Abstract
Background: Bloodstream infections (BSI) caused by highly resistant pathogens in non-ICU COVID-19 departments pose important challenges. Methods: We performed a comparative analysis of incidence and microbial epidemiology of BSI in COVID-19 vs. non-COVID-19, non-ICU departments between 1 September 2020-31 October 2021. Risk factors for BSI and its impact on outcome were evaluated by a case-control study which included COVID-19 patients with/without BSI. Results: Forty out of 1985 COVID-19 patients developed BSI. The mean monthly incidence/100 admissions was 2.015 in COVID-19 and 1.742 in non-COVID-19 departments. Enterococcus and Candida isolates predominated in the COVID-19 group (p < 0.001 and p = 0.018, respectively). All Acinetobacter baumannii isolates were carbapenem-resistant (CR). In the COVID-19 group, 33.3% of Klebsiella pneumoniae was CR, 50% of Escherichia coli produced ESBL and 19% of Enterococcus spp. were VRE vs. 74.5%, 26.1% and 8.8% in the non-COVID-19 group, respectively. BSI was associated with prior hospitalization (p = 0.003), >2 comorbidities (p < 0.001), central venous catheter (p = 0.015), severe SARS-CoV-2 pneumonia and lack of COVID-19 vaccination (p < 0.001). In the multivariate regression model also including age and multiple comorbidities, only BSI was significantly associated with adverse in-hospital outcome [OR (CI95%): 21.47 (3.86–119.21), p < 0.001]. Conclusions: BSI complicates unvaccinated patients with severe SARS-CoV-2 pneumonia and increases mortality. BSI pathogens and resistance profiles differ among COVID-19/non-COVID-19 departments, suggesting various routes of pathogen acquisition.
1. Introduction
The COVID-19 pandemic emerged as the most serious public health threat worldwide since the influenza pandemic of the last century. Millions of cases of infection and a subsequent proportion of attributed deaths were caused by this novel disease, which has seriously affected global healthcare systems along with social, administrative and economic life [,]. The causative virus, SARS-CoV-2, a novel coronavirus belonging to the subfamily of Coronavirinae in the family Coronaviridae, causes severe pneumonia often requiring hospital care []. Moreover, COVID-19 critical illness led to an increase in intensive care unit (ICU) admissions and a devastating rate of mortality []. Severe SARS-CoV-2 pneumonia leads to prolonged hospitalization and can be complicated by secondary bacterial and fungal infections with an increased negative impact on the patients’ outcome [,]. Co-infections are much more frequent in critically ill patients in the ICU (31.5%) than in hospitalized patients in standard medical COVID-19 wards (9%) []. Rates of bacterial co-infection are low at admission (3.5%), rising to more than 15% during hospitalization; overall, 7% of hospitalized patients will develop bacterial superinfection [,,]. Both community-acquired and nosocomial bacterial co-infections were caused by Gram-negative bacteria such as Pseudomonas aeruginosa and Escherichia coli, while Acinetobater baumannii and Klebsiella pneumoniae were linked to a higher need for invasive mechanical ventilation (IMV) and increased in-hospital mortality [,]. Moreover, a high risk for secondary fungal infections among COVID-19 patients has been described []. Bloodstream infections are a significant subset of infections following acute SARS-CoV-2 pneumonia and a driver for sepsis and in-hospital mortality [,]. Current data suggest that diagnosis and subsequent prompt treatment of BSI might be delayed due to a focalized effort for controlling the primary infection (SARS-CoV-2 pneumonia) []. Moreover, nosocomial BSI is often caused by difficult-to-treat multidrug-resistant organisms (MDRO), especially in countries with high prevalence of antimicrobial resistance, posing additional therapeutic concerns [,].
The impact of BSI in patients hospitalized for COVID-19 has been studied through national or local cohorts; however, most studies were performed in critically ill patients in the ICU who present a higher mortality from a BSI event [,,,,]. Clinical characteristics and outcome of patients with BSI and COVID-19 in medical wards might differ from patients hospitalized in the ICUs, and they are not fully investigated. For this purpose, we conducted a case-control study on risk factors for BSI and on the patients’ clinical outcome in COVID-19 medical wards, outside the ICU department. The incidence of BSI, the microbial epidemiology and antimicrobial resistance patterns were also compared between COVID-19 and non-COVID-19, non-ICU departments.
2. Materials and Methods
2.1. Study Protocol
We retrospectively analyzed prospectively collected data on the incidence, clinical characteristics and outcome of BSI in patients hospitalized for COVID-19 in the medical, non-ICU department of our tertiary-care hospital from 1 September 2020–31 October 2021. The incidence of BSI per 100 admissions, BSI pathogens and susceptibility profiles were also assessed in COVID-19 vs. non-COVID-19 departments of our hospital. Microbiological and clinical data from the ICU were not included in the study.
Potential risk factors for BSI and its impact on mortality were evaluated through a case-control study. Patients with BSI were matched for age and gender at a 1:1 ratio with a randomly selected control group of patients without BSI hospitalized for COVID-19 pneumonia during the study period. Analysis of factors associated with adverse outcome defined as IMV and/or death was performed. Demographics, comorbidities, prior recent hospitalization or residency to a healthcare setting or nursing home, vaccination against SARS-CoV-2, the placement of a central venous catheter (CVC) during hospitalization and the length of hospital stay were recorded. Time from patients’ admission to the onset of BSI (date of collecting the first positive blood culture) was also assessed. Anonymized patients’ data were analyzed.
2.2. Definitions
A BSI episode was documented by at least one positive blood culture and consistent clinical presentation. Common skin contaminants (such as coagulase-negative staphylococci (CoNs), Micrococcus spp., viridans group streptococci, Propionibacterium spp., Corynebacterium spp. and Bacillus spp.) were excluded unless they were recovered from at least two blood culture sets. Catheter-related BSI (CRBSI) was detected by catheter tip culture and/or differential time to positivity according to the CRBSI definitions by IDSA guidelines []. Multiple positive blood cultures for the same organism in the same patient were considered as one BSI episode in the present analysis. BSI episodes were categorized as community-acquired or hospital-acquired, depending on the time of blood sampling, within or after 48 h from admission.
Diagnosis of SARS-CoV-2 pneumonia relied upon clinical and radiological characteristics of the disease on lung X-ray or CT scan along with a positive nasopharyngeal swab test for SARS-CoV-2 (RT-PCR) according to current guidelines []. Severe SARS-CoV-2 pneumonia was classified according to the WHO clinical progression scale of the disease []. All hospitalized patients received remdesivir. Dexamethasone was administered only in patients with severe pneumonia.
In-hospital outcome was defined as “cure” when the patient was discharged at home or any other permanent hosting place and “non-cure” if the patient had either died or been intubated and transferred to the ICU.
2.3. Microbiology
Blood cultures were incubated using the BD BACTEC™ FX Automated Blood Culture System (Becton and Dickinson, Franklin Lakes, NJ, USA). Identification and susceptibility testing of blood isolates were performed by the Vitek 2 automated system (bioMerieux, Marcy l’ Etoile, France). MIC values for colistin were determined by broth microdilution (MIC-Strip Colistin, Merlin Diagnostika GmbH, Bornheim, Germany). EUCAST breakpoints were applied for interpretation of MIC values []. Phenotypic detection of carbapenemases and differentiation of carbapenemase type among Enterobacterales and P. aeruginosa isolates was performed by immunochromatography (NG-Test® CARBA 5, NG Biotech, Guipry, France).
Gram-negative multidrug resistant (MDR) pathogens were defined according to Magiorakos et al. criteria []. MDR Gram-positive bacteria (GPB) included methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcocus spp. (VRE).
2.4. Statistical Analysis
Pearson chi square and Fischer exact tests were applied for categorical and the Mann–Whitney test for continuous variables in comparisons between BSI and non-BSI COVID-19 groups. Factors of in-hospital adverse outcome (“non-cured patients”) were identified via univariate and multivariate regression analysis. The primary dependent variable was outcome (cure vs. non-cure). The primary independent variable was BSI (either bacteremia or fungemia). Fisher’s exact test along with the odds ratio (OR) and 95% confidence interval (CI) were applied. The level of significance was set at 0.05. Statistical analyses were performed with SPSS version 22.0.
3. Results
3.1. Microbial Epidemiology
During the study period, a total of 470 blood culture (BC) sets were obtained from 321 non-ICU hospitalized patients with COVID-19; 102 BC sets were detected as positive (26.9%). True pathogens were recovered from 49 BC sets. Contamination rate was 52%. A total of 45 BSI episodes in 40 patients were identified among 1985 non-ICU COVID-19 hospitalized patients (2%). Most patients were female, (n = 24, 60%) and the median age was 73.5 years [IQR (25–75): 64.5–85.25 years]. Multiple (more than 2) comorbidities, were found in 31 patients (77.5%) while 11 patients (27.5%) were either transferred from a nursing care center or had recent prior hospitalizations. The mean (±SD) time of hospitalization from admission to the onset of the BSI episode was 11 ± 7.84 days. All but 3 BSI cases fulfilled the definition of nosocomial infection. A CVC was inserted in 18 patients (90% into the femoral vein); CRBSI was documented in 4 patients (22.2%). Overall adverse outcome (in-hospital mortality or IMV) was noted in 60% of BSI cases. Sixteen patients required IMV; 14 of them died. Eight non-ventilated patients died in the non-ICU COVID department.
During the same period, a total of 2570 BC sets were obtained from 1422 patients hospitalized in non-COVID-19 departments. Of them, 692 BC sets (26.9%) were detected as positive. True pathogens were recovered from 416 BC sets. Contamination rate was 39.9%. A total of 343 BSI episodes in 294 patients (male 48%, median age 77.5 years [IQR (25–75): 65–86]) were recorded among 19,973 patients hospitalized in non-COVID-19 departments (1.5%). The median time of hospitalization from admission to the onset of the BSI episode was 4 days [IQR (25–75): 0–21]. Nosocomial BSI accounted for 55.7% of all episodes.
Monthly number of BSI episodes and monthly admissions in the COVID-19 department and all (except ICU) medical and surgical departments of the hospital are shown in Figure 1. The mean monthly incidence of BSI per 100 admissions was 2.015 (range: 0–6.349) in COVID-19 departments vs. 1.742 (range: 1.319–2.437) in non-COVID-19 departments.
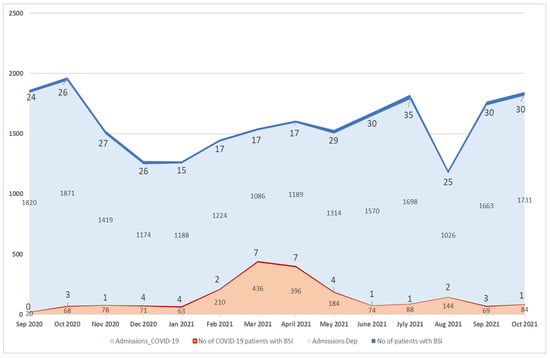
Figure 1.
Monthly number of BSI episodes and monthly admissions in COVID-19 and non-COVID-19 departments, except ICU.
Blood isolates in COVID-19 and non-COVID-19 departments are presented in Table 1.

Table 1.
Blood isolates in COVID-19 and non-COVID-19 departments. Bold means statistical significant values.
Of note, blood-culture contamination rate was lower in non-COVID-19 departments (39.9%) than in COVID-19 ones (52%). In the COVID-19 group, Gram-positive bacteria accounted for 46.4%, Gram-negative bacteria for 33.9% and fungi (candida isolates) for 19.6% of all pathogens. Gram-negatives prevailed in the non-COVID-19 group (59%). The rate of polymicrobial BSI did not differ between groups (24.4% vs. 20.7%). Candidemia and enterococcal bacteremia were significantly more frequent in COVID-19 than in non-COVID-19 patients (p = 0.018 and p < 0.001, respectively) (Table 1). Among Candida isolates, Candida parapsilosis was more frequently identified in the COVID-19 vs. the non-COVID-19 group (Supplementary Table S2). Candidemia was more often detected in patients with a CVC in place compared to patients without a CVC [n = 8 (44.4%) vs. n = 2 (9.1%), p = 0.025]. The outcome of COVID-19 patients with BSI did not significantly differ among pathogens (data in Supplementary Table S1).
3.2. Antimicrobial Resistance
Resistance profiles of blood isolates among the COVID-19 department and all other non-ICU departments of the hospital are presented in Figure 2. Multidrug resistance was detected in 46% of pathogens in the COVID-19 group vs. 36.6% in the non-COVID-19 group (p = 0.769). All A. baumannii isolates were carbapenem-resistant in both groups and high rates of colistin resistance were noted (87% in the COVID-19 group and 77.1% in the non-COVID-19 group, p = 0.666). All S. aureus isolates were MRSA in the COVID-19 group vs. 42.9% in the non-COVID-19 cohort (p = 0.042). VRE accounted for 19% of enterococcal bacteremia in COVID-19 vs. 8.8% in the non-COVID-19 patients (p = 0.231). Half of E. coli isolates were ESBL producers in the COVID-19 group vs. 26.1% in the non-COVID-19 group (p = 0.3). Carbapenem-resistant K. pneumonia (CRKP) and P. aeruginosa predominated in the non-COVID-19 cohort vs. COVID-19 (74.5% vs. 33.3%, p = 0.047 and 18.8% vs. 0%, p = 0.002, respectively). The single carbapenemase-producing K. pneumoniae isolate detected in the COVID-19 group was a KPC and VIM co-producer. In the non-COVID-19 group, among all CRKP, KPC producers predominated (66.7%).

Figure 2.
Resistance profiles of blood isolates among the COVID-19 department and all other non-ICU departments. Footnotes: CRAB—carbapenem resistant Acinetobacter baumannii; CRKP—carbapenem-resistant Klebsiella pneumoniae; P. aeruginosa CR (carbapenem-resistant); VRE—vancomycin resistant Enterococci; MRSA—methicillin-resistant Staphylococcus aureus; statistical significance * p = 0.047, ** p = 0.002, *** p = 0.042.
3.3. Case-Control Analysis for Clinical Data and Outcome
Multiple comorbidities (i.e., heart disease, dyslipidemia and neuropsychiatric disorders), presence of CVC and prior hospitalization were significantly more frequent in the BSI group. Severe pneumonia and lack of vaccination against SARS-CoV-2 predominated in the BSI group. Patients with BSI were more frequently intubated (40% vs. 2.5% in the non-BSI group, p < 0.001) and had a longer length of hospital stay than non-BSI patients [mean days ±SD: 19.92 (9.56) vs. 9.57 (6.58), p = 0.001] (Table 2).

Table 2.
Case-control analysis of clinical characteristics of patients with COVID-19 between BSI and non-BSI groups. Bold means statistical significant values.
All cases of adverse outcome of COVID-19 patients—both in BSI and non-BSI group—were related to severe SARS-CoV-2 pneumonia [OR (95% CI): 2.238 (1.628–3.076), p < 0.001] and lack of vaccination [OR (95% CI): 0.606 (0.499–0.736), p = 0.003] (Table 3). In the multivariate-adjusted model including BSI, age > 60 years, and neurological and psychiatric disease, only BSI and neurological disease were associated with adverse outcome (Table 3). The same regression analysis including age >60 years, BSI and more than two of any comorbidity revealed BSI as the unique independent predictor of death/and or IMV during hospitalization [OR (95% CI): 21.47 (3.86–119.21), p < 0.001].

Table 3.
Analysis of factors related to adverse outcome of COVID-19 patients in medical wards.
4. Discussion
We conducted a comparative analysis of microbial epidemiology and antimicrobial resistance profiles in BSI among COVID-19 and non-COVID-19 patients. Moreover, we analyzed the clinical characteristics and the outcome of patients with BSI and COVID-19 compared to non-BSI COVID-19 patients hospitalized in medical wards. The novelty of our study is a clear incorporation of a COVID-19 population outside the ICU environment. The incidence of BSI in COVID-19 patients and their outcome has been studied mostly in ICU or in mixed populations of both ICU and medical wards []. Furthermore, epidemiology of bacterial and fungal pathogens in BSI of patients with COVID-19 was mostly described in ICU departments with endemicity of multidrug antimicrobial resistance, but not in non-ICU COVID-19 settings [].
In our cohort, the mean incidence of BSI (2.015 per 100 admissions) was lower than the reported rates in COVID-19 wards outside the ICU [,]. However, our results confirmed previous reports of higher BSI incidence in COVID-19 vs. non-COVID-19 patients [].
The role of BSI contaminants in COVID-19 departments has been highlighted. The rate of blood-culture contamination in our study was high (52%), being higher than that observed in non-COVID-19 departments. High percentages of CoNS without evidence of increased catheter-related BSI (CRBSI), raise a higher suspicion of contamination and may result in additional unnecessary antimicrobial use. BSI contaminants were more frequently detected during the pandemic vs. the pre-pandemic era [,,]. A double rate of BSI contaminants in COVID-19 vs. non-COVID-19 patients was also described []. During early hospitalization for COVID-19, the rates of bacterial and fungal co-infection are low, therefore overutilization of blood cultures is not justified [,]. During the COVID-19 waves, reduced compliance with blood sampling protocols and guidelines for the placement and management of CVCs have been observed [,,]. Moreover, antimicrobial stewardship is required as empirical antimicrobial treatment in patients with COVID-19 for unjustified diagnosis of bacterial co-infection is high, raising concerns regarding the emergence of antimicrobial resistance [,,].
As epidemiology of BSI in COVID-19 patients, similar to non-COVID-19 patients, varies among countries and continents, continuous surveillance is required []. Our study demonstrated high rates of MDR pathogens, as also reported from another hospital in our country []; both studies mirror the endemic spread of MDRO, especially Gram-negatives. Protonotariou et al. reported a higher rate of MDRO (58% vs. 46% in our study) possibly because ICU patients prevailed in that cohort. In our study, all A. baumannii isolates detected in both COVID-19 and non-COVID-19 BSI episodes were carbapenem-resistant, suggesting a nosocomial horizontal spread throughout hospital departments. A. baumannii outbreaks during the initial surge of COVID-19 could be partly explained by the shortages in personnel, personal protective equipment (PPE) and dedicated medical equipment, which had affected the conventional infection prevention and control (IPC) practices []. MDR A. baumannii was the main BSI pathogen in a COVID-19 ICU (44%) in Spain []. The increased prevalence of A. baumannii either as a colonizer or as a pathogen during the COVID-19 pandemic has not been fully explored. In our cohort, colonization screening among COVID-19 patients was not routinely performed. Contrary to A. baumannii, other carbapenem-resistant Gram-negatives, such as K. pneumoniae and P. aeruginosa, were more frequently found in the non-ICU non-COVID-19 wards. Current published data suggest that bacteremia caused by K. pneumoniae and A. baumannii are mainly ICU-acquired in COVID-19 patients [].
MRSA bacteremia in COVID-19 patients was mostly detected upon admission [,]. In our study, all S. aureus isolates were MRSA, though detected in only four cases; these episodes did not clearly originate from the healthcare environment and had no impact on mortality. On the contrary, mortality of S. aureus bacteremia during the COVID-19 pandemic was reported to be high, over 60% in larger studies [,].
Enterococci are a major cause of BSI in COVID-19 patients hospitalized in ICUs. This may be due to enteric involvement in patients with severe disease, colonization pressure by antibiotics and/or limitations in controlling the patient-to-patient transmission in extremely challenging circumstances []. However, molecular analysis of Enterococcus spp. clinical isolates from COVID-19 patients did not always confirm a horizontal nosocomial transmission []. In our study, enterococcal bacteremia was significantly more frequent in COVID-19 patients than in non-COVID-19 patients with a high rate of VRE. We have no data on genetic relatedness of the isolates. It is presumed that prior antibiotic consumption in the community by outpatient physicians or over the counter, can alter gut flora and facilitate VRE colonization and subsequent infection.
Candidemia is increasingly reported in COVID-19 patients usually associated with ICU stay, need for IMV, use of corticosteroids or other immunosuppressive drugs for COVID-19 [,]. Mortality has been reported to be higher in patients with candidemia and COVID-19 than in patients without COVID-19 []. Our study corroborates the higher incidence in COVID-19 vs. non-COVID-19 patients in non-ICU wards, demonstrating that other factors might interact with the host during the course of the disease, allowing invasive candidiasis. The alterations of microbiota from enteritis of COVID-19 or/and the prior use of antimicrobials and subsequent gut or mucosal colonization by Candida spp. might be implicated in the pathogenesis of candidemia [].
High mortality rates (57%) have been reported among COVID-19 patients with BSI following an increased need for IMV (74%) []. Several risk factors for the development of BSI have been described in COVID-19 patients, similarly to influenza infection cohorts []. Bacteremia has been strongly correlated with ICU stay, the presence of an indwelling device, underlying comorbidities and adverse clinical outcome []. In a large cohort of 13,007 patients originally recruited followed by a propensity-score-matching cohort of 6520 patients, 3.74% and a 3.97% were diagnosed with clinically significant BSI, respectively. COVID-19 patients with BSI had significantly longer hospitalizations, more frequent ICU admission and higher mortality rates compared to those without BSI []. In our study, lack of vaccination for SARS-COV2 was not only correlated to severe pneumonia but also to an increased frequency of BSI, further hampering the patients’ outcome. As already published [], BSI in hospitalized patients with COVID-19 has the most significant impact on mortality.
Standards of care during the COVID-19 pandemic seemed to be altered, shifting to a less frequent and per protocol oral care and/or washing/dressing and CVC management, further complicated by the prone position of the patient. In addition, the rapidly increasing burden of workload and the pressure on healthcare professionals has a negative impact on infection-control practices []. Therefore, during the first wave of COVID-19, the rates of CRBSI had increased in a Dutch hospital but dropped again to less than 9% after implementation of infection-control measures. The decrease in CRBSI rates following the adaptation of the infection-control policies, to meet the needs of the new hospital environment for COVID-19, proves that the key to avoid life-threatening BSI episodes is the application of feasible and effective infection-control interventions []. This is only an example of how surveillance data on nosocomial infections can improve actions for infection control. Maintaining IPC best practices (e.g., MDRO surveillance, hand hygiene, standard and contact precautions and environmental cleaning) could mitigate the spread of infections [].
5. Conclusions
In conclusion, our cohort demonstrated a relatively high incidence of MDRO in BSI complicating COVID-19 infection in patients hospitalized in medical wards outside the ICU environment. Neither cross-transmission nor gut pre-colonization—especially for VRE, ESLB-producing E. coli and Candida spp.—as a portal of the pathogen can be excluded. A limitation of our study is the lack of information on colonization rates and molecular analysis for clusters of BSI isolates. Additionally, our study is a single-center study in a highly endemic area for MDRO; therefore, our results cannot be generalized. However, via a case-control analysis, we highlighted the conditions that correlate with BSI in COVID-19 patients in medical wards. We confirmed that BSI is the strongest predictor of IMV and in-hospital mortality in a non-ICU population of patients with COVID-19, considered to be less prone to severe complications and adverse outcome.
The COVID-19 pandemic revealed in a dramatic way the urgent necessity for implementation of efficacious infection-control policies with a bundle of interventions in order to reduce BSI and other co-infection in COVID-19 units. Compliance with guidelines for infection control and standards of care are imperative [,].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10071314/s1, Supplementary Table S1: Identification of Candida spp.; Table S2: Pathogen-related in-hospital outcome in patients with BSI.
Author Contributions
Conceptualization, E.G. and O.Z.; methodology, E.G. and O.Z.; software, G.L. (Georgios Loizos), P.K., P.M., C.S., I.S. and E.K.; validation: L.V., G.K. and V.M.; formal analysis, E.K. and C.L.; investigation, E.G., O.Z., C.L., V.M., E.K., V.P., N.R., I.D., P.M. and I.S.; resources, C.L., V.P., N.R., I.D., L.V., G.K., P.M., G.L. (Georgios Loizos) and P.K.; data curation, L.V., G.K., A.S., G.L. (Garifallia Linardaki) and V.M.; writing—original draft preparation, E.G. and O.Z.; writing—review and editing, E.G. and O.Z.; visualization, E.G. and O.Z.; supervision; K.T.-D., S.G., G.C. and O.Z.; project administration, K.T.-D., S.G., G.C., E.G. and O.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of TZANEIO General Hospital, (protocol code 17425/17-12-2020 and date of approval 21-12-2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Markhvida, M.; Hallegatte, S.; Walsh, B. Socio-Economic Impacts of COVID-19 on Household Consumption and Poverty. Econ. Disasters Clim. Chang. 2020, 4, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, R.; Urgelés, S.; Rodríguez, A.; Bodí, M.; Martín-Loeches, I.; Sole-Violan, J.; Díaz, E.; Gómez, J.; Trefler, S.; Vallverdú, M.; et al. Mortality comparison between the first and second/third waves among 3795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg. Health-Europe 2021, 11, 100243. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Zamora-Cintas, M.I.; López, D.J.; Blanco, A.C.; Rodriguez, T.M.; Segarra, J.M.; Novales, J.M.; Ferriol, M.F.R.; Maestre, M.M.; Sacristán, M.S. Coinfections among hospitalized patients with covid-19 in the first pandemic wave. Diagn. Microbiol. Infect. Dis. 2021, 101, 115416. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2020, 27, 83–88. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Wang, Q.; Mei, H.; Zheng, H.; Liang, G.; She, X.; Liu, W. Fungal co-infection in COVID-19 patients: Evidence from a systematic review and meta-analysis. Aging 2021, 13, 7745–7757. [Google Scholar] [CrossRef]
- Pasquini, Z.; Barocci, I.; Brescini, L.; Candelaresi, B.; Castelletti, S.; Valentina, I.; Mazzanti, S.; Procaccini, G.; Orsetti, E.; Pallotta, F.; et al. Bloodstream infections in the COVID-19 era: Results from an Italian multicenter study. Int. J. Infect. Dis. 2021, 111, 31–36. [Google Scholar] [CrossRef]
- Ippolito, M.; Simone, B.; Filisina, C.; Catalanotto, F.R.; Catalisano, G.; Marino, C.; Misseri, G.; Giarratano, A.; Cortegiani, A. Bloodstream Infections in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Microorganisms 2021, 9, 2016. [Google Scholar] [CrossRef] [PubMed]
- Miyagami, T.; Uehara, Y.; Harada, T.; Watari, T.; Shimizu, T.; Nakamura, A.; Ogura, N.; Kushiro, S.; Masuyama, K.; Kanai, Y.; et al. Delayed treatment of bacteremia during the COVID-19 pandemic. Diagnosis 2020, 8, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Soubirou, J.F.; Voiriot, G.; Chemam, S.; Neuville, M.; Mourvillier, B.; Sonneville, R.; Mariotte, E.; Bouadma, L.; Wolff, M. Treatment of bloodstream infections in ICUs. BMC Infect. Dis. 2014, 14, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, V.; Corona, A.; Vitale, D.; Nencini, C.; Potalivo, A.; Prete, A.; Zani, G.; Malfatto, A.; Tritapepe, L.; Taddei, S.; et al. Bacterial infections in critically ill patients with SARS-2-COVID-19 infection: Results of a prospective observational multicenter study. Infection 2022, 50, 139–148. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.A.; Sherertz, R.J.; Warren, D.K. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 49, 1–45. [Google Scholar] [CrossRef]
- Hanson, K.E.; Caliendo, A.M.; Arias, C.A.; Hayden, M.K.; Englund, J.A.; Lee, M.J.; Loeb, M.; Patel, R.; Alayli, A.E.; Altayar, O.; et al. The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing. Available online: https://www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/diagnostics/idsa-covid-19-gl-dx-v2.0.0.pdf (accessed on 1 April 2021).
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Bailli, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf (accessed on 23 January 2022).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Protonotariou, E.; Mantzana, P.; Meletis, G.; Tychala, A.; Kassomenaki, A.; Vasilaki, O.; Kagkalou, G.; Gkeka, I.; Archonti, M.; Kati, S.; et al. Microbiological characteristics of bacteremias among COVID-19 hospitalized patients in a tertiary referral hospital in Northern Greece during the second epidemic wave. FEMS Microbes 2021, 2, xtab021. [Google Scholar] [CrossRef]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Katiyar, C.P.; Jain, R.; Aldrich, M.; Weston, G.; et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect. Control Hosp. Epidemiol. 2020, 42, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Granda, M.J.; Carrillo, C.S.; Rabadán, P.M.; Valerio, M.; Olmedo, M.; Muñoz, P.; Bouza, E. Increase in the frequency of catheter-related bloodstream infections during the COVID-19 pandemic: A plea for control. J. Hosp. Infect. 2021, 119, 149–154. [Google Scholar] [CrossRef]
- Van Der Kooi, T.; Altorf-Van Der Kuil, W.; Schoffelen, A.; Van Rooden, S.; De Greeff, S. First COVID-19 wave in Dutch hospitals: Effects on central venous catheter care and distribution of bacteremia pathogens. Antimicrob. Resist. Infect. Control 2021, 10 (Suppl. 1), covidwho-1448434. [Google Scholar]
- Ohki, R.; Fukui, Y.; Morishita, N.; Iwata, K. Increase of blood culture contamination during COVID-19 pandemic. A retrospective descriptive study. Am. J. Infect. Control 2021, 49, 1359–1361. [Google Scholar] [CrossRef]
- Bayo, S.M.; Ruíz, M.P.P.; Hijazo, M.M.; Usón, M.C.V. Bacteremia during COVID-19 pandemic in a tertiary hospital in Spain. Enferm. Infecc. Microbiol. Clin. 2021, 40, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.; Westblade, L.F.; Whittier, S.; Satlin, M.J.; Greendyke, W.G.; Aaron, J.G.; Zucker, J.; Dietz, D.; Sobieszczyk, M.; Choi, J.J.; et al. Bacteremia and Blood Culture Utilization during COVID-19 Surge in New York City. J. Clin. Microbiol. 2020, 58, e00875-20. [Google Scholar] [CrossRef] [PubMed]
- Denny, S.; Rawson, T.M.; Hart, P.; Satta, G.; Abdulaal, A.; Hughes, S.; Gilchrist, M.; Mughal, N.; Moore, L.S.P. Bacteraemia variation during the COVID-19 pandemic; a multi-centre UK secondary care ecological analysis. BMC Infect. Dis. 2021, 21, 556. [Google Scholar] [CrossRef]
- Guisado-Gil, A.; Infante-Domínguez, C.; Peñalva, G.; Praena, J.; Roca, C.; Navarro-Amuedo, M.; Aguilar-Guisado, M.; Espinosa-Aguilera, N.; Poyato-Borrego, M.; Romero-Rodríguez, N.; et al. Impact of the COVID-19 Pandemic on Antimicrobial Consumption and Hospital-Acquired Candidemia and Multidrug-Resistant Bloodstream Infections. Antibiotics 2020, 9, 816. [Google Scholar] [CrossRef]
- Langford, B.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; Macfadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Innes, G.K.; Walters, M.S.; Mehr, J.; Arias, J.; Greeley, R.; Chew, D. Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions—New Jersey, February–July 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1827–1831. [Google Scholar] [CrossRef]
- Nebreda-Mayoral, T.; Miguel-Gómez, M.A.; March-Rosselló, G.A.; Puente-Fuertes, L.; Cantón-Benito, E.; Martínez-García, A.M.; Muñoz-Martín, A.B.; Orduña-Domingo, A. Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y León, Spain. Enferm. Infecc. Y Microbiol. Clin. (Engl. Ed.) 2022, 40, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Riederer, K.; Sharma, M.; Fukushima, E.A.; Johnson, L.; Saravolatz, L. High rate of Multidrug-Resistant Organisms (MDROs) among COVID-19 patients presenting with bacteremia upon hospital admission. Am. J. Infect. Control 2021, 49, 1441–1442. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, J.A.; Dupper, A.C.; Malik, Y.; Gavioli, E.M.; Banga, J.; Berbel Caban, A.; Nadkarni, D.; Obla, A.; Vasa, C.V.; Mazo, D.; et al. Staphylococcus aureus Bacteremia in Patients Infected with COVID-19: A Case Series. Open Forum Infect. Dis. 2020, 7, ofaa518. [Google Scholar] [CrossRef] [PubMed]
- Adalbert, J.R.; Varshney, K.; Tobin, R.; Pajaro, R. Clinical outcomes in patients co-infected with COVID-19 and Staphylococcus aureus: A scoping review. BMC Infect. Dis. 2021, 21, 985. [Google Scholar] [CrossRef]
- Bonazzetti, C.; Morena, V.; Giacomelli, A.; Oreni, L.; Casalini, G.; Galimberti, L.R.; Bolis, M.; Rimoldi, M.; Ballone, E.; Colombo, R.; et al. Unexpectedly High Frequency of Enterococcal Bloodstream Infections in Coronavirus Disease 2019 Patients Admitted to an Italian ICU: An Observational Study. Crit. Care Med. 2020, 49, e31–e40. [Google Scholar] [CrossRef]
- DeVoe, C.; Segal, M.R.; Wang, L.; Stanley, K.; Madera, S.; Fan, J.; Schouest, J.; Graham-Ojo, R.; Nichols, A.; Prasad, P.A.; et al. Increased rates of secondary bacterial infections, including Enterococcus bacteremia, in patients hospitalized with coronavirus disease 2019 (COVID-19). Infect. Control Hosp. Epidemiol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2020, 64, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; Czaja, C.A.; Kayalioglu, H.; et al. The Landscape of Candidemia During the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin. Infect. Dis. 2021, 74, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Thelen, J.M.; Buenen, A.G.N.; van Apeldoorn, M.; Wertheim, H.F.; Hermans, M.H.A.; Wever, P.C. Community-acquired bacteraemia in COVID-19 in comparison to influenza A and influenza B: A retrospective cohort study. BMC Infect. Dis. 2021, 21, 199. [Google Scholar] [CrossRef]
- Rajni, E.; Garg, V.K.; Bacchani, D.; Sharma, R.; Vohra, R.; Mamoria, V. Prevalence of bloodstream infections and their etiology in covid-19 patients admitted in a tertiary care hospital in Jaipur. Indian J. Crit. Care Med. 2021, 25, 369–373. [Google Scholar] [CrossRef]
- Khatri, A.; Malhotra, P.; Izard, S.; Kim, A.; Oppenheim, M.; Gautam-Goyal, P.; Chen, T.; Doan, T.-L.; Berlinrut, I.; Niknam, N.; et al. Hospital-Acquired Bloodstream Infections in Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 Infection (Coronavirus Disease 2019): Association With Immunosuppressive Therapies. Open Forum Infect. Dis. 2021, 8, ofab339. [Google Scholar] [CrossRef]
- Abelenda-Alonso, G.; Rombauts, A.; Gudiol, C.; Oriol, I.; Simonetti, A.; Coloma, A.; Rodríguez-Molinero, A.; Izquierdo, E.; Díaz-Brito, V.; Sanmartí, M.; et al. Immunomodulatory therapy, risk factors and outcomes of hospital-acquired bloodstream infection in patients with severe COVID-19 pneumonia: A Spanish case–control matched multicentre study (BACTCOVID). Clin. Microbiol. Infect. 2021, 27, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.; Al Mohajer, M. Discontinuation of Contact Precautions in Patients with Nosocomial MRSA and VRE Infections During the COVID-19 Pandemic. Antimicrob. Steward. Healthc. Epidemiol. 2021, 1, s24. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Frank, G.; Kahlmeter, A.; Pan, N.; Petrosillo, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20 (Suppl. 1), 1–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).