Comparison of Lipid Content in Nine Dinoflagellate Species Using Flow Cytometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Organisms
2.2. Staining of Algal Cells Using Fluorescent Dyes
2.3. Flow Cytometric Analysis
3. Results and Discussion
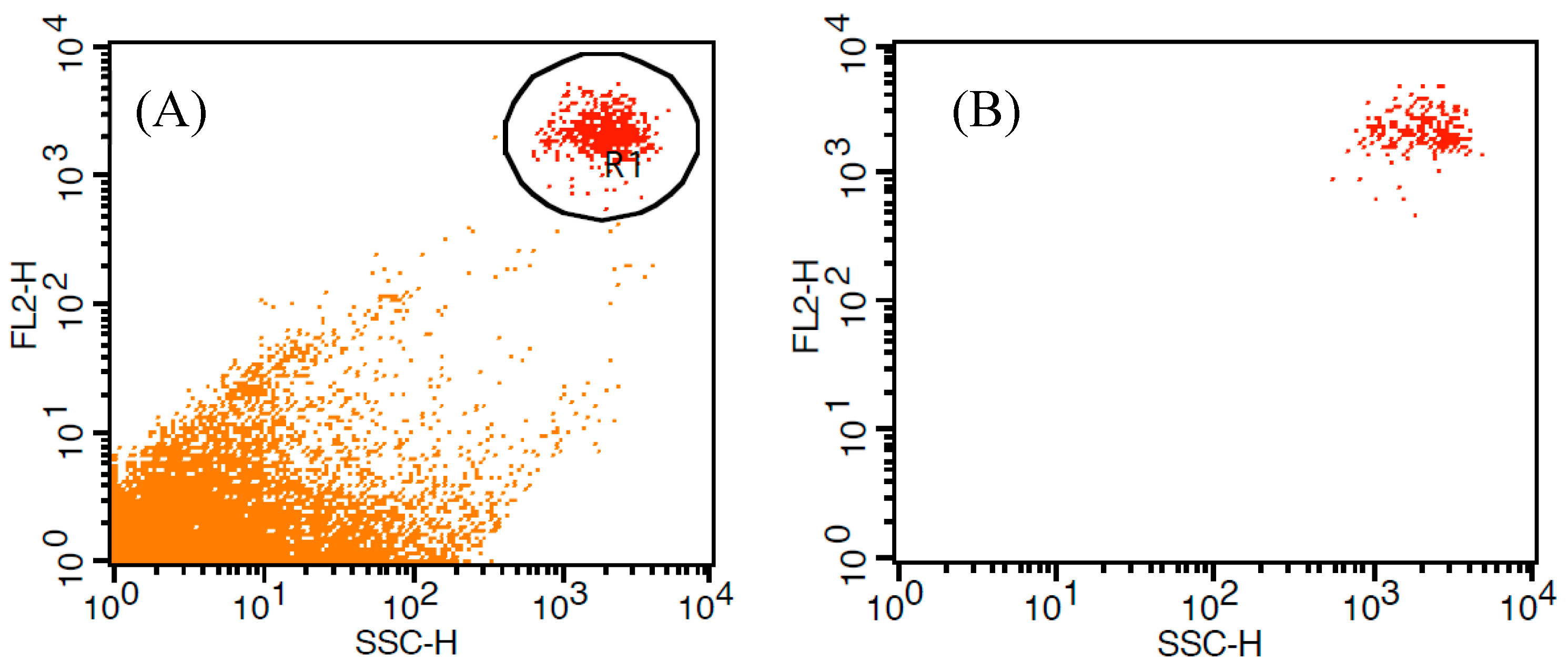
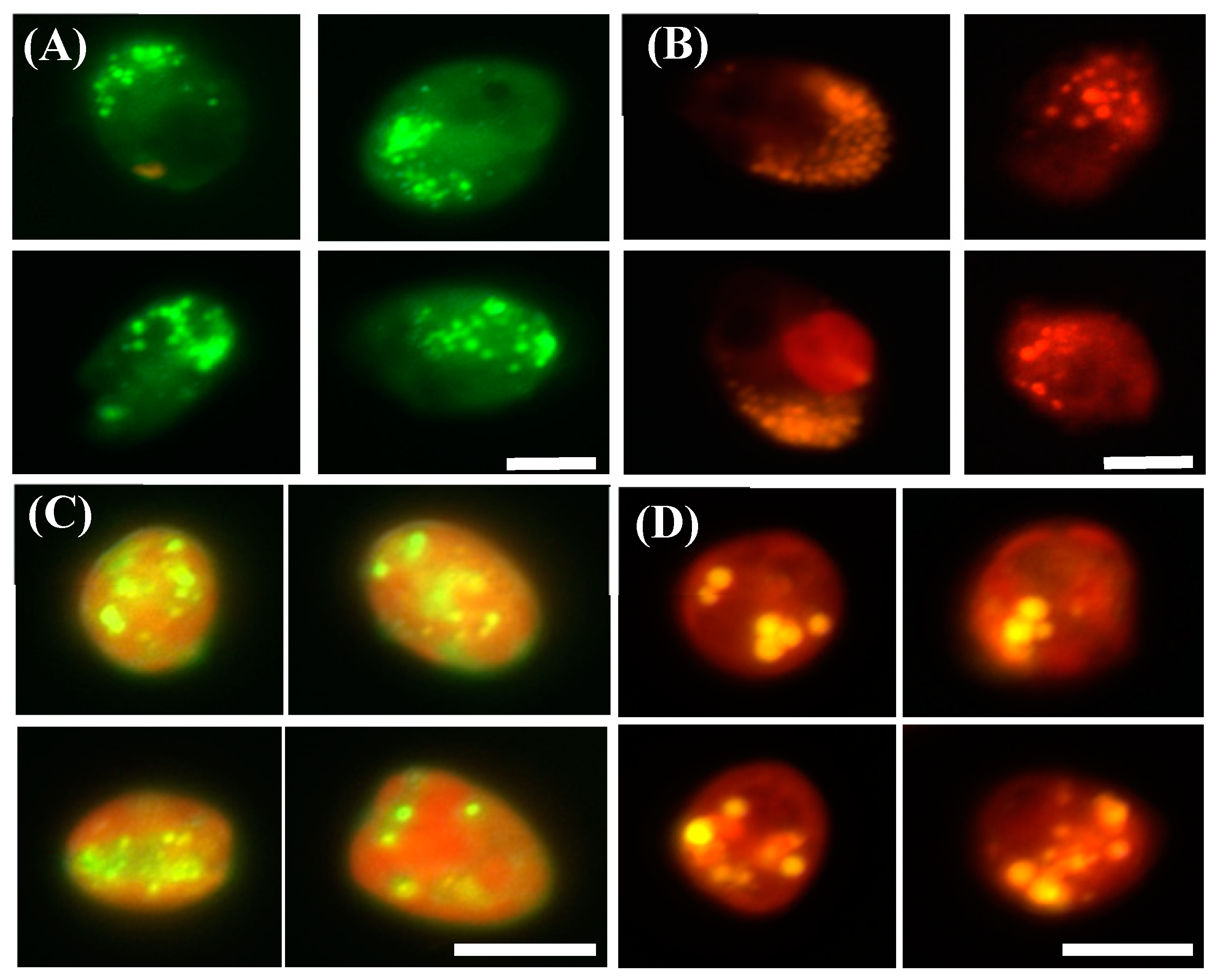
3.1. Flow Cytometric Analysis of Lipid Contents Using BODIPY 505/515
3.2. Comparision of Fluorescent Dyes (BODIPY 505/515 vs. Nile Red)
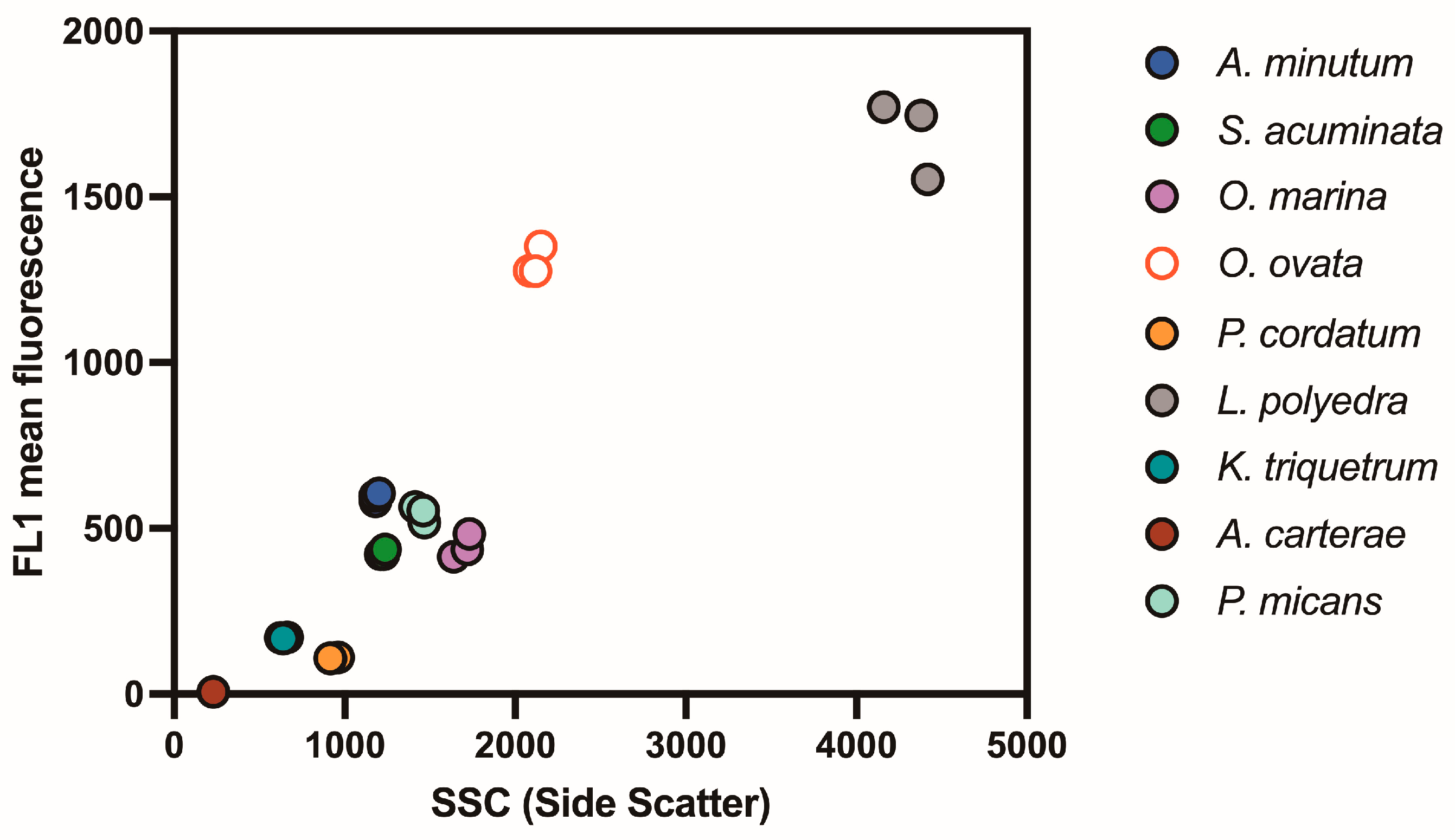
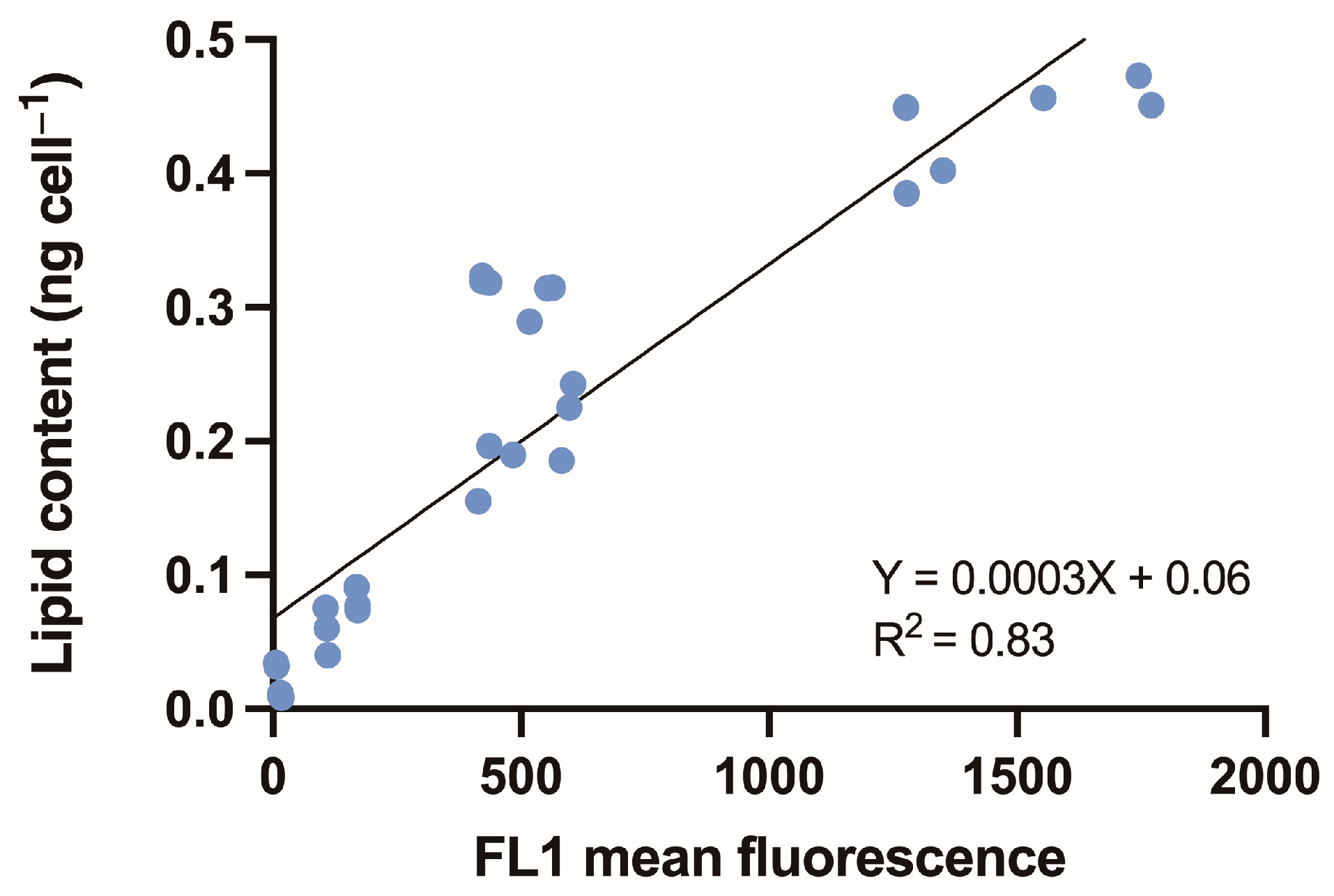
3.3. Estimating Lipid Content Using Relative Fluorescence
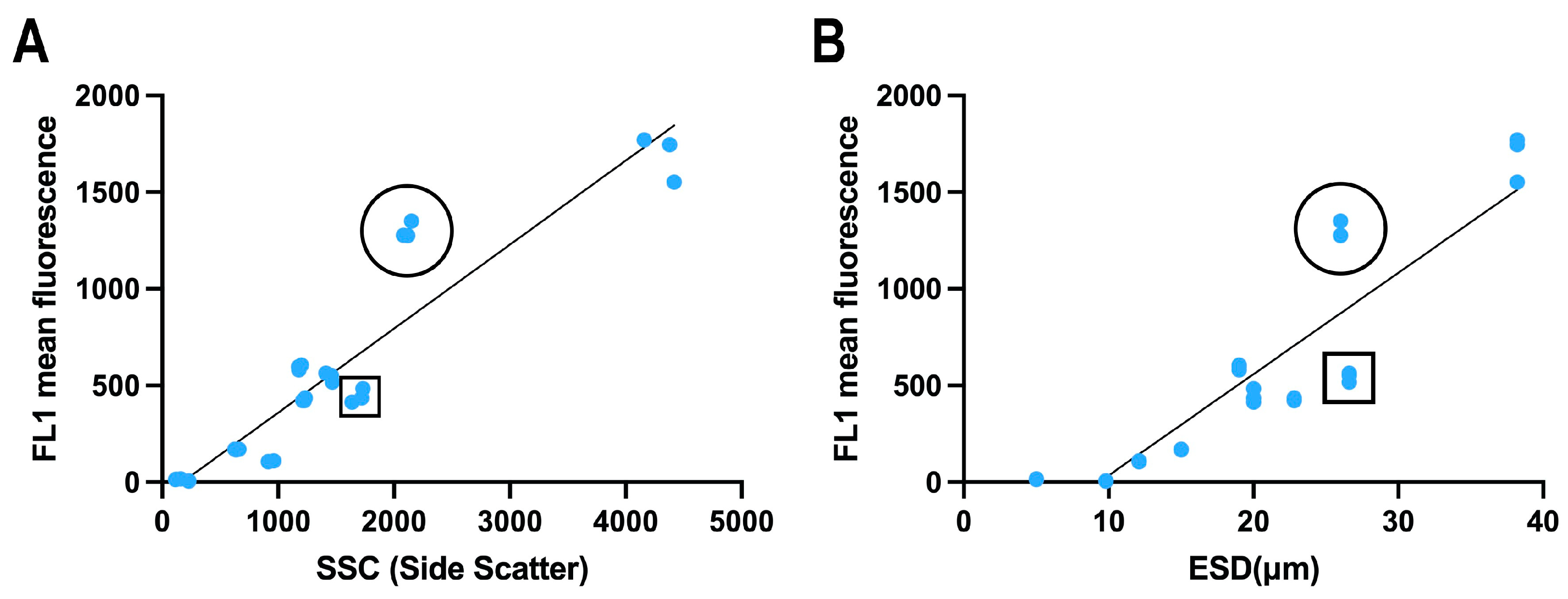
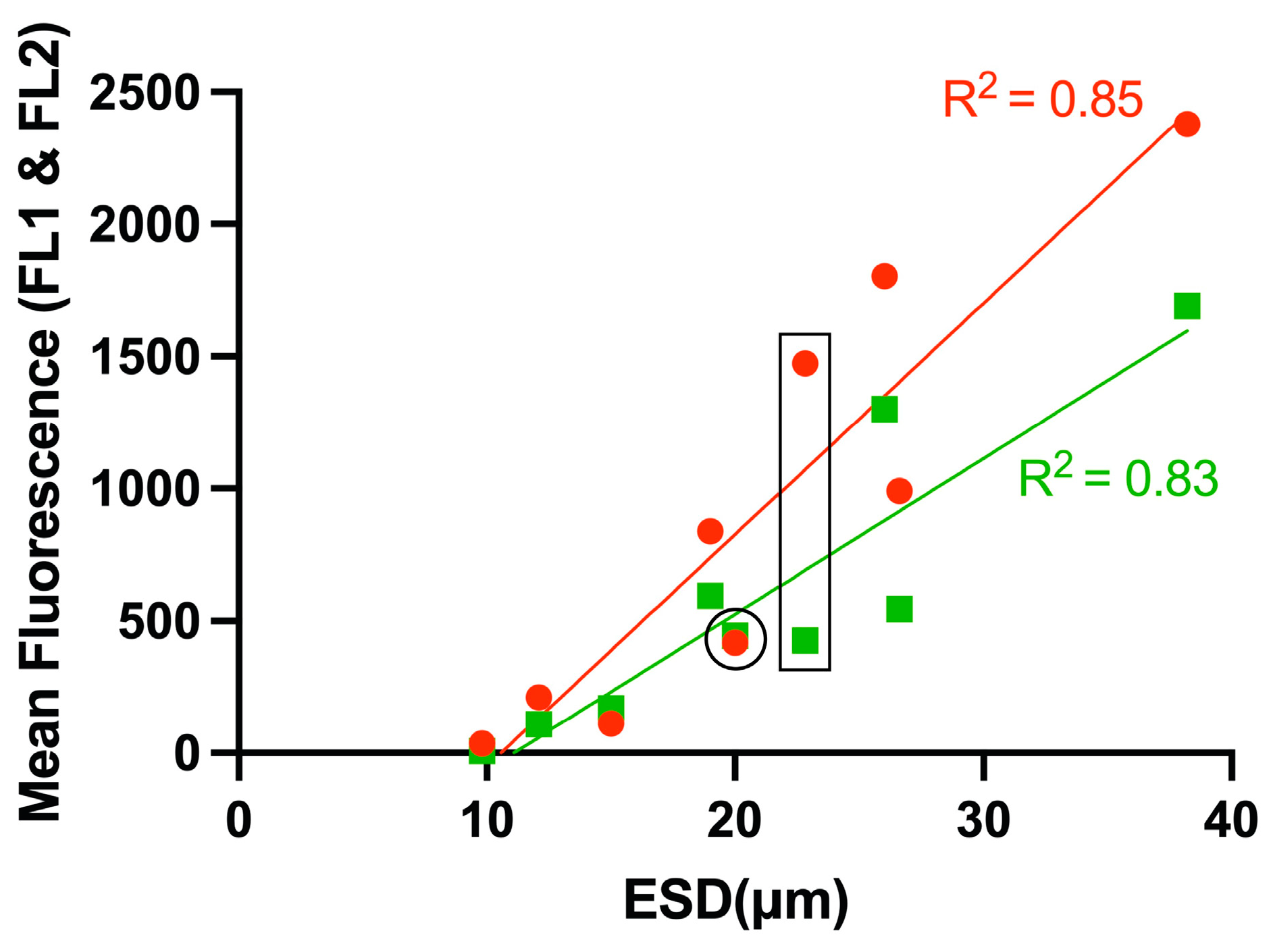
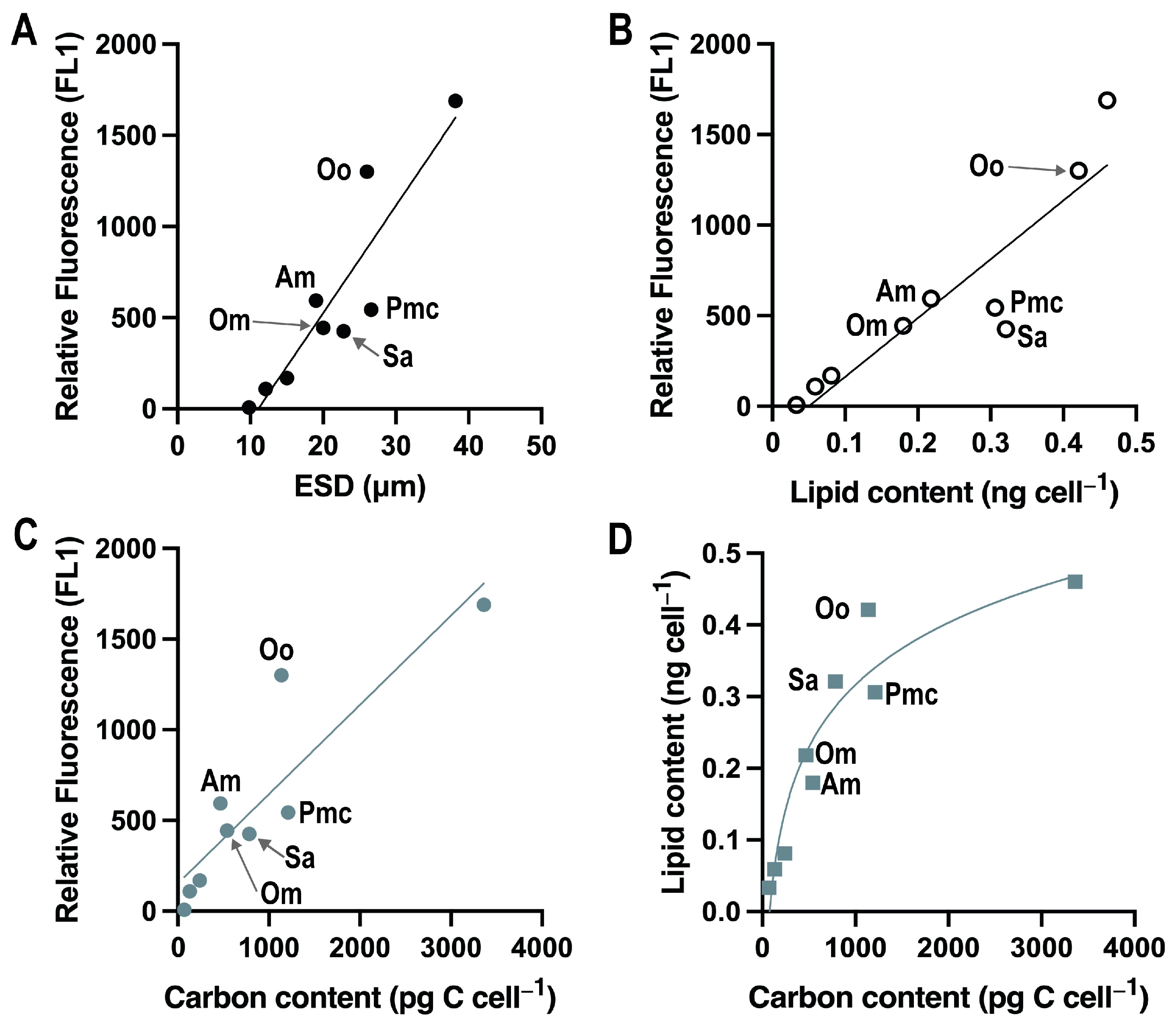
3.4. Comparison of Relative Fluorescence with Size, Lipid Concentration and Carbon Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piretti, M.V.; Pagliuca, G.; Boni, L.; Pistocchi, R.; Diamante, M.; Gazzotti, T. Investigation of 4-methyl sterols from cultured dinoflagellate algal strains. J. Phycol. 1997, 33, 61–67. [Google Scholar] [CrossRef]
- Mansour, M.P.; Volkman, J.K.; Jackson, A.E.; Blackburn, S.I. The fatty acid and sterol composition of five marine dinoflagellates. J. Phycol. 1999, 35, 710–720. [Google Scholar] [CrossRef]
- Muscatine, L. The role of symbiotic algae in carbon and energy flux in reef corals. In Coral Reefs: Ecosystems of the World; Dubinski, Z., Ed.; Elsevier: New York, NY, USA, 1990; pp. 75–87. [Google Scholar]
- Jeong, H.J.; Yoo, Y.D.; Kim, J.S.; Seong, K.A.; Kang, N.S.; Kim, T.H. Growth, feeding and ecological roles of mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci. J. 2010, 45, 65–91. [Google Scholar] [CrossRef]
- de la Jara, A.; Mendoza, H.; Martel, A.; Molina, C.; Nordströn, L.; de la Rosa, V.; Díaz, R. Flow cytometric determination of lipid content in a marine dinoflagellate, Crypthecodinium cohnii. J. Appl. Phycol. 2003, 15, 433–438. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Garcés, E.; Rossi, S.; Camp, J. Use of the dinoflagellate Karlodinium veneficum as a sustainable source of biodiesel production. J. Ind. Microbiol. Biotechnol. 2009, 36, 1215–1224. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Anderson, D.M. Turning back to red tide. Nature 1997, 388, 513–514. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeong, H.J.; Yoo, Y.D.; Yoon, E.Y. Mixotrophic dinoflagellate red tides in Korean waters: Distribution and ecophysiology. Harmful Algae 2013, 30, S28–S40. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Garcés, E.; Alacid, E.; Rossi, S.; Camp, J. Biomass and lipid production of dinoflagellates and raphidophytes in indoor and outdoor photobioreactors. Mar. Biotechnol. 2013, 15, 37–47. [Google Scholar] [CrossRef]
- Xu, S.J.; Wu, K.C.; Chan, S.C.Y.; Yau, Y.H.; Chan, K.K.; Lee, F.W.F. Investigation of growth, lipid productivity, and fatty acid profiles in marine bloom-forming dinoflagellates as potential feedstock for biodiesel. J. Mar. Sci. Eng. 2020, 8, 381. [Google Scholar] [CrossRef]
- Gui, J.; Chen, S.; Luo, G.; Wu, Z.; Fan, Y.; Yao, L.; Xu, H. Nutrient deficiency and an algicidal bacterium improved the lipid profiles of a novel promising oleaginous dinoflagellate, Prorocentrum donghaiense, for biodiesel production. Appl. Environ. Microbiol. 2021, 87, e01159-21. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Zheng, Y.; VanderGheynst, J.S. Rapid quantitative analysis of lipids using a colorimetric method in a microplate format. Lipids 2011, 46, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.D.; Liu, P.H.; Yang, X.; Shi, J.; Zhang, S. Screening method for lipid-content microalgae based on sulfo-phospho-vanillin reaction. Adv. Mater. Res. 2013, 610, 3532–3535. [Google Scholar] [CrossRef]
- Byreddy, A.R.; Gupta, A.; Barrow, C.J.; Puri, M. A quick colorimetric method for total lipid quantification in microalgae. J. Microbiol. Methods 2016, 125, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeong, H.J.; Yoon, E.Y.; Moon, S.J. Easy and rapid quantification of lipid contents of marine dinoflagellates using the sulpho-phospho-vanillin method. Algae 2016, 31, 391–401. [Google Scholar] [CrossRef]
- Marie, D.; Simon, N.; Vaulot, D. Phytoplankton cell counting by flow cytometry. In Algal Culturing Techniques; Anderson, R.A., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 253–267. [Google Scholar]
- Cirulis, J.T.; Strasser, B.C.; Scott, J.A.; Ross, G.M. Optimization of staining conditions for microalgae with three lipophilic dyes to reduce precipitation and fluorescence variability. Cytometry A 2012, 81, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Hyka, P.; Lickova, S.; Přibyl, P.; Melzoch, K.; Kovar, K. Flow cytometry for the development of biotechnological processes with microalgae. Biotechnol. Adv. 2013, 31, 2–16. [Google Scholar] [CrossRef]
- Cooksey, K.E.; Guckert, J.B.; Williams, S.A.; Callis, P.R. Fluorometric determination of the neutral lipid content of microalgal cells using Nile red. J. Microbiol. Methods 1987, 6, 333–345. [Google Scholar] [CrossRef]
- Elsey, D.; Jameson, D.; Raleigh, B.; Cooney, M.J. Fluorescent measurement of microalgal neutral lipids. J. Microbiol. Methods 2007, 68, 639–642. [Google Scholar] [CrossRef]
- Govender, T.; Ramanna, L.; Rawat, I.; Bux, F. BODIPY staining, an alternative to the Nile Red fluorescence method for the evaluation of intracellular lipids in microalgae. Bioresour. Technol. 2012, 114, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.S.; Hardin, W.R.; Petersen, T.W.; Cattolico, R.A. Visualizing “green oil” in live algal cells. J. Biosci. Bioeng. 2010, 109, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessand, E. Carbon to volume relationships for dinoflagelaltes, diatoms, and other protest plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Dempster, T.A.; Sommerfeld, M.R. Effects of environmental conditions on growth and lipid accumulation in Nitzschia communis (Bacillariophyceae). J. Phycol. 1998, 34, 712–721. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Huang, A.; Huan, L.; He, L.; Lin, A.; Wang, G. Detection of intracellular neutral lipid content in the marine microalgae Prorocentrum micans and Phaeodactylum tricornutum using Nile red and BODIPY 505/515. J. Appl. Phycol. 2014, 26, 1659–1668. [Google Scholar] [CrossRef]
- Laurens, L.M.; Wolfrum, E.J. Rapid compositional analysis of microalgae by NIR spectroscopy. NIR News 2012, 23, 9–11. [Google Scholar] [CrossRef]
- De la Hoz Siegler, H.; Ayidzoe, W.; Ben-Zvi, A.; Burrell, R.E.; McCaffrey, W.C. Improving the reliability of fluorescence-based neutral lipid content measurements in microalgal cultures. Algal Res. 2012, 1, 176–184. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef]
- Seo, S.H.; Ha, J.S.; Yoo, C.; Srivastava, A.; Ahn, C.Y.; Cho, D.H.; Oh, H.M. Light intensity as major factor to maximize biomass and lipid productivity of Ettlia sp. in CO2-controlled photoautotrophic chemostat. Bioresour. Technol. 2017, 244, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Pancha, I.; Chokshi, K.; Mishra, S. Enhanced biofuel production potential with nutritional stress amelioration through optimization of carbon source and light intensity in Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 179, 565–572. [Google Scholar] [CrossRef]
- Mendoza Guzmán, H.; de la Jara Valido, A.; Carmona Duarte, L.; Freijanes Presmanes, K. Analysis of interspecific variation in relative fatty acid composition: Use of flow cytometry to estimate unsaturation index and relative polyunsaturated fatty acid content in microalgae. J. Appl. Phycol. 2011, 23, 7–15. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Hansen, P.J.; Caron, D.A.; Mitra, A. Mixotrophy in the marine plankton. Ann. Rev. Mar. Sci. 2017, 9, 311–335. [Google Scholar] [CrossRef]
- Ser-Giacomi, E.; Zinger, L.; Malviya, S.; De Vargas, C.; Karsenti, E.; Bowler, C.; De Monte, S. Ubiquitous abundance distribution of non-dominant plankton across the global ocean. Nat. Ecol. Evol. 2018, 2, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.F.; Hagen, W.; Kattner, G. Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser. 2006, 307, 273–306. [Google Scholar] [CrossRef]
- Jónasdóttir, S.H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 2019, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Bi, R.; Zhang, C.; Cui, J.; Li, L.; Ding, Y.; Zhao, M. Quantification of multiple environmental controls on lipid biomarkers in common marine diatoms and dinoflagellates. Mar. Biol. 2023, 170, 136. [Google Scholar] [CrossRef]
- Jeong, H.J.; Yoo, Y.D.; Lee, K.; Kang, H.C.; Kim, J.S.; Kim, K.Y. Annual carbon retention of a marine-plankton community in the eutrophic Masan Bay, based on daily measurements. Mar. Biol. 2021, 168, 69. [Google Scholar] [CrossRef]

| Class | Species Name | Trophic Mode | Origin |
|---|---|---|---|
| Dinoflagellates | Amphidinium carterae | Mixotroph | USA |
| Kryptoperidinium triquetrum | Mixotroph | Masan bay, Republic of Korea | |
| Prorocentrum cordatum | Mixotroph | Kunsan, Republic of Korea | |
| Alexandrium minutum | Mixotroph | CCMP 113 | |
| Oxyrrhis marina | Heterotroph | Kunsan, Republic of Korea | |
| Scrippsiella acuminata | Mixotroph | Jeju, Republic of Korea | |
| Ostreopsis ovata | Autotroph | Jeju, Republic of Korea | |
| Prorocentrum micans | Mixotroph | Shiwha, Republic of Korea | |
| Lingulaulax polyedra | Mixotroph | USA |
| Species Name | ESD (μm) | Relative Fluorescence (Average) | Lipid Conc. (ng Cell−1) | Carbon Contents (pg Cell−1) | ||
|---|---|---|---|---|---|---|
| SSC | FL1 (BODIPY 505/515) | FL2 (Nile Red) | [19] | |||
| Amphidinium carterae | 9.8 | 229.4 | 6.8 | 36.8 | 0.033 ± 0.004 | 72.8 |
| Prorocentrum cordatum | 12.1 | 930.5 | 108.6 | 210.8 | 0.059 ± 0.018 | 131.8 |
| Krytoperidinium triquetrum | 15 | 641.6 | 169.7 | 111.0 | 0.081 ± 0.009 | 241.4 |
| Alexandrium minutum | 19 | 1185.8 | 594.4 | 838.4 | 0.218 ± 0.029 | 469.9 |
| Oxyrrhis marina | 20 | 1696.2 | 444.6 | 417.1 | 0.180 ± 0.022 | 542.9 |
| Scrippsiella acuminata | 22.8 | 1224.5 | 426.6 | 1474.8 | 0.321 ± 0.002 | 785.3 |
| Ostreopsis ovata | 26 | 2117.2 | 1301.4 | 1803.0 | 0.421 ± 0.033 | 1136.9 |
| Prorocentrum micans | 26.6 | 1447.1 | 544.7 | 991.2 | 0.306 ± 0.015 | 1212.4 |
| Lingulaulax polyedra | 38.2 | 4319.7 | 1689.7 | 2378.4 | 0.460 ± 0.012 | 3360.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Yoon, E.Y.; Moon, S.J.; Hyung, J.-H.; Lee, H. Comparison of Lipid Content in Nine Dinoflagellate Species Using Flow Cytometry. Microorganisms 2025, 13, 44. https://doi.org/10.3390/microorganisms13010044
Park J, Yoon EY, Moon SJ, Hyung J-H, Lee H. Comparison of Lipid Content in Nine Dinoflagellate Species Using Flow Cytometry. Microorganisms. 2025; 13(1):44. https://doi.org/10.3390/microorganisms13010044
Chicago/Turabian StylePark, Jaeyeon, Eun Young Yoon, Seung Joo Moon, Jun-Ho Hyung, and Hangy Lee. 2025. "Comparison of Lipid Content in Nine Dinoflagellate Species Using Flow Cytometry" Microorganisms 13, no. 1: 44. https://doi.org/10.3390/microorganisms13010044
APA StylePark, J., Yoon, E. Y., Moon, S. J., Hyung, J.-H., & Lee, H. (2025). Comparison of Lipid Content in Nine Dinoflagellate Species Using Flow Cytometry. Microorganisms, 13(1), 44. https://doi.org/10.3390/microorganisms13010044






