Abstract
Leishmaniasis is considered to be one of the most neglected tropical diseases affecting humans and animals around the world. Due to the absence of an effective vaccine, current treatment is based on chemotherapy. However, the continuous appearance of drug resistance and therapeutic failure (TF) lead to an early obsolescence of treatments. Identification of the factors that contribute to TF and drug resistance in leishmaniasis will constitute a useful tool for establishing future strategies to control this disease. In this manuscript, we evaluated the transcriptomic changes in the intracellular amastigotes of the Leishmania infantum parasites isolated from patients with leishmaniasis and TF at 96 h post-infection of THP-1 cells. The adaptation of the parasites to their new environment leads to expression alterations in the genes involved mainly in the transport through cell membranes, energy and redox metabolism, and detoxification. Specifically, the gene that codes for the prostaglandin f2α synthase seems to be relevant in the pathogenicity and TF since it appears substantially upregulated in all the L. infantum lines. Overall, our results show that at the late infection timepoint, the transcriptome of the parasites undergoes significant changes that probably improve the survival of the Leishmania lines in the host cells, contributing to the TF phenotype as well as drug therapy evasion.
1. Introduction
Leishmaniasis is a disease caused by the protozoan parasite Leishmania that involves a wide variety of clinical manifestations, ranging from self-healing cutaneous lesions to visceral disease, the latter of which can be fatal if left untreated. Treatment options vary between regions but are generally limited to only a few first-line drugs, including miltefosine (Mil), paromomycin (PMM), amphotericin B (AmB), and antimonials (Sb). All have limitations in terms of toxicity, efficacy, price, and a significant increase in resistance and therapeutic failure (TF). TF has a multifactorial origin, involving a considerable number of factors concerning (i) the host (immunity or nutritional status), (ii) the parasite (drug resistance, infectivity, parasite localization, accessibility to drugs and coinfection with other pathogens), (iii) the drug (quality, pharmacokinetics), and (iv) the environment (global warming and the expansion of the disease to new geographical areas). Thus, strategies for confronting these drawbacks must include new efficient treatment options [1,2].
Changes at the molecular level in Leishmania parasites associated with drug resistance have been described [1], including, among others, decreased drug entry in host cells infected by Leishmania lines [3,4,5,6,7,8,9]. Additionally, it has been shown that there is an amplification of the ABC transporter PGPA gene in Leishmania tarentolae that leads to a sequestration of the metal–thiol conjugates into vesicular membranes, conferring resistance to arsenites and antimonies [10,11]. Furthermore, an increased drug efflux due to the overexpression of ABC transporters has been described [8,9,12,13,14,15]. However, reports of molecular changes in Leishmania lines associated with TF are significantly scarce, and more studies exploring this aspect are necessary [1]. A Leishmania infantum genetic marker associated with Mil TF has been associated with the absence of a miltefosine sensitivity locus (MSL); this MSL has been found in the genomes of L. infantum and Leishmania donovani lines that have a cure rate above 93% [16]. The MSL has been considered to be a potential molecular marker for predicting the efficacy of Mil treatment”. An additional factor that may also affect clinical efficacy and lead to treatment failure is the presence of Leishmania RNA virus 1 (LRV-1) in parasites of the Viannia subgenus. Recent data have indicated that the virus may subvert the host immune response and affect the clinical response to drugs [17,18].
Knowledge of molecular changes associated with drug resistance and TF could help to increase the efficiency of new therapeutic strategies to be used in patients with leishmaniasis and TF. However, these studies have been undertaken principally in promastigote forms of Leishmania lines that are experimentally resistant to the common drugs, with very few studies including the use of clinical drug-resistant isolates and/or clinical isolates from patients with leishmaniasis and TF [19,20,21]. Molecular approaches to characterize the mechanisms of drug resistance or TF in these Leishmania lines include microarray studies [22], proteomic analysis [23], and, more recently, RNA-seq [24,25,26,27].
Leishmania are parasites whose life cycle requires them to infect and replicate within host cells as intracellular amastigote forms, the most important clinical forms of this organism. Consequently, the use of intracellular amastigotes may be useful for studies directed to know the transcriptomic changes associated with the parasite’s ability to survive, evade the host defenses, and elude the toxic effects of anti-leishmanial drugs. During a host–pathogen interaction, global changes in the gene expression pattern occur both in the host (macrophages) [28,29,30,31,32] and in the infecting pathogens [33,34,35,36,37,38,39,40,41,42].
Recently, based on the dual RNA-seq method, we identified host-specific genes that were modulated after the host macrophages interacted with clinical isolates of L. infantum with different levels of drug susceptibility to the anti-leishmanial drugs associated, or not, with TF [28,29]. In this article, we analyzed the transcriptomic changes in these intracellular amastigotes of L. infantum parasites at 96 h post-infection of THP-1 cells.
Our results contribute to the identification of genes with an altered expression pattern in several L. infantum clinical isolates in the amastigote form after a late infection. The association between these genes and TF is described in this work and expands the knowledge of TF and drug resistance in leishmaniasis.
2. Materials and Methods
2.1. Chemical Compounds
Triton X-100, 4′,6-diamidino-2-phenylindole dilactate (DAPI), phorbol 12-myristate 13-acetate (PMA), and sodium dodecyl sulfate (SDS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). L-glutamine and penicillin/streptomycin were obtained from Gibco. All chemicals were of the highest quality available.
2.2. Culture of Leishmania infantum Lines and THP-1 Cells
We used promastigotes of L. infantum lines: (i) JPC-M5 (MCAN/ES/98/LLM-877) (LJPC) is a reference line for genomics sequence and for transcriptomic studies that have been employed in several publications [27,43] (in this way, this line is sensitive to all the drugs tested) [30]; (ii) LEM3323 and LEM5159, an Sb-resistant line and a Mil-resistant line, respectively, isolated from human immunodeficiency virus (HIV) patients with visceral leishmaniasis (VL) [44] from Dr. L. Lachaud, France; (iii) LEM2126, a PMM-resistant line isolated from an HIV patient with nephrotic syndrome [45]; (iv) LLM2070; (v) LLM2165; (vi) LLM2255; and (vii) LLM2221. The last four L. infantum lines were isolated from TF HIV patients with VL unsuccessfully treated with liposomal AmB (from the WHO Collaborating Center for Leishmaniasis, Instituto de Salud Carlos III; Dr. F. Javier Moreno). The sensitivity of all the above Leishmania lines to the different anti-leishmanial drugs has been recently published [28,29].
All these L. infantum lines were grown at 28 °C in an RPMI 1640-modified medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (hiFBS) (Invitrogen) as described [30]. Human myelomonocytic cells THP-1 were grown at 37 °C and 5% CO2 in an RPMI-1640 medium supplemented with 10% hiFBS, 2 mM glutamate, 100 U/mL penicillin, and 100 mg/mL streptomycin as described [46]. The THP-1 cell line was isolated from a patient with acute monocytic leukemia [47], and these macrophage-differentiated-THP-1 cells have been widely used in the scientific community as a suitable host model for infection of Leishmania lines [48].
2.3. In Vitro Macrophage Infection
THP-1 cells (3 × 106 cells in 25 cm2 flasks or 5 × 105 cells/well in 24-well plates) were differentiated to macrophages and infected with different L. infantum stationary-phase promastigotes incubated for 72 h in an acid medium plus 10% hiFBS at a macrophage/parasite ratio of 1:10 [28,29]. Infected macrophages were incubated in an RPMI-1640 medium plus 10% hiFBS at 37 °C and 5% CO2 for 96 h. Finally, the samples were collected in Qiazol (Qiagen, Hilden, Germany), and total RNA was isolated at the Genomic Unit of GENyO facilities (Granada, Spain), as described later. To determine the percentage of infection and the average number of amastigotes per cell, the macrophages were infected with the same L. infantum lines and treated in parallel with the same conditions as those employed for RNA extraction. For microscopy visualization, the cells were fixed for 30 min at 4 °C with 2.5% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100 in PBS for 30 min. Intracellular parasites were detected by nuclear staining with DAPI (Invitrogen, Carlsbad, CA, USA). We observed a percentage of infection of up to 76% in the L. infantum lines, with a number of amastigotes per cell of up to 11.
2.4. RNA Isolation and cDNA Library Preparation
The protocols used for the RNA isolation of the samples and the generation of cDNA libraries were described previously [28,29]. Briefly, eight different group of samples, defined as LEM2126, LEM3323, LEM5159, LLM2221, LLM2255, LLM2165, LLM2070, and LJPC, with three independent biological replicate experiments each, were mRNA-extracted using a QIAamp RNA miRNeasy Micro Kit (Qiagen/Qiacube), followed by treatment with a DNase kit. RNA integrity was evaluated using an Agilent 2100 Bioanalyzer system with an RNA 6000 Nano Lab Chip kit (Agilent Technologies). Poly(A)-enriched cDNA libraries were generated using a TruSeq Stranded mRNA kit (Illumina). All these analyses and sequencing were performed at GENyO facilities (Granada, Spain).
2.5. RNA-seq Data Generation, Pre-Processing
The RNA-seq protocol carried out in this manuscript was previously described [28,29]. In summary, a next-generation sequencing run for whole transcriptome sequencing was performed using a paired-end 2 × 75 nt library on a NextSeq 500/550 Illumina sequencer and using a high-output Kit v2.5 (150 cycles), which generated an average of 18.9 million reads per sample. Raw data were generated for each of the libraries by the genomic core service at GENyO (Granada, Spain). The RNA-seq data are available at NCBI Short Read Archive (SRA) under accession number PRJNA836366.
2.6. Data Analysis
The transcriptomic samples were analyzed using the miARma-Seq pipeline [49,50]. This software performs automatically all the required steps to obtain differentially expressed genes (DEGs) from raw data files. Firstly, fastq files were evaluated using the FastQC software to analyze the quality of the reads [51]. On average, 91% of the reads have a mean quality over Q30, and no adapter sequence was found. Subsequently, after filtering sequences by quality (lower than Q30) and homogenizing the number of reads per sample using the Seqtk software [52], miARma-Seq aligns all sequences using HISAT2 [53]. With this aim, Leishmania_infantum: LinfantumJPCM5 was used as the reference genome from the TriTrypDB version 38 database. The average percentage of aligned reads against this reference genome was 20.36% (the remaining 60.30% aligned against the human genome). After that, the featureCounts software [52] was used to assign sequence reads with a minimum mapping quality score over 10 genes.
2.7. Differential Expression Analysis
To perform the differential expression analysis, the edgeR package was used [54]. Low-expressed genes were removed, and the remaining genes were normalized using the trimmed mean of M-values (TMM) method [55]. Counts per million (CPM) and log2 counts per million (log-CPM) were used for exploratory plots [54] to check the consistency of the replicates. In that sense, mutilBamSummary and PlotCorrelation from deepTools were used to obtain the Pearson correlation of the triplicates: 0.997 on average. Besides, principal component analysis (PCA) and hierarchical clustering of normalized reads per kilobase per million mapped reads (RPKM) were used to get a general overview on the similarity of RNA-sequencing samples [56,57].
DEGs were calculated between LEM2126, LEM3323, LEM5159, LLM2221, LLM2255, LLM2165, LLM2070 compared to LJPC. All genes having a false discovery rate (FDR) value ≤ 0.05 and log2 FC ≥ 1 (upregulated) or log2 FC ≤ −1 (downregulated) were marked as DEGs. Log2 FC was used to evaluate the significance and the change in expression of a gene between both types of samples.
2.8. Enrichment Analysis
In order to identify the effects of differential gene expression, a functional enrichment study was carried out using the clusterProfiler Bioconductor package [58]. To this end, DEGs were compared against all the expressed genes in the RNA-seq assay. We obtained Gene Ontology (GO) terms per gene from the TriTrypDB version 56 database, and they were associated to Entrez gene identifiers in an orgDB R object through the AnnotationForge package to be used with clusterProfiler. Therefore, GO enrichment analysis was calculated for biological process ontology terms, defining a statistically enriched term to those having a p-adjust value ≤ 0.05.
2.9. Protein–Protein Interaction Network Analysis
The DEGs common to all Leishmania lines with an FDR value lower than 0.05 were submitted to Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) (https://version-11-5.string-db.org/cgi/network?networkId=bZzuqfjJ3nB4; accessed on 1 May 2022) database version 11.0 [59].
3. Results and Discussion
3.1. Transcriptomic Profile of L. infantum Lines after Infection of THP-1 Cells
We analyzed the transcriptomic profile of seven L. infantum lines isolated from patients suffering from leishmaniasis and experiencing TF (associated or not with drug resistance). Specifically, we examined (i) three clinical isolates (LEM3323, LEM5159m and LEM2126) resistant to Sb, Mil, and PMM, respectively, and (ii) four lines that led to TF (LLM2070, LLM2165, LLM2255, and LLM2221). We compared the transcriptome from these clinical isolates with a genomic reference line (LJPC) at a late timepoint post-infection of THP-1 cells. Those genes having an FDR value ≤ 0.05 and log2 FC ≥ 1 (upregulated) or ≤−1 (downregulated) were considered as DEGs.
Our study on the transcriptome of these lines revealed that the number of DEGs varied according to the Leishmania line studied; e.g., LLM2070 with 256 genes was the line with the highest number of upregulated genes (Figure 1). On the contrary, the Sb-resistant line LEM3323 presented the largest number of downregulated genes, 307 (Figure 1), among all the lines. LLM2255 had the fewest changes, 84 upregulated and 149 downregulated genes, in contrast with the 402 DEGs from LEM3323. In the remaining lines, the number of upregulated genes ranged from 84 to 222 genes compared to 43–201 downregulated genes (Figure 1). Additionally, we examined the relative number of hypothetical transcripts altered in all the Leishmania lines. Hypothetical genes are experimentally uncharacterized genes whose functions cannot be deduced from simple sequence comparisons as they lack sequence similarity with known proteins or domains. The results revealed 30–40% of hypothetical genes among the DEGs in each line. However, the LEM2126 line contained around 50% of the DEGs annotated as hypothetical (Figure 1). Other authors have shown similar values of differentially expressed hypothetical genes in their Leishmania transcriptome analyses [27]. One important consideration is that there was no correlation between the DEGs and the corresponding group of Leishmania lines (drug-resistant lines or lines that led to TF).
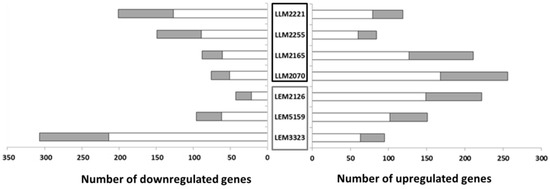
Figure 1.
Differentially expressed genes (DEGs) in the Leishmania lines after infection of THP-1 cells. The number of DEGs in comparison with the reference line (LJPC) is shown. The gray section of each bar represents the number of DEGs that code for hypothetical proteins. Therapeutic failure lines are included in the black box, drug-resistant lines in the gray box.
Venn diagrams were used to reveal the DEGs shared by the different L. infantum lines, including both the upregulated and downregulated genes. For that purpose, the drug-resistant isolates (Figure 2A) and the TF lines (Figure 2B) were studied separately. A total of 25 DEGs were commonly modulated in the drug-resistant lines LEM3323, LEM5159, and LEM2129 (Figure 2A). Moreover, we found eight commonly modulated genes for the TF lines LLM2070, LLM2165, LLM2255, and LLM2221 (Figure 2B). However, the most significant finding was that the two groups (resistant and TF lines, Figure 2C) shared five DEGs, known as LINF_32000970, LINF_320009800, LINF_200016760, LINF_010013400, and LINF_26SNORNA3. LINF_320009800 and LINF_200016760 both corresponded to hypothetical proteins, while LINF_010013400 coded for a peptidyl dipeptidase. LINF_26SNORNA3 is a small nuclear RNA, i.e., a noncoding RNA located in the nucleolus that is involved in rRNA modification. However, the most important commonly modulated gene was LINF_320009700, which codes for a prostaglandin F synthase, a protein involved in essential lipid metabolism pathways in protozoan parasites [60].
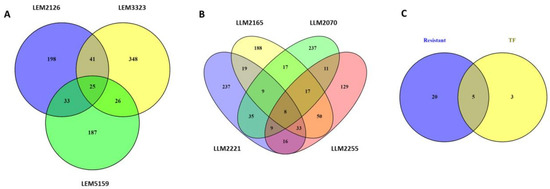
Figure 2.
Venn diagrams of differentially expressed genes (DEGs) in L. infantum lines after THP-1 infection. Venn diagrams of (A) resistant lines, (B) therapeutic failure (TF) lines, and the combination of both (C). The number of exclusive and common genes in each comparison, including total upregulated and downregulated DEGs, are represented.
We also drew up volcano plots to compare the fold changes in expression (log2) with the corresponding adjusted p-values (−log10) (Figure 3). The plots represent the gene expression profiles subtracting the statistically nonsignificant genes from the clinical isolates of L. infantum lines. The DEGs involved in the most relevant functions linked to TF and based on the bibliography are indicated (Figure 3).
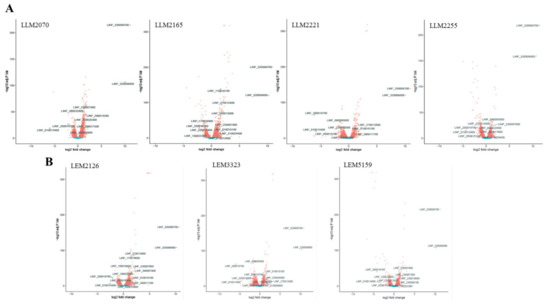
Figure 3.
Volcano plots from the RNA-seq data of clinical isolates from the TF (A) and drug-resistant (B) L. infantum lines. The log2 FC is plotted on the x-axis, and the negative log10 FDR (adjusted p-value) is plotted on the y-axis. Genes are colored according to their FDR: red, FDR ≤ 0.05; blue, FDR ≥ 0.05. Most relevant DEGs based on bibliography and linkage to TF/drug resistance of L. infantum lines are shown.
3.2. Gene Ontology (GO) Enrichment Analysis of the DEGs
We analyzed the GO-enriched terms of the DEGs from the TriTrypDB version 56 database for the biological process category in clinical isolates of L. infantum infecting THP-1 cells at a late timepoint of infection (96 h), focusing on the changes observed in the gene expression of the parasites, to identify common altered processes in Leishmania. The individual profiles of each line are shown in Supplementary Tables S1–S6. This analysis indicated that the drug-resistant parasites LEM2126 and LEM5159 presented a higher number of GO-enriched terms, 28 and 22, respectively (Supplementary Tables S1 and S3). In contrast, TF lines showed a lower number of GO-enriched terms, with zero in the case of LLM2255 and six for LLM2221 (Supplementary Table S6).
On the other hand, the analysis of the common GO terms in the different Leishmania lines revealed seven GO-enriched categories comprising up- and downregulated genes, as well as hypothetical proteins (Table 1). The GO-enriched terms were those primarily related to macromolecular modification and phosphorylation. Interestingly, the presence of similar pathways reinforces the involvement of their genes in TF/drug resistance. In this way, in Leishmania line LEM2126 (a PMM-resistant line), the GO-enriched terms related to macromolecular modification and phosphorylation were due to the upregulation of most of the DEGs; in contrast, in Leishmania line LEM3323 (an Sb-resistant line), these GO-enriched terms were due to the downregulation of most of the DEGs (Table 1). In other Leishmania lines, like LLM2070 and LLM2221, the GO-enriched terms were due to DEGs’ upregulation or downregulation, respectively (Table 1).

Table 1.
Common GO-enriched terms of DEGs for the biological process category in several lines of L. infantum.
3.3. Most Relevant DEGs of L. infantum Lines after Late-Timepoint THP-1 Cell Infection
Chemotherapy is the only effective weapon against leishmaniasis, and this effectiveness is limited by the frequent appearance of drug resistance and TF (1); it is, therefore, crucial to identify potential drug targets able to prevent or reverse such mechanisms. For this purpose, we used L. infantum isolates from leishmaniasis patients to study by RNA-seq the transcriptomic changes after late infection in THP-1 cells. Our analysis of the top DEGs in the different L. infantum lines after late-timepoint THP-1 cell infection (96 h) showed pathways associated with (i) transport, uptake, and efflux across cellular membranes; (ii) energy metabolism; (iii) redox metabolism and detoxification; and (iv) hypothetical proteins (Table 2).

Table 2.
Top DEGs in the different L. infantum lines after late THP-1 cell infection.
In the category “transport, uptake, and efflux through cell membranes”, we included several DEGs, such as mitochondrial ornithine transporter 1-like protein LINF_160007200 that was downregulated in the LLM2221 (−1.38 log2 FC) line (Table 2). This transporter catalyzes the translocation of ornithine and related substrates, such as arginine, across the inner mitochondrial membrane [61].
Other differentially expressed transcripts identified in this study were LINF_130020800, phospholipid-transporting ATPase 1 which was downregulated with a -1.13 log2 FC in the LEM3323 line, and LINF_320010400, a ligand effect modulator 3 (LEM3) family/CDC50 family which was 1.01 log2 FC and 2.34 log2 FC upregulated in LEM5159 and LEM2126, Mil- and PMM-resistant lines, respectively (Table 2). Phospholipid-transporting ATPase 1 and its beta subunit LdRos3 (CDC50/Lem3) are involved in phospholipid translocation at the plasma membrane of Leishmania parasites. Thus, phospholipid-transporting ATPase 1 and LdRos3 form part of the same translocation machinery that determines flippase activity and Mil sensitivity in Leishmania [62]. Therefore, changes in expression of the transporter could contribute to the appearance of differences in the uptake of Mil.
Furthermore, the aquaporin LINF_220020300 was overexpressed in the LLM2070 line (1.20 log2 FC) (Table 2). Aquaporins are membrane proteins responsible for permeating water, ions, dissolved gases, and other small molecular weight compounds through the protective cell membranes of living organisms [63]. LmAQP1 is an aquaporin from L. major, which has proven permeable to glycerol and acts as a drug delivery route for Sb compounds, in addition to playing an important role in osmotaxis [64].
Interestingly, in our study, several transcripts belonging to ATP-binding cassette (ABC) transporters were upregulated in Leishmania lines (Table 2). Thus, ABCC1 and ABCC2 (LINF_230007200 and LINF_230007300, respectively) were upregulated in the LEM5159 line (1.04 log2 FC and 1.01 log2 FC) and ABCA2 (LINF_110018150) was upregulated with a 1.67 log2 FC in the LLM2165 line (Table 2). ABC transporters comprise a superfamily of integral membrane proteins involved in the ATP-dependent transport of a variety of molecules across biological membranes, including amino acids, sugars, peptides, lipids, ions, and chemotherapy drugs [65]. They have been associated with drug resistance in various diseases. In Leishmania, the first ABC protein identified was MRPA (multidrug resistance protein, PgpA) [54], which is a member of the ABCC subfamily, able to confer resistance to Sb by sequestering thiol–metal conjugates into an intracellular vesicle [14,66]. Meanwhile, the ABCA2 transporter plays a role in phospholipid trafficking, which may modify the vesicular trafficking and the infectivity of Leishmania [67]. Thus, overexpression of these ABC transporters could be associated with drug resistance and, as a consequence, TF in these lines.
On the other hand, the category “energy metabolism” also showed differentially expressed transcripts. For example, the argininosuccinate synthase LINF_230007900 was upregulated in all Leishmania lines, except LEM3323 and LLM2221, with a log2 FC from 1.37 to 2.12 (Table 2). Argininosuccinate synthase is a key enzyme in the urea cycle that catalyzes the rate-limiting step in the conversion of L-citrulline to L-arginine and has a rate-limiting role in high-output nitric oxide synthesis [68,69]. This protein is overexpressed in L. infantum axenic antimony-resistant amastigotes and is related to parasite pathogenesis and oxidative stress [70,71].
Another transcript included in the category “energy metabolism” and upregulated (1.43 to 1.43 log2 FC) in several Leishmania lines was the fatty-acyl-CoA synthetase 2 LINF_010010100 (Table 2). This enzyme catalyzes the formation of fatty-acyl-CoA, occupying a pivotal role in cellular homeostasis, particularly lipid metabolism. Fatty acyl-CoA synthetase is differentially expressed by L. donovani amastigotes resistant to Sb [72].
Additionally, the cytochrome c oxidase subunit 10 LINF_230009100 was upregulated (1.02 log2 FC) in the LEM5159 line (Table 2). This enzyme is critical for generating the mitochondrial proton gradient for cellular ATP production. It has been observed that Mil inhibits cytochrome c oxidase in Leishmania promastigotes [73].
Furthermore, the ATP-dependent phosphofructokinase LINF_290032900 was upregulated (1.44 log2 FC) in the LLM2070 line (Table 2). Glycolysis is a central metabolic pathway in all organisms, and a key enzyme of this pathway is 6-phosphofructokinase, which catalyzes the third step of glycolysis: the phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate via the conversion of ATP to ADP. This enzyme has been widely studied in Leishmania [74,75].
One of the categories studied with several DEGs present in the Leishmania lines was that referring to “redox metabolism and detoxification”. A proper intracellular redox balance is vital for the survival and proliferation of all living organisms and depends on reactions based on thiol groups. Most eukaryotic and prokaryotic organisms use glutathione (GSH) or thioredoxin supplemented with the relevant reductases to maintain their reducing capacity. However, the redox metabolism of trypanosomatids is based on the molecule trypanothione, T(SH)2. Thus, this molecule would be involved in functions as diverse as DNA replication, defense against oxidizing agents, assembly of iron–sulfur clusters, and detoxification of ketoaldehydes, xenobiotics, and heavy metals.
The gene that codes for the prostaglandin F2α synthase (PGF2S) (LINF_320009700) was found differentially expressed in all the L. infantum lines studied, with a log2 FC of more than 10 (Table 2). In mammals, prostaglandin synthases catalyze the production of prostaglandins (PGs) using arachidonic acid metabolites as substrates. PGs are lipid mediators involved in cellular processes such as inflammation and tissue homeostasis. PG production is not restricted to multicellular organisms. Trypanosomatids also synthesize several metabolites of arachidonic acid. Nevertheless, their biological role in these early-branching parasites as well as in host–parasite interactions are not well-elucidated. PGF2S has been observed in the Leishmania braziliensis-secreted proteome and in L. donovani extracellular vesicles; it is an enzyme constitutively expressed throughout promastigote development whose overexpression leads to an increase of infectivity in vitro. Furthermore, a positive correlation between L. braziliensis PGF2S expression and pathogenicity in mice has been described [60]. Other researchers have shown PGF2S homologs in L. major, L. tropica, L. donovani, L. infantum, Trypanosoma cruzi, and Trypanosoma brucei [59]. Additionally, it has been described that PGF2S in T. cruzi plays critical roles in oxidative stress and susceptibility to benznidazole [76].
On the other hand, tryparedoxin peroxidases (TXNPx) were differentially expressed in the LLM2070 (LINF_290017300), LLM2165 (LINF_260013000), and LEM2126 (LINF_150018800) lines (1.30, 1.58, and 1.20 log2 FC, respectively) (Table 2). These are peroxiredoxins belonging to a ubiquitous family of antioxidant enzymes which use redox-active cysteines to reduce their substrates. TXNPxs are broad-spectrum peroxidases which can detoxify hydroperoxides, peroxynitrite, and organic hydroperoxides. Different approaches to trypanosomatids have demonstrated that TXNPxs contribute to parasite survival and the establishment and maintenance of infection [77]. Thioredoxin (Trx) genes are present in the genomes of trypanosomatids and are differentially expressed in LLM2070 (LINF_350008900) and LLM2221 (LINF_260011700) (1.21 and 1.02 log2 FC, respectively) (Table 2); however, few studies have been performed on these proteins. The reducing substrate for Trx is T(SH)2 [78]. Recently, a new Trx has been described in T. brucei (TbTrx2) located in the mitochondria and proposed to be essential for parasite growth in both mammalian and insect stages, as well as relevant for in vivo infectivity [79]. Furthermore, glutaredoxins (Grxs, formerly called thiol transferases) are ubiquitous small thiol–disulfide proteins that catalyze the reduction of proteins that are thiolated by GSH (glutathionylated, PSSG). We found that LINF_010006900 was a DEG in LEM3323 (1.10 log2 FC) (Table 2). It is involved in multiple processes, including resistance to oxidative damage, intracellular replication of amastigotes, apoptotic-like cell death, thermotolerance, and proliferation, among other things.
One of the most important genes that codes for gamma-glutamylcysteine synthetase (γ-GCS) (LINF_180022300) (Table 2) was found differentially expressed in LEM5159, LEM2126, and LLM2165 (1.03, 1.65, and −1.04 log2 FC, respectively). It is the first enzyme in the glutathione pathway that produces γ-glutamylcysteine, a direct precursor of glutathione [80]. It has been shown to be essential for L. infantum, where it confers protection against oxidative stress and SbV.
The relationship of the overexpression of cysteine leucine-rich proteins (LINF_340011100 and LINF_320009800) (Table 2), which our RNA-seq analysis revealed to be in LEM3323 and LEM2126 in the first case and in all the L. infantum lines in the second case, with Sb resistance in clinical isolates of L. donovani was recently described [81].
Other relevant genes (LINF_050015100, LINF_220010000, LINF_330034900, LINF_080005000, LINF_170016000, LINF_230016800, LINF_290015200, LINF_290007800, LINF_310025400, and LINF_220017900) related to glutathione metabolism as well as the transfer of different chemical groups for detoxification were altered in the various Leishmania lines studied in this work (Table 2).
Finally, one gene (LINF_200016760) that codifies for an unspecified protein was differentially expressed (downregulated) in all the clinical Leishmania lines with a high log2 FC value (Table 2). Future studies focusing on this gene of unknown function, which has such a high level of downregulation, could pave the way to new therapeutic pathways or provide knowledge about drug resistance and TF in leishmaniasis.
3.4. Functional and Physical Protein Associations
Based on STRING database version 11.5, we established a functional and physical association map using the only gene (PGF2S) that was differentially expressed (upregulated and with an FDR value lower than 0.05) of the five genes common to all the clinical Leishmania lines used in this study that encoded for a protein present in the mentioned database (Figure 4, Table 3).
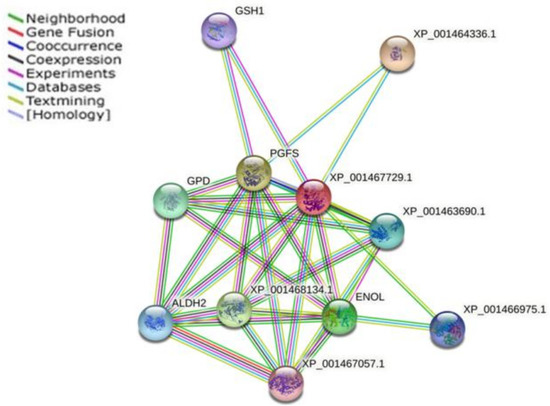
Figure 4.
Functional and physical protein associations interaction map of prostaglandin F2α synthase (PGFS). The nodes are representative of protein species, and different line colors show the types of evidence for the association. The STRING tool (http://www.string-db.org; accessed on 1 May 2022) was used to construct the interaction network. The related proteins are listed in Table 3.

Table 3.
List of proteins directly related to prostaglandin f synthase based on the STRING database.
As described in the previous section, PGs are involved in cellular processes such as inflammation, tissue homeostasis and cellular defense. In this regard, this protein could be considered as an interesting potential drug target taking into account the metabolic functions in which it is involved as well as the overexpression found in the TF Leishmania lines studied. In this way, previous studies confirmed the presence of the PGF2S protein in the secretome of L. braziliensis and the exosome of L. donovani. Additionally, a positive correlation between Leishmania PGF2S expression and pathogenicity has been observed in mice [60]. Using the TDR Targets Database (tdrtargets.org), PGF2S was identified as a drug target with a high potential, having a 0.8 druggability index (range: 0.0 to 1.0) [82].
The structure of the LmPGF2S protein has been resolved by crystallization [83], facilitating future studies for drug design using this protein. The structure of this protein could be useful as a template for structure-based drug design efforts for therapeutics that target both L. major and T. cruzi. However, the high structural similarity of human PGF represents a potential hurdle for the design of selective molecules. Thus, the rational screening of compounds that specifically inhibit the PGF2S could be a way for extending the scarce arsenal of drugs for the treatment of leishmaniasis. One protein intrinsically related to PGF2S is γ-glutamylcysteine synthetase (GSH1) (Figure 4). An increase in GSH1 mRNA levels has been reported in some Leishmania samples with in vitro-induced resistance to Sb; it is one of the most significant proteins in the parasite’s redox metabolism [84] and in some Sb-resistant L. donovani field isolates [85]. Another protein pinpointed in the interaction analysis, related to redox metabolism, and important for TF in leishmaniasis was putative glutathione-S-transferase/glutaredoxin (described above). From the energetic metabolism category, the analysis highlighted the enzyme known as enolase; this is considered to be an important molecule for parasite survival owing to its role in energy production and, more specifically, in the processes of glycolysis and gluconeogenesis. It also contributes to parasite infectivity, as evidenced by its abundant expression in Sb- and AmB-resistant Leishmania parasites [23,86,87], and the protein glycerol-3-phosphate dehydrogenase (glycosomal) (GAPDH). Changes in GAPDH expression may be responsible, at least in part, for the natural resistance to nitric oxide reported in human and canine leishmaniasis [88]. We also located the dihydroxyacetone kinase 1-like protein, which is important for growth and survival and essential for virulence [89].
Lastly, the differential expression pattern of the PGF2S found in all the Leishmania lines analyzed at a late timepoint post-infection could have affected the different pathways in the transcriptome of the parasites due to the relationship established between PGF2S and proteins that facilitate the survival and growth of the parasites, partially explaining the TF in the patients infected with these Leishmania lines.
4. Conclusions
In this work, we evaluated the transcriptomic changes in intracellular amastigotes of L. infantum parasites isolated from patients with leishmaniasis and TF (associated, or not, with drug resistance) 96 h after infection of THP-1 cells. The relevant altered functional pathways and genes include transport through cell membranes, energy and redox metabolism, and detoxification. These modifications determine distinct parasite behaviors that are likely to account for parasite survival and TF in patients. Specifically, the gene that codes for the prostaglandin f2α synthase seems to play an important role in pathogenicity and TF since it appears significantly upregulated in all the L. infantum lines studied. This gene could be a relevant drug target for leishmaniasis for increasing the efficacy of chemotherapy in cases of leishmaniasis; additionally, its combination with a host-directed therapy including molecules that interfere with certain genes/proteins from the host cells required for the survival of these parasites could represent a new therapeutic strategy for increasing the efficacy of chemotherapy and decreasing TF in patients with leishmaniasis. In general, these results show that at a late timepoint post-infection, the transcriptome of these Leishmania lines experiences significant changes that probably contribute to the survival of these parasites in the host cells, as well as to TF. Future works will focus on the proteome of the infecting parasites of L. infantum from TF in order to establish a correlation with the transcriptomic data presented in this manuscript; in this way, we will deepen our understanding of the involvement of PGF2S in the TF and their potential as drug targets.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/microorganisms10071304/s1, Table S1: DEGs for GO-enriched terms for the biological process category in LEM2126 L. infantum line; Table S2: DEGs for GO-enriched terms for the biological process category in LEM3323 L. infantum line; Table S3: DEGs for GO-enriched terms for the biological process category in LEM5159 L. infantum line; Table S4: DEGs for GO-enriched terms for the biological process category in LLM2165 L. infantum line; Table S5: DEGs for GO-enriched terms for the biological process category in LLM2070 L. infantum line; Table S6: DEGs for GO-enriched terms for the biological process category in LLM2221 L. infantum line.
Author Contributions
Conceptualization, F.G., R.G.-H., J.I.M. and A.P.-M.; methodology, R.G.-H., J.I.M. and A.P.-M.; formal analysis, R.G.-H., J.I.M., A.P.-M., L.C.T.-C. and E.A.-L., writing—original draft preparation, R.G.-H., J.I.M., A.P.-M. and F.G.; supervision, F.G.; project administration, F.G.; funding acquisition, F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant RTI2018-097210-B-100 (Francisco Gamarro), funded by MCIN/AEI/10.13039/501100011033 and by ERDF (“A way of making Europe”). E.A.L. was a recipient of a postdoctoral fellowship from the regional Andalusian Government (2020_DOC_00541). L.C.T. was a recipient of a postdoctoral fellowship from the regional Andalusian Government (POSTDOC_21_00394).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Acknowledgments
We thank Laurence Lachaud from Centre National de Référence des Leishmanioses, Université Montpellier (Montpellier, France), for providing the LEM3323 (L3323) and LEM5159 (L5159) L. infantum lines used in this work. Furthermore, we thank F. Javier Moreno from the WHO Collaborating Center for Leishmaniasis, Instituto de Salud Carlos III (ISCIII), for providing the LEM-2126 L. infantum line and the L. infantum lines from therapeutic failure used in this study. RNA-seq were carried out at the Genomics Core at GENyO (Granada, Spain), whereas all bioinformatics analyses were performed at Instituto de Parasitología y Biomedicina “López-Neyra” (IPBLN-CSIC).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Vanaerschot, M.; Dumetz, F.; Roy, S.; Ponte-Sucre, A.; Arevalo, J.; Dujardin, J.C. Treatment failure in leishmaniasis: Drug-resistance or another (epi-) phenotype? Expert Rev. Anti-Infect. Ther. 2014, 12, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Gourbal, B.; Sonuc, N.; Bhattacharjee, H.; Legare, D.; Sundar, S.; Ouellette, M.; Rosen, B.P.; Mukhopadhyay, R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J. Biol. Chem. 2004, 279, 31010–31017. [Google Scholar] [CrossRef] [PubMed]
- Marquis, N.; Gourbal, B.; Rosen, B.P.; Mukhopadhyay, R.; Ouellette, M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Mol. Microbiol. 2005, 57, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Baba, E.H.; Machado-de-Avila, R.A.; Chavez-Olortegui, C.; Demicheli, C.P.; Frézard, F.; Monte-Neto, R.L.; Murta, S.M. Silver and Nitrate Oppositely Modulate Antimony Susceptibility through Aquaglyceroporin 1 in Leishmania (Viannia) Species. Antimicrob. Agents Chemother. 2016, 60, 4482–4489. [Google Scholar] [CrossRef]
- Jhingran, A.; Chawla, B.; Saxena, S.; Barrett, M.P.; Madhubala, R. Paromomycin: Uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2009, 164, 111–117. [Google Scholar] [CrossRef]
- Seifert, K.; Pérez-Victoria, F.J.; Stettler, M.; Sánchez-Cañete, M.P.; Castanys, S.; Gamarro, F.; Croft, S.L. Inactivation of the miltefosine transporter, LdMT, causes miltefosine resistance that is conferred to the amastigote stage of Leishmania donovani and persists in vivo. Int. J. Antimicrob. Agents 2007, 30, 229–235. [Google Scholar] [CrossRef]
- Pérez-Victoria, F.J.; Sánchez-Cañete, M.P.; Seifert, K.; Croft, S.L.; Sundar, S.; Castanys, S.; Gamarro, F. Mechanisms of experimental resistance of Leishmania to miltefosine: Implications for clinical use. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2006, 9, 26–39. [Google Scholar] [CrossRef]
- Barrera, M.C.; Rojas, L.J.; Weiss, A.; Fernandez, O.; McMahon-Pratt, D.; Saravia, N.G.; Gomez, M.A. Profiling gene expression of antimony response genes in Leishmania (Viannia) panamensis and infected macrophages and its relationship with drug susceptibility. Acta Trop. 2017, 176, 355–363. [Google Scholar] [CrossRef]
- Haimeur, A.; Brochu, C.; Genest, P.; Papadopoulou, B.; Ouellette, M. Amplification of the ABC transporter gene PGPA and increased trypanothione levels in potassium antimonyl tartrate (SbIII) resistant Leishmania tarentolae. Mol. Biochem. Parasitol. 2000, 108, 131–135. [Google Scholar] [CrossRef]
- Légaré, D.; Richard, D.; Mukhopadhyay, R.; Stierhof, Y.D.; Rosen, B.P.; Haimeur, A.; Papadopoulou, B.; Ouellette, M. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J. Biol. Chem. 2001, 276, 26301–26307. [Google Scholar] [CrossRef] [PubMed]
- Perea, A.; Manzano, J.I.; Castanys, S.; Gamarro, F. The LABCG2 Transporter from the Protozoan Parasite Leishmania Is Involved in Antimony Resistance. Antimicrob. Agents Chemother. 2016, 60, 3489–3496. [Google Scholar] [CrossRef] [PubMed]
- Castanys-Muñoz, E.; Pérez-Victoria, J.M.; Gamarro, F.; Castanys, S. Characterization of an ABCG-like transporter from the protozoan parasite Leishmania with a role in drug resistance and transbilayer lipid movement. Antimicrob. Agents Chemother. 2008, 52, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.; Légaré, D.; Haimeur, A.; Grondin, K.; Roy, G.; Brochu, C.; Papadopoulou, B. ABC transporters in Leishmania and their role in drug resistance. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 1998, 1, 43–48. [Google Scholar] [CrossRef]
- Coelho, A.C.; Messier, N.; Ouellette, M.; Cotrim, P.C. Role of the ABC transporter PRP1 (ABCC7) in pentamidine resistance in Leishmania amastigotes. Antimicrob. Agents Chemother. 2007, 51, 3030–3032. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Crouch, K.; Forrester, S.; Silva, V.C.; Carvalho, S.F.G.; Damasceno, J.D.; Brown, E.; Dickens, N.J.; Costa, D.L.; Costa, C.H.N.; et al. A Leishmania infantum genetic marker associated with miltefosine treatment failure for visceral leishmaniasis. EBioMedicine 2018, 36, 83–91. [Google Scholar] [CrossRef]
- Adaui, V.; Lye, L.F.; Akopyants, N.S.; Zimic, M.; Llanos-Cuentas, A.; Garcia, L.; Maes, I.; De Doncker, S.; Dobson, D.E.; Arevalo, J.; et al. Association of the Endobiont Double-Stranded RNA Virus LRV1 With Treatment Failure for Human Leishmaniasis Caused by Leishmania braziliensis in Peru and Bolivia. J. Infect. Dis. 2016, 213, 112–121. [Google Scholar] [CrossRef]
- Bourreau, E.; Ginouves, M.; Prévot, G.; Hartley, M.A.; Gangneux, J.P.; Robert-Gangneux, F.; Dufour, J.; Sainte-Marie, D.; Bertolotti, A.; Pratlong, F.; et al. Presence of Leishmania RNA Virus 1 in Leishmania guyanensis Increases the Risk of First-Line Treatment Failure and Symptomatic Relapse. J. Infect. Dis. 2016, 213, 105–111. [Google Scholar] [CrossRef]
- Singh, N. Drug resistance mechanisms in clinical isolates of Leishmania donovani. Indian J. Med. Res. 2006, 123, 411–422. [Google Scholar]
- Ghosh, S.; Biswas, S.; Mukherjee, S.; Pal, A.; Saxena, A.; Sundar, S.; Dujardin, J.C.; Das, S.; Roy, S.; Mukhopadhyay, R.; et al. A Novel Bioimpedance-Based Detection of Miltefosine Susceptibility Among Clinical Leishmania donovani Isolates of the Indian Subcontinent Exhibiting Resistance to Multiple Drugs. Front. Cell. Infect. Microbiol. 2021, 11, 768830. [Google Scholar] [CrossRef]
- Purkait, B.; Kumar, A.; Nandi, N.; Sardar, A.H.; Das, S.; Kumar, S.; Pandey, K.; Ravidas, V.; Kumar, M.; De, T.; et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2012, 56, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Do Monte-Neto, R.L.; Coelho, A.C.; Raymond, F.; Légaré, D.; Corbeil, J.; Melo, M.N.; Frézard, F.; Ouellette, M. Gene expression profiling and molecular characterization of antimony resistance in Leishmania amazonensis. PLoS Negl. Trop. Dis. 2011, 5, e1167. [Google Scholar] [CrossRef]
- Matrangolo, F.S.; Liarte, D.B.; Andrade, L.C.; de Melo, M.F.; Andrade, J.M.; Ferreira, R.F.; Santiago, A.S.; Pirovani, C.P.; Silva-Pereira, R.A.; Murta, S.M. Comparative proteomic analysis of antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum chagasi lines. Mol. Biochem. Parasitol. 2013, 190, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Rastrojo, A.; García-Hernández, R.; Vargas, P.; Camacho, E.; Corvo, L.; Imamura, H.; Dujardin, J.C.; Castanys, S.; Aguado, B.; Gamarro, F.; et al. Genomic and transcriptomic alterations in Leishmania donovani lines experimentally resistant to antileishmanial drugs. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Patino, L.H.; Imamura, H.; Cruz-Saavedra, L.; Pavia, P.; Muskus, C.; Méndez, C.; Dujardin, J.C.; Ramírez, J.D. Major changes in chromosomal somy, gene expression and gene dosage driven by Sb(III) in Leishmania braziliensis and Leishmania panamensis. Sci. Rep. 2019, 9, 9485. [Google Scholar] [CrossRef] [PubMed]
- Patino, L.H.; Muskus, C.; Ramírez, J.D. Transcriptional responses of Leishmania (Leishmania) amazonensis in the presence of trivalent sodium stibogluconate. Parasites Vectors 2019, 12, 348. [Google Scholar] [CrossRef]
- Andrade, J.M.; Gonçalves, L.O.; Liarte, D.B.; Lima, D.A.; Guimarães, F.G.; de Melo Resende, D.; Santi, A.M.M.; de Oliveira, L.M.; Velloso, J.P.L.; Delfino, R.G.; et al. Comparative transcriptomic analysis of antimony resistant and susceptible Leishmania infantum lines. Parasites Vectors 2020, 13, 600. [Google Scholar] [CrossRef]
- Perea-Martínez, A.; García-Hernández, R.; Manzano, J.I.; Gamarro, F. Transcriptomic Analysis in Human Macrophages Infected with Therapeutic Failure Clinical Isolates of Leishmania infantum. ACS Infect. Dis. 2022, 8, 800–810. [Google Scholar] [CrossRef]
- García-Hernández, R.; Manzano, J.I.; Perea-Martínez, A.; Gamarro, F. New Insights on Drug-Resistant Clinical Isolates of Leishmania infantum-Infected Human Macrophages as Determined by Comparative Transcriptome Analyses. Omics J. Integr. Biol. 2022, 26, 165–177. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, C.C.; Batra, S.; McKerrow, J.H.; Loke, P. Delineation of diverse macrophage activation programs in response to intracellular parasites and cytokines. PLoS Negl. Trop. Dis. 2010, 4, e648. [Google Scholar] [CrossRef]
- Chaussabel, D.; Semnani, R.T.; McDowell, M.A.; Sacks, D.; Sher, A.; Nutman, T.B. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 2003, 102, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.; Díaz-Toro, Y.; Tellez, J.; Castilho, T.M.; Rojas, R.; Ettinger, N.A.; Tikhonova, I.; Alexander, N.D.; Valderrama, L.; Hager, J.; et al. Human macrophage response to L. (Viannia) panamensis: Microarray evidence for an early inflammatory response. PLoS Negl. Trop. Dis. 2012, 6, e1866. [Google Scholar] [CrossRef] [PubMed]
- Rastrojo, A.; Carrasco-Ramiro, F.; Martín, D.; Crespillo, A.; Reguera, R.M.; Aguado, B.; Requena, J.M. The transcriptome of Leishmania major in the axenic promastigote stage: Transcript annotation and relative expression levels by RNA-seq. BMC Genom. 2013, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Rastrojo, A.; Corvo, L.; Lombraña, R.; Solana, J.C.; Aguado, B.; Requena, J.M. Analysis by RNA-seq of transcriptomic changes elicited by heat shock in Leishmania major. Sci. Rep. 2019, 9, 6919. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.I.; Muxel, S.M.; Zampieri, R.A.; Müller, K.E.; Nerland, A.H.; Floeter-Winter, L.M. Differential immune response modulation in early Leishmania amazonensis infection of BALB/c and C57BL/6 macrophages based on transcriptome profiles. Sci. Rep. 2019, 9, 19841. [Google Scholar] [CrossRef]
- Shadab, M.; Das, S.; Banerjee, A.; Sinha, R.; Asad, M.; Kamran, M.; Maji, M.; Jha, B.; Deepthi, M.; Kumar, M.; et al. RNA-Seq Revealed Expression of Many Novel Genes Associated With Leishmania donovani Persistence and Clearance in the Host Macrophage. Front. Cell. Infect. Microbiol. 2019, 9, 17. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Dillon, L.A.; Belew, A.T.; Bravo, H.C.; Mosser, D.M.; El-Sayed, N.M. Dual Transcriptome Profiling of Leishmania-Infected Human Macrophages Reveals Distinct Reprogramming Signatures. mBio 2016, 7, e00027-16. [Google Scholar] [CrossRef]
- Dillon, L.A.; Suresh, R.; Okrah, K.; Corrada Bravo, H.; Mosser, D.M.; El-Sayed, N.M. Simultaneous transcriptional profiling of Leishmania major and its murine macrophage host cell reveals insights into host-pathogen interactions. BMC Genom. 2015, 16, 1108. [Google Scholar] [CrossRef]
- Inbar, E.; Hughitt, V.K.; Dillon, L.A.; Ghosh, K.; El-Sayed, N.M.; Sacks, D.L. The Transcriptome of Leishmania major Developmental Stages in Their Natural Sand Fly Vector. mBio 2017, 8, e00029-17. [Google Scholar] [CrossRef]
- Verma, A.; Bhandari, V.; Deep, D.K.; Sundar, S.; Dujardin, J.C.; Singh, R.; Salotra, P. Transcriptome profiling identifies genes/pathways associated with experimental resistance to paromomycin in Leishmania donovani. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Dumetz, F.; Imamura, H.; Sanders, M.; Seblova, V.; Myskova, J.; Pescher, P.; Vanaerschot, M.; Meehan, C.J.; Cuypers, B.; De Muylder, G.; et al. Modulation of Aneuploidy in Leishmania donovani during Adaptation to Different In Vitro and In Vivo Environments and Its Impact on Gene Expression. mBio 2017, 8, e00599-17. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, M.; Kelly, S.; Gluenz, E. Comparative Life Cycle Transcriptomics Revises Leishmania mexicana Genome Annotation and Links a Chromosome Duplication with Parasitism of Vertebrates. PLoS Pathog. 2015, 11, e1005186. [Google Scholar] [CrossRef]
- González-de la Fuente, S.; Peiró-Pastor, R.; Rastrojo, A.; Moreno, J.; Carrasco-Ramiro, F.; Requena, J.M.; Aguado, B. Resequencing of the Leishmania infantum (strain JPCM5) genome and de novo assembly into 36 contigs. Sci. Rep. 2017, 7, 18050. [Google Scholar] [CrossRef]
- Lachaud, L.; Bourgeois, N.; Plourde, M.; Leprohon, P.; Bastien, P.; Ouellette, M. Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin. Infect. Dis. 2009, 48, e16–e22. [Google Scholar] [CrossRef] [PubMed]
- Enríquez, R.; Sirvent, A.E.; Padilla, S.; Toro, P.; Sánchez, M.; Millán, I. Membranoproliferative glomerulonephritis due to visceral leishmaniasis in an HIV patient. Am. J. Case Rep. 2015, 16, 8–11. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; García-Moreno, M.I.; Arroba, A.I.; Aguilar-Diosdado, M.; Padrón, J.M.; García-Hernández, R.; Gamarro, F.; Fustero, S.; Sánchez-Aparicio, J.E.; Masgrau, L.; et al. Synthesis of polyfluoroalkyl sp(2)-iminosugar glycolipids and evaluation of their immunomodulatory properties towards anti-tumor, anti-leishmanial and anti-inflammatory therapies. Eur. J. Med. Chem. 2019, 182, 111604. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Hendrickx, S.; Van Bockstal, L.; Caljon, G.; Maes, L. In-depth comparison of cell-based methodological approaches to determine drug susceptibility of visceral Leishmania isolates. PLoS Negl. Trop. Dis. 2019, 13, e0007885. [Google Scholar] [CrossRef]
- Andrés-León, E.; Núñez-Torres, R.; Rojas, A.M. miARma-Seq: A comprehensive tool for miRNA, mRNA and circRNA analysis. Sci. Rep. 2016, 6, 25749. [Google Scholar] [CrossRef]
- Andrés-León, E.; Rojas, A.M. miARma-Seq, a comprehensive pipeline for the simultaneous study and integration of miRNA and mRNA expression data. Methods 2019, 152, 31–40. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A quality control tool for high throughput sequence data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 1 May 2022).
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Nikolayeva, O.; Robinson, M.D. edgeR for differential RNA-seq and ChIP-seq analysis: An application to stem cell biology. Methods Mol. Biol. 2014, 1150, 45–79. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Reeb, P.D.; Bramardi, S.J.; Steibel, J.P. Assessing Dissimilarity Measures for Sample-Based Hierarchical Clustering of RNA Sequencing Data Using Plasmode Datasets. PLoS ONE 2015, 10, e0132310. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Alves-Ferreira, E.V.C.; Ferreira, T.R.; Walrad, P.; Kaye, P.M.; Cruz, A.K. Leishmania braziliensis prostaglandin F(2α) synthase impacts host infection. Parasites Vectors 2020, 13, 9. [Google Scholar] [CrossRef]
- Monné, M.; Miniero, D.V.; Daddabbo, L.; Palmieri, L.; Porcelli, V.; Palmieri, F. Mitochondrial transporters for ornithine and related amino acids: A review. Amino Acids 2015, 47, 1763–1777. [Google Scholar] [CrossRef]
- Pérez-Victoria, F.J.; Sánchez-Cañete, M.P.; Castanys, S.; Gamarro, F. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J. Biol. Chem. 2006, 281, 23766–23775. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef]
- Figarella, K.; Uzcategui, N.L.; Zhou, Y.; LeFurgey, A.; Ouellette, M.; Bhattacharjee, H.; Mukhopadhyay, R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: Possible role in volume regulation and osmotaxis. Mol. Microbiol. 2007, 65, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.S.; Monte Neto, R.L.; Andrade, J.M.; Santi, A.M.; Reis, P.G.; Frézard, F.; Murta, S.M. Molecular characterization of the MRPA transporter and antimony uptake in four New World Leishmania spp. susceptible and resistant to antimony. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Santos, J.M.; Parodi-Talice, A.; Castanys, S.; Gamarro, F. The overexpression of an intracellular ABCA-like transporter alters phospholipid trafficking in Leishmania. Biochem. Biophys. Res. Commun. 2005, 330, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.; Höhne, W.; Häberle, J. Mutations and polymorphisms in the human argininosuccinate synthetase (ASS1) gene. Hum. Mutat. 2009, 30, 300–307. [Google Scholar] [CrossRef]
- Xie, L.; Gross, S.S. Argininosuccinate synthetase overexpression in vascular smooth muscle cells potentiates immunostimulant-induced NO production. J. Biol. Chem. 1997, 272, 16624–16630. [Google Scholar] [CrossRef]
- El Fadili, K.; Drummelsmith, J.; Roy, G.; Jardim, A.; Ouellette, M. Down regulation of KMP-11 in Leishmania infantum axenic antimony resistant amastigotes as revealed by a proteomic screen. Exp. Parasitol. 2009, 123, 51–57. [Google Scholar] [CrossRef]
- Lakhal-Naouar, I.; Jardim, A.; Strasser, R.; Luo, S.; Kozakai, Y.; Nakhasi, H.L.; Duncan, R.C. Leishmania donovani argininosuccinate synthase is an active enzyme associated with parasite pathogenesis. PLoS Negl. Trop. Dis. 2012, 6, e1849. [Google Scholar] [CrossRef]
- Kaur, J.; Tiwari, R.; Kumar, A.; Singh, N. Bioinformatic Analysis of Leishmania donovani Long-Chain Fatty Acid-CoA Ligase as a Novel Drug Target. Mol. Biol. Int. 2011, 2011, 278051. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luque-Ortega, J.R.; Rivas, L. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- López, C.; Chevalier, N.; Hannaert, V.; Rigden, D.J.; Michels, P.A.; Ramirez, J.L. Leishmania donovani phosphofructokinase. Gene characterization, biochemical properties and structure-modeling studies. Eur. J. Biochem. 2002, 269, 3978–3989. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.M.; Kinkead, J.; McNae, I.W.; Vásquez-Valdivieso, M.; Wear, M.A.; Michels, P.A.M.; Walkinshaw, M.D. Kinetic and structural studies of Trypanosoma and Leishmania phosphofructokinases show evolutionary divergence and identify AMP as a switch regulating glycolysis versus gluconeogenesis. FEBS J. 2020, 287, 2847–2861. [Google Scholar] [CrossRef] [PubMed]
- García-Huertas, P.; Mejía-Jaramillo, A.M.; Machado, C.R.; Guimarães, A.C.; Triana-Chávez, O. Prostaglandin F2α synthase in Trypanosoma cruzi plays critical roles in oxidative stress and susceptibility to benznidazole. R. Soc. Open Sci. 2017, 4, 170773. [Google Scholar] [CrossRef]
- Piñeyro, M.D.; Arias, D.; Parodi-Talice, A.; Guerrero, S.; Robello, C. Trypanothione Metabolism as Drug Target for Trypanosomatids. Curr. Pharm. Des. 2021, 27, 1834–1846. [Google Scholar] [CrossRef]
- Hillebrand, H.; Schmidt, A.; Krauth-Siegel, R.L. A second class of peroxidases linked to the trypanothione metabolism. J. Biol. Chem. 2003, 278, 6809–6815. [Google Scholar] [CrossRef]
- Currier, R.B.; Ulrich, K.; Leroux, A.E.; Dirdjaja, N.; Deambrosi, M.; Bonilla, M.; Ahmed, Y.L.; Adrian, L.; Antelmann, H.; Jakob, U.; et al. An essential thioredoxin-type protein of Trypanosoma brucei acts as redox-regulated mitochondrial chaperone. PLoS Pathog. 2019, 15, e1008065. [Google Scholar] [CrossRef]
- Mukherjee, A.; Roy, G.; Guimond, C.; Ouellette, M. The gamma-glutamylcysteine synthetase gene of Leishmania is essential and involved in response to oxidants. Mol. Microbiol. 2009, 74, 914–927. [Google Scholar] [CrossRef]
- Das, S.; Shah, P.; Tandon, R.; Yadav, N.K.; Sahasrabuddhe, A.A.; Sundar, S.; Siddiqi, M.I.; Dube, A. Over-Expression of Cysteine Leucine Rich Protein Is Related to SAG Resistance in Clinical Isolates of Leishmania donovani. PLoS Negl. Trop. Dis. 2015, 9, e0003992. [Google Scholar] [CrossRef][Green Version]
- Alves-Ferreira, E.V.; Toledo, J.S.; De Oliveira, A.H.; Ferreira, T.R.; Ruy, P.C.; Pinzan, C.F.; Santos, R.F.; Boaventura, V.; Rojo, D.; López-Gonzálvez, Á.; et al. Differential Gene Expression and Infection Profiles of Cutaneous and Mucosal Leishmania braziliensis Isolates from the Same Patient. PLoS Negl. Trop. Dis. 2015, 9, e0004018. [Google Scholar] [CrossRef] [PubMed]
- Moen, S.O.; Fairman, J.W.; Barnes, S.R.; Sullivan, A.; Nakazawa-Hewitt, S.; Van Voorhis, W.C.; Staker, B.L.; Lorimer, D.D.; Myler, P.J.; Edwards, T.E. Structures of prostaglandin F synthase from the protozoa Leishmania major and Trypanosoma cruzi with NADP. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Guimond, C.; Trudel, N.; Brochu, C.; Marquis, N.; El Fadili, A.; Peytavi, R.; Briand, G.; Richard, D.; Messier, N.; Papadopoulou, B.; et al. Modulation of gene expression in Leishmania drug resistant mutants as determined by targeted DNA microarrays. Nucleic Acids Res. 2003, 31, 5886–5896. [Google Scholar] [CrossRef]
- Mukherjee, A.; Padmanabhan, P.K.; Singh, S.; Roy, G.; Girard, I.; Chatterjee, M.; Ouellette, M.; Madhubala, R. Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J. Antimicrob. Chemother. 2007, 59, 204–211. [Google Scholar] [CrossRef]
- Kumar, A.; Sisodia, B.; Misra, P.; Sundar, S.; Shasany, A.K.; Dube, A. Proteome mapping of overexpressed membrane-enriched and cytosolic proteins in sodium antimony gluconate (SAG) resistant clinical isolate of Leishmania donovani. Br. J. Clin. Pharmacol. 2010, 70, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, M.C.; Bourassa, S.; Légaré, D.; Poirier, G.G.; Droit, A.; Ouellette, M. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.C.; Silva, W.R.; Azevedo, A.F.; Santos, P.L.; Teixeira, S.A.; Muscará, M.N.; Thomazzi, S.M.; Almeida, R.P.; Fernandes, R.P.; Scher, R. Expression of glyceraldehyde 3-phosphate dehydrogenase is enhanced in Leishmania spp naturally resistant to nitric oxide. Genet. Mol. Res. GMR 2015, 14, 7113–7121. [Google Scholar] [CrossRef]
- Zufferey, R.; Ben Mamoun, C. Leishmania major expresses a single dihydroxyacetone phosphate acyltransferase localized in the glycosome, important for rapid growth and survival at high cell density and essential for virulence. J. Biol. Chem. 2006, 281, 7952–7959. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).