Abstract
Radiation protection is an important field of study, as it relates to human health and environmental safety. Radiation-resistance mechanisms in extremophiles are a research hotspot, as this knowledge has great application value in bioremediation and development of anti-radiation drugs. Mount Everest, an extreme environment of high radiation exposure, harbors many bacterial strains resistant to radiation. However, owing to the difficulties in studying them because of the extreme terrain, many remain unexplored. In this study, a novel species (herein, S7-12T) was isolated from the moraine of Mount Everest, and its morphology and functional and genomic characteristics were analyzed. The strain S7-12T is white in color, smooth and rounded, non-spore-forming, and non-motile and can survive at a UV intensity of 1000 J/m2, showing that it is twice as resistant to radiation as Deinococcus radiodurans. Radiation-resistance genes, including IbpA and those from the rec and CspA gene families, were identified. The polyphasic taxonomic approach revealed that the strain S7-12T (=KCTC 59114T =GDMCC 1.3458T) is a new species of the genus Knoellia and is thus proposed to be named glaciei. The in-depth study of the genome of strain S7-12T will enable us to gain further insights into its potential use in radiation resistance. Understanding how microorganisms resist radiation damage could reveal potential biomarkers and therapeutic targets, leading to the discovery of potent anti-radiation compounds, thereby improving human resistance to the threat of radiation.
1. Introduction
As the main peak of the Himalayas, Mount Everest, the highest mountain in the world, is subject to extreme environmental conditions, such as high radiation, low-oxygen concentrations [1], and high-temperature variations [2]. Although such extreme settings do not appear to be conducive to life, they are rich in microbial organisms that are resistant to radiation and oxidation [3] and can tolerate extreme pH and salinity levels [4]. Many microorganisms dwelling in these harsh conditions have developed survival and adaptive mechanisms to overcome these stresses [5]. For example, Flavobacterium sp. LB2P22T, isolated from the Laiku glacier on the Tibetan Plateau in China by Zhang et al., can degrade alpha-cypermethrin and has a certain degree of salt tolerance [6]. Moreover, collected from the high Arctic glacier near the settlement of Nova Oresund (Svalbard, Norway) by Xie et al., Pengzhenrongella M0-14T was not only hydrolytically active but also grew in media containing 1–5% (w/v) NaCl [7]. Valenzuela-Ibaceta et al. isolated Arthrobacter EH-1B-1T from Union Glacier soil in the Ellsworth Mountains, and the strain exhibited antioxidant activity and cold-acclimation response [8]. Thus, glacial ice serves as a viable ecosystem to support the survival of several microorganisms with anti-radiation and anti-oxidative activities [2]. Therefore, an in-depth study of the genomes of these strains would be helpful for developing radiation-, salt-, and low-temperature-resistant strains.
To date, only six strains have been identified in the genus Knoellia, including Knoellia aerolata DSM 18566T, isolated from air samples from Suwon, Republic of Korea, which could grow normally in high-salt concentrations and adapt to a wide range of pH and temperature [9]; salt-tolerant Knoellia locipacati DMZ1T from soil in the Korean Demilitarized Zone [10]; Knoellia remsis ATCC BAA-1496T from the air of the Regenerative Closed Life Support Module simulator system [11]; Knoellia flava TL1T from pig feces [12]; and Knoellia sinensis KCTC 19936T and Knoellia subterranea KCTC 19937T from a cave in China [13]. However, none of these strains have been studied in detail in terms of their functioning, and knowledge on the genus Knoellia remains very sparse.
These known strains of Knoellia were collected from a wide range of sources: from everyday air to off-the-beaten-track caves. However, only strain S7-12T, which is the focus of this paper, was isolated from extreme environments, and it was more resistant to radiation than Deinococcus radiodurans [14], which has a high resistance to radiation and antioxidants [15]. Because strain S7-12T is resistant to UV radiation, we performed an in-depth analysis of its genome and identified many genes with the drug-resistance function. Moreover, we found genes with cold tolerance and adaptation to a high-radiation environment. This is a very important discovery and proves why the strain can be isolated from extreme environmental conditions, such as those with strong radiation and oxidation in glaciers.
In May 2019, we collected moraine samples from the north slope of Mount Everest. After culturing the samples on the R2A medium, strain S7-12T was obtained, purified, and cultured. To determine whether S7-12T can resist radiation and oxidation, we performed experiments with various radiation and oxidation gradients. Lastly, we sequenced and analyzed its genome to understand the uniqueness of this strain and its specific functions.
2. Materials and Methods
2.1. Bacterial Isolation and Culture
On 8 May 2019, strain S7-12T was collected from moraine samples on the northern slope of Mount Everest (28.02° N, 86.56° E) at 5800 m above sea level. After sampling using a sterile shovel to collect about 200 g of samples into a sterile bag, the collected samples were placed in a 4 °C incubator and transported to Everest Base Camp before the experiment samples were stored in the −20 °C refrigerator. The ecological niche is characterized by high altitude, high ultraviolet and cosmic ray radiation, low temperature, and low concentration of atmospheric oxygen [16]. Briefly, a moraine sample (5 g) was placed in a 50-mL sterile centrifuge tube with 30 mL of sterile saline (0.85%) and shaken at 180 rpm at 30 °C for 40 min. The supernatant (100 µL) was diluted to 10−4, dissolved in Reasoner’s 2A (R2A) agar medium [17], and incubated at 30 °C for 15 days. Strain S7-12T was purified and cultured on R2A agar medium for 72 h. Reference strains K. flava TL1T, K. sinensis KCTC 19936T, and K. subterranea KCTC 19937T were purchased from the Korean Collection for Type Cultures (KCTC) and K. locipacati NBRC 109775T from the Biological Resource Center, NITE (NBRC).
2.2. Morphological, Physiological, and Biochemical Analysis
After 72 h of incubation on R2A agar medium, the morphological characteristics of strain S7-12T were observed using an electron microscope (JSM-5600, JEOL (BEIJING) Co., Ltd., Beijing, China). The Gram reaction was determined using Solarbio’s Gram staining kit (Solarbio Cat# G1132, Beijing, China). Growth temperature tests were performed on the R2A liquid medium in the range of 10–45 °C at 5 °C intervals. NaCl tolerance tests were performed on the R2A liquid medium containing 0–10% (w/v) at 1% intervals. The growth pH range was determined using the R2A liquid medium with pH 4.0–12.0 at 1.0 pH-unit intervals. Carbohydrate utilization tests, nitrogen utilization tests, and hydrolysis tests were determined according to the methods of Shirling and Gottlieb, Williams, and Kurup and Schmitt, respectively [18,19,20]. Other enzyme activities were assayed using API ZYM strips according to the manufacturer’s instructions (biome Rieux, Lyon, France).
2.3. Chemotaxonomic Analysis
For the analysis of the chemical taxonomic characteristics of strain S7-12T and its closely related strains K. flava TL1T, K. sinensis KCTC 19936T, K. subterranea KCTC 19937T, and K. locipacati DMZ1T, the strains were incubated in R2A liquid medium at 30 °C for 72 h to obtain the required cell biomass. The contents of respiratory quinones, polar lipids, and fatty acids were determined. Respiratory quinones were extracted from the dried organisms (100 mg) using a chloroform/methanol (2:1, v/v) solution and analyzed using HPLC (<37 °C) [21]. The diaminoacrylic acid isomers of the cell wall and whole-cell sugars were analyzed using Lechevalier and Lecheyalier’s [22] and Staneck and Roberts’s methods, respectively [23]. The polar lipids were extracted using a chloroform/methanol/water system via two-dimensional TLC and identified according to Minnikin et al.’s method. The samples were tested and analyzed using the Sherlock MIDI standard protocol (microbial identification system 6.2b). The peak results were determined by comparison with the database TSBA 6 (version 6.21).
2.4. Phylogenetic Analysis
The bacterial genomic DNA extraction kit (Omega) was used to extract the DNA from cells of strain S7-12T according to the manufacturer’s instructions. The whole genome was sequenced on the Illumina Hiseq 2000 platform with >fold coverage. The genome assembly was performed using the short sequence assembly software SOAPdenovo2 v2.04-r241 [24]. The completed genome was mapped using Unicycler version 0.4.8 [25] to assemble the third-generation sequence. During assembly, the sequence was corrected and polished with long reads using Pilon version 1.22. The assembly results of the scanning maps and chromosomal genomes were predicted using Glimmer. Based on comparisons with the genome data of strain S7-12T, the average nucleotide identity (ANI) was calculated based on OrthoANIu (OrthoANI), BLAST (ANIb), and MUMmer (ANIm) algorithms [26,27,28,29]. The average amino acid identity (AAI) was calculated using the online resource from the Konstantinidis group (http://enve-omics.ce.gatech.edu/aai/, accessed on 19 February 2024) [30]. The genome distances were calculated using the Genome-To-Genome Distance Calculator (http://ggdc.dsmz.de/, accessed on 19 February 2024) [31]. The dDDH results were obtained from the recommended formula 2, which was independent from the genome length and robust against the utilization of incomplete draft genomes.
The 16S rRNA gene sequencing was performed by polymerase chain reaction (PCR) using the universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) [32]. The sequences of both strands of the PCR-amplified 16S DNA were determined by Tsingke Company (Xi’an, China) using the dideoxy chain termination method with an ABI 3730XL Analyzer (Applied Biosystems, Waltham, MA, USA). The almost complete sequence of the 16S rRNA gene was compiled using the SeqMan software v12.3 (Lasergene) and analyzed using EzBioCloud (https://www.ezbiocloud.net/, accessed on 19 February 2024). The consensus sequences of strains belonging to the same phylogenetic group and those of other representatives of the Alphaproteobacteria were aligned using the ClustalW multiple alignment program [33]. Phylogenetic trees were constructed using the neighbor-joining [34], minimum-evolution, and maximum-likelihood [35] methods, followed by bootstrap analysis with 1000 resamplings [35] using MEGA 11 [36].
Kimura’s two-parameter model [37] was used for nucleotide substitution to estimate genetic differences. The phylogenomic tree was reconstructed based on the up-to-date bacterial core gene set according to the pipeline suggested by Na et al. [38].
2.5. Genomic Analysis and Prediction
The Prokaryotic Genome Annotation System (Prokka) was used to generate the protein and nucleotide sequences of the genes and annotation files (GFF3, GBK) to ensure the consistency and reliability of genome annotations and gene predictions and perform downstream genome analysis.
The gene prediction of plasmids was performed after the sequencing of strain S7-12T using Glimmer version 3.02 and GeneMarkS version 4.30. The rRNAs and tRNAs contained in the genome of the strain were predicted using Barrnap version 0.4.2 and tRNAscan-SE version 1.3.1. The 16sRNAs in the genome were predicted using the 16s database and compared with the housekeeping gene database.
The NCBI prokaryotic genome annotation pipeline [39] was used to predict the tRNA genes, rRNA genes, and noncoding rRNA genes of strain S7-12T. The genomes were annotated using Rapid Annotation of Subsystem Technology [40]. The Kyoto Encyclopedia of Genes and Genomes (KEGG) [41], Clusters of Orthologous Groups (COG) of proteins [42], NCBI Non-Redundant Protein [43], Protein Families [44], Swiss-Prot [45], and Carbohydrate Active Enzymes databases were selected for retrieval to improve functional annotation [46]. The biosynthetic gene cluster of secondary metabolites was predicted by silicon calculation using AntiSMASH 6.0.1 (https://antismash.secondarymetabolites.org/, accessed on 19 February 2024) [47]. The statistical analyses were performed in SPSS version 16.0 [48]. The pan-genome was constructed using the Bacterial Pan Genome Analysis software [49]. The genome sequencing data of the strain S7-12T was deposited in the GenBank database with the accession number.
2.6. Radiation-Resistance Analysis
Escherichia coli BL21 was used as a control strain. First, 0.5 mL of strain inoculum in the exponential growth phase (OD600 = 0.6) was grown in 10 mL of R2A liquid medium, placed in a 50-mL triangle bottle, and incubated at 30 °C with shaking at 200 rpm. The inoculum was diluted with saline to 10–4 once OD600 = 1.0 was reached. As radiation-free control, 100 μL of inoculum was diluted to 10–4 and grown on the R2A liquid medium, the other medium receiving 100 J/m2 UVC radiation, respectively. After 24 h of incubation at 30 °C, the number of colonies on the R2A liquid medium was counted. The radiation survival rate was calculated as follows: (Ns/Nc) × 100%, where Ns is the number of colonies spreading irradiated inoculum on the R2A agar substrate, and Nc is the number of colonies spreading radiation-free inoculum on the R2A agar substrate. All the experiments were performed in triplicate.
3. Results and Discussion
3.1. Phylogenetic Characterization Based on 16S rRNA Gene Sequencing
The full-length 16S rRNA gene sequencing and genome data of strain S7-12T were stored in the JCM/GDMCC/GenBank with accession numbers GDMCC 1.3458 and GCA_040518285.1, respectively.
By comparing the 16S rRNA gene sequences of strain S7-12T in the EzTaxon database, this species was identified and classified into phylum Actinobacteria. The highest similarity values to strain S7-12T were found with members of the genus Knoellia. The species closely related to strain S7-12T are K. locipacati DMZ1T, K. sinensis KCTC 19936T, K. subterranea KCTC 19937T, K. aerolata DSM 18566T, K. flava TL1T, and K. remsis ATCC BAA-1496T, with 16S rRNA gene sequence similarity levels of 98.61%, 98.55%, 98.41%, 98.13%, 97.58%, and 97.16%, respectively.
The dDDH and ANI values of S7-12T with other similar strains in the genus Knoellia reached 22.3–24.3% and 79.31–81.60%, respectively, which were lower than the thresholds for the identification of a new species (70% for dDDH and 95% for ANI). This confirms that S7-12T is a novel species [50]. The phylogenetic tree was reconstructed using four algorithms with sixteen type strains that are highly related to strain S7-12T and four Knoellia species isolated from the northern slope of Mount Everest. A neighbor-joining dendrogram with Marihabitans asiaticum DSM 18935T as an outgroup shows the phylogenetic position of strain S7-12T (Figure 1, Figures S1 and S2). The three phylogenetic trees based on 16S rRNA gene sequences showed that S7-12T forms a stable branch, suggesting that it is a member of the genus Knoellia. The UBCG phylogenetic tree showed that strains S7-12T and K. aerolata DSM 18566T clustered together to form a stable branch (Figure 2). This also indicates that strain S7-12T belongs to the genus Knoellia.
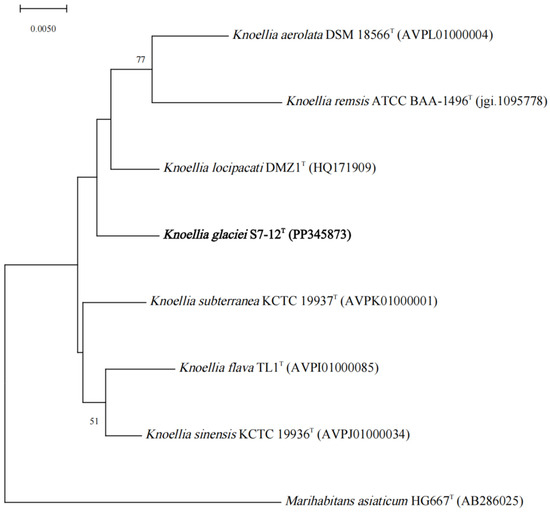
Figure 1.
Neighbor-joining phylogenetic tree based on 16S rRNA gene sequences of the strain S7-12T and the type strains of other closely related species in the genus Knoellia and Marihabitans. Marihabitans asiaticum HG667T (AB286025) was used as an outgroup. Bar, 0.005 substitutions per nucleotide position.
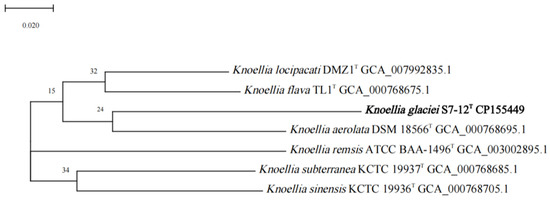
Figure 2.
UBCG phylogenetic tree based on the up-to-date core gene set and pipeline of strain S7-12T and the type strains of other closely related species in the genus Knoellia and Marihabitans. Marihabitans asiaticum HG667T (AB286025) was used as an outgroup.
For the phylogenetic tree generated with UBCG using the amino acids sequences, the numbers at the nodes indicate the gene support index. Bar, 0.02 substitutions per nucleotide position.
3.2. Phenotypic Characterization
After 72 h of incubation on the R2A liquid medium at 30 °C, strain S7-12T formed rounded colonies with regular, raised edges and globular cells. It was colorless and opaque. The strain was Gram-negative, aerobic, non-motile, and non-budding (0.4 μm × 0.6 μm, 0.6 μm × 1.0 μm) (Figure 3).

Figure 3.
Scanning electron microscope photos of the cells of strain S7-12T.
The growth temperature range was 10–35 °C (optimum temperature at 30 °C), and the growth pH range was pH 6.0–8.0 (optimum pH at 7.0). It could tolerate 3.0% (w/v) NaCl conditions (optimum growth under 1% NaCl). Compared to K. flava TL1T, K. aerolata DSM 18566T, K. locipacati DMZ1T, K. remsis ATCC BAA-1496T, K. sinensis KCTC 19936T, and K. subterranea KCTC 19937T, strain S7-12T grew in the same temperature range but a narrower pH range (Table 1).

Table 1.
Phenotypic characteristics of strain S7-12T and their closely related type strains in the genus Knoellia. Strains: 1. S7-12T; 2. K. flava TL1T; 3. K. aerolata DSM 18566T; 4. K. locipacati DMZ1T; 5. K. remsis ATCC BAA-1496T; 6. K. sinensis KCTC 19936T; 7. K. subterranea KCTC 19937T. ND, not detected. +, reacts positively. −, reacts negatively.
3.3. Chemotaxonomic Characteristics
The major fatty acids of strain S7-12T were iso-C16:0, iso-C16:1H, and C17:1ω8c. Of these, iso-C16:0 had the highest concentration in other Knoellia strains. By contrast, in strain S7-12T iso-C16:1H and C17:1ω8c had the highest concentration (Table 2). The cell wall amino acids of strain S7-12T mainly include meso-diaminopimelic acid, and the cell wall sugar components are mainly ribose (rib), glucose (glu), arabinose (ara), rhamnose (rha), xylose (xyl), mannose (man), and galactose (gal). The main polar lipids are diphosphatidylglycerol (DPG), phosphatidylglycerol (PG), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phospholipids (PL1-8), an unidentified glycolipid (GL), and aminophosphoglycolipid (APGL) (Figure S3).

Table 2.
Whole cellular fatty acids composition of S7-12T and the closely related type strains of the genus Knoellia. Strains: 1. S7-12T; 2. K. flava TL1T; 3. K. aerolata DSM 18566T; 4. K. locipacati DMZ1T; 5. K. remsis ATCC BAA-1496T; 6. K. sinensis KCTC 19936T; 7. K. subterranea KCTC 19937T. ND, not detected. All data were obtained in this study.
3.4. Radiation Resistance
To assess the resistance of strain S7-12T to UV-NIR (UVC 254 nm) radiation, the most commonly used radiation-resistant strain, D. radiodurans, was chosen as reference (Figure 4). To control the variables, a 10−4 concentration gradient was used for both strains, and the irradiation gradient was set at 0–2000 J/m2. After irradiation, the survival rate of both strains decreased with an increase in irradiation dose, and strain S7-12T had a higher radiation resistance than D. radiodurans. Notably, S7-12T could survive even after irradiation with 1000 J/m2, whereas the control bacteria no longer survived at irradiation intensity higher than 500 J/m2. These results confirm the high radiation resistance of S7-12T.
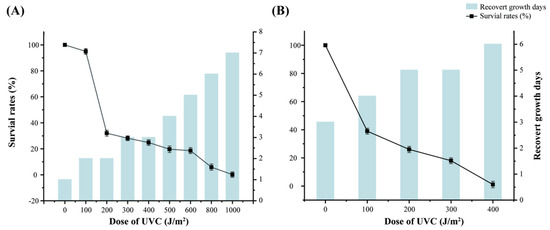
Figure 4.
Comparison of UV irradiation resistance and days to recovery of growth between strain S7-12T (A) and strain D. radiodurans (B).
The growth period of strain S7-12T and the reference strain after irradiation was 7 days (7 d). By contrast, under normal growth conditions, the strain required only three days (3 d) to resume growth following irradiation. This indicates that a higher irradiation dose corresponds with a longer time for the strain to resume growth. The survival of the irradiated strains was lower than that of the non-irradiated strains, implying that although irradiation killed some strains, most of the strains exhibited radiation resistance.
3.5. Genomic Analysis
3.5.1. General Genome Features
The complete genome of strain S7-12T contained 4,163,720 bp, with a guanine–cytosine (GC) content of 67.81 mol%. The total number of coding sequences (CDSs) was 3955, and there were 50 RNAs, including 44 tRNAs and two sets of 5S rRNA, 16S rRNA, and 23S rRNA (Table S1). Only one plasmid was presented in strain S7-12T.
The AAI, ANIb, ANIm, dDDH, and OrthoANI values were calculated to identify the genomic similarities of strain S7-12T to its closely related strains. The sequencing similarity values of the 16S rRNA gene were lower than 98.61% (threshold for proteobacteria) to that of the phylogenetically proximate type strain (Figure 5) [51]. The highest OrthoANI values between strain S7-12T and the related strains were 81.60% (K. locipacati DMZ1T), 81.25% (K. aerolata DSM 18566T), 81.11% (K. flava TL1T), 80.86% (K. subterranea KCTC 19937T), 80.31% (K. sinensis KCTC 19936T), and 79.31% (K. remsis ATCC BAA-1496T), which were all lower than the 95% threshold as defined by prokaryotes [52]. The highest ANIb and ANIm values between strain S7-12T and the related strains were 81.36% and 84.97% (K. aerolata DSM 18566T), 81.74% and 85.31% (K. locipacati DMZ1T), 79.76% and 84.74% (K. remsis ATCC BAA-1496T), 80.59% and 84.80% (K. sinensis KCTC 19936T), 80.82% and 84.87% (K. subterranea KCTC 19937T), and 81.20% and 85.17% (K. flava TL1T), respectively, which were all lower than the 95% threshold as defined by prokaryotes [52]. The dDDH values between strain S7-12T and other Knoellia species were 24.20% (K. aerolata DSM 18566T), 24.30% (K. locipacati DMZ1T), 22.30% (K. remsis ATCC BAA-1496T), 23.10% (K. sinensis KCTC 19936T), 23.20% (K. subterranea KCTC 19937T), and 23.80% (K. flava TL1T), which were lower than the 70% threshold as defined by prokaryotes [53]. These results indicate that strain S7-12T is a novel species clustered in the genus Knoellia.
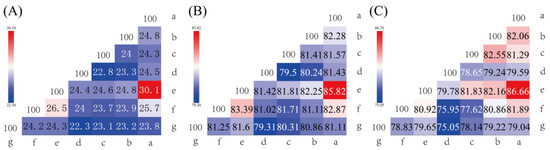
Figure 5.
Genome comparisons of strain S7-12T and its related reference strains including the dDDH value (A), OrthoANI value (B), and AAI value (C). Furthermore, a–g represent S7-12T, K. flava TL1T, K. subterranea KCTC 19937T, K. sinensis KCTC 19936T, K. remsis ATCC BAA-1496T, K. locipacati DMZ1T, K. aerolata DSM 18566T, respectively.
3.5.2. COG Analysis
COGs are databases of homologous protein clusters (Table S2). COG annotation can functionally annotate unknown sequences with known proteins, identify conserved sites, and analyze their evolutionary relationships by performing multiple sequence comparisons between the sequences to be analyzed and the proteins in the COG number for comparison. A total of 3955 CDSs are distributed into 24 COG functional categories in strain S7-12T (Figure 6). The major functional category includes genes that contain translation, ribosomal structure, and biogenesis (COG-J, 208 genes); transcription (COG-K, 332 genes); replication, recombination, and repair (COG-L, 146 genes); defense mechanisms (COG-V, 104 genes); signal transduction mechanisms (COG-T, 193 genes); cell wall/membrane/envelope biogenesis (COG-M, 189 genes); posttranslational modification, protein turnover, and chaperones (COG-O, 144 genes); energy production and conversion (COG-C, 197 genes); transportation of drugs/metabolites and carbohydrates (COG-G, 286 genes); amino acid transport and metabolism (COG-E, 282 genes); coenzyme transport and metabolism (COG-H, 221 genes); lipid transport and metabolism (COG-I, 222 genes); inorganic ion transport and metabolism (COG-P, 155 genes); general function prediction only (COG-R, 328 genes); and unknown function (COG-S, 130 genes). The detailed annotation results of COGs containing less than 100 genes are shown in Figure 6. Within these gene sequences, we have identified those that can form multidrug transporter proteins, resistance proteins, and hydrogen peroxide reductase.
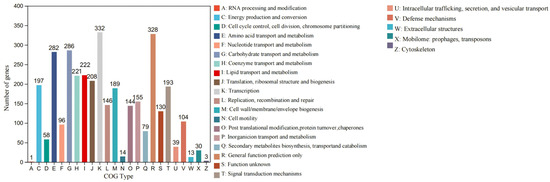
Figure 6.
Distribution of CDS in 24 COG functional categories in strain S7-12T.
The analysis of the COGs of S7-12 and similar strains within the genus Knoellia revealed significant differences in some functional abundances (Figure S4).
3.5.3. Pan-Genome Analysis
The pan-genome represents the entire genetic composition of a species and is the gene pool of all strains of the species. It consists of three main components: core genes, dispensable genes, and unique genes. Pan-genomes can be further categorized into closed-type or open-type pan-genomes [54]. When the number of the sequenced genomes increases with an increase in the size of the pan-genome of a species, the genus has an open-type pan-genome; otherwise, when the number of sequenced genomes increases and the size of the pan-genome of a species increases only up to a certain extent and then converges to a certain value, the genus has a closed-type pan-genome. As a branch of comparative genomics, pan-genome analysis examines the bacterial genome from the perspective of the population and the characteristics of bacterial genome dynamics so as to evaluate the dynamic changes in bacterial genomes during evolution. The file GFF3 derived from Prokka allows for pan-genomic analysis using the Roray [55] pipeline.
We compared the core genes, dispensable genes, and unique genes from the pan-genome of this strain with the database of the essential gene (DEG) (http://www.essentialgene.org/, accessed on 19 February 2024) using the BLASTN (E − value = 1 × 10−5) [56]. Data accessed on 29 February 2024. The overlap between the genes and underlying genes was assessed using homogeneity scores and bits [57].
To gain a more detailed understanding of the genomic characterization and function of S7-12T, we performed the pan-genomic analysis after COG analysis. The Heaps’ law modeling analysis can be used to obtain an estimate of the parameter α to determine whether the pan-genome is open or closed [58]. From the equation in Figure S5 and the trend of the curve, parameter α equals 0.550, which is less than the threshold value of 1.00; thus, it is an open-type pan-genome (Figure S5).
Generally, gene clusters are classified as core, dispensable, or unique. A core gene cluster is a conserved gene family common to all samples of the same genus; a dispensable gene cluster refers to a gene cluster present in two or more samples at the same time; and a unique gene cluster refers to a gene cluster present only in one sample (Figure 7A). A comparative analysis based on homologous proproteomes identified 1903 core genes present in all seven Knoellia genomes (Figure 7B), accounting for the largest proportion. Among them, K. subterranea KCTC 19937T has the highest core genome content, reaching up to 56% (Figure 7A). This indicates that the percentage of common functional proteins is relatively high in all types of species. The proportion of unique genomes in strain S7-12T was relatively large compared to that of the other genomes in the genus Knoellia, which accounted for approximately 22%, the highest proportion of unique genomes among the remaining six, excluding K. remsis ATCC BAA-1496T (Figure 7A).
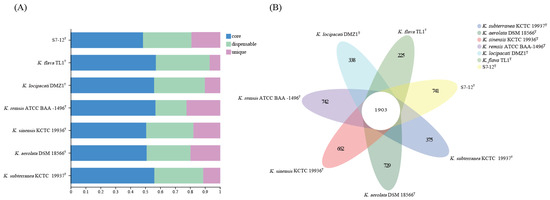
Figure 7.
Comparisons of orthologous protein groups in S7-12T and six related Knoellia genomes. (A) Percentage of core, dispensable, and unique genes in each of all eight genomes. (B) Venn diagram displaying the number of core and unique genes for each of the S7-12T and related type strains.
Unique gene clusters play an essential role in predicting potential gene clusters that cannot be identified by traditional methods [48]. In a strain, the core genes represent the commonality in the strain, whereas the unique genes represent the distinct characteristics. Functional differences across strains can be compared based on the unique genes [59].
The seven species belonging to the genus Knoellia also have a different proportion of unique genes. The differences in the size of different genomes may affect the number of unique genomes. As can be seen in Figure 6, most of the COG functions of all strains in the genus Knoellia are expressed as metabolic functions. Among these pan-genomes, strain S7-12T has the largest number of unique genomes compared to other similar strains in the same genus, which proves the importance of studying it. Based on the literature, the other six strains in the genus Knoellia originated from environmental samples of air [9], feces [12], soil [13,60], and fildes bay [61], suggesting that the genus can grow in specific environments. Furthermore, the core genome of strain S7-12T is enriched in genes involved in metabolic functions, which likely contributes to its ability to utilize a wide range of nutrient sources and adapt to diverse ecological niches. This metabolic versatility could be a significant factor in the competitive advantage of strain S7-12T over other strains in the genus Knoellia (Figure 8).
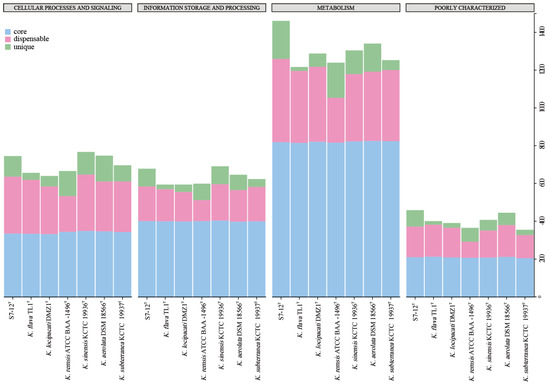
Figure 8.
Classification of COG functions annotated to different pan-genomes in the genus Knoellia.
We also performed a systematic gene function analysis of all strains of the genus Knoellia using the KEGG database (Figure S6). A large proportion of biological processes are in the metabolism, organismal systems, human diseases, environmental information processing, cellular processes, and genetic information processing. Strain S7-12T has a higher proportion of biological processes, especially involving biometabolic pathways, than the other strains in the genus Knoellia. This suggests that the energy drive from biometabolism is high in S7-12T. We speculate that this helps in DNA repair and provides higher resistance to radiation [62].
Homologous proteins are defined as proteins with similar amino acid sequences and exercise similar or identical functions. To elucidate the similarities and differences between strain S7-12T and the other species in its genus, we classified the number of pan-genomes and functional genes among different Knoellia strains (Figure 7). A total of 1620 proteins associated with annotated genes form the core genome in all members of the genus Knoellia, and each member has its unique genes, except for unknown genes. In the core genome, 739 genes are responsible for functional categories and metabolism-related functions, 342 for information storage and processing machinery processing functions, 371 for cellular processes and signaling functions, and 222 are poorly characterized. Among these genes, we identified several radiation-resistant DNA repair genes, including the recombinational DNA repair protein RecO, a critical component of the RecF pathway [63]; the multifunctional RadA/RecA recombinase; and the alkylated DNA repair dioxygenase AlkB [64,65]. These findings suggest that strain S7-12T possesses a robust DNA damage-response mechanism, which is essential for its survival in environments with elevated levels of ionizing radiation. Among the DNA repair genes we also found the ssb gene, which binds to and repairs broken single-stranded DNA in the early stages of damage repair [15]. Similarly, the genes expressing DNA repair function in strain S7-12T are UvrA, UvrB, and UvrC, and the UvrABC pathway, in which these three genes are involved, provides a great help for nucleotide excision repair (NER) in the strain [66]. The gene encoding the mismatch repair enzyme MutL [67] also plays a large role in DNA repair in S7-12T, as do mutS, and mutH, but unfortunately, we did not find these genes in this strain.
We also identified gene sequences associated with heat-shock response proteins, such as IbpA [68], HSP-20 [69], HSP-70 [70], HSP-90 [71], and the CspA family [72,73], and gene fragments that may express antioxidant capacity, such as choD, which expresses the oxidoreductase capacity of GMC [74], the DyP-type peroxidase family, DyP [75]; the cytochrome bd family, cydA/cydB [76], and cytochrome C [77]. Their presence indicates that strain S7-12T has evolved a sophisticated stress-response system to adapt to a broad range of environmental stresses.
Overall, the unique genome of strain S7-12T revealed a diverse set of gene sequences, including 520 genes related to cellular processes and signal transduction, 513 genes involved in information storage and processing, 1107 genes dedicated to metabolic functions, and 325 genes of unknown function. We also predicted 329 virulence genes and 240 resistance genes in the genome of S7-12T (Figure S7). As seen from the two prediction maps, the genes related to nutrient/metabolic factors, immunomodulatory factors, peptide antibiotics, and macrocyclic endolipid antimicrobials account for a relatively large number of genes.
A further comparison of the virulence genes with those of the other bacteria within the same genus reveals a distinct family of MntABC genes [78] that express metal-transporter proteins, an L-methionine-binding lipoprotein (MetQ) [79] linked to immune evasion in gonococcal pathogenesis, and a specific SpoVK [80] phage motif sequence. In addition, the number of genes responsible for metabolism is the largest for all bacteria in the genus Knoellia, and strain S7-12T has the highest number of genes with metabolism and human disease functions among all the other strains (Figure 9). These findings prove why we can find more fragments of genes concerning disease resistance and drug resistance in the genome of S7-12T.
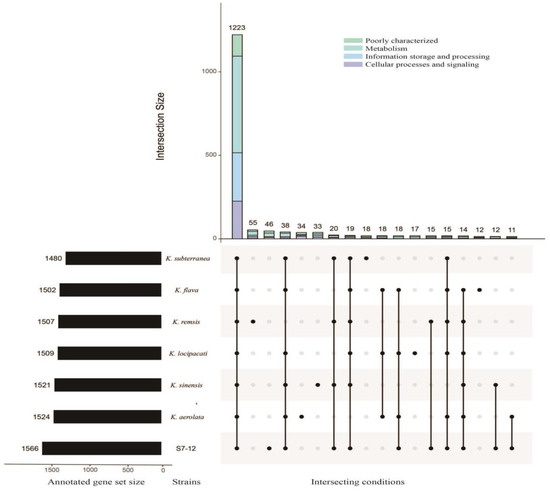
Figure 9.
The number and functional gene classification of pan genomes between different Knoellia strains. The upset plot shows the number and functional classification of the core and unique genes in different Knoellia strains. The bar chart above represents the number of core and unique genes contained in each type of group. The strip at the bottom left represents the total number of genes in different Knoellia strains. The dot and line at the bottom right represent the types of different combinations (where only values above 10 and annotated genes are shown; further, unknown genes were not shown).
3.5.4. Horizontal Gene Transfer Analysis
Genomic islands (GIs) are a common type of horizontally transferred element (Table S3). They are classified according to the functions of the genes they contain, such as virulence islands, resistance islands, metabolic islands, and symbiotic islands. In addition to several core and homologous proteins that have similar functions and structures to proteins in other known strains, S7-12T contains non-homologous proteins that may not have directly corresponding homologues in other known strains. The presence of non-homologous proteins may represent unique biological properties or adaptations, allowing strategic survival or competitive advantage in a particular environment.
The identification of non-homologous proteins indicates the occurrence of horizontal gene transfer events in S7-12T. We identified 13 GIs in S7-12T, containing 269 genes ranging from 73–4233 bp in length. According to the gene function analysis of GIs, most of these known functional genes are involved in cellular metabolism and membrane transport functions. Predictive gene function annotation of the GIs of S7-12T revealed that S7-12T has several gene sequences that can be expressed as multidrug resistance proteins sugE [81,82], cysE [83], nisC [84], dinB [85], sprC [86], csoR [87], trkA [88], lanthionine synthetase C-like protein nisC [84], proteins that can bind potential drug-binding targets cysK [89], and related cation transporter proteins.
In addition to these horizontally transferred genes with drug-resistance function, S7-12T contains fragments of the genes cspA, rpoE [90], sigB [91], resB [92], and recF, indicating its potential antioxidant capacity. These genes may contribute to the survival of the bacterium in extreme environmental conditions. This gene sequence is not found in other bacteria of the same genus, suggesting that it was acquired through horizontal transfer to adapt to extreme environments.
We also found genes cphA and cphB [93] that can synthesize cyanobactin, gene family aroK and aroL [94] that can synthesize mangiferic acid, and genes FitA and FitB [95] that can produce toxin–antitoxin factors. In addition, we identified a unique gene family, namely, the BtpA/SgcQ, that can be used as reference for the treatment of drug-resistant bacterial infections [96]. Mangiferic acid is not only an intermediate metabolite in the synthesis of aromatic amino acids in E. coli but is also a synthetic precursor of anti-influenza drugs [97]. The antitoxin usually acts in conjunction with the toxin, which exerts toxic effects to inhibit bacterial growth, while the antitoxin can neutralize the toxicity. The interaction between the two can play a role in regulating the bacterial growth state [98]. Overall, the analysis of the predicted gene function showed that S7-12T contains many drug-resistance and toxicity genes, implying its application value for drug development.
The radiation resistance of strains can be harnessed for bioremediation, significantly mitigating long-term hazards to human health and ecosystems. Amidst the escalating global challenge of antibiotic resistance, the study of bacterial resistance mechanisms in strains is pivotal for the development of novel antibiotics and therapeutic strategies, which is crucial to combat resistant infections and safeguard public health. In summary, the radiation and antibiotic resistance capacities of strains are at the forefront of biological research. By thoroughly investigating and judiciously applying these strains, breakthroughs in various fields are anticipated, contributing substantially to the advancement of human society.
4. Conclusions
This is the first study to describe the novel bacterial strain Knoellia S7-12T isolated from the north slope of Mount Everest. To date, only six bacterial strains from this genus have been reported. Its mechanism of radiation resistance and genomic function were investigated under extreme environmental stresses. Multidrug-resistance, pathogenicity, and antimicrobial genes, including cysE, nisC, sugE, dinB, sprC, csoR, and trkA, were identified. In addition, strain S7-12T contains many genes for radiation protection and cold tolerance that are not expressed in other species in the genus Knoellia, including rpoE, sigB, resB, CspA, and other gene families. Therefore, we speculate that this is why this strain can be isolated at high altitude in a cold-, radiation-, and oxidation-resistant environment. Owing to the expression of radiation-resistant genes, this bacterium has an increased chance of survival in a high-radiation environment. Thus, this novel strain provides opportunities for developing radiation-resistant drugs.
In addition, the gene family BtpA/SgcQ can be used as a reference for the treatment of drug-resistant bacterial infections. This gene family plays an important role in cell physiological processes and may be a potential target for the development of drugs in the future.
Overall, the experimental and genomic analysis demonstrated that the strain S7-12T can resist radiation. Our findings provide the theoretical foundation for the development and application of anti-radiation drugs.
Description of Knoellia glaciei sp. nov.
Knoellia glaciei sp. nov. (gla.ci. e’i. L. gen. n. glaciei of ice, referring to the frozen environment from which the type strain was isolated).
Cells are non-motile and non-spore-forming, growing as irregular spheres (0.5–0.7 µm in diameter) or rods (0.4–1.0 µm in diameter), appearing singly, in pairs, or clusters. Single colonies appear yellowish, round, smooth, and were raised in R2A medium for 72 h. Grow aerobically at 10–45 °C (with optimum growth at 30 °C), pH 6.0–8.0 and in 10% NaCl (with optimum growth at pH 7.0 and 1% NaCl). Positive for peroxidase but negative for oxidase. Can reduce nitrate to nitrite. Tween20, Tween80, gelatin, and starch are hydrolyzed, but urea is not. Assimilated L-arabinose, D-fructose, D-galactose, D-glucose, D-mannitol, D-raffinose, and sucrose and can weakly utilize D-xylose. Alkaline phosphatase, esterase (C4), N-acetyl-β-alkaline phosphatase, esterase (C4), N-acetyl-β-glucosaminase, and esterase lipase (C8) are detected but not lipase (C14) and acid phosphatase. The major cellular fatty acids are iso-C16:0H, isoC16:0, and C17:1ω8c. Characteristic cell wall sugar components are ribose and glucose. Polar lipids include diphosphatidylglycerol (DPG), phosphatidylglycerol (PG), phosphatidylethanolamine (PE), phosphatidylinositol (PI), eight phospholipids (PL), aminophosphoglycolipid (APGL), and an unidentified glycolipid (GL).
The K. glaciei type strain S7-12T (=KCTC 59114T =GDMCC 1.3458T) was isolated from the moraine of the north slope area of Mount Everest (28.02° N, 86.56° E), PR China. The G+C content of the genomic DNA of strain S7-12T was 67.8 mol%. The full-length 16S rRNA gene sequence and genome data of strain S7-12T were stored in JCM/GDMCC/GenBank with accession numbers KCTC 59114, GDMCC 1.3458, and GCA_040518285.1, respectively.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12091748/s1, Figure S1: Minimum-evolution phylogenetic tree based on 16S rRNA gene sequences of the strain S7-12T, and the type strains of other closely related species in the genus Knoellia and Marihabitans; Figure S2: Maximum-likelihood phylogenetic tree based on 16S rRNA gene sequences of the strain S7-12T, and the type strains of other closely related species in the genus Knoellia and Marihabitans; Figure S3: Polar lipids profile of strain S7-12T; Figure S4: Comparison of COGs functional abundance between strain S7-12T and similar strains in its genus; Figure S5: Characteristic curves of the pan-genome and core genome of S7-12T; Figure S6: Phylogenetic functional analysis of all strains of the genus Knoellia using the KEGG database; Figure S7: Virulence genes (A) and resistance gene prediction (B) of strain S7-12T; Table S1: General genomic characteristics comparison of strain S7-12T, and its closely related species; Table S2: The description of COG type; Table S3: Features of the GIs found in the genome of S7-12T.
Author Contributions
Conceptualization, B.Z. and X.W.; Methodology, Y.L.; Software, Z.C.; Validation, X.W., K.W. and Z.C.; Formal Analysis, X.W.; Investigation, X.W.; Resources, Y.L.; Data Curation, K.W.; Writing—Original Draft Preparation, X.W.; Writing—Review & Editing, B.Z.; Visualization, B.Z.; Supervision, G.L.; Project Administration, T.C.; Funding Acquisition, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Science Foundation of China (No. 42071099) and Gansu Province Talent Project in 2024.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matthews, T.; Perry, L.B.; Lane, T.P.; Elmore, A.C.; Khadka, A.; Aryal, D.; Shrestha, D.; Tuladhar, S.; Baidya, S.K.; Gajurel, A.; et al. Into Thick(er) Air? Oxygen Availability at Humans’ Physiological Frontier on Mount Everest. iScience 2020, 23, 101718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, X.; Yang, R.; Zhang, Y.; Xu, Y.; Liu, G.; Zhang, B.; Wang, J.; Wang, X.; Zhang, W.; et al. Genomic Insights into the Radiation-Resistant Capability of Sphingomonas qomolangmaensis S5-59T and Sphingomonas glaciei S8-45T, Two Novel Bacteria from the North Slope of Mount Everest. Microorganisms 2022, 10, 2037. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, Y.; Xu, Y.; Chen, T.; Zhang, S.; Wang, J.; Yang, R.; Liu, G.; Zhang, W.; Zhang, G. Paracoccus everestensis sp. nov., a novel bacterium with great antioxidant capacity isolated from the north slope of Mount Everest. Int. J. Syst. Evol. Microbiol. 2022, 72, 005562. [Google Scholar] [CrossRef]
- Sood, U.; Dhingra, G.G.; Anand, S.; Hira, P.; Kumar, R.; Kaur, J.; Verma, M.; Singhvi, N.; Lal, S.; Rawat, C.D.; et al. Microbial Journey: Mount Everest to Mars. Indian. J. Microbiol. 2022, 62, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Kong, W.; Jia, H.; Delgado-Baquerizo, M.; Zhou, T.; Liu, X.; Ferrari, B.C.; Malard, L.; Liang, C.; Xue, K.; et al. Polar soils exhibit distinct patterns in microbial diversity and dominant phylotypes. Soil. Biol. Biochem. 2022, 166, 108550. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Liu, Q.; Liu, H.-C.; Zhou, Y.-G.; Xin, Y.-H. Flavobacterium ranwuense sp. nov., isolated from glacier. Int. J. Syst. Evol. Microbiol. 2019, 69, 3812–3817. [Google Scholar] [CrossRef]
- Xie, J.; Ren, L.; Wei, Z.; Peng, X.; Qin, K.; Peng, F. Pengzhenrongella phosphoraccumulans sp. nov., isolated from high Arctic glacial till, and emended description of the genus Pengzhenrongella. Int. J. Syst. Evol. Microbiol. 2024, 74, 006368. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Ibaceta, F.; Carrasco, V.; Lagos-Moraga, S.; Dietz-Vargas, C.; Navarro, C.A.; Pérez-Donoso, J.M. Arthrobacter vasquezii sp. nov., isolated from a soil sample from Union Glacier, Antarctica. Int. J. Syst. Evol. Microbiol. 2023, 73, 006095. [Google Scholar] [CrossRef]
- Weon, H.-Y.; Kim, B.-Y.; Schumann, P.; Kroppenstedt, R.M.; Noh, H.-J.; Park, C.-W.; Kwon, S.-W. Knoellia aerolata sp nov., isolated from an air sample in Korea. Int. J. Syst. Evol. Microbiol. 2007, 57, 2861–2864. [Google Scholar] [CrossRef][Green Version]
- Shin, N.-R.; Roh, S.W.; Kim, M.-S.; Jung, M.-J.; Whon, T.W.; Bae, J.-W. Knoellia locipacati sp. nov., from soil of the Demilitarized Zone in South Korea. Int. J. Syst. Evol. Microbiol. 2012, 62, 342–346. [Google Scholar] [CrossRef]
- Osman, S.; Moissl, C.; Hosoya, N.; Briegel, A.; Mayilraj, S.; Satomi, M.; Venkateswaran, K. Tetrasphaera remsis sp. nov., isolated from the Regenerative Enclosed Life Support Module Simulator (REMS) air system. Int. J. Syst. Evol. Microbiol. 2007, 57, 2749–2753. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Du, Y.; Wang, G. Knoellia flava sp. nov., isolated from pig manure. Int. J. Syst. Evol. Microbiol. 2012, 62, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Groth, I.; Schumann, P.; Schütze, B.; Augsten, K.; Stackebrandt, E. Knoellia sinensis gen. nov., sp. nov. and Knoellia subterranea sp. nov., two novel actinobacteria isolated from a cave. Int. J. Syst. Evol. Microbiol. 2002, 52, 77–84. [Google Scholar] [CrossRef]
- Battista, J.R. Against all odds: The survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 1997, 51, 203–224. [Google Scholar] [CrossRef]
- Liu, F.; Li, N.; Zhang, Y. The radioresistant and survival mechanisms of Deinococcus radiodurans. Radiat. Med. Prot. 2023, 4, 70–79. [Google Scholar] [CrossRef]
- Lieberman, P.; Morey, A.; Hochstadt, J.; Larson, M.; Mather, S. Mount Everest: A space analogue for speech monitoring of cognitive deficits and stress. Aviat. Space Env. Med. 2005, 76, B198–B207. [Google Scholar]
- Reasoner, D.J.; Geldreich, E.E. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Env. Microbiol. 1985, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species1. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Williams, S.T.; Goodfellow, M.; Alderson, G.; Wellington, E.M.H.; Sneath, P.H.A.; Sackin, M.J. Numerical Classification of Streptomyces and Related Genera. Microbiology 1983, 129, 1743–1813. [Google Scholar] [CrossRef]
- Kurup, P.V.; Schmitt, J.A. Numerical taxonomy of Nocardia. Can. J. Microbiol. 1973, 19, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Pirouz, T.; Goodfellow, M.; Minnikin, D.E. Distribution of menaquinones in actinomycetes and corynebacteria. J. Gen. Microbiol. 1977, 100, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Lechevalier, H.A.; Lechevalier, M.P.; Gerber, N.N. Chemical Composition as a Criterion in the Classification of Actinomycetes. In Advances in Applied Microbiology; Perlman, D., Ed.; Academic Press: Cambridge, MA, USA, 1971; Volume 14, pp. 47–72. [Google Scholar]
- Staneck, J.L.; Roberts, G.D. Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl. Microbiol. 1974, 28, 226–231. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2012, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Lee, I.; Chalita, M.; Ha, S.M.; Na, S.I.; Yoon, S.H.; Chun, J. ContEst16S: An algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2017, 67, 2053–2057. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Oliver Glöckner, F.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Rodriguez-R, L.M.; Konstantinidis, K.T. Bypassing Cultivation To Identify Bacterial Species: Culture-independent genomic approaches identify credibly distinct clusters, avoid cultivation bias, and provide true insights into microbial species. Microbe Mag. 2014, 9, 111–118. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 Enhances Tolerance of Wheat to Salt Stress through Improvement of Antioxidative Defense System and Gene Expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nishimaki, T.; Sato, K. An Extension of the Kimura Two-Parameter Model to the Natural Evolutionary Process. J. Mol. Evol. 2019, 87, 60–67. [Google Scholar] [CrossRef]
- Na, S.I.; Kim, Y.O.; Yoon, S.H.; Ha, S.M.; Baek, I.; Chun, J. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol. 2018, 56, 280–285. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Bairoch, A. The ENZYME database in 2000. Nucleic Acids Res 2000, 28, 304–305. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Yang, T.; Gao, F. High-quality pan-genome of Escherichia coli generated by excluding confounding and highly similar strains reveals an association between unique gene clusters and genomic islands. Brief. Bioinform. 2022, 23, bbac283. [Google Scholar] [CrossRef]
- Chaudhari, N.M.; Gupta, V.K.; Dutta, C. BPGA- an ultra-fast pan-genome analysis pipeline. Sci. Rep. 2016, 6, 24373. [Google Scholar] [CrossRef]
- Yang, X.; Garuglieri, E.; Van Goethem, M.W.; Marasco, R.; Fusi, M.; Daffonchio, D. Mangrovimonas cancribranchiae sp. nov., a novel bacterial species associated with the gills of the fiddler crab Cranuca inversa (Brachyura, Ocypodidae) from Red Sea mangroves. Int. J. Syst. Evol. Microbiol. 2024, 74, 006415. [Google Scholar] [CrossRef]
- Molina-Menor, E.; Gimeno-Valero, H.; Pascual, J.; Peretó, J.; Porcar, M. High Culturable Bacterial Diversity from a European Desert: The Tabernas Desert. Front. Microbiol. 2020, 11, 583120. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- International Committee on Systematic Bacteriology announcement of the report of the ad hoc Committee on Reconciliation of Approaches to Bacterial Systematics. J. Appl. Bacteriol. 1988, 64, 283–284. [CrossRef]
- Costa, S.S.; Guimaraes, L.C.; Silva, A.; Soares, S.C.; Barauna, R.A. First Steps in the Analysis of Prokaryotic Pan-Genomes. Bioinform. Biol. Insights 2020, 14, 1177932220938064. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lin, Y.; Gao, F.; Zhang, C.T.; Zhang, R. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res. 2014, 42, D574–D580. [Google Scholar] [CrossRef]
- Wu, H.; Wang, D.; Gao, F. Toward a high-quality pan-genome landscape of Bacillus subtilis by removal of confounding strains. Brief. Bioinform. 2021, 22, 1951–1971. [Google Scholar] [CrossRef]
- Tettelin, H.; Riley, D.; Cattuto, C.; Medini, D. Comparative genomics: The bacterial pan-genome. Curr. Opin. Microbiol. 2008, 11, 472–477. [Google Scholar] [CrossRef]
- Fang, X.; Qin, K.; Li, S.; Han, S.; Zhu, T.; Fang, X.; Qin, K. Whole genome sequence of Diaporthe capsici, a new pathogen of walnut blight. Genomics 2020, 112, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Lim, J.-M.; Hamada, M.; Ahn, J.-H.; Weon, H.-Y.; Suzuki, K.-i.; Ahn, T.-Y.; Kwon, S.-W. Oryzobacter terrae gen. nov., sp nov., isolated from paddy soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 3190–3195. [Google Scholar] [CrossRef]
- Lamilla, C.; Pavez, M.; Santos, A.; Hermosilla, A.; Llanquinao, V.; Barrientos, L. Bioprospecting for extracellular enzymes from culturable Actinobacteria from the South Shetland Islands, Antarctica. Polar Biol. 2017, 40, 719–726. [Google Scholar] [CrossRef]
- Veech, R.L.; Todd King, M.; Pawlosky, R.; Kashiwaya, Y.; Bradshaw, P.C.; Curtis, W. The “great” controlling nucleotide coenzymes. IUBMB Life 2019, 71, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Nirwal, S.; Czarnocki-Cieciura, M.; Chaudhary, A.; Zajko, W.; Skowronek, K.; Chamera, S.; Figiel, M.; Nowotny, M. Mechanism of RecF-RecO-RecR cooperation in bacterial homologous recombination. Nat. Struct. Mol. Biol. 2023, 30, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Del Val, E.; Nasser, W.; Abaibou, H.; Reverchon, S. RecA and DNA recombination: A review of molecular mechanisms. Biochem. Soc. Trans. 2019, 47, 1511–1531. [Google Scholar] [CrossRef]
- Sedgwick, B.; Bates, P.A.; Paik, J.; Jacobs, S.C.; Lindahl, T. Repair of alkylated DNA: Recent advances. DNA Repair. 2007, 6, 429–442. [Google Scholar] [CrossRef]
- Xiumin, L.I.U.; Jing, W.U.; Wei, Z.; Wei, L.U.; Shuzhen, P.; Min, L.I.N.; Ming, C. Disruption and characterization of the excision repair pathway in the extremely radioresistant bacterium Deinococcus SP. BR501. Acta Agric. Nucleatae Sin. 2007, 21, 357–361. [Google Scholar]
- Mennecier, S.; Coste, G.; Servant, P.; Bailone, A.; Sommer, S. Mismatch repair ensures fidelity of replication and recombination in the radioresistant organism Deinococcus radiodurans. Mol. Genet. Genom. 2004, 272, 460–469. [Google Scholar] [CrossRef]
- Piróg, A.; Cantini, F.; Nierzwicki, Ł.; Obuchowski, I.; Tomiczek, B.; Czub, J.; Liberek, K. Two Bacterial Small Heat Shock Proteins, IbpA and IbpB, Form a Functional Heterodimer. J. Mol. Biol. 2021, 433, 167054. [Google Scholar] [CrossRef] [PubMed]
- Turan, M. Genome-wide analysis and characterization of HSP gene families (HSP20, HSP40, HSP60, HSP70, HSP90) in the yellow fever mosquito (Aedes aegypti) (Diptera: Culicidae). J. Insect Sci. 2023, 23, 27. [Google Scholar] [CrossRef]
- Yu, E.-m.; Yoshinaga, T.; Jalufka, F.L.; Ehsan, H.; Mark Welch, D.B.; Kaneko, G. The complex evolution of the metazoan HSP70 gene family. Sci. Rep. 2021, 11, 17794. [Google Scholar] [CrossRef] [PubMed]
- Genest, O.; Wickner, S.; Doyle, S.M. Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling. J. Biol. Chem. 2019, 294, 2109–2120. [Google Scholar] [CrossRef]
- Yamanaka, K.; Fang, L.; Inouye, M. The CspA family in Escherichia coli: Multiple gene duplication for stress adaptation. Mol. Microbiol. 1998, 27, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.C.; Joshi, G.K.; Mishra, P.K. CspA encodes a major cold shock protein in Himalayan psychrotolerant Pseudomonas strains. Interdiscip. Sci. 2014, 6, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Sützl, L.; Foley, G.; Gillam, E.M.J.; Bodén, M.; Haltrich, D. The GMC superfamily of oxidoreductases revisited: Analysis and evolution of fungal GMC oxidoreductases. Biotechnol. Biofuels 2019, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Sugano, Y. A structural and functional perspective of DyP-type peroxidase family. Arch. Biochem. Biophys. 2015, 574, 49–55. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Paiardini, A.; Hoogewijs, D.; Forte, E.; Giuffrè, A.; Poole, R.K. Bacterial Oxidases of the Cytochrome bd Family: Redox Enzymes of Unique Structure, Function, and Utility as Drug Targets. Antioxid. Redox Signal 2021, 34, 1280–1318. [Google Scholar] [CrossRef]
- Yin, V.; Shaw, G.S.; Konermann, L. Cytochrome c as a Peroxidase: Activation of the Precatalytic Native State by H2O2-Induced Covalent Modifications. J. Am. Chem. Soc. 2017, 139, 15701–15709. [Google Scholar] [CrossRef]
- Kehl-Fie, T.E.; Zhang, Y.; Moore, J.L.; Farrand, A.J.; Hood, M.I.; Rathi, S.; Chazin, W.J.; Caprioli, R.M.; Skaar, E.P. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect. Immun. 2013, 81, 3395–3405. [Google Scholar] [CrossRef]
- Sikora, A.E.; Gomez, C.; Le Van, A.; Baarda, B.I.; Darnell, S.; Martinez, F.G.; Zielke, R.A.; Bonventre, J.A.; Jerse, A.E. A novel gonorrhea vaccine composed of MetQ lipoprotein formulated with CpG shortens experimental murine infection. Vaccine 2020, 38, 8175–8184. [Google Scholar] [CrossRef] [PubMed]
- Kohm, K.; Floccari, V.A.; Lutz, V.T.; Nordmann, B.; Mittelstädt, C.; Poehlein, A.; Dragoš, A.; Commichau, F.M.; Hertel, R. The Bacillus phage SPβ and its relatives: A temperate phage model system reveals new strains, species, prophage integration loci, conserved proteins and lysogeny management components. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bay, D.C.; Rommens, K.L.; Turner, R.J. Small multidrug resistance proteins: A multidrug transporter family that continues to grow. Biochim. Biophys. Acta 2008, 1778, 1814–1838. [Google Scholar] [CrossRef]
- Cruz, A.; Micaelo, N.; Félix, V.; Song, J.Y.; Kitamura, S.; Suzuki, S.; Mendo, S. sugE: A gene involved in tributyltin (TBT) resistance of Aeromonas molluscorum Av27. J. Gen. Appl. Microbiol. 2013, 59, 39–47. [Google Scholar] [CrossRef]
- Verma, D.; Gupta, V. New insights into the structure and function of an emerging drug target CysE. 3 Biotech 2021, 11, 373. [Google Scholar] [CrossRef]
- Siegers, K.; Heinzmann, S.; Entian, K.D. Biosynthesis of lantibiotic nisin—Posttranslational modification of its prepeptide occurs at a multimeric membrane-associated lanthionine synthetase complex. J. Biol. Chem. 1996, 271, 12294–12301. [Google Scholar] [CrossRef] [PubMed]
- Fahey, D.; O’Brien, J.; Pagnon, J.; Page, S.; Wilson, R.; Slamen, N.; Roddam, L.; Ambrose, M. DinB (DNA polymerase IV), ImuBC and RpoS contribute to the generation of ciprofloxacin-resistance mutations in Pseudomonas aeruginosa. Mutat. Res. 2023, 827, 111836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, H.; Yang, H.; He, C.; Shu, W.; Cui, Z.; Liu, Q. Insights Into the Impact of Small RNA SprC on the Metabolism and Virulence of Staphylococcus aureus. Front. Cell Infect. Microbiol. 2022, 12, 746746. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.A.; Sidiropoulos, S.W.; Steinberg, H.; Talaat, A.M. CsoR Is Essential for Maintaining Copper Homeostasis in Mycobacterium tuberculosis. PLoS ONE 2016, 11, e0151816. [Google Scholar] [CrossRef]
- Hirose, M.; Kuroda, Y.; Murata, E. NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain. Pr. 2016, 16, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Schnell, R.; Sriram, D.; Schneider, G. Pyridoxal-phosphate dependent mycobacterial cysteine synthases: Structure, mechanism and potential as drug targets. Biochim. Biophys. Acta 2015, 1854, 1175–1183. [Google Scholar] [CrossRef]
- Fan, Y.; Bai, J.; Xi, D.; Yang, B. RpoE Facilitates Stress-Resistance, Invasion, and Pathogenicity of Escherichia coli K1. Microorganisms 2022, 10, 879. [Google Scholar] [CrossRef]
- Tran, H.T.; Bonilla, C.Y. SigB-regulated antioxidant functions in gram-positive bacteria. World J. Microbiol. Biotechnol. 2021, 37, 38. [Google Scholar] [CrossRef]
- Cabrejos, M.E.; Zhao, H.L.; Guacucano, M.; Bueno, S.; Levican, G.; Garcia, E.; Jedlicki, E.; Holmes, D.S. IST1 insertional inactivation of the resB gene: Implications for phenotypic switching in Thiobacillus ferrooxidans. FEMS Microbiol. Lett. 1999, 175, 223–229. [Google Scholar] [CrossRef]
- Hübschmann, T.; Jorissen, H.; Börner, T.; Gärtner, W.; de Marsac, N.T. Phosphorylation of proteins in the light-dependent signalling pathway of a filamentous cyanobacterium. Eur. J. Biochem. 2001, 268, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Whipp, M.J.; Pittard, A.J. A reassessment of the relationship between arok-encoded and arol-encoded shikimate kinase enzymes of Escherichia-coli. J. Bacteriol. 1995, 177, 1627–1629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munavar, M.H.; Madhavi, K.; Jayaraman, R. Aberrant transcription in fit mutants of Escherichia-coli and its alleviation by suppressor mutations. J. Biosci. 1993, 18, 37–45. [Google Scholar] [CrossRef]
- Skotnicová, P.; Srivastava, A.; Aggarwal, D.; Talbot, J.; Karlínová, I.; Moos, M.; Mares, J.; Bucinská, L.; Koník, P.; Simek, P.; et al. A thylakoid biogenesis BtpA protein is required for the initial step of tetrapyrrole biosynthesis in cyanobacteria. New Phytol. 2024, 241, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Gao, C.; Chen, X.; Liu, L. Using dynamic molecular switches for shikimic acid production in Escherichia coli. Sheng Wu Gong Cheng Xue Bao 2020, 36, 2104–2112. [Google Scholar] [CrossRef]
- He, Z.; Wang, H. Functions of bacterial Toxin-Antitoxin systems. Sheng Wu Gong Cheng Xue Bao 2018, 34, 1270–1278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).