Abstract
Background: Limited data are available in the existing literature regarding the seroepidemiology of T. gondii infection among cardiovascular patients. We aimed to comprehensively assess the prevalence of T. gondii infection and associated risk factors among Romanian cardiovascular patients. Methods: Serologic testing was conducted in 1205 patients with cardiovascular diseases to demonstrate the presence of T. gondii antibodies. An avidity test was performed in patients with detectable IgG and IgM antibodies. A structured questionnaire was designed to identify the potential risk factors associated with T. gondii. Results: The overall seroprevalence of T. gondii antibodies was 52.1%, with the highest value observed in patients diagnosed with dilated cardiomyopathy (66.66%) and the lowest in patients with myopericarditis (30.0%). The 11 patients found with detectable IgM and IgG antibodies had a high avidity test result. A patient’s area of residence, gender, educational level, owning dogs, owning any pet, and toxoplasmosis awareness were significantly associated with T. gondii seropositivity in multiple logistic regression analyses. Conclusions: This study provides novel and valuable insights into the seroprevalence and risk factors associated with T. gondii among Romanian cardiovascular patients. Our findings reiterate the importance of toxoplasmosis awareness and health education for better control and prevention of infection with T. gondii.
1. Introduction
Toxoplasma gondii, an obligate intracellular protozoan, is one of the most successful and well-adapted parasites due to its ability to spread within all ecosystems and across different hosts (humans and domestic and wild animals and birds) and to infect different types of cells [1,2,3,4].
Approximately 2 billion people worldwide are chronically infected with T. gondii, and the prevalence varies widely (between countries and within a country) due to differences in host susceptibility, hygiene, diet, habits, and climate (higher values are observed in low-altitude areas with warm and humid climates) [3,5].
The life cycle of T. gondii is complex (functioning in a prey–predator system) and involves a definitive host that harbours sexual reproduction (domestic and wild cats) and an intermediate host that harbours asexual reproduction (most warm-blooded mammals, birds, and humans) [6,7]. All three developmental stages of T. gondii can infect humans: (i) tachyzoites (the rapid-reproducing forms that can penetrate any nucleated cell); (ii) bradyzoites (the slow-replicating forms, found in tissue cysts), and (iii) sporozoites (found in oocysts released via felid faeces) [3,6,8,9]. Humans can become infected with T. gondii in four ways: (i) foodborne transmission (the consumption of raw/undercooked meat and primary offal containing bradyzoites inside tissue cysts); (ii) zoonotic transmission (the consumption of water and food contaminated with sporulated oocysts containing sporozoites); (iii) vertical transmission (tachyzoites transmitted through the placenta to the foetus); (iv) transmission via blood transfusion (in the case of a recently infected donor, parasitemic at the time of blood sampling) or organ transplant (if the organs contain tissue cysts or tachyzoites) [3,6,9] (Figure 1).
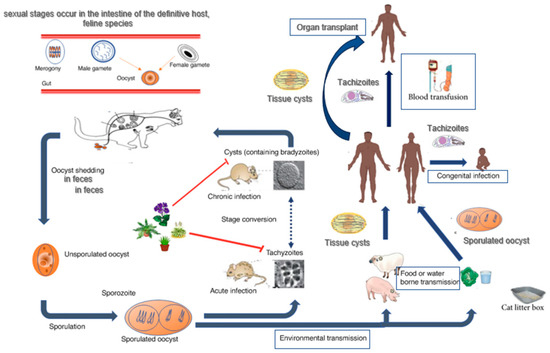
Figure 1.
Toxoplasma gondii—life cycle. Adapted from Niveria et al., 2023 [10], Ahmadpour et al., 2023 [11], and Mose et al., 2020 [12].
In humans, T. gondii exists either in the form of tachyzoites (found in acute infections) or bradyzoites (characteristic of chronic infection). Tissue cysts, found predominantly in the brain, retina, skeletal muscles, and cardiac muscles, may persist throughout life due to their ability to elude immunomediated destruction and their long-term survival [6,7].
Accurate diagnosis (through indirect and direct methods) plays a crucial role in monitoring and preventing T. gondii infection. In immunocompetent individuals, the presence of specific T. gondii antibodies (IgG, IgM, IgA) is evaluated through indirect serological methods. Anti-T. gondii IgG antibodies persist throughout a person’s life, and anti-T. gondii IgM antibodies may be detected for months or even years after infection [13]. Therefore, IgG avidity test results are highly valuable in identifying the moment of infection in pregnant women, in patients with retinochoroiditis or uveitis, and in organ donors or transplant recipients [1,7].
Top of Form
With a loss of 2–8 million disability-adjusted life years, toxoplasmosis is one of the most damaging zoonotic diseases in the world [5]. The course of infection and its severity are influenced by different factors: (i) the inoculated dose and the stage of the parasite (bradyzoites vs. sporozoites); (ii) the parasite genotype; (iii) the genetic characteristics of the host; and (iv) the host’s immune status [1]. In immunocompetent individuals, a generally asymptomatic infection (rarely requiring intervention) may be fatal in the case of an atypical strain [2,5]. In immunocompromised patients (those with HIV/AIDS or undergoing cancer treatment or organ transplant), toxoplasmosis may be life-threatening, while in pregnant women, it may lead to miscarriage and can cause significant disease in congenitally infected newborns and infants [2,3,14].
Over time, there has been increasing interest in evaluating the potential association between T. gondii infection and other pathologies: (i) psychiatric disorders (major depression, schizophrenia, bipolar disorder, epilepsy, personality changes) [4,15,16,17]; (ii) neurologic diseases (Parkinson’s and Alzheimer’s diseases) [18,19]; and (iii) different types of cancer, including brain tumours [20,21,22].
The potential cardiac damage associated with T. gondii infection presents as atrial and ventricular arrhythmias, pericardial effusion, constrictive pericarditis, myocarditis, and acute heart failure [23]. The World Health Organization (WHO) states that cardiovascular diseases (CVDs) are the leading cause of mortality worldwide, with approximately 17.9 million deaths each year (an estimated 32% of all global deaths) [24]. The cardiovascular involvement in toxoplasmosis is often asymptomatic or overshadowed by neurological manifestations. Limited data are available in the existing international literature regarding the seroepidemiology of T. gondii infection among cardiovascular patients [25,26]. We have previously assessed the seroprevalence of toxoplasmosis in cardiovascular patients from Western Romania [27]. However, no evaluation regarding the potential risk factors associated with T. gondii in cardiovascular patients has previously been conducted. Therefore, in this study, we aimed to comprehensively assess the risk factors associated with the seroprevalence of T. gondii in patients with cardiovascular diseases.
2. Materials and Methods
2.1. Study Design and Population
We enrolled 1205 consecutive volunteer patients diagnosed with cardiovascular diseases in the order that they were admitted to the Institute of Cardiovascular Diseases in Timisoara, between July and October 2019. The Institute of Cardiovascular Diseases provides specialized healthcare services to the inhabitants of Western Romania (with a total population of 2,110,963 located in 5 counties: Arad, Bihor, Caras-Severin, Hunedoara, and Timis) and is considered a reference medical institution, with an average of 5000 treated patients annually. Clinical diagnoses were established based on the International Classification of Diseases 10th Revision (ICD-10) [28].
At study enrolment, venous blood samples were collected into Clot Activator Vacuum and Serum Separation Gel Tubes and centrifugated at 4000× g for 10 min, in maximum 30 min after collection. The sera were then transferred into sterile Centrifuge Eppendorf Tubes and stored at −20 °C until they were tested for IgG and/or IgM T. gondii antibodies. Serum samples with detectable T. gondii antibodies were further tested to evaluate the presence of T. gondii IgM antibodies only. In cases of positive serologic IgG and IgM test results, a specific IgG avidity test was performed.
The study participants’ demographic data (age, gender, area of residence) were extracted from the electronic database of the Institute of Cardiovascular Diseases, using a code (without their identification). All data have been processed with utmost confidentiality. An interviewer-administered, structured questionnaire was designed for this study to identify the potential risk factors associated with T. gondii: educational level (elementary/middle school, high school, university), employment status, owning cat(s), number of cats owned, cleaning cat litter, owning dog(s), number of dogs owned, owning cat(s) and/or dog(s), contact with soil (through agriculture and/or gardening activities), gardening with gloves, eating unwashed raw vegetables/fruits, the consumption of raw/undercooked meat, drinking raw/unpasteurised milk, drinking alcohol, smoking habits and drinking water. Study participants were also questioned regarding toxoplasmosis awareness and their personal medical history: previous blood transfusions and associated chronic diseases (such as diabetes, neurological diseases, gastrointestinal diseases, liver diseases, or cancer). Regarding smoking status, current and/or former smokers were recorded as a yes and those who had never smoked were recorded as a no. Study participants were grouped according to their age (at the time of enrolment in the study) in 7 age groups: 19–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, and 80 years and over.
2.2. Serologic Tests
All serum samples were tested at the Center for Diagnosis and Study of Parasitic Diseases, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania. A Pastorex Toxo kit (Bio-Rad, Marnes-la-Coquette, France) was used to simultaneously detect the presence of IgG and/or IgM antibodies to T. gondii. Previous reports showed an excellent ability to detect T. gondii antibodies in patients with acute and chronic toxoplasmosis for this latex particle agglutination test [29,30,31].
To identify the presence of serum anti-T. gondii IgM antibodies and evaluation of IgG avidity, the enzyme-linked fluorescent assay (ELFA) designed for VIDAS (bioMérieux, Marcy-l’Etoile, France) was used. ELFA IgM (VIDAS Toxo IgM kit) has a sensitivity of 100% and a specificity of 98.6% [32,33]. An accuracy of 93.4% in detecting a T. gondii infection dating more than 4 months was recently found for IgG avidity Vidas [34]. Quality controls and testing and interpretation of results were based on the manufacturer’s criteria.
2.3. Interpretation of the Serologic Test Results
T. gondii IgM test results were interpreted as follows: <0.55, negative; ≥0.55 to 0.65, equivocal, and >0.65, positive [35]. For the purposes of this study, equivocal test results were considered negative.
The Vidas IgG avidity test was interpreted as follows: <0.2, low avidity; ≥0.2 to 0.29, equivocal result; >0.3%, high avidity [35]. In case of low or equivocal test results, the T. gondii infection may have occurred within the 4 months before testing. The possibility of a primary infection within the previous 4 months was excluded by a high avidity test result [36].
2.4. Data Management and Statistical Analysis
All collected data were introduced in a Microsoft Excel database, version 2011 (Microsoft Corp., Redmond, WA, USA). Statistical analyses were performed using MedCalc for Windows, version 19.4 (MedCalc Software, Ostend, Belgium) and Epi Info statistical package, version 3.3.2 (Centers for Disease Control and Prevention, Atlanta, GA, USA). Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. To identify the significant association between T. gondii seroprevalence and risk factors and to compare proportions between groups, we used Mantel–Haenszel chi-square test and Fisher’s 2-tailed exact test. p-values < 0.05 were considered to be of statistical significance. Variables that reached a significance level in the univariable analyses were further analysed using the multivariable logistic regression model.
2.5. Ethical Consideration
The protocol of this study was conducted in accordance with the Helsinki Declaration and approved by the Victor Babes University Ethics Committee, Timisoara, Romania (no. 6 from 16 March 2018). All study participants were thoroughly informed about the study goals and procedures and provided written informed consent.
3. Results
The 1205 adults diagnosed with cardiovascular diseases enrolled in the study were aged between 19 and 94 years (mean age = 64.34 ± 12.06 years), 703 (58.3%) were residents of urban areas, and 779 (64.6%) were males (Table 1).

Table 1.
Seroprevalence of Toxoplasma gondii infection in patients with cardiovascular diseases from Western Romania according to age, area of residence, and gender.
T. gondii IgG and/or IgM antibodies were demonstrated in 52.1% (628/1205) of study participants (95% CI: 49.29–54.93). Of the 628 study participants with detectable T. gondii antibodies, 11 (1.75%) were identified as having T. gondii IgM antibodies, and the IgG avidity test was subsequently performed in those 11 samples. A high avidity test result (≥0.3) was obtained for all samples tested. In the 617 (98.25%) of the 628 cases in which the IgG avidity test was not performed, the diagnosis of chronic infection was based on negative test results for T. gondii IgM antibodies.
The T. gondii seroprevalence showed a significant age-associated increase, from 26.32% (5/19) in the age group of 19–29 years to 54.55% (54/99) in the age group of 40–49 years (p = 0.04), to 53.37% (222/416) in the age group of 60–69 years (p = 0.03), to 54.49% (194/356) in the age group of 70–79 years (p = 0.01), and 59.77% (52/87) in patients aged 80 years and over (p = 0.01) (Table 1).
The prevalence of T. gondii infection was significantly higher in patients residing in rural areas (57.57%, 289/502) compared to those from urban areas (48.22%, 339/703) (p = 0.001) and in females (56.34%, 240/426) compared to males (49.81%, 388/779) (p = 0.03) (Table 1).
When data were analysed according to the level of education, the T. gondii seroprevalence decreased with an increase in level of education, from 58.04% in patients who graduated from elementary/middle school to 33.33% in those who attended university. Moreover, the seroprevalence was significantly higher in study participants who attended primary/middle school (58.04%, 260/448) and those who attended high school (51.41%, 329/640) compared to patients who attended university (33.33%, 39/117) (p < 0.001) (Table 2).

Table 2.
Factors associated with Toxoplasma gondii seroprevalence in patients with cardiovascular diseases from Western Romania.
We found that retired study participants were 1.37 times more likely to test positive for T. gondii antibodies compared to employed ones (p = 0.009) (Table 2).
Owning cats, owning dogs, and owning any pets (cats and/or dogs) were variables that were significantly associated with T. gondii seroprevalence in our study group (p < 0.001, p = 0.01, and p < 0.001, respectively). However, there was no significant relationship between the number of cats owned, the cleaning of cat litter, the number of dogs owned, and seropositivity for T. gondii (Table 2).
Contact with soil (through agriculture or gardening activities), gardening with gloves, eating unwashed raw vegetables and/or fruits, the consumption of raw/undercooked meat, drinking raw/unpasteurized milk, drinking alcohol, smoking habits, type of water consumed were all found not to be risk factors for T. gondii infection in patients with cardiovascular diseases in univariate analysis (Table 2).
In our study group, only 166 (13.77%) patients had an awareness of toxoplasmosis. The T. gondii seroprevalence was significantly higher in study participants who had never heard or read about toxoplasmosis (53.99%, 561/1039) compared to those who had (40.36%, 67/166) (p = 0.001) (Table 2).
No significant differences in T. gondii seroprevalence were observed in univariate analysis regarding patients’ history of blood transfusions and associated chronic diseases (Table 2).
When the variables identified as risk factors for T. gondii infection in the univariate analysis (age, area of residence, gender, educational level, employment status, owning cats, owning dogs, owning any pets, and awareness of toxoplasmosis) were evaluated using a multiple logistic regression model, only the area of residence, gender, educational level, owning dogs, owning any pet, and toxoplasmosis awareness remained significantly associated with T. gondii seropositivity (Table 3).

Table 3.
Risk factors for Toxoplasma gondii infection in patients with cardiovascular diseases from Western Romania (multiple logistic regression analysis).
When data were analysed according to the ICD-10 Diagnosis Code, the highest T. gondii seroprevalence was observed in patients diagnosed with dilated cardiomyopathy (66.66%, 20/30) and the lowest in patients with myopericarditis (30.0%, 3/10) (Table 4).

Table 4.
Seroprevalence of Toxoplasma gondii antibodies in patients with cardiovascular diseases from Western Romania, according to their ICD-10 Diagnosis Code (univariate analysis).
4. Discussion
There is little information in the international literature regarding the epidemiology of T. gondii infection in patients with cardiovascular diseases. Most of the currently available data comprise the case reports of immunocompromised patients (transplant recipients or individuals infected with HIV) [26]. Moreover, to the best of our knowledge, this is the first study that has evaluated the potential risk factors associated with T. gondii infection in Romanian cardiovascular patients.
When we compared our results with those from studies conducted in similar groups of patients with cardiovascular diseases, we found that the 52.1% T. gondii seroprevalence found in our study is higher than the 13.8% seroprevalence found in Mexico [25], but lower than the 63.1% and 63.73% prevalences reported in Egypt [37] and Iran [38], respectively. It is well known that the prevalence of T. gondii infection varies widely across the globe [4,7,39], and there are several factors that can explain this phenomenon: (i) host susceptibility; (ii) cultural habits; (iii) cooking habits and diet; (iv) hygiene; (v) the presence of cats and their number; (vi) the conditions in the external environment that may or may not favour the survival of the infecting oocysts of T. gondii; and (vii) socioeconomic conditions [1,4,39]. Additionally, the differences observed may be explained by variations in the sample size of the study groups and different assays (with different specificities and/or sensitivities) used to evaluate the presence of T. gondii antibodies [40].
The univariate analysis revealed that the prevalence of T. gondii antibodies increased with age in our study group, due to a prolonged length of exposure to the parasite [40]. However, when multiple logistic regression was performed, we noticed that age was no longer a significant risk factor for toxoplasmosis. In their study, Alvarado-Esquivel and colleagues also found no association between age and seropositivity for T. gondii [25]. The diverse backgrounds of study participants and individual circumstances may lead to different ages of exposure to the parasite and may explain the result [41].
Area of residence and gender were both found to be associated with T. gondii infection in our survey, suggesting that residing in a rural area and being female lead to a greater risk of becoming infected. Activities carried out in rural areas (gardening, farming, handling animals) may expose individuals to the main sources of infection with T. gondii: (i) oocysts excreted with cat faeces and (ii) tissue cysts found in meat. Feral cats defecate in sandboxes or gardens, posing a risk of infection for some individuals, regardless of whether they own a cat [42,43]. The finding that T. gondii seroprevalence is significantly higher in females compared to males in Romanian cardiovascular patients represents important information for public health. Women spend more time cooking and therefore handle raw meat more frequently [44]. Additionally, they typically take care of, and tend to play more with, animals (including cats) [45].
Educational level was found to be an important risk factor for the occurrence of T. gondii infection in our study group: the multiple logistic regression analysis revealed that a lower educational level significantly increases the seropositivity for T. gondii. In patients with heart diseases from Durango City, Mexico, a low educational level was not associated with the prevalence of T. gondii infection [25]. However, the present study validates our previous findings [40,44] suggesting that a high level of education (university) increases the awareness and understanding of T. gondii infection and its preventive measures, therefore reducing the likelihood of exposure [40].
In Romanian patients with cardiovascular diseases, retired individuals were more likely to present anti-T. gondii antibodies compared to employed ones when using univariate analysis. However, when multiple logistic regression was performed, employment status was no longer identified as a risk factor for T. gondii, and this is in agreement with previous findings [25].
Similar to another report [25], in our study group, owning cats was found to be associated with T. gondii seropositivity when using univariate analysis. However, after performing the multiple logistic regression, contact with cats was no longer identified as a risk factor for T. gondii, and this validates our previous findings in Romanian blood donors [40]. Interestingly, owning dogs and owning any pet (cats and/or dogs) may increase the risk for T. gondii infection in our study group. Previous reports revealed that 25% of dogs from Central Romania (Cluj-Napoca) [46] and 63% from Southern Romania are infected with T. gondii [47]. Dogs may act as mechanical carriers for T. gondii oocysts and could play an important role in the transmission of this parasite (i) through it contaminating their fur and (ii) through feeding on cat faces (accidentally ingesting T. gondii oocysts and defecating them, probably after passive gastrointestinal transport) [48,49,50]. The identification of owning pets (cats and/or dogs) being a risk factor for T. gondii infection in patients with cardiovascular diseases confirms our previous result found in Romanian pregnant women [44]. The T. gondii seroprevalence in household cats from Central and North-Western Romania was 47% [51]. The close contact between cats and dogs (the most popular pet animals worldwide) and their owner may explain the potential risk factor for T. gondii infection in humans represented by these animals [52].
In this study, T. gondii seroprevalence was not found to be associated with the consumption of raw or undercooked meat; this is similar to recently data published by other authors [25]. Moreover, this result confirms our previous findings in Romanian blood donors and pregnant women [40,44]. This outcome could potentially be explained by several factors: (i) the increase in the utilization of frozen and industrially processed meat and meat products; (ii) the implementation of modern systems and enhanced hygiene conditions in animal farms; (iii) improved sanitary measures during meat processing [40,53].
Contact with soil, eating unwashed raw vegetables/fruits, drinking raw/unpasteurized milk, and the type of water used for drinking were not found to be risk factors for T. gondii infection in Romanian patients with cardiovascular diseases. Previous reports found no association between seropositivity for T. gondii and the consumption of untreated water [25,38] or of unwashed raw vegetables/fruits [25] in patients with heart diseases. Khademvatan and colleagues found that exposure to soil and drinking raw milk were significantly associated with T. gondii infection in their study [38].
In contrast with findings observed by Alvarado-Esquivel and colleagues [25], we found no association between T. gondii seropositivity and the consumption of alcohol. Further studies are needed to elucidate the potential role of alcohol consumption in T. gondii infection.
Our results revealed that the vast majority of study participants (86.22%) were not aware of T. gondii infection. Moreover, toxoplasmosis awareness was found to be associated with T. gondii seropositivity in multiple logistic regression analysis. The present study underlines the importance of formal education and literacy in increasing the awareness of T. gondii infection and methods for its prevention.
Previous reports have documented the possibility of T. gondii transmission via the transfusion of leukocytes or platelets and blood transfusion from asymptomatic seropositive individuals in the early stages of acute infection (T. gondii can survive for more than 50 days in citrated blood at 5 °C) [40,54]. However, we noticed that the prevalence of T. gondii was not associated with a history of blood transfusion in our study group; this is similar to the results previously published by Alvarado-Esquivel and colleagues [25].
The present study has several limitations. Despite the large size of the study sample, the number of patients with detectable T. gondii IgM antibodies was small, and, consequently, the number of serum samples tested for avidity was limited. Serum samples with negative results for T. gondii IgG antibodies were not tested further to assess the presence of T. gondii IgM antibodies. However, it is exceptionally uncommon to detect IgM antibodies in the absence of IgG antibodies [55]. Therefore, it is highly unlikely that the results of this study would have significantly changed had negative IgG sera been tested for IgM. Female patients are less represented in our study, and this can be listed as another limitation. Gender differences are increasingly recognized as influencing susceptibility and pathology in cardiovascular diseases due to several factors: sex chromosomes, gonadal hormones, different cardiac structure and function, intrinsic variations in cardiac and vascular aging, differences in myocardial substrate metabolism, and cultural and social behaviours [56,57]. Properly evaluating T. gondii’s seroprevalence and the influence of the parasite on cardiac function was difficult in patients diagnosed with dilated cardiomyopathy, aortic aneurysm, hypertension, congestive heart failure, pulmonary oedema, pulmonary embolism, and myopericarditis due to the limited number of study participants. Further studies involving a larger participant pool will be needed to validate the results of the present survey.
5. Conclusions
This study provides novel and valuable insights into the seroprevalence and risk factors associated with T. gondii among Romanian patients with cardiovascular diseases. Area of residence, gender, educational level, owning dogs, owning any pet, and toxoplasmosis awareness were found to be significantly associated with T. gondii seropositivity in a multiple logistic regression analysis. Our findings reiterate the importance of toxoplasmosis awareness and health education for better control and prevention of infection with T. gondii.
Author Contributions
Conceptualization, A.D., M.A.L. and T.R.O.; methodology, A.D., M.A.L. and T.R.O.; software, A.D., M.A.L. and T.R.O.; validation, A.D., M.A.L. and T.R.O.; formal analysis, A.D., M.A.L., C.G.M., L.M.C. and T.R.O.; investigation, A.D., M.A.L. and T.R.O.; resources, A.D., M.A.L. and T.R.O.; data curation, A.D., M.A.L. and T.R.O.; writing—original draft preparation, A.D., M.A.L. and T.R.O.; writing—review and editing, A.D., M.A.L., C.G.M., L.M.C. and T.R.O.; visualization, A.D., M.A.L., C.G.M., L.M.C. and T.R.O.; supervision, A.D., M.A.L. and T.R.O.; project administration, A.D., M.A.L. and T.R.O.; funding acquisition, T.R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We gratefully thank Elena Curelea and Mihaela Dorothea Badescu for their technical assistance during the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Barros, R.A.M.; Torrecilhas, A.C.; Marciano, M.A.M.; Mazuz, M.L.; Pereira-Chioccola, V.L.; Fux, B. Toxoplasmosis in Human and Animals around the World. Diagnosis and Perspectives in the One Health Approach. Acta Trop. 2022, 231, 106432. [Google Scholar] [CrossRef] [PubMed]
- Nasiru Wana, M.; Mohd Moklas, M.A.; Watanabe, M.; Nordin, N.; Zasmy Unyah, N.; Alhassan Abdullahi, S.; Ahmad Issa Alapid, A.; Mustapha, T.; Basir, R.; Abd Majid, R. A Review on the Prevalence of Toxoplasma gondii in Humans and Animals Reported in Malaysia from 2008–2018. Int. J. Environ. Res. Public Health 2020, 17, 4809. [Google Scholar] [CrossRef] [PubMed]
- Madireddy, S.; Rivas Chacon, E.D.; Mangat, R. Toxoplasmosis; StatPearls: Tampa, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563286/ (accessed on 2 February 2024).
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar] [PubMed]
- Smith, N.C.; Goulart, C.; Hayward, J.A.; Kupz, A.; Miller, C.M.; van Dooren, G.G. Control of human toxoplasmosis. Int. J. Parasitol. 2021, 51, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Attias, M.; Teixeira, D.E.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The life-cycle of Toxoplasma gondii reviewed using animations. Parasit. Vectors 2020, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F.; Dardé, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [PubMed]
- Poulle, M.L.; Forin-Wiart, M.A.; Josse-Dupuis, É.; Villena, I.; Aubert, D. Detection of Toxoplasma gondii DNA by qPCR in the feces of a cat that recently ingested infected prey does not necessarily imply oocyst shedding. Parasite 2016, 23, 29. [Google Scholar] [CrossRef]
- Babekir, A.; Mostafa, S.; Obeng-Gyasi, E. The Association of Toxoplasma gondii IgG and Cardiovascular Biomarkers. Int. J. Environ. Res. Public Health 2021, 18, 4908. [Google Scholar] [CrossRef]
- Niveria, K.; Yadav, M.; Dangi, K.; Singh, P.; Verma, A.K.; Kanwar, J.R. Recent Approaches to Combat Toxoplasma gondii with Plant-Derived Alternatives. In Natural Product Based Drug Discovery against Human Parasites; Singh, A., Rathi, B., Verma, A.K., Singh, I.K., Eds.; Springer: Singapore, 2023; pp. 307–327. [Google Scholar]
- Ahmadpour, E.; Babaie, F.; Kazemi, T.; Mehrani Moghaddam, S.; Moghimi, A.; Hosseinzadeh, R.; Nissapatorn, V.; Pagheh, A.S. Overview of Apoptosis, Autophagy, and Inflammatory Processes in Toxoplasma gondii Infected Cells. Pathogens 2023, 12, 253. [Google Scholar] [CrossRef]
- Mose, J.M.; Kagira, J.M.; Kamau, D.M.; Maina, N.W.; Ngotho, M.; Karanja, S.M. A Review on the Present Advances on Studies of Toxoplasmosis in Eastern Africa. BioMed Res. Int. 2020, 2020, 7135268. [Google Scholar] [CrossRef]
- Vargas-Villavicencio, J.A.; Cañedo-Solares, I.; Correa, D. Anti-Toxoplasma gondii IgM Long Persistence: What Are the Underlying Mechanisms? Microorganisms 2022, 10, 1659. [Google Scholar] [CrossRef] [PubMed]
- Olariu, T.R.; Press, C.; Talucod, J.; Olson, K.; Montoya, J.G. Congenital toxoplasmosis in the United States: Clinical and serologic findings in infants born to mothers treated during pregnancy. Toxoplasmose congénitale aux États-Unis: Observations cliniques et sérologiques chez les nourrissons nés de mères traitées pendant la grossesse. Parasite 2019, 26, 13. [Google Scholar]
- Cossu, G.; Preti, A.; Gyppaz, D.; Gureje, O.; Carta, M.G. Association between toxoplasmosis and bipolar disorder: A systematic review and meta-analysis. J. Psychiatr. Res. 2022, 153, 284–291. [Google Scholar] [CrossRef]
- Martinez, V.O.; de Mendonça Lima, F.W.; de Carvalho, C.F.; Menezes-Filho, J.A. Toxoplasma gondii infection and behavioral outcomes in humans: A systematic review. Parasitol. Res. 2018, 117, 3059–3065. [Google Scholar] [CrossRef]
- Vittecoq, M.; Thomas, F. Toxoplasmose et cancer: Connaissances actuelles et perspectives de recherche [Toxoplasmosis and cancer: Current knowledge and research perspectives]. Bull. Soc. Pathol. Exot. 2017, 110, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, S.; Pinto, B.; Bonuccelli, U.; Bruschi, F. Neurobiological studies on the relationship between toxoplasmosis and neuropsychiatric diseases. J. Neurol. Sci. 2015, 351, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sugden, K.; Moffitt, T.E.; Pinto, L.; Poulton, R.; Williams, B.S.; Caspi, A. Is Toxoplasma gondii Infection Related to Brain and Behavior Impairments in Humans? Evidence from a Population-Representative Birth Cohort. PLoS ONE 2016, 11, e0148435. [Google Scholar] [CrossRef] [PubMed]
- Ngô, H.M.; Zhou, Y.; Lorenzi, H.; Wang, K.; Kim, T.K.; Zhou, Y.; El Bissati, K.; Mui, E.; Fraczek, L.; Rajagopala, S.V.; et al. Toxoplasma Modulates Signature Pathways of Human Epilepsy, Neurodegeneration & Cancer. Sci. Rep. 2017, 7, 11496, Erratum in Sci. Rep. 2019, 9, 8110. [Google Scholar]
- Thomas, F.; Lafferty, K.D.; Brodeur, J.; Elguero, E.; Gauthier-Clerc, M.; Missé, D. Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biol. Lett. 2012, 8, 101–103. [Google Scholar] [CrossRef]
- Vittecoq, M.; Elguero, E.; Lafferty, K.D.; Roche, B.; Brodeur, J.; Gauthier-Clerc, M.; Missé, D.; Thomas, F. Brain cancer mortality rates increase with Toxoplasma gondii seroprevalence in France. Infect. Genet. Evol. 2012, 12, 496–498. [Google Scholar] [CrossRef]
- Akella, P.; Bhatt, I.; Serhan, M.; Giri, D.D.; Pastores, S.M. Toxic ‘Toxo’ in the heart: Cardiac toxoplasmosis following a hematopoietic stem cell transplant- a case report. IDCases 2021, 25, e01217. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 2 February 2024).
- Alvarado-Esquivel, C.; Salcedo-Jaquez, M.; Sanchez-Anguiano, L.F.; Hernandez-Tinoco, J.; Rabago-Sanchez, E.; Beristain-Garcia, I.; Liesenfeld, O.; Estrada-Martinez, S.; Perez-Alamos, A.R.; Alvarado-Soto, E. Association between Toxoplasma gondii Exposure and Heart Disease: A Case-Control Study. J. Clin. Med. Res. 2016, 8, 402–409. [Google Scholar] [CrossRef]
- Zhou, Z.; Ortiz Lopez, H.I.A.; Pérez, G.E.; Burgos, L.M.; Farina, J.M.; Saldarriaga, C.; Lopez-Santi, R.; Cotella, H.I.; Sauce Pérez, A.L.; Baranchuk, A. Toxoplasmosis and the Heart. Curr. Probl. Cardiol. 2021, 46, 100741. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, A.; Lupu, M.A.; Lighezan, R.; Paduraru, A.A.; Olariu, T.R. Toxoplasma gondii Infection in Patients with Cardiovascular Diseases from Western Romania: A Case–Control Study. Life 2023, 13, 1575. [Google Scholar] [CrossRef] [PubMed]
- International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). Available online: https://icd.who.int/browse10/2019/en (accessed on 2 February 2024).
- Olariu, T.R.; Darabus, G.; Cretu, O.; Jurovits, O.; Giura, E.; Erdelean, V.; Marincu, I.; Iacobiciu, I.; Petrescu, C.; Koreck, A. Prevalence of Toxoplasma gondii antibodies among women of childbearing age in Timis County. Lucr. Stiintifice Med. Vet. Timis. 2008, 41, 367–371. [Google Scholar]
- Villard, O.; Cimon, B.; Franck, J.; Fricker-Hidalgo, H.; Godineau, N.; Houze, S.; Paris, L.; Pelloux, H.; Villena, I.; Candolfi, E. Network from the French National Reference Center for Toxoplasmosis. Evaluation of the usefulness of six commercial agglutination assays for serologic diagnosis of toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2012, 73, 231–235. [Google Scholar] [PubMed]
- Olariu, T.R.; Petrescu, C.; Darabus, G.; Lighezan, R.; Mazilu, O. Seroprevalence of Toxoplasma gondii in Western Romania. Infect. Dis. 2015, 47, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Remington, J.S.; Clavet, C.; Varney, G.; Press, C.; Ware, D. Evaluation of Six Commercial Kits for Detection of Human Immunoglobulin M Antibodies to Toxoplasma gondii. The FDA Toxoplasmosis Ad Hoc Working Group. J. Clin. Microbiol. 1997, 35, 3112–3115. [Google Scholar]
- Gharavi, M.J.; Oormazdi, H.; Roointan, E.S. A Comparative Study on Sensitivity and Specificity of Conventional and Unconventional IgG and IgM Assays for Diagnosis of Toxoplasmosis. Iran. J. Public Health 2008, 37, 42–45. [Google Scholar]
- Smets, A.; Fauchier, T.; Michel, G.; Marty, P.; Pomares, C. Comparison of Toxoplasma gondii IgG Avidity Architect and Vidas Assays with the Estimated Date of Infection in Pregnant Women. Parasite 2016, 23, 45. [Google Scholar] [CrossRef]
- Murat, J.B.; Dard, C.; Fricker Hidalgo, H.; Dardé, M.L.; Brenier-Pinchart, M.P.; Pelloux, H. Comparison of the Vidas system and two recent fully automated assays for diagnosis and follow-up of toxoplasmosis in pregnant women and newborns. Clin. Vaccine Immunol. 2013, 20, 1203–1212. [Google Scholar] [CrossRef]
- Olariu, T.R.; Blackburn, B.G.; Press, C.; Talucod, J.; Remington, J.S.; Montoya, J.G. Role of Toxoplasma IgA as Part of a Reference Panel for the Diagnosis of Acute Toxoplasmosis during Pregnancy. J. Clin. Microbiol. 2019, 57, e01357-18. [Google Scholar] [CrossRef]
- Alhusseiny, S.M.; Saleh, N.E.; El-Zayady, W.M.; Hussein, M.S.; El-Beshbishi, S.N. Association between Toxoplasma gondii infection and coronary atherosclerosis. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1190–1197. [Google Scholar] [CrossRef]
- Khademvatan, S.; Khademvatani, K.; Tappeh, K.H.; Asadi, N.; Khezri, P.; Abasi, E. Association of Toxoplasma gondii infection with cardiovascular diseases: A cross-sectional study among patients with heart failure diseases in Urmia, North-West of Iran. Ann. Parasitol. 2020, 66, 193–199. [Google Scholar] [PubMed]
- Sanchez, S.G.; Besteiro, S. The pathogenicity and virulence of Toxoplasma gondii. Virulence 2021, 12, 3095–3114. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.A.; Lighezan, R.; Paduraru, A.A.; Dragomir, A.; Pavel, R.; Grada, S.; Mihu, A.G.; Ursoniu, S.; Olariu, T.R. Seroepidemiology of Toxoplasma gondii Infection in Blood Donors from Western Romania. Microorganisms 2022, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.; Shetty, N. Seroprevalence and risk factors for toxoplasmosis among antenatal women in London: A re-examination of risk in an ethnically diverse population. Eur. J. Public Health 2013, 23, 648–652. [Google Scholar] [CrossRef]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef]
- Mihu, A.G.; Balta, C.; Marti, D.T.; Paduraru, A.A.; Lupu, M.A.; Olariu, T.R. Seroprevalence of Toxoplasma gondii infection among women of childbearing age in an endemic region of Romania, 2016–2018. Parasite 2020, 27, 59. [Google Scholar] [CrossRef]
- Olariu, T.R.; Ursoniu, S.; Hotea, I.; Dumitrascu, V.; Anastasiu, D.; Lupu, M.A. Seroprevalence and Risk Factors of Toxoplasma gondii Infection in Pregnant Women from Western Romania. Vector Borne Zoonotic Dis. 2020, 20, 763–767. [Google Scholar] [CrossRef]
- Xiao, Y.; Yin, J.; Jiang, N.; Xiang, M.; Hao, L.; Lu, H.; Sang, H.; Liu, X.; Xu, H.; Ankarklev, J.; et al. Seroepidemiology of human Toxoplasma gondii infection in China. BMC Infect. Dis. 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Cozma, V.; ¸Suteu, O.; Titilincu, A.; Osztian, R.M. Seroprevalence of Toxoplasma gondii antibodies in dogs from Cluj Napoca. Rev. Romana Parazitol. 2007, 17, 23–26. [Google Scholar]
- Fernoaga, C.; Codreanu, M.D.; Cornila, M.; Nae, R.T.; Ionita, M.; Mitrea, I.L. Clinical follow-up of dogs with neurological disorders and positive for antibodies against Toxoplasma gondii. Sci. Works C Ser. Vet. Med. 2015, 61, 131–134. [Google Scholar]
- Frenkel, J.K.; Lindsay, D.S.; Parker, B.B.; Dobesh, M. Dogs as possible mechanical carriers of toxoplasma, and their fur as a source of infection of young children. Int. J. Infect. Dis. 2003, 7, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Fábrega, L.; Restrepo, C.M.; Torres, A.; Smith, D.; Chan, P.; Pérez, D.; Cumbrera, A.; Caballero, E.Z. Frequency of Toxoplasma gondii and Risk Factors Associated with the Infection in Stray Dogs and Cats of Panama. Microorganisms 2020, 8, 927. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P.; Butler, J.M.; Blagburn, B.L. Mechanical transmission of Toxoplasma gondii oocysts by dogs. Vet. Parasitol. 1997, 73, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Györke, A.; Opsteegh, M.; Mircean, V.; Iovu, A.; Cozma, V. Toxoplasma gondii in Romanian household cats: Evaluation of serological tests, epidemiology and risk factors. Prev. Vet. Med. 2011, 102, 321–328. [Google Scholar] [CrossRef]
- Oi, M.; Yoshikawa, S.; Maruyama, S.; Nogami, S. Comparison of Toxoplasma gondii Seroprevalence in Shelter. Cats and Dogs during 1999–2001 and 2009–2011 in Tokyo, Japan. PLoS ONE 2015, 10, e0135956. [Google Scholar] [CrossRef]
- Stopić, M.; Štajner, T.; Marković-Denić, L.; Nikolić, V.; Djilas, I.; Srzentić, S.J.; Djurković-Djaković, O.; Bobić, B. Epidemiology of Toxoplasmosis in SERBIA: A Cross-Sectional Study on Blood Donors. Microorganisms 2022, 10, 492. [Google Scholar] [CrossRef]
- Foroutan-Rad, M.; Majidiani, H.; Dalvand, S.; Daryani, A.; Kooti, W.; Saki, J.; Hedayati-Rad, F.; Ahmadpour, E. Toxoplasmosis in Blood Donors: A Systematic Review and Meta-Analysis. Transfus. Med. Rev. 2016, 30, 116–122. [Google Scholar] [CrossRef]
- Jones, J.L.; Kruszon-Moran, D.; Elder, S.; Rivera, H.N.; Press, C.; Montoya, J.G.; McQuillan, G.M. Toxoplasma gondii Infection in the United States, 2011–2014. Am. J. Trop. Med. Hyg. 2018, 98, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Reue, K.; Wiese, C.B. Illuminating the Mechanisms Underlying Sex Differences in Cardiovascular Disease. Circ. Res. 2022, 130, 1747–1762. [Google Scholar] [CrossRef] [PubMed]
- Majidi, M.; Eslami, V.; Ghorbani, P.; Foroughi, M. Are women more susceptible to ischemic heart disease compared to men? A literature overview. J. Geriatr. Cardiol. 2021, 18, 289–296. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).