Interplay between the Human Microbiome and Biliary Tract Cancer: Implications for Pathogenesis and Therapy
Abstract
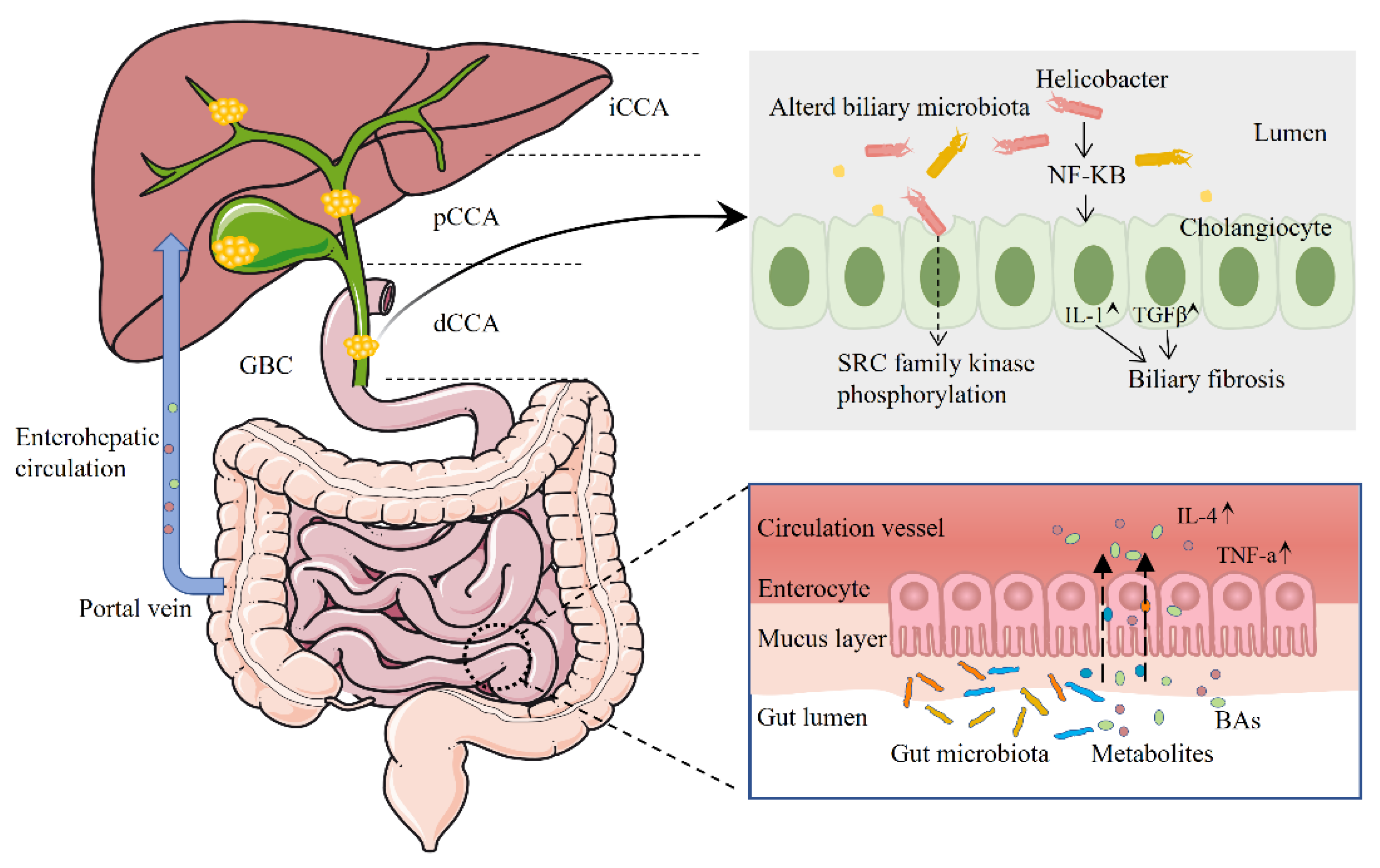
:1. Introduction
2. Biliary Tract Cancer and the Human Microbiome
2.1. The Microbiome and Intrahepatic Cholangiocarcinoma
2.2. The Microbiome and Extrahepatic Cholangiocarcinoma
2.3. The Microbiome and Gallbladder Cancer
| Author, Year | Biological Specimens | Sampling Methods | Tumor Site and Size | Main Conclusion |
|---|---|---|---|---|
| Chen, 2019 [30] | bile | ERCP | dCCA, 8 | Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria are the most dominant phyla in the bile. Gemmatimonadetes, Nitrospirae, Chloroflexi, Latescibacteria, and Planctomycetes in the phylum increase in dCCA patients compared with the onset of common bile duct stones patients. |
| Avilés-Jiménez, 2016 [34] | biliary duct epithelial cells | Brushing during ERCP | eCCA, 100 | Phylum Proteobacteria dominated all samples (60.4% average). Nesterenkonia decreased, whereas Methylophilaceae, Fusobacterium, Prevotella, Actinomyces, Novosphingobium, and H. pylori increased in eCCA. Predicted associated functions showed an increased abundance of H. pylori virulence genes in eCCA. |
| Saab, 2021 [31] | bile | ERCP | eCCA, 28 | Proteobacteria did not significantly differ between eCCA patients and controls. The most abundant genera were Enterococcus, Streptococcus, Bacteroides, Klebsiella, and Pyramidobacter in eCCA’s biliary microbiota. Levels of Bacteroides, Geobacillus, Meiothermus, and Anoxybacillus genera were significantly higher in eCCA patients’ biliary microbiota, without an associated disease, in comparison with controls. |
| Li, 2022 [32] | bile | ERCP | pCCA, 14 dCCA, 9 | The top three biomarkers for pCCA at the genus level were Pseudomonas, Sphingomonas, and Halomonas; for dCCA, they were Streptococcus, Prevotella, and Halomonas. |
| Miyabe, 2022 [35] | Bile and stool | ERCP | CCA (mainly pCCA), 49 | Increased species richness and abundance of Fusobacteria were correlated with the duration of PSC and characterized the biliary microbiota in CCA. |
| Ito, 2022 [10] | Bile and stool | ERCP | iCCA, 12 eCCA, 12 GBC, 6 | A higher Enterobacteriaceae abundance and a lower Clostridia abundance, including that of Faecalibacterium and Coprococcus, in the BTC patients than in the other subjects. A bile-isolated strain possessed the carcinogenic bacterial colipolyketide synthase-encoding gene. |
| Di Carlo, 2019 [28] | bile | ERCP | CCA, 42 GBC, 5 | E. coli and P. aeruginosa were significant negative predictors of CCA. About GBC, there were no significant correlations with E. coli, K. pneumoniae, or P. aeruginosa. |
| Pomyen, 2023 [51] | stool | - | iCCA, 19 | Two Veillonella species were found to be more abundant in iCCA samples and could distinguish iCCA from HCC and healthy controls. Ruminococcus gnavus was depleted in iCCA patients and could distinguish HCC from iCCA samples. High Veillonella genus counts in the iCCA group were associated with enriched amino acid biosynthesis and glycolysis pathways. |
| Chai, 2023 [23] | tissues | surgery | iCCA, 99 | The most abundant bacterial orders include Burkholderiales, Pseudomonadales, Xanthomonadales, Bacillales, and Clostridiales. The content of Paraburkholderia fungorum was significantly higher in the paracancerous tissues. |
| Deng, 2022 [19] | fecal | - | CCA, 46 | Gammaproteobacteria were significantly higher in both gemcitabine- and cisplatin-resistance groups compared to sensitive groups. |
| Jia, 2020 [18] | stool and blood | - | iCCA, 28 | The abundances of four genera (Lactobacillus, Actinomyces, Peptostreptococcaceae, and Alloscardovia) were increased in patients with ICC compared with those in patients with hepatocellular carcinoma or liver cirrhosis and in healthy individuals. The glycoursodeoxycholic acid and tauroursodeoxycholic acid (TUDCA) plasma-stool ratios were obviously increased in patients with ICC. |
| Chng, 2016 [24] | tissue | - | CCA, 60 | A distinct, tissue-specific microbiome dominated by the bacterial families Dietziaceae, Pseudomonadaceae, and Oxalobacteraceae was observed in bile duct tissues. Several bacterial families, with a significant increase in Stenotrophomonas species distinguishing tumors from paired normals. |
3. The Effect of Dysbiosis on Biliary Tract Cancer and Its Precancerous Lesions
4. Potential Role of Microbes in Chemotherapy and Immunotherapy for Biliary Tract Cancer
5. The Role of Bacterial Metabolites in the Progression of Biliary Tract Cancer
6. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int 2019, 39 (Suppl. S1), 98–107. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. S1), 19–31. [Google Scholar] [CrossRef]
- Sripa, B.; Tangkawattana, S.; Brindley, P.J. Update on Pathogenesis of Opisthorchiasis and Cholangiocarcinoma. Adv. Parasitol. 2018, 102, 97–113. [Google Scholar]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Wheatley, R.C.; Kilgour, E.; Jacobs, T.; Lamarca, A.; Hubner, R.A.; Valle, J.W.; McNamara, M.G. Potential influence of the microbiome environment in patients with biliary tract cancer and implications for therapy. Br. J. Cancer 2022, 126, 693–705. [Google Scholar] [CrossRef]
- Ito, Z.; Koido, S.; Kato, K.; Odamaki, T.; Horiuchi, S.; Akasu, T.; Saruta, M.; Hata, T.; Kumagai, Y.; Fujioka, S.; et al. Dysbiosis of the Fecal and Biliary Microbiota in Biliary Tract Cancer. Cancers 2022, 14, 5379. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, T.; Wu, M.; Shen, F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016, 379, 198–205. [Google Scholar] [CrossRef]
- Wu, L.; Tsilimigras, D.I.; Paredes, A.Z.; Mehta, R.; Hyer, J.M.; Merath, K.; Sahara, K.; Bagante, F.; Beal, E.W.; Shen, F.; et al. Trends in the Incidence, Treatment and Outcomes of Patients with Intrahepatic Cholangiocarcinoma in the USA: Facility Type is Associated with Margin Status, Use of Lymphadenectomy and Overall Survival. World J. Surg. 2019, 43, 1777–1787. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Herraez, E.; Romero, M.R.; Macias, R.I.R.; Monte, M.J.; Marin, J.J.G. Clinical relevance of the relationship between changes in gut microbiota and bile acid metabolism in patients with intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2020, 9, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Ren, T.; Wang, X.; Wang, H.; Zou, Y.; Sun, Y.; Liu, S.; Ren, Z.; Yu, Z. Dysbiosis in the Human Microbiome of Cholangiocarcinoma. Front. Physiol. 2021, 12, 715536. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, J.; Jin, C.; Yang, J.; Zheng, C.; Chen, K.; Xie, Y.; Yang, Y.; Bo, Z.; Wang, J.; et al. Association of gut microbiome and primary liver cancer: A two-sample Mendelian randomization and case-control study. Liver Int. 2022, 43, 221–233. [Google Scholar] [CrossRef]
- Jia, X.; Lu, S.; Zeng, Z.; Liu, Q.; Dong, Z.; Chen, Y.; Zhu, Z.; Hong, Z.; Zhang, T.; Du, G.; et al. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 71, 893–906. [Google Scholar] [CrossRef]
- Deng, T.; Li, J.; He, B.; Chen, B.; Liu, F.; Chen, Z.; Zheng, J.; Shi, Z.; Zhang, T.; Deng, L.; et al. Gut microbiome alteration as a diagnostic tool and associated with inflammatory response marker in primary liver cancer. Hepatol. Int. 2022, 16, 99–111. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, S.; Jin, C.; Lin, Z.; Deng, T.; Xie, X.; Deng, L.; Li, X.; Ma, J.; Ding, X.; et al. A Predictive Model Based on the Gut Microbiota Improves the Diagnostic Effect in Patients with Cholangiocarcinoma. Front. Cell. Infect. Microbiol. 2021, 11, 751795. [Google Scholar] [CrossRef]
- Rao, B.C.; Zhang, G.Z.; Zou, Y.W.; Ren, T.; Ren, H.Y.; Liu, C.; Yu, Z.J.; Ren, Z.G. Alterations in the human oral microbiome in cholangiocarcinoma. Mil. Med. Res. 2022, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, C.; Duan, Y.; Heinrich, B.; Rosato, U.; Diggs, L.P.; Ma, L.; Roy, S.; Fu, Q.; Brown, Z.J.; et al. Gut Microbiome Directs Hepatocytes to Recruit MDSCs and Promote Cholangiocarcinoma. Cancer Discov. 2021, 11, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Wang, J.; Li, H.; Gao, C.; Li, S.; Wei, C.; Huang, J.; Tian, Y.; Yuan, J.; Lu, J.; et al. Intratumor microbiome features reveal antitumor potentials of intrahepatic cholangiocarcinoma. Gut Microbes 2023, 15, 2156255. [Google Scholar] [CrossRef] [PubMed]
- Chng, K.R.; Chan, S.H.; Ng, A.H.Q.; Li, C.; Jusakul, A.; Bertrand, D.; Wilm, A.; Choo, S.P.; Tan, D.M.Y.; Lim, K.H.; et al. Tissue Microbiome Profiling Identifies an Enrichment of Specific Enteric Bacteria in Opisthorchis viverrini Associated Cholangiocarcinoma. EBioMedicine 2016, 8, 195–202. [Google Scholar] [CrossRef]
- Sitthirak, S.; Suksawat, M.; Phetcharaburanin, J.; Wangwiwatsin, A.; Klanrit, P.; Namwat, N.; Khuntikeo, N.; Titapun, A.; Jarearnrat, A.; Sangkhamanon, S.; et al. Chemotherapeutic resistant cholangiocarcinoma displayed distinct intratumoral microbial composition and metabolic profiles. PeerJ 2022, 10, e13876. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.K.; Min, S.K.; Lee, W.H. 16S rDNA microbiome composition pattern analysis as a diagnostic biomarker for biliary tract cancer. World J. Surg. Oncol. 2020, 18, 19. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Heij, L.R.; Luedde, T.; van Dam, R.; Lang, S.A.; Ulmer, T.F.; Hornef, M.W.; Neumann, U.P. Bacterial bile duct colonization in perihilar cholangiocarcinoma and its clinical significance. Sci. Rep. 2021, 11, 2926. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, P.; Serra, N.; D’Arpa, F.; Agrusa, A.; Gulotta, G.; Fasciana, T.; Rodolico, V.; Giammanco, A.; Sergi, C. The microbiota of the bilio-pancreatic system: A cohort, STROBE-compliant study. Infect. Drug Resist. 2019, 12, 1513–1527. [Google Scholar] [CrossRef]
- Kim, I.H.; Choi, J.K.; Lee, D.G.; Lee, I.S.; Hong, T.H.; You, Y.K.; Chun, H.J.; Lee, M.A. Clinical significance of isolated biliary candidiasis in patients with unresectable cholangiocarcinoma. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 533–539. [Google Scholar] [CrossRef]
- Chen, B.; Fu, S.W.; Lu, L.; Zhao, H. A Preliminary Study of Biliary Microbiota in Patients with Bile Duct Stones or Distal Cholangiocarcinoma. Biomed. Res. Int. 2019, 2019, 1092563. [Google Scholar] [CrossRef] [PubMed]
- Saab, M.; Mestivier, D.; Sohrabi, M.; Rodriguez, C.; Khonsari, M.R.; Faraji, A.; Sobhani, I. Characterization of biliary microbiota dysbiosis in extrahepatic cholangiocarcinoma. PLoS ONE 2021, 16, e0247798. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chu, J.; Su, F.; Ding, X.; Zhang, Y.; Dou, L.; Liu, Y.; Ke, Y.; Liu, X.; Liu, Y.; et al. Characteristics of bile microbiota in cholelithiasis, perihilar cholangiocarcinoma, distal cholangiocarcinoma, and pancreatic cancer. Am. J. Transl. Res. 2022, 14, 2962–2971. [Google Scholar]
- Dangtakot, R.; Intuyod, K.; Ahooja, A.; Wongwiwatchai, J.; Hanpanich, P.; Lulitanond, A.; Chamgramol, Y.; Pinlaor, S.; Pinlaor, P. Profiling of Bile Microbiome Identifies District Microbial Population between Choledocholithiasis and Cholangiocarcinoma Patients. Asian Pac. J. Cancer Prev. 2021, 22, 233–240. [Google Scholar] [CrossRef]
- Avilés-Jiménez, F.; Guitron, A.; Segura-López, F.; Méndez-Tenorio, A.; Iwai, S.; Hernández-Guerrero, A.; Torres, J. Microbiota studies in the bile duct strongly suggest a role for Helicobacter pylori in extrahepatic cholangiocarcinoma. Clin. Microbiol. Infect. 2016, 22, 178.e111–178.e122. [Google Scholar] [CrossRef]
- Miyabe, K.; Chandrasekhara, V.; Wongjarupong, N.; Chen, J.; Yang, L.; Johnson, S.; Chia, N.; Walther-Antonio, M.; Yao, J.Z.; Harrington, S.C.; et al. Potential Role of Inflammation-Promoting Biliary Microbiome in Primary Sclerosing Cholangitis and Cholangiocarcinoma. Cancers 2022, 14, 2120. [Google Scholar] [CrossRef] [PubMed]
- Roa, J.C.; García, P.; Kapoor, V.K.; Maithel, S.K.; Javle, M.; Koshiol, J. Gallbladder cancer. Nat. Rev. Dis. Primers 2022, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Yadav, M.; Tripathi, G.; Mathew, B.; Bindal, V.; Falari, S.; Pamecha, V.; Maras, J.S. Bile multi-omics analysis classifies lipid species and microbial peptides predictive of carcinoma of gallbladder. Hepatology 2022, 76, 920–935. [Google Scholar] [CrossRef]
- Sharma, V.; Chauhan, V.S.; Nath, G.; Kumar, A.; Shukla, V.K. Role of bile bacteria in gallbladder carcinoma. Hepatogastroenterology 2007, 54, 1622–1625. [Google Scholar]
- Nath, G.; Gulati, A.K.; Shukla, V.K. Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J. Gastroenterol. 2010, 16, 5395–5404. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Loza, E.; Villa-Gomez, G.; Trujillo, C.C.; Baez, S.; Asai, T.; Ikoma, T.; Endoh, K.; Nakamura, K. Metagenomics of Microbial Communities in Gallbladder Bile from Patients with Gallbladder Cancer or Cholelithiasis. Asian Pac. J. Cancer Prev. 2018, 19, 961–967. [Google Scholar] [PubMed]
- Choi, S.J.; Kim, Y.; Jeon, J.; Gwak, H.J.; Kim, M.; Kang, K.; Kim, Y.; Jeong, J.; Jung, Y.K.; Lee, K.G.; et al. Association of Microbial Dysbiosis with Gallbladder Diseases Identified by Bile Microbiome Profiling. J. Korean Med. Sci. 2021, 36, e189. [Google Scholar] [CrossRef] [PubMed]
- Walawalkar, Y.D.; Gaind, R.; Nayak, V. Study on Salmonella Typhi occurrence in gallbladder of patients suffering from chronic cholelithiasis-a predisposing factor for carcinoma of gallbladder. Diagn. Microbiol. Infect. Dis. 2013, 77, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Wozniak, A.; Cook, P.; Adaniel, C.; Acevedo, J.; Azócar, L.; Hsing, A.W.; Roa, J.C.; Pasetti, M.F.; Miquel, J.F.; et al. Salmonella enterica serovar Typhi and gallbladder cancer: A case-control study and meta-analysis. Cancer Med. 2016, 5, 3310–3235. [Google Scholar] [CrossRef]
- Nagaraja, V.; Eslick, G.D. Systematic review with meta-analysis: The relationship between chronic Salmonella typhi carrier status and gall-bladder cancer. Aliment. Pharmacol. Ther. 2014, 39, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L.E.; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H.; et al. Salmonella Manipulation of Host Signaling Pathways Provokes Cellular Transformation Associated with Gallbladder Carcinoma. Cell Host Microbe 2015, 17, 763–774. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, J.D.; Weng, M.Z.; Zhang, Y.; Wang, X.F.; Gong, W.; Quan, Z.W. Infections of Helicobacter spp. in the biliary system are associated with biliary tract cancer: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 447–454. [Google Scholar] [CrossRef]
- Murphy, G.; Michel, A.; Taylor, P.R.; Albanes, D.; Weinstein, S.J.; Virtamo, J.; Parisi, D.; Snyder, K.; Butt, J.; McGlynn, K.A.; et al. Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology 2014, 60, 1963–1971. [Google Scholar] [CrossRef]
- Takayama, S.; Takahashi, H.; Matsuo, Y.; Okada, Y.; Takeyama, H. Effect of Helicobacter bilis infection on human bile duct cancer cells. Dig. Dis. Sci. 2010, 55, 1905–1910. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Hu, Y.; Li, H.; Ren, T.; Li, Y.; Liu, L.; Li, L.; Li, X.; Wang, Z.; et al. A metagenomic study of biliary microbiome change along the cholecystitis-carcinoma sequence. Clin. Transl. Med. 2020, 10, e97. [Google Scholar] [CrossRef]
- Kirishima, M.; Yokoyama, S.; Matsuo, K.; Hamada, T.; Shimokawa, M.; Akahane, T.; Sugimoto, T.; Tsurumaru, H.; Ishibashi, M.; Mataki, Y.; et al. Gallbladder microbiota composition is associated with pancreaticobiliary and gallbladder cancer prognosis. BMC Microbiol. 2022, 22, 147. [Google Scholar] [CrossRef]
- Pomyen, Y.; Chaisaingmongkol, J.; Rabibhadana, S.; Pupacdi, B.; Sripan, D.; Chornkrathok, C.; Budhu, A.; Budhisawasdi, V.; Lertprasertsuke, N.; Chotirosniramit, A.; et al. Gut dysbiosis in Thai intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Sci. Rep. 2023, 13, 11406. [Google Scholar] [CrossRef] [PubMed]
- Chagani, S.; Kwong, L.N. Cholangiocarcinoma Risk Factors Open the Floodgates for Gut Microbes and Immunosuppressive Myeloid Cells. Cancer Discov. 2021, 11, 1014–1015. [Google Scholar] [CrossRef]
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary sclerosing cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Horsley-Silva, J.L.; Carey, E.J.; Lindor, K.D. Advances in primary sclerosing cholangitis. Lancet Gastroenterol. Hepatol. 2016, 1, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Özdirik, B.; Müller, T.; Wree, A.; Tacke, F.; Sigal, M. The Role of Microbiota in Primary Sclerosing Cholangitis and Related Biliary Malignancies. Int. J. Mol. Sci. 2021, 22, 6975. [Google Scholar] [CrossRef] [PubMed]
- Little, R.; Wine, E.; Kamath, B.M.; Griffiths, A.M.; Ricciuto, A. Gut microbiome in primary sclerosing cholangitis: A review. World J. Gastroenterol. 2020, 26, 2768–2780. [Google Scholar] [CrossRef]
- Liwinski, T.; Zenouzi, R.; John, C.; Ehlken, H.; Ruhlemann, M.C.; Bang, C.; Groth, S.; Lieb, W.; Kantowski, M.; Andersen, N.; et al. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut 2020, 69, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Grigor’eva, I.N.; Romanova, T.I. Gallstone Disease and Microbiome. Microorganisms 2020, 8, 835. [Google Scholar] [CrossRef]
- Fremont-Rahl, J.J.; Ge, Z.; Umana, C.; Whary, M.T.; Taylor, N.S.; Muthupalani, S.; Carey, M.C.; Fox, J.G.; Maurer, K.J. An analysis of the role of the indigenous microbiota in cholesterol gallstone pathogenesis. PLoS ONE 2013, 8, e70657. [Google Scholar] [CrossRef]
- Wang, H.H.; Portincasa, P.; Afdhal, N.H.; Wang, D.Q. Lith genes and genetic analysis of cholesterol gallstone formation. Gastroenterol. Clin. N. Am. 2010, 39, 185–207. [Google Scholar] [CrossRef]
- Xu, M.Y.; Ma, J.H.; Yuan, B.S.; Yin, J.; Liu, L.; Lu, Q.B. Association between Helicobacter pylori infection and gallbladder diseases: A retrospective study. J. Gastroenterol. Hepatol. 2018, 33, 1207–1212. [Google Scholar] [CrossRef]
- Fatemi, S.M.; Doosti, A.; Shokri, D.; Ghorbani-Dalini, S.; Molazadeh, M.; Tavakoli, H.; Minakari, M.; Tavakkoli, H. Is There a Correlation between Helicobacter Pylori and Enterohepatic Helicobacter Species and Gallstone Cholecystitis? Middle East J. Dig. Dis. 2018, 10, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Urdaneta, V.; Casadesús, J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Trifylli, E.M.; Koustas, E.; Papadopoulos, N.; Sarantis, P.; Aloizos, G.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Karamouzis, M.V. An Insight into the Novel Immunotherapy and Targeted Therapeutic Strategies for Hepatocellular Carcinoma and Cholangiocarcinoma. Life 2022, 12, 665. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; Bilodeau, S.; Greten, T.F.; Wang, X.W.; Trinchieri, G. The gut-liver axis: Host microbiota interactions shape hepatocarcinogenesis. Trends Cancer 2022, 8, 583–597. [Google Scholar] [CrossRef]
- Koustas, E.; Sarantis, P.; Papavassiliou, A.G.; Karamouzis, M.V. The Resistance Mechanisms of Checkpoint Inhibitors in Solid Tumors. Biomolecules 2020, 10, 666. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Liao, W.; Overman, M.J.; Boutin, A.T.; Shang, X.; Zhao, D.; Dey, P.; Li, J.; Wang, G.; Lan, Z.; Li, J.; et al. KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell 2019, 35, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 2014, 6, 237ra267. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, D.; Long, J.; Yang, X.; Lin, J.; Song, Y.; Xie, F.; Xun, Z.; Wang, Y.; Wang, Y.; et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer 2021, 9, e003334. [Google Scholar] [CrossRef] [PubMed]
- Elvevi, A.; Laffusa, A.; Gallo, C.; Invernizzi, P.; Massironi, S. Any Role for Microbiota in Cholangiocarcinoma? A Comprehensive Review. Cells 2023, 12, 370. [Google Scholar] [CrossRef]
- Beyoğlu, D.; Idle, J.R. The metabolomic window into hepatobiliary disease. J. Hepatol. 2013, 59, 842–858. [Google Scholar] [CrossRef]
- Miolo, G.; Muraro, E.; Caruso, D.; Crivellari, D.; Ash, A.; Scalone, S.; Lombardi, D.; Rizzolio, F.; Giordano, A.; Corona, G. Pharmacometabolomics study identifies circulating spermidine and tryptophan as potential biomarkers associated with the complete pathological response to trastuzumab-paclitaxel neoadjuvant therapy in HER-2 positive breast cancer. Oncotarget 2016, 7, 39809–39822. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Murakami, Y.; Kubo, S.; Tamori, A.; Itami, S.; Kawamura, E.; Iwaisako, K.; Ikeda, K.; Kawada, N.; Ochiya, T.; Taguchi, Y.H. Comprehensive analysis of transcriptome and metabolome analysis in Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma. Sci. Rep. 2015, 5, 16294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Z.; Shi, Z.; Zhu, Z.; Li, C.; Du, Z.; Zhang, Y.; Wang, Z.; Jiao, Z.; Tian, X.; et al. Analysis of bile acid profile in plasma to differentiate cholangiocarcinoma from benign biliary diseases and healthy controls. J. Steroid Biochem. Mol. Biol. 2021, 205, 105775. [Google Scholar] [CrossRef]
- Banales, J.M.; Iñarrairaegui, M.; Arbelaiz, A.; Milkiewicz, P.; Muntané, J.; Muñoz-Bellvis, L.; La Casta, A.; Gonzalez, L.M.; Arretxe, E.; Alonso, C.; et al. Serum Metabolites as Diagnostic Biomarkers for Cholangiocarcinoma, Hepatocellular Carcinoma, and Primary Sclerosing Cholangitis. Hepatology 2019, 70, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, R.; Zhou, X.; Liang, X.; Campbell, D.J.; Zhang, X.; Zhang, L.; Shi, R.; Wang, G.; Pandak, W.M.; et al. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology 2014, 60, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, J.; Lv, S.; Sun, S.; Liu, C.; Xu, F.; Sun, H.; Yang, J.; Wang, X.; Zhong, X.; et al. Linoleic acid pathway disturbance contributing to potential cancerization of intrahepatic bile duct stones into intrahepatic cholangiocarcinoma. BMC Gastroenterol. 2022, 22, 269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Dong, C.; Lin, Y.; Shi, H.; Zhou, W. Interplay between the Human Microbiome and Biliary Tract Cancer: Implications for Pathogenesis and Therapy. Microorganisms 2023, 11, 2598. https://doi.org/10.3390/microorganisms11102598
Ye C, Dong C, Lin Y, Shi H, Zhou W. Interplay between the Human Microbiome and Biliary Tract Cancer: Implications for Pathogenesis and Therapy. Microorganisms. 2023; 11(10):2598. https://doi.org/10.3390/microorganisms11102598
Chicago/Turabian StyleYe, Cheng, Chunlu Dong, Yanyan Lin, Huaqing Shi, and Wence Zhou. 2023. "Interplay between the Human Microbiome and Biliary Tract Cancer: Implications for Pathogenesis and Therapy" Microorganisms 11, no. 10: 2598. https://doi.org/10.3390/microorganisms11102598
APA StyleYe, C., Dong, C., Lin, Y., Shi, H., & Zhou, W. (2023). Interplay between the Human Microbiome and Biliary Tract Cancer: Implications for Pathogenesis and Therapy. Microorganisms, 11(10), 2598. https://doi.org/10.3390/microorganisms11102598







