Abstract
Melipona quadrifasciata anthidioides and Scaptotrigona depilis are species of stingless bees capable of producing propolis, which has considerable bioprospecting potential. In this context, the objective of this study was to determine the chemical compositions and evaluate the antimicrobial activity of propolis produced by M. q. anthidioides and S. depilis. The ethanolic extracts of propolis of M. q. anthidioides (EEP-M) and S. depilis (EEP-S) were prepared, and their chemical constituents were characterized by HPLC-ESI-MS. The antimicrobial activity was evaluated against bacteria and fungi, isolated from reference strains and hospital origin resistant to the action of antibiotics. From EEP-M, phenolic compounds were annotated, including gallic acid, ellagic acid, and flavonoids, as well as diterpenes and triterpenes. EEP-S showed mainly triterpene in its chemical composition. Both extracts inhibited the growth of medically relevant bacteria and fungi, including hospital-acquired and antimicrobial-resistant. In general, EEP-S showed better antimicrobial activity compared to EEP-M. The MIC of EEP-S against vancomycin-resistant Enterococcus faecalis was 3.50 mg/mL, while the MIC of EEP-M was 5.33 ± 0.16 mg/mL. In conclusion, this study shows that propolis produced by M. q. anthidioides and S. depilis has the potential to be used for the prevention or treatment of microbial infections.
1. Introduction
Melipona quadrifasciata anthidioides (Lepeletier, 1836) and Scaptotrigona depilis (Moure, 1942) are species of stingless bees found in South America, distributed in Argentina, Paraguay, Bolivia, and Brazil [1]. These bees belong to the Meliponini tribe and are efficient pollinators of native plants [2]. Additionally, they can produce honey as a nutritional source for offspring in addition to cerumen and propolis, which provide mechanical and biological protection to the bees of the hive [3].
Among bee products, propolis has been widely studied because it is a complex bioactive mixture known for its high chemical diversity [3,4,5] and important pharmacological activities [6,7]. Propolis is formed by mixing plant exudates with salivary enzymes from bees, resulting in a viscous material with variable color and flavor [8,9].
These unique characteristics render propolis a product of commercial interest and great pharmacological potential, since qualitative and quantitative changes in the chemical compounds found in propolis modify its therapeutic properties [10,11,12]. Some studies describe the chemical composition of propolis from M. q. anthidioides and S. depilis, reporting a predominance of diterpenes [13,14] in addition to phytosterols, phenolic compounds, and tocopherol [15]. These compounds may be related to the biological activities already described for these products, such as antibacterial [9,13], antioxidant [14,15], and cytotoxic activities [15].
Given the therapeutic potential of the propolis from M. q. anthidioides and S. depilis, this study aimed to investigate the chemical composition of propolis from these species and evaluate its antimicrobial activity against different bacteria and yeasts, isolated from reference strains and hospital origin resistant to the action of antibiotics.
2. Materials and Methods
2.1. Preparation of the Ethanol Extract of Propolis
Propolis samples from M. q. anthidioides and S. depilis were collected from the state of Mato Grosso do Sul (22°13′12″ S–54°49′2″ W), in the Midwest region of Brazil, with a total of seven collections being performed for each species. The ethanol extract of propolis was prepared according to the method described by Bonamigo et al. [15], using 4.5 mL of 80% ethanol per 1 g of propolis. The extraction was performed at 70 °C until total dissolution, and, subsequently, this material was filtered by filter paper qualitative 80 g/m2 (Prolab, São Paulo, SP, Brazil) to obtain the ethanolic extracts of propolis of M. q. anthidioides (EEP-M) and S. depilis (EEP-S). After the preparation of the extracts, they were kept at a temperature of −20 °C until analysis.
2.2. Analyses by High-Performance Liquid Chromatography Coupled to Diode Array Detector and Mass Spectrometry (HPLC-DAD-MS)
Five microliters of each sample, EEP-M or EEP-S (1 mg/mL), were injected into an LC-20AD ultra-fast liquid chromatograph (UFLC) (Shimadzu) coupled to a diode array detector (DAD) and a mass spectrometer micrOTOF-Q III (Bruker Daltonics) with electrospray ionization source (ESI) and quadrupole and time-of-flight analyzers. A column Kinetex C-18 (150 mm × 2.2 mm inner diameter, 2.6 μm) was used in the analyses and maintained at 50 °C during the analyses. The mobile phase consisted of deionized water (A) and acetonitrile (B), both containing 0.1% formic acid, and the following elution gradient profile was applied: 0–2 min-3% B; 2–25 min-3–25% B; 25–40 min-25–80% B; and 40–43 min-80% B. The gradient was followed by reconditioning of the column (5 min). The flow rate was 0.3 mL/min. The samples were analyzed in negative and positive ion mode (m/z 120–1300). Nitrogen was applied as a nebulizer (4 Bar), drying (9 mL/min), and collision gas. The capillary voltage was 4500 kV.
2.3. Antimicrobial Activity
The antimicrobial activity of EEP-M and EEP-S was investigated in microorganisms collected from biological fluids at the Hospital Center and identified in the Microbiology Laboratory of Escola Superior Agrária (ESA) de Bragança, Portugal. Reference strains were obtained from the authorized ATCC distributor (LGC Standards SLU, Barcelona, Spain), as listed in Table 1.

Table 1.
Strains of microorganisms used to test the antimicrobial activity of EEP-M and EEP-S.
The microorganisms were stored in a Mueller–Hinton broth supplemented with 20% glycerol at −70 °C before experimental use. The inoculum was then prepared by dilution of the cell mass in 0.85% NaCl solution, adjusted to 0.5 on the MacFarland scale, as confirmed by spectrophotometric readings at 580 and 640 nm, for bacteria and yeast, respectively. Antimicrobial assays were performed as described by Silva et al. [16] using nutrient broth (NB) for bacteria or yeasts peptone dextrose (YPD) for yeast in microplates of 96 wells. The extracts were diluted in dimethylsulfoxide (DMSO) and transferred to the first well, followed by serial dilution (0.625–160 mg/mL). The inoculum was added to all wells (104 colony forming units (CFU)/mL), and the plates were incubated at 37 °C for 24 h for bacteria and 25 °C for 48 h for yeast. Media controls were conducted with and without inoculum, and 0.27% DMSO alone was used as a solvent control in the inoculated medium. In addition, gentamicin and amphotericin B were used as antibacterial and antifungal positive controls, respectively. After the incubation period, the antimicrobial activity was detected by the addition of 20 μL of 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) solution (5 mg/mL). The minimum inhibitory concentration (MIC) was defined as the lowest concentration of EEP-M and EEP-S that visibly inhibited the growth of microorganisms, as indicated by TTC staining, which marks viable cells in red color, due to the formation of formazan. To determine the minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC), 20 μL of the last well where growth was observed and from each well where no color changes were seen was seeded in NB or YPD and incubated for 24 h at 37 °C for bacteria growth and 48 h for yeast growth. The lowest concentration that did not result in growth (<10 CFU/plate) after this subculture process was considered the MBC or MFC. The experiments were performed in triplicate, and the results were expressed in mg/mL. The data are shown as the mean ± standard error of the mean (SEM).
2.4. Statistical Analysis
Statistical analysis was performed for statistically significant differences between groups using one-way analysis of variance (ANOVA) followed by the Newman–Keuls test for the comparison of more than two groups using the Prism 5 GraphPad Software (GraphPad Software Inc., San Diego, CA, USA). The results were considered significant when p < 0.05.
3. Results
3.1. Chemical Composition by HPLC-DAD-MS
The extracts EEP-M and EEP-S were analyzed by HPLC-DAD-MS, and their constituents could be identified by UV, MS (accurate mass), and MS/MS data compared with data reported in the literature. The molecular formulas were determined considering errors and m-Sigma up 8 ppm and 30, respectively. In addition, some compounds were confirmed by injection of authentic standards. Thus, forty-seven compounds were detected and summarized in Table 2, and the chromatograms are illustrated in Figure 1. Chemical differences between EEP-M and EEP-S were evidenced, such as the presence of nonpolar compounds in EEP-S, which are not present in EEP-M. Additionally, EEP-M revealed mainly phenolic compounds in its composition.

Table 2.
Chemicals constituents identified from ethanolic extracts of Melipona quadrifasciata anthidiodes (EEP-M) and Scaptotrigona depilis (EEP-S) propolis by LC-DAD-MS.
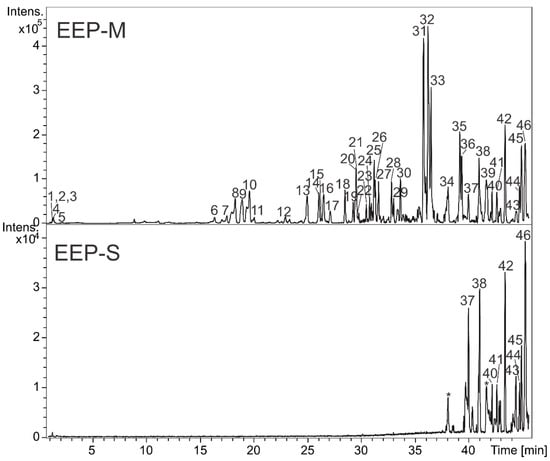
Figure 1.
Base peak chromatogram (negative ion mode) from ethanolic extracts of Melipona quadrifasciata anthidiodes (EEP-M) and Scaptotrigona depilis (EEP-S) propolis by LC-DAD-MS. (* contaminant peaks from the chromatographic system.)
Compounds 5 and 6 were confirmed by injection of authentic standards and identified as gallic acid and ellagic acid, respectively. In addition, peaks 1–4 revealed an absorption band with λmax at 270 nm in their UV spectra, which is compatible with the chromophore of gallic acid [17]. For these components, the fragment ions at m/z 169 were observed, indicating the presence of galloyl substituent, while the ion m/z 301 suggested the hexahydroxydiphenoyl group. These components were annotated as hydrolysable tannins O-galloyl hexoside (1), di-O-galloyl hexoside (2 and 4), and O-galloyl- hexahydroxydiphenoyl hexoside (3). Their spectral data are compatible with the data described in the literature [17,18].
The compounds 7–8, 10, 15–16, 18–19, and 27 showed two absorption bands at the wavelength ≈280 and 310 nm, which are compatible and suggested, together with MS/MS data, the chromophores relative to galloyl and coumaroyl substituents [19]. Beyond fragment ions at m/z 169 [gallic acid-H]-, losses of 146 or 164 u (146 + H2O) suggested the coumaroyl substituents [17]. These metabolites were putatively annotated as O-coumaroyl O-galloyl hexoside (7 and 8), O-coumaroyl di-O-galloyl hexoside (10), O-coumaroyl tetra-O-galloyl hexoside (15), di-O-coumaroyl hexoside (16), di-O-coumaroyl O-galloyl hexoside (18), O-coumaroyl O-galloyl O-benzoyl hexoside (19), and O-coumaroyl O-cynnamoyl O-galloyl hexoside (27). The compounds 13 and 14 also showed losses of 148 u relative to losses of a cinnamoyl and subsequently a water molecule, as reported by Jin et al. [20], and they were annotated as O-cinnamoyl O-galloyl hexoside (13) and O-cinnamoyl di-O-galloyl hexoside (14).
The chromatographic peaks 9, 17, 21, and 28 presented UV spectra (λmax ≈ 290 and 330 nm—shoulder) compatible with flavanones [19]. The MS/MS data were compared to fragmentations reported in the literature, and they revealed relevant fragments to annotate them such as losses of CO, retro-Diels–Alder fission of the C ring, and radical methyl [21,22]. Thus, these components were annotated as eriodictyol (9), naringenin (17), O-methyl eriodictyol (21), and O-methyl naringenin (28) [19,22,23].
The compounds 31–34 and 39 revealed deprotonated ions compatible with a molecular formula that suggested diterpenes, while 37–38 and 41 were similar for triterpenes. The compound 39 revealed a fragmentation pathway similar to the diterpene abietic acid, which is a component already described from propolis of M. quadrifasciata [21].
3.2. Antimicrobial Activity
Investigation of the antimicrobial activity of the propolis extracts of M. q. anthidioides and S. depilis revealed both to be effective against the microorganisms evaluated; EEP-S was more effective than EEP-M. Inhibitory and bactericidal activity against gram-positive and gram-negative bacteria were observed, including hospital-acquired strains resistant to methicillin and vancomycin (Table 3). The extracts also showed inhibitory and fungicidal activity against Cryptococcus neoformans and Candida albicans, in both reference strains and amphotericin-B-resistant strains (Table 4).

Table 3.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for the studied bacteria, gram-negative and gram-positive.

Table 4.
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) for the studied fungi.
4. Discussion
Propolis is a bee product known for centuries for its medicinal properties, including its antiseptic, healing, anti-inflammatory, and anticancer properties [3,5,24]. These activities are related to the chemical composition of propolis, which varies according to the local vegetation, season, and bee species that generate this product [25,26,27]. In this study, the chemical composition of propolis from stingless bees M. q. anthidioides and S. depilis varied among the evaluated samples. The extracts showed bactericidal and fungicidal activity against reference strains and hospital origin resistant to the action of antimicrobial agents.
The EEP-M presented in its composition 46 compounds, among them phenolic compounds, including gallic acid, ellagic acid, and flavonoids such as naringenin and eriodictyol. In addition, the EEP-M presented triterpenes, which were also detected in the EEP-S. Interestingly, EEP-S showed still unknown lipophilic compounds, which ratifies bee products as sources of new bioactive molecules, since this extract has proved to be a more potent antimicrobial in inhibiting the growth of medically relevant microorganisms, including the bacteria Staphylococcus aureus and Pseudomonas aeruginosa and the yeast C. albicans.
Przybyłek and Karpinski [9] reported that propolis promotes antibacterial activity by increasing the permeability of the cell membrane, disruption of membrane potential and adenosine triphosphate (ATP) production, and by decreasing bacterial motility. These mechanisms of action of propolis are correlated with the chemical profile, which may correspond to the different proportions of terpenes and phenolic compounds.
Lipophilic compounds such as terpenes, present in EEP-M and EEP-S, are described in the literature because they present antimicrobial action [28,29].
Cornara et al. [25] emphasized that the antimicrobial activity of different samples of propolis is related to the presence of terpenes such as α-pinene, β-pinene, δ-cadinene, farnesol, and dihydroeudesmol. Terpenes can cross the cell membrane and promote the loss of essential intracellular components, resulting in the death of microorganisms such as bacteria and fungi [30].
In addition to terpenes, in other studies with propolis extracts, antimicrobial activity against different strains of Staphylococcus was attributed to the presence of phenolic compounds such as caffeic acid and its derivatives and flavonoids such as pinostrobin, pinocembrin, chrysin, and galangin [31].
Phenolic compounds as flavonoids can act by inhibiting the activity of the enzymes RNA polymerase [25], DNA gyrase, and ATP synthase and by inhibiting virulence factors such as lipopolysaccharides present in the outer membrane of gram-negative bacteria [32]. Flavonoids are the largest group of phenolic compounds, totaling approximately 6500 compounds [33], and are widely known for their biological activities.
Additionally, flavonoids identified in different propolis extracts, such as quercetin, myricetin, kaempferol, pinocembrin, and naringenin, have antifungal activity against Candida spp., acting mainly in the inhibition of the development of this microorganism [34]. Haghdoost et al. [35] reported that propolis decreases the formation of germ tubes, one of the main virulence factors of fungi, such as C. albicans.
Gucwa et al. [36] reported the antifungal action of Polish propolis extract and attributed the depolarization of the fungal membrane and inhibition of hyphae formation in C. albicans as the main mechanisms of action. The authors also highlight that of the 50 propolis samples evaluated, the ones with the highest antifungal activity had higher flavones and flavonols content than extracts with the lowest antifungal activity [36].
In conclusion, this study demonstrates that despite their very different compositions, propolis extracts produced by both M. q. anthidioides and S. depilis stingless bees were active, showing that these bee products have the potential to be used for the prevention or treatment of microbial infections.
Author Contributions
Conceptualization: J.F.C., T.B., P.d.S.d.R., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; methodology: J.F.C., T.B., P.d.S.d.R., V.M.B.P., U.P.d.S., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; software: J.F.C., T.B., P.d.S.d.R., D.B.S., L.M.E., K.d.P.S. and E.L.d.S.; validation, J.F.C., T.B., P.d.S.d.R., J.B.P.B., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; formal analysis: J.F.C., P.d.S.d.R., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; investigation, J.F.C., T.B., P.d.S.d.R., J.B.P.B., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; resources: D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; data curation: J.F.C., T.B., P.d.S.d.R., J.B.P.B., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; writing—original draft preparation: J.F.C., T.B., P.d.S.d.R., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; writing—review and editing: J.F.C., T.B., P.d.S.d.R., V.M.B.P., U.P.d.S., J.B.P.B., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; visualization: J.F.C., T.B., P.d.S.d.R., J.B.P.B., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; supervision: D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; project administration: J.F.C., D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S.; funding acquisition: D.B.S., C.A.C., L.M.E., K.d.P.S. and E.L.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pró-Reitoria de Ensino de Pós-Graduação e Pesquisa da Universidade Federal da Grande Dourados (PROPP-UFGD); Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT: 275/2016); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [process nº 313047/2020-0]; Financiadora de Estudos e Projetos (Finep) and PRODER [24.073-Â, Portugal].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Camargo, J.M.F.; Pedro, S.R.M.; Melo, G.A.R. Meliponini Lepeletier, 1836. Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region-Online Version. Moure, J.S., Urban, D., Melo, G.A.R., Eds.; 2013. Available online: http://moure.cria.org.br/catalogue (accessed on 12 February 2021).
- Slaa, E.J.; Chaves, L.A.S.; Malagodi-Braga, K.S.; Hofstede, F.E. Stingless bees in applied pollination: Practice and perspectives. Apidologie 2006, 37, 293–315. [Google Scholar] [CrossRef]
- Lavinas, F.C.; Macedo, E.H.B.; Sá, G.B.; Amaral, A.C.F.; Silva, J.; Azevedo, M.; Vieira, B.A.; Domingos, T.F.S.; Vermelho, A.B.; Carneiro, C.S.; et al. Brazilian stingless bee propolis and geopropolis: Promising sources of biologically active compounds. Rev. Bras. Farm. 2018, 29, 389–399. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.-M.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of stingless bees: A phytochemist’s guide through the jungle of tropical biodiversity. Phytomedicine 2019, 86, 153098. [Google Scholar] [CrossRef]
- Osés, S.M.; Marcos, P.; Azofra, P.; De Pablo, A.; Fernández-Muíño, M.Á.; Sancho, M.T. Phenolic Profile, Antioxidant Capacities and Enzymatic Inhibitory Activities of Propolis from Different Geographical Areas: Needs for Analytical Harmonization. Antioxidants 2020, 9, 75. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Salatino, A.; Teixeira, E.; Negri, G.; Message, D. Origin and Chemical Variation of Brazilian Propolis. Evid. -Based Complement. Altern. Med. 2005, 2, 33–38. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Marcucci, M. Antibacterial ent-kaurene from Brazilian propolis of native stingless bees. Fitoterapia 2000, 71, 693–696. [Google Scholar] [CrossRef]
- Sawaya, A.C.H.F. Composition and antioxidant activity of propolis from three species of Scaptotrigona stingless bees. J. ApiProduct ApiMedical Sci. 2009, 1, 37–42. [Google Scholar] [CrossRef]
- Bonamigo, T.; Campos, J.F.; Alfredo, T.M.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; de Picoli Souza, K.; Dos Santos, E.L. Antioxidant, Cytotoxic, and Toxic Activities of Propolis from Two Native Bees in Brazil: Scaptotrigona depilis and Melipona quadrifasciata anthidioides. Oxidative Med. Cell. Longev. 2017, 2017, 1038153. [Google Scholar] [CrossRef]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef]
- Reichert, C.L.; Silva, D.; Carollo, C.A.; Weffort-Santos, A.M.; Santos, C. Metabolic profiling and correlation analysis for the determination of killer compounds of proliferating and clonogenic HRT-18 colon cancer cells from Lafoensia pacari. J. Ethnopharmacol. 2018, 224, 541–552. [Google Scholar] [CrossRef]
- Dutra, R.P.; Abreu, B.V.D.B.; Cunha, M.S.; Batista, M.C.A.; Torres, L.M.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Phenolic Acids, Hydrolyzable Tannins, and Antioxidant Activity of Geopropolis from the Stingless Bee Melipona fasciculata Smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef]
- Silva, D.B.; Okano, L.T.; Lopes, N.P.; de Oliveira, D.C. Flavanone glycosides from Bidens gardneri Bak. (Asteraceae). Phytochemistry 2013, 96, 418–422. [Google Scholar] [CrossRef]
- Jin, W.; Wang, Y.-F.; Ge, R.-L.; Shi, H.-M.; Jia, C.-Q.; Tu, P.-F. Simultaneous analysis of multiple bioactive constituents inRheum tanguticum Maxim. ex Balf. by high-performance liquid chromatography coupled to tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2351–2360. [Google Scholar] [CrossRef]
- Cisilotto, J.; Sandjo, L.P.; Faqueti, L.G.; Fernandes, H.; Joppi, D.; Biavatti, M.W.; Creczynski-Pasa, T.B. Cytotoxicity mechanisms in melanoma cells and UPLC-QTOF/MS2 chemical characterization of two Brazilian stingless bee propolis: Uncommon presence of piperidinic alkaloids. J. Pharm. Biomed. Anal. 2018, 149, 502–511. [Google Scholar] [CrossRef]
- Rubinho, M.P.; de Carvalho, P.L.N.; Reis, A.L.L.E.; Ern; Reis, E.; de Alencar, S.M.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Ikegaki, M. A comprehensive characterization of polyphenols by LC-ESI–QTOF-MS from Melipona quadrifasciata anthidioides geopropolis and their antibacterial, antioxidant and antiproliferative effects. Nat. Prod. Res. 2019, 34, 3139–3144. [Google Scholar] [CrossRef] [PubMed]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2019, 129, 108756. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.F.; dos Santos, H.F.; Bonamigo, T.; Domingues, N.L.C.; de Picoli Souza, K.; Dos Santos, E.L. Stingless Bee Propolis: New Insights for Anticancer Drugs. Oxidative Med. Cell. Longev. 2021, 2021, 2169017. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.R.; Sandjo, L.P.; Friedemann, M.T.; Tomazzoli, M.M.; Maraschin, M.; Mello, C.F.; Santos, A.R.S. Chemical characterization, antioxidant and antimicrobial activity of propolis obtained from Melipona quadrifasciata quadrifasciata and Tetragonisca angustula stingless bees. Brazilian J. Med. Biol. Res. 2018, 51, e7118. [Google Scholar] [CrossRef]
- Negri, G.; Salatino, A.; Pereira, L.L.R.; Salatino, M.L.F.; Nascimento, R.M.; Mendonça, R.Z. A highly complex stingless bee propolis: Composition and influence of the period of collection. JSFA Rep. 2022, 2, 64–80. [Google Scholar] [CrossRef]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef]
- Chimshirova, R.; Popova, M.; Chakir, A.; Valcheva, V.; Dimitrov, S.; Trusheva, B.; Romane, A.; Bankova, V. Antimicrobial Triterpenoids and Ingol Diterpenes from Propolis of Semi-Arid Region of Morocco. Molecules 2022, 27, 2206. [Google Scholar] [CrossRef]
- Islam, M.T.; da Mata, A.M.; de Aguiar, R.P.; Paz, M.F.; de Alencar, M.V.; Ferreira, P.M.; de Carvalho Melo-Cavalcante, A.A. Therapeutic Potential of Essential Oils Focusing on Diterpenes. Phytother. Res. 2016, 30, 1420–1444. [Google Scholar] [CrossRef]
- Grecka, K.; Kuś, P.M.; Okińczyc, P.; Worobo, R.W.; Walkusz, J.; Szweda, P. The Anti-Staphylococcal Potential of Ethanolic Polish Propolis Extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef]
- Seleem, D.; Pardi, V.; Murata, R.M. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity in vitro. Arch. Oral Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef]
- Haghdoost, N.S.; Salehi, T.Z.; Khosravi, A.; Sharifzadeh, A. Antifungal activity and influence of propolis against germ tube formation as a critical virulence attribute by clinical isolates of Candida albicans. J. Mycol. Med. 2016, 26, 298–305. [Google Scholar] [CrossRef]
- Gucwa, K.; Kusznierewicz, B.; Milewski, S.; Van Dijck, P.; Szweda, P. Antifungal Activity and Synergism with Azoles of Polish Propolis. Pathogens 2018, 7, 56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).