Abstract
Antimicrobial resistance (AMR) in non-typhoidal Salmonella is a pressing public health concern in the United States, necessitating continuous surveillance. We conducted a retrospective analysis of 251 Salmonella isolates from 11 animal species recovered between 1982 and 1999, utilizing serotyping, antimicrobial susceptibility testing, and whole-genome sequencing (WGS). Phenotypic resistance was observed in 101 isolates, with S. Typhimurium, S. Dublin, S. Agona, and S. Muenster prevailing among 36 identified serovars. Notably, resistance to 12 of 17 antibiotics was detected, with ampicillin being most prevalent (79/251). We identified 38 resistance genes, primarily mediating aminoglycoside (n = 13) and β-lactamase (n = 6) resistance. Plasmid analysis unveiled nine distinct plasmids associated with AMR genes in these isolates. Chromosomally encoded blaSCO-1 was present in three S. Typhimurium and two S. Muenster isolates from equine samples, conferring resistance to amoxicillin/clavulanic acid. Phylogenetic analysis revealed three distinct clusters for these five isolates, indicating evolutionary divergence. This study represents the first report of blaSCO-1 in the USA, and our recovered isolates harboring this gene as early as 1989 precede those of all other reports. The enigmatic nature of blaSCO-1 prompts further research into its function. Our findings highlight the urgency of addressing antimicrobial resistance in Salmonella for effective public health interventions.
1. Introduction
Salmonella, a Gram-negative Enterobacteriaceae family member, stands as a predominant etiological agent of gastroenteritis [1]. Non-typhoidal Salmonella (NTS), an extensive diversity within the Salmonella genus, which is evidenced by the documentation of more than 2600 serovars, is the most common enteric pathogen in animals and humans [2]. This poses a substantial and persistent threat to public health and is estimated to cause about 150 million illnesses and 60,000 deaths globally yearly [3]. These different NTS serovars are prevalent across diverse animal hosts, emphasizing the critical intersection between animal and human health [1].
The development of antimicrobial resistance (AMR) within Salmonella is attributed to various factors, including chromosomally encoded genes, chromosomal mutations, and the acquisition and recombination of mobile genetic elements such as transposons and plasmids [4,5,6,7]. For instance, Salmonella harbors naturally occurring chromosomally mediated β-lactamases, believed to have evolved from penicillin-binding proteins with shared sequence homology, and they have been disseminated by mobile genetic elements [8,9].
The increasing global incidence of AMR in Salmonella strains emphasizes the necessity for comprehensive investigations into their genomic complexities to facilitate effective public health interventions [10]. Whole genome sequencing (WGS) has become more accessible in recent years, enabling molecular characterization studies [11]. Determination of the antimicrobial resistance gene (AMG) through WGS complements traditional laboratory-based surveillance, offering insight into Salmonella serovars in a high-resolution manner. This approach provides direct insights into their evolutionary changes, strain relatedness, gene location, and detailed gene arrangements [5,12]. Consequently, several studies have established a strong correlation between antimicrobial genotypes and phenotypes in non-typhoidal Salmonella [13,14].
Most Salmonella genomic surveillance focuses on food animals as carriers, with very few studies addressing diseased animals, creating a general gap in research. One study examined samples from diseased animals due to reported concerns about antimicrobial resistance (AMR) in animals from clinical settings [15,16].
The history of Salmonella epidemiology has relied on various features to categorize strains. In vitro antibiotic susceptibility testing remains crucial for monitoring antibiotic resistance trends and guiding effective anti-infective therapy [17]. Thus, this study focused on 251 Salmonella clinical isolates collected over 18 years (1982–1999) in the United States from 11 different animal host species. We employed a multidimensional approach encompassing phenotypic characterization and genotypic profiling to understand the complex interplay between genomics and antimicrobial resistance.
The insights garnered from this study are anticipated to inform public health policies and interventions, addressing the diverse evolutionary patterns of antimicrobial resistance in Salmonella strains.
2. Materials and Methods
2.1. Bacterial Isolates
A total of 251 non-typhoidal Salmonella enterica clinical isolates of animal origin, recovered over 18 years (1982–1999), were included in this study. All isolates were sourced from the Bacteriology and Mycology Diagnostic Laboratory, College of Veterinary Medicine, at Auburn University. Pure isolates were reactivated by streaking on Tryptic Soy Agar and incubating at 37 °C.
2.2. Antimicrobial Susceptibility Testing
The isolates underwent phenotypic characterization via microbroth dilution antimicrobial susceptibility testing using the Vitek® 2 system. The tested antimicrobials (μg/mL) included ampicillin, amoxicillin/clavulanic acid, cefalexin, cefpodoxime, cefovecin, ceftazidime, ceftiofur, imipenem, amikacin, gentamicin, ciprofloxacin, enrofloxacin, marbofloxacin, doxycycline, nitrofurantoin, chloramphenicol, and trimethoprim/sulfamethoxazole. Minimum inhibitory concentration (MIC) values were interpreted using standard guideline breakpoints from the Clinical and Laboratory Standards Institute [18,19], National Antimicrobial Resistance Monitoring System for Enteric Bacteria [20], and IDEXX [21].
2.3. Whole-Genome Sequencing (WGS) and Genome Analysis
Genomic DNA extraction was performed on isolates obtained from 1.0 mL of 16–24 h culture prepared in Tryptic Soy Broth using the DNeasy Blood & Tissue Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol. The quality of isolated DNA was analyzed using NanoDrop™One and quantified using a Qubit® 3.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The extracted DNA was shipped from Auburn University College of Veterinary Medicine to South Dakota University to perform WGS. A concentration of 0.3 ng/μL of DNA was used for library preparation using Illumina DNA Sample Prep Kit (Illumina Inc., San Diego, CA, USA). After bead normalization, the pooled library was denatured, and sequencing was performed on the Illumina Miseq platform using V3 reagents with 2 × 300 paired-end chemistry. Sequencing data’s basic quality statistics were analyzed by fast QC, and assembly quality details were analyzed by QUAST using the Galaxytrakr platform. The raw sequences were uploaded into NCBI Sequence Read Archive (SRA), and the de novo assembly was generated by running SKESA under the NCBI BioProject PRJNA280335. (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA280335).
Antimicrobial resistance genotypes were predicted using the NCBI Pathogen Detection website (https://www.ncbi.nlm.nih.gov/pathogens/isolates; [22]) and ResFinder (https://cge.food.dtu.dk/services/ResFinder/, [23]). Individual genetic element locations were accessed through Pathogen Detection Microbial Browser for Identification of Genetic and Genomic Elements (MicroBIGG-E) (https://www.ncbi.nlm.nih.gov/pathogens/microbigge/). Plasmids were identified using the PlasmidFinder database on ABRicate (https://github.com/tseemann/abricate, [24,25]) and the plasmid-mediated AMR genes were regarded as genes found sharing the same contigs with identified plasmids [26]. The BlastP analysis was used to identify protein similarities (http://www.ncbi.nlm.nih.gov/BLAST, [27]) and conserved domains of proteins were generated on the NCBI conserved domain platform. (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, [28]).
2.4. Genotypic and Phenotypic Correlation Analysis
Agreement between phenotypic MIC data and genotypic WGS data was statistically evaluated using Cohen’s kappa (κ) test (https://idostatistics.com/cohen-kappa-free-calculator/#risultati, [29]). Isolates with intermediate resistance were not considered in this analysis. The results were interpreted as 0.01–0.20 slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement, 0.81–1.00 almost perfect or perfect agreement [30].
2.5. Genome Visualization and Analysis
Contigs for the genomes of interest were concatenated, and circular genomes were generated using BLAST Ring Image Generator (BRIG) software (https://sourceforge.net/projects/brig/). Phylogenetic analysis based on Average Nucleotide Identity (ANI) was calculated using MASH clustering to determine percentage similarities among isolates, and the FastANI tool (https://github.com/ParBLiSS/FastANI) was used for the analysis.
3. Results
3.1. Identification of 36 Salmonella Serovars in 11 Animal Species
Amongst the 251 analyzed isolates of Salmonella recovered from 11 animal host species, we identified 36 distinct serovars. Notably, S. Typhimurium appeared as the most prevalent, constituting 36.2% (n = 91) of the isolates, followed by S. Anatum (9.1%, n = 23), S. Dublin (8.3%, n = 21), and S. Agona (7.1%, n = 18). Other identified serovars were S. Newport (4.3%, n = 11), S. Give (3.2%, n = 8), S. Mbandaka (3.1%, n = 8), S. Muenster (3.1%, n = 8), S. Muenchen (2.7%, n = 7), S. Enteritidis (2.3%, n = 6), and S. Meleagridis (2.0%, n = 5). Additionally, several serovars, such as S. Montevideo, S. Tennessee, S. Worthington, S. Cholerasuis, S. Uganda, S. Berta, and others, exhibited lower prevalence rates (below 2%) (Table S1).
3.2. Salmonella Antimicrobial Resistance (AMR) Phenotypes
In this study, phenotypic resistance was most notable against ampicillin (79/251), doxycycline (64/251), gentamicin (33/251), trimethoprim/sulfamethoxazole (25/251), and chloramphenicol (16/251), among other antimicrobials (Figure 1). One hundred and one isolates (n = 101) displayed resistance to at least one of the 17 tested antimicrobials, of which none of the isolates exhibited resistance to imipenem, amikacin, ciprofloxacin, enrofloxacin, or marbofloxacin. Among the 101 resistant isolates, S. Typhimurium serovars were the most prevalent, constituting 49.5% (50/101), followed by S. Dublin 15.8% (16/101), S. Agona 8.9% (9/101), and S. Muenster 5.9% (6/101). Lower resistance prevalence was observed in S. Anatum, S. Enteritidis, S. Newport, S. Mbandaka, S. Choleraesuis, S. Meleagridis, and S. Worthington, while other identified isolates did not show antimicrobial resistance (Figure 2).
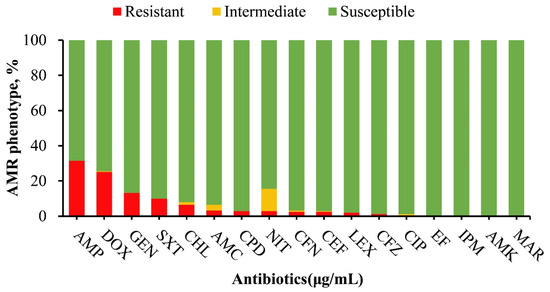
Figure 1.
Antimicrobial susceptibility profile of Salmonella isolates (n = 251) in this study. The drugs that are mostly resistant are ampicillin (31.5%), doxycycline (25.5%), gentamicin (13.1%), trimethoprim/sulfamethoxazole (10%), and chloramphenicol (6.4%). Other antimicrobials with lower resistance frequencies are AMC (3.2%), CPD (2.8%), NIT (2.8%), CFN (2.8%), CEF (2.8%), LEX (2.0%), CFZ (1.2%). No resistance was observed against CIP, EF, IPM, AMK, MAR. Abbreviations; AMP = ampicillin, AMC = amoxicillin/clavulanic acid, LEX = cephalexin, CPD = cefpodoxime, CFN = cefovecin, CFZ = ceftazidime, CEF = ceftiofur, IPM = imipenem, AMK = amikacin, GEN = gentamicin, CIP = ciprofloxacin, EF = enrofloxacin, MAR = marbofloxacin, DOX = doxycycline, NIT = nitrofurantoin, CHL = chloramphenicol, SXT = trimethoprim/sulfamethoxazole.
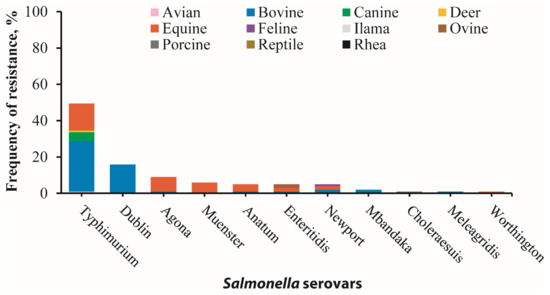
Figure 2.
Antimicrobial resistance in 101 Salmonella isolates across 11 serovars and 11 animal species exhibiting resistance to at least one antimicrobial tested. The highest frequency of resistance was observed in S. Typhimurium (49.5%), followed by S. Dublin (15.8%), S. Agona (8.9%), S. Muenster (5.9%), S. Anatum (5.0%), S. Enteriditis (5.0%), S. Newport (5.0%), S. Mbandaka (2.0%), S. Choleraesuis (1.0%), S. Maleagridis (1.0%), and S. Worthington (1.0%). Additionally, bovine-recovered serovars exhibited the highest frequency of resistance (51.5% total resistance) with S. Typhimurium 27.7%, S. Dublin 15.8%, S. Newport 2.0%, S. Mbandaka 2.0%, S. Anatum 1.0%, and S. Enteritidis 1.0%, followed by equine-recovered serovars (37.6% total resistance), which include S. Typhimurium 14.9%, S. Agona 7.9%, S. Muenster 5.9%, S. Anatum 4.0%, S. Enteritidis 2.0%, S. Newport 2.0%, and S. Worthington 1.0%. Other animal-recovered serovars with lower resistance are canine-recovered serovars (5% total resistance in S. Typhimurium), ovine-recovered serovars (2% total resistance in S. Enteritidis), avian-recovered serovars (1% total resistance S. Typhimurium), deer-recovered serovars (1% total resistance S. Typhimurium), feline-recovered serovars (1% S. Newport), and porcine-recovered serovars (1% total resistance S. Choleraesuis). Note: animal species of resistant serovars are distinguished by distinct color shades.
Furthermore, bovine-recovered serovars displayed the highest overall frequency of antimicrobial resistance at 51.5%, primarily driven by resistance in S. Typhimurium (27.7%) and S. Dublin (15.8%). Equine isolates showed a resistance rate of 37.6%, where S. Typhimurium (14.9%), S. Agona (7.9%), and S. Muenster (5.9%) were the predominant contributors (Figure 2; Tables S2 and S3). Conversely, serovars from other animal host species showed lower resistance rates (≤5%) (Figure 2).
3.3. Antimicrobial Resistance Genes in Salmonella Isolates
Genome analysis identified thirty-eight different antimicrobial resistance genes distributed across our isolates, and aminoglycosides exhibited the largest gene diversity with 13 distinct resistance genes. We also found six β-lactamase-producing genes, including the rarely reported gene blaSCO-1. AMGs were also identified to mediate resistance to tetracyclines (n = 4), phenicol (n = 3), trimethoprim (n = 3), sulfonamides (n = 2), quinolones (n = 2), fosfomycin (n = 1), macrolides (n = 1), bleomycin (n = 1), and the multi efflux genes (n = 2) (Table 1).

Table 1.
Antimicrobial resistance genes (AMGs) identified in Salmonella isolates.
3.4. Phenotypic and Genotypic Resistance Correlation in Salmonella Isolates
Varying levels of phenotypic and genotypic correlations were identified in this study. An isolate was classified as genotypically resistant if it carried at least one resistant gene or mutation related to the specific antibiotic. Notably, there was almost perfect agreement between resistant phenotypes and genotypes for ampicillin (k = 0.98) and for gentamicin, doxycycline, trimethoprim/sulfamethoxazole, and chloramphenicol (k = 0.93–1). Moderate to perfect correlations (k = 0.59–1) were observed for cephalosporins (cephalexin, cefpodoxime, cefovecin, ceftazidime, ceftiofur), whereas only a slight correlation (k = 0.13) was observed for amoxicillin/clavulanic (Figure 3; Table S4).
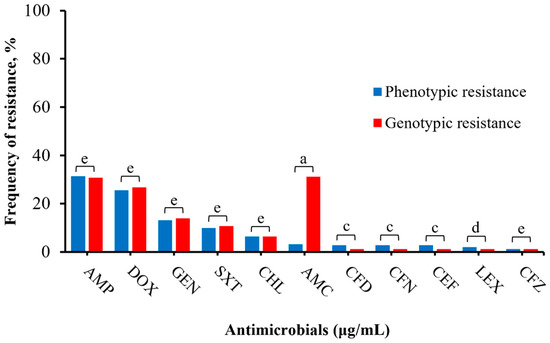
Figure 3.
Correlation between phenotype and genotype of Salmonella isolates (n = 251). Antimicrobials with no phenotypic resistance profile (imipenem, amikacin, ciprofloxacin, enrofloxacin, marbofloxacin) and nitrofurantoin (no specific resistant genotype) were excluded in this comparison. The total number of phenotypic resistance to genotypic resistance per antimicrobial are AMP: (79/251, 77/251); DOX: (64/251, 67/251), GEN: (33/251, 35/251), SXT: (25/251, 27/251), CHL: (16/251, 16/251), AMC: (8/251, 77/251), CFD: (7/251, 3/251), CFN: (7/251, 3/251), CEF: (7/251, 3/251), LEX: (5/251, 3/251), CFZ: (3/251, 3/251), respectively. The total genotypic resistance was determined by summating the number of isolates positive for at least one common resistant gene per antimicrobial. Results were interpreted as follows: a: 0.01–0.20 slight agreement, b: 0.21–0.40 fair agreement, c: 0.41–0.60 moderate agreement, d: 0.61–0.80 substantial agreement, e: 0.81–1.00 almost perfect or perfect agreement.
Interestingly, we observed that isolates with the blaSCO-1 gene showed intermittent (MIC = 16 μg/mL) to complete resistance (MIC ≥ 32 μg/mL) to amoxicillin/clavulanic acid (Table 1). In this phenotypic and genotypic comparison, we excluded nitrofurantoin (n = 7) due to absence of a corresponding resistance gene and quinolones-resistant genes gyrA(S83F) and parC(T57S) (n = 249) as no phenotypic resistance was observed.
3.5. Plasmid-Mediated AMR Genes
Plasmid analysis revealed nine different plasmids associated with AMR genes in Salmonella isolates. The identified plasmids include ColE10_1, IncA/C_1, IncA/C2_1, IncFIA(HI1)_1_HI1, IncFIA_1, IncFII(S)_1, IncI1_1_Alpha, IncN_1, and IncQ1_1, while IncN_1 (39/115) and (IncQ1_1) (24/115) were the most prevalent plasmids (Figure 4A). Subsequently, we identified fourteen (n = 14) different plasmid-mediated AMR genes across several isolates (Figure 4B).
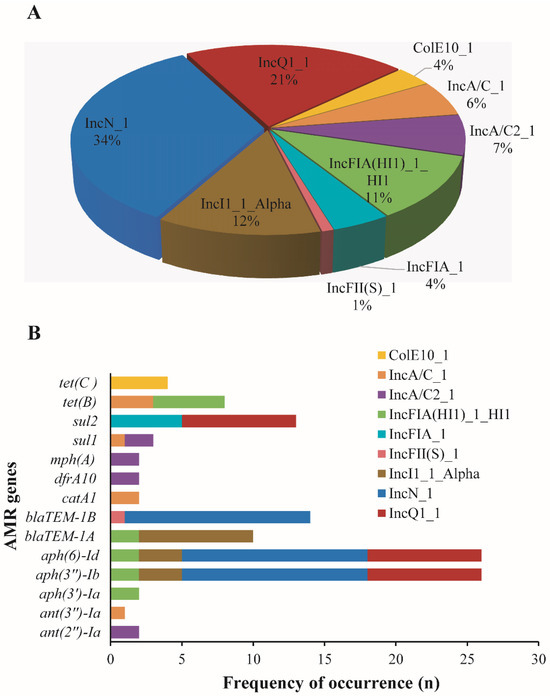
Figure 4.
Plasmids harboring antimicrobial resistance genes in Salmonella isolates. (A): Pie chart highlighting the frequency occurrence of different plasmids (n = 9) associated with antimicrobial resistance (AMR) genes in Salmonella isolates investigated in this study. (B): Bar chart showing the distribution of different AMR genes (n = 14) on the detected plasmids in different Salmonella isolates. Note: These genes were co-located on similar contigs harboring the plasmids.
In these three S. Typhimurium isolates, only the IncA/C_1 plasmid of a 417-nucleotide sequence representing a replication protein was found to be associated with AMR genes. These genes were identified as tetB for ADRDL-S60, catA1, sul1, tet(B), and ant(3″)-Ia for ADRDL-S178 and catA1 and tet(B) for ADRDL-S179. In contrast, two S. Muenster isolates did not harbor any plasmid-mediated genes.
3.6. Characteristics of blaSCO-1 Positive Isolates
Genomic analysis showed that three S. Typhimurium isolates (ADRDL-S60, ADRDL-S178, ADRDL-S179) revealed similar features with 5.2–5.3 Mb genome size, 52% GC content, and 11 antimicrobial resistance (AMR) genes with similar phenotypic characteristics (Figure 5A, Table 1). The S. Muenster isolates (ADRDL-S235, ADRDL-S241) exhibited similar phenotypic and genotypic attributes with a 5.1 Mb genome size and 52% GC content (Figure 5B, Table 1). All isolates carried two copies of blaSCO-1. Phylogenetic analysis revealed three distinct clusters showing 100% nucleotide identity between ADRDL-S178 and ADRDL-S179, and ADRDL-S235 and ADRDL-S241 (Figure 5C).
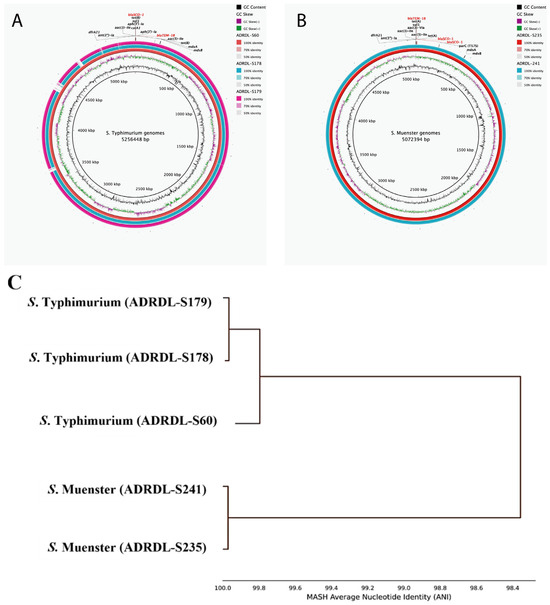
Figure 5.
Genomic characterization of blaSCO-1 -positive Salmonella isolates. (A): Circular genome representation comparing S. Typhimurium genomes (ADRDL-S60, -S178, -S179) that harbored similar AMR genes (n = 11). ADRDL-S60 served as the reference genome (inner ring) and its AMR gene annotation was used for labeling. Each gene was present in two copies except mdsA and mdsB (B): Circular genome representation comparing S. Muenster genomes (ADRDL-S235, -S241) with similar AMR genes (n = 11). ADRDL-S235 served as the reference sequence and its AMR gene annotation was used for labeling. Each gene was present in two copies except mdsA, mdsB, parC(T57S). Legends on the right of each map indicate GC content and GC skew and percentage identity of regions among isolates. The circular maps were generated with BLAST Ring Image Generator (BRIG) software. (https://sourceforge.net/projects/brig/). (C): Phylogenetic tree showing the average nucleotide identity of all the Salmonella isolates (n = 5), constructed by MASH clustering to determine percentage similarities among isolates. This was calculated using the FastANI pipeline version 1.34 (https://github.com/ParBLiSS/FastANI).
Amino acid analysis revealed that blaSCO-1 gene comprised 288 amino acids with a conserved penicillin-binding protein transpeptidase domain. In ADRDL-S178, ADRDL-S179, and ADRDL-S60, one gene copy spanned nucleotides 14,692 to 15,558 and the other from 9831 to 10,697 (except in ADRDL-S178, where it was 1862 to 2728). The ADRDL-S60 isolate had distinct coordinates of 1662 to 2528 and 1703 to 2569.
3.7. Comparison of the Genetic Environment of blaSCO-1 Gene
First of all, the blaSCO-1 gene in all these five isolates was not associated with any plasmid. The BLASTP analysis with the gene sequence blaSCO-1 showed a 100% identity with proteins found in isolates with the accession numbers EF104648.1, EF063111.1, and AM939421.1. Furthermore, we comparatively investigated the genetic environment of our isolates and other blaSCO-1 positive isolates (Figure 6A). Upstream of all copies of the blaSCO-1 gene in our isolates was occupied by a gene encoding the cellulase family glycosyl hydrolase, comprising 399 amino acids (aa), situated 161 base pairs away from the gene in opposite orientation, while the serine recombinase family protein gene of varying nucleotide lengths (108–546 nucleotides) was located 29 base pairs away from the blaSCO-1 gene, also in the opposite orientation. Notably, the shortest copy of this gene encoded a specific transposon DNA-invertase of 35 amino acids and was found in the ADRDL-S178 isolate (Figure 6A). Comparison with the identified isolates from NCBI showed some similarities upstream and variations downstream of the blaSCO-1 gene (Figure 6B).
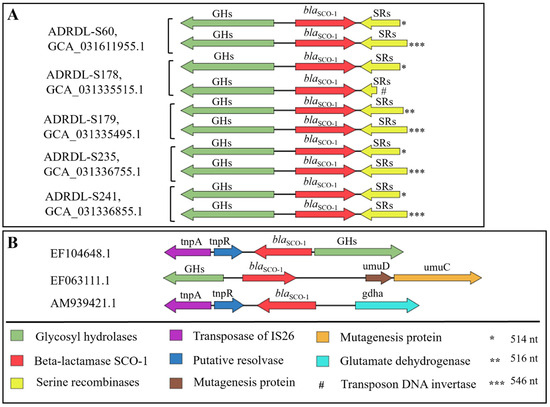
Figure 6.
Comparison of the genetic environment of blaSCO-1 gene. (A): Genetic environment of copies of blaSCO-1 gene (n = 2) found in five Salmonella isolates in this study. (B): The genetic environment of other isolates was identified to have harbored blaSCO-1 gene from NCBI database. The direction of the arrows indicates the orientation of the genes, while the length is a representation of their open reading frames (ORFs) All isolates were identified using their accession numbers from NCBI. Asterisks were used to show the varying nucleotide (nt) length of the serine recombinase sequence. Note: Sequence lengths and distances are not drawn to scale.
4. Discussion
The occurrence of antimicrobial resistance in Salmonella poses a significant threat to public health, despite efforts in recent decades to decrease the use of antibiotics [31,32]. The initiation of the National Antimicrobial Resistance Monitoring Program (NARMS) in 1996 marked the beginning of systematic surveillance for antimicrobial resistance in zoonotic enteric pathogens [33]. However, potential gaps in antimicrobial resistance data since its establishment highlight the need for ongoing and retrospective surveillance in animal populations. A wide range of domestic and wild animals can serve as reservoirs for Salmonella, thereby facilitating the dissemination of this pathogen to other animals, environments, and humans [32]. Our retrospective analysis of Salmonella isolates recovered from 1982 to 1999 fills a crucial temporal gap, offering insights into resistance trends before NARMS. This historical dataset is a valuable reference for comparing and comprehending recent antimicrobial surveillance data.
The observed phenotypic resistance against the tested antimicrobials showed the highest resistance against ampicillin, which is consistent with previous retrospective reports [34,35]. Ampicillin resistance was initially detected in 1949 but became a significant problem in the early 1980s due to its use in treating salmonellosis, resulting in a notable increase in ampicillin resistance between the 1980s and 2000s [34,35,36]. This shift led to changes in the clinical approach towards the utilization of extended spectrum cephalosporins and quinolones [35]. Our analysis indicated low to no resistance to these antimicrobials. However, recent documentation has shown increasing resistance against both classes of antimicrobials among Salmonella serovars recovered from animal and human sources [37,38]. Similarly, high resistance against doxycycline following ampicillin was expected due to its extensive use in animals, owing to its low toxicity and affordability, as reported by Galarce et al. [39].
Serovar-specific variations in resistance frequency were noted in this study, with S. Typhimurium displaying the highest resistance among serovars, predominantly distributed in animal hosts. This observation aligns with previous findings that S. Typhimurium consistently exhibits greater antimicrobial resistance compared to other common serovars [17,40]. Notably, most of the antimicrobial resistance serovars were recovered from bovine sources, particularly S. Typhimurium and S. Dublin, which corresponds with previous reports and emphasizes their significance in bovines due to both their zoonotic potential and clinical impact in cattle herds [17,41]. Similarly, in this study, S. Typhimurium from equine sources exhibited a high resistance, a trend supported by Spier et al. [42], who documented a high fatality rate of horses associated with S. Typhimurium.
In this study, we evaluated the correlation between phenotypic and genotypic resistance, assessing the predictive capability of WGS [43]. Almost perfect to perfect agreement (k = 0.93–1) for ampicillin, doxycycline, chloramphenicol, gentamicin, and trimethoprim/sulfamethoxazole suggests WGS as a reliable predictor. Moderate to substantial agreement (k = 0.59–1) with cephalosporins indicates minor discrepancies. Slight agreement (k = 0.11) for amoxicillin/clavulanic acid may result from clavulanic acid inhibiting beta-lactamase [8]. A false positive was observed in nitrofurantoin, possibly due to unknown resistance mechanisms such as the expression of efflux pumps [44,45]. Conversely, a false negative occurred with quinolone-resistant genes (gyrA(S83F), parC(T57S)), indicating phenotypic susceptibility despite the associated genotype, suggesting silenced resistance genes [43].
A significant finding of our study was the identification of blaSCO-1, an uncommon gene, in S. Typhimurium and S. Muenster obtained from equine sources between 1989 and 1999, marking the first report of such occurrence in the United States. blaSCO-1, characterized as a class A beta-lactamase in Escherichia coli and Acinetobacter species in Greece and Argentina [46,47], was reported in diverse locations and bacterial strains, including in Salmonella enterica serovar Livingstone, Serratia marcescens, Aeromonas salmonicida, Acinetobacter radioresistens, and Klebsiella pneumoniae in Tunisia, Japan, Switzerland, Antarctica, and Belgium [48,49,50,51,52]. To the best of our knowledge, our recovered isolates harboring this gene as early as 1989 precede those of all other reports.
The blaSCO-1 positive isolates in this study exhibited resistance to amoxicillin/clavulanic acid, potentially attributed to the blaSCO-1 gene, as documented by Ruppé et al. [50]. However, this finding contradicts earlier reports suggesting clavulanic acid’s inhibition of narrow-spectrum hydrolysis towards beta-lactams mediated by blaSCO-1 [46,47]. Nevertheless, we also observed intermediate resistance (MIC = 16 μg/mL) towards amoxicillin/clavulanic acid in two S. Muenster isolates harboring this gene. This implies diverse genetic expression or phenotypic impact across strains or species, challenging a uniform interpretation of blaSCO-1 functional behavior.
The phylogenetic relationship of these five blaSCO-1 positive isolates in this study revealed distinct clustering patterns, indicating that relationships may be more influenced by genome sequence content, and strain type, than by antimicrobial resistance profiles [53].
While prior studies have reported blaSCO-1 as plasmid-mediated [46,47,52,54], the non-plasmid association of this gene in this study prompted a closer examination of its genetic environment. blaSCO-1 occurred as part of a contiguous chromosomal sequence, as indicated by its association with a glycosidase gene upstream, in line with other reports [46,47,50]. The presence of serine recombinases downstream of blaSCO-1 accompanied by transposon DNA invertase could facilitate site-specific DNA rearrangement, potentially enhancing the mobility of this gene [47,50,55,56].
Prior studies have also emphasized the lack of homology across these plasmids due to the absence of the blaSCO-1 gene in highly similar plasmids [46,47,52,54]. This raises questions about the intrinsic or acquired nature of this resistance gene.
It is important to highlight that blaSCO-1 has predominantly been identified in clinical isolates obtained from both human and animal hosts, including isolates associated with nosocomial outbreaks [46,48,49,50]. In our study, the serovars S. Muenster and S. Typhimurium, which harbor this gene, have previously been implicated in human salmonellosis outbreaks originating from zoonotic sources [57,58].
While the in silico PlasmidFinder web tool utilized in this study has been validated to identify known plasmids by searching for conserved replicon sites and comparing them to a curated database of plasmid replicons, it was designed to identify at least 80% nucleotide identity with those currently included in the database and will not adequately cover plasmid diversity outside this scope [25]. Nevertheless, this method was sufficient for other authors who reported blaSCO-1 as plasmid-mediated [52].
Overall, our study contributes to the ongoing discourse on the genomic landscape of antibiotic resistance in Salmonella, providing crucial insights for public health efforts. The complexities surrounding blaSCO-1 identified in this study warrant further investigation to decipher its implications for the evolution of antibiotic resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12030528/s1, Table S1: Frequency of distribution of Salmonella serovars (n = 251) across 11 animal host species; Table S2: Antimicrobial susceptibility profile of Salmonella isolates (n = 251) in this study; Table S3: Antimicrobial resistance in 101 Salmonella isolates across 11 serovars and 11 animal species exhibiting resistance to at least one antimicrobial tested; Table S4: Correlation between phenotype and genotype of Salmonella isolates (n = 251) in this study.
Author Contributions
Conceptualization, C.W.; Methodology, N.V.I., D.R., A.C., J.S., J.N., L.R., S.P., M.Y. and J.G.; Formal analysis, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the USDA Agricultural Research Service Program (58-6040-9-017), and by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award U19FD007117. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
Data Availability Statement
The data presented in this study are openly available online in NCBI data repository at: https://www.ncbi.nlm.nih.gov/bioproject/ (accessed on 25 February 2024) with a BioProject accession number: PRJNA280335. The isolates can be found with identifiers ranging from ADRDL S1—ADRDL S251.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Syed Abu Thahir, S.; Rajendiran, S.; Shaharudin, R.; Veloo, Y. Multidrug-resistant Salmonella species and their mobile genetic elements from poultry farm environments in Malaysia. Antibiotics 2023, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Kijima, M.; Shirakawa, T.; Uchiyama, M.; Kawanishi, M.; Ozawa, M.; Koike, R. Trends in the serovar and antimicrobial resistance in clinical isolates of Salmonella enterica from cattle and pigs between 2002 and 2016 in Japan. J. Appl. Microbiol. 2019, 127, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). CDC Yellow Book: Salmonellosis, Nontyphoidal. 2024. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/salmonellosis-nontyphoidal (accessed on 25 February 2024).
- Eng, S.-K.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Li, C.; Tyson, G.H.; Hsu, C.-H.; Harrison, L.; Strain, E.; Tran, T.-T.; Tillman, G.E.; Dessai, U.; McDermott, P.F.; Zhao, S. Long-read sequencing reveals evolution and acquisition of antimicrobial resistance and virulence genes in Salmonella enterica. Front. Microbiol. 2021, 12, 777817. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Maddamsetti, R.; Weiss, A.; Ha, Y.; Wang, T.; Wang, S.; You, L. Intra- and interpopulation transposition of mobile genetic elements driven by antibiotic selection. Nat. Ecol. Evol. 2022, 6, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Wang, Z.; Huang, C.; Teng, L.; Zhou, H.; An, H.; Liao, S.; Liu, Y.; Huang, L.; Tang, B.; et al. Mobilome-driven partitions of the resistome in Salmonella. mSystems 2023, 8, e0088323. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Vale, A.P.; Shubin, L.; Cummins, J.; Leonard, F.C.; Barry, G. Detection of blaOXA-1, blaTEM-1, and virulence factors in E. coli isolated from seals. Front. Vet. Sci. 2021, 8, 583759. [Google Scholar] [CrossRef]
- Mather, A.E.; Reid, S.W.; Maskell, D.J.; Parkhill, J.; Fookes, M.C.; Harris, S.R.; Brown, D.J.; Coia, J.E.; Mulvey, M.R.; Gilmour, M.W.; et al. Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science 2013, 341, 1514–1517. [Google Scholar] [CrossRef]
- Shivani, C.; Abha, K.; Alka, G.; Sampat, N. Comparative genome analysis of three pathogenic strains of E. coli, Salmonella, and Shigella. Int. J. Sch. Res. Rev. 2015, 4, 68–80. [Google Scholar]
- Katiyar, A.; Sharma, P.; Dahiya, S.; Singh, H.; Kapil, A.; Kaur, P. Genomic profiling of antimicrobial resistance genes in clinical isolates of Salmonella Typhi from patients infected with Typhoid fever in India. Sci. Rep. 2020, 10, 8299. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S. Whole-genome sequencing for detecting antimicrobial resistance in nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515–5520. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zeng, X.; Zhang, P.; Zhang, D.; Wang, C.; Lin, J. Characterization of the emerging multidrug-resistant Salmonella enterica serovar Indiana strains in China. Emerg. Microbes Infect. 2019, 8, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, X.; Ed-Dra, A.; Zhou, X.; Jia, C.; Müller, A.; Liu, Y.; Kehrenberg, C.; Yue, M. Genome-based assessment of antimicrobial resistance and virulence potential of isolates of non-Pullorum/Gallinarum Salmonella serovars recovered from dead poultry in China. Microbiol. Spectr. 2022, 10, e0096522. [Google Scholar] [CrossRef] [PubMed]
- Elbediwi, M.; Tang, Y.; Yue, M. Genomic characterization of ESBL-producing Salmonella Thompson isolates harboring mcr-9 from dead chick embryos in China. Vet. Microbiol. 2023, 278, 109634. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Biswas, S.; Paudyal, N.; Pan, H.; Li, X.; Fang, W.; Yue, M. Antibiotic resistance in Salmonella Typhimurium isolates recovered from the food chain through National Antimicrobial Resistance Monitoring System between 1996 and 2016. Front. Microbiol. 2019, 10, 985. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2023. [Google Scholar]
- National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS). 2019. Available online: https://www.cdc.gov/narms/antibiotics-tested.html (accessed on 25 February 2024).
- IDEXX. Diagnostic Update: Microbiology Guide to Interpreting Minimum Inhibitory Concentration (MIC). 2019. Available online: https://www.idexx.com/files/microbiology-guide-interpreting-mic.pdf (accessed on 25 February 2024).
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.H.; McDermott, P.F.; et al. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob. Agents Chemother. 2019, 63, e00483-19. [Google Scholar] [CrossRef]
- Florensa, A.F.; Kaas, R.S.; Clausen, P.T.L.C.; Aytan-Aktug, D.; Aarestrup, F.M. ResFinder—An open online resource for identification of antimicrobial resistance genes in next-generation sequencing data and prediction of phenotypes from genotypes. Microb. Genom. 2022, 8, 000748. [Google Scholar] [CrossRef]
- Seemann, T. ABRicate: Mass Screening of Contigs for Antimicrobial Resistance Genes. 2016. Available online: https://github.com/tseemann/abricate (accessed on 25 February 2024).
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Khezri, A.; Avershina, E.; Ahmad, R. Plasmid identification and plasmid-mediated antimicrobial gene detection in Norwegian isolates. Microorganisms 2020, 9, 52. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, G. Idostatistics: Cohen’s Kappa Free Calculator. 2022. Available online: https://idostatistics.com/cohen-kappa-free-calculator/ (accessed on 25 February 2024).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Gargano, V.; Gambino, D.; Migliore, S.; Vitale, M.; Sciortino, S.; Costa, A.; Vicari, D. Can human handling increase the presence of multidrug resistance (MDR) in Salmonella spp. isolated from food sources? Microorganisms 2021, 9, 2018. [Google Scholar] [CrossRef] [PubMed]
- Galán-Relaño, Á.; Valero Díaz, A.; Huerta Lorenzo, B.; Gómez-Gascón, L.; Mena Rodríguez, M.Á.; Carrasco Jiménez, E.; Pérez Rodríguez, F.; Astorga Márquez, R.J. Salmonella and salmonellosis: An update on public health implications and control strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, L.; Fedorka-Cray, P.J.; Angulo, F.J. Public health aspects of antibiotic resistance monitoring in the USA. Acta Vet. Scand. Suppl. 1999, 92, 67–75. [Google Scholar] [PubMed]
- Smith, S.M.; Palumbo, P.E.; Edelson, P.J. Salmonella strains resistant to multiple antibiotics: Therapeutic implications. Pediatr. Infect Dis. 1984, 3, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, D.A.; Singh, A.; Zhao, S.; Bartholomew, M.; Womack, N.; Ayers, S.; Fields, P.I.; McDermott, P.F. Antimicrobial resistance in Salmonella in the United States from 1948 to 1995. Antimicrob. Agents Chemother. 2016, 60, 2567–2571. [Google Scholar] [CrossRef]
- Cohen, M.L.; Tauxe, R.V. Drug-resistant Salmonella in the United States: An epidemiologic perspective. Science 1986, 234, 964–969. [Google Scholar] [CrossRef]
- Zamudio, R.; Boerlin, P.; Beyrouthy, R.; Madec, J.Y.; Schwarz, S.; Mulvey, M.R.; Zhanel, G.G.; Cormier, A.; Chalmers, G.; Bonnet, R.; et al. Dynamics of extended-spectrum cephalosporin resistance genes in Escherichia coli from Europe and North America. Nat. Commun. 2022, 13, 7490. [Google Scholar] [CrossRef]
- Yin, X.; Dudley, E.G.; Pinto, C.N.; M’ikanatha, N.M. Fluoroquinolone sales in food animals and quinolone resistance in non-typhoidal Salmonella from retail meats: United States, 2009–2018. J. Glob. Antimicrob. Resist. 2022, 29, 163–167. [Google Scholar] [CrossRef]
- Galarce, N.; Arriagada, G.; Sánchez, F.; Escobar, B.; Miranda, M.; Matus, S.; Vilches, R.; Varela, C.; Zelaya, C.; Peralta, J.; et al. Phenotypic and genotypic antimicrobial resistance in Escherichia coli strains isolated from household dogs in Chile. Front. Vet. Sci. 2023, 10, 1233127. [Google Scholar] [CrossRef]
- Lee, L.A.; Puhr, N.D.; Maloney, E.K.; Bean, N.H.; Tauxe, R.V. Increase in antimicrobial-resistant Salmonella infections in the United States, 1989–1990. J. Infect Dis. 1994, 170, 128–134. [Google Scholar] [CrossRef]
- Otto, S.J.G.; Ponich, K.L.; Cassis, R.; Goertz, C.; Peters, D.; Checkley, S.L. Antimicrobial resistance of bovine Salmonella enterica ssp. enterica isolates from the Alberta Agriculture and Forestry Disease Investigation Program (2006–2014). Can. Vet. J. 2018, 59, 1195–1201. [Google Scholar]
- Spier, S.J. Salmonellosis. Vet. Clin. North Equine Pract. 1993, 9, 385–397. [Google Scholar] [CrossRef]
- Alzahrani, K.O.; AL-Reshoodi, F.M.; Alshdokhi, E.A.; Alhamed, A.S.; Al Hadlaq, M.A.; Mujallad, M.I.; Mukhtar, L.E.; Alsufyani, A.T.; Alajlan, A.A.; Al Rashidy, M.S.; et al. Antimicrobial resistance and genomic characterization of Salmonella enterica isolates from chicken meat. Front. Microbiol. 2023, 14, 1104164. [Google Scholar] [CrossRef]
- Webber, M.A.; Piddock, L.J.V. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, T.J.; Nguyen, S.; El Garach, F.; Miossec, C.; Cuinet, E.; Woehrlé, F.; Fanning, S.; Niedziela, D.A. A study of the correlation between phenotypic antimicrobial susceptibility testing methods and the associated genotypes determined by whole genome sequencing for a collection of Escherichia coli of bovine origin. bioRxiv. 2021. [Google Scholar] [CrossRef]
- Papagiannitsis, C.C.; Loli, A.; Tzouvelekis, L.S.; Tzelepi, E.; Arlet, G.; Miriagou, V. SCO-1, a novel plasmid-mediated class A β-lactamase with carbenicillinase characteristics from Escherichia coli. Antimicrob. Agents Chemother. 2007, 51, 2185–2188. [Google Scholar] [CrossRef][Green Version]
- Poirel, L.; Corvec, S.; Rapoport, M.; Mugnier, P.; Petroni, A.; Pasteran, F.; Faccone, D.; Galas, M.; Drugeon, H.; Cattoir, V.; et al. Identification of the novel narrow-spectrum beta-lactamase SCO-1 in Acinetobacter spp. from Argentina. Antimicrob. Agents Chemother. 2007, 51, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Ktari, S.; Arlet, G.; Verdet, C.; Jaoua, S.; Kachrid, A.; Ben Redjeb, S.; Mahjoubi-Rhimi, F.; Hammami, A. Molecular epidemiology and genetic environment of acquired bla ACC-1 in Salmonella enterica serotype Livingstone causing a large nosocomial outbreak in Tunisia. Microbial Drug Resistance (Larchmont, N.Y.). Microb. Drug Resist. 2009, 15, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wachino, J.-I.; Kimura, K.; Yamada, K.; Arakawa, Y. New plasmid-mediated aminoglycoside 6′-N-acetyltransferase, AAC(6′)-Ian, and ESBL, TLA-3, from a Serratia marcescens clinical isolate. J. Antimicrob. Chemother. 2015, 70, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Ruppé, E.; Cherkaoui, A.; Wagner, N.; La Scala, G.C.; Beaulieu, J.-Y.; Girard, M.; Frey, J.; Lazarevic, V.; Schrenzel, J. In vivo selection of a multidrug-resistant Aeromonas salmonicida during medicinal leech therapy. New Microbes New Infect. 2018, 21, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Opazo-Capurro, A.; Higgins, P.G.; Wille, J.; Seifert, H.; Cigarroa, C.; González-Muñoz, P.; Quezada-Aguiluz, M.; Domínguez-Yévenes, M.; Bello-Toledo, H.; Vergara, L.; et al. Genetic features of Antarctic Acinetobacter radioresistens strain A154 harboring multiple antibiotic-resistance genes. Front. Cell Infect. Microbiol. 2019, 9, 328. [Google Scholar] [CrossRef]
- Debergh, H.; Maex, M.; Garcia-Graells, C.; Boland, C.; Saulmont, M.; Van Hoorde, K.; Saegerman, C. First Belgian report of ertapenem resistance in an ST11 Klebsiella pneumoniae strain isolated from a dog carrying blaSCO-1 and blaDHA-1 combined with permeability defects. Antibiotics 2022, 11, 1253. [Google Scholar] [CrossRef]
- Monte, D.F.; Lincopan, N.; Berman, H.; Cerdeira, L.; Keelara, S.; Thakur, S.; Fedorka-Cray, P.J.; Landgraf, M. Genomic features of high-priority Salmonella enterica serovars circulating in the food production chain, Brazil, 2000–2016. Sci. Rep. 2019, 9, 11058. [Google Scholar] [CrossRef]
- Papagiannitsis, C.C.; Tzouvelekis, L.S.; Kotsakis, S.D.; Tzelepi, E.; Miriagou, V. Sequence of pR3521, an IncB plasmid from Escherichia coli encoding ACC-4, SCO-1, and TEM-1 beta-lactamases. Antimicrob. Agents Chemother. 2011, 55, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.C. Site-specific DNA inversion by serine recombinases. Microbiol. Spectr. 2015, 3, 1–36. [Google Scholar] [CrossRef]
- Nicolas, E.; Lambin, M.; Dandoy, D.; Galloy, C.; Nguyen, N.; Oger, C.A.; Hallet, B. The Tn3-family of replicative transposons. Microbiol. Spectr. 2015, 3, 693–726. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Outbreak of Salmonella Infections Linked to Pet Bearded Dragons. 2020. Available online: https://www.cdc.gov/Salmonella/muenster-10-20/index.html (accessed on 25 February 2024).
- Patel, K.; Stapleton, G.S.; Trevejo, R.T.; Tellier, W.T.; Higa, J.; Adams, J.K.; Hernandez, S.M.; Sanchez, S.; Nemeth, N.M.; Debess, E.E.; et al. Human salmonellosis outbreak linked to Salmonella Typhimurium epidemic in wild songbirds, United States, 2020–2021. Emerg. Infect. Dis. 2023, 29, 2298–2306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).