Molecular Mechanisms of the Biological Control of Pine Wilt Disease Using Microorganisms
Abstract
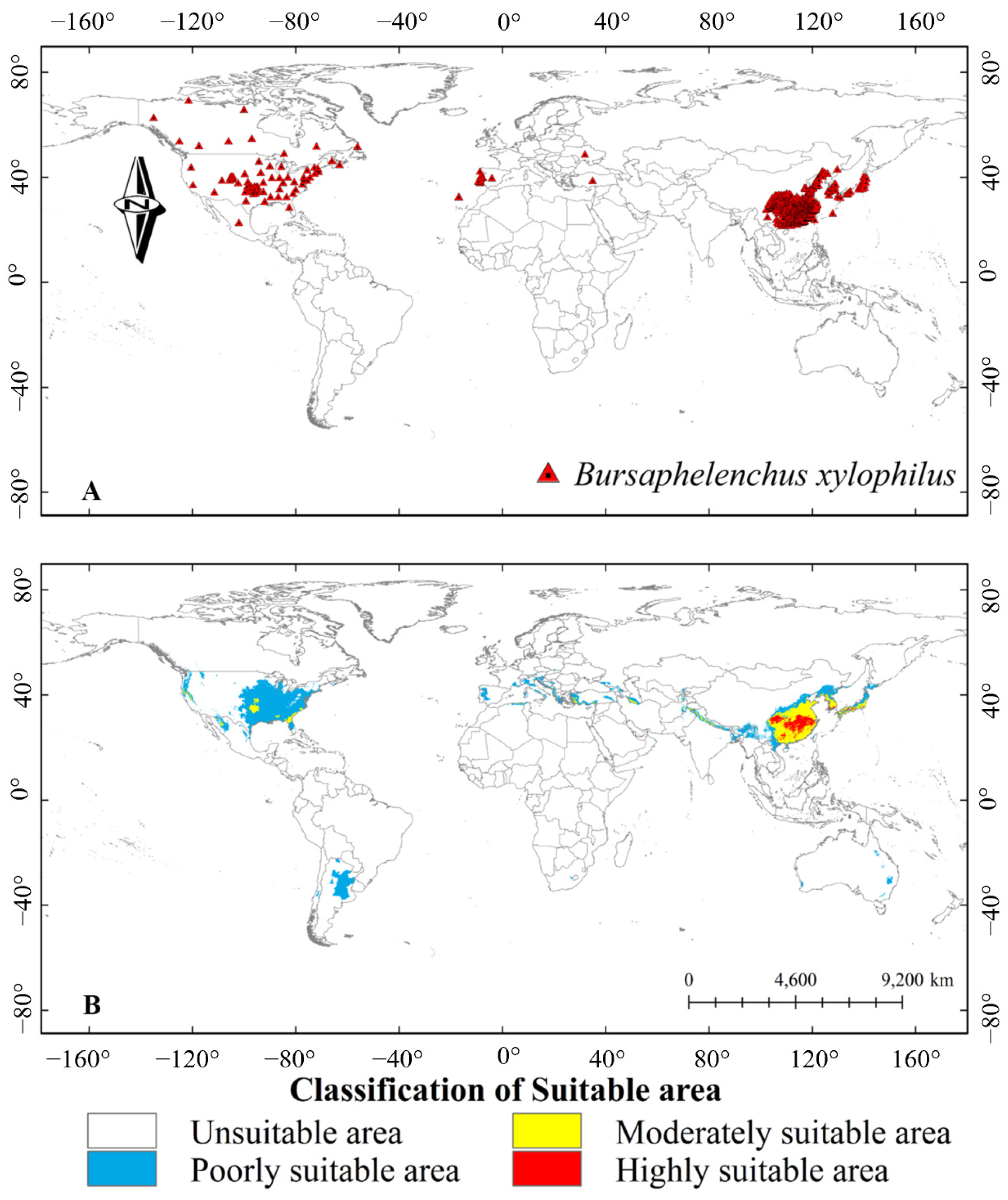
1. Introduction
2. Methodology
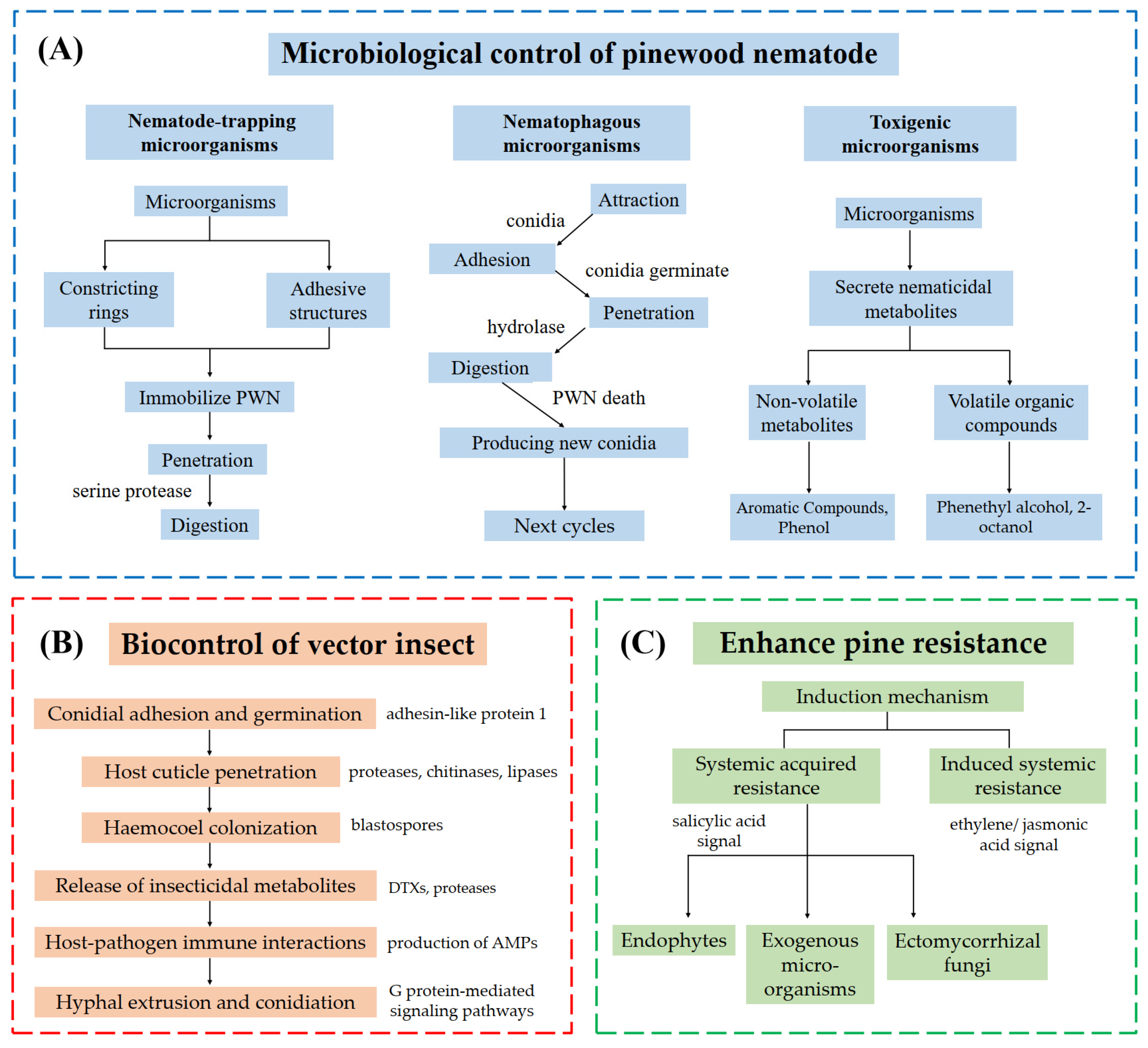
3. Molecular Mechanisms of Microbiological Control of PWN
3.1. Nematode-Trapping Microorganisms
3.2. Nematophagous Microorganisms
3.3. Toxigenic Microorganisms
3.3.1. Nematicidal Proteins from Toxigenic Microorganisms
3.3.2. Nematicidal Metabolites from Toxigenic Microorganisms
| Strain | Substance Class | Bioactive Substance | Reference |
|---|---|---|---|
| Bacteria | |||
| Brevundimonas diminuta LCB-3 | Alcohols | (R)-(-)-2-ethylhexan-1-ol | [61] |
| Serratia marcescens AHPC29 | Alkaloids | salsolinol | [59] |
| Bacillus sp. SMrs28 | Alkenes | 5,8-triene | [62] |
| Streptomyces sp. AN091965 | Antibiotics | Spectinabilin | [63] |
| Streptomyces ahygroscopicus | Antibiotics | tetramycin B3 | [64] |
| Streptomyces sp. AE170020 | Aromatic Compounds | alloaureothin | [60] |
| Streptomyces sp. 680560 | Aromatic Compounds | Teleocidin B4 | [59] |
| Bacillus sp. SMrs28 | Aromatic Compounds | phenylacetamide | [62] |
| Lysinimonas M4 | Aromatic Compounds | 2-coumaranone | [65] |
| Streptomyces sp. AE170020 | Benzopyranones | Aureothin | [60] |
| Bacillus sp. SMrs28 | Cyclic Compounds | 4-Oxabicyclo [3.2.2] nona-1 | [62] |
| Stenotrophomonas maltophilia G2 | Enzymes | serine protease | [66] |
| Bacillus sp. SMrs28 | Ester | methyl elaidate | [62] |
| Bacillus sp. SMrs28 | Fatty Acids | lauric acid | [62] |
| Bacillus sp. SMrs28 | Ketones | 4-dione | [62] |
| Streptomyces avermitilis AVE-H39 | Lactone | 13α-Hydroxymilbemycinβ13 | [67] |
| Streptomyces avermitilis AVE-H39 | Lactone | 26-methyl-13α-hydroxymilbemycin β13 | [67] |
| Bacillus pumilus LYMC-3 | Nitrogen Compounds | 2-{3-[(3S,8aS)-1,4-dioxooctahydropyrrolo [1,2-a] pyrazin-3-yl] propyl} guanidine | [54] |
| Bacillus sp. SMrs28 | Nitrogen Compounds | (3S, 8aS)-hexahydro-3methylpyrro [1,2-a] pyrazine-1 | [62] |
| Bacillus amyloliquefaciens JK-JS3 | Nitrogen Compounds | 2,2-dimethyl-N-phenylpropanethioamide | [68] |
| Bacillus amyloliquefaciens JK-JS3 | Nitrogen Compounds | Hexahydro-5-methyl-1-phenyl-1,3,5-triazine-2-thione | [68] |
| Bacillus amyloliquefaciens JK-JS3 | Nitrogen Compounds | [(4,7,7-trimethyl-3-bicyclo [2.2.1] heptanylidene) amino] urea | [68] |
| Streptomyces sp. C611 | Nitrogen Compounds | Furaltadone | [69] |
| Bacillus sp. SMrs28 | Peptides | cyclo(L-Pro-L-Val) | [62] |
| Lysinimonas M4 | Peptides | cyclo-(Phe-Pro) | [65] |
| Erwinia sp. A41C3 | Siderophores | Catecholate-typesiderophore | [70] |
| Rouxiella sp. Arv20#4.1 | Siderophores | hydroxamate-type siderophore | [70] |
| Streptomyces sp. TCS19-048 | Sulfur compounds | S-3-1 | [71] |
| Pseudoduganella violaceinigra G5-3 | VOCs | 2,5-dimethyl pyrazine | [72] |
| Pseudoduganella violaceinigra G5-3 | VOCs | 4-dimethylaminopyridine | [72] |
| Pseudoduganella violaceinigra G5-3 | VOCs | benzyl acetate | [72] |
| Pseudoduganella violaceinigra G5-3 | VOCs | phenethyl butyrate | [72] |
| Pseudoduganella violaceinigra G5-3 | VOCs | phenethyl alcohol | [72] |
| Stenotrophomonas maltophilia | VOCs | phenol | [73] |
| Bacillus subtilis | VOCs | 2-octanol | [73] |
| Serratia marcescens | VOCs | benzaldchyde | [73] |
| Stenotrophomonas maltophilia | VOCs | benzeneacetaldehyde | [73] |
| Bacillus subtilis | VOCs | decanal | [73] |
| Bacillus subtilis | VOCs | 2-nonanone | [73] |
| Stenotrophomonas maltophilia | VOCs | 2-undecanone | [73] |
| Bacillus subtilis | VOCs | cyclohexene | [73] |
| Stenotrophomonas maltophilia | VOCs | dimethyl disulfide | [73] |
| Vibrio atlanticus S-16 and Pseudoalteromonas marina H-42 | VOCs | dimethyl disulfide | [74] |
| Vibrio atlanticus S-16 and Pseudoalteromonas marina H-42 | VOCs | benzaldehyde | [74] |
| Vibrio atlanticus S-16 and Pseudoalteromonas marina H-42 | VOCs | dimethyl trisulfide | [74] |
| Vibrio atlanticus S-16 | VOCs | tert-butylamine | [74] |
| Vibrio atlanticus S-16 | VOCs | acetone | [74] |
| Pseudoalteromonas marina H-42 | VOCs | Dimethylamine | [74] |
| Pseudoalteromonas marina H-42 | VOCs | N(diisopropylphosphino)methyl- | [74] |
| Fungi | |||
| Geotrichum sp. AL4 | Alcohols | [2,3-dihydro-2-(1-methylethenyl)-1-benzofuran-5-yl] methanol | [75] |
| Alternaria sp. Samif01 | Aromatic Compounds | Alternariol 9-methyl ether | [76] |
| Aspergillus fumigatus | Aromatic Compounds | Fumiquinones A and B | [25] |
| Caryospora callicarpa YMF1.01026 | Aromatic Compounds | 4,8-Dihydroxy-3,4-dihydronaphthalen-1(2H)-one | [77] |
| Caryospora callicarpa YMF1.01026 | Aromatic Compounds | 4,6-dihydroxy-3,4-dihydronaphthalen-1(2H)-one | [77] |
| Caryospora callicarpa YMF1.01026 | Aromatic Compounds | 4,6,8-trihydroxy-3,4-dihydronaphthalen-1(2H)-one) | [77] |
| Caryospora callicarpa YMF1.01026 | Aromatic Compounds | 3,4,6,8-tetrahydroxy-3,4-dihydronaphthalen-1(2H)-one(cis-4-hydroxyscytalone) | [77] |
| Oidiodendron sp. | Aromatic Compounds | 4-Hydroxyphenylacetic acid | [78] |
| Gliocladium roseum YMF1.00133 | Aromatic Compounds | 5-n-heneicosylresorcinol | [79] |
| Geotrichum sp. AL4 | Aromatic Compounds | 1-(2,4-dihydroxyphenyl) ethanone | [75] |
| Caryospora callicarpa YMF1.01026 | Aromatic Compounds | caryospomycins A–C | [80,81] |
| Coelomycetes sp. YMFl.01029 | Aromatic Compounds | Preussomerin C | [82] |
| Coelomycetes sp. YMFl.01029 | Aromatic Compounds | preussomerin E | [82] |
| Coelomycetes sp. YMFl.01029 | Aromatic Compounds | preussomerin D | [82,83] |
| Coelomycetes sp. YMFl.01029 | Aromatic Compounds | 4,6,8-trjhydfoxy-3,4-dihydronaphthalen-1(2H)-one | [82,83] |
| Coelomycetes sp. YMFl.01029 | Aromatic Compounds | (4RS)4,8-dihydroxy-3,4-dihydronaphthalen-1(2H)-one | [82,83] |
| Chaetomium ascotrichoides 1-24-2 | Aromatic Compounds | 4,5,6-trihydroxy-7-methylphthalide | [84] |
| Chaetomium ascotrichoides 1-24-2 | Aromatic Compounds | 2-chlorobenzothiazole | [84] |
| Fusarium oxysporum EF119 | Benzopyranones | Bikaverin | [85] |
| Aspergillus sp. | Carboxylic Acids | 5-Hydroxymethyl-2-furoic acid | [86] |
| Fusarium oxysporum EF119 | Carboxylic Acids | fusaric acid | [85] |
| Pseudohalonectria adversaria YMF1.01019 | Cyclic Compounds | pseudohalonectrin A and B | [80] |
| Fusarium bulbicola | Cyclic Esters | Beauvericin | [87] |
| Beauveria bassiana and Beauveria pseudobassiana | Cyclic Esters | Beauvericin | [87] |
| Paraniesslia sp. YMF1.01400 | Glycosides | (2S,2‘R,3R,3′E,4E,8E)-1-O-(β-D-glucopyranosyl)-3-hydroxyl-2-[N-2′-hydroxyl-3′-eicosadecenoyl] amino-9-methyl-4,8-octadecadiene | [80] |
| Oidiodendron sp. | Lactone | oidiolactone D | [78] |
| Ophioceras dolichostomum YMF1.00988 | Lipids | Ophiocerol | [88] |
| Geotrichum sp. AL4 | Nitrogen Compounds | 1-[(2R*,4S*,5S*)-2-chloro-4-methyl-1,3-oxazinan-5-yl] ethenone | [75] |
| Chaetomium ascotrichoides 1-24-2 | Nitrogen Compounds | O-methylisourea | [84] |
| Gliocladium roseum YMF1.00133 | Peptides | Gliocladin C | [79] |
| Gliocladium roseum 1A | Peptides | Gliocladines A–D | [89] |
| Trichoderma sp. | VOCs | 1β-vinylcyclopentane-1α,3α-diol | [83,90] |
| Trichoderma sp. | VOCs | 6-pentyl-2H-pyran-2-one (2) | [83,90] |
| Annulohypoxylon sp. FPYF3050 | VOCs | 1,8-cineole | [91] |
| Annulohypoxylon sp. FPYF3050 | VOCs | (+)-sativene | [91] |
| Annulohypoxylon sp. FPYF3050 | VOCs | isocaryophyllene | [91] |
4. Molecular Mechanisms of Microbial Control of Vector Insects
4.1. Biological Control Agents of M. alternatus
| Strain | Bioactive Substance | Killed Insects | Source | Reference |
|---|---|---|---|---|
| B. bassiana | N/A | Monochamus alternatus | Monochamus alternatus | [100,107] |
| B. bassiana F-263 | N/A | Monochamus alternatus | Monochamus alternatus | [108,109,110] |
| B. bassiana ERL836 | N/A | Monochamus alternatus | Entomology Research Laboratory, University of Vermont, USA | [106] |
| B. bassiana B7/B9 | N/A | Monochamus alternatus | Monochamus alternatus | [111] |
| B. brongniartii F-877 | N/A | Monochamus alternatus | Monochamus alternatus | [108] |
| B. brongniartii #879 | N/A | Monochamus alternatus | Psacothea hilaris | [108] |
| B. pseudobassiana | N/A | Monochamus galloprovincialis | Monochamus galloprovincialis | [97] |
| M. anisopliae JEF-279 | Destruxin and protease | Monochamus alternatus | Soil | [112] |
| M. anisopliae 1291 | N/A | Monochamus alternatus | M. alternatus larva | [113] |
| M. anisopliae 1349 | N/A | Monochamus alternatus | M. alternatus adult | [113] |
| M. anisopliae 2049 | N/A | Monochamus alternatus | Cydnid bug adult | [113] |
| M. anisopliae JEF-197 | N/A | Monochamus alternatus | Soil | [114] |
| M. anisopliae JEF-271 | N/A | Monochamus alternatus | Soil | [114] |
| M. anisopliae JEF-279 | N/A | Monochamus alternatus | Soil | [114] |
| M. anisopliae Ma789 | N/A | Monochamus alternatus | Chinese Academy of Forestry | [115] |
| M. anisopliae MaYTTR-03 | N/A | Monochamus alternatus | Soil | [116] |
| M. anisopliae MaYTTR-04 | N/A | Monochamus alternatus | Soil | [116] |
| M. anisopliae MaZPTR-01 | N/A | Monochamus alternatus | Soil | [116] |
| M. anisopliae var. anisopliae | N/A | Monochamus alternatus | Monochamus alternatus | [107] |
| M. anisopliae var. major CQMa117 | N/A | Monochamus alternatus | Monochamus alternatus | [117] |
| M. robertsii GQH6 | N/A | Monochamus alternatus | Soil | [98] |
| Aspergillus austwickii | N/A | Monochamus alternatus | Monochamus alternatus | [100] |
| Aspergillus ruber | N/A | Monochamus alternatus | Monochamus alternatus | [100] |
| Bacillus thuringiensis Cry3Aa | Coleopteran-specific Cry3Aa toxin | Monochamus alternatus | Not mentioned | [118] |
| Lecanicillium attenuatum | N/A | Monochamus alternatus | Monochamus alternatus | [100] |
| Paecilomyces farinosus | N/A | Monochamus alternatus | Monochamus alternatus | [107] |
| Penicillium citrinum | N/A | Monochamus alternatus | Monochamus alternatus | [100] |
| Scopulariopsis alboflavescens | N/A | Monochamus alternatus | Monochamus alternatus | [100] |
| Serratia marcescens | N/A | Monochamus alternatus | Monochamus alternatus | [107] |
| Serratia marcescens AHPC29 | N/A | M. alternatus and M. saltuarius | M. alternatus and M. saltuarius | [99] |
| Trichoderma dorotheae | N/A | Monochamus alternatus | Monochamus alternatus | [100] |
4.2. Infection Mechanisms of Metarhizium and Beauveria Against M. alternatus
5. Molecular Mechanisms of Microbial Enhancement of Pine Resistance Against PWN
5.1. Mechanisms of Microorganisms Improve Pine SAR Against PWN
5.1.1. Improvement of SAR by Exogenous Microorganisms
5.1.2. Improvement of SAR by Endophytes
5.1.3. Improvement of SAR by Ectomycorrhizal Fungi
5.2. Mechanisms of Microorganisms Induce Pine ISR Against PWN
6. The Application of Microbial BCAs
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kusunoki, M. Symptom development of pine wilt disease-histopathological observations with electron microscopes. Jpn. J. Phytopathol. 1987, 53, 622–629. [Google Scholar] [CrossRef][Green Version]
- Mamiya, Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Guo, Q.; Xie, N.; Yuan, G.; Liao, M.; Gui, Q.; Ding, G. Predicting the global potential distribution of Bursaphelenchus xylophilus using an ecological niche model: Expansion trend and the main driving factors. BMC Ecol. Evol. 2024, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C. Pine Wilt Disease in Korea. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 26–32. [Google Scholar]
- Hao, Z.; Huang, J.; Li, X.; Sun, H.; Fang, G. A multi-point aggregation trend of the outbreak of pine wilt disease in China over the past 20 years. For. Ecol. Manag. 2022, 505, 119890. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine wilt disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Back, M.A.; Bonifácio, L.; Inácio, M.L.; Mota, M.; Boa, E. Pine wilt disease: A global threat to forestry. Plant Pathol. 2024, 73, 1026–1041. [Google Scholar] [CrossRef]
- Mamiya, Y. History of pine wilt disease in Japan. J. Nematol. 1988, 20, 219. [Google Scholar]
- Yang, B.J. The history, dispersal and potential threat of pine wood nematode in China. In The Pinewood Nematode, Bursaphelenchus xylophilus; Brill: Leiden, The Netherlands, 2004; pp. 21–24. [Google Scholar]
- Zhang, X.; Zhao, J.; Yan, J.; Fang, G.; Huang, J. Economic loss assessment of pine wilt disease in mainland China in 2017. J. Beijing For. Univ. 2020, 42, 96–106. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, X.; Li, J.; Ren, J.; Ren, L.; Luo, Y. Pine wilt disease in Northeast and Northwest China: A comprehensive risk review. Forests 2023, 14, 174. [Google Scholar] [CrossRef]
- Zhao, H.; Xian, X.; Yang, N.; Guo, J.; Zhao, L.; Shi, J.; Liu, W. Risk assessment framework for pine wilt disease: Estimating the introduction pathways and multispecies interactions among the pine wood nematode, its insect vectors, and hosts in China. Sci. Total Environ. 2023, 905, 167075. [Google Scholar] [CrossRef]
- Ryss, A.Y.; Kulinich, O.A.; Sutherland, J.R. Pine wilt disease: A short review of worldwide research. For. Stud. China. 2011, 13, 132–138. [Google Scholar] [CrossRef]
- Bi, Z.; Gong, Y.; Huang, X.; Yu, H.; Bai, L.; Hu, J. Efficacy of four nematicides against the reproduction and development of pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 2015, 47, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Si, G.; Chen, L.; Hu, L.; Cui, G.; Wang, M.; Zhao, D. Current Status and Prospects of Pine Wilt Disease Management with Phytochemicals—A Review. Plants 2024, 13, 2129. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.; Pang, H.; Yin, S.; Liu, H.; Tian, Y.; Gao, S.; Zhou, C.; Wu, P.; Miao, S.; et al. Sustainable DMSNs nano-biopesticide platform built by a “one-pot” method focusing on injury-free drug demonstration of pine wood nematodes. Environ. Sci. Nano 2024, 11, 363–372. [Google Scholar] [CrossRef]
- Hao, X.; Wang, B.; Chen, J.; Wang, B.; Xu, J.; Pan, J.; Ma, L. Molecular characterization and functional analysis of multidrug resistance-associated genes of Pinewood nematode (Bursaphelenchus xylophilus) for nematicides. Pestic. Biochem. Physiol. 2021, 177, 104902. [Google Scholar] [CrossRef]
- Pires, D.; Vicente, C.S.L.; Menendez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inacio, M.L. The Fight against Plant-Parasitic Nematodes: Current Status of Bacterial and Fungal Biocontrol Agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef]
- Ning, T.; Fang, Y.; Tang, J.; Sun, J. Advances in research on Bursaphelenchus xyophilus and its key vector Monochamus spp. Chin. J. Appl. Entomol. 2004, 41, 97–104. [Google Scholar] [CrossRef]
- Ye, J. Epidemic status of pine wilt disease in China and its prevention and control techniques and counter measures. Sci. Silvae Sin. 2019, 55, 1–10. [Google Scholar] [CrossRef]
- Jeon, H.W.; Park, A.R.; Sung, M.; Kim, N.; Mannaa, M.; Han, G.; Kim, J.; Koo, Y.; Seo, Y.S.; Kim, J.C. Systemic Acquired Resistance-Mediated Control of Pine Wilt Disease by Foliar Application with Methyl Salicylate. Front. Plant Sci. 2021, 12, 812414. [Google Scholar] [CrossRef]
- Xu, M.; Xu, F.Y.; Liu, Y.P.; Pan, Y.S.; Wu, X.Q. Assessment of Metarhizium anisopliae (Clavicipitaceae) and its vector, Scleroderma guani (Hymenoptera: Bethylidae), for the control of Monochamus alternatus (Coleoptera: Cerambycidae). Can. Entomol. 2015, 147, 628–634. [Google Scholar] [CrossRef]
- Xu, F.; Xu, K.; Xie, C.; Zhang, P.; Shin, S.; Cheong, Y. Studies on Scleroderma guani to Control the Pine Sawyer Beetle, Monochamus alternatus. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Mota, M.M., Vieira, P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 379–388. [Google Scholar]
- Dou, G.; Yan, D. Research Progress on Biocontrol of Pine Wilt Disease by Microorganisms. Forests 2022, 13, 1047. [Google Scholar] [CrossRef]
- Hayashi, A.; Fujioka, S.; Nukina, M.; Kawano, T.; Shimada, A.; Kimura, Y. Fumiquinones A and B, nematicidal quinones produced by Aspergillus fumigatus. Biosci. Biotechnol. Biochem. 2007, 71, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Stamps, W. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Zhao, L.; Mota, M.; Vieira, P.; Butcher, R.A.; Sun, J. Interspecific communication between pinewood nematode, its insect vector, and associated microbes. Trends Parasitol. 2014, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jung, J.K.; Hong, K.J.; Kim, C.J.; Lee, B.W.; Kim, I.K. Discovery and biology of Spathius verustus Chao (Hymenoptera: Braconidae), a potential biological agent on two Monochamus vectors of the pinewood nematode. Forests 2022, 13, 955. [Google Scholar] [CrossRef]
- Liu, X.; Song, J.; Li, X.; Xu, H.; Wang, Z. Current Status of Research on Control Methods of Monochamus Alternatus Hope (Co-leoptera: Cerambycidae). Inst. Contin. Judic. Educ. 2022, 8, 21–29. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Liang, L.; Tian, B.; Zhang, Y.; Cheng, C.; Zhang, K.Q. Cloning and characterization of an extracellular serine protease from the nematode-trapping fungus Arthrobotrys conoides. Arch. Microbiol. 2007, 188, 167–174. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.Y.; Gu, L.J.; Sun, B.S.; Zhang, D.L.; Liu, L.; Lee, M.R.; Wang, C.L.; Li, Z.; Mo, E.K.; et al. Variabilities of two Drechslerella dactyloides isolates in Korea and high predacity against Bursaphelenchus xylophilus. Curr. Microbiol. 2011, 62, 472–478. [Google Scholar] [CrossRef]
- Jinyan, D.; Ru, L.; Keqin, Z. Advances in biological control of Bursaphelenchus xylophilus by natural enemies and nematocidal compounds from plants. Plant Prot. 2005, 31, 9–15. [Google Scholar]
- Zhang, X.; Zhang, H.; Jiang, Z.; Bai, Q.; Wu, S.; Wang, Y.; Li, C.; Zeng, X.; Gan, X.; Xie, X. A new strain of Volutella citrinella with nematode predation and nematicidal activity, isolated from the cysts of potato cyst nematodes in China. BMC Microbiol. 2021, 21, 323. [Google Scholar] [CrossRef]
- Zhu, M.; Li, X.; Zhao, N.; Yang, L.; Zhang, K.; Yang, J. Regulatory mechanism of trap formation in the nematode-trapping fungi. J. Fungi 2022, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Tunlid, A.; Jansson, H.; Nordbring-Hertz, B. Fungal attachment to nematodes. Mycol. Res. 1992, 96, 401–412. [Google Scholar] [CrossRef]
- Wang, D.; Ma, N.; Rao, W.; Zhang, Y. Recent advances in life history transition with nematode-trapping fungus Arthrobotrys oligospora and its application in sustainable agriculture. Pathogens 2023, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Zhang, G.; Wang, W.; Feng, H.; Yang, X.; Zheng, Y.; Yang, L.; Xie, M.; Zhang, K.-Q.; Yang, J. Ric8 acts as a regulator of G-protein signalling required for nematode-trapping lifecycle of Arthrobotrys oligospora. Environ. Microbiol. 2022, 24, 1714–1730. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Sakagami, H. Molecular mechanisms underlying Ca2+/calmodulin-dependent protein kinase kinase signal transduction. Int. J. Mol. Sci. 2022, 23, 11025. [Google Scholar] [CrossRef]
- Zhen, Z.; Zhang, G.; Yang, L.; Ma, N.; Li, Q.; Ma, Y.; Niu, X.; Zhang, K.; Yang, J. Characterization and functional analysis of calcium/calmodulin-dependent protein kinases (CaMKs) in the nematode-trapping fungus Arthrobotrys oligospora. Appl. Microbiol. Biotechnol. 2019, 103, 819–832. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, N.; Zhang, K. Extracellular enzymes serving as virulence factors in nematophagous fungi involved in infection of the host. Res. Microbiol. 2004, 155, 811–816. [Google Scholar] [CrossRef]
- Yang, J.; Tian, B.; Liang, L.; Zhang, K. Extracellular enzymes and the pathogenesis of nematophagous fungi. Appl. Microbiol. Biotechnol. 2007, 75, 21–31. [Google Scholar] [CrossRef]
- Vidal-Diez de Ulzurrun, G.; Hsueh, Y. Predator-prey interactions of nematode-trapping fungi and nematodes: Both sides of the coin. Appl. Microbiol. Biotechnol. 2018, 102, 3939–3949. [Google Scholar] [CrossRef]
- Veenhuis, M.; Van Wijk, C.; Wyss, U.; Nordbring-Hertz, B.; Harder, W. Significance of electron dense microbodies in trap cells of the nematophagous fungus Arthrobotrys oligospora. Antonie Van Leeuwenhoek 1989, 56, 251–261. [Google Scholar] [CrossRef]
- Paiva, G.; Proenca, D.N.; Francisco, R.; Verissimo, P.; Santos, S.S.; Fonseca, L.; Abrantes, I.M.; Morais, P.V. Nematicidal bacteria associated to pinewood nematode produce extracellular proteases. PLoS ONE 2013, 8, e79705. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.S.L.; Soares, M.; Faria, J.M.S.; Ramos, A.P.; Inacio, M.L. Insights into the Role of Fungi in Pine Wilt Disease. J. Fungi 2021, 7, 780. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.; Shih, J.; Tzean, S. Esteya, a new nematophagous genus from Taiwan, attacking the pinewood nematode (Bursaphelenchus xylophilus). Mycol. Res. 1999, 103, 242–248. [Google Scholar] [CrossRef]
- Lin, F.; Ye, J.; Wang, H.; Zhang, A.; Zhao, B. Host deception: Predaceous fungus, Esteya vermicola, entices pine wood nematode by mimicking the scent of pine tree for nutrient. PLoS ONE 2013, 8, e71676. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, Z.; Fang, Z.M.; Zhang, D.L.; Gu, L.J.; Liu, L.; Sung, C.K. Attraction of pinewood nematode to endoparasitic nematophagous fungus Esteya vermicola. Curr. Microbiol. 2010, 60, 387–392. [Google Scholar] [CrossRef]
- Wang, H.H.; Wang, Y.B.; Yin, C.; Gao, J.; Tao, R.; Sun, Y.L.; Wang, C.Y.; Wang, Z.; Li, Y.X.; Sung, C.K. In vivo infection of Bursaphelenchus xylophilus by the fungus Esteya vermicola. Pest Manag. Sci. 2020, 76, 2854–2864. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.Y.; Yang, Z.H.; Fang, Z.M.; Moon, Y.J.; Sun, B.S.; Lee, M.R.; Sung, C.K. Viability and pathogenicity of Esteya vermicola in pine trees. Biocontrol Sci. Technol. 2011, 21, 387–393. [Google Scholar] [CrossRef]
- Yin, C.; Wang, Y.; Zhang, Y.; Wang, H.; Duan, B.; Tao, R.; Gao, J.; Sung, C. Hypothesized mechanism of biocontrol against pine wilt disease by the nematophagous fungus Esteya vermicola. Eur. J. Plant Pathol. 2020, 156, 811–818. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Araújo, J.P.M.; Zhang, X.; Ji, Y.; Hulcr, J. Esteya floridanum sp. nov.: An Ophiostomatalean nematophagous fungus and its potential to control the pine wood nematode. Phytopathology 2021, 111, 304–311. [Google Scholar] [CrossRef]
- Guo, Y.; Weng, M.; Sun, Y.; Carballar-Lejarazú, R.; Wu, S.; Lian, C. Bacillus thuringiensis toxins with nematocidal activity against the pinewood nematode Bursaphelenchus xylophilus. J. Invertebr. Pathol. 2022, 189, 107726. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Chen, F. Bacillus pumilus strain LYMC-3 shows nematicidal activity against Bursaphelenchus xylophilus via the production of a guanidine compound. Biocontrol Sci. Technol. 2018, 28, 1128–1139. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, L.; Wu, J.; Zhang, L. Detection and characterisation of a Bacillus thuringiensis crystal protein with nematicidal activity against the pinewood nematode Bursaphelenchus xylophilus. Biocontrol Sci. Technol. 2012, 22, 1143–1153. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Chen, F.; Hao, D.; Tan, J. An alkaline protease from Bacillus cereus NJSZ-13 can act as a pathogenicity factor in infection of pinewood nematode. BMC Microbiol. 2023, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Wang, X.; Zhang, W.; Wen, X.; Liu, Z.; Feng, Y.; Zhang, X. Engineered pine endophytic Bacillus toyonensis with nematocidal and colonization abilities for pine wilt disease control. Front. Microbiol. 2023, 14, 1240984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, S.; Lu, F.; Guo, K.; Huang, L.; Su, X.; Chen, Y. Nematotoxicity of a Cyt-like protein toxin from Conidiobolus obscurus (Entomophthoromycotina) on the pine wood nematode Bursaphelenchus xylophilus. Pest Manag. Sci. 2021, 77, 686–692. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, M.H.; Liu, M.J.; Jin, C.Z.; Park, S.H.; Lee, J.M.; Kim, J.; Park, D.; Park, H.R.; Kim, Y.H.; et al. Nematicidal activity of teleocidin B4 isolated from Streptomyces sp. against pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2021, 77, 1607–1615. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, J.H.; Liu, M.J.; Jin, C.Z.; Park, D.J.; Kim, J.; Son, K.H.; Kim, C.J. New discovery on the nematode activity of aureothin and alloaureothin isolated from endophytic bacteria Streptomyces sp. AE170020. Sci Rep. 2022, 12, 3947. [Google Scholar] [CrossRef]
- Zheng, L.; Li, G.; Wang, X.; Pan, W.; Li, L.; Hua, L.; Liu, F.; Dang, L.; Mo, M.; Zhang, K. Nematicidal endophytic bacteria obtained from plants. Ann. Microbiol. 2008, 58, 569–572. [Google Scholar] [CrossRef]
- Zeng, L.; Jin, H.; Lu, D.; Yang, X.; Pan, L.; Cui, H.; He, X.; Qiu, H.; Qin, B. Isolation and identification of chemical constituents from the bacterium Bacillus sp. and their nematicidal activities. J. Basic Microbiol. 2015, 55, 1239–1244. [Google Scholar] [CrossRef]
- Liu, M.J.; Hwang, B.S.; Jin, C.Z.; Li, W.J.; Park, D.J.; Seo, S.T.; Kim, C.J. Screening, isolation and evaluation of a nematicidal compound from actinomycetes against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2019, 75, 1585–1593. [Google Scholar] [CrossRef]
- Sun, S.; Li, W.; Ju, K.; Xiong, X.; Li, J.; Yu, C.; Tian, Y.; Liu, H. Tetramycin B3: An Effective and Biological Nematicide for Bursaphelenchus xylophilus. Forests 2024, 15, 1699. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Du, G.; Deng, W.; Yang, H.; Li, R.; Xu, Q.; Guo, Q. Two nematicidal compounds from Lysinimonas M4 against the pine wood nematode, Bursaphelenchus xylophilus. Forests 2022, 13, 1191. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Ding, J.; He, Q.; Xiong, R.; Zhang, K. The investigation of nematocidal activity in Stenotrophomonas maltophilia G2 and characterization of a novel virulence serine protease. Can. J. Microbiol. 2009, 55, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.; Qi, H.; Zhang, J.; Li, J.S.; Zhang, S.Y.; Hao, Z.K.; Zhang, L.Q.; Xiang, W.S. Two new 13-hydroxylated milbemycin metabolites from the genetically engineered strain Streptomyces avermitilis AVE-H39. J. Asian Nat. Prod. Res. 2021, 23, 837–843. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, X.; Hu, F.; Xu, H. Extraction and structure analysis of the nematicidal active substances produced by Bacillus amyloliquefaciens JK-JS3. Sci. Silvae Sin. 2011, 47, 133–137. [Google Scholar]
- Huang, B.; Chen, J.; Li, W.; Song, X.; Wang, B.; Zhang, C.; Zhao, S.; Niu, Q. Screening and identification of actinomycetes for biological control of Bursaphelenchus xylophilus and preliminary study on their toxicity factors. J. Beijing For. Univ. 2019, 41, 99–106. [Google Scholar] [CrossRef]
- Proença, D.N.; Heine, T.; Senges, C.H.; Bandow, J.E.; Morais, P.V.; Tischler, D. Bacterial metabolites produced under iron limitation kill pinewood nematode and attract Caenorhabditis elegans. Front. Microbiol. 2019, 10, 2166. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, C.; Zhang, S.; Chen, F.; Pei, F.; Zhou, S.; Lin, H. Screening, isolation and mechanism of a nematicidal extract from actinomycetes against the pine wood nematode Bursaphelenchus xylophilus. Heliyon 2022, 8, e11713. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Q.; Wang, L.; Ma, Y.; Zhang, T.; Li, R. Nematicidal activities of bacterial volatiles from Pseudoduganella violaceinigra G5-3 and Novosphingobium pokkalii G8-2 against the pine wood nematode Bursaphelenchus xylophilus. Chiang Mai J. Sci. 2019, 46, 236–246. [Google Scholar]
- Gu, Y.Q.; Mo, M.H.; Zhou, J.P.; Zou, C.S.; Zhang, K.Q. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Yu, J.; Du, G.; Li, R.; Li, L.; Li, Z.; Zhou, C.; Chen, C.; Guo, D. Nematicidal activities of bacterial volatiles and components from two marine bacteria, Pseudoalteromonas marina strain H-42 and Vibrio atlanticus strain S-16, against the pine wood nematode, Bursaphelenchus xylophilus. Nematology 2015, 17, 1011–1025. [Google Scholar] [CrossRef]
- Li, G.H.; Yu, Z.F.; Li, X.; Wang, X.B.; Zheng, L.J.; Zhang, K.Q. Nematicidal metabolites produced by the endophytic fungus Geotrichum sp. AL4. Chem. Biodivers. 2007, 4, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Yu, R.; Wang, X.; Mao, Z.; Fu, L.; Liu, Y.; Zhou, L. Alternariol 9-methyl ether from the endophytic fungus Alternaria sp. Samif01 and its bioactivities. Braz. J. Microbiol. 2016, 47, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dong, J.; Wang, L.; Zhou, W.; Li, L.; He, H.; Liu, H.; Zhang, K. Screening and isolation of antinematodal metabolites against Bursaphelenchus xylophilus produced by fungi. Ann. Microbiol. 2008, 58, 375–380. [Google Scholar] [CrossRef]
- Ohtani, K.; Fujioka, S.; Shimada, A.; Kimura, Y. Nematicidal activities of 4-hydroxyphenylacetic acid and oidiolactone D produced by the fungus Oidiodendron sp. Z. Naturforsch. C 2011, 66, 31–34. [Google Scholar] [CrossRef]
- Song, H.; Shen, W.; Dong, J. Nematicidal metabolites from Gliocladium roseum YMF1. 00133. Appl. Biochem. Microbiol. 2016, 52, 324–330. [Google Scholar] [CrossRef]
- Dong, J.; Li, G.; Zhang, K. Screening and isolation of anti-nematodal metabolites against Bursaphelenchus xylophilus produced by fungi and plant. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Mota, M.M., Vieira, P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 347–358. [Google Scholar]
- Dong, J.Y.; Li, R.; He, H.P.; Zhang, K.Q. Nematicidal sphingolipids from the freshwater fungus Paraniesslia sp. YMF1. 01400. Eur. J. Lipid Sci. Technol. 2005, 107, 779–785. [Google Scholar] [CrossRef]
- Dong, J.Y.; Song, H.C.; Li, J.H.; Tang, Y.S.; Sun, R.; Wang, L.; Zhou, Y.P.; Wang, L.M.; Shen, K.Z.; Wang, C.R.; et al. Ymf 1029A−E, Preussomerin Analogues from the Fresh-Water-Derived Fungus YMF 1.01029. J. Nat. Prod. 2008, 71, 952–956. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, K.; Dong, J.; Wang, L.; Sun, R.; Wang, C.; Wang, L.; Zhang, K. Nematicidal metabolites of the aquatic fungus Coelomycetes sp. YMF1. 01029. Chin. J. Antibiot. 2009, 34, 74–78. [Google Scholar]
- Kamaruzzaman, M.; Zheng, L.; Zhou, S.; Ye, W.; Yuan, Y.; Qi, Q.; Gao, Y.; Tan, J.; Wang, Y.; Chen, B. Evaluation of the novel endophytic fungus Chaetomium ascotrichoides 1-24-2 from Pinus massoniana as a biocontrol agent against pine wilt disease caused by Bursaphelenchus xylophilus. Pest Manag. Sci. 2024, 80, 4924–4940. [Google Scholar] [CrossRef]
- Kwon, H.R.; Son, S.W.; Han, H.R.; Choi, G.J.; Jang, K.S.; Choi, Y.H.; Lee, S.; Sung, N.D.; Kim, J.C. Nematicidal activity of bikaverin and fusaric acid isolated from Fusarium oxysporum against pine wood nematode, Bursaphelenchus xylophilus. Plant Pathol. J. 2007, 23, 318–321. [Google Scholar] [CrossRef]
- Kimura, Y.; Tani, S.; Hayashi, A.; Ohtani, K.; Fujioka, S.; Kawano, T.; Shimada, A. Nematicidal activity of 5-hydroxymethyl-2-furoic acid against plant-parasitic nematodes. Z. Naturforsch. C 2007, 62, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, T.; Harte, S.J.; Zamora, P.; Bareyre, M.; Díez, J.J.; Herrero, B.; Niño-Sánchez, J.; Martín-García, J. Nematicidal effect of Beauveria species and the mycotoxin beauvericin against pinewood nematode Bursaphelenchus xylophilus. Front. For. Glob. Chang. 2023, 6, 1229456. [Google Scholar] [CrossRef]
- Dong, J.Y.; Wang, L.; Song, H.C.; Wang, L.M.; Shen, K.Z.; Sun, R.; Li, G.H.; Li, L.; Zhang, K.Q. Ophiocerol, a novel macrocylic neolignan from the aquatic fungus Ophioceras dolichostomum YMF1.00988. Nat. Prod. Res. 2010, 24, 1004–1012. [Google Scholar] [CrossRef]
- Dong, J.Y.; He, H.P.; Shen, Y.M.; Zhang, K.Q. Nematicidal Epipolysulfanyldioxopiperazines from Gliocladium roseum. J. Nat. Prod. 2005, 68, 1510–1513. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Z.; Lei, L.; Xia, Z.; Shao, L.; Zhang, K.; Li, G. Nematicidal effect of volatiles produced by Trichoderma sp. J. Asia-Pac. Entomol. 2012, 15, 647–650. [Google Scholar] [CrossRef]
- Li, H.; Dou, G.; Gao, M.; Ren, F.; Li, R.; Zhang, X.; Yan, D.H. Annulohypoxylon sp. FPYF3050 produces volatile organic compounds against the pine wood nematode, Bursaphelenchus xylophilus. Nematology 2020, 22, 245–255. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Z.; Cai, L.; Liu, S.; Zhang, H.; Duan, M.; Zhang, K. Nematicidal effect of freshwater fungal cultures against the pine-wood nematode, Bursaphelenchus xylophilus. Fungal Divers. 2004, 15, 125–135. [Google Scholar]
- Shimada, A.; Fujioka, S.; Koshino, H.; Kimura, Y. Nematicidal activity of beauvericin produced by the fungus Fusarium bulbicola. Z. Naturforsch. C 2010, 65, 207–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, X.; Huang, C.; Zhou, J.; Shi, J.; Zhao, L. Temperature-regulated metabolites of Serratia marcescens inhibited reproduction of pinewood nematode Bursaphelenchus xylophilus. iScience 2023, 26, 107082. [Google Scholar] [CrossRef]
- Wang, S.; Han, M.; Li, H.; Xie, J.; Wei, K.; Wang, X. Testing the host range of Cyanopterus ninghais (Hymenoptera: Braconidae), a candidate for the biological control of Monochamus alternatus, the vector of pine wilt disease in Asia. Biol. Control. 2024, 195, 105547. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, S.J.; Kim, S.; Lee, M.R.; Baek, S.; Park, S.E.; Kim, J.; Shin, T.Y.; Kim, J.S. Management of pine wilt disease vectoring Monochamus alternatus adults using spray and soil application of Metarhizium anisopliae JEF isolates. J. Asia-Pac. Entomol. 2020, 23, 224–233. [Google Scholar] [CrossRef]
- Álvarez-Baz, G.; Fernández-Bravo, M.; Pajares, J.; Quesada-Moraga, E. Potential of native Beauveria pseudobassiana strain for biological control of Pine Wood Nematode vector Monochamus galloprovincialis. J. Invertebr. Pathol. 2015, 132, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Shi, H.L.; Wang, D. A New Strain of Metarhizium robertsii Isolated from Loess Plateau and Its Virulence and Pathological Characteristics against Monochamus alternatus. Microorganisms 2024, 12, 514. [Google Scholar] [CrossRef]
- Tian, H.; Koski, T.M.; Zhao, L.; Liu, Z.; Sun, J. Invasion history of the pinewood nematode Bursaphelenchus xylophilus influences the abundance of Serratia sp. in pupal chambers and tracheae of insect-vector Monochamus alternatus. Front. Plant Sci. 2022, 13, 856841. [Google Scholar] [CrossRef]
- Wu, S.; Wu, J.; Wang, Y.; Qu, Y.; He, Y.; Wang, J.; Cheng, J.; Zhang, L.; Cheng, C. Discovery of entomopathogenic fungi across geographical regions in southern China on pine sawyer beetle Monochamus alternatus and implication for multi-pathogen vectoring potential of this beetle. Front. Plant Sci. 2022, 13, 1061520. [Google Scholar] [CrossRef]
- Hussein, K.A.; Abdel-Rahman, M.A.; Abdel-Mallek, A.Y.; El-Maraghy, S.S.; Joo, J.H. Climatic factors interference with the occurrence of Beauveria bassiana and Metarhizium anisopliae in cultivated soil. Afr. J. Biotechnol. 2010, 9, 7674–7682. [Google Scholar]
- Fernandes, E.K.; Rangel, D.E.; Moraes, A.M.; Bittencourt, V.R.; Roberts, D.W. Cold activity of Beauveria and Metarhizium, and thermotolerance of Beauveria. J. Invertebr. Pathol. 2008, 98, 69–78. [Google Scholar] [CrossRef]
- Omuse, E.R.; Niassy, S.; Wagacha, J.M.; Ong’amo, G.O.; Azrag, A.G.; Dubois, T. Suitable models to describe the effect of temperature on conidial germination and mycelial growth of Metarhizium anisopliae and Beauveria bassiana. Biocontrol Sci. Technol. 2022, 32, 281–298. [Google Scholar] [CrossRef]
- McGuire, A.V.; Northfield, T.D. Tropical occurrence and agricultural importance of Beauveria bassiana and Metarhizium anisopliae. Front. Sustain. Food Syst. 2020, 4, 6. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, M.R.; Kim, S.; Lee, S.J.; Park, S.E.; Baek, S.; Gasmi, L.; Shin, T.Y.; Kim, J.S. Long-term storage stability of Beauveria bassiana ERL836 granules as fungal biopesticide. J. Asia-Pac. Entomol. 2019, 22, 537–542. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, M.R.; Yu, J.S.; Park, S.E.; Ha, P.; Kim, J.S. Management of overwintering pine sawyer beetle, Monochamus alternatus with colonized Beauveria bassiana ERL836. PLoS ONE 2022, 17, e0274086. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, L.; Lin, H.; Mao, S. Investigation of pathogens of Monochamus alternatus in East China and virulence. Chin. J. Biol. Control. 2009, 25, 220–225. [Google Scholar] [CrossRef]
- Shimazu, M. Potential of the cerambycid-parasitic type of Beauveria brongniartii (Deuteromycotina: Hyphomycetes) for microbial control of Monochamus alternatus Hope (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1994, 29, 127–130. [Google Scholar] [CrossRef][Green Version]
- Maehara, N.; He, X.; Shimazu, M. Maturation feeding and transmission of Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae) by Monochamus alternatus (Coleoptera: Cerambycidae) inoculated with Beauveria bassiana (Deuteromycotina: Hyphomycetes). J. Econ. Entomol. 2007, 100, 49–53. [Google Scholar] [CrossRef]
- Takatsuka, J. Specific PCR assays for the detection of DNA from Beauveria bassiana F-263, a highly virulent strain affecting the Japanese pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae), by a sequence-characterized amplified region (SCAR) marker. Appl. Entomol. Zool. 2007, 42, 619–628. [Google Scholar] [CrossRef][Green Version]
- He, R.; Cui, X.; Ying, Y.; Qu, L.; Wang, R.; Zhang, Y. Screening and identification of Beauveria bassiana strains for biocontrol of Monochamus alternatus adults (Coleoptera: Cerambycidae). Sci. Silvae Sin. 2020, 56, 129–134. [Google Scholar] [CrossRef]
- Kim, H.M.; Choi, I.S.; Lee, S.; Hwang, I.M.; Chun, H.H.; Wi, S.G.; Kim, J.C.; Shin, T.Y.; Kim, J.C.; Kim, J.S. Advanced strategy to produce insecticidal destruxins from lignocellulosic biomass Miscanthus. Biotechnol. Biofuels 2019, 12, 188. [Google Scholar] [CrossRef]
- He, X.; Chen, S.; Huang, J. Preliminary screening of virulent strains of Metarhizium anisopliae against Monochamus alternatus. Acta Entomol. Sin. 2005, 6, 975–981. [Google Scholar] [CrossRef]
- Kim, J.C.; Baek, S.; Park, S.E.; Kim, S.; Lee, M.R.; Jo, M.; Im, J.S.; Ha, P.; Kim, J.S.; Shin, T.Y. Colonization of Metarhizium anisopliae on the surface of pine tree logs: A promising biocontrol strategy for the Japanese pine sawyer, Monochamus alternatus. Fungal Biol. 2020, 124, 125–134. [Google Scholar] [CrossRef]
- Pan, Y.S.; Xu, F.Y.; Han, Z.M. Controlling on Monochamus alternatus larva with Scleroderma guani and carried fungus. J. Jiangsu For. Sci. Technol. 2009, 36, 11–14. [Google Scholar]
- He, X.; Chen, S.; Yang, X.; Huang, J.; Huang, B.; Cai, S. The investigation of Metarhizium anisopliae in forest soil in Fujian and Jiangxi provinces and pathogenicity against Monochamus alternatus. Mycosystema 2007, 2, 289–294. [Google Scholar] [CrossRef]
- Yin, Y.; Song, Z.; Xie, N.; Wang, Z. Nutrition requirement and culture characteristic of Metarhizium anisopliae strain CQMa117. Chin. J. Biol. Control. 2010, 26, 206. [Google Scholar] [CrossRef]
- Guo, Y.; Carballar-Lejarazú, R.; Sheng, L.; Fang, Y.; Wang, S.; Liang, G.; Hu, X.; Wang, R.; Zhang, F.; Wu, S. Identification and characterization of aminopeptidase-N as a binding protein for Cry3Aa in the midgut of Monochamus alternatus (Coleoptera: Cerambycidae). J. Econ. Entomol. 2020, 113, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Valero-Jimenez, C.A.; Wiegers, H.; Zwaan, B.J.; Koenraadt, C.J.; van Kan, J.A. Genes involved in virulence of the entomopathogenic fungus Beauveria bassiana. J. Invertebr. Pathol. 2016, 133, 41–49. [Google Scholar] [CrossRef]
- Peng, Z.; Huang, S.; Chen, J.; Li, N.; Wei, Y.; Nawaz, A.; Deng, S. An update of a green pesticide: Metarhizium anisopliae. All Life 2022, 15, 1141–1159. [Google Scholar] [CrossRef]
- Cai, N.; Liu, R.; Yan, D.; Zhang, N.; Zhu, K.; Zhang, D.; Nong, X.; Tu, X.; Zhang, Z.; Wang, G. Bioinformatics Analysis and Functional Characterization of the CFEM Proteins of Metarhizium anisopliae. J. Fungi 2022, 8, 661. [Google Scholar] [CrossRef]
- Wang, C.; St Leger, R.J. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot. Cell 2007, 6, 808–816. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Fine structure of the fungus Metarrhizium anisopliae infecting three species of larval Elateridae (Coleoptera) II. Conidial Germ tubes and appressoria. J. Invertebr. Pathol. 1970, 15, 81–91. [Google Scholar] [CrossRef]
- Wen, Z.; Tian, H.; Xia, Y.; Jin, K. MaPmt1, a protein O-mannosyltransferase, contributes to virulence through governing the appressorium turgor pressure in Metarhizium acridum. Fungal Genet. Biol. 2020, 145, 103480. [Google Scholar] [CrossRef]
- Krieger de Moraes, C.; Schrank, A.; Vainstein, M.H. Regulation of extracellular chitinases and proteases in the entomopathogen and acaricide Metarhizium anisopliae. Curr. Microbiol. 2003, 46, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Jeong, S.G.; Choi, I.S.; Yang, J.E.; Lee, K.H.; Kim, J.; Kim, J.C.; Kim, J.S.; Park, H.W. Mechanisms of Insecticidal Action of Metarhizium anisopliae on Adult Japanese Pine Sawyer Beetles (Monochamus alternatus). ACS Omega 2020, 5, 25312–25318. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wu, H.; Liu, Z.; Wang, Z.; Huang, B. G-Protein Subunit Galpha(i) in Mitochondria, MrGPA1, Affects Conidiation, Stress Resistance, and Virulence of Entomopathogenic Fungus Metarhizium robertsii. Front. Microbiol. 2020, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Widemann, E.; Bernard, G.; Lesot, A.; Pinot, F.; Pedrini, N.; Keyhani, N.O. CYP52X1, representing new cytochrome P450 subfamily, displays fatty acid hydroxylase activity and contributes to virulence and growth on insect cuticular substrates in entomopathogenic fungus Beauveria bassiana. J. Biol. Chem. 2012, 287, 13477–13486. [Google Scholar] [CrossRef]
- Wanchoo, A.; Lewis, M.W.; Keyhani, N.O. Lectin mapping reveals stage-specific display of surface carbohydrates in in vitro and haemolymph-derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiology 2009, 155, 3121–3133. [Google Scholar] [CrossRef]
- James, P.; Kershaw, M.; Reynolds, S.; Charnley, A. Inhibition of desert locust (Schistocerca gregaria) Malpighian tubule fluid secretion by destruxins, cyclic peptide toxins from the insect pathogenic fungus Metarhizium anisopliae. J. Insect Physiol. 1993, 39, 797–804. [Google Scholar] [CrossRef]
- Pucheta Díaz, M.; Flores Macías, A.; Rodríguez Navarro, S.; De la Torre, M. Mechanism of action of entomopathogenic fungi. Interciencia 2006, 31, 856–860. [Google Scholar]
- Kim, J.C.; Lee, M.R.; Kim, S.; Park, S.E.; Lee, S.J.; Shin, T.Y.; Kim, W.J.; Kim, J. Transcriptome Analysis of the Japanese Pine Sawyer Beetle, Monochamus alternatus, Infected with the Entomopathogenic Fungus Metarhizium anisopliae JEF-197. J. Fungi 2021, 7, 373. [Google Scholar] [CrossRef]
- Zhang, W.; Meng, J.; Ning, J.; Qin, P.; Zhou, J.; Zou, Z.; Wang, Y.; Jiang, H.; Ahmad, F.; Zhao, L.; et al. Differential immune responses of Monochamus alternatus against symbiotic and entomopathogenic fungi. Sci. China Life Sci. 2017, 60, 902–910. [Google Scholar] [CrossRef][Green Version]
- Yokoi, K.; Koyama, H.; Minakuchi, C.; Tanaka, T.; Miura, K. Antimicrobial peptide gene induction, involvement of Toll and IMD pathways and defense against bacteria in the red flour beetle, Tribolium castaneum. Results Immunol. 2012, 2, 72–82. [Google Scholar] [CrossRef]
- Chu, X.; Yang, M.; Yu, L.; Xie, H.; Liu, J.; Wu, S.; Zhang, F.; Hu, X. Double-strand RNAs targeting MaltRelish and MaltSpz reveals potential targets for pest management of Monochamus alternatus. Pestic. Biochem. Physiol. 2023, 194, 105495. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xu, W.; Lv, G.; Yuan, H.; Zhang, Q.H.; Wickham, J.D.; Xu, L.; Zhang, L. Associated bacteria of a pine sawyer beetle confer resistance to entomopathogenic fungi via fungal growth inhibition. Environ. Microbiome 2022, 17, 47. [Google Scholar] [CrossRef]
- Wang, C.; St Leger, R.J. A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 6647–6652. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Peng, G.; Xia, Y. The adenylate cyclase gene MaAC is required for virulence and multi-stress tolerance of Metarhizium acridum. BMC Microbiol. 2012, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.B.; Yu, S.F.; Wang, C.L.; Wang, L. cAMP Signalling Pathway in Biocontrol Fungi. Curr. Issues Mol. Biol. 2022, 44, 2622–2634. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, D.Y.; Ying, S.H.; Feng, M.G. A novel Ras GTPase (Ras3) regulates conidiation, multi-stress tolerance and virulence by acting upstream of Hog1 signaling pathway in Beauveria bassiana. Fungal Genet. Biol. 2015, 82, 85–94. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Ying, S.H.; Feng, M.G. Adenylate cyclase orthologues in two filamentous entomopathogens contribute differentially to growth, conidiation, pathogenicity, and multistress responses. Fungal Biol. 2014, 118, 422–431. [Google Scholar] [CrossRef]
- Modesto, I.; Mendes, A.; Carrasquinho, I.; Miguel, C.M. Molecular defense response of pine trees (Pinus spp.) to the parasitic nematode Bursaphelenchus xylophilus. Cells 2022, 11, 3208. [Google Scholar] [CrossRef]
- Van Loon, L. Systemic Induced Resistance. In Mechanisms of Resistance to Plant Diseases; Slusarenko, A.J., Fraser, R.S.S., van Loon, L.C., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 521–574. [Google Scholar]
- Ross, A.F. Localized acquired resistance to plant virus infection in hypersensitive hosts. Virology 1961, 14, 329–339. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic acquired resistance (SAR) and induced systemic resistance (ISR): Role and mechanism of action against phytopathogens. In Fungal Biotechnology and Bioengineering; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, C.; Wang, H.; Chen, H.; Tang, M. Pine wilt disease alters soil properties and root-associated fungal communities in Pinus tabulaeformis forest. Plant Soil 2016, 404, 237–249. [Google Scholar] [CrossRef]
- Romera, F.J.; Garcia, M.J.; Lucena, C.; Martinez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcantara, E.; Angulo, M.; Perez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, J.; Xia, R.; Li, D.; Wang, F. Antioxidant processes involving epicatechin decreased symptoms of pine wilt disease. Front. Plant Sci. 2022, 13, 1015970. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Vicente, C.S.L.; Barbosa, P.; Espada, M.; Glick, B.R.; Mota, M.; Oliveira, S. Evidence for the involvement of ACC deaminase from Pseudomonas putida UW4 in the biocontrol of pine wilt disease caused by Bursaphelenchus xylophilus. BioControl 2012, 58, 427–433. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, Y.; Li, D.; Ye, J. The Growth-Promoting and Colonization of the Pine Endophytic Pseudomonas abietaniphila for Pine Wilt Disease Control. Microorganisms 2024, 12, 1089. [Google Scholar] [CrossRef]
- Sun, M.; Liang, C.; Fu, X.; Liu, G.; Zhong, Y.; Wang, T.; Tang, G.; Li, P. Nematocidal activity and biocontrol efficacy of endophytic Bacillus velezensis Pt-RP9 from Pinus tabuliformis against pine wilt disease caused by Bursaphelenchus xylophilus. Biol. Control. 2024, 196, 105579. [Google Scholar] [CrossRef]
- Boller, T. The plant hormone ethylene. In Ethylene in Pathogenesis and Disease Resistance; Mattoo, A.K., Suttle, J.C., Eds.; CRC: Boca Raton, FL, USA, 1991; pp. 297–301. [Google Scholar]
- Trobacher, C.P. Ethylene and programmed cell death in plants. Botany 2009, 87, 757–769. [Google Scholar] [CrossRef]
- Nunes da Silva, M.; Pintado, M.E.; Sarmento, B.; Stamford, N.P.; Vasconcelos, M.W. A biofertilizer with diazotrophic bacteria and a filamentous fungus increases Pinus pinaster tolerance to the pinewood nematode (Bursaphelenchus xylophilus). Biol. Control 2019, 132, 72–80. [Google Scholar] [CrossRef]
- Ponpandian, L.N.; Rim, S.O.; Shanmugam, G.; Jeon, J.; Park, Y.H.; Lee, S.K.; Bae, H. Phylogenetic characterization of bacterial endophytes from four Pinus species and their nematicidal activity against the pine wood nematode. Sci. Rep. 2019, 9, 12457. [Google Scholar] [CrossRef] [PubMed]
- Robinet, C.; Raffin, A.; Jactel, H.; Kersaudy, E.; Deuffic, P.; Clopeau, A.; Hotte, H.; Kleinhentz, M.; Robin, C.; Roux, G. Improving monitoring and management methods is of the utmost importance in countries at risk of invasion by the pinewood nematode. Ann. For. Sci. 2024, 81, 16. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Li, Y.; Wei, P.; Sun, N.; Wen, X.; Liu, Z.; Li, D.; Feng, Y.; Zhang, X. Differences between microbial communities of Pinus species having differing level of resistance to the pine wood nematode. Microb. Ecol. 2022, 84, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Liu, X.; Chen, J.; Wang, B.; Li, Y.; Ye, Y.; Ma, W.; Ma, L. Effects on community composition and function Pinus massoniana infected by Bursaphelenchus xylophilus. BMC Microbiol. 2022, 22, 157. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Liang, S.; Zhu, W.; Li, H. Changes in Phyllosphere Microbial Communities of Pinus tabuliformis after Infestation by Bursaphelenchus xylophilus. Forests 2023, 14, 179. [Google Scholar] [CrossRef]
- Jia, J.; Chen, L.; Yu, W.; Cai, S.; Su, S.; Xiao, X.; Tang, X.; Jiang, X.; Chen, D.; Fang, Y. The novel nematicide chiricanine A suppresses Bursaphelenchus xylophilus pathogenicity in Pinus massoniana by inhibiting Aspergillus and its secondary metabolite, sterigmatocystin. Front. Plant Sci. 2023, 14, 1257744. [Google Scholar] [CrossRef]
- Vicente, C.S.; Nascimento, F.; Espada, M.; Barbosa, P.; Mota, M.; Glick, B.R.; Oliveira, S. Characterization of bacteria associated with pinewood nematode Bursaphelenchus xylophilus. PLoS ONE 2012, 7, e46661. [Google Scholar] [CrossRef]
- Xue, Q.; Xiang, Y.; Wu, X.; Li, M. Bacterial communities and virulence associated with pine wood nematode Bursaphelenchus xylophilus from different Pinus spp. Int. J. Mol. Sci. 2019, 20, 3342. [Google Scholar] [CrossRef]
- Park, A.R.; Jeong, S.I.; Jeon, H.W.; Kim, J.; Kim, N.; Ha, M.T.; Mannaa, M.; Kim, J.; Lee, C.W.; Min, B.S.; et al. A Diketopiperazine, Cyclo-(L-Pro-L-Ile), Derived From Bacillus thuringiensis JCK-1233 Controls Pine Wilt Disease by Elicitation of Moderate Hypersensitive Reaction. Front. Plant Sci. 2020, 11, 1023. [Google Scholar] [CrossRef]
- Nakashima, H.; Eguchi, N.; Uesugi, T.; Yamashita, N.; Matsuda, Y. Effect of ectomycorrhizal composition on survival and growth of Pinus thunbergii seedlings varying in resistance to the pine wilt nematode. Trees 2015, 30, 475–481. [Google Scholar] [CrossRef]
- Chu, H.; Tang, M.; Wang, H.; Wang, C. Pinewood nematode infection alters root mycoflora of Pinus tabulaeformis Carr. J. Appl. Microbiol. 2018, 125, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, C.; Li, Z.; Wang, H.; Xiao, Y.; Chen, J.; Tang, M. The dark septate endophytes and ectomycorrhizal fungi effect on Pinus tabulaeformis Carr. seedling growth and their potential effects to pine wilt disease resistance. Forests 2019, 10, 140. [Google Scholar] [CrossRef]
- Chu, H.; Wang, H.; Zhang, Y.; Li, Z.; Wang, C.; Dai, D.; Tang, M. Inoculation With Ectomycorrhizal Fungi and Dark Septate Endophytes Contributes to the Resistance of Pinus spp. to Pine Wilt Disease. Front. Microbiol. 2021, 12, 687304. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- Van Wees, S.C.; Luijendijk, M.; Smoorenburg, I.; Van Loon, L.C.; Pieterse, C.M. Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis is not associated with a direct effect on expression of known defense-related genes but stimulates the expression of the jasmonate-inducible gene Atvsp upon challenge. Plant Mol. Biol. 1999, 41, 537–549. [Google Scholar] [CrossRef]
- Pieterse, C.M.; van Wees, S.C.; Hoffland, E.; van Pelt, J.A.; van Loon, L.C. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 1996, 8, 1225–1237. [Google Scholar] [CrossRef]
- De Meyer, G.; Capieau, K.; Audenaert, K.; Buchala, A.; Métraux, J.; Höfte, M. Nanogram amounts of salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 activate the systemic acquired resistance pathway in bean. Mol. Plant-Microbe Interact. 1999, 12, 450–458. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Tang, J.; Gu, X.; Liu, J.; He, Z. Roles of small RNAs in crop disease resistance. Stress Biol. 2021, 1, 6. [Google Scholar] [CrossRef]
- Yan, J.; Qiu, R.; Wang, K.; Liu, Y.; Zhang, W. Enhancing alfalfa resistance to Spodoptera herbivory by sequestering microRNA396 expression. Plant Cell Rep. 2023, 42, 805–819. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, B.; Gao, C.; Zhang, H.; Wen, F.; Tao, L.; Fu, G.; Xiong, J. The evolution and functional roles of miR408 and its targets in plants. Int. J. Mol. Sci. 2022, 23, 530. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, S.D.; Veselova, S.V.; Burkhanova, G.F.; Alekseev, V.Y.; Maksimov, I.V. Bacillus subtilis 26D triggers induced systemic resistance against Rhopalosiphum padi L. by regulating the expression of genes AGO, DCL and microRNA in bread spring wheat. Microorganisms 2023, 11, 2983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Fan, Z.; Li, Z.; Niu, D.; Li, Y.; Zheng, M.; Wang, Q.; Jin, H.; Guo, J. Bacillus cereus AR156 triggers induced systemic resistance against Pseudomonas syringae pv. tomato DC3000 by suppressing miR472 and activating CNLs-mediated basal immunity in Arabidopsis. Mol. Plant Pathol. 2020, 21, 854–870. [Google Scholar] [CrossRef] [PubMed]
- Rosli, R.; Amiruddin, N.; Ab Halim, M.A.; Chan, P.; Chan, K.; Azizi, N.; Morris, P.E.; Leslie Low, E.; Ong-Abdullah, M.; Sambanthamurthi, R. Comparative genomic and transcriptomic analysis of selected fatty acid biosynthesis genes and CNL disease resistance genes in oil palm. PLoS ONE 2018, 13, e0194792. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Kanzaki, N.; Futai, K. How different is induced host resistance against the pine wood nematode, Bursaphelenchus xylophilus, by two avirulent microbes? Nematology 2006, 8, 435–442. [Google Scholar] [CrossRef]
- Linthorst, H.J.; Van Loon, L. Pathogenesis-related proteins of plants. Crit. Rev. Plant Sci. 1991, 10, 123–150. [Google Scholar] [CrossRef]
- Kim, N.; Jeon, H.W.; Mannaa, M.; Jeong, S.I.; Kim, J.; Kim, J.; Lee, C.; Park, A.R.; Kim, J.C.; Seo, Y.S. Induction of resistance against pine wilt disease caused by Bursaphelenchus xylophilus using selected pine endophytic bacteria. Plant Pathol. 2018, 68, 434–444. [Google Scholar] [CrossRef]
- Han, G.; Mannaa, M.; Kim, N.; Jeon, H.W.; Jung, H.; Lee, H.H.; Kim, J.; Park, J.; Park, A.R.; Kim, J.C.; et al. Response of Pine Rhizosphere Microbiota to Foliar Treatment with Resistance-Inducing Bacteria against Pine Wilt Disease. Microorganisms 2021, 9, 688. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.; Ali, Q.; Zhao, W.; Chi, Y.; Shafiq, M.; Ali, F.; Yu, X.; Yu, Q.; Zhao, J. Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challenges, and global perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Wang, R.; Dong, L.; Chen, Y.; Wang, S.; Qu, L. Third generation genome sequencing reveals that endobacteria in Nematophagous fungi Esteya vermicola contain multiple genes encoding for Nematicidal proteins. Front. Microbiol. 2022, 13, 842684. [Google Scholar] [CrossRef]
- Cheng, C.; Qin, J.; Wu, C.; Lei, M.; Wang, Y.; Zhang, L. Suppressing a plant-parasitic nematode with fungivorous behavior by fungal transformation of a Bt cry gene. Microb. Cell Factories 2018, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Geng, C.; Li, M.; Wang, Y.; Liu, H.; Zheng, J.; Peng, D.; Sun, M. Whole-genome analysis of Bacillus thuringiensis revealing partial genes as a source of novel Cry toxins. Appl. Environ. Microbiol. 2018, 84, e00277-18. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Choi, B.Y.; Kim, D.S.; Han, H.; Kim, Y.-H.; Shim, D. Temporal transcriptome profiling of Pinus densiflora infected with pine wood nematode reveals genetically programmed changes upon pine wilt disease. Phytopathology 2024, 114, 982–989. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, Y.; Ma, L.; Li, D.; Zhang, W.; Feng, Y.; Liu, Z.; Wang, X.; Wen, X.; Zhang, X. Transcriptomic response of Pinus massoniana to infection stress from the pine wood nematode Bursaphelenchus xylophilus. Stress Biol. 2023, 3, 50. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Ye, L.; Liu, N.; Wang, F. Methyl jasmonate induced tolerance effect of Pinus koraiensis to Bursaphelenchus xylophilus. Pest Manag. Sci. 2025, 81, 80–92. [Google Scholar] [CrossRef]
- Cardoso, J.M.; Manadas, B.; Abrantes, I.; Robertson, L.; Arcos, S.C.; Troya, M.T.; Navas, A.; Fonseca, L. Pine wilt disease: What do we know from proteomics? BMC Plant Biol. 2024, 24, 98. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Zou, C.; Ji, X.; Liu, T.; Zhao, P.; Liang, L.; Xu, J.; An, Z.; Zheng, X. Bacteria can mobilize nematode-trapping fungi to kill nematodes. Nat. Commun. 2014, 5, 5776. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, T.; Li, W.; Hong, J.; Xu, J.; Yu, Z. Endosymbiotic bacteria within the nematode-trapping fungus Arthrobotrys musiformis and their potential roles in nitrogen cycling. Front. Microbiol. 2024, 15, 1349447. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Z.; Zhang, J.; Liu, X. Classification of dendrocola nematode-trapping fungi. J. For. Res. 2021, 32, 1295–1304. [Google Scholar] [CrossRef]
- Jia, H.; Xia, R.; Zhang, R.; Liang, G.; Zhuang, Y.; Zhou, Y.; Li, D.; Wang, F. Transcriptome analysis highlights the influence of temperature on hydrolase and traps in nematode-trapping fungi. Front. Microbiol. 2024, 15, 1384459. [Google Scholar] [CrossRef] [PubMed]
- Saiki, H.; Saito, T.; Yoneda, K.; Umi, M.; Uchida, K.; Yamanaka, K. Biological control of the pine-wood nematode by spraying a nematode-trapping fungus. J. Jpn. For. Soc. 1984, 66, 30–32. [Google Scholar] [CrossRef]
- Wingfield, M.J. Fungi associated with the pine wood nematode, Bursaphelenchus xylophilus, and cerambycid beetles in Wisconsin. Mycologia 1987, 79, 325–328. [Google Scholar] [CrossRef]
- Kano, S.; Aimi, T.; Masumoto, S.; Kitamoto, Y.; Morinaga, T. Physiology and molecular characteristics of a pine wilt nematode-trapping fungus, Monacrosporium megalosporum. Curr. Microbiol. 2004, 49, 158–164. [Google Scholar] [CrossRef]
- Kubátová, A.; Novotný, D.; Práil, K.; Mrácek, Z. The nematophagous hyphomycete Esteya vermicola found in the Czech Republic. Czech Mycol. 2000, 52, 227–235. [Google Scholar] [CrossRef]
- Wang, C.Y.; Fang, Z.M.; Sun, B.S.; Gu, L.J.; Zhang, K.Q.; Sung, C.K. High infectivity of an endoparasitic fungus strain, Esteya vermicola, against nematodes. J. Microbiol. 2008, 46, 380–389. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Wang, J.; Guan, T.; Li, H. Morphological, molecular and biological characterization of Esteya vermicola, a nematophagous fungus isolated from intercepted wood packing materials exported from Brazil. Mycoscience 2014, 55, 367–377. [Google Scholar] [CrossRef]
- Meng, Q.; Shi, X.; Meng, F.; Feng, X.; Sun, J. Isolation of an Acremonium sp. from a screening of 52 seawater fungal isolates and preliminary characterization of its growth conditions and nematicidal activity. Biotechnol. Lett. 2012, 34, 1847–1850. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, Y.; Li, R.; Zhou, W.; Li, L.; Zhu, Y.; Huang, R.; Zhang, K. New nematicidal azaphilones from the aquatic fungus Pseudohalonectria adversaria YMF1. 01019. FEMS Microbiol. Lett. 2006, 264, 65–69. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, M.; Xi, P.; Jiang, Z. Screening of nematicidal isolates from the endophytic fungi of Quisqualis indica and optimization of culture conditions for the isolate. Chin. J. Biol. Control 2010, 26, 474. [Google Scholar] [CrossRef]
- Proença, D.N.; Francisco, R.; Santos, C.V.; Lopes, A.; Fonseca, L.; Abrantes, I.M.; Morais, P.V. Diversity of bacteria associated with Bursaphelenchus xylophilus and other nematodes isolated from Pinus pinaster trees with pine wilt disease. PLoS ONE 2010, 5, e15191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ponpandian, L.N.; Kim, H.; Jeon, J.; Hwang, B.S.; Lee, S.K.; Park, S.C.; Bae, H. Distribution and diversity of bacterial endophytes from four Pinus species and their efficacy as biocontrol agents for devastating pine wood nematodes. Sci. Rep. 2019, 9, 12461. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, C.; Guo, Q.; Li, L.; Guo, D. Identification and culture condition study of marine actinomycete HT-8 with nematicidal activity against pine wood nematode. J. Microbiol. 2016, 6, 18. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, X.; Wang, Y. Nematicidal activity of bacteria against Bursaphelenchus xylophilus and its fermentation and culture characteristics. Biotechnol. Bull. 2019, 35, 76. [Google Scholar] [CrossRef]
- Xu, H.; Xu, J.; Zhang, L.; Lin, H. Nematicidal activity of Bacillus thuringiensis to Bursaphelenchus xylophilus. Chin. J. Biol. Control 2010, 26, 85. [Google Scholar]
- Zhao, Y.; Yuan, Z.; Wang, S.; Wang, H.; Chao, Y.; Sederoff, R.R.; Sederoff, H.; Yan, H.; Pan, J.; Peng, M.; et al. Gene sdaB is involved in the Nematocidal activity of Enterobacter ludwigii AA4 against the pine wood nematode Bursaphelenchus xylophilus. Front. Microbiol. 2022, 13, 870519. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Luo, Y.; Hu, J.; Xia, Y.; Liu, M.; Li, Y.; Wang, H. Molecular Mechanisms of the Biological Control of Pine Wilt Disease Using Microorganisms. Microorganisms 2025, 13, 1215. https://doi.org/10.3390/microorganisms13061215
Su X, Luo Y, Hu J, Xia Y, Liu M, Li Y, Wang H. Molecular Mechanisms of the Biological Control of Pine Wilt Disease Using Microorganisms. Microorganisms. 2025; 13(6):1215. https://doi.org/10.3390/microorganisms13061215
Chicago/Turabian StyleSu, Xiaotian, Yimou Luo, Jingfei Hu, Yixin Xia, Min Liu, Yongxia Li, and Haihua Wang. 2025. "Molecular Mechanisms of the Biological Control of Pine Wilt Disease Using Microorganisms" Microorganisms 13, no. 6: 1215. https://doi.org/10.3390/microorganisms13061215
APA StyleSu, X., Luo, Y., Hu, J., Xia, Y., Liu, M., Li, Y., & Wang, H. (2025). Molecular Mechanisms of the Biological Control of Pine Wilt Disease Using Microorganisms. Microorganisms, 13(6), 1215. https://doi.org/10.3390/microorganisms13061215







