Abstract
Visceral leishmaniasis (VL) is a chronic systemic disease. In Brazil this infection is caused by Leishmania (Leishmania) infantum. Extracellular vesicles (EVs) released by Leishmania species have different functions like the modulation of host immune systems and inflammatory responses, among others. This study evaluated the participation of EVs from L. (L.) infantum (Leish-EVs) in recognition of the humoral and cellular immune response of hosts with VL. Promastigotes were cultivated in 199 medium and, in the log phase of growth, they were centrifuged, washed, resus-pended in RPMI medium, and incubated for 2 to 24 h, at 25 °C or 37 °C to release Leish-EVs. This dynamic was evaluated using transmission (TEM) and scanning (SEM) electron microscopies, as well as nanoparticle tracking analysis (NTA). The results suggested that parasite penetration in mammal macrophages requires more Leish-EVs than those living in insect vectors, since promastigotes incubated at 37 °C released more Leish-EVs than those incubated at 25 °C. Infected THP-1 cells produced high EV concentration (THP-1 cells-EVs) when compared with those from the control group. The same results were obtained when THP-1 cells were treated with Leish-EVs or a crude Leishmania antigen. These data indicated that host–EV concentrations could be used to distinguish infected from uninfected hosts. THP-1 cells treated with Leish-EVs expressed more IL-12 than control THP-1 cells, but were unable to express IFN-γ. These same cells highly expressed IL-10, which inhibited TNF-α and IL-6. Equally, THP-1 cells treated with Leish-EVs up-expressed miR-21-5p and miR-146a-5p. In conclusion, THP-1 cells treated with Leish-EVs highly expressed miR-21-5p and miR-146a-5p and caused the dysregulation of IL-10. Indirectly, these results suggest that high expression of these miRNAs species is caused by Leish-EVs. Consequently, this molecular via can contribute to immunosuppression causing enhanced immunopathology in infected hosts.
1. Introduction
Visceral leishmaniasis (VL) is caused by protozoans classified in Leishmania (Leishmania) donovani complex. They are transmitted by infected phlebotomine sandflies (Diptera: Psychodidae). VL is by far the most severe form of the leishmaniases, since it compromises internal organs and is highly lethal for untreated hosts. Severe infection affects humans and domestic dogs in peridomestic and domestic transmission foci, and is included in the neglected diseases. If adequate treatment is not initiated in a timely manner, infected hosts progress to death in 90% of cases [1,2,3,4,5,6,7].
Globally, VL is among the top-ten neglected tropical diseases, with more than 12 million people and around 1 billion people at risk of infection [7]. This disease has spread worldwide and, at the present time, is found in the Americas, Africa, Southern Europe, and Asia. The majority of cases occur in India, Bangladesh, Sudan, Nepal, and Brazil [1,3,7,8]. Around 90% of the cases in Latin America occur in Brazil. VL in Brazil is caused by Leishmania (Leishmania) infantum, transmitted by the main vector, Lutzomyia longipalpis [8,9,10,11]. Currently, human and canine infections are present in wild and rural environments as well as in urban centers. Unfortunately, the control strategies against the disease, vectors, and reservoirs of VL have been ineffective [1,2,3,4]
The life cycle of L. (L.) infantum is heteroxenic with two main life forms. Promastigotes, which live in infected female sandflies and are regurgitated during the blood meal, and amastigotes, which live inside the phagocytic cells of the vertebrate host [2,8]. In the beginning of infection, promastigotes interact with a complex extracellular matrix, immune system molecules, and phagocytic tissue cells. Next, macrophages eliminate the phagocytosed parasites by production of microbicidal molecules, and they stimulate the effector immune response to release immunoregulatory and proinflammatory cytokines, such as TNF-α, IL-10, and IL-6 [12,13].
Despite the complex host system, all Leishmania species have several virulence factors that are capable of surviving the macrophage effector response, causing Leishmaniasis pathogenesis [14,15]. In order to guarantee the invasion in the host cells, pathogens release effector molecules through the cellular membrane, and the via extracellular vesicles (EVs) are transported to host cells. These compounds cause the modulation of cytokines and microRNAs (miRNAs) for preparing the cells for parasite infection [16,17,18,19]. In vivo studies, analyzing mice and macrophages, complement these findings, reporting that EVs released by Leishmania (Leish-EVs) can modulate the immune-regulating and signaling pathways [19,20,21,22,23,24].
EVs represent all particle populations. The largest are the apoptotic bodies that arise as blogging from the apoptotic cell membranes. They are phosphatidylserine-rich vesicles with 500–5000 nm in diameter. The microvesicles are particles of 100–1000 nm in diameter that shed from the plasma membrane. They are enriched with phosphatidylserine and cholesterol. The exosomes are the smallest EVs (30–150 nm) and are formed by the exocytosis of multivesicular bodies (MVBs). They are liberated intraluminal vesicles upon fusion with the plasma membrane [25,26,27,28]. Studies have shown that the different cell types use EVs as vehicles for delivery of modulatory proteins, lipids, and nucleic acids to other short- and long-distance cells. In addition, EVs are critical mediators of intercellular communication potentiated organ cross-talk [27,28,29,30,31,32].
miRNAs are small endogenous non-coding RNAs. They are produced by all cells and a part of them is transported by EVs to biological fluids to promote different biological functions. Each miRNA can target hundreds of mRNAs within a given cell type, and a single mRNA is often the target of multiple miRNAs [33,34]. Each molecule contains around 22 nucleotides and post-transcriptionally regulates the expression of several tar-gets [35]. Studies have been shown that miRNAs are promising biomarkers for many hu-man diseases, and they can be investigated through non-invasive methods. Furthermore, miRNAs are able to assess the prognosis of a disease and the response to treatment [36].
Based on these findings, the goal of this study was to evaluate the participation of Leish-EVs, released by L. (L.) infantum, in the recognition of the humoral and cellular immune response of hosts with VL. The investigations described throughout this article in-directly suggest that Leish-EVs trigger in infected hosts a molecular via that can contribute to the immunosuppression and production of cytokines.
2. Methods
2.1. Ethical Statements
This study was performed in accordance with the recommendations of the Ethics Committee for Animal Use of the Adolfo Lutz Institute (Comissão de Ética no Uso de Animais do Instituto Adolfo Lutz, CEUA-IAL) and the National Experimentation Control Council (Conselho Nacional de Controle de Experimentação, CONCEA). Both committees approved this study (Protocol: A-CEUA-003).
2.2. L. (L.) infantum Cultures and Production of Crude Leishmania Antigen (CLA) and Leish-EVs
The protocols were prepared as described before [19]. Promastigotes of L. (L.) infantum (MHOM/BR/1972/LD) were maintained at 25 °C, in 199 medium, pH 7.2 containing fetal bovine serum (FBS) 10%, gentamicin 30 μg/mL, hemin 50 μL/mL, and human urine 50 μL/mL.
For use in experiments, promastigotes were collected from culture medium in the log phase of growth, counted, washed 3 times, and concentrated through centrifugation (3000× g for 10 min) with sterile phosphate-buffered saline (PBS).
For CLA preparation, promastigotes dissolved in sterile in PBS (2 mL) were ruptured in Disruptor (Tissue Lyser, Qiagen, Hilden, Germany) through agitation by 8 cycles of 4 min (50 oscillations/second) with 2 min intervals between cycles and sonicated for 10 min. After certifying the lysis of the parasites through optical microscopy, the CLA was dissolved in 0.3 M NaCl.
For Leish-EVs, promastigotes were concentrated through centrifugation (2000× g for 15 min) and washed (5 times) with sterile PBS. Next, parasites were resuspended in RPMI medium (1 mL) and incubated for 2 h at 25 °C to release vesicles in the culture medium. The mixture, containing promastigotes and Leish-EVs, was filtered (0.22 μm filter) to remove the parasites.
The protein concentrations of Leish-EVs and CLA were estimated using BCA protein kit (Pierce) according to the manufacturer’s instructions and by a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 280 nm. Next, aliquots were treated with 10 μg/mL of a cocktail of protease inhibitors containing, per mL, 20 µM AEBSF (4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride), 10 µM EDTA, 1.3 µM Bestatin, 0.14 µM E-64, 10 nM Leupeptin, and 3 nM Aprotinin (Sigma-Aldrich, St. Louis, MO, USA). Thereafter, each aliquot was stored at –20 °C until analysis.
For immunological reactions, the supernatants containing Leish-EVs were transferred to Ultra-Clear centrifuge tubes (6 mL tube for SW-55 rotor), (Beckman Coulter, Brea, CA, USA) and the volume (6 mL) was completed with filtered PBS. Samples were ultracentrifuged at 100,000× g for 60 min at 25 °C in a Beckman® Coulter L8-80M centrifuge. The pellet containing Leish-EVs was suspended in 100 µL of filtered PBS. For ultrastructural analyses, promastigotes were analyzed in 5 moments with incubation times of T0, T2, T4, T6, and T24 h.
2.3. Ultrastructural Analyses of Promastigotes Releasing Leish-EVs through Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
For ultrastructural analyses in TEM and SEM of promastigotes releasing Leish-EVs, 5 sets of parasites were analyzed based on the incubation time for EV release at temperatures of 25 °C or 37 °C. They were: 0 (in the beginning), 2, 4, 6, and 24 h.
For analysis of Leish-EVs through TEM, supernatants containing Leish-EVs were transferred to Ultra-Clear centrifuge tubes (6 mL tube for SW-55 rotor, Beckman Coulter, Brea, CA, USA), and the volume was completed to 6 mL with filtered PBS. Tubes were ultracentrifuged at 100,000× g for 60 min at 25 °C in a Beckman Coulter L8-80M centrifuge. Next, pellets containing Leish-EVs were suspended in filtered PBS (100 µL).
Promastigotes releasing Leish-EVs or only Leish-EVs were fixed with 2.5% glutaraldehyde, 4% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, for at least 2 h. Then, pellets were rinsed in the same buffer and post-fixed in a solution containing 1% osmium tetroxide, 0.8% ferrocyanide, and 5 mM calcium chloride; washed in 0.1 M sodium cacodylate buffer; dehydrated in graded acetone; and embedded in Epon resin. Ultrathin sections (100 nm) were obtained in a Sorvall ultramicrotome; stained with uranyl acetate and lead citrate; and observed under a Transmission Electron Microscope JEOL (model JEM 1011, Peabody, MA, USA) operating at 80 kV. Images were captured on a charge-coupled device camera (model 785 ES1000W, Gatan, Pleasanton, CA, USA) and the Gatan version 1.6 programs.
For high-resolution SEM analysis, after incubations, 1 × 106/mL promastigotes were washed twice in filtered PBS and adhered on coverslips previously coated with 0.1% aqueous poly-L-lysine (Sigma) for at least 2 h at room temperature. Next, coverslips were fixed in 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.2, containing 0.146 mol/L sucrose and 5 mmol/L CaCl2 for 1 h at room temperature. The parasites were post-fixed in 1% osmium tetroxide (OsO4) for 15 min and then dehydrated in increasing concentration series of ethanol, 7.5%, 15%, 30%, 50%, 70%, 90%, and 100% (v/v), for 15 min in each step. Next, the samples were critical point-dried with CO2 and sputter-coated with a thin layer (2 nm) of platinum. The images were recorded at accelerating voltages of 1 Kv on microscopes equipped with the Field Emission Gun (FEG): a Quattro ESEM (Thermo Fisher, Waltham, MA, USA) or an Auriga High-Resolution SEM (Zeiss, Jena, Germany).
2.4. THP-1 Cell Line and THP-1 Released EVs (THP-1-EVs) Production
The cell line used in experiments was the THP-1 cell line (ATCC nº TIB 202–The Global Bioresource Center, Manassas, VA, USA), a human pro-monocyte cell line derived from an acute monocytic leukemia patient. THP-1 cell line was maintained in culture bottles containing RPMI medium, 20% FBS, 2 mM glutamine, 10% gentamicin, at 37 °C in 5% CO2. For experiments, THP-1 cells (5 × 105 cells/well) were plated, in duplicate, in 24-well plates containing the same medium, stimulated and differentiated by Phorbol 12-Myristate 13-Acetate (PMA) 160 ng/mL, and non-adherent cells were removed after macrophage adhesion.
After the multiplicity of infection (MOI) calculation, macrophages were infected with promastigotes at proportion 5:1. In parallel, THP-1-cells were treated with CLA or Leish-EVs at concentration 20 µg/well.
For each experiment, a normal control was included, which constituted the same macrophage concentration but without infection or treatment. THP-1 cells were incubated at 37 °C for 24 h, in 5% CO2 to release THP-1-EVs. All experiments were performed in duplicate. The supernatants were collected (500 μL/well) without any treatment. Next, macrophages and supernatants were kept at −70 °C until experiments. Subsequently, supernatants were used for determination of THP-1-EV concentrations by NTA, and miRNAs expression. The macrophages were used for cytokine determinations.
2.5. Concentration Determination of Leish-EVs and THP-1-EVs through Nanoparticle Tracking Analysis (NTA)
Each experiment was performed twice to confirm the results. Leish-EVs and THP-1-EVs were evaluated for concentration/mL through nanoparticle tracking analysis (NTA) on the NanoSight NS300 equipment (Malvern- NanoSight™, NTA 3.0), coupled to an automatic sCMOS camera with a wavelength of 532 nm. NanoSight computes size and particle number based on the measured Brownian motion. The coefficient and hydrodynamic radius were determined using the Stokes–Einstein equation and the results were exhibited as particle size distribution. Captures and analyses were performed according to the equipment protocol. Data were shown as the average and standard deviation of three video recordings of 30 s per sample. Since NTA is accurate between particle concentrations in the range of 2 × 107 to 2 × 109/mL, the aliquots (EVs) were diluted before analysis in filtered PBS that was used as a control. Next the relative concentration was calculated according to dilution factor.
2.6. Immunological Investigations
The experiments were carried out to investigate whether Leish-EVs could recognize the humoral response in infected hosts. For this purpose, five serum samples from dogs with canine visceral leishmaniasis (Can-VL) were tested by Immunoblotting and Dot blot, using Leish-EVs as antigen and CLA, as a positive antigen control. Five healthy dogs were used for negative control. The canine sera have been previously tested for Can-VL through ELISA, Fast test, and real-time PCR [19,37].
For immunoblotting, CLA or Leish-EVs (20 µg/mL for both) were solubilized in lysis buffer (2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 60 mM Tris-HCl, pH 6.8, and 0.002% bromophenol blue), boiled, and run in 12% polyacrylamide-SDS gels. After visualization through silver staining, Leish-EVs and CLA proteins were transferred to nitrocellulose membranes, blocked with 5% skim milk-PBS (at room temperature/60 min), and washed with PBS (5 times/5 min). Membranes containing proteins were incubated for 18 h (4 °C) with sera (diluted 1:50 in PBS-5% skimmed milk). After another five washes and drying phases (around 2 h), the membranes were incubated at room temperature, under agitation (2 h) with a peroxidase-conjugated rabbit anti-dog IgG (diluted 1:1500 in 5% skim milk-PBS) and washed again.
For the Dot blots, strips of nitrocellulose membranes (0.45 μm in diameter) were incubated with CLA or Leish-EVs (1.5 μg/mL). After five washes with PBS (5 min), the strips were incubated for 18 h (4 °C) with each serum (diluted 1:50 in 5% skim milk-PBS). After five more washes with PBS (5 min) and drying (at least 2 h), strips were incubated at room temperature, under agitation (2 h) with peroxidase-conjugated rabbit anti-dog IgG (diluted 1:4000 in 5% skim milk-PBS) and washed again.
Bound antibodies from Dot blots and immunoblotting were visualized after treatment with chemiluminescence Western blotting substrate (Pierce ECL Western Solution, Thermo Scientific, Waltham, MA, USA). The images were captured on documenting gel with a chemiluminescence filter (UVITEC, Cleaver Scientific, Rugby, UK) in a Blot Scanner (C-DiGit).
2.7. Cytokine and miRNA Analysis by Gene Expression in Quantitative Real-Time PCR (qPCR)
To evaluate whether Leish-EVs could stimulate the cellular immune response, THP-1 cells (5 × 105 cells/well) were incubated for 24 h with Leish-EVs (20 µg/mL). For each experiment, a normal control was included, which constituted the same cell concentration but without treatment. Prior to the experimental process and handling samples, all materials and working surfaces were cleaned with RNase decontamination solution (RNaseZap—Thermo Fisher) in order to minimize the RNA degradation.
Reactions for cytokine and miRNA expression were performed as described before [38,39]. For cytokine investigation, RNA molecules were extracted from THP-1 cells using RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Isolated RNA molecules were treated with RNA later to remove residual genomic DNA. Next, 10 μL of each sample was reverse-transcribed (RT) using a high-capacity cDNA reverse transcription kit in a Veriti 96-Well Thermal Cycler, according to the manufacturer’s instructions under the following thermal conditions: 10 min at 25 °C, 120 min at 37 °C, followed by 5 min at 85 °C. cDNA samples were stored at −70 °C until use in qPCR. Each qPCR amplification mixture contained 5 μL of 2× TaqMan Universal PCR Master Mix and 0.5 μL TaqMan Gene Expression Assays were mixture with the following genes: Interferon gamma (IFN-γ), Interleukin 10 (IL-10), Interleukin 12 (IL-12), Interleukin 6 (IL-6), Transforming growth factor beta (TGF-β), Tumor necrosis factor-alpha (TNF-α), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Assay IDs are shown in Table 1. Template cDNA (2 µL) and 2.5 µL of RNAse-free water were added to a total volume of 10 µL. Reactions were prepared in duplicate for each sample and all assays. Samples were amplified and detected using a StepOne Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) using the following thermal profile: 2 min, 50 °C, and 95 °C for 10 min, followed by 40 cycles performed at 95 °C for 15 s and 60 °C for 1 min. The results with no amplification in qPCR were repeated at least two times.

Table 1.
Cytokines assayed in this study.
In parallel, miRNA expression profiles were assessed in supernatants of the same samples using the miRNeasy Serum/Plasma Kit (Qiagen, Valencia, CA, USA) and miRNAs were eluted into 30 µL of nuclease-free water. The normalization of miRNA expression started in miRNA extractions, since each sample was spiked with 5 µL (25 fmol) of the spike-in control (Caenorhabditis elegans miRNA, the synthetic molecule cel-miR-39, Ambion). Therefore, cel-miR-39-3p was chosen as an exogenous gene. The success of the extractions was confirmed through qPCR amplification of the exogenous miRNA. For cDNA synthesis, 2 μL of total RNA was used in an RT-PCR using TaqMan Advanced miRNA cDNA synthesis kit (Applied Biosystems). RT was performed according to manufacturer’s instructions, in four steps, under the following thermal conditions: 45 min at 37 °C, 10 min at 65 °C for poly (A) tailing reaction; 60 min at 16 °C for ligation reaction; 15 min at 42 °C, 5 min at 85 °C for reverse transcription reaction; 5 min at 95 °C, followed by 14 cycles at 95 °C for 3 s and 60 °C for 30 s; stop reaction at 99 °C for 10 min for miR-Amp reaction. After cDNA synthesis, samples were diluted 1:10 according to the manufacturer’s instructions. qPCR was performed in a customized assay produced by Applied Biosystems. Each qPCR amplification mix contained TaqMan Fast Advanced Master Mix (5 µL), cDNA (2.5 µL), RNAse-free water (2 µL), and TaqMan Advanced miRNA Assays (0.5 µL) for each gene: miR-21-5p, miR-146a-5p, miR-125b-5p, and miR-144-3p. Table 2 describes, in detail, the characterization of each miRNA species. Reactions were performed in duplicate in a final volume of 10 µL and included a negative control (amplification mix only). The qPCRs were performed in the StepOne Real-Time PCR Systems equipment (Applied Biosystems) using the cycling program in Fast mode: one cycle of 95 °C at 20 s, 40 cycles of 95 C at 1 s, and 60 °C for 20 s.

Table 2.
miRNAs species assayed in this study.
2.8. Data Analysis
Statistical analyses and graphics were performed using Graph Pad Prism software version 8.4.3 (San Diego, CA, USA). Comparisons of concentration and size of Leish-EVs and THP-1-EVs were determined through an unpaired one-tailed test based on a critical value of p ≤ 0.05. Values are presented as mean ± SEM (standard error of the mean). The indicated data were representative of at least two individual experiments.
For cytokine and miRNA expressions, the data were based in amplification plot, which represented the cycle threshold (CT) values for each sample. The values of expression (cytokines and miRNAs) are showed as relative quantification (RQ) and were calculated through the comparative CT method (2−ΔΔCT) as described before [40]. THP-1 cells without infection or treatment (control) were considerate calibrators, which values were always 1.0 according to the comparative CT method. The use of a calibrator is recommended to describe how many times the studied gene is expressed (more or less than the calibrator).
In order to carry out this formula, the variation in cDNA concentration between samples (ΔCT value) was corrected by subtracting the CT value obtained from the exogenous gene (cel-miR-39-3p) or gene endogenous for cytokines (GAPDH). Next, ΔCT values from the tested samples were subtracted from the ΔCT value of the calibrator.
3. Results
The initial experiments were performed to define the ideal in vitro conditions for production of Leish-EVs and THP-1-EVs: promastigote number for each cell (MOI), ideal temperature, incubation time, and transformation of THP-1 monocytes in macrophages.
3.1. Biological Characteristics of Leish-EVs Released by L. (L.) infantum
The aim of these experiments was to investigate how L. (L.) infantum promastigotes release EVs over time in different environments. Figure 1 shows images captured by TEM and SEM of promastigotes releasing Leish-EVs, at 25 °C (vector temperature), in five periods. Figure 1A–F show promastigotes at the onset of Leish-EV releasing (T = 0). The images show the Leish-EVs inside the promastigotes and preparing to be released by the plasma membrane. After 2 h (Figure 1B,G), 4 h (Figure 1C,H), 6 h (Figure 1D,I), and 24 h (Figure 1E,J) incubation, it was possible to note different moments of Leish-EV release through the plasma membrane and those already released to the external environment. Figure 2 also shows images captured through TEM and SEM in the same five periods of promastigotes releasing Leish-EVs, but at 37 °C (mammal body temperature). Despite the fact that these images do not clearly show that promastigotes released more Leish-EVs than those promastigotes from Figure 1, in the NTA results (concentration of Leish-EVs/mL) allow us to note that promastigotes incubated at 37 °C released more Leish-EVs than promastigotes incubated at 25 °C (Figure 3A). However, the mean sizes of Leish-EVs were similar in both incubation periods and the majority of them had a size which was compatible with that of microvesicles (Figure 3B).
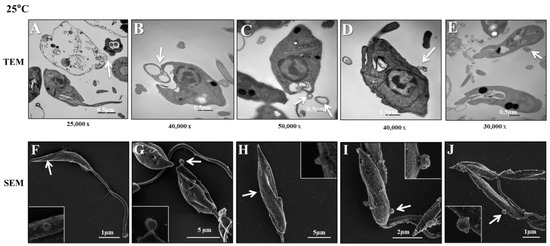
Figure 1.
L. (L.) infantum releasing Leish-EVs by membrane surface at 25 °C. (A–E) Images captured through TEM and (F–J) SEM of promastigotes shedding Leish-EVs by membranes in the beginning of incubation in culture medium at 25 °C (A,F): after 2 h (B,G), 4 h (C,H), 6 h (D,I), and 24 h (E,J). The arrows indicate the Leish-EVs. Bars = 2 µm (F–J).

Figure 2.
L. (L.) infantum releasing Leish-EVs by membrane surface at 37 °C. (A–E) Images captured through TEM and (F–J) SEM of promastigotes shedding Leish-EVs by membranes in the beginning of incubation in culture medium at 37 °C (A,F): after 2 h (B,G), 4 h (C,H), 6 h (D,I), and 24 h (E,J). The arrows indicate the Leish-EVs. Bars = 2 µm (F–J).

Figure 3.
Biological characteristics of Leish-EVs. Distribution of concentration/mL (A) and size (nm) (B) of Leish-EVs released by 1 × 108 promastigotes incubated in culture medium at 25 °C or 37 °C for 2, 4, 6, and 24 h. The data represent three reads per aliquot and each experiment was performed at least 3 times. TEM image of Leish-EVs released by 1 × 108 promastigotes incubated in culture medium at 25 °C for 2 h with size compatible with microvesicles (C).
Promastigotes incubated in RPMI medium at 25 °C for 2, 4, 6, and 24 h released the mean concentrations (and sizes) of 2.54 × 108 (150.8 nm), 7.15 × 108 (195.9 nm), 9.45 × 108 (205.5 nm), and 2.45 × 109 (142.3 nm) Leish-EVs/mL, respectively. For those incubated at 37 °C, the mean concentrations (and sizes) were 1.75 × 109 (142.3), 6.71 × 109 (170.5), 1.71 × 1010 (198.4 nm), and 1.71 × 1010 (150.8 nm) Leish-EVs/mL, respectively.
3.2. THP-1-EVs Releasing Was Stimulated by L. (L.) infantum Antigens and Leish-EVs
The intention of these experiments was to define whether non-infected and infected macrophages with promastigotes can release the same amount of THP-1-EVs. For this purpose, THP-1 cells were infected with promastigotes at proportion 5:1 and incubated for 6 and 24 h. Next, the concentrations of THP-1-EVs in the supernatants were analyzed using NTA. The values represent the means of three reads in NanoSight equipment and the mean concentration ± SEM (standard error of the mean) of each aliquot, prepared in duplicate. The comparison between aliquots was statistically determined through unpaired, one-tailed t-tests. As shown in Figure 4, the mean concentration released by control aliquot (THP-1) was 1.6 × 109 ± 1.9 × 108 THP-1-EVs/mL. The mean concentration released by THP-1 aliquot, incubated with promastigotes for 6 h, increased to 2.7 × 109 ± 9.5 × 107 THP-1-EVs/mL. THP-1 cells incubated with promastigotes for 24 h released 3.8 × 109 ± 1.5 × 108 THP-1-EVs/mL. The THP-1 cells without infection (control) released, during 24 h, 1.8 × 109 ± 3.8 × 108 THP-1-EVs/mL. The increase in THP-1-EV production in aliquots from infected THP-1 cells (incubations with 6 and 24 h) compared with control aliquots was statistically different at * p < 0.018 and * p < 0.042, respectively.
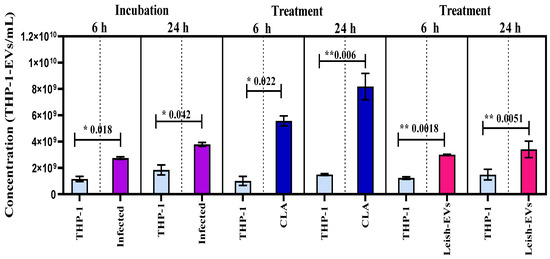
Figure 4.
Stimulation of THP-1 cells for releasing EVs. Mean concentration of THP-1-EVs in supernatants analyzed through NTA after incubations during 6 h and 24 h (purple bars) with promastigotes; after CLA treatment (20 µg/well) (dark blue bars); and Leish-EVs (20 µg/well) (dark pink bars). THP-1 cells received only culture medium in controls (light blue bars). The data represent three reads per sample.
In parallel, THP-1 cells were treated with CLA (20 µg/well) and were incubated for 6 or 24 h. This experiment was performed to understand whether the excess of EVs was released by promastigotes or not. The mean concentration released by control aliquot and incubated for 6 h was 1.0 × 109 ± 3.4 × 108 THP-1-EVs/mL. The mean concentration released by THP-1 aliquots treated with CLA increased to 5.6 × 109 ± 3.8 × 108 THP-1-EVs/mL. THP-1 cells incubated at 24 h released 1.5 × 109 ± 7.0 × 107 THP-1-EVs/mL (control). Those treated with CLA and incubated for 24 h released 8.2 × 109 ± 1.0 × 109 THP-1-EVs/mL. The increase in THP-1-EV production compared with both control aliquots was statistically different at * p < 0.022 and ** p < 0.006, respectively.
Next, THP-1 cells were treated with Leish-EVs at a concentration of 20 µg/well and incubated for 6 h or 24 h. The mean concentration released by the control aliquot incubated for 6 h was 1.2 × 109 ± 9.0 × 107 THP-1-EVs/mL. The mean concentration of THP-1 aliquots treated with Leish-EVs was 3.1 × 109 ± 2.9 × 107 THP-1-EVs/mL. For those without treatment (controls) incubated for 24 h, the mean concentration was 1.5 × 109 ± 4.0 × 108 THP-1-EVs/mL. In THP-1 aliquots treated with Leish-EVs, the mean concentration was 3.4 × 109 ± 6.2 × 108 THP-1-EVs/mL. The increase in THP-1-EV production compared with both control aliquots was statistically different at ** p < 0.018 and ** p < 0.051, respectively.
3.3. Immunological Experiments
The aim of the immunological assays was to evaluate whether Leish-EVs were recognized by sera from infected hosts. The assays were performed using Leish-EVs as antigen. CLA was used as a positive antigen control. Figure 5A shows the electrophoretic profile Leish-EVs on 12% SDS-PAGE after silver nitrate staining (strip 1). Next, Leish-EVs were analyzed as antigen in immunoblot using 2 canine sera. One was positive (strip 2) and the other was negative (strip 3) for Can-VL. As positive antigen control, CLA was used for the same sera for Leish-EVs, strips 4 and 5, respectively. Leish-EVs and CLA were recognized by the positive sera (strips 2 and 4) and were not recognized by negative sera (strips 3 and 5).

Figure 5.
Recognition of Leish-EVs by canine sera. (A) Electrophoretic analysis of Leish-EVs on 12% SDS-PAGE stained with silver nitrate (strip 1). Immunoblotting using Leish-EVs as antigen against a positive (strip 2) and negative (strip 3) canine sera for Can-LV. CLA, as positive antigen control, was used against the same sera used for Leish-EVs. Positive (strip 4) and negative (strip 5) sera. (B) Dot blot using Leish-EVs as antigen against five positive and five negative sera for Can-LV. CLA was used as positive antigen control.
Equally, Figure 5B shows the same results of Dot blot using Leish-EVs as antigen and five positive and five negative canine sera for Can-VL. CLA was used as the antigen control.
3.4. Leish-EVs Stimulated the THP-1 Cells to Produce Cytokines and miRNAs
The results of the gene expression of cytokines produced by THP-1 cells after treatment with Leish-EVs are shown in Figure 6A.

Figure 6.
Interaction of THP-1 cells and Leish-EVs. Relative quantifications of the cytokines IL-10, IL-12, TGF-β, TNF-α, IL-6 (A), and the miRNAs species miR-21-5p, miR-146a-5p, miR-125b-5p, and miR-144-3p (B) in THP-1 cells treated with Leish-EVs. Values are expressed as mean ± SEM of relative quantification after calculation through the comparative CT method (2−∆∆CT) as described in the Section 2.
IL-10 and IL-12 were expressed in the THP-1 cells treated with Leish-EVs by around 7 and 4 times more than the THP-1 cells without treatment (control), respectively. TGF-β, TNF-α and IL-6 also were up-expressed, although with low intensity. The values were 1.51, 1.20, and 1.11 times more expressed than controls (THP-1 cells without treatment), respectively. IFN-γ was not expressed in the tested samples.
The relative quantifications of the four miRNA species (miR-21-5p, miR-146a-5p, miR-125b-5p, miR-144-3p) were estimated from miRNAs purified from THP-1 cells (5 × 105 cells/well) treated with Leish-EVs (20 µg) for 24 h. THP-1 cells without Leish-EV treatment were considered calibrators with a fixed value of 1.0. Thus, the values of treated cells inform us of how many times they were more expressed. miR-21-5p and miR-146a-5p were up-expressed in THP-1 cells treated with Leish-EVs (RQ means of 6.97 and 2.73, respectively). These values represent around 7 and 3 times more expression than the THP-1 cells without treatment (control), respectively. miR-125b-5p and miR-144-3p were slightly expressed, with RQ mean of 1.40 and 0.42, respectively. Figure 6B shows the RQ mean of each miRNA species.
4. Discussion
Diverse studies related with EVs and pathogenic protozoa interactions have shown that these nanoparticles play an important role in parasite survival, facilitating their infection in experimental models [41] and adapting the parasite to the host environment [42]. EVs carry virulence factors that impact the modulation of the cellular immune response, as has been shown in Leishmania species, such as L. (L.) mexicana [24], L. (L.) major [43], L. (L.) amazonensis [33], and L. (L.) donovani [34].
Similar to other Leishmania species, the images captured through electronic microscopy and NTA analyses suggest that L. (L.) infantum promastigotes released more Leish-EVs at 37 °C than those incubated at 25 °C. These results suggest that parasite penetration in mammal macrophages require more Leish-EV than those living in insect vectors. Probably, the virulence factors carried by Leish-EVs contribute to parasite adaptation and maintenance in host cells. These data are in concordance with previous studies [20,35]. Regarding Leish-EVs, the structures shown in NTA and TEM had the size and shape characteristics of microvesicles.
The macrophages play a dual role during infections caused by the different Leishmania species. They are responsible for internalizing and destroying parasites, and, at the same time, macrophages are safe places for promastigote multiplication. Thus, these cells are the key to the failure or success of the infection [44].
The experiments using THP-1 cells were performed to understand the relationship between the release of macrophages L. (L.) infantum and THP-1-EV during infection. The first investigations revealed that THP-1 cells infected with promastigotes were capable of producing an increase in the release of THP-1-EVs. The results demonstrated an increase in the concentrations of THP-1-EVs released from 6 to 24 h. Interestingly, THP-1 cells treated with the CLA or Leish-EVs had the same results. Similar results were observed in EVs from bone marrow in L. (L.) amazonensis [45]. The comparison between groups (control versus infected) were statistically different. These data indicate that the concentration of EVs may be able to distinguish infected from uninfected hosts [19].
The immunological analyses were performed to investigate the ability of the immune system from infected hosts to recognize the Leish-EVs. The immunoblots and Dot blots used in this study indicated a high capacity of Leish-EVs to be used as biomarkers in serological diagnosis of the Can-VL. Similar results have been seen in patients with VL (manuscript in preparation). These data confirm the findings of our previous study [19], in which we showed that Leish-EVs were highly antigenic for infected hosts (dogs with Can-VL).
In natural Can-VL infection, the protective cellular immunity is related to increase in Th1 mediated by IFN-γ and TNF-α. Thus, the macrophage activation and cytotoxic T lymphocytes exhibit apparent resistance to infection. In vitro studies have demonstrated that IFN-γ plays an essential role in combating infectious diseases. Induction of IFN-γ secretion by T and natural killer cells through synergistic stimulation with IL-12 and IL-18 in the adaptive immune responses against pathogens is well established. In the same way, naturally activated macrophages with treatment with IL-12 and IL-18 immediately secreted IFN-γ [46].
In symptomatic infection, the roles of IL-4 and IL-10 advance, reducing the effects of Th1 cytokines. In this case, the production of nitric oxide in macrophages decreases and prevents the destruction of the parasite [44]. Here, THP-1 cells treated with Leish-EVs expressed four times more IL-12 than the THP-1 cells without treatment, but they were unable to produce IFN-γ.
Leish-EVs are released to extracellular cargo, targeting the host cells; consequently, they cause immunosuppression in these cells to facilitate the parasite invasion [47].
IL-10 is an anti-inflammatory cytokine that prevents damage to the host. However, the dysregulation of IL-10 is associated with enhanced immunopathology in response to infection as well as increased risk for development of autoimmune diseases [48]. A previous study demonstrated that patients with VL had high IL-10 production that blocked the production of IL-6 and TNF α [49]. THP-1 cells treated with Leish-EVs expressed around four times more IL-12 than normal THP-1 cells, but they were unable to produce IFN-γ. However, these same cells highly expressed IL-10; this expression was around seven times higher than that of normal macrophages. At the same time, they inhibited TNF-α and IL-6. Although TGF-β had low expression, it cooperated with IL-10 in causing immunosuppression [44,45,46,47].
One of the functions of miRNAs is to directly activate cytokine production, regulating functions correlated with cellular immune responses [50,51,52,53]. Thus, the expression of miRNAs was analyzed to determine whether Leish-EVs could stimulate the expression of some miRNAs in macrophages. miR-21-5p and miR-146a-5p were up-expressed in THP-1 cells treated with Leish-EVs. Previous studies demonstrated that up-expression of miR-21-5p in tissue samples from dogs with Can-VL promoted an anti-inflammatory reaction, reducing the Th1 immune response among T cells and phagocytes [54]. In humans, the up-expression of miR-21-5p caused an imbalance between Th1 and Th2 responses [55,56,57,58]. Equally, miR-146a-5p contributed to the continuous suppression of the Th1 response, inducing cytokine expression, IL-17, and IL-2 [59,60]. After L. (L.) infantum infection with clinical evolution, dogs were found to produce high Leish-EVs concentrations in blood and high expression of miR21-5p and miR146a-5p [19]. These studies suggest that miR-21-5p and miR-146a-5p can cooperate in the suppression of the protective Th1 response in VL [19,54,55,56,57,58,59,60].
In conclusion, these findings, including this study, originated from in vitro and in vivo analyses and the results are concordant. Here, the THP-1 cells treated with Leish-EVs showed high expression of miR-21-5p and miR-146a-5p and caused the dysregulation of IL-10. Indirectly, these results can suggest that high expression of these miRNAs species is caused by the presence of Leish-EVs. Consequently, this molecular via can contribute to immunosuppression and dysregulation of IL-10, causing enhanced immunopathology in infected hosts.
Author Contributions
V.L.P.-C. coordinated the experiments and designed the study. V.L.P.-C., F.M.C. and A.B.d.C. wrote the manuscript. R.G. and R.M.H. performed the serological and molecular diagnoses for visceral leishmaniasis. R.M.H. performed the selection of dog samples. V.L.P.-C., A.B.d.C., F.M.C. and M.M.M. performed EVs isolation and particle quantifications in NanoSight-NTA, SDS-PAGE, and immunoblot. F.M.C. and I.d.S.P. performed the genic expression of cytokines and miRNAs. N.N.T. and G.M.N. performed experiments using transmission electron microscopy. R.M.M., V.M. and B.V. performed the experiments using high-resolution scanning electron microscopy. All authors contributed substantially to the data and to the manuscript. All authors contributed substantially to the data and to the manuscript, approved the final version submitted, published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the FAPESP (Fundação de Amparo à Pesquisa do Estado de Sao Paulo, Brazil) 2021/02217-3. VLP-C was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) 303566/2021-3. ABC, FMC, and MMM were supported by scholarship from CAPES (Coordination for the Improvement of Higher Education Personnel) Process SCBA nº 88881.689557/2022-0, Program PROAP/ CAPES—AUXPE nº 115/2022.
Data Availability Statement
Data supporting reported results can be requested from the authors.
Acknowledgments
We thank the Graduate Program in Science, Coordinator for Disease Control, Ministry of Health (PPG-CCD-SES/SP), and Coordination for the Improvement of Higher Education Personnel (CAPES). Furthermore, the authors would like to thank the teams from the Rudolf Barth Electron Microscopy Platform (Oswaldo Cruz Institute, Fiocruz, Brazil) and the Advanced Microscopy Unit of the National Center for Structural Biology and Bioimaging (CENABIO, UFRJ) for their technical support.
Conflicts of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript. There are no financial relationships that could be construed as potential conflicts of interest.
References
- World Health Organization. Leishmaniasis. 2022. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab (accessed on 2 October 2023).
- Matsumoto, P.S.S.; Taniguchi, H.H.; Pereira, V.B.R.; Hiramoto, R.M.; Seviero Rampazzi, K.L.; de Raeffray Barbosa, J.E.; Puci Neto, R.A.; Camprigher, V.M.; de Barros Cortez, L.R.P.; Rahaman, K.R.; et al. Efficacies of insecticide dog collars against visceral leishmaniasis in low and high-income areas and the effects for non-collared neighbor dogs. Acta Trop. 2022, 235, 106626. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Yactayo, S.; Bern, C. Leishmaniasis and poverty. Trends Parasitol. 2006, 22, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet. Parasitol. 2007, 149, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Best practices for preventing vector-borne diseases in dogs and humans. Trends Parasitol. 2016, 32, 43–55. [Google Scholar] [CrossRef] [PubMed]
- PAHO–Pan American Health Organization. Visceral Leishmaniasis. 2022. Available online: https://www.paho.org/en/topics/leishmaniasis/visceral-leishmaniasis; https://www.paho.org/en/topics/leishmaniasis (accessed on 10 October 2023).
- Grimaldi, G., Jr.; Tesh, R.B. Leishmaniases of the New World: Current concepts and implications for future research. Clin. Microbiol. Rev. 1993, 6, 230–250. [Google Scholar] [CrossRef] [PubMed]
- Degrave, W.; Fernandes, O.; Campbell, D.; Bozza, M.; Lopes, U. Use of molecular probes and PCR for detection and typing of Leishmania-a mini-review. Mem. Inst. Oswaldo Cruz 1994, 89, 463–469. [Google Scholar] [CrossRef]
- Lainson, R.; Shaw, J.J. New world leishmaniasis. The neotropical Leishmania species. In Microbiology and Microbial Infectious Diseases; Collier, L., Balows, A., Sussman, M., Eds.; Topley & Wilson’s 9th: London, UK, 1998; Volume 5. [Google Scholar]
- Lainson, R.; Rangel, E.F. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: A review. Mem. Inst. Oswaldo Cruz 2005, 100, 811–827. [Google Scholar] [CrossRef]
- de Menezes, J.P.; Saraiva, E.M.; da Rocha-Azevedo, B. The site of the bite: Leishmania interaction with macrophages, neutrophilsand the extracellular matrix in the dermis. Parasit. Vectors 2016, 9, 264. [Google Scholar] [CrossRef]
- Costa-da-Silva, A.C.; Nascimento, D.O.; Ferreira, J.R.M.; Guimarães-Pinto, K.; Freire-de-Lima, L.; Morrot, A.; Decote-Ricardo, D.; Filardy, A.A.; Freire-de-Lima, C.G. Immune Responses in Leishmaniasis: An Overview. Trop. Med. Infect. Dis. 2022, 7, 5. [Google Scholar] [CrossRef]
- Orikaza, C.M.; Pessoa, C.C.; Paladino, F.V.; Florentino, P.T.V.; Barbiéri, C.L.; Goto, H.; Ramos-Sanchez, E.M.; Franco da Silveira, J.; Rabinovitch, M.; Mortara, R.A.; et al. Dual Host-Intracellular Parasite Transcriptome of Enucleated Cells Hosting Leishmania amazonensis: Control of half-life of host cell transcripts by the parasite. Infect. Immun. 2020, 88, 11. [Google Scholar] [CrossRef] [PubMed]
- Atayde, V.D.; Hassani, K.; da Silva Lira Filho, A.; Borges, A.R.; Adhikari, A.; Martel, C.; Olivier, M. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell. Immunol. 2016, 309, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Filho, A.L.; Olivier, M. Modulation of Host-Pathogen Communication by Extracellular Vesicles (EVs) of the Protozoan Parasite Leishmania. Front. Cell. Infect. Microbiol. 2019, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Clos, J.; de’Oliveira, C.C.; Shirvani, O.; Fang, Y.; Wang, C.; Foster, L.J.; Reiner, N.E. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci. 2010, 123, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Wagner, V.; Minguez-Menendez, A.; Fernandez-Prada, C.; Olivier, M. Extracellular vesicles and leishmaniasis: Current knowledge and promising avenues for future development. Mol. Immunol. 2021, 135, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.B.; Carneiro, F.M.; Maia, M.M.; Pereira, I.S.; Taniwaki, N.N.; Namiyama, G.M.; Gava, R.; Hiramoto, R.M.; Pereira-Chioccola, V.L. Dogs with canine visceral leishmaniasis have a boost of extracellular vesicles and miR-21-5p up-expression. Parasite Immunol. 2023, 45, e13004. [Google Scholar] [CrossRef]
- Hassani, K.; Antoniak, E.; Jardim, A.; Olivier, M. Temperature-induced protein secretion by Leishmania mexicana modulates macrophage signaling and function. PLoS ONE 2011, 3, e18724. [Google Scholar] [CrossRef]
- Zauli, R.C.; de Souza Perez, I.C.; de Morais, A.C.C.; Ciaccio, A.C.; Vidal, A.S.; Soares, R.P.; Torrecilhas, A.C.; Batista, W.L.; Xander, P. Extracellular Vesicles Released by Leishmania (Leishmania) amazonensis Promastigotes with Distinct Virulence Profile Differently Modulate the Macrophage Functions. Microorganisms 2023, 11, 2973. [Google Scholar] [CrossRef]
- Vasconcelos, C.I.; Cronemberger-Andrade, A.; Souza-Melo, N.; Maricato, J.T.; Xander, P.; Batista, W.L.; Soares, R.P.; Schenkma, S.; Torrecilhas, A.C. Stress Induces Release of Extracellular Vesicles by Trypanosoma cruzi Trypomastigotes. J. Immunol. Res. 2021, 2021, 2939693. [Google Scholar] [CrossRef]
- Nogueira, P.M.; de Menezes-Neto, A.; Borges, V.M.; Descoteaux, A.; Torrecilhas, A.C.; Xander, P.; Revach, O.Y.; Regev-Rudzki, N.; Soares, R.P. Immunomodulatory properties of Leishmania extracellular vesicles during host-parasite interaction: Differential activation of TLRs and NF-kB translocation by dermotropic and viscerotropic species. Front. Cell Infect. Microbiol. 2020, 10, 380. [Google Scholar] [CrossRef]
- Reis, N.F.C.; Dupin, T.V.; Costa, C.R.; Toledo, M.D.S.; de Oliveira, V.C.; Popi, A.F.; Torrecilhas, A.C.; Xander, P. Leishmania amazonensis Promastigotes or Extracellular Vesicles Modulate B-1 Cell Activation and Differentiation. Front. Cell. Infect. Microbiol. 2020, 10, 573813. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Torrecilhas, A.C.; Soares, R.P.; Schenkman, S.; Fernandez-Prada, C.; Olivier, M. Extracellular vesicles in Trypanosomatids: Host cell communication. Front. Cell. Infect. Microbiol. 2020, 10, 602502. [Google Scholar] [CrossRef]
- Marti, M.; Johnson, P.J. Emerging roles for extracellular vesicles in parasitic infections. Curr. Opin. Microbiol. 2016, 32, 66–70. [Google Scholar] [CrossRef]
- Fleming, A.; Sampey, G.; Chung, M.C.; Bailey, C.; van Hoek, M.L.; Kashanchi, F.; Hakami, R.M. The carrying pigeons of the cell: Exosomes and their role in infectious diseases caused by human pathogens. Pathog. Dis. 2014, 71, 109–120. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. Review of exosomal isolation methods: Is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Szempruch, A.J.; Sykes, S.E.; Kieft Dennison, L.; Becker, A.C.; Gartrell, A.; Martin, W.J.; Nakayasu, E.S.; Almeida, I.C.; Hajduk, S.L.; Harrington, J.M. Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 2016, 164, 246–257. [Google Scholar] [CrossRef]
- Turchinovich, A.; Weiz, L.; Burwinkel, B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem. Sci. 2012, 37, 460–465. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen Lee, M.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.A.; Odorizzi, R.M.; Laurenti, M.D.; Galati, E.A.; Canavez, F.; Pereira-Chioccola, V.L. Detection of Leishmania (Leishmania) infantum RNA in fleas and ticks collected from naturally infected dogs. Parasitol. Res. 2011, 109, 267–274. [Google Scholar] [CrossRef]
- Hippólito, D.D.C.; Gomes, A.H.S.; Maia, M.M.; Meira-Strejevitch, C.D.S.; Kanamura, C.T.; Lauletta Lindoso, J.A.; Pereira-Chioccola, V.L. Gene expression profile of cytokines produced in biopsies from patients with American cutaneous leishmaniasis. Acta Trop. 2019, 189, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.B.; Maia, M.M.; Pereira, I.S.; Taniwaki, N.N.; Namiyama, G.M.; Telles, J.P.M.; Vidal, J.E.; Spegiorin, L.C.J.F.; Brandão de Mattos, C.C.; Mattos, L.C.; et al. Human extracellular vesicles and correlation with two clinical forms of toxoplasmosis. PLoS ONE 2020, 15, e0229602. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nogueira, P.M.; Ribeiro, K.; Silveira, A.C.O.; Campos, J.H.; Martins-Filho, A.O.; Bela, S.R.; Campos, M.A.; Pessoa, N.L.; Colli, W.; Alves, M.J.M.; et al. Vesicles from differential innate and chronic imune responses. J. Extracell. Vesicles 2015, 26, 28734. [Google Scholar] [CrossRef]
- Lambertz, U.; Silverman, J.M.; Nandan, D.; McMaster, W.R.; Clos, J.; Foster, L.J.; Reiner, N.E. Secreted virulence factors and immune evasion in visceral leishmaniasis. J. Leukoc. Biol. 2012, 91, 887–899. [Google Scholar] [CrossRef]
- Hassani, K.; Shio, M.T.; Martel, C.; Faubert, D.; Olivier, M. Absence of metalloprotease GP63 alters the protein content of Leishmania exosomes. PLoS ONE 2014, 15, e95007. [Google Scholar] [CrossRef]
- Barbiéri, C.L. Immunology of canine leishmaniasis. Parasite Immunol. 2006, 28, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Reiner, N.E. Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front. Cell. Infect. Microbiol. 2012, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Darwich, L.; Coma, G.; Peña, R.; Bellido, R.; Blanco, E.J.; Este, J.A.; Borras, F.E.; Clotet, B.; Ruiz, L.; Rosell, A.; et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology 2009, 126, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, A.; Chandrasekaran, S.; Kuchipudi, S.V.; Kalangi, S.K. Cytokines: Key Determinants of Resistance or Disease Progression in Visceral Leishmaniasis: Opportunities for Novel Diagnostics and Immunotherapy. Front. Immunol. 2019, 10, 670. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Maksoud, S.; El Hokayem, J. The cytokine/chemokine response in Leishmania/HIV infection and co-infection. Heliyon 2023, 9, e15055. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 23, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110, 3234–3244. [Google Scholar] [CrossRef]
- Amado, T.; Schmolka, N.; Metwally, H.; Silva-Santos, B.; Gomes, A.Q. Cross-regulation between cytokine and microRNA pathways in T cells. Eur. J. Immunol. 2015, 45, 1584–1595. [Google Scholar] [CrossRef]
- Alexander, M.; Hu, R.; Runtsch, M.C.; Kagele, D.A.; Mosbruger, T.L.; Tolmachova, T.; Seabra, M.C.; Round, J.L.; Ward, D.M.; O’Connell, R.M. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Commun. 2015, 6, 7321. [Google Scholar] [CrossRef]
- Bragato, J.P.; Rebech, G.T.; Freitas, J.H.; Santos, M.O.D.; Costa, S.F.; Eugênio, F.R.; Santos, P.S.P.D.; de Lima, V.M.F. miRNA-21 regulates CD69 and IL-10 expression in canine leishmaniasis. PLoS ONE 2022, 17, e0265192. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pandey, R.K.; Prajapati, P.; Prajapati, V.K. Circulating MicroRNAs: Potential and Emerging Biomarkers for Diagnosis of Human Infectious Diseases. Front. Microbiol. 2016, 15, 1274. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Lin, X.; Zhou, F.; Li, C.; Wang, X.; Yu, H.; Pan, Y.; Fei, H.; Ma, L.; Zhang, S. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020, 113, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Lakhter, A.J.; Pratt, R.E.; Moore, R.E.; Doucette, K.K.; Maier, B.F.; DiMeglio, L.A.; Sims, E.K. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia. 2018, 61, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Varikuti, S.; Verma, C.; Holcomb, E.; Jha, B.K.; Viana, A.; Maryala, R.; Lamenza, F.; McElwain, B.K.; Doni, N.Y.; Papenfuss, T.; et al. MicroRNA-21 Deficiency Promotes the Early Th1 Immune Response and Resistance toward Visceral Leishmaniasis. J. Immunol. 2021, 1, 1322–1332. [Google Scholar] [CrossRef]
- Yang, L.; Boldin, M.P.; Yu, Y. miR-146a controls the resolution of T cell responses in mice. J. Exp. Med. 2012, 209, 1655–1670. [Google Scholar] [CrossRef]
- Ganguly, S.; Ghoshal, B.; Banerji, I.; Bhattacharjee, S.; Chakraborty, S.; Goswami, A.; Mukherjee, K.; Bhattacharyya, S.N. Leishmania survives by exporting miR-146a from infected to resident cells to subjugate inflammation. Life Sci. Alliance 2022, 24, e202101229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).