Abstract
Bacterial symbionts play an important role in insect survival by contributing to key metabolic and defensive functions. While stingless bees are known to harbor diverse microbial communities, their core bacterial symbionts remain poorly characterized. In this study, we analyzed the gut microbiota of sixteen stingless bee species collected from different regions of Mexico using 16S rRNA gene sequencing on the Illumina® MiSeq™ platform. Our results revealed that Proteobacteria, Firmicutes, and Actinobacteria are the most abundant bacterial phyla across species. Among the dominant genera, lactic acid bacteria, such as Lactobacillus spp., Bifidobacterium, and Fructobacillus spp., were the most prevalent. These bacteria are responsible for developing biochemical functions in metabolic processes like lactic fermentation and the biotransformation of complex organic compounds into molecules that are more easily assimilated by bees. This study offers a novel perspective on the diversity and predicted composition of gut microbiota in Mexican stingless bees. By highlighting differences in microbial communities among species with different feeding habits, our results emphasize the importance of preserving microbial biodiversity in these pollinators.
1. Introduction
The tribe Meliponini (Hymenoptera: Apidae), commonly known as stingless bees, comprises over 60 genera and hundreds of species, including Melipona, Trigona, Tetragonula, and Scaptotrigona and is distributed across tropical and subtropical regions worldwide [1,2]. In the Neotropics, stingless bees are key pollinators that contribute to ecosystem resilience and biodiversity maintenance [1]. Their evolutionary history dates back approximately 80 million years to the Cretaceous period; thus, a long trajectory has shaped their remarkable taxonomic and ecological diversification [2]. Phylogenetic reconstructions have revealed how historical biogeographic and climatic changes have driven lineage diversification within Meliponini [2,3].
The ecological and agricultural relevance of stingless bees extends beyond pollination. Their social organization enhances foraging efficiency, especially for crops like coffee and cacao, while their honey is culturally significant and has been used for its therapeutic properties, including antibacterial and anti-inflammatory effects [4]. However, despite their importance, stingless bees face significant threats from habitat degradation, agrochemical exposure, emerging pathogens, and interspecific competition, especially with the invasive Apis mellifera, which affects native pollinator populations by competing for nesting and floral resources [5,6,7,8,9,10].
While numerous studies have characterized the gut microbiota of the Western honeybee, A. mellifera, uncovering a consistent core composed of Spodgrassella alvi, Gillamella apicola, Frischella perrara, Bifidobacterium, and clades of Lactobacillus (Firm-4 and Firm-5), as well as other lesser-known symbionts like Apibacter and Parasaccaribacter [11,12,13,14,15], the gut microbial communities of stingless bees remain poorly characterized. Notably, Snodgrassella and Gilliamella, which are tightly associated with corbiculate bee linage, appear to be absent or only sporadically detected in stingless bees [11,13,14].
Recent studies have begun to address this gap. Investigations on Australian stingless bees revealed that although lactic acid bacteria (LAB) are commonly found across genera, such as Teragonula and Austroplebeia, the composition of LAB is shaped by phylogenetic lineage and environmental context, suggesting both host-specific and regionally influenced patterns [16]. LAB isolated from Mexican stingless bees, including Melipona beecheii, Scaptotrigona pectoralis, and Plebeia jatiformis, have been shown to include members of Apilactobacillus, Lactiplantibacillus, Weissella, and Leuconostoc, highlighting a diverse and functionally relevant symbiotic assemblage [17].
Given the ecological relevance of stingless bees, their diversity in Mexico, and the limited data available on their microbial symbionts, this study aims to characterize the gut microbiota of 16 stingless bees using high throughput 16S rRNA sequencing. Readers must be aware that the scope of our inferences is mainly limited by an unreplicated design. Notwithstanding, this is, to our knowledge, the first exploration study in Mexico to profile the microbiota of Meliponini using next-generation sequencing (NGS), providing a baseline for future research on microbial ecology, conservation, and bee health.
2. Materials and Methods
2.1. Stingless Bee Collection and Identification
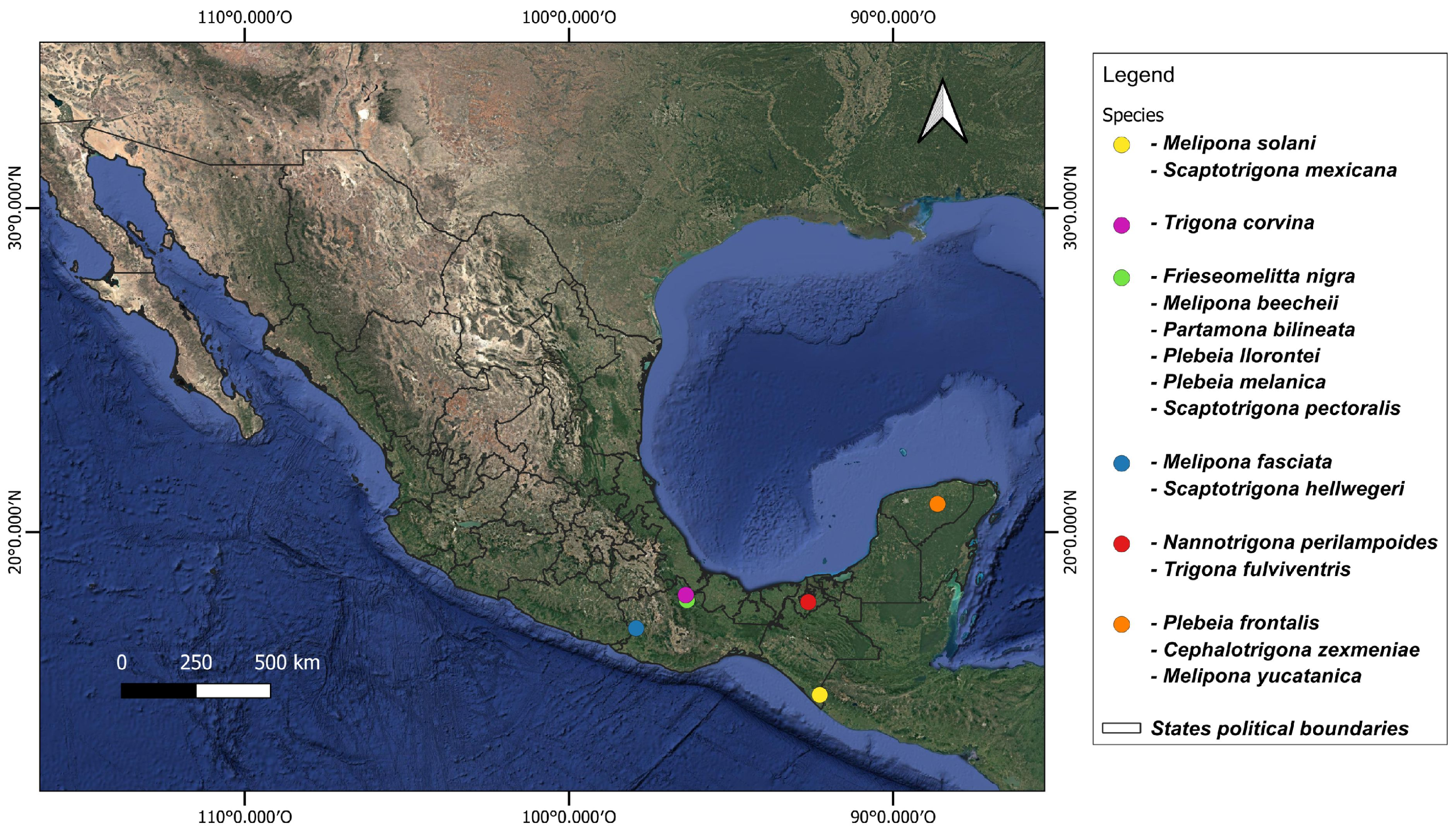
Adult worker bees were obtained from different states in the south of Mexico between May 2021 and October 2023 (Figure 1, Table S1). Specimens were identified based on morphological characteristics with the keys of Ayala-Barajas [18,19] and Arnold et al. [20]. Ten worker individuals per species were sampled directly from hives and stored in a DNA shield until processing.
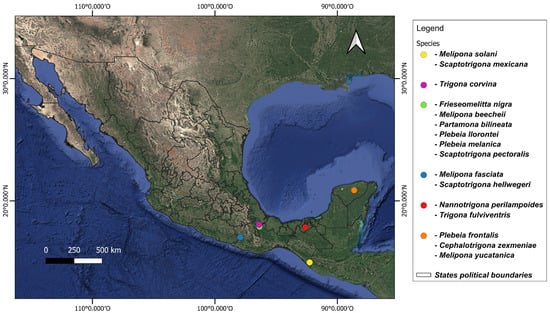
Figure 1.
Sampling sites of stingless bees collected from southern Mexico.
2.2. Gut Collection
The guts from ten worker stingless bees per species were used for tissue isolation. Prior to dissection, adult bees were surface-sterilized with 96% ethanol for three minutes and then rinsed with sterile deionized water. Dissections were performed under a stereomicroscope (Leica MZ16, 1.6X, Wetzlar, Germany) on sterile Petri dishes containing 2 mL of sterile phosphate-buffered solution (10 mM, pH 7.4, Ambion, Thermo Fisher Scientific, Waltham, MA, USA), using a pair of flamed-sterilized needles cooled in 96% ethanol. The dissected guts were transferred into 1.5 mL Eppendorf® tubes containing a DNA shield and stored at 70 °C until DNA extraction and sequencing.
2.3. DNA Extraction and Sequencing
The gut samples were processed using the ZymoBIOMICS® service provided by Zymo Research (Irvine, CA, USA). DNA was extracted using the ZymoBIOMICS®-96 MagBead DNA Kit (Zymo Research). Library preparation followed the protocol described by Gómez-Govea et al. [21], and sequencing was performed on an Illumina® MiSeq™ platform as part of the ZymoBIOMICS™ Service Targeted Metagenomic Sequencing service. Bacterial 16S rRNA gene sequencing targeted the V3–V4 region using the Quick-16S™ NGS Library Prep Kit (Zymo Research).
2.4. Bioinformatic Analysis
The processing of the 16S rRNA gene libraries was carried out using the DADA2 pipeline. Reads were filtered based on quality, and singletons, chimeric sequences, and low-quality reads were removed. Amplicon Sequence Variants (ASVs) were inferred using default parameters [22]. Metadata was then imported to create a phyloseq object (v1.46.0) for downstream analysis in R [23]. ASVs were filtered based on a prevalence threshold of 0.05, retaining only those with a relative abundance greater than 5%. Samples were rarefied to 10,000 reads, and the read counts were transformed into relative abundances. Alpha diversity (Shannon and Chao indexes) and beta diversity (Principal Coordinate Analysis, PCoA) were performed using the microbiome v1.22.0, vegan v2.6-4, stats v4.3.2, and ggplot2 v3.4.4 packages in Rstudio v12.1. Statistical differences in alpha diversity were assessed using a standard t-test (stats v4.3.2). Pairwise comparisons were conducted only among bee genera represented by more than one species: Melipona, Plebeia, Trigona, and Scaptotrigona.
The top 20 bacterial taxa at the phylum, family, genus, and species levels were visualized using stacked bar plots. PCoA based on Bray–Curtis distances was performed to visualize differences between groups in beta diversity. Additionally, differences in taxa abundance between the genera Melipona and Trigona were explored through marker bacteria analysis, motivated by their distinct feeding behaviors. Differences between M. beecheii (an obligate pollinivore) and T. corvina (a facultative necrophage) were tested. Differentially abundant ASVs were identified using DEseq2, fold changes (FC > 1), and the False Discovery Rate (FDR) > −log (0.05) was visualized via volcano plots generated with EnhancedVolcano v1.18.0 [24,25].
3. Results
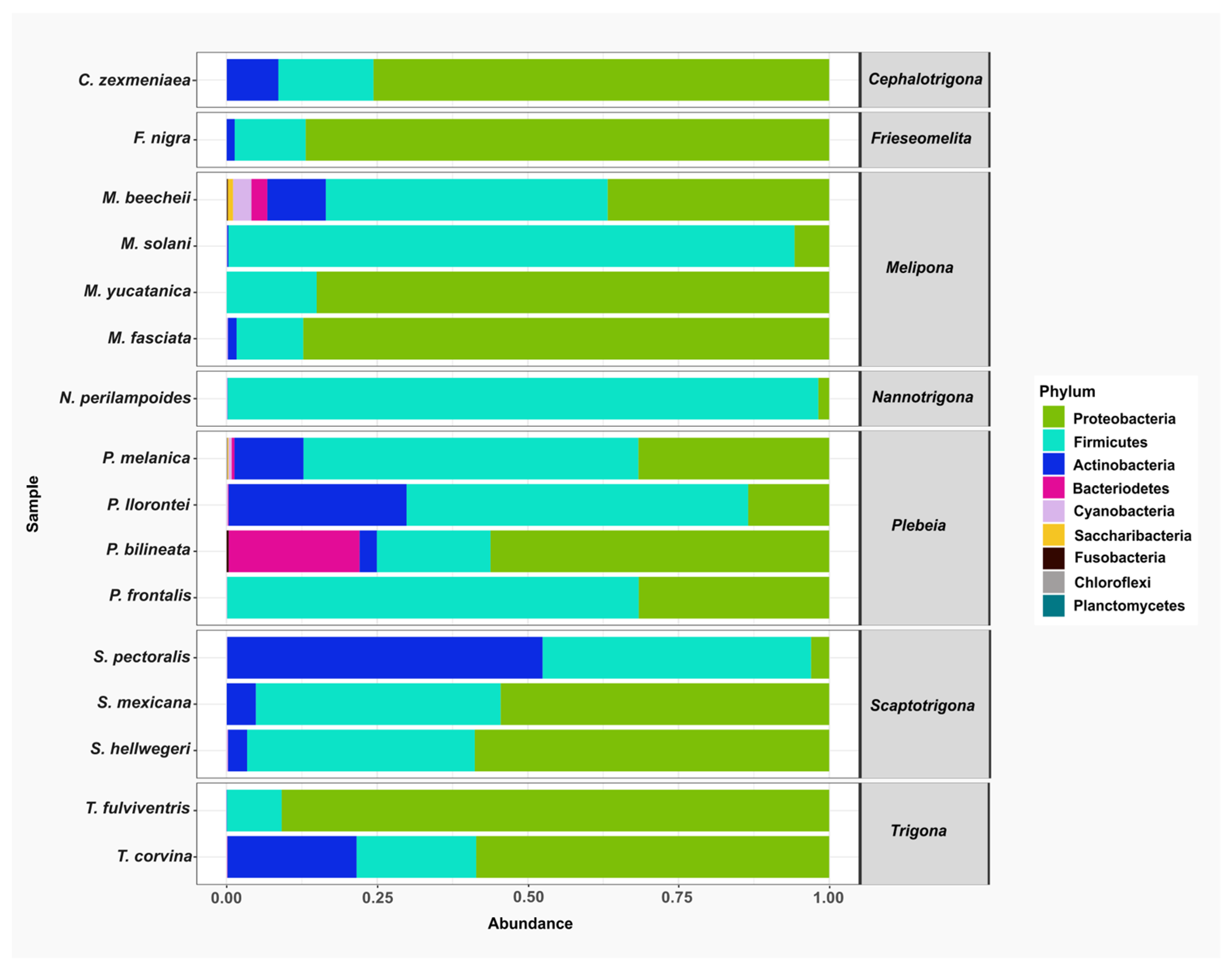
A total of nine bacterial phyla were identified across all stingless bee species: Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, Cyanobacteria, Saccaribacteria, Fusobacteria, Chloroflexi. and Planctomycetes. Among these, Proteobacteria, Firmicutes, and Actinobacteria were the most abundant and were detected in nearly all the species analyzed. In contrast, Chloroflexi, Planctomycetes, Saccharibacteria, and Fusobacteria were found in only three species and represented the least abundant phyla (Figure 2).
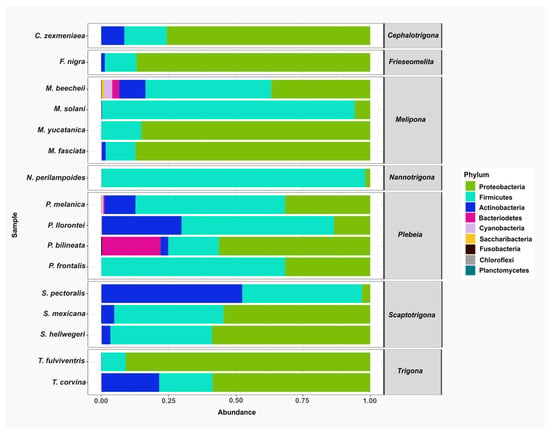
Figure 2.
The relative abundance of bacterial phyla in gut samples from stingless bee species. Bar plots represent the composition of the most abundant phyla across the bee species analyzed. The predominant phyla were Proteobacteria (green), Firmicutes (aqua), and Actinobacteria (blue).
At the class level, Bacilli was among the top 20 most abundant classes and was consistently detected in all the species analyzed. Other frequently observed classes included Actinobacteria, which were present in Scaptotrigona pectoralis, S. mexicana, S. hellwegeri, Plebeia melanica, P. llorontei, P. bilineata, Melipona beechii, M. solani, M. fasciata, Trigona corvina, Frieseomelitta nigra, and Cephalotrigona zexmeniae. Within the Proteobacteria group, Alphaproteobacteria was found in all stingless bee species analyzed.
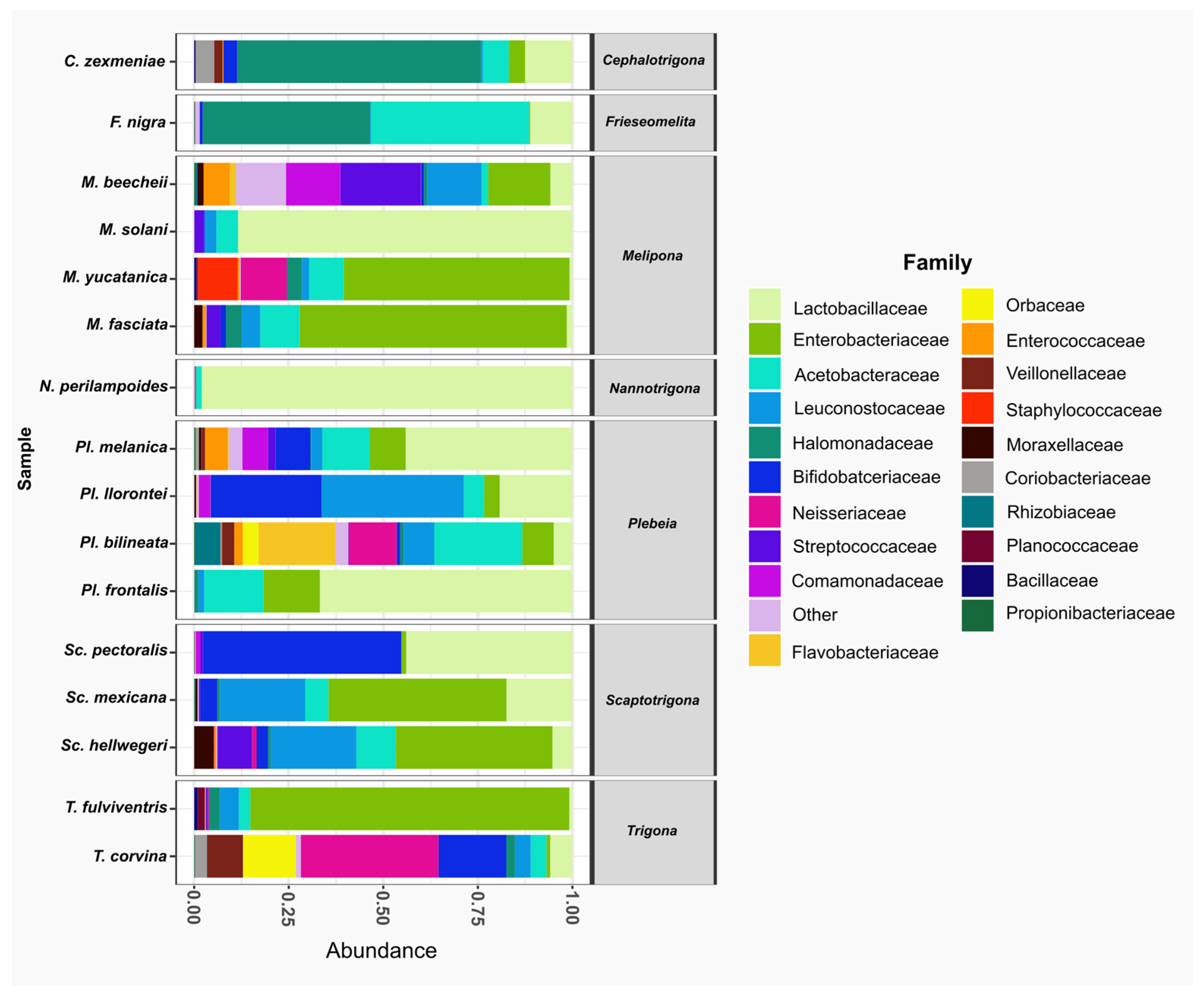
At the family level, Lactobacillaceae was the most abundant family, present in all species. It was the dominant family in Nannotrigona perilampoides and Melipona solani, while in M. yucatanica and Trigona fulviventris, it was the least abundant. The second most abundant family was Enterobacteriaceae, also present in all bee species, followed by Acetobacteraceae, which was absent only in Scaptotrigona pectoralis. In general, within the top 20 families, T. corvina, P. bilineata, and M. beecheii had microbiomes composed of 18, 17, and 16 families, respectively (Figure 3).
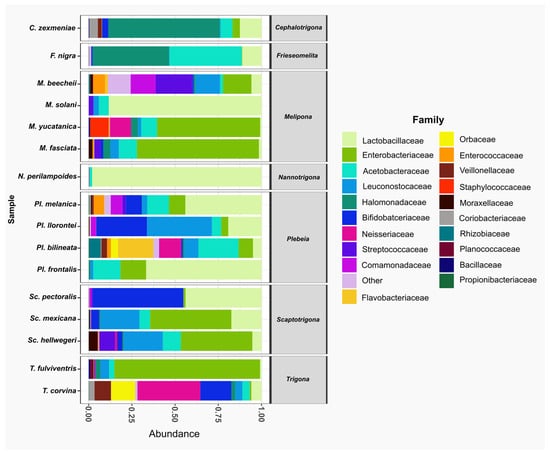
Figure 3.
The relative abundance of bacterial families in gut samples from stingless species. Bar plots represent the composition of the most abundant families across the bee species analyzed. The predominant family was Lactobacillaceae (light green), present in all species. Other frequent families included Enterobacteriaceae (green) and Acetobacteraceae (aqua).
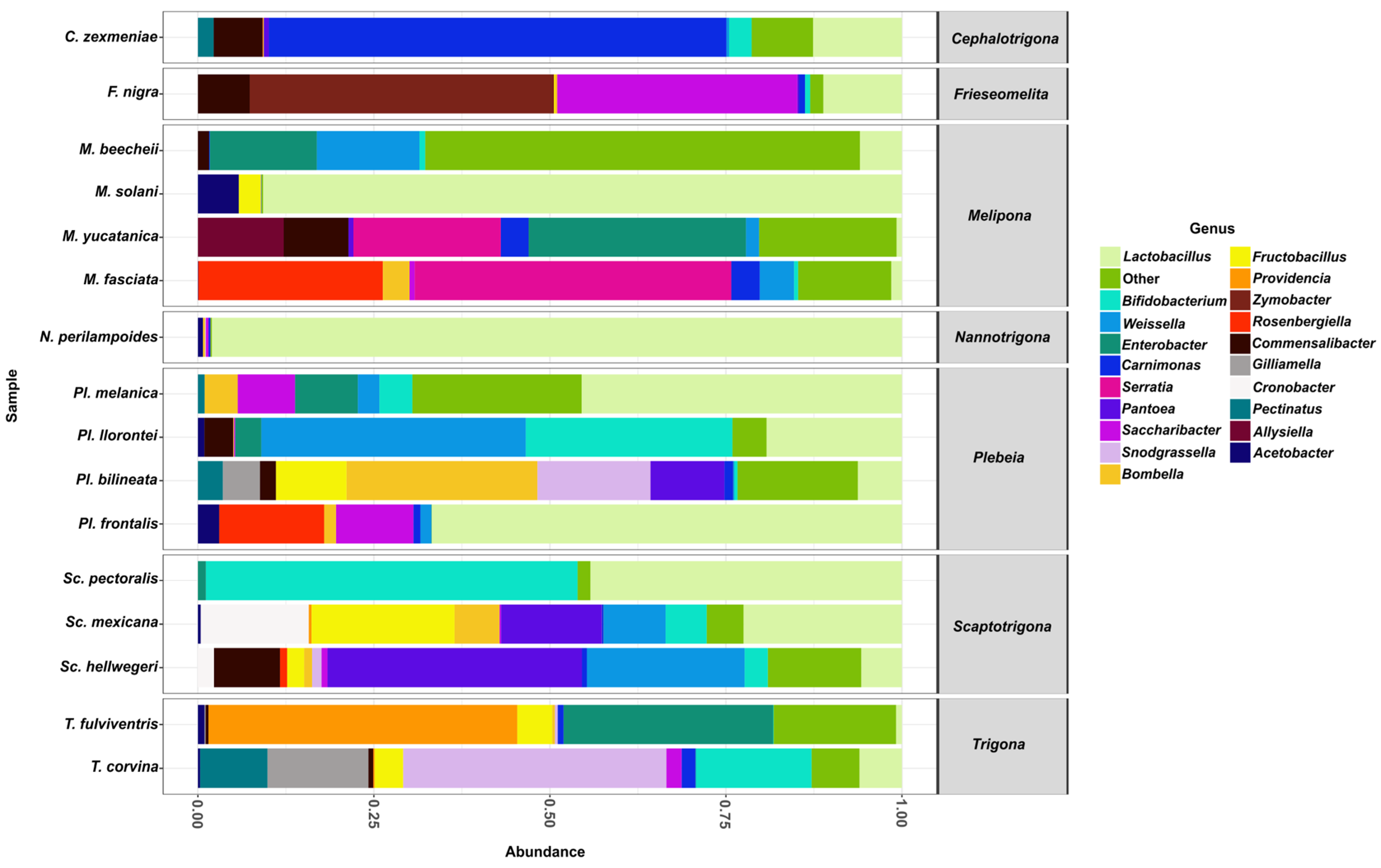
From the aforementioned families, the 20 most abundant genera were Lactobacillus, Bifidobacterium, Weissella, Enterobacter, Carnimonas, Serratia, Pantoea, Saccharibacter, Snodgrassella, Bombella, Fructobacillus, Providencia, Zymobacter, Rosenbergiella, Commensalibacter, Gilliamella, Cronobacter, Pectinatus, Alysiella, and Acetobacter.
Among these genera, Lactobacillus was found in all stingless bee species analyzed (Figure 4) and was represented by five distinct species: Lactobacillus sp29216, Lactobacillus suebicus, Lactobacillus sp29206, Lactobacillus oris, and Lactobacillus vaccinostercus. L. sp29216 was present in all 16 bee species, whereas L. vaccinostercus was found only in P. llorontei, M. solani, N. perilampoides, F. nigra, P. frontalis, and M. fasciata.
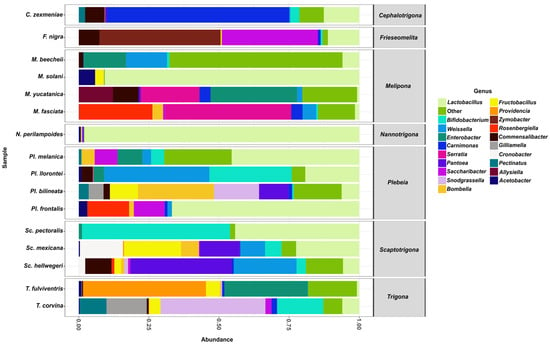
Figure 4.
The relative abundance of bacterial genera in gut samples from stingless bee species. Bar plots represent the composition of the most abundant bacterial genera across all bee species analyzed. Lactobacillus (light green) was the dominant genus in most species.
The second most abundant genus was Bifidobacterium, which was detected in most species except for N. perilampoides, M. yucatanica, and P. frontalis. This genus was represented by a single species, Bifidobacterium sp4969, which was found to be present in most species except for N. perilampoides, M. yucatanica, and P. frontalis. The third most abundant genus was Weissella, present in most species except S. pectoralis, T. fulviventris, M. solani, N. perilampoides, and F. nigra. It was represented by the species Weissella paramesenteroides, found in several species, including P. melanica, M. beecheii, P. llorontei, S. mexicana, P. bilineata, M. yucatanica, C. zexmeniae, P. frontalis, M. fasciata, and S. hellwegeri.
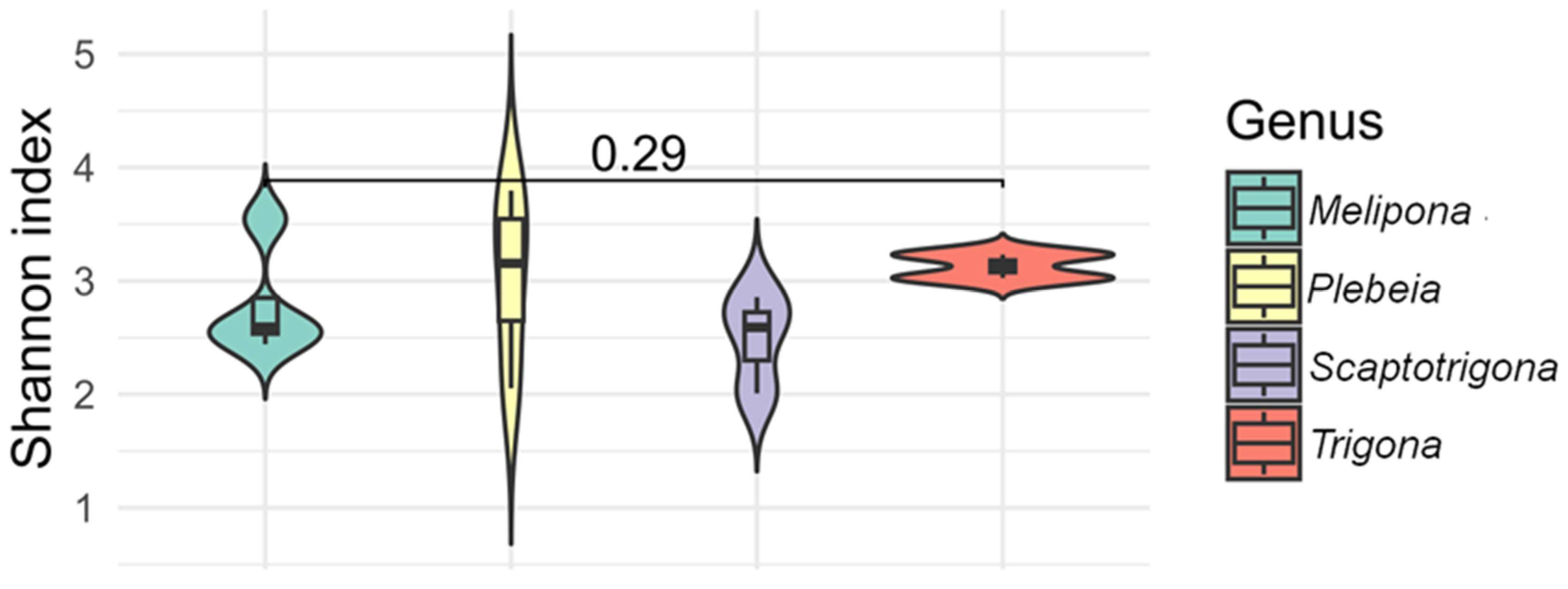
The alpha diversity index (Shannon) indicated that species from the genera Melipona, Plebeia, Scaptotrigona, and Trigona exhibited the most diverse bacterial communities, with values ranging from 1.9 to 3.7. Within Melipona, M. beecheii exhibited the highest diversity, while M. solani, M. yucatanica, and M. fasciata showed similar values. In Plebeia, P. melanica and P. bilineata had similar values (3.4 and 3.7, respectively), as did P. llorontei and P. frontalis (2.0 and 2.8, respectively). Alpha diversity values in Scaptotrigona species were approximately 2.0, and values in Trigona species were around 3.0 (Figure 5). No statistically significant differences were observed for alpha diversity (p > 0.05). The species with the highest observed bacterial richness were Partamona bilineata, followed by M. beecheii, Plebeia melanica, Trigona fulviventris, and T. corvina, whereas Scaptotrigona pectoralis and Frieseomelitta nigra exhibited the lowest diversity.
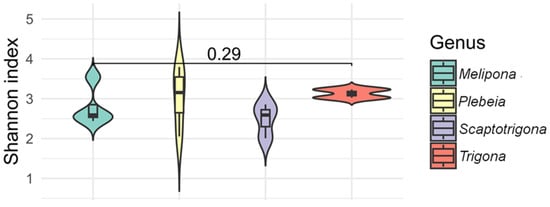
Figure 5.
The alpha diversity (Shannon index) of gut bacterial communities across stingless bee genera. Violin plots show the distribution of Shannon index values for species within the genera Melipona, Plebeia, Scaptotrigona, and Trigona. All four genera exhibited relatively high bacterial diversity, with no statistical differences observed between them (p = 0.29).
The Chao index revealed that P. bilineata had the highest estimated bacterial richness, followed by T. corvina and M. beecheii. In contrast, S. pectoralis and P. frontalis exhibited the lowest richness, with 39 and 53 observed species, respectively.
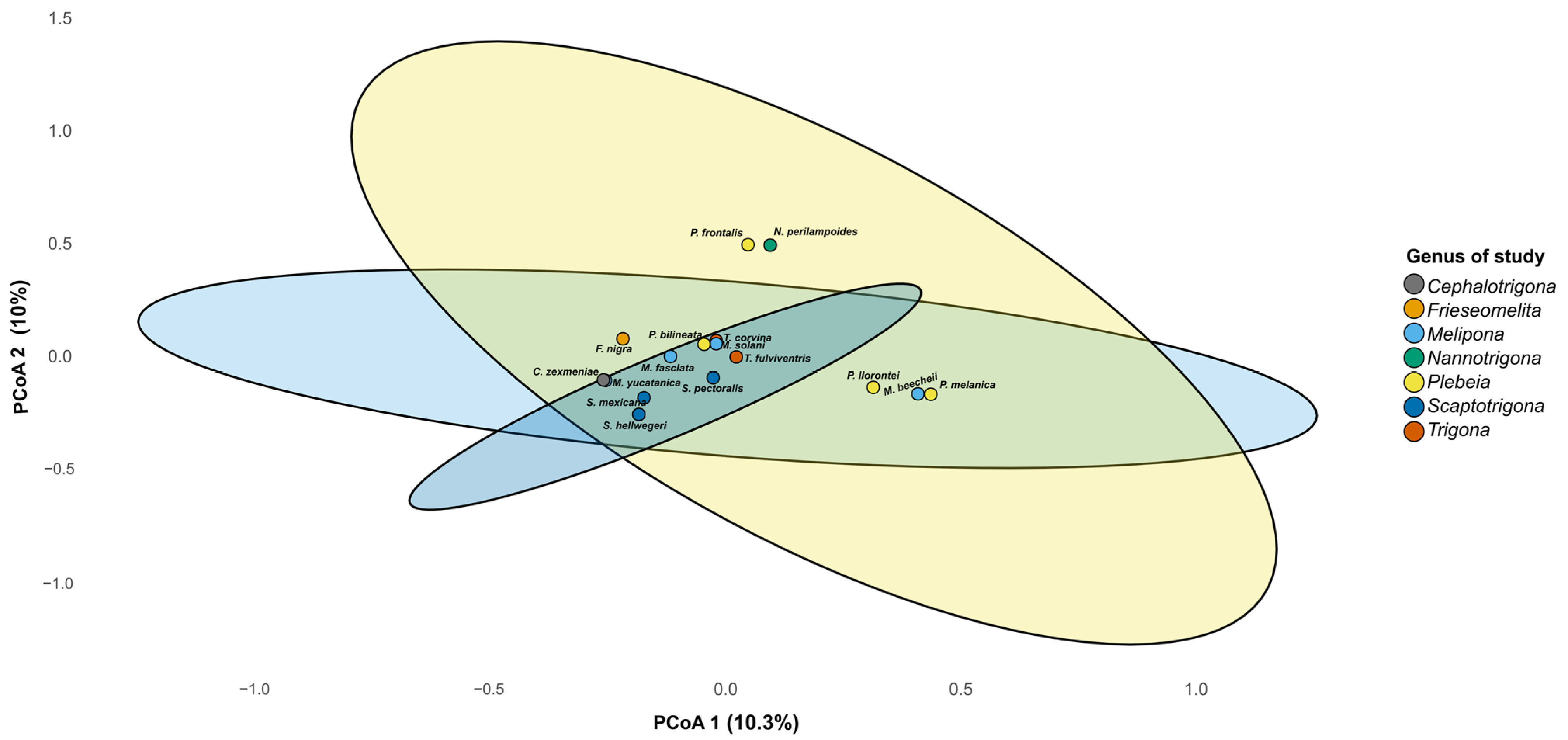
Beta diversity analysis revealed differences in microbiome composition among bee genera, with three clustering patterns observed on PCoA 1 in the plot (Figure 6). One cluster was composed of P. llorontei, P. melanica, and M. beecheii; a second cluster was composed of P. frontalis and N. perilampoides; and a third cluster included the rest of the taxa. These differences were statistically supported by the PERMANOVA (R2 = 0.43, F = 1.13, p = 0.009). On PCoA 2, P. frontalis and N. perilampoides were grouped and separated from the rest of the taxa.
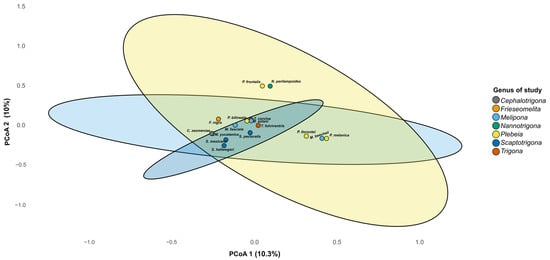
Figure 6.
Principal coordinate analysis (PCoA) based on Bray–Curtis distances of gut microbiota from stingless bee genera. The PCoA plot shows the partial clustering of bacterial community composition according to bee genus. Each point represents the microbiome of one species, and ellipses indicate the 95% confidence interval for each genus. The first two axes explain 20.3% of the total variance (PCo1 = 10.3%, PCo2 = 10.0%).
Bacteria Marker Analysis of Bacterial Communities in Melipona and Trigona
A comparative analysis of the bacterial communities of Melipona and Trigona species was carried out to identify marker bacteria, considering differences in their foraging behavior. For example, M. beecheii forages exclusively on plants and is managed for honey production, whereas T. corvina is a facultative species that also feed on carrion. Melipona and Trigona share several bacterial genera, including Lactobacillus spp., Citrobacter spp., Enterobacter spp., and Klebsiella spp. However, species-level comparisons revealed distinct patterns of abundance.
In Melipona, the most abundant bacterial species included Acinetobacter apisnectaris, Actinobacillus capsulatus, Aquabacterium parvum, Asaia astilbis, Bergeyella zoohelcum, Citrobacter rodentium, Delftia lacustris-tsuruhatensis, Enterobacter ludwigii, Fluviicola sp16240, Lactobacillus sp29166, Lactobacillus sp29206, Lactobacillus sp29216, Lactobacillus suebicus, Lactobacillus vaccinostercus, Moraxella cuniculi, Pedobacter insulae, Prevotella timonensis, Pseudomonas otitidis, Ralstonia pickettii, Roseburia sp33131, Serratia marcescens and Weissella paramesenteroides. These taxa were less abundant in Trigona.
In contrast, the most abundant bacterial species in Trigona included Bifidobacterium callitrichos, Bifidobacterium sp4969, Bifidobacterium sp4969, Citrobacter amalonaticus, Enterobacter aerogenes, Escherichia coli, Fructobacillus ficulneus, Fructobacillus pseudoficulneus, Gilliamella apicola, Gluconobacter oxydans-uchimurae, Klebsiella pneumonia, Lactobacillus mellis, Lactobacillus sp29206, Pectinatus frisingensis, Proteus mirabilis, Providencia rettgeri-vermicola, Saccharibacter sp45729 and Snodgrassella alvi.
4. Discussion
In this study, we report for the first time the characterization of the gut microbiota of sixteen Mexican stingless bees (Apidae: Meliponini). Despite the fact that differences in alpha diversity were not found, beta diversity suggested that microbiome composition differed significantly for some taxa. For example, the grouping of P. llorontei, P. melanica, and M. beecheii may indicate differences linked to diet, as they were reared by the same beekeeper. On the other hand, P. frontalis and N. perilampoides were collected at different locations on the side of the Gulf of Mexico. Moreover, the exploratory analysis contrasting feeding habits suggests differences in microbiome composition. Because our hypothesis is limited by the lack of replicates, which may, for instance, lead to hidden natural biological variation, a higher risk of statistical errors, or weak conclusions [26], our exploratory results must be used with caution. Despite this, they serve as a reference for further studies on the microbiome of Mexican stingless bees.
The gut microbiota plays essential roles in digestion, the synthesis of bioactive compounds, and immune protection and is key to individual well-being and colony cohesion [12]. However, anthropogenic factors such as habitat fragmentation, pollution, and pesticides have been shown to significantly alter the composition and functionality of this microbiota in social bees, affecting their ability to metabolize nutrients, resist infections, and adapt to environmental changes [27]. We found that Firmicutes, Actinobacteria, and Proteobacteria were the most dominant and conserved phyla across all species analyzed.
Within the order Hymenoptera, most studies on gut bacterial communities have focused on Apis mellifera, where five core species of bacteria have been consistently identified in the intestinal tract: Snodgrassella alvi, Gilliamella apicola, Lactobacillus spp., and Bifidobacterium spp. These bacteria are commonly found in the adult worker A. mellifera worldwide [27].
It has been suggested that the emergence of eusocial corbiculate bees (a group of bees that have pollen baskets on their hind legs) coincided with the acquisition of the following key gut bacterial lineages: Snodgrassella, Gilliamella, Lactobacillus (Firm-4 and Firm-5), and Bifidobacterium. However, our findings indicate that not all stingless bee species harbor these genera. For example, Snodgrasella and Gilliamella were found in Trigona corvina and Partamona bilineata but not in Melipona species. This observation is consistent with previous reports describing the absence of Snodgrassella and Gilliamella in members of the Meliponini tribe [15,28,29,30].
Recently, Cerqueira et al. [15] demonstrated that the gut microbiota of Partamona helleri, Plebeia droyana, Trigona spinipes, Tetragonula angustula, and T. carbonaria included both Snodgrassella and Gilliamella, whereas Melipona spp. lacked these bacteria, consistent with our observations. Hall et al. [31] suggest that these genera have been replaced in Melipona spp. by new symbionts that fulfill equivalent or even enhanced metabolic functions. However, a study by Sarton-Lohéach et al. [32] reported the presence of Snodgrasella in some Melipona colonies. This suggests that the genus is not entirely absent in Melipona but may be occasionally acquired from other bees, with its prevalence potentially varying according to season, bee age, and colony development. The microbiota of Meliponini is highly variable, which may reflect the evolutionary diversity of their life histories, morphologies, and behaviors. No single bacterial phylotype has been consistently reported across all bee species [33].
One of the earliest microbiome studies on the intestinal tract of stingless bees was conducted on Melipona quadrifasciata, where Bacillus meliponotrophicus was found to be associated with Trigona and Melipona but not with Apis or Bombus, despite their phylogenetic proximity to stingless bees [32]. Later, Nogueira-Neto [34] suggested that B. meliponotrophicus was likely involved in the pre-digestion of honey and pollen in M. quadrifasciata. Additional studies also identified Bacillus species in M. quadrifasciata [35,36,37].
Among the few studies conducted in Mexico on the effects of lactic acid bacteria (LAB), Torres-Moreno et al. [17] found that LAB can provide beneficial effects, including reducing bacterial and parasitic infections and increasing honey production in hives. They analyzed the gut microbiota of Melipona beecheii, Scaptotrigona pectoralis, Plebeia llorentei, and Plebeia jatiformis, identifying the presence of Lactiplantibacillus plantarum, Weissella paramesenteroides, Leuconostoc citreum, and Apilactobacillus. These authors demonstrated that L. plantarum, W. paramesenteroides, and L. citreum are heterofermentative and exhibit a strong capacity to hydrolyze complex plant-derived carbohydrates present in pollen and other plant structures, such as arabinose, cellobiose, fructose, maltose, mannitol, melibiose, raffinose, sucrose, sorbitol, and xylose [17,38,39]. This metabolic capacity has been associated with the feeding habits of bees, which provide bacteria with a nutrient-reach environment derived from pollen and nectar [40]. The ability to ferment a wide variety of carbohydrates is considered a desirable trait for potential zootechnical probiotic applications in bees, as LAB can enhance the assimilation of nutrients from plant-based sources [38,40].
Torres-Moreno et al. [17] determined that these bees possess a resilient microbiota related to their specific environmental conditions and diet. Furthermore, the isolated bacterial strains may contribute to honey production or fermentation, making them potentially valuable for fermented food processing or as zootechnical probiotics for bees. Among the bacteria reported by these authors, W. paramesenteroides was also identified in our study in several species, including Plebeia spp., Scaptotrigona hellwegeri, S. pectoralis, Melipona beecheii, and M. yucatanica. It is possible that this bacterium performs similar functions in these species as previously described in other stingless bees. Another species found in our study was Bombella intestine, which was found in Partamona bilineata, Plebeia melanica, and Scaptotrigona mexicana. B. intestini has been reported to produce acids from various carbohydrates, including sucrose, d-fructose, d-glucose, d-mannitol, d-galactose, d-mannose, and l-arabinose [41].
Several studies suggest that lactic acid bacteria, such as Lactobacillus spp., Bifidobacterium, and Fructobacillus spp., are among the most abundant groups in the hindgut of stingless bees. These bacteria are responsible for developing biochemical functions in metabolic processes, including lactic fermentation and the biotransformation of complex organic compounds into molecules that are more easily assimilated by bees. In addition, they act as probiotics, helping to prevent digestive infections [16,27,28,30,42,43,44,45]. In our study, Lactobacillus was present in all species analyzed; Bifidobacterium was the second most abundant genus; and Weissella ranked third. Other studies on the microbiome of stingless bees have been conducted in species such as Lepidotrigona terminata, Lepidotrigona ventralis, and Tetragonula pagdeni, where bacteria including Acetobacter, Snodgrassella, Lactobacillus, Psychrobacter, Pseudomonas, and Bifidobacterium have been identified. The relative abundance of these bacterial groups varied among communities within the same bee species, likely influenced by multiple factors such as dietary preferences, age, and genetic differences [15].
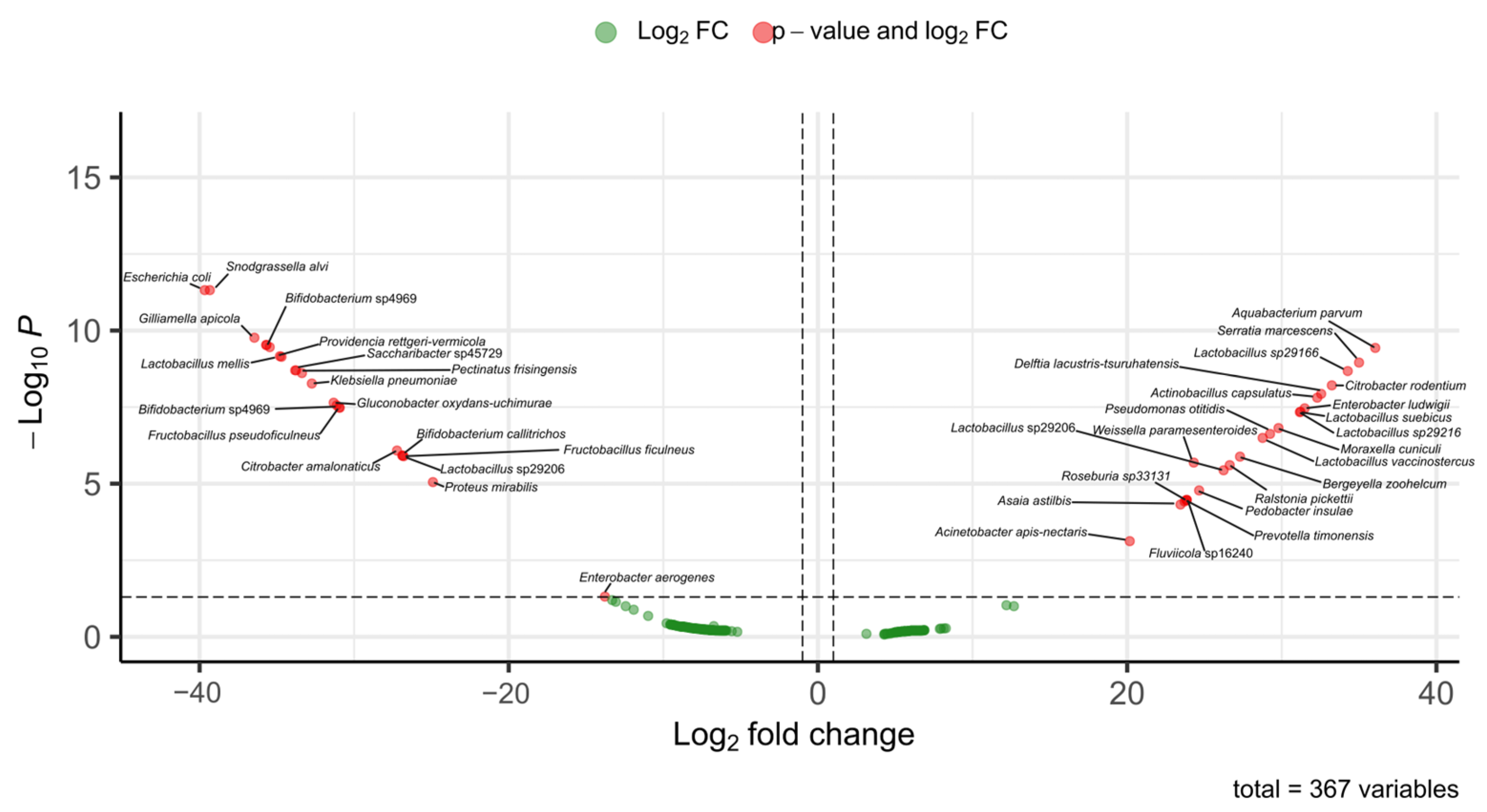
Among stingless bees, vulture bees are specialized in the consumption of rotten meat. Studies conducted to determine whether the gut microbiota of vulture bees differs from that of bees that feed on nectar and pollen have shown that their microbiome composition is different, likely due to their diet types. It has been suggested that bacteria capable of producing lactic and acetic acids may play a role in digestion and pathogen defense [45]. In our study, two species identified as vulture bees, Trigona corvina and P. bilineata, were included. These species are facultative necrophages, and the relative abundance of their gut microbiota differs from that of pollinivorous species such as M. beecheii (Figure 7).
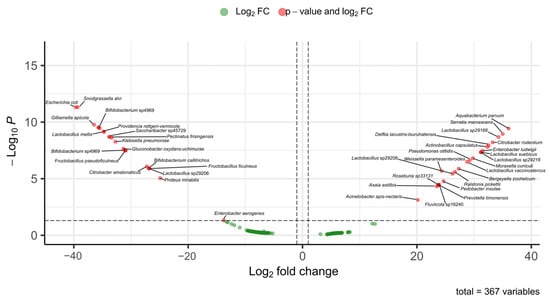
Figure 7.
Differentially abundant bacterial taxa between Melipona and Trigona based on fold-change analysis. The volcano plot shows ASVs with significant differences in abundance between the microbiomes of Melipona and Trigona. Red dots indicate bacterial taxa with both significant p-values and log2 FC, while green dots correspond to taxa with fold change values that did not reach statistical significance. Positive log2 FC values indicated enrichment in Trigona, whereas negative values indicated enrichment in Melipona. A total of 367 bacterial variables were analyzed.
In previous studies on these species, Maccaro et al. [46] reported the presence of bacteria such as Commensalibacter, Pseudomonas, Escherichia, and Enterobacter in facultative necrophagous bees. In our study, we identified some of these bacterial genera in Trigona spp. classified as facultative necrophages. Several of the bacteria found in high abundance in these bees are considered opportunistic human pathogens and are known for their antibiotic resistance and ability to form biofilms, including Pseudomonas, Myroides, Bacteroides, Serratia, Enterococcus, Klebsiella, Salmonella, and Enterobacter [47,48,49]. The ability to form biofilms in response to environmental stress or antimicrobial compounds from competing microbes may confer a symbiotic advantage to bees [46].
In another study, Ntougias et al. [49] found that the obligate necrophagous bee harbored several bacterial taxa associated with fatty or salted meat, including Carnimonas nigrificans, Lactobacillus sp., Halomonas sp., and Hatalea alkaline. In our study, we also detected C. nigrificans in T. corvina. In this context, Maccaro et al. [46] found that several bacteria are likely acquired from the environment but selectively retained based on dietary needs. Similarly, Figueroa et al. [45] suggest that the vulture bee microbiome adapts to the host’s novel diet through a combination of novel symbiont recruitment, the loss of some ancestral microbes, and the possible adaptation of others.
Overall, the observed differences in microbiota composition among species and genera likely reflect a combination of dietary specialization, environmental exposure, and phylogenetic history. However, one limitation of our exploratory study is the absence of independent biological replicates for each species, which restricts our ability to assess species-specific microbiome variability. Although we included 10 individuals per species, all were sampled from a single colony or locality, limiting the scope of ecological and geographic comparisons within species. Future studies, including multiple colonies and environmental contexts, will be essential to validate the observed taxonomic patterns and to further understand the factors shaping gut microbiota diversity in stingless bees.
5. Conclusions
This study offers a novel perspective on the diversity and predicted composition of gut microbiota in Mexican stingless bees. By revealing differences in microbial communities among species with different feeding habits, our findings emphasize the importance of preserving microbial biodiversity in these bees. Considering that many stingless bee species are threatened by environmental pressures such as habitat loss and competition from introduced species, conserving their associated microbiota could be key to ensuring their survival.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms13071645/s1. Table S1: Collection dates and localities of stingless bee species (species, locality, coordinates, date, altitude, and identifier). File Genus-Species. File sv. Sequences.
Author Contributions
First conceived the idea: M.A.G.-G., M.d.L.R.-A. and I.P.R.-S.; carried out the experiments: M.d.L.R.-A., M.A.G.-G., K.I.P.-C., M.L.J.-M., G.d.J.T.-R., M.E.-R., A.G.V., J.I.G.-R., D.R.-P. and I.P.R.-S. collected the data: M.A.G.-G., K.I.P.-C., A.G.V., A.E.F., M.d.L.R.-A. and I.P.R.-S. conducted the data analysis: M.d.L.R.-A., M.A.G.-G., K.I.P.-C., M.L.J.-M., G.d.J.T.-R., M.E.-R., A.G.V., J.I.G.-R., D.R.-P. and I.P.R.-S., supervised the findings of the work: A.E.F., A.G.V. and I.P.R.-S., discussed the results: M.d.L.R.-A., M.A.G.-G., K.I.P.-C., M.L.J.-M., G.d.J.T.-R., M.E.-R., A.G.V., J.I.G.-R., D.R.-P. and I.P.R.-S., wrote the final manuscript version: M.d.L.R.-A., M.A.G.-G., K.I.P.-C., M.L.J.-M., G.d.J.T.-R., M.E.-R., A.G.V., J.I.G.-R., D.R.-P. and I.P.R.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The first author gives thanks for the support they received from the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI) for her postdoctoral scholarship. Also, many thanks go to Benjamin Gregorio Ramírez for providing us with his stingless bees and sharing his knowledge of Meliponiculture. We also thank José Cortés Larriva and Rodrigo Navarro Meneses of RESERBALAM and Luis Miguel Canseco Avila (Universidad Autónoma de Chiapas) for providing the bee specimens from their meliponaries.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Heard, T.A. The role of stingless bees in crop pollination. Annu. Rev. Entomol. 1999, 44, 183–206. [Google Scholar] [CrossRef]
- Rasmussen, C.; Cameron, S.A. Global stingless bee phylogeny supports ancient divergence, vicariance, and long-distance dispersal. Biol. J. Linn. Soc. 2010, 99, 206–232. [Google Scholar] [CrossRef]
- Engel, M.S. A monograph of the Baltic amber bees and evolution of the Apoidea (Hymenoptera). Bull. Am. Mus. Nat. Hist. 2001, 259, 1–192. [Google Scholar] [CrossRef]
- Vit, P.; Medina, M.; Enriquez, M.E. Quality standards for medicinal uses of meliponinae honey in Guatemala, Mexico and Venezuela. Bee World 2004, 85, 2–5. [Google Scholar] [CrossRef]
- Brown, J.C.; Albrecht, C. The effect of tropical deforestation on stingless bees in Central America and Brazil. Biol. Conserv. 2001, 97, 83–89. [Google Scholar] [CrossRef]
- Roubik, D.W. Stingless bee nesting biology. Apidologie 2006, 37, 124–143. [Google Scholar] [CrossRef]
- Slaa, E.J.; Sánchez Chaves, L.A.; Malagodi-Braga, K.S.; Hofstede, F.E. Stingless bees in applied pollination: Practice and perspectives. Apidologie 2006, 37, 293–315. [Google Scholar] [CrossRef]
- Brosi, B.J.; Daily, G.C.; Ehrlich, P.R. Bee community shifts with landscape context in a tropical countryside. Ecol. Appl. 2008, 18, 1821–1832. [Google Scholar] [CrossRef]
- Elliott, B.; Wildon, R.; Shapcott, A.; Keller, A.; Newis, R.; Cannizarro, C.; Burwell, C.; Smith, T.; Leonhardt, S.D.; Kämper, W.; et al. Pollen diets and niche overlap of honey bees and native bees in protected areas. Basic Appl. Ecol. 2021, 50, 169–180. [Google Scholar] [CrossRef]
- Heard, T.A.; Dollin, A.E. Stingless bee keeping in Australia: Snapshot of an infant industry. Bee World 2000, 81, 116–125. [Google Scholar] [CrossRef]
- Bonilla-Rosso, G.; Engel, P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr. Opin. Microbiol. 2018, 43, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.A. Genomics of the honey bee microbiome. Curr. Opin. Insect Sci. 2015, 10, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.; Ratnayeke, N.; Moran, N.A. Strain diversity and host specificity in a specialized gut symbiont of honeybees and bumblebees. Mol. Ecol. 2016, 25, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.; Nastasa, A.; Chapman, A.; Kwong, W.K.; Foster, L.J. The honey bee gut microbiota: Strategies for study and characterization. Insect Mol. Biol. 2019, 28, 455–472. [Google Scholar] [CrossRef]
- Tang, Q.H.; Miao, C.H.; Chen, Y.F.; Dong, Z.X.; Cao, Z.; Liao, S.Q.; Wang, J.X.; Wang, Z.W.; Guo, J. The composition of bacteria in gut and beebread of stingless bees (Apidae: Meliponini) from tropics Yunnan, China. Antonie Leeuwenhoek 2021, 114, 1293–1305. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Kaltenpoth, M. Microbial communities of three sympatric Australian stingless bee species. PLoS ONE 2014, 9, e105718. [Google Scholar] [CrossRef]
- Torres-Moreno, R.; Humberto, S.H.S.; Méndez-Tenorio, A.; Palmeros-Sánchez, B.; Melgar-Lalanne, G. Characterization and identification of lactic acid bacteria from Mexican stingless bees (Apidae: Meliponini). IOP Conf. Ser. Earth Environ. Sci. 2021, 858, 012010. [Google Scholar] [CrossRef]
- Ayala, R. Revisión de las abejas sin Aguijón de México (Hymenoptera: Apidae: Meliponini). Folia Entomol. Mex. 1999, 106, 1–123. [Google Scholar]
- Ayala-Barajas, R. Las abejas del género Plebeia Schwarz (Apidae: Meliponini) de México. Entomol. Mex. 2016, 3, 937–942. [Google Scholar]
- Arnold, N.; Zepeda, R.; Vásquez, V.D.; Maya, E.M.A. Las Abejas sin Aguijón y su Cultivo en Oaxaca; ECOSUR El Colegio de la Frontera Sur: San Cristóbal de Las Casas, Chiapas, Mexico, 2019; pp. 1–193. [Google Scholar]
- Gómez-Govea, M.A.; Ramírez-Ahuja, M.D.L.; Contreras-Perera, Y.; Jiménez-Camacho, A.J.; Ruiz-Ayma, G.; Villanueva-Segura, O.K.; Trujillo-Rodríguez, G.J.; Delgado-Enciso, I.; Martínez-Fierro, M.L.; Manrique-Saide, P.; et al. Suppression of midgut microbiota impact pyrethroid susceptibility in Aedes aegypti. Front. Microbiol. 2022, 13, 761459. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. R Package Version 1.26.0. 2025. Available online: https://bioconductor.org/packages/EnhancedVolcano (accessed on 30 May 2024).
- Larios Serrato, V.; Meza, B.; Gonzalez-Torres, C.; Gaytan-Cervantes, J.; González Ibarra, J.; Santacruz Tinoco, C.E.; Torres, J. Diversity, composition, and networking of saliva microbiota distinguish the severity of COVID-19 episodes as revealed by an analysis of 16S rRNA variable V1–V3 region sequences. mSystems 2023, 8, e01062-22. [Google Scholar] [CrossRef]
- Raymann, K.; Moran, N.A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 2018, 26, 97–104. [Google Scholar] [CrossRef]
- Botina, L.L.; Vélez, M.; Barbosa, W.F.; Mendonça, A.C.; Pylro, V.S.; Tótola, M.R.; Martins, G.F. Behavior and gut bacteria of Partamona helleri under sublethal exposure to a bioinsecticide and a leaf fertilizer. Chemosphere 2019, 234, 187–195. [Google Scholar] [CrossRef]
- Cerqueira, A.E.S.; Hammer, T.J.; Moran, N.A.; Santana, W.C.; Kasuya, M.C.M.; da Silva, C.C. Extinction of anciently associated gut bacterial symbionts in a clade of stingless bees. ISME J. 2021, 15, 2813–2816. [Google Scholar] [CrossRef]
- Cerqueira, A.E.S.; Lima, H.S.; Silva, L.C.F.; Veloso, T.G.R.; de Paula, S.O.; Santana, W.C.; da Silva, C.C. Melipona stingless bees and honey microbiota reveal the diversity, composition, and modes of symbionts transmission. FEMS Microbiol. Ecol. 2024, 100, fiae063. [Google Scholar] [CrossRef]
- Hall, M.A.; Brettell, L.E.; Liu, H.; Nacko, S.; Spooner-Hart, R.; Riegler, M.; Cook, J.M. Temporal changes in the microbiome of stingless bee foragers following colony relocation. FEMS Microbiol. Ecol. 2021, 97, fiaa236. [Google Scholar] [CrossRef]
- Sarton-Lohéac, G.; Nunes da Silva, C.G.; Mazel, F.; Baud, G.; de Bakker, V.; Das, S.; El Chazli, Y.; Ellegaard, K.; Garcia-Garcera, M.; Glover, N.; et al. Deep divergence and genomic diversification of gut symbionts of neotropical stingless bees. mBio 2023, 14, e03538-22. [Google Scholar] [CrossRef]
- Machado, J.O. Symbiosis between Brazilian social bees (Meliponinae) and a species of bacteria. Ciênc. Cult. 1971, 23, 625–633. [Google Scholar]
- Kwong, W.K.; Medina, L.A.; Koch, H.; Sing, K.W.; Soh, E.J.Y.; Ascher, J.S.; Jaffe, R.; Moran, N.A. Dynamic microbiome evolution in social bees. Sci. Adv. 2017, 3, e1600513. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Neto, P. Vida e Criação de Abelhas Indígenas Sem Ferrão; Nogueirapis: São Paulo, Brazil, 1997; p. 446. [Google Scholar]
- Cruz-Landim, C.D.; Silva-de-Moraes, R.L.M.; Serrão, J.E. Ultrastructural aspects of epithelial renewal in the midgut of adult worker bees (Hymenoptera, Apidae). J. Comp. Biol. 1996, 1, 29–40. [Google Scholar]
- Morais, P.B.; Calaça, P.S.S.T.; Rosa, C.A. Microorganisms associated with stingless bees. In Pot-Honey: A Legacy of Stingless Bees; Springer: New York, NY, USA, 2012; pp. 173–186. [Google Scholar]
- Lee, F.J.; Miller, K.I.; McKinlay, J.B.; Newton, I.L. Differential carbohydrate utilization and organic acid production by honey bee symbionts. FEMS Microbiol. Ecol. 2018, 94, fiy113. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Rahman, R.N.Z.R.A.; Yusof, M.T.; Syahir, A.; Sabri, S. Characterisation of bacteria isolated from the stingless bee, Heterotrigona itama, honey, bee bread and propolis. PeerJ 2019, 7, e7478. [Google Scholar] [CrossRef]
- Li, L.; Praet, J.; Borremans, W.; Nunes, O.C.; Manaia, C.M.; Cleenwerck, I.; Vandamme, P. Bombella intestini gen. nov., sp. nov., an acetic acid bacterium isolated from bumble bee crop. Int. J. Syst. Evol. Microbiol. 2015, 65, 267–273. [Google Scholar] [CrossRef]
- Tamarit, D.; Ellegaard, K.M.; Wikander, J.; Olofsson, T.; Vasquez, A.; Andersson, S.G. Functionally structured genomes in Lactobacillus kunkeei colonizing the honey crop and food products of honeybees and stingless bees. Genome Biol. Evol. 2015, 7, 1455–1473. [Google Scholar] [CrossRef]
- Paludo, C.R.; Ruzzini, A.C.; Silva-Junior, E.A.; Pishchany, G.; Currie, C.R.; Nascimento, F.S.; Pupo, M.T. Whole-genome sequence of Bacillus sp. SDLI1, isolated from the social bee Scaptotrigona depilis. Genome Announc. 2016, 4, 174-16. [Google Scholar] [CrossRef]
- Díaz, S.; de Souza Urbano, S.; Caesar, L.; Blochtein, B.; Sattler, A.; Zuge, V.; Haag, K.L. Report on the microbiota of Melipona quadrifasciata affected by a recurrent disease. J. Invertebr. Pathol. 2017, 143, 35–39. [Google Scholar] [CrossRef]
- Figueroa, L.L.; Maccaro, J.J.; Krichilsky, E.; Yanega, D.; McFrederick, Q.S. Why did the bee eat the chicken? Symbiont gain, loss, and retention in the vulture bee microbiome. mBio 2021, 12, e0231721. [Google Scholar] [CrossRef]
- Maccaro, J.J.; Figueroa, L.L.; McFrederick, Q.S. From pollen to putrid: Comparative metagenomics reveals how microbiomes support dietary specialization in vulture bees. Mol. Ecol. 2024, 33, e17421. [Google Scholar] [CrossRef] [PubMed]
- Goullet, P.; Picard, B. An epidemiological study of Serratia marcescens isolates from nosocomial infections by enzyme electrophoresis. J. Med. Microbiol. 1997, 46, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Sevjahova, L.; Gorman, R.; White, S. The emergence of the genus as important opportunistic pathogens. Pathogens 2022, 11, 1032. [Google Scholar] [CrossRef]
- Theocharidi, N.A.; Balta, I.; Houhoula, D.; Tsantes, A.G.; Lalliotis, G.P.; Polydera, A.C.; Stamatis, H.; Halvatsiotis, P. High prevalence in Greek meat products: Detection of virulence and anti-microbial resistance genes by molecular techniques. Foods 2022, 11, 708. [Google Scholar] [CrossRef]
- Ntougias, S.; Lapidus, A.; Copeland, A.; Reddy, T.B.K.; Pati, A.; Ivanova, N.N.; Zervakis, G.I. High-quality permanent draft genome sequence of the extremely osmotolerant diphenol degrading bacterium Halotalea alkalilenta AW-7T, and emended description of the genus Halotalea. Stand. Genom. Sci. 2015, 10, 52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).