Bioactive Antimicrobial Peptides: A New Weapon to Counteract Zoonosis
Abstract
:1. Introduction
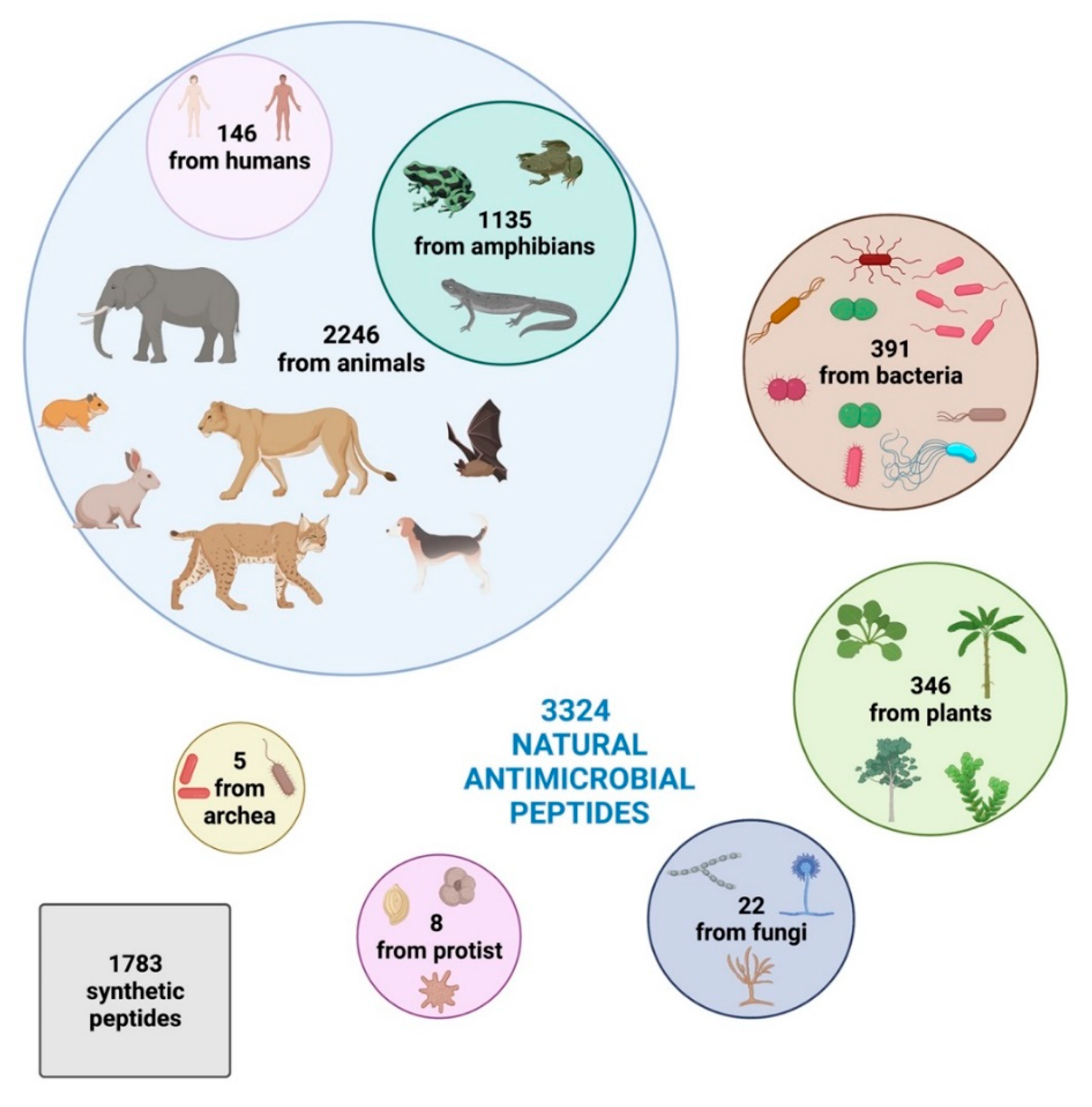
2. Increasing Interest in New Bioactive Antimicrobial Peptides
3. Evidence of the Potentiality of Synthetic Antimicrobial Peptides against Zoonoses
3.1. Viral Zoonosis
3.2. Bacterial Zoonosis
3.3. Fungal zoonosis
3.4. Zoonotic parasites
4. Clinical Development of SAMPs
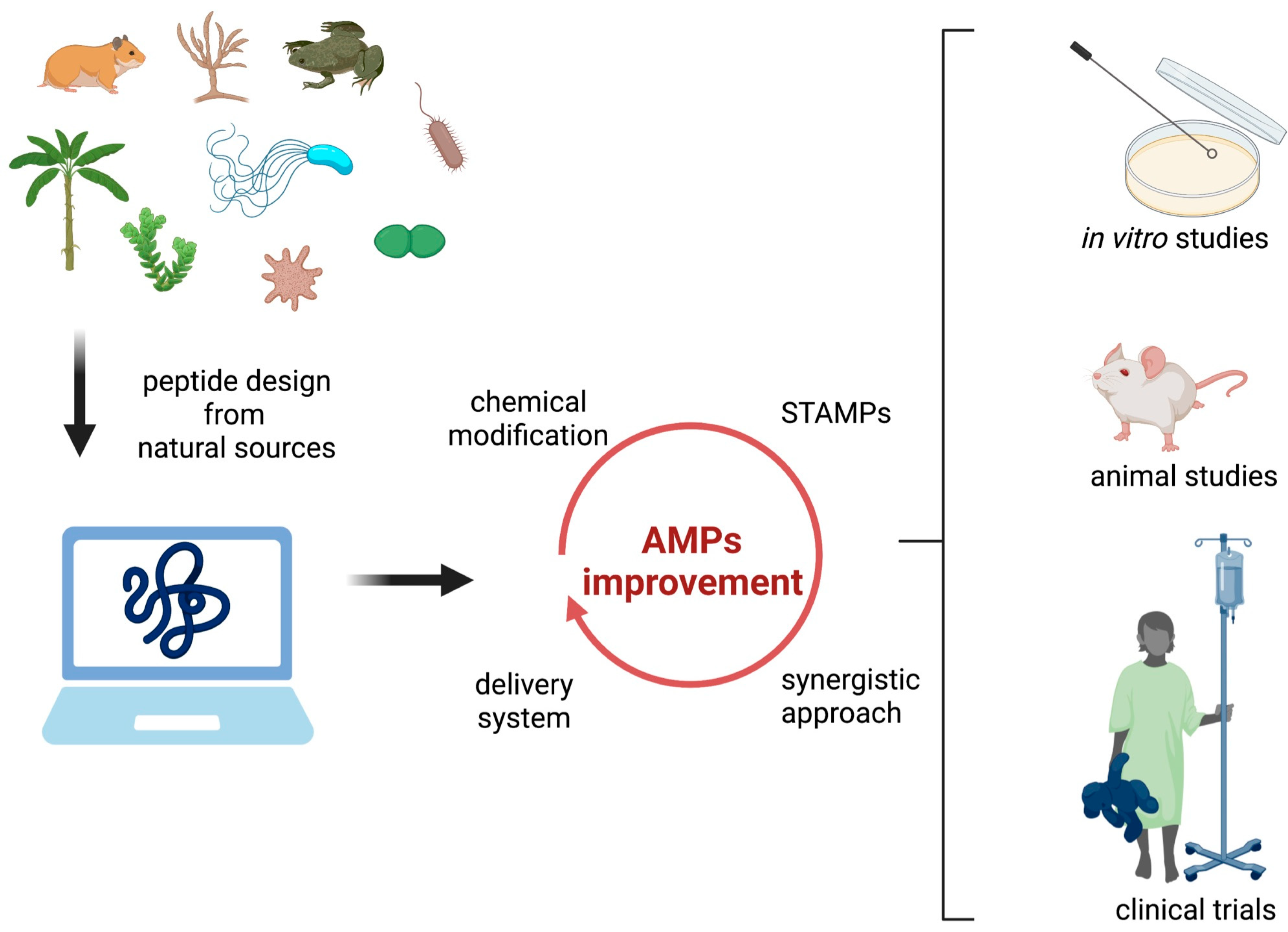
5. Prospects for Future Development of Therapeutic Antimicrobial Peptides
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO-Convened Global Study of Origins of SARS-CoV-2: China Part. Available online: https://www.who.int/publications-detail-redirect/who-convened-global-study-of-origins-of-sars-cov-2-china-part (accessed on 9 June 2022).
- World Health Organization Zoonoses. Available online: https://www.who.int/news-room/fact-sheets/detail/zoonoses (accessed on 9 June 2022).
- CDC. Centers for Disease Control and Prevention Zoonotic Diseases|One Health|CDC. Available online: https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html (accessed on 9 June 2022).
- World Health Organization. Eastern Mediterranean Regional Office Zoonotic Disease: Emerging Public Health Threats in the Region. Available online: http://www.emro.who.int/about-who/rc61/zoonotic-diseases.html (accessed on 9 June 2022).
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global Hotspots and Correlates of Emerging Zoonotic Diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Magouras, I.; Brookes, V.J.; Jori, F.; Martin, A.; Pfeiffer, D.U.; Dürr, S. Emerging Zoonotic Diseases: Should We Rethink the Animal–Human Interface? Front. Vet. Sci. 2020, 7, 582743. [Google Scholar] [CrossRef] [PubMed]
- Kemunto, N.; Mogoa, E.; Osoro, E.; Bitek, A.; Kariuki Njenga, M.; Thumbi, S.M. Zoonotic Disease Research in East Africa. BMC Infect. Dis. 2018, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Neiderud, C.-J. How Urbanization Affects the Epidemiology of Emerging Infectious Diseases. Infect. Ecol. Epidemiol. 2015, 5, 27060. [Google Scholar] [CrossRef]
- Khan, A.; Khan, M.; Ullah, S.; Wei, D.-Q. Hantavirus: The Next Pandemic We Are Waiting For? Interdiscip. Sci. Comput. Life Sci. 2021, 13, 147–152. [Google Scholar] [CrossRef]
- Zhong, S.; Crang, M.; Zeng, G. Constructing Freshness: The Vitality of Wet Markets in Urban China. Agric. Hum. Values 2020, 37, 175–185. [Google Scholar] [CrossRef]
- Rahman, T.; Sobur, A.; Islam, S.; Ievy, S.; Hossain, J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for South-East Asia. A Brief Guide to Emerging Infectious Diseases and Zoonoses; WHO Regional Office for South-East Asia: New Delhi, India, 2014; ISBN 978-92-9022-458-7. [Google Scholar]
- Otte, J.; Pica-Ciamarra, U. Emerging Infectious Zoonotic Diseases: The Neglected Role of Food Animals. One Health 2021, 13, 100323. [Google Scholar] [CrossRef]
- King, L. Neglected Zoonotic Diseases. In The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Opportunities for Integrated Intervention Strategies; National Academies Press (US): Washington, DC, USA, 2011; p. A13. [Google Scholar]
- World Health Organization. Smallpox. Available online: https://www.who.int/health-topics/smallpox (accessed on 9 June 2022).
- European Food Safety Authority. European Centre for Disease Prevention and Control The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- World Health Organization. Multi-Country Monkeypox Outbreak: Situation Update. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390 (accessed on 9 June 2022).
- Bidaisee, S.; Macpherson, C.N.L. Zoonoses and One Health: A Review of the Literature. J. Parasitol. Res. 2014, 2014, 874345. [Google Scholar] [CrossRef]
- Stephen, C.; Artsob, H.; Bowie, W.R.; Drebot, M.; Fraser, E.; Leighton, T.; Morshed, M.; Ong, C.; Patrick, D. Perspectives on Emerging Zoonotic Disease Research and Capacity Building in Canada. Can. J. Infect. Dis. Med. Microbiol. 2004, 15, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, M.L.; Doty, J.B.; Maghlakelidze, G.; Morgan, J.; Khmaladze, E.; Parkadze, O.; Donduashvili, M.; Wemakoy, E.O.; Muyembe, J.-J.; Mulumba, L.; et al. Frameworks for Preventing, Detecting, and Controlling Zoonotic Diseases. Emerg. Infect. Dis. 2017, 23, S71–S76. [Google Scholar] [CrossRef] [PubMed]
- Dubal, Z.; Barbuddhe, S.; Singh, N. Important Zoonotic Diseases: Prevention and Control; Technical Bulletin No. 39; ICAR Research Complex for Goa (Indian Council of Agricultural Research): Ela, India, 2014; p. 48. Available online: https://ccari.icar.gov.in/Technical%20Bulletin%20No.%2039.pdf (accessed on 9 June 2022).
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Hemeg, H. Nanomaterials for Alternative Antibacterial Therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [PubMed]
- Golkar, Z.; Bagasra, O.; Pace, D.G. Bacteriophage Therapy: A Potential Solution for the Antibiotic Resistance Crisis. J. Infect. Dev. Ctries. 2014, 8, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Dai, T. The Antimicrobial Effect of Blue Light: What Are Behind? Virulence 2017, 8, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Dasilva, D.; Haranahalli, K.; McCarthy, J.B.; Mallamo, J.; Ojima, I.; Del Poeta, M. The Future of Antifungal Drug Therapy: Novel Compounds and Targets. Antimicrob. Agents Chemother. 2021, 65, e01719-20. [Google Scholar] [CrossRef]
- Hoenigl, M.; Sprute, R.; Egger, M.; Arastehfar, A.; Cornely, O.A.; Krause, R.; Lass-Flörl, C.; Prattes, J.; Spec, A.; Thompson, G.R.; et al. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs 2021, 81, 1703–1729. [Google Scholar] [CrossRef]
- Adamson, C.S.; Chibale, K.; Goss, R.J.M.; Jaspars, M.; Newman, D.J.; Dorrington, R.A. Antiviral Drug Discovery: Preparing for the next Pandemic. Chem. Soc. Rev. 2021, 50, 3647–3655. [Google Scholar] [CrossRef]
- Aschrafi, A.; Zupin, L.; Vilela, L.M.B.; dos Santos Silva, C.A.; Filho, R.S.R.; de Lima, L.M.; de Andrade Lima, C.S.; Petix, V.; Tossi, A.; Amorim, L.L.B.; et al. Antimicrobial and Cytotoxic Properties of Extracts from Plants Traditionally Used in North-East Brazil. Int. J. Pharmacol. Phytochem. Ethnomedicine 2021, 16, 21–32. [Google Scholar] [CrossRef]
- Hollmann, A.; Cardoso, N.P.; Espeche, J.C.; Maffía, P.C. Review of Antiviral Peptides for Use against Zoonotic and Selected Non-Zoonotic Viruses. Peptides 2021, 142, 170570. [Google Scholar] [CrossRef] [PubMed]
- Benko-Iseppon, A. Plant Response to Biotic Stress: Insights from Transcriptomics and Structural Genomics|Plant Genomics 2016|Conferenceseries Ltd. 2016. Available online: https://www.omicsonline.org/proceedings/plant-response-to-biotic-stress-insights-from-transcriptomics-and-structural-genomics-105039.html (accessed on 26 March 2021).
- Benko-Iseppon, A.M.; Crovella, S. Ethnobotanical Bioprospection of Candidates for Potential Antimicrobial Drugs from Brazilian Plants: State of Art and Perspectives. Curr. Protein Pept. Sci. 2010, 11, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Benko-Iseppon, A.; Crovella, S. Editorial (Thematic Issue: Plant Immunity and Beyond: Signals from Proteins & Peptides). Curr. Protein Pept. Sci. 2017, 18, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Belarmino, L.C.; Benko-Iseppon, A.M. Data Bank Based Mining on the Track of Antimicrobial Weapons in Plant Genomes. Curr. Protein Pept. Sci. 2010, 11, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-K.; Kim, C.; Seo, C.H.; Park, Y. The Therapeutic Applications of Antimicrobial Peptides (AMPs): A Patent Review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Govender, T.; Kruger, H.G.; de la Torre, B.G.; Albericio, F. Short AntiMicrobial Peptides (SAMPs) as a Class of Extraordinary Promising Therapeutic Agents: Short AntiMicrobial Peptides. J. Pept. Sci. 2016, 22, 438–451. [Google Scholar] [CrossRef]
- Benko-Iseppon, A.M.; Galdino, S.L.; Calsa, T., Jr.; Kido, E.A.; Tossi, A.; Belarmino, L.C.; Crovella, S. Overview on Plant Antimicrobial Peptides. Curr. Protein Pept. Sci. 2010, 11, 181–188. [Google Scholar] [CrossRef]
- de Jesús-Pires, C.; Ferreira-Neto, J.R.C.; Pacifico Bezerra-Neto, J.; Kido, E.A.; de Oliveira Silva, R.L.; Pandolfi, V.; Wanderley-Nogueira, A.C.; Binneck, E.; da Costa, A.F.; Pio-Ribeiro, G.; et al. Plant Thaumatin-like Proteins: Function, Evolution and Biotechnological Applications. Curr. Protein Pept. Sci. 2020, 21, 36–51. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A Review of Antimicrobial Peptides and Their Therapeutic Potential as Anti-Infective Drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef]
- dos Santos-Silva, C.A.; Zupin, L.; Oliveira-Lima, M.; Vilela, L.M.B.; Bezerra-Neto, J.P.; Ferreira-Neto, J.R.; Ferreira, J.D.C.; de Oliveira-Silva, R.L.; de Jesús Pires, C.; Aburjaile, F.F.; et al. Plant Antimicrobial Peptides: State of the Art, In Silico Prediction and Perspectives in the Omics Era. Bioinform. Biol. Insights 2020, 14, 117793222095273. [Google Scholar] [CrossRef] [PubMed]
- Polesello, V.; Segat, L.; Crovella, S.; Zupin, L. Candida Infections and Human Defensins. Protein Pept. Lett. 2017, 24, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Kang, X.; Dong, F.; Liu, Y.; Zhu, N.; Hu, Y.; Xu, H.; Lao, X.; Zheng, H. DRAMP 3.0: An Enhanced Comprehensive Data Repository of Antimicrobial Peptides. Nucleic Acids Res. 2022, 50, D488–D496. [Google Scholar] [CrossRef]
- Oliveira-Lima, M.; Benko-Iseppon, A.; Neto, J.; Rodriguez-Decuadro, S.; Kido, E.; Crovella, S.; Pandolfi, V. Snakin: Structure, Roles and Applications of a Plant Antimicrobial Peptide. Curr. Protein Pept. Sci. 2017, 18, 368–374. [Google Scholar] [CrossRef]
- Haney, E.F.; Mansour, S.C.; Hancock, R.E.W. Antimicrobial Peptides: An Introduction. In Antimicrobial Peptides; Hansen, P.R., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1548, pp. 3–22. ISBN 978-1-4939-6735-3. [Google Scholar]
- Pelegrini, P.B.; del Sarto, R.P.; Silva, O.N.; Franco, O.L.; Grossi-de-Sa, M.F. Antibacterial Peptides from Plants: What They Are and How They Probably Work. Biochem. Res. Int. 2011, 2011, 250349. [Google Scholar] [CrossRef]
- Jeżowska-Bojczuk, M.; Stokowa-Sołtys, K. Peptides Having Antimicrobial Activity and Their Complexes with Transition Metal Ions. Eur. J. Med. Chem. 2018, 143, 997–1009. [Google Scholar] [CrossRef]
- López-Meza, J.E.; Aguilar, A.O.-Z.J.A.; Loeza-Lara, P.D. Antimicrobial Peptides: Diversity and Perspectives for Their Biomedical Application. In Biomedical Engineering, Trends, Research and Technologies; IntechOpen: London, UK, 2011; p. 658. ISBN 978-953-307-514-3. Available online: https://www.intechopen.com/books/482 (accessed on 29 June 2022). [CrossRef]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant Antimicrobial Peptides. Folia Microbiol. 2014, 59, 181–196. [Google Scholar] [CrossRef]
- Mardirossian, M.; Grzela, R.; Giglione, C.; Meinnel, T.; Gennaro, R.; Mergaert, P.; Scocchi, M. The Host Antimicrobial Peptide Bac71-35 Binds to Bacterial Ribosomal Proteins and Inhibits Protein Synthesis. Chem. Biol. 2014, 21, 1639–1647. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef]
- He, S.; Zhang, J.; Li, N.; Zhou, S.; Yue, B.; Zhang, M. A TFPI-1 Peptide That Induces Degradation of Bacterial Nucleic Acids, and Inhibits Bacterial and Viral Infection in Half-Smooth Tongue Sole, Cynoglossus Semilaevis. Fish Shellfish. Immunol. 2017, 60, 466–473. [Google Scholar] [CrossRef]
- Subbalakshmi, C.; Sitaram, N. Mechanism of Antimicrobial Action of Indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96. [Google Scholar] [CrossRef]
- Lutkenhaus, J. Regulation of Cell Division in E. coli. Trends Genet. 1990, 6, 22–25. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. The Human Salivary Peptide Histatin 5 Exerts Its Antifungal Activity through the Formation of Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2001, 98, 14637–14642. [Google Scholar] [CrossRef]
- De Cesare, G.B.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20. [Google Scholar] [CrossRef]
- Torrent, M.; Pulido, D.; Rivas, L.; Andreu, D. Antimicrobial Peptide Action on Parasites. Curr. Drug Targets 2012, 13, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral Peptides as Promising Therapeutic Drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, H.; Fang, L.; Liu, D.; Liu, J.; Su, M.; Fang, Z.; Ren, W.; Jiao, H. The Modification and Design of Antimicrobial Peptide. Curr. Pharm. Des. 2018, 24, 904–910. [Google Scholar] [CrossRef]
- Albarano, L.; Esposito, R.; Ruocco, N.; Costantini, M. Genome Mining as New Challenge in Natural Products Discovery. Mar. Drugs 2020, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- Amador, V.C.; dos Santos-Silva, C.A.; Vilela, L.M.B.; Oliveira-Lima, M.; de Santana Rêgo, M.; Roldan-Filho, R.S.; de Oliveira-Silva, R.L.; Lemos, A.B.; de Oliveira, W.D.; Ferreira-Neto, J.R.C.; et al. Lipid Transfer Proteins (LTPs)—Structure, Diversity and Roles beyond Antimicrobial Activity. Antibiotics 2021, 10, 1281. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Mahendran, A.S.K.; Lim, Y.S.; Fang, C.-M.; Loh, H.-S.; Le, C.F. The Potential of Antiviral Peptides as COVID-19 Therapeutics. Front. Pharmacol. 2020, 11, 575444. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, A.; Marin, M.; Honnen, W.; Ramasamy, S.; Porter, E.; Subbian, S.; Pinter, A.; Melikyan, G.B.; Lu, W.; et al. Human Defensins Inhibit SARS-CoV-2 Infection by Blocking Viral Entry. Viruses 2021, 13, 1246. [Google Scholar] [CrossRef]
- Diamond, G.; Molchanova, N.; Herlan, C.; Fortkort, J.; Lin, J.; Figgins, E.; Bopp, N.; Ryan, L.; Chung, D.; Adcock, R.; et al. Potent Antiviral Activity against HSV-1 and SARS-CoV-2 by Antimicrobial Peptoids. Pharmaceuticals 2021, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Ling, R.; Dai, Y.; Huang, B.; Huang, W.; Yu, J.; Lu, X.; Jiang, Y. In Silico Design of Antiviral Peptides Targeting the Spike Protein of SARS-CoV-2. Peptides 2020, 130, 170328. [Google Scholar] [CrossRef]
- Mahmud, S.; Biswas, S.; Paul, G.K.; Mita, M.A.; Afrose, S.; Hasan, R.; Shimu, S.S.; Uddin, M.A.R.; Uddin, S.; Zaman, S.; et al. Antiviral Peptides against the Main Protease of SARS-CoV-2: A Molecular Docking and Dynamics Study. Arab. J. Chem. 2021, 14, 103315. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Talukder, S.A.; Khan, A.M.; Afrin, N.; Ali, M.A.; Islam, R.; Parves, R.; Al Mamun, A.; Sufian, A.; Hossain, M.N.; et al. Antiviral Peptides as Promising Therapeutics against SARS-CoV-2. J. Phys. Chem. B 2020, 124, 9785–9792. [Google Scholar] [CrossRef]
- Kindhauser, M.K.; Allen, T.; Frank, V.; Santhana, R.S.; Dye, C. Zika: The Origin and Spread of a Mosquito-Borne Virus. Bull. World Health Organ. 2016, 94, 675–686. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, H.; Li, Y.; Wang, G.; Tang, B.; Zhao, J.; Huang, Y.; Zheng, J. Cathelicidin-Derived Antimicrobial Peptides Inhibit Zika Virus Through Direct Inactivation and Interferon Pathway. Front. Immunol. 2018, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Ji, M.; Hu, J.; Zhu, T.; Chen, Y.; Bai, X.; Mwangi, J.; Mo, G.; Lai, R.; Jin, L. Snake Cathelicidin Derived Peptide Inhibits Zika Virus Infection. Front. Microbiol. 2020, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-S.; Zhou, Y.; Takagi, T.; Kameoka, M.; Kawashita, N. Dengue Virus and Its Inhibitors: A Brief Review. Chem. Pharm. Bull. 2018, 66, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.M.C.; Oliveira, M.D.; Dias, R.S.; Nacif-Marçal, L.; Feio, R.N.; Ferreira, S.O.; Oliveira, L.L.; Silva, C.C.; Paula, S.O. The Antimicrobial Peptide HS-1 Inhibits Dengue Virus Infection. Virology 2018, 514, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Tambunan, U.S.F.; Alamudi, S. Designing Cyclic Peptide Inhibitor of Dengue Virus NS3-NS2B Protease by Using Molecular Docking Approach. Bioinformation 2010, 5, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Songprakhon, P.; Thaingtamtanha, T.; Limjindaporn, T.; Puttikhunt, C.; Srisawat, C.; Luangaram, P.; Dechtawewat, T.; Uthaipibull, C.; Thongsima, S.; Yenchitsomanus, P.-T.; et al. Peptides Targeting Dengue Viral Nonstructural Protein 1 Inhibit Dengue Virus Production. Sci. Rep. 2020, 10, 12933. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef]
- Siegers, J.Y.; Dhanasekaran, V.; Xie, R.; Deng, Y.-M.; Patel, S.; Ieng, V.; Moselen, J.; Peck, H.; Aziz, A.; Sarr, B.; et al. Genetic and Antigenic Characterization of an Influenza A(H3N2) Outbreak in Cambodia and the Greater Mekong Subregion during the COVID-19 Pandemic, 2020. J. Virol. 2021, 95, e01267-21. [Google Scholar] [CrossRef]
- Fujita, D.M.; Dos Santos Soares, G.; Sartori, G.P.; Henrique da Silva Nali, L. COVID-19 and Influenza Coinfection: The Rise of Ômicron and H3N2 in Brazil—2022. Travel Med. Infect. Dis. 2022, 46, 102262. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Yuan, N.; Zeng, M.; Zhao, Y.; Yu, R.; Liu, Z.; Wu, H.; Dong, S. A Novel Natural Influenza A H1N1 Virus Neuraminidase Inhibitory Peptide Derived from Cod Skin Hydrolysates and Its Antiviral Mechanism. Mar. Drugs 2018, 16, 377. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, P.K.; Turner, K.L.; Crockett, T.M.; Lu, X.; Morris, C.F.; Konkel, M.E. Inhibitory Effect of Puroindoline Peptides on Campylobacter Jejuni Growth and Biofilm Formation. Front. Microbiol. 2021, 12, 702762. [Google Scholar] [CrossRef] [PubMed]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as Zoonotic Foodborne Diseases: A Review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Aarestrup, F.M.; Hansen, E.B. Dissection of the Antimicrobial and Hemolytic Activity of Cap18: Generation of Cap18 Derivatives with Enhanced Specificity. PLoS ONE 2018, 13, e0197742. [Google Scholar] [CrossRef] [PubMed]
- Sijbrandij, T.; Ligtenberg, A.J.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Bikker, F.J. Effects of Lactoferrin Derived Peptides on Simulants of Biological Warfare Agents. World J. Microbiol. Biotechnol. 2017, 33, 3. [Google Scholar] [CrossRef]
- Sijbrandij, T.; Ligtenberg, A.J.; Nazmi, K.; van den Keijbus, P.A.M.; Veerman, E.C.I.; Bolscher, J.G.M.; Bikker, F.J. LFchimera Protects HeLa Cells from Invasion by Yersinia Spp. in Vitro. Biometals 2018, 31, 941–950. [Google Scholar] [CrossRef]
- Hunt, J.M. Shiga Toxin–Producing Escherichia Coli (STEC). Clin. Lab. Med. 2010, 30, 21–45. [Google Scholar] [CrossRef]
- Lino, M.; Kus, J.V.; Tran, S.L.; Naqvi, Z.; Binnington, B.; Goodman, S.D.; Segall, A.M.; Foster, D.B. A Novel Antimicrobial Peptide Significantly Enhances Acid-Induced Killing of Shiga Toxin-Producing Escherichia Coli O157 and Non-O157 Serotypes. Microbiology 2011, 157, 1768–1775. [Google Scholar] [CrossRef]
- Hokken, M.W.J.; Zwaan, B.J.; Melchers, W.J.G.; Verweij, P.E. Facilitators of Adaptation and Antifungal Resistance Mechanisms in Clinically Relevant Fungi. Fungal Genet. Biol. 2019, 132, 103254. [Google Scholar] [CrossRef]
- Bermas, A.; Geddes-McAlister, J. Combatting the Evolution of Antifungal Resistance in Cryptococcus Neoformans. Mol. Microbiol. 2020, 114, 721–734. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global Burden of Disease of HIV-Associated Cryptococcal Meningitis: An Updated Analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Liu, Y.; Zhao, X.; Lian, X.; Zhang, J.; Zhao, D.; Wang, Y.; Zhong, J.; Wang, J.; et al. A Peptide from Budding Yeast GAPDH Serves as a Promising Antifungal against Cryptococcus Neoformans. Microbiol. Spectr. 2022, 10, e00826-21. [Google Scholar] [CrossRef]
- Specht, C.A.; Homan, E.J.; Lee, C.K.; Mou, Z.; Gomez, C.L.; Hester, M.M.; Abraham, A.; Rus, F.; Ostroff, G.R.; Levitz, S.M. Protection of Mice against Experimental Cryptococcosis by Synthesized Peptides Delivered in Glucan Particles. mBio 2022, 13, e03367-21. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global Epidemiology of Sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef]
- Toriello, C.; Brunner-Mendoza, C.; Ruiz-Baca, E.; Duarte-Escalante, E.; Pérez-Mejía, A.; Del Rocío Reyes-Montes, M. Sporotrichosis in Mexico. Braz. J. Microbiol. 2021, 52, 49–62. [Google Scholar] [CrossRef]
- Rossow, J.A.; Queiroz-Telles, F.; Caceres, D.H.; Beer, K.D.; Jackson, B.R.; Pereira, J.G.; Ferreira Gremião, I.D.; Pereira, S.A. A One Health Approach to Combatting Sporothrix Brasiliensis: Narrative Review of an Emerging Zoonotic Fungal Pathogen in South America. J. Fungi 2020, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Li, F.; Li, J.; Chen, F. Antifungal Activity of ToAP2D Peptide Against Sporothrix Globosa. Front. Bioeng. Biotechnol. 2021, 9, 761518. [Google Scholar] [CrossRef]
- Smith, N.C.; Goulart, C.; Hayward, J.A.; Kupz, A.; Miller, C.M.; van Dooren, G.G. Control of Human Toxoplasmosis. Int J Parasitol 2021, 51, 95–121. [Google Scholar] [CrossRef]
- de Assis, D.R.R.; de Oliveira Pimentel, P.M.; dos Reis, P.V.M.; Rabelo, R.A.N.; Vitor, R.W.A.; do Nascimento Cordeiro, M.; Felicori, L.F.; Olórtegui, C.D.C.; Resende, J.M.; Teixeira, M.M.; et al. Tityus Serrulatus (Scorpion): From the Crude Venom to the Construction of Synthetic Peptides and Their Possible Therapeutic Application Against Toxoplasma Gondii Infection. Front. Cell. Infect. Microbiol. 2021, 11, 706618. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Y.; Tang, X.; Wu, M.; Hou, S.; Liu, X.; Li, J.; Deng, M.; Huang, S.; Jiang, L. Anti-Toxoplasma Gondii Effects of a Novel Spider Peptide XYP1 In Vitro and In Vivo. Biomedicines 2021, 9, 934. [Google Scholar] [CrossRef] [PubMed]
- Ness, T.E.; Agrawal, V.; Bedard, K.; Ouellette, L.; Erickson, T.A.; Hotez, P.; Weatherhead, J.E. Maternal Hookworm Infection and Its Effects on Maternal Health: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2020, 103, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Diemert, D.; Campbell, D.; Brelsford, J.; Leasure, C.; Li, G.; Peng, J.; Zumer, M.; Younes, N.; Bottazzi, M.E.; Mejia, R.; et al. Controlled Human Hookworm Infection: Accelerating Human Hookworm Vaccine Development. Open Forum Infect. Dis. 2018, 5, ofy083. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.M.S.; Alencar, L.M.R.; Dias, L.P.; Lima, R.C.; Silva, C.R.; Santos-Oliveira, R.; Oliveira, J.T.A.; Junior, L.M.C.; Souza, P.F.N. New Insights into Anthelmintic Mechanisms of Action of a Synthetic Peptide: An Ultrastructural and Nanomechanical Approach. Polymers 2021, 13, 2370. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Kotze, A.C.; Kopp, S.; McCarthy, J.S.; Coleman, G.T.; Craik, D.J. Anthelmintic Activity of Cyclotides: In Vitro Studies with Canine and Human Hookworms. Acta Trop. 2009, 109, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Symeonidou, I.; Arsenopoulos, K.; Tzilves, D.; Soba, B.; Gabriël, S.; Papadopoulos, E. Human Taeniasis/Cysticercosis: A Potentially Emerging Parasitic Disease in Europe. Ann. Gastroenterol. 2018, 31, 406–412. [Google Scholar] [CrossRef]
- CystiTeam Group for Epidemiology and Modelling of Taenia solium Taeniasis/Cysticercosis The World Health Organization 2030 Goals for Taenia Solium: Insights and Perspectives from Transmission Dynamics Modelling: CystiTeam Group for Epidemiology and Modelling of Taenia Solium Taeniasis/Cysticercosis. Gates Open Res. 2019, 3, 1546. [CrossRef]
- Dixon, M.A.; Winskill, P.; Harrison, W.E.; Basáñez, M.-G. Taenia Solium Taeniasis/Cysticercosis: From Parasite Biology and Immunology to Diagnosis and Control. Adv. Parasitol. 2021, 112, 133–217. [Google Scholar] [CrossRef]
- Landa, A.; Jiménez, L.; Willms, K.; Jiménez-García, L.F.; Lara-Martínez, R.; Robert, L.; Cirioni, O.; Barańska-Rybak, W.; Kamysz, W. Antimicrobial Peptides (Temporin A and Iseganan IB-367): Effect on the Cysticerci of Taenia Crassiceps. Mol. Biochem. Parasitol. 2009, 164, 126–130. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where Are We and Where Are We Heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Robles-Loaiza, A.A.; Pinos-Tamayo, E.A.; Mendes, B.; Teixeira, C.; Alves, C.; Gomes, P.; Almeida, J.R. Peptides to Tackle Leishmaniasis: Current Status and Future Directions. Int. J. Mol. Sci. 2021, 22, 4400. [Google Scholar] [CrossRef]
- Kumar, V.; Chugh, A. Peptide-Mediated Leishmaniasis Management Strategy: Tachyplesin Emerges as an Effective Anti-Leishmanial Peptide against Leishmania Donovani. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183629. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, W.; Cao, S.; Zhao, P.; Liu, J.; Dong, H.; Guo, Y.; Liu, Q.; Gong, P. In Vitro Leishmanicidal Activity of Antimicrobial Peptide KDEL against Leishmania Tarentolae. Acta Biochim. Biophys. Sin. 2019, 51, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- D’Aloisio, V.; Dognini, P.; Hutcheon, G.A.; Coxon, C.R. PepTherDia: Database and Structural Composition Analysis of Approved Peptide Therapeutics and Diagnostics. Drug Discov. Today 2021, 26, 1409–1419. [Google Scholar] [CrossRef]
- Wild, C.; Greenwell, T.; Matthews, T. A Synthetic Peptide from HIV-1 Gp41 Is a Potent Inhibitor of Virus-Mediated Cell—Cell Fusion. AIDS Res. Hum. Retrovir. 1993, 9, 1051–1053. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- Bacalum, M.; Radu, M. Cationic Antimicrobial Peptides Cytotoxicity on Mammalian Cells: An Analysis Using Therapeutic Index Integrative Concept. Int. J. Pept. Res. Ther. 2015, 21, 47–55. [Google Scholar] [CrossRef]
- Eckert, R.; Sullivan, R.; Shi, W. Targeted Antimicrobial Treatment to Re-Establish a Healthy Microbial Flora for Long-Term Protection. Adv Dent. Res. 2012, 24, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Alni, R.H.; Tavasoli, F.; Barati, A.; Badarbani, S.S.; Salimi, Z.; Babaeekhou, L. Synergistic Activity of Melittin with Mupirocin: A Study against Methicillin-Resistant S. Aureus (MRSA) and Methicillin-Susceptible S. Aureus (MSSA) Isolates. Saudi J. Biol. Sci. 2020, 27, 2580–2585. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Bonten, M.; Koopmans, M. Pandemics—One Health Preparedness for the Next. Lancet Reg. Health-Eur. 2021, 9, 100210. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.G.; Oliveira, J.T.A.; Amaral, J.L.; Freitas, C.D.T.; Souza, P.F.N. Synthetic Antimicrobial Peptides: Characteristics, Design, and Potential as Alternative Molecules to Overcome Microbial Resistance. Life Sci. 2021, 278, 119647. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.B.; Seo, J. Antimicrobial Peptides under Clinical Investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The Multifaceted Nature of Antimicrobial Peptides: Current Synthetic Chemistry Approaches and Future Directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef] [PubMed]

| Microorganisms | Target Microorganisms | Peptide and Source | Mechanism of Action | Reference |
|---|---|---|---|---|
| Viruses | SARS-CoV-2 | θ-defensin analog RC101 (human) | Affected viral fusion and entry, possibly with a direct impact on the virions | [66] |
| MXB-4 and MXB-9 peptoids | Membrane disruptive | [67] | ||
| HR1 and HR2 (SARS-CoV-2) | Binding of the spike protein | [68] | ||
| Circulin A from Chassalia parviflora Piscidin 4 from Morone chrysops/Morone saxatilis Neutrophil defensin 1 from Pan troglodytes (chimpanzee) Corticostatin-3 from Oryctolagus cuniculus (rabbit) | Inhibition of Mpro | [69] | ||
| S2P25 and S2P26 (synthetic) | Binding of the RBD spike | [70] | ||
| ZIKA virus | GF-17 (human cathelicidins) BMAP-18 (bovine cathelicidins) | Direct virus inactivation | [72] | |
| ZY13 (snake venom cathelicidin-30) | Direct virus inactivation | [73] | ||
| Dengue virus | HS-1 (anuran Hypsiboas semilineatus) | Block of virus binding and internalization | [76] | |
| Synthetic peptides targeting | Block active sites of viral proteins (NS2B-NS3 protease) Competitive inhibitors of viral entry and viral replication | [77] | ||
| Synthetic peptides | Inhibition of DENV through targeting NS1 protease | [78] | ||
| Influenza A H3N2 | Fish-skin-derived SAMPs | Inhibitor of influenza A neuraminidase | [82] | |
| Bacteria | Campylobacter | Puroindoline A (PinA) from puroindolines (Triticum aestivum) | Inhibiting bacterial growth by disrupting their cellular membranes while also blocking biofilm formation | [84] |
| Salmonella | Cap-18 derivatives (from rabbit neutrophils, analog to the human LL-37) | Inhibition of bacterial growth | [86] | |
| Puroindoline A (PinA) from puroindolines (Triticum aestivum) | Inhibiting bacterial growth by disrupting their cellular membranes while also blocking biofilm formation | [84] | ||
| Yersinia enterocolitica | Antimicrobial peptides derived from bovine lactoferrin | Bactericidal effect due to permeabilization and depolarization; inhibition of host cell invasion by the bacteria | [87,88] | |
| Listeria monocytogenes | Puroindoline A (PinA) from puroindolines (Triticum aestivum) | Inhibiting bacterial growth by disrupting their cellular membranes while also blocking biofilm formation | [84] | |
| Shiga-toxin-producing Escherichia coli | Hexapeptide WRWYCR against STEC | Inhibition of bacterial DNA repair, reducing STEC survival, with no increase in Shiga toxin production in an acidic environment | [90] | |
| Fungi | Cryptococcus neoformans | SP1(derived from Saccharomyces cerevisiae) | Interaction with the pathogen’s membrane ergosterol and enters the vacuole, causing calcium ion homeostasis imbalance, increased reactive oxygen, exposure to phosphatidylserine, and nuclear fragmentation | [96] |
| Sporothrix | ToAP2A, ToAP2C, and ToAP2D | Inhibition of the growth membrane deformation and rupture | [101] | |
| Parasites | Toxoplasma gondii | Peptides derived from the venom of the yellow scorpion Tityus serrulatus | Reduced the replication of tachyzoites | [103] |
| Peptide (XYP1) derived from the venom gland of the spider Lycosa coelestis | Inhibited the viability, invasion, and proliferation of tachyzoites through membrane disruption | [104] | ||
| Ancylostoma caninum Necator americanus | Kalata B1, kalata B6, and cycloviolacin O14 | Reduction of the viability of larval (Ancylostoma caninum, Necator americanus) and adult stages (Ancylostoma caninum) | [108] | |
| Taenia | Temporin A (TA, from frog Rana temporaria) Iseganan IB-367 (IB-367, a synthetic analog of porcine protegrin | Cysticerci shrinkage, loss of motility, formation of macrovesicles in the tegument, decrease in evagination properties | [112] | |
| Leishmania | KDEL, based on the Pseudomonas aeruginosa exotoxin PE | Disruption of the integrity of the parasite’s surface membrane and cellular apoptosis | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupin, L.; Santos-Silva, C.A.d.; Al Mughrbi, A.R.H.; Vilela, L.M.B.; Benko-Iseppon, A.M.; Crovella, S. Bioactive Antimicrobial Peptides: A New Weapon to Counteract Zoonosis. Microorganisms 2022, 10, 1591. https://doi.org/10.3390/microorganisms10081591
Zupin L, Santos-Silva CAd, Al Mughrbi ARH, Vilela LMB, Benko-Iseppon AM, Crovella S. Bioactive Antimicrobial Peptides: A New Weapon to Counteract Zoonosis. Microorganisms. 2022; 10(8):1591. https://doi.org/10.3390/microorganisms10081591
Chicago/Turabian StyleZupin, Luisa, Carlos André dos Santos-Silva, Aya R. Hamad Al Mughrbi, Livia Maria Batista Vilela, Ana Maria Benko-Iseppon, and Sergio Crovella. 2022. "Bioactive Antimicrobial Peptides: A New Weapon to Counteract Zoonosis" Microorganisms 10, no. 8: 1591. https://doi.org/10.3390/microorganisms10081591
APA StyleZupin, L., Santos-Silva, C. A. d., Al Mughrbi, A. R. H., Vilela, L. M. B., Benko-Iseppon, A. M., & Crovella, S. (2022). Bioactive Antimicrobial Peptides: A New Weapon to Counteract Zoonosis. Microorganisms, 10(8), 1591. https://doi.org/10.3390/microorganisms10081591







