Abstract
Eusocial wasps are represented in the Vespidae by the subfamilies Stenogastrinae, Vespinae and Polistinae. These wasps present colonies that are sometimes composed of thousands of individuals which live in nests built with paper materials. The high density of the adult and larval population, as well as the stable micro environment of the nests, make very favourable conditions for the flourishing of various types of microorganisms. These microorganisms, which may be pathogens, are beneficial and certainly contribute to model the sociality of these insects. The mutualistic relationships that we observe in some species, especially in Actinomycete bacteria and yeasts, could have important fallouts for the development of new medicines and for the use of these insects in agricultural environments.
Keywords:
social wasps; viruses; bacteria; fungi; yeasts; antimicrobial secretions; mutualistic symbioses 1. Introduction
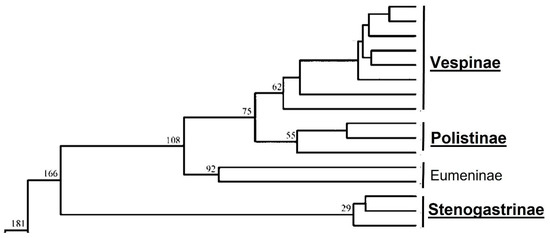
Eusociality, the condition in which a group of individuals presents communal cares of immature brood, generations overlap and, above all, some members of the group renounce exploiting their reproductive capacity to rear the offspring of other (often related) individuals, is not so represented in nature [1]. In insects, we can find it especially in Termites and Aculeate Hymenoptera (ants, bees and wasps). Considering the wasps (family Vespidae), it has been asserted, in light of phylogenetic studies (based on data from four nuclear genes fragments [2] on sequence data generated by 378 loci across 136 vespid species [3] and on mtgenome PCG12R datasets [4]), that eusociality originated twice: once in the subfamily Stenogastrinae and the other in the ancestor of the group composed of the subfamilies Polistinae and Vespinae.
The relationship between wasps and microorganisms of various types (viruses, bacteria and unicellular fungi) is present in solitary species, but, during evolution, it acquired a special and diverse significance in social species. The main characteristic of the latter is the formation of colonies that can have populations spanning from a few individuals to large superorganisms. Adults and immature broods live in nests that are mainly built with materials collected in the field and treated with a gluing secretion of the adults before being used for construction. The colony (represented by the nest, adults and immature brood) forms special environments where microorganisms can proliferate, which can present challenges to the lives of these insects, meaning that we can expect the presence of the various systems evolved by the hosts to limit or influence the pathogens and commensals in their nests [5,6]. Recently, the focus on the microbiome has led to us considering an individual as the product of the interaction of its genes and the genes of the microorganisms inhabiting its body. The hologenome theory [7,8], which regards all microorganisms and the host as the unique subject exposed to selective pressure, has been extensively described in insects. Among these, Aphids and Buchnera have improved our understanding of the evolutionary dynamics in host–microorganism interactions [9], microorganism symbiosis studies on Nasonia highlighted the role of the microbiota in the speciation process [10], while studies on termites were crucial for the synthesis and degradation of nutrients from plant polymers [11].
Social insects are a perfect example of the holobiont theory of evolution since they depend significantly on commensal yeasts, fungi and bacteria (Guerrero et al., 2013) [12]. With respect to other social insects, however, reports and experiments on the symbiotic relationships between social wasps and microorganisms are quite limited. Recently, Mayorga et al., 2021 [13], published an excellent review that is part of a book on South American social wasps, which focuses on the published contributions about the presence of microorganisms in the colonies of these insects. The review also presents synoptic tables which list the viruses, bacteria and fungi found mainly in Vespinae and Polistinae wasps. At present, more than 150 species of microorganisms have been reported to be present in the colonies of social wasps, with a vast majority of fungi (almost 70%) (Majorga et al., 2021 [13].
The purpose of this short review is to give an account of the principal examples of the mutualistic symbiosis between social wasps and microorganisms reported in the literature. First, however, we must mention at least one of the kinds of defence evolved by social wasps against pathogenic microorganisms.
2. Defence against Pathogens: Antimicrobial Secretions
The defence of social wasps against pathogens evolved in various ways both at the individual and social level [14]: the choice of where to build the nests, the organisation of nest architecture, the presence of hygienic behaviours and the production of antimicrobial substances. The last ones are particularly important as they can be effective for the individuals and the whole colony [15], and can be present in the secretions of special glands of the larvae and the adults, or produced by mutualistic microorganisms. In all the cases, the products can be of great interest for the development of antimicrobial agents [16]. All the species in which the presence of these substances was searched for had a colony defence based on active substances which can be secreted by the larvae ([17] for Vespula (Vla) sp. and [18] for Polistes dominula). The venom, however, was especially found to be the source of important compounds. The venom of social insects is a very complex secretion which contains components of various molecular weights; the medium weight components are mainly formed by short peptides, of a few residues spanning from 12 to 15, that are called mastoparans [19,20]. Mastoparans can cause many different effects on biological organisms and possess cytolytic and antimicrobial activity. Various types have been described in the venom of social wasps belonging to several species of Stenogastrinae [15], Vespinae (Vespa tropica—[21]; V. magnifica, V. orientalis, V. nigrithorax—[22]; Vespula vulgaris—[23]; Dolichovespula saxonica—[24]) and Polistinae (Agelaia pallipes pallipes—[25]; Polybia paulista—[26]; Polybia dymorpha—[27]; Chartergellus communis—[28]; Sinoeca surinama—[29]; Polistes dominula [30]; P. major major and P. dorsalis dorsalis—[31]; and P. wattii—[32]). Moreover, targets of the antimicrobial activity span from bacteria to fungi and even viruses. Hoggard et al. in 2011 [33] noted that the antimicrobial activity of social species, which build paper nests, tends to be the highest with respect to that of solitary species, with an increment also related to group size and social complexity.
4. Conclusions
Table 1 summarises the mutualistic relationships observed and experimentally confirmed, at present, between microorganisms and social wasps. We can observe that the role of microorganisms in the various associations is principally defensive, with the production of antimicrobial substances, while the insects provide a house and propagation means to the organisms. Future research will probably discover other interesting characteristics of this symbiosis.

Table 1.
Characteristics of mutual symbiosis between social wasps and microorganisms. Only the experimental confirmed researches are reported.
In conclusion, these insects, given the different stages of sociality they reached in the course of evolution, the characteristics of their nests which favour the presence of microorganisms, the production of antimicrobial compounds, the easy handling of the colonies of some species and other important ecosystem services they furnish [82], are crucial for the study of the interactions between different levels of biological entities (holobionts), the discovery of new medicines and the convey of useful microorganisms in the environment.
Author Contributions
S.T. conceptualised the problems concerning the evolution of sociality in wasps; D.C. conceptualised the microbiological aspects of symbiosis between insects and microorganisms; N.M. discussed the relationships between wasps and yeasts. S.T., D.C. and N.M. wrote and discussed the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Joint Laboratory for the Research on Medical, Microbiological and Environmental Entomotherapy (LABREMMA) at the University of Florence.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilson, E.O. Genesis: The Deep Origin of Societies; Penguin: London, UK, 2019. [Google Scholar]
- Hines, H.M.; Hunt, J.H.; O’Connor, T.K.; Gillespie, J.J.; Cameron, S.A. Multigene phylogeny reveals eusociality evolved twice in vespid wasps. Proc. Natl. Acad. Sci. USA 2007, 104, 3295–3299. [Google Scholar] [CrossRef]
- Piekarski, P.K.; Carpenter, J.M.; Lemmon, A.R.; Moriarty Lemmon, E.; Sharanowski, B.J. Phylogenomic evidence overturns current conceptions of social evolution in wasps (Vespidae). Mol. Biol. Evol. 2018, 35, 2097–2109. [Google Scholar] [CrossRef]
- Huang, P.; Carpenter, J.M.; Chen, B.; Li, T.J. The first divergence time estimation of the subfamily Stenogastrinae (Hymenoptera: Vespidae) based on mitochondrial phylogenomics. Int. J. Biol. Macromol. 2019, 137, 767–773. [Google Scholar] [CrossRef]
- Boomsma, J.J.; Schmid-Hempel, P. Pressure across the Major Groups of Social Insects. In Insect Evolutionary Ecology, Proceedings of the Royal Entomological Society’s 22nd Symposium, Reading, UK, 2005; CABI International: Wallingford, UK, 2005; p. 139. [Google Scholar]
- Hughes, D.P.; Pierce, N.E.; Boomsma, J.J. Social insect symbionts: Evolution in homeostatic fortresses. Trends Ecol. Evol. 2008, 23, 672–677. [Google Scholar] [CrossRef]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution ndof animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef]
- Rosenberg, E. Evolution of holobionts: The hologenome concept. In Microbiomes: Current Knowledge and Unanswered Questions; Springer: Cham, Switzerland, 2021; pp. 317–352. [Google Scholar]
- Shigenobu, S.; Yorimoto, S. Aphid hologenomics: Current status and future challenges. Curr. Opin. Insect Sci. 2022, 50, 100882. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Hu, H.; Wang, G.H. Nasonia–microbiome associations: A model for evolutionary hologenomics research. Trends Parasitol. 2022, 39, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. The holobiont concept: The case of xylophagous termites and cockroaches. Symbiosis 2016, 68, 49–60. [Google Scholar] [CrossRef]
- Guerrero, R.; Margulis, L.; Berlanga, M. Symbiogenesis: The holobiont as a unit of evolution. Int. Microbiol. 2013, 16, 133–143. [Google Scholar]
- Mayorga-Ch, D.; Rodríguez-C, C.; Ortíz-Reyes, A.; Romero-Tabarez, M.; Sarmiento, C.E. Interactions of Social Wasps in Microorganisms. In Neotropical Social Wasps: Basic and Applied Aspects; Prezoto, F., Nascimento, F.S., Barbosa, B.C., Somavilla, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 405–434. [Google Scholar]
- Cremer, S.; Armitage, S.A.; Schmid-Hempel, P. Social immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D.; Mazza, G.; Turillazzi, S. From individual to collective immunity: The role of the venom as antimicrobial agent in the Stenogastrinae wasp societies. J. Insect Physiol. 2012, 58, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Erturk, Ö.; Bagdatli, E. A comprehensive study on nest materials of Vespa crabro and Polistes dominula: Chemical properties and biological characterization with antioxidant and antimicrobial activity. Biologia 2019, 74, 797–812. [Google Scholar] [CrossRef]
- Gambino, P. Antibiotic activity of larval saliva of Vespula wasps. J. Invertebr. Pathol. 1993, 61, 110. [Google Scholar] [CrossRef]
- Turillazzi, S.; Perito, B.; Pazzagli, L.; Pantera, B.; Gorfer, S.; Tancredi, M. Antibacterial activity of larval saliva of the European paper wasp Polistes dominulus (Hymenoptera, Vespidae). Insectes Sociaux 2004, 51, 339–341. [Google Scholar] [CrossRef]
- Hirai, Y.; Yasuhara, T.; Yoshida, H.; Nakajima, T.; Fujino, M.; Kitada, C. A New Mast Cell Degranulating Peptide “Mastoparan” in the Venom of Vespula lewisii. Chem. Pharm. Bull. 1979, 27, 1942–1944. [Google Scholar] [CrossRef]
- Choi, M.B.; Lee, Y.H. The structure and antimicrobial potential of wasp and hornet (Vespidae) mastoparans: A review. Entomol. Res. 2020, 50, 369–376. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Lee, W.H.; Zhang, Y. Antimicrobial peptides from the venom gland of the social wasp Vespa tropica. Toxicon 2013, 74, 151–157. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.; Lee, Y.J.; Kim, H.R.; Nam, J.O.; Choi, M.B.; Hahn, D. Antibacterial potential of Nidus vespae built by invasive alien hornet, Vespa velutina nigrithorax, against food-borne pathogenic bacteria. Entomol. Res. 2020, 50, 28–33. [Google Scholar] [CrossRef]
- Kim, Y.; Son, M.; Noh, E.-Y.; Kim, S.; Kim, C.; Yeo, J.-H.; Park, C.; Lee, K.W.; Bang, W.Y. MP-V1 from the Venom of Social Wasp Vespula vulgaris Is a de Novo Type of Mastoparan that Displays Superior Antimicrobial Activities. Molecules 2016, 21, 512. [Google Scholar] [CrossRef]
- Ertürk, Ö.; Başkan, C.; Koleren, Z. Antimicrobial Activities of Nest Materials Dolichovespula saxonica (Fabricius, 1793) (Hymenoptera: Vespidae) in Turkey. Arıcılık Araştırma Derg. 2018, 10, 9–14. [Google Scholar]
- Wang, K.; Dang, W.; Xie, J.; Zhu, R.; Sun, M.; Jia, F.; Zhao, Y.; An, X.; Qiu, S.; Li, X.; et al. Antimicrobial peptide protonectin (Agelaya pallipes pallipes) disturbs the membrane integrity and induces ROS production in yeast cells. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 2365–2373. [Google Scholar] [CrossRef]
- Ribeiro, S.P.; Mendes, M.A.; Dos Santos, L.D.; de Souza, B.M.; Marques, M.R.; de Azevedo, W.F., Jr.; Palma, M.S. Structural and functional characterization of N-terminally blocked peptides isolated from the venom of the social wasp Polybia paulista. Peptides 2004, 25, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, R.C.; Trentini, M.M.; de Castro e Silva, J.; Simon, K.S.; Bocca, A.L.; Silva, L.P.; Mortari, M.R.; Kipnis, A.; Junqueira-Kipnis, A.P. Antimycobacterial activity of a new peptide polydim-I isolated from neotropical social wasp Polybia dimorpha. PLoS ONE 2016, 11, e0149729. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.S.; Campos, G.A.A.; Camargo, L.C.; de Souza, A.C.B.; Ibituruna, B.V.; Magalhães, A.C.M.; da Rocha, L.F.; Garcia, A.B.; Rodrigues, M.C.; Ribeiro, D.M.; et al. Characterization of two peptides isolated from the venom of social wasp Chartergellus communis (Hymenoptera: Vespidae): Influence of multiple alanine residues and C-terminal amidation on biological effects. Peptides 2017, 95, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Freire, D.O.; da Cunha, N.B.; Leite, M.L.; Kostopoulos, A.G.; da Silva, S.N.; de Souza, A.C.; Nolasco, D.O.; Franco, O.L.; Mortari, M.R.; Dias, S.C. Wasp venom peptide, synoeca-MP, from Synoeca surinama shows antimicrobial activity against human and animal pathogenic microorganisms. Pept. Sci. 2020, 112, e24141. [Google Scholar] [CrossRef]
- Turillazzi, S.; Mastrobuoni, G.; Dani, F.R.; Moneti, G.; Pieraccini, G.; la Marca, G.; Nolasco, D.O.; Franco, O.L.; Mortari, M.R.; Dias, S.C. Dominulin A and B: Two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-ion trap. J. Am. Soc. Mass Spectrom. 2006, 17, 376–383. [Google Scholar] [CrossRef]
- Čeřovský, V.; Slaninová, J.; Fučík, V.; Hulačová, H.; Borovičková, L.; Ježek, R.; Bednárová, L. New potent antimicrobial peptides from the venom of Polistinae wasps and their analogs. Peptides 2008, 29, 992–1003. [Google Scholar] [CrossRef]
- Al-Shammery, K.; Hozzein, W.N. Antibacterial activities of two potential peptides extracted from Polistes wattii Cameron, 1900 (Vespidae: Polistinae) wasp venom collected at Eastern Province, Saudi Arabia. PLoS ONE 2022, 17, e0264035. [Google Scholar] [CrossRef]
- Hoggard, S.J.; Wilson, P.D.; Beattie, A.J.; Stow, A.J. Social complexity and nesting habits are factors in the evolution of antimicrobial defences in wasps. PLoS ONE 2011, 6, e21763. [Google Scholar] [CrossRef]
- Turillazzi, S. The Biology of Hover Wasps; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Krombein, K.V. Biosystematic Studies of Ceylonese Wasps, XIX: Natural History Notes in Several Families (Hymenoptera: Eumenidae, Vespidae, Pompilidae and Crabronidae); Smithsonian Contributions to Zoology; Smithsonian Institution Press: Washington, DC, USA, 1991. [Google Scholar]
- Hansell, M.H.; Turillazzi, S. Nest structure and building material of three species of Anischnogaster (Vespidae Stenogastrinae) from Papua New Guinea. Trop. Zool. 1995, 8, 203–219. [Google Scholar] [CrossRef]
- Turillazzi, S. Function and characteristics of the abdominal substance secreted by wasps of the genus Parischnogaster (Hymenoptera Stenogastrinae). Monit. Zool. Ital. J. Zool. 1985, 19, 91–99. [Google Scholar]
- Silveira, O.T.; Andena, S.R.; Somavilla, A.; Carpenter, J.M. Phylogeny and classification of the Neotropical social wasps. In Neotropical Social Wasps: Basic and Applied Aspects; Springer: Cham, Switzerland, 2021; pp. 267–291. [Google Scholar]
- Kaltenpoth, M.; Engl, T. Defensive microbial symbionts in Hymenoptera. Funct. Ecol. 2014, 28, 315–327. [Google Scholar] [CrossRef]
- Roossinck, M.J. The good viruses: Viral mutualistic symbioses. Nat. Rev. Microbiol. 2011, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Morel, G.; Fouillaud, M. Presence of microorganisms and viral inclusion bodies in the nests of the paper wasp Polistes hebraeus Fabricius (Hymenoptera, Vespidae). J. Invertebr. Pathol. 1992, 60, 210–212. [Google Scholar] [CrossRef]
- Fouillaud, M.; Morel, G. Characterization of cytoplasmic and nuclear polyhedrosis viruses recovered from the nest of Polistes hebraeus F. (Hymenoptera; Vespidae). J. Invertebr. Pathol. 1994, 64, 89–95. [Google Scholar] [CrossRef]
- Madden, A.A.; Boyden, S.D.; Soriano, J.A.N.; Corey, T.B.; Leff, J.W.; Fierer, N.; Starks, P.T. The emerging contribution of social wasps to grape rot disease ecology. PeerJ 2017, 5, e3223. [Google Scholar] [CrossRef] [PubMed]
- Dalmon, A.; Gayral, P.; Decante, D.; Klopp, C.; Bigot, D.; Thomasson, M.; Herniou, E.A.; Alaux, C.; Le Conte, Y. Viruses in the invasive hornet Vespa velutina. Viruses 2019, 11, 1041. [Google Scholar] [CrossRef]
- Currie, C.R. A community of ants, fungi, and bacteria: A multilateral approach to studying symbiosis. Annu. Rev. Microbiol. 2001, 55, 357–380. [Google Scholar] [CrossRef]
- Little, A.E.; Currie, C.R. Symbiotic complexity: Discovery of a fifth symbiont in the attine ant–microbe symbiosis. Biol. Lett. 2007, 3, 501–504. [Google Scholar] [CrossRef]
- Moreau, C.S. Symbioses among ants and microbes. Curr. Opin. Insect Sci. 2020, 39, 1–5. [Google Scholar] [CrossRef]
- Kim, E.; Seo, J.; Yang, S.H.; Kim, I.S.; Koo, Y. Intestine bacterial microbiota of Asian hornet (Vespa velutina nigrithorax) and honeybee. Korean J. Environ. Agric. 2018, 37, 135–140. [Google Scholar] [CrossRef]
- Fang, C.; Achal, V. Physico-Chemical Aspects and Complete Bacterial Community Composition Analysis of Wasp Nests. Sustainability 2020, 12, 2652. [Google Scholar] [CrossRef]
- Cini, A.; Meriggi, N.; Bacci, G.; Cappa, F.; Vitali, F.; Cavalieri, D.; Cervo, R. Gut microbial composition in different castes and developmental stages of the invasive hornet Vespa velutina nigrithorax. Sci. Total Environ. 2020, 745, 140873. [Google Scholar] [CrossRef] [PubMed]
- Ishay, J.S.; Riabinin, K.; Pertsis, V. Symbiotic bacteria in hornet pupal silk. Naturwissenschaften 2003, 90, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Tempestini, A. Caratterizzazione di Batteri Associati a Larve di Polistes Dominulus; Facoltà di Scienze Matematiche Fisiche Naturali, Corso di Laurea in Scienze Biologiche, Università degli Studi di Firenze: Florence, Italy, 2006; p. 86. [Google Scholar]
- Madden, A.A.; Grassetti, A.; Soriano, J.A.N.; Starks, P.T. Actinomycetes with antimicrobial activity isolated from paper wasp (Hymenoptera: Vespidae: Polistinae) nests. Environ. Entomol. 2013, 42, 703–710. [Google Scholar] [CrossRef]
- Cervo, R.; Zacchi, F.; Turillazzi, S. Polistes dominulus (Hymenoptera, Vespidae) invading North America: Some hypotheses for its rapid spread. Insectes Sociaux 2000, 47, 155–157. [Google Scholar] [CrossRef]
- Mhlongwe, T.R. The Search for a Biological Control Agent to Control Invasive Polistes Dominula Wasps in the Western Cape Region, South Africa. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2018. [Google Scholar]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef]
- Baranova, A.A.; Zakalyukina, Y.V.; Ovcharenko, A.A.; Korshun, V.A.; Tyurin, A.P. Antibiotics from Insect-Associated Actinobacteria. Biology 2022, 11, 1676. [Google Scholar] [CrossRef]
- Matarrita-Carranza, B.; Moreira-Soto, R.D.; Murillo-Cruz, C.; Mora, M.; Currie, C.R.; Pinto-Tomas, A.A. Evidence for widespread associations between neotropical hymenopteran insects and Actinobacteria. Front. Microbiol. 2017, 8, 2016. [Google Scholar] [CrossRef]
- Chavarría-Pizarro, L. Los insectos y la biotecnología: Avispas sociales como fuente de nuevos compuestos antibióticos. Rev. Tecnol. Marcha 2019, g-114. [Google Scholar] [CrossRef]
- Matarrita-Carranza, B.; Murillo-Cruz, C.; Avendaño, R.; Ríos, M.I.; Chavarría, M.; Gómez-Calvo, M.L.; Tamayo-Castillo, G.; Araya, J.J.; Pinto-Tomás, A.A. Streptomyces sp. M54: An actinobacteria associated with a neotropical social wasp with high potential for antibiotic production. Antonie Van Leeuwenhoek 2021, 114, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Araya, M.; Núñez-Montero, K.; Pizarro-Cerdá, J.; Chavarría-Pizarro, L. Draft Genome Sequences of Saccharopolyspora sp. Strains and Streptomyces sp. Strains, Isolated from Social Wasps (Vespidae; Polistinae: Epiponini). Microbiol. Resour. Announc. 2022, 11, e00935-21. [Google Scholar] [CrossRef] [PubMed]
- Glare, T.R.; Harris, R.J.; Donovan, B.J. Aspergillus flavus as a pathogen of wasps, Vespula spp., in New Zealand. N. Z. J. Zool. 1996, 23, 339–344. [Google Scholar] [CrossRef]
- Harris, R.J.; Harcourt, S.J.; Glare, T.R.; Rose, E.A.F.; Nelson, T.J. Susceptibility of Vespula vulgaris (Hymenoptera: Vespidae) to generalist entomopathogenic fungi and their potential for wasp control. J. Invertebr. Pathol. 2000, 75, 251–258. [Google Scholar] [CrossRef]
- Durrell, L.W. Fungi in nests of paper wasps. Am. Midl. Nat. 1965, 73, 501–503. [Google Scholar] [CrossRef]
- Fouillaud, M.; Morel, G. Fungi associated with nests of the paper wasp Polistes hebraeus (Hymenoptera: Vespidae) on La Reunion Island. Environ. Entomol. 1995, 24, 298–305. [Google Scholar] [CrossRef]
- Jayaprakash, A.; Ebenezer, P. A new report on mycobiota associated with Ropalidia marginata paper nests. Indian J. Sci. Technol. 2010, 3, 6–8. [Google Scholar] [CrossRef]
- Madden, A.A.; Stchigel, A.M.; Guarro, J.; Sutton, D.; Starks, P.T. Mucor nidicola sp. nov., a fungal species isolated from an invasive paper wasp nest. Int. J. Syst. Evol. Microbiol. 2012, 62, 1710–1714. [Google Scholar] [CrossRef]
- Davis, T.S.; Boundy-Mills, K.; Landolt, P.J. Volatile emissions from an epiphytic fungus are semiochemicals for eusocial wasps. Microb. Ecol. 2012, 64, 1056–1063. [Google Scholar] [CrossRef]
- Blackwell, M. Made for each other: Ascomycete yeasts and insects. Microbiol. Spectr. 2017, 5, 5.3.13. [Google Scholar] [CrossRef]
- Stefanini, I.; Dapporto, L.; Legras, J.L.; Calabretta, A.; Di Paola, M.; De Filippo, C.; Viola, R.; Capretti, P.; Polsinelli, M.; Turillazzi, S.; et al. Role of social wasps in Saccharomyces cerevisiae ecology and evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 13398–13403. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, I.; Dapporto, L.; Berná, L.; Polsinelli, M.; Turillazzi, S.; Cavalieri, D. Social wasps are a Saccharomyces mating nest. Proc. Natl. Acad. Sci. USA 2016, 113, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Dapporto, L.; Stefanini, I.; Rivero, D.; Polsinelli, M.; Capretti, P.; De Marchi, P.; Viola, R.; Turillazzi, S.; Cavalieri, D. Social wasp intestines host the local phenotypic variability of Saccharomyces cerevisiae strains. Yeast 2016, 33, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Alarco, A.M.; Marcil, A.; Chen, J.; Suter, B.; Thomas, D.; Whiteway, M. Immune-deficient Drosophila melanogaster: A model for the innate immune response to human fungal pathogens. J. Immunol. 2004, 172, 5622–5628. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S. Drosophila and Galleria insect model hosts: New tools for the study of fungal virulence, pharmacology and immunology. Virulence 2011, 2, 521–527. [Google Scholar] [CrossRef]
- Bergin, D.; Murphy, L.; Keenan, J.; Clynes, M.; Kavanagh, K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006, 8, 2105–2112. [Google Scholar] [CrossRef]
- Meriggi, N.; Di Paola, M.; Vitali, F.; Rivero, D.; Cappa, F.; Turillazzi, F.; Gori, A.; Dapporto, L.; Beani, L.; Turillazzi, S.; et al. Saccharomyces cerevisiae induces immune enhancing and shapes gut microbiota in social wasps. Front. Microbiol. 2019, 10, 2320. [Google Scholar] [CrossRef]
- Rizzetto, L.; Ifrim, D.C.; Moretti, S.; Tocci, N.; Cheng, S.C.; Quintin, J.; Renga, G.; Oikonomou, V.; De Filippo, C.; Weil, T.; et al. Fungal chitin induces trained immunity in human monocytes during cross-talk of the host with Saccharomyces cerevisiae. J. Biol. Chem. 2016, 291, 7961–7972. [Google Scholar] [CrossRef]
- Jimenez, S.I.; Carroll, C.; Babcock, T.; Derstine, N.; Hadwin, A.; Moore, M.; Gries, G. Yeasts harbored by vespine wasps in the Pacific Northwest. Environ. Entomol. 2017, 46, 217–225. [Google Scholar] [CrossRef]
- Valentini, B.; Barbero, F.; Casacci, L.P.; Luganini, A.; Stefanini, I. Forests influence yeast populations vectored by insects into vineyards. Front. Microbiol. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Madden, A.A.; Epps, M.J.; Fukami, T.; Irwin, R.E.; Sheppard, J.; Sorger, D.M.; Dunn, R.R. The ecology of insect–yeast relationships and its relevance to human industry. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172733. [Google Scholar] [CrossRef] [PubMed]
- Meriggi, N.; Di Paola, M.; Cavalieri, D.; Stefanini, I. Saccharomyces cerevisiae–insects association: Impacts, biogeography, and extent. Front. Microbiol. 2020, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Brock, R.E.; Cini, A.; Sumner, S. Ecosystem services provided by aculeate wasps. Biol. Rev. 2021, 96, 1645–1675. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).