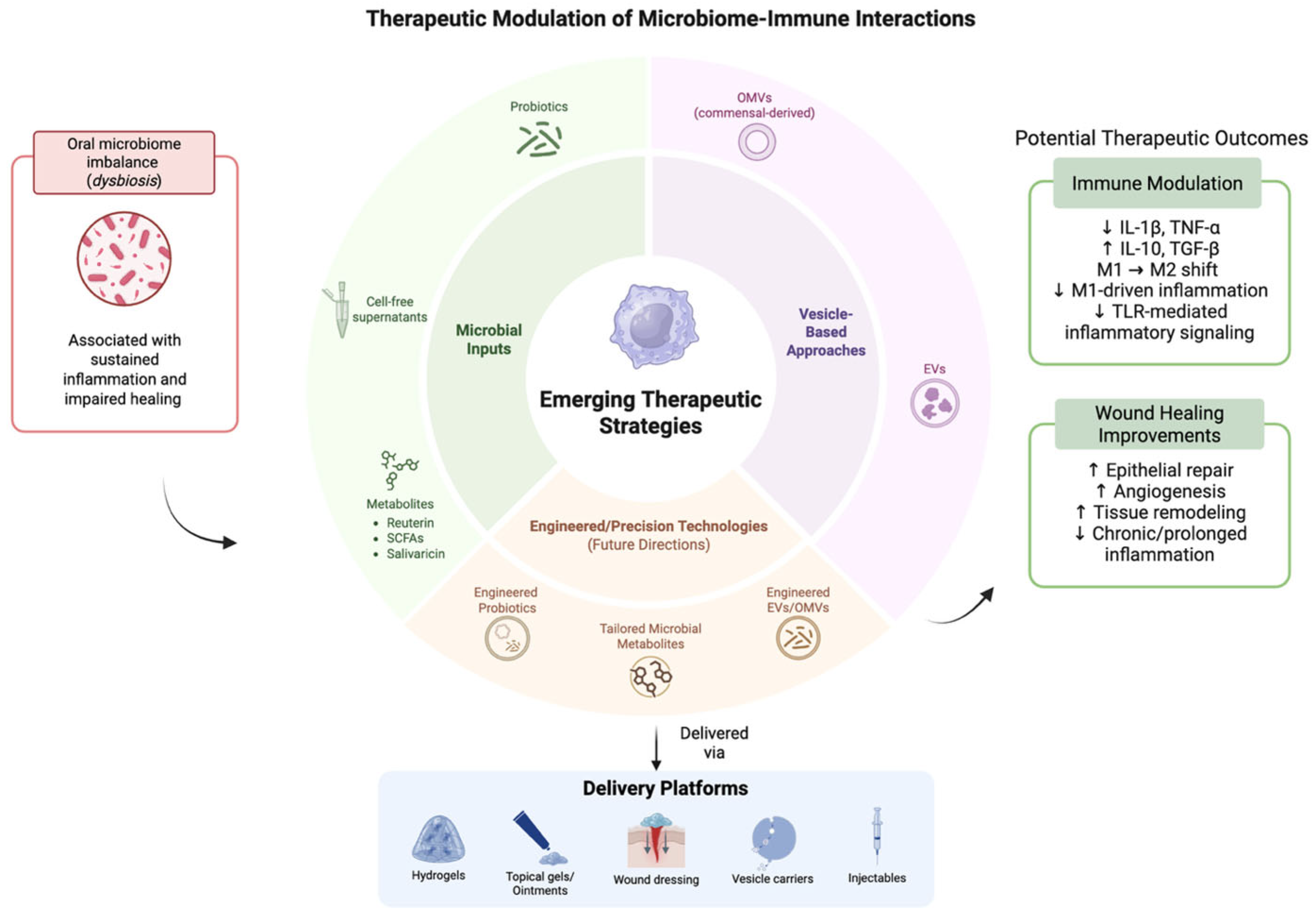
By combining metagenomic, metatranscriptomic, proteomic and metabolomic profiling of microbial communities with host single-cell and spatial transcriptomics/omics, researchers can simultaneously characterize which microbial taxa are present, what genes and metabolites they express, and how host immune cells respond at high resolution [185]. Recognizing the tissue architecture while examining both host and microbial molecular states enables the linkage of microbial signals to define the phenotypes of immune cells within their native contexts, thus going beyond traditional single-cell profiling techniques that disrupt samples through spatial multi-omics. Single-cell RNA sequencing of oral mucosa revealed macrophage dynamics in clearing of microbes by macrophage subpopulations in the inflammatory and remodeling phase [186]. On the computational side, sophisticated AI and machine learning models tailored for high-dimensional multi-omics data can combine these diverse datasets (microbial genomics/transcriptomics/metabolomics + host transcriptomics/epigenomics + spatial context) to deduce networks of interactions between microbes and hosts, pinpoint crucial metabolites or signals derived from microbes that are linked to immune activation or suppression, and differentiate individual variations that might affect disease susceptibility or healing responses [187]. Combining multi-omics, spatial or single-cell sequencing and AI-powered integrative analytics creates a robust, detailed approach to unravel the mechanisms of microbe–immune interactions [188]. This holds the potential to reveal new strategies for microbiome-driven modulation of macrophage activity and enhanced oral health treatments (Figure 4).
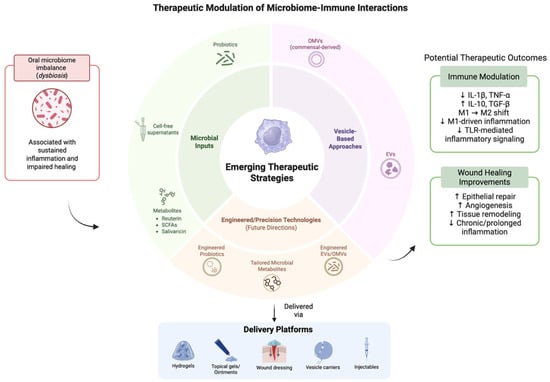
Figure 4.
Emerging therapeutic approaches to modulate microbiome–immune crosstalk. The above figure was created with BioRender.com. Goudy, S. (2026) https://BioRender.com/gfoio8n.
Macrophages are highly responsive to local environmental cues, including cytokines and microbial signals, which shape their functional phenotypes during wound healing. These observations underscore the potential of microbiome-targeted immunomodulatory strategies as promising therapeutic approaches for managing chronic and impaired oral wounds.
Bringing scientific insights into clinical therapies is further challenged by the ever-changing oral environment, where saliva, chewing, and microbial competition reduce how well therapeutic agents stay in place. This has led to a focus on new delivery systems, including mucoadhesive hydrogels, nanofiber scaffolds, nanoparticles, and smart biomaterials, that can provide sustained, targeted release of immune modulators at the fistula site. New approaches such as 3D bioprinting and tissue-engineered constructs with built-in immune cues also offer promising solutions for repairing complex defects and achieving long-term tissue integration. In the end, combining multi-omics analysis, advanced biomaterials, and targeted immune therapies could pave the way for personalized, biologically informed treatments that lower recurrence rates and improve outcomes for people with oronasal fistula.
Further investigations are warranted to define the specific microbial components and host signaling pathways that optimize macrophage function and promote regenerative healing. Future therapies may involve precision probiotic formulations, microbial-derived metabolites or engineered extracellular vesicles to specifically modulate immune response in chronic wound conditions.
This schematic illustrates emerging microbiome-informed therapeutic approaches, including microbial inputs, vesicle-based strategies, and engineered modalities, along with their delivery platforms. These interventions aim to modulate immune responses and support wound healing processes in the oral environment. Potential therapeutic outcomes shown are based on preliminary experimental findings. Their efficacy remains variable and is influenced by complex host–microbiome–immune interactions.
8. Conclusions
Advancing targeted therapies for oral wound healing requires a comprehensive understanding of the dynamic crosstalk between the oral microbiome, host immune responses, and tissue regeneration pathways. This review highlights key mechanistic insights demonstrating how microbial composition and microbe-derived metabolites regulate inflammatory resolution, macrophage polarization, epithelial barrier restoration, and angiogenesis, all critical determinants of effective wound repair. While emerging preclinical evidence supports microbiome-based and immune-targeted therapeutic strategies, clinical translation remains limited by wound heterogeneity, inter-individual microbiome variability, and the absence of standardized regulatory and clinical trial frameworks. Future research should prioritize inclusion of underexplored microbial components such as the oral virome and mycobiome, development of personalized and precision probiotic formulations, and well-designed longitudinal clinical trials to establish efficacy, safety, and dosing strategies. Integrating advances in microbiology, immunology, biomaterials, and systems biology will be essential to overcome current gaps and unlock the full therapeutic potential of microbiome-based interventions for oral wound healing.
Author Contributions
K.P.C.S.: conceptualization, writing—original draft, writing—review and editing, visualization. T.C., P.E.P., B.S., H.A.P., P.K.: figures, editing, writing—review and editing. H.R., R.J. and D.T.W.: editing. S.G.: writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication is supported by the National Institutes of Health, National Institute of Dental and Craniofacial Research (NIDCR), under award number R01DE031271.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
During the preparation of this manuscript, the author(s) used BioRender for figure creation. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| AIM2 | Absent in Melanoma 2 |
| ATP | Adenosine Triphosphate |
| BCN | Bacterial Cellulose Nanocomposite |
| BMM | Bone Marrow-Derived Macrophages |
| BV | Bacterial Vaginosis |
| CAR-T | Chimeric Antigen Receptor T cells |
| CCR7 | C-C Chemokine Receptor Type 7 |
| CD | Cluster of Differentiation |
| CF | Cystic Fibrosis |
| CX3CR1 | C-X3-C Motif Chemokine Receptor 1 |
| CXCL | C-X-C Motif Chemokine Ligand |
| ECM | Extracellular Matrix |
| EGF | Epidermal Growth Factor |
| FGF | Fibroblast Growth Factor |
| GM-CSF | Granulocyte–Macrophage Colony-Stimulating Factor |
| HAEPS | Hyaluronic Acid-Encapsulated Probiotic System |
| HIF-1α | Hypoxia-Inducible Factor-1 Alpha |
| IBS | Irritable Bowel Syndrome |
| IFN-β | Interferon Beta |
| IFN-γ | Interferon Gamma |
| IGF-1 | Insulin-Like Growth Factor-1 |
| IL | Interleukin |
| IL-1RA | Interleukin-1 Receptor Antagonist |
| iNOS | Inducible Nitric Oxide Synthase |
| LPS | Lipopolysaccharide |
| MHC | Major Histocompatibility Complex |
| MDM | Monocyte-Derived Macrophages |
| MMP | Matrix Metalloproteinase |
| MVs | Membrane Vesicles |
| NETs | Neutrophil Extracellular Traps |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NGF | Nerve Growth Factor |
| NO | Nitric Oxide |
| OMVs | Outer Membrane Vesicles |
| ONF | Oro-Nasal Fistula |
| OSCC | Oral Squamous Cell Carcinoma |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PD-L1 | Programmed Death-Ligand 1 |
| PD-L2 | Programmed Death-Ligand 2 |
| PDGF | Platelet-Derived Growth Factor |
| PEG | Polyethylene Glycol |
| PG | Peptidoglycan |
| PRRs | Pattern Recognition Receptors |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-Chain Fatty Acids |
| SNPs | Single-Nucleotide Polymorphisms |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| TAMs | Tumor-Associated Macrophages |
| TGF-α | Transforming Growth Factor Alpha |
| TGF-β | Transforming Growth Factor Beta |
| Th17 | T Helper 17 Cells |
| TIMPs | Tissue Inhibitors of Metalloproteinases |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor Alpha |
| VEGF | Vascular Endothelial Growth Factor |
References
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Pawłowska, A.; Orzeł, A.; Sulej, L.; Muzyka-Placzyńska, K.; Baran, A.; Filipecka-Tyczka, D.; Pawłowska, P.; Nowińska, A.; Bogusławska, J.; et al. Wound Microbiota and Its Impact on Wound Healing. Int. J. Mol. Sci. 2023, 24, 17318. [Google Scholar] [CrossRef] [PubMed]
- Brand, H.S.; Ligtenberg, A.J.; Veerman, E.C. Saliva and wound healing. Monogr. Oral Sci. 2014, 24, 52–60. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Perez-Mongiovi, D.; Araujo, R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Khalel, A.M. Using the Immune System for Effective Periodontal and Caries Management. BioMed Res. Int. 2025, 2025, 6385469. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Wang, L.; Yang, K.; Xie, X.; Wang, S.; Gan, H.; Wang, X.; Wei, H. Macrophages as Multifaceted Orchestrators of Tissue Repair: Bridging Inflammation, Regeneration, and Therapeutic Innovation. J. Inflamm. Res. 2025, 18, 8945–8959. [Google Scholar] [CrossRef]
- Zenobia, C.; Herpoldt, K.-L.; Freire, M. Is the oral microbiome a source to enhance mucosal immunity against infectious diseases? npj Vaccines 2021, 6, 80. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, L.; Dinu, C.A.; Gurau, G.; Maftei, N.-M.; Matei, M.N.; Hincu, M.-A.; Radu, M.; Mehedinti, M.-C. The Role of Probiotics in Healing Burns and Skin Wounds; An Integrative Approach in the Context of Regenerative Medicine. Life 2025, 15, 1434. [Google Scholar] [CrossRef] [PubMed]
- Juarez, V.M.; Montalbine, A.N.; Singh, A. Microbiome as an immune regulator in health, disease, and therapeutics. Adv. Drug Deliv. Rev. 2022, 188, 114400. [Google Scholar] [CrossRef]
- Kutschera, U. Antonie van Leeuwenhoek (1632–1723): Master of Fleas and Father of Microbiology. Microorganisms 2023, 11, 1994. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Sanketh, D.S.; Amrutha, N. Microbial flora in oral diseases. J. Contemp. Dent. Pract. 2013, 14, 1202–1208. [Google Scholar] [CrossRef]
- Ezaura, E.; Nicu, E.A.; Krom, B.P.; Keijser, B.J.F. Acquiring and maintaining a normal oral microbiome: Current perspective. Front. Cell. Infect. Microbiol. 2014, 4, 85. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Deo, P.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ahmed, R.; Ramadan, Y.N.; Fathy, H.; Khorshid, M.; Mabrouk, M.M.; Hashem, M. Gut virome: New key players in the pathogenesis of inflammatory bowel disease. World J. Methodol. 2025, 15, 92592. [Google Scholar] [CrossRef] [PubMed]
- Pinana, M.; Rapoport, C.; Champtiaux, N.; Lescaille, G.; Allenbach, Y.; Rochefort, J. Cytomegalovirus-induced oral ulcers: A case report and literature review. Clin. Case Rep. 2023, 11, e7459. [Google Scholar] [CrossRef] [PubMed]
- Piperi, E.; Papadopoulou, E.; Georgaki, M.; Dovrat, S.; Bar Illan, M.; Nikitakis, N.G.; Yarom, N. Management of oral herpes simplex virus infections: The problem of resistance. A narrative review. Oral Dis. 2024, 30, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, P.; Banks, J.M.; Rahat, R.; Brandini, D.A.; Naqvi, A.R. Viruses of the oral cavity: Prevalence, pathobiology and association with oral diseases. Rev. Med. Virol. 2022, 32, e2311. [Google Scholar] [CrossRef]
- Crimi, S.; Fiorillo, L.; Bianchi, A.; D’amico, C.; Amoroso, G.; Gorassini, F.; Mastroieni, R.; Marino, S.; Scoglio, C.; Catalano, F.; et al. Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data. Viruses 2019, 11, 463. [Google Scholar] [CrossRef]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. JNCI Monogr. 2001, 2001, 7–15. [Google Scholar] [CrossRef]
- DiPietro, L.A. Oral Stem Cells: The Fountain of Youth for Epithelialization and Wound Therapy? Adv. Wound Care 2014, 3, 465–467. [Google Scholar] [CrossRef]
- Komori, T.; Ono, M.; Hara, E.S.; Ueda, J.; Nguyen, H.T.T.; Nguyen, H.T.; Yonezawa, T.; Maeba, T.; Kimura-Ono, A.; Takarada, T.; et al. Type IV collagen α6 chain is a regulator of keratin 10 in keratinization of oral mucosal epithelium. Sci. Rep. 2018, 8, 2612. [Google Scholar] [CrossRef]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Yang, B.; Hang, S.; Xu, S.; Gao, Y.; Yu, W.; Zang, G.; Zhang, L.; Wang, Z. Macrophage polarisation and inflammatory mechanisms in atherosclerosis: Implications for prevention and treatment. Heliyon 2024, 10, e32073. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef]
- Chibly, A.M.; Aure, M.H.; Patel, V.N.; Hoffman, M.P. Salivary gland function, development, and regeneration. Physiol. Rev. 2022, 102, 1495–1552. [Google Scholar] [CrossRef]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The power of saliva: Antimicrobial and beyond. PLoS Pathog. 2019, 15, e1008058. [Google Scholar] [CrossRef]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Brass, L.F.; Zhu, L.; Stalker, T.J. Minding the gaps to promote thrombus growth and stability. J. Clin. Investig. 2005, 115, 3385–3392. [Google Scholar] [CrossRef] [PubMed]
- Fuster, V.; Fayad, Z.A.; Moreno, P.R.; Poon, M.; Corti, R.; Badimon, J.J. Atherothrombosis and high-risk plaque: Part II: Approaches by noninvasive computed tomographic/magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.S. Leukocytosis and ischemic vascular disease morbidity and mortality: Is it time to intervene? Arterioscler. Thromb. Vasc. Biol. 2005, 25, 658–670. [Google Scholar] [CrossRef]
- Goren, I.; Allmann, N.; Yogev, N.; Schurmann, C.; Linke, A.; Holdener, M.; Waisman, A.; Pfeilschifter, J.; Frank, S. A transgenic mouse model of inducible macrophage depletion: Effects of diphtheria toxin-driven lysozyme m-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am. J. Pathol. 2009, 175, 132–147. [Google Scholar] [CrossRef]
- Willenborg, S.; Lucas, T.; van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Clark, R.A. Fibrin and wound healing. Ann. N. Y. Acad. Sci. 2001, 936, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Saarialho-Kere, U.K.; Pentland, A.P.; Birkedal-Hansen, H.; Parks, W.C.; Welgus, H.G. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J. Clin. Investig. 1994, 94, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Barbul, A. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004, 187, 11S–16S. [Google Scholar] [CrossRef]
- Rada, B. Neutrophil Extracellular Traps. Methods Mol. Biol. 2019, 1982, 517–528. [Google Scholar] [CrossRef]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Hamilton, J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008, 8, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Tsourouktsoglou, T.-D.; Branzk, N.; Wang, Q.; Reincke, S.; Herbst, S.; Gutierrez, M.; Papayannopoulos, V. Reactive Oxygen Species Localization Programs Inflammation to Clear Microbes of Different Size. Immunity 2017, 46, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, S.M.; Edmisson, J.S.; Jimenez-Flores, E. Human neutrophils and oral microbiota: A constant tug-of-war between a harmonious and a discordant coexistence. Immunol. Rev. 2016, 273, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Alimova, Y.; Ebersole, J.L. Macrophage polarization in response to oral commensals and pathogens. Pathog. Dis. 2016, 74, ftw011. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Kaci, G.; Goudercourt, D.; Dennin, V.; Pot, B.; Doré, J.; Ehrlich, S.D.; Renault, P.; Blottière, H.M.; Daniel, C.; Delorme, C. Anti-Inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl. Environ. Microbiol. 2014, 80, 928–934. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Luo, G.; Zhao, J.; Bai, G.; Xu, D. Innovative strategies targeting oral microbial dysbiosis: Unraveling mechanisms and advancing therapies for periodontitis. Front. Cell. Infect. Microbiol. 2025, 15, 1556688. [Google Scholar] [CrossRef]
- Aleksijević, L.H.; Aleksijević, M.; Škrlec, I.; Šram, M.; Šram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef]
- Michalak, K.P.; Michalak, A.Z. Understanding chronic inflammation: Couplings between cytokines, ROS, NO, Cai2+, HIF-1α, Nrf2 and autophagy. Front. Immunol. 2025, 16, 1558263. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, P.; Gao, W. Microbial dysbiosis in periodontitis and peri-implantitis: Pathogenesis, immune responses, and therapeutic. Front. Cell. Infect. Microbiol. 2025, 15, 1517154. [Google Scholar] [CrossRef]
- Leonov, G.E.; Varaeva, Y.R.; Livantsova, E.N.; Starodubova, A.V. The Complicated Relationship of Short-Chain Fatty Acids and Oral Microbiome: A Narrative Review. Biomedicines 2023, 11, 2749. [Google Scholar] [CrossRef]
- Wan, J.; Shan, Y.; Fan, Y.; Fan, C.; Chen, S.; Sun, J.; Zhu, L.; Qin, L.; Yu, M.; Lin, Z. NF-κB inhibition attenuates LPS-induced TLR4 activation in monocyte cells. Mol. Med. Rep. 2016, 14, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, T.; Tang, Y.; Luo, G.; Liang, G.; He, W. Epigenetic regulation of macrophage polarization in wound healing. Burn. Trauma. 2023, 11, tkac057. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2021, 298, 101530. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Asiri, F.Y.I.; Mali, M.; Khan, R.S.; Sahibzada, H.A.; Zafar, M.S.; Moin, S.F.; Khan, E. Significance and Diagnostic Role of Antimicrobial Cathelicidins (LL-37) Peptides in Oral Health. Biomolecules 2017, 7, 80. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.F.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Mah, W.; Jiang, G.; Olver, D.; Cheung, G.; Kim, B.; Larjava, H.; Häkkinen, L. Human gingival fibroblasts display a non-fibrotic phenotype distinct from skin fibroblasts in three-dimensional cultures. PLoS ONE 2014, 9, e90715. [Google Scholar] [CrossRef] [PubMed]
- Chuhuaicura, P.; Rodríguez-Niklitschek, C.; Oporto, G.H.; Salazar, L.A. Distinct Molecular Mechanisms in Oral Mucosal Wound Healing: Translational Insights and Future Directions. Int. J. Mol. Sci. 2025, 26, 10660. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Bădăluță, V.A.; Curuțiu, C.; Dițu, L.M.; Holban, A.M.; Lazăr, V. Probiotics in Wound Healing. Int. J. Mol. Sci. 2024, 25, 5723. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Chiu, H.-T.; Alshawsh, M.A. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines 2022, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Shi, C.-W.; Cheng, M.-Y.; Yang, X.; Lu, Y.-Y.; Yin, H.-D.; Zeng, Y.; Wang, R.-Y.; Jiang, Y.-L.; Yang, W.-T.; Wang, J.-Z.; et al. Probiotic Lactobacillus rhamnosus GG Promotes Mouse Gut Microbiota Diversity and T Cell Differentiation. Front. Microbiol. 2020, 11, 607735. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhao, Z.; Wang, W.; Liu, X. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. J. Immunol. Res. 2021, 2021, 8030297. [Google Scholar] [CrossRef]
- Heavey, M.K.; Hazelton, A.; Wang, Y.; Garner, M.; Anselmo, A.C.; Arthur, J.C.; Nguyen, J. Targeted delivery of the probiotic Saccharomyces boulardii to the extracellular matrix enhances gut residence time and recovery in murine colitis. Nat. Commun. 2024, 15, 3784. [Google Scholar] [CrossRef]
- Martinez, E.; Taminiau, B.; Rodriguez, C.; Daube, G. Gut Microbiota Composition Associated with Clostridioides difficile Colonization and Infection. Pathogens 2022, 11, 781. [Google Scholar] [CrossRef]
- Repoila, F.; Le Bohec, F.; Guérin, C.; Lacoux, C.; Tiwari, S.; Jaiswal, A.K.; Santana, M.P.; Kennedy, S.P.; Quinquis, B.; Rainteau, D.; et al. Adaptation of the gut pathobiont Enterococcus faecalis to deoxycholate and taurocholate bile acids. Sci. Rep. 2022, 12, 8485. [Google Scholar] [CrossRef]
- Li, N.; Tan, G.; Xie, Z.; Chen, W.; Yang, Z.; Wang, Z.; Liu, S.; He, M. Distinct enterotypes and dysbiosis: Unraveling gut microbiota in pulmonary and critical care medicine inpatients. Respir. Res. 2024, 25, 304. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’addona, A. Oral microbiota in human health and disease: A perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef]
- Babina, K.; Salikhova, D.; Polyakova, M.; Svitich, O.; Samoylikov, R.; El-Abed, S.A.; Zaytsev, A.; Novozhilova, N. The Effect of Oral Probiotics (Streptococcus Salivarius k12) on the Salivary Level of Secretory Immunoglobulin A, Salivation Rate, and Oral Biofilm: A Pilot Randomized Clinical Trial. Nutrients 2022, 14, 1124. [Google Scholar] [CrossRef] [PubMed]
- Widyarman, A.S.; Udawatte, N.S.; Roeslan, M.O.; Rizal, M.I.; Richi, M.; Kusnoto, J.; Seneviratne, C.J. Short- term effect of probiotic Lactobacillus reuteri consumption on the salivary microbiome profile of subjects undergoing orthodontic treatment with fixed appliances. J. Oral Microbiol. 2022, 14, 2067103. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiao, J.; Niu, Y. Editorial: The Pivotal Role of Oral Microbiota Dysbiosis and Microbiota-Host Interactions in Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 947638. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Santonocito, S.; Polizzi, A.; Mauceri, R.; Troiano, G.; Lo Giudice, A.; Romano, A.; Mascitti, M.; Isola, G. A Reciprocal Link between Oral, Gut Microbiota during Periodontitis: The Potential Role of Probiotics in Reducing Dysbiosis-Induced Inflammation. Int. J. Mol. Sci. 2023, 24, 1084. [Google Scholar] [CrossRef]
- Su, S.-C.; Chang, L.-C.; Huang, H.-D.; Peng, C.-Y.; Chuang, C.-Y.; Chen, Y.-T.; Lu, M.-Y.; Chiu, Y.-W.; Chen, P.-Y.; Yang, S.-F. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 2021, 42, 127–135. [Google Scholar] [CrossRef]
- D’arcangelo, S.; Di Fermo, P.; Diban, F.; Ferrone, V.; D’ercole, S.; Di Giulio, M.; Di Lodovico, S. Staphylococcus aureus/Staphylococcus epidermidis from skin microbiota are balanced by Pomegranate peel extract: An eco-sustainable approach. PLoS ONE 2024, 19, e0308211. [Google Scholar] [CrossRef]
- Rozas, M.; de Ruijter, A.H.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Chou, C.-H.; Chiang, Y.-J.; Lin, C.-G.; Lee, C.-H. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef]
- Delanghe, L.; Spacova, I.; Van Malderen, J.; Oerlemans, E.; Claes, I.; Lebeer, S. The role of lactobacilli in inhibiting skin pathogens. Biochem. Soc. Trans. 2021, 49, 617–627. [Google Scholar] [CrossRef]
- Kobayashi, T.; Glatz, M.; Horiuchi, K.; Kawasaki, H.; Akiyama, H.; Kaplan, D.H.; Kong, H.H.; Amagai, M.; Nagao, K. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity 2015, 42, 756–766. [Google Scholar] [CrossRef]
- Schmid, B.; Künstner, A.; Fähnrich, A.; Bersuch, E.; Schmid-Grendelmeier, P.; Busch, H.; Glatz, M.; Bosshard, P. Dysbiosis of skin microbiota with increased fungal diversity is associated with severity of disease in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1811–1819. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Fan, S.; Fang, C.; Ge, L.; Lyu, J.; Huang, Z.; Zhao, S.; Zou, Y.; Huang, L.; Liu, X.; et al. Orally administrated Lactobacillus gasseri TM13 and Lactobacillus crispatus LG55 can restore the vaginal health of patients recovering from bacterial vaginosis. Front. Immunol. 2023, 14, 1125239. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Reid, G.; ter Haar, J.A. Lacticaseibacillus rhamnosus GR-1, a.k.a. Lactobacillus rhamnosus GR-1: Past and Future Perspectives. Trends Microbiol. 2021, 29, 747–761. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyu, J.; Ge, L.; Huang, L.; Peng, Z.; Liang, Y.; Zhang, X.; Fan, S. Probiotic Lacticaseibacillus rhamnosus GR-1 and Limosilactobacillus reuteri RC-14 as an Adjunctive Treatment for Bacterial Vaginosis Do Not Increase the Cure Rate in a Chinese Cohort: A Prospective, Parallel-Group, Randomized, Controlled Study. Front. Cell. Infect. Microbiol. 2021, 11, 669901. [Google Scholar] [CrossRef]
- Velloza, J.; Heffron, R. The Vaginal Microbiome and its Potential to Impact Efficacy of HIV Pre-exposure Prophylaxis for Women. Curr. HIV/AIDS Rep. 2017, 14, 153–160. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Chen, T. Role of Vaginal Microbiota Dysbiosis in Gynecological Diseases and the Potential Interventions. Front. Microbiol. 2021, 12, 643422. [Google Scholar] [CrossRef]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef]
- Shi, Z.; Yao, C.; Shui, Y.; Li, S.; Yan, H. Research progress on the mechanism of angiogenesis in wound repair and regeneration. Front. Physiol. 2023, 14, 1284981. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.-D.; Gao, J.; Tang, A.-F.; Feng, C. Shaping the immune landscape: Multidimensional environmental stimuli refine macrophage polarization and foster revolutionary approaches in tissue regeneration. Heliyon 2024, 10, e37192. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in cardiovascular diseases: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Soria, S.A.; Pires, J.R.; Sant’aNa, A.C.P.; Freire, M. Natural and induced immune responses in oral cavity and saliva. BMC Immunol. 2025, 26, 34. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, F.; Cheng, H.; Su, M.; Wang, Y. Macrophage polarization: An important role in inflammatory diseases. Front. Immunol. 2024, 15, 1352946. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Su, D.; Tsai, H.-I.; Xu, Z.; Yan, F.; Wu, Y.; Xiao, Y.; Liu, X.; Wu, Y.; Parvanian, S.; Zhu, W.; et al. Exosomal PD-L1 functions as an immunosuppressant to promote wound healing. J. Extracell. Vesicles 2019, 9, 1709262. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, Z.-L.; Liu, S.-Y.; Wu, J.-J.; Zhao, B.-H.; Lv, G.-Z.; Du, Y.; Yu, S.; Yang, M.-L.; Yuan, F.-L.; et al. Direct and Indirect Roles of Macrophages in Hypertrophic Scar Formation. Front. Physiol. 2019, 10, 1101. [Google Scholar] [CrossRef]
- Pizzurro, G.A.; Miller-Jensen, K. Reframing macrophage diversity with network motifs. Trends Immunol. 2023, 44, 965–970. [Google Scholar] [CrossRef]
- Yan, L.; Wang, J.; Cai, X.; Liou, Y.; Shen, H.; Hao, J.; Huang, C.; Luo, G.; He, W. Macrophage plasticity: Signaling pathways, tissue repair, and regeneration. Medcomm 2024, 5, e658. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Huang, X.; Huang, X.; Huang, Y.; Zheng, J.; Lu, Y.; Mai, Z.; Zhao, X.; Cui, L.; Huang, S. The oral microbiome in autoimmune diseases: Friend or foe? J. Transl. Med. 2023, 21, 211. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xin, J.; Hu, M.; Xue, C.; Dong, N. Programmable microbial therapeutics: Advances in engineered bacteria for targeted in vivo delivery and precision medicine. J. Adv. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Calderon, G.; Susapto, H.H.; Hauser, C.A.E. Delivery of Endothelial Cell-Laden Microgel Elicits Angiogenesis in Self-Assembling Ultrashort Peptide Hydrogels In Vitro. ACS Appl. Mater. Interfaces 2021, 13, 29281–29292. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Sheng, T.; Zhang, W.; Feng, H.; Yu, J.; Gu, Z.; Zhang, Y. Microneedle-Mediated Cell Therapy. Adv. Sci. 2024, 11, 2304124. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, H.; Weinans, H.; van der Wal, B.; Rios, J.L. Novel Aptamer Strategies in Combating Bacterial Infections: From Diagnostics to Therapeutics. Pharmaceutics 2024, 16, 1140. [Google Scholar] [CrossRef]
- Engevik, M.A.; Danhof, H.A.; Ruan, W.; Engevik, A.C.; Chang-Graham, A.L.; Engevik, K.A.; Shi, Z.; Zhao, Y.; Brand, C.K.; Krystofiak, E.S.; et al. Fusobacterium nucleatum Secretes Outer Membrane Vesicles and Promotes Intestinal Inflammation. mBio 2021, 12, 10-1128. [Google Scholar] [CrossRef]
- Rockel, C.; Hartung, T. Systematic review of membrane components of gram-positive bacteria responsible as pyrogens for inducing human monocyte/macrophage cytokine release. Front. Pharmacol. 2012, 3, 56. [Google Scholar] [CrossRef]
- Cosseau, C.; Devine, D.A.; Dullaghan, E.; Gardy, J.L.; Chikatamarla, A.; Gellatly, S.; Yu, L.L.; Pistolic, J.; Falsafi, R.; Tagg, J.; et al. The Commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect. Immun. 2008, 76, 4163–4175. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, X.; Wang, Y.; Li, D.; Xie, L.; Liang, S.; Zhang, Y.; Li, W.; Fu, A.; Zhan, X. Bacterial Metabolite Reuterin Attenuated LPS-Induced Oxidative Stress and Inflammation Response in HD11 Macrophages. Antioxidants 2022, 11, 1662. [Google Scholar] [CrossRef]
- Trombetta, A.C.; Soldano, S.; Contini, P.; Tomatis, V.; Ruaro, B.; Paolino, S.; Brizzolara, R.; Montagna, P.; Sulli, A.; Pizzorni, C.; et al. A circulating cell population showing both M1 and M2 monocyte/macrophage surface markers characterizes systemic sclerosis patients with lung involvement. Respir. Res. 2018, 19, 186. [Google Scholar] [CrossRef]
- Kwiecień, I.; Polubiec-Kownacka, M.; Dziedzic, D.; Wołosz, D.; Rzepecki, P.; Domagała-Kulawik, J. CD163 and CCR7 as markers for macrophage polarisation in lung cancer microenvironment. Cent. Eur. J. Immunol. 2019, 44, 395–402. [Google Scholar] [CrossRef]
- Burgess, M.; Wicks, K.; Gardasevic, M.; A Mace, K. Cx3CR1 Expression Identifies Distinct Macrophage Populations That Contribute Differentially to Inflammation and Repair. Immuno Horiz. 2019, 3, 262–273. [Google Scholar] [CrossRef]
- Sun, X.; Gao, J.; Meng, X.; Lu, X.; Zhang, L.; Chen, R. Polarized Macrophages in Periodontitis: Characteristics, Function, and Molecular Signaling. Front. Immunol. 2021, 12, 763334. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, C.; Tao, R.; Yong, X.; Jiang, Q.; Feng, C. PD-L1-Mediated Immunosuppression in Oral Squamous Cell Carcinoma: Relationship With Macrophage Infiltration and Epithelial to Mesenchymal Transition Markers. Front. Immunol. 2021, 12, 693881. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, N.; Mizuno-Kamiya, M.; Umemura, N.; Takayama, E.; Kawaki, H.; Mitsudo, K.; Muramatsu, Y.; Sumitomo, S. Immunomodulatory aspects in the progression and treatment of oral malignancy. Jpn. Dent. Sci. Rev. 2019, 55, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nie, F.; Zhao, J.; Li, S.; Liu, W.; Guo, H.; Yang, P. PGRN is involved in macrophage M2 polarization regulation through TNFR2 in periodontitis. J. Transl. Med. 2024, 22, 407. [Google Scholar] [CrossRef]
- Chaudhari, N.; Prakash, N.; Pradeep, G.; Mahajan, A.; Lunawat, S.; Salunkhe, V. Evaluation of density of tumor-associated macrophages using CD163 in histological grades of oral squamous cell carcinoma, an immunohistochemical study. J. Oral Maxillofac. Pathol. 2020, 24, 577. [Google Scholar] [CrossRef]
- Xu, Z.-J.; Gu, Y.; Wang, C.-Z.; Jin, Y.; Wen, X.-M.; Ma, J.-C.; Tang, L.-J.; Mao, Z.-W.; Qian, J.; Lin, J. The M2 macrophage marker CD206: A novel prognostic indicator for acute myeloid leukemia. Oncoimmunology 2020, 9, 1683347. [Google Scholar] [CrossRef]
- Corliss, B.A.; Azimi, M.S.; Munson, J.M.; Peirce, S.M.; Murfee, W.L. Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis. Microcirculation 2016, 23, 95–121. [Google Scholar] [CrossRef]
- Shigeoka, M.; Koma, Y.-I.; Nishio, M.; Akashi, M.; Yokozaki, H. Alteration of Macrophage Infiltrating Compartment: A Novel View on Oral Carcinogenesis. Pathobiology 2021, 88, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, I.; Rutkowska, E.; Polubiec-Kownacka, M.; Raniszewska, A.; Rzepecki, P.; Domagała-Kulawik, J. Identification of PD-1 ligands: PD-L1 and PD-L2 on macrophages in lung cancer milieu by flow cytometry. Transl. Lung Cancer Res. 2021, 10, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Kuraji, R.; Ito, H.; Numabe, Y. Wound healing in periodontal disease induces macrophage polarization characterized by different arginine-metabolizing enzymes. J. Periodontal Res. 2022, 57, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Goudy, S.L.; Bradley, H.L.; Gacasan, C.A.; Toma, A.; Sekar, K.P.C.; Wuest, W.M.; Tomov, M.; Serpooshan, V.; Coskun, A.; Jones, R.M. Microbial Changes Occurring During Oronasal Fistula Wound Healing. Microorganisms 2025, 13, 327. [Google Scholar] [CrossRef]
- Novak, M.L.; Koh, T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 2013, 183, 1352–1363. [Google Scholar] [CrossRef]
- Cho, Y.-D.; Kim, K.-H.; Lee, Y.-M.; Ku, Y.; Seol, Y.-J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef]
- Ryu, H.S.; Lim, N.K.; Padalhin, A.R.; Abueva, C.; Park, S.Y.; Chung, P.; Woo, S.H. Improved healing and macrophage polarization in oral ulcers treated with photobiomodulation (PBM). Lasers Surg. Med. 2022, 54, 600–610. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Zhang, S.; Lu, E.; Qiao, W.; Chen, H.; Liu, P.; Tang, X.; Cheng, T.; Chen, H. Proteomic and single-cell analysis shed new light on the anti-inflammatory role of interferonβ in chronic periodontitis. Front. Pharmacol. 2023, 14, 1232539. [Google Scholar] [CrossRef]
- Bootun, R. Effects of immunosuppressive therapy on wound healing. Int. Wound J. 2013, 10, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Saaoud, F.; Shao, Y.; Cornwell, W.; Wang, H.; Rogers, T.J.; Yang, X. Cigarette Smoke Modulates Inflammation and Immunity via Reactive Oxygen Species-Regulated Trained Immunity and Trained Tolerance Mechanisms. Antioxid. Redox Signal. 2023, 38, 1041–1069. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Guo, D.; Meng, L.; Feng, X.; Zhang, Y.; Pan, S. Immune dysregulation and macrophage polarization in peri-implantitis. Front. Bioeng. Biotechnol. 2024, 12, 1291880. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, E.M.; Tosios, K.I.; Christopoulos, P.F. The Role of Macrophages in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 611115. [Google Scholar] [CrossRef]
- Wälivaara, D.Å.; Sjögren, I.; Gerasimcik, N.; Yucel-Lindberg, T.; Twetman, S.; Abrahamsson, P. Effects of Lactobacillus reuteri-containing lozenges on healing after surgical removal of mandibular third molars: A randomised controlled trial. Benef Microbes 2019, 10, 653–659. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Qiao, Q.; Zhao, N.; Yang, Y.; Wang, L.; Wang, Y.; Guo, C.; Guo, Y. Oral administration of Bifidobacterium breve improves anti-angiogenic drugs-derived oral mucosal wound healing impairment via upregulation of interleukin-10. Int. J. Oral Sci. 2023, 15, 56. [Google Scholar] [CrossRef]
- Han, N.; Jia, L.; Guo, L.; Su, Y.; Luo, Z.; Du, J.; Mei, S.; Liu, Y. Balanced oral pathogenic bacteria and probiotics promoted wound healing via maintaining mesenchymal stem cell homeostasis. Stem Cell Res. Ther. 2020, 11, 61. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Song, J.; Zhou, L.; Wu, K.; Lu, X.; Zhai, X.; Wan, Z.; Gao, J. Highly active probiotic hydrogels matrixed on bacterial EPS accelerate wound healing via maintaining stable skin microbiota and reducing inflammation. Bioact. Mater. 2024, 35, 31–44. [Google Scholar] [CrossRef]
- Li, W.; Yu, J.; Li, Q.; Wang, H.; Liu, X.; Li, P.; Jiang, X.; Yang, J. Bacterial cellulose nanofiber reinforced self-healing hydrogel to construct a theranostic platform of antibacterial and enhanced wound healing. Int. J. Biol. Macromol. 2024, 281, 136336. [Google Scholar] [CrossRef]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef]
- Vågesjö, E.; Öhnstedt, E.; Mortier, A.; Lofton, H.; Huss, F.; Proost, P.; Roos, S.; Phillipson, M. Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Öhnstedt, E.; Vågesjö, E.; Fasth, A.; Lofton Tomenius, H.; Dahg, P.; Jönsson, S.; Tyagi, N.; Åström, M.; Myktybekova, Z.; Ringstad, L.; et al. Engineered bacteria to accelerate wound healing: An adaptive, randomised, double-blind, placebo-controlled, first-in-human phase 1 trial. EClinicalMedicine 2023, 60, 102014. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Na, H.S.; Park, E.; Park, M.H.; Lee, H.A.; Chung, J. Streptococcus mutans activates the AIM2, NLRP3 and NLRC4 inflammasomes in human THP-1 macrophages. Int. J. Oral Sci. 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Baik, J.E.; Kang, S.-S.; Yun, C.-H.; Seo, D.-G.; Han, S.H. Lipoteichoic acid of Streptococcus mutans interacts with Toll-like receptor 2 through the lipid moiety for induction of inflammatory mediators in murine macrophages. Mol. Immunol. 2014, 57, 284–291. [Google Scholar] [CrossRef]
- Song, G.; Li, M.; Zhou, B.; Qi, H.; Guo, J. Streptococcus mutans outer membrane vesicles affect inflammasome activation and the glycolysis of macrophages. Microb. Pathog. 2024, 196, 106994. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Liu, S.; Zhang, S.; Pan, Y. The Role of Porphyromonas gingivalis Outer Membrane Vesicles in Periodontal Disease and Related Systemic Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 585917. [Google Scholar] [CrossRef]
- Park, E.; Na, H.S.; Song, Y.-R.; Shin, S.Y.; Kim, Y.-M.; Chung, J. Activation of NLRP3 and AIM2 Inflammasomes by Porphyromonas gingivalis Infection. Infect. Immun. 2014, 82, 112–123. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Lee, M.K.; Singleton, W.; Achuthan, A.; Lee, M.-C.; O’BRien-Simpson, N.M.; Cook, A.D.; Murphy, A.J.; Dashper, S.G.; Reynolds, E.C.; et al. Metabolic Remodeling, Inflammasome Activation, and Pyroptosis in Macrophages Stimulated by Porphyromonas gingivalis and Its Outer Membrane Vesicles. Front. Cell. Infect. Microbiol. 2017, 7, 351. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Kong, X.; Wu, R.; Peng, Q.; Zhang, Y.; Zhou, L.; Duan, L. Fusobacterium nucleatum Facilitates M2 Macrophage Polarization and Colorectal Carcinoma Progression by Activating TLR4/NF-κB/S100A9 Cascade. Front. Immunol. 2021, 12, 658681. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, D.-J.; Han, S.-H.; Kang, M.-J.; Lee, J.-Y.; Jeong, Y.-J.; Lee, S.-J.; Kim, T.-H.; Ahn, S.-G.; Yoon, J.-H.; et al. Diverse toll-like receptors mediate cytokine production by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Infect. Immun. 2014, 82, 1914–1920. [Google Scholar] [CrossRef]
- Groeger, S.; Zhou, Y.; Ruf, S.; Meyle, J. Pathogenic Mechanisms of Fusobacterium nucleatum on Oral Epithelial Cells. Front. Oral Health 2022, 3, 831607. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Fu, X. Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity. Transl. Oncol. 2019, 12, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Noh, E.-J.; Kang, M.-J.; Jeong, Y.-J.; Lee, J.-Y.; Choi, H.-J.; Oh, S.-M.; Lee, K.-B.; Kim, D.-J.; Shin, J.-A.; Cho, S.-D.; et al. Withaferin A inhibits inflammatory responses induced by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Mol. Med. Rep. 2016, 14, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, X.; Liu, A.; Fan, S.; Liu, S.; Li, Z.; Yang, X.; Guo, H.; Wu, M.; Liu, M.; et al. Lactobacillus Reuteri Vesicles Regulate Mitochondrial Function of Macrophages to Promote Mucosal and Cutaneous Wound Healing. Adv. Sci. 2024, 11, e2309725. [Google Scholar] [CrossRef] [PubMed]
- Okahashi, N.; Nakata, M.; Kuwata, H.; Kawabata, S. Streptococcus oralis Induces Lysosomal Impairment of Macrophages via Bacterial Hydrogen Peroxide. Infect. Immun. 2016, 84, 2042–2050. [Google Scholar] [CrossRef]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef]
- Almubarak, A.; Tanagala, K.K.K.; Papapanou, P.N.; Lalla, E.; Momen-Heravi, F. Disruption of Monocyte and Macrophage Homeostasis in Periodontitis. Front. Immunol. 2020, 11, 330. [Google Scholar] [CrossRef]
- Wu, X.; He, W.; Mu, X.; Liu, Y.; Deng, J.; Liu, Y.; Nie, X. Macrophage polarization in diabetic wound healing. Burn. Trauma 2022, 10, tkac051. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Madsen, D.H.; Leonard, D.; Masedunskas, A.; Moyer, A.; Jürgensen, H.J.; Peters, D.E.; Amornphimoltham, P.; Selvaraj, A.; Yamada, S.S.; Brenner, D.A.; et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor–mediated pathway. J. Cell Biol. 2013, 202, 951–966. [Google Scholar] [CrossRef]
- Min, Z.; Yang, L.; Hu, Y.; Huang, R. Oral microbiota dysbiosis accelerates the development and onset of mucositis and oral ulcers. Front. Microbiol. 2023, 14, 1061032. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, C.; Lan, D.; Chen, Y.; Wang, Y.; Qi, S. Porphyromonas gingivalis promote microglia M1 polarization through the NF-κB signaling pathway. Heliyon 2024, 10, e35340. [Google Scholar] [CrossRef]
- Sardar, P.; Almeida, A.; Pedicord, V.A. Integrating functional metagenomics to decipher microbiome–immune interactions. Immunol. Cell Biol. 2024, 102, 680–691. [Google Scholar] [CrossRef]
- Zhu, B.; Bai, Y.; Yeo, Y.Y.; Lu, X.; Rovira-Clavé, X.; Chen, H.; Yeung, J.; Nkosi, D.; Glickman, J.; Delgado-Gonzalez, A.; et al. A multi-omics spatial framework for host-microbiome dissection within the intestinal tissue microenvironment. Nat. Commun. 2025, 16, 1230. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, J.; Jung, S.-H.; Woerner, J.; Suh, E.H.; Lee, D.-G.; Shivakumar, M.; Lee, M.E.; Kim, D. Harnessing Artificial Intelligence in Multimodal Omics Data Integration: Paving the Path for the Next Frontier in Precision Medicine. Annu. Rev. Biomed. Data Sci. 2024, 7, 225–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Thomas, J.P.; Korcsmaros, T.; Gul, L. Integrating multi-omics to unravel host-microbiome interactions in inflammatory bowel disease. Cell Rep. Med. 2024, 5, 101738. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.