Abstract
A novel myxobacterial strain ZKHCc1 1396T was isolated in 2017 from a soil sample collected along Chalus Road connecting Tehran and Mazandaran, Iran. It was a Gram-negative, rod-shaped bacterial strain that displayed the general features of Corallococcus, including gliding and fruiting body formation on agar and microbial lytic activity. Strain ZKHCc1 1396T was characterized as an aerobic, mesophilic, and chemoheterotrophic bacterium resistant to many antibiotics. The major cellular fatty acids were branched-chain iso-C17:0 2-OH, iso-C15:0, iso-C17:1, and iso-C17:0. The strain showed the highest 16S rRNA gene sequence similarity to Corallococcus terminator CA054AT (99.67%) and C. praedator CA031BT (99.17%), and formed a novel branch both in the 16S rRNA gene sequence and phylogenomic tree. The genome size was 9,437,609 bp, with a DNA G + C content of 69.8 mol%. The strain had an average nucleotide identity (ANI) value lower than the species cut-off (95%), and with the digital DNA–DNA hybridization (dDDH) below the 70% threshold compared to the closest type strains. Secondary metabolite and biosynthetic gene cluster analyses revealed the strain’s potential to produce novel compounds. Based on polyphasic taxonomic characterization, we propose that strain ZKHCc1 1396T represents a novel species, Corallococcus soli sp. nov. (NCCB 100659T = CIP 111634T).
1. Introduction
Myxobacteria are Gram-negative, rod-shaped bacteria belonging to the phylum Myxococcota [1] and are considered unique for their social behavior and complex developmental growth stages. In many myxobacteria, nutrient-limiting conditions enable the vegetative cells to swarm, aggregate, and form multicellular fruiting bodies [2,3,4,5]. Within the fruiting bodies, resistant and dormant myxospores are contained to ensure the next generation of cells. Myxobacteria are widely and commonly distributed in nature, including in topsoil, animal dung, decaying plants, bark of trees [2,5], and even in halophilic environments [6,7,8,9]. Isolation of myxobacteria in the past decades was mainly driven by natural product application, including the discovery of new antibiotics, and anticancer and antiviral compounds [10,11,12,13].
Myxobacteria form a relatively homogeneous cluster based on 16S rRNA gene-based phylogenetic analysis [14,15,16]. In the last decade, many new genera and families of myxobacteria with unprecedented characteristics were discovered [17,18,19,20,21]. The discovery of several new Corallococcus species in the previous year [22] was not a surprise, since they seem common and widespread in the environment. In the ten described Corallococcus species with validated names, eight were primarily taxonomically described based on the draft genomic data [22]. The genus Corallococcus is known for its rippling swarm; hard, non-sporangiole-type fruiting body; and rounded myxospores [23], which makes morphology an important component in strain characterization. These combined growth features help distinguish this group of bacteria at the very early stage of strain isolation.
Corallococcus appears to gain relatively more attention due to the bioactive compounds discovered in this genus. The strains of C. coralloides had been described for several compounds, including corallopyronins A, B, and C [24], corallorazine A [25], and coralmycins A and B [26], which are known to have antibacterial properties. Of these antibiotics, only the corallopyronin BGC information from Corallococcus coralloides strain B035 [27] is available in the MIBiG database (https://mibig.secondarymetabolites.org/, accessed on 15 December 2021).
The present study describes a novel species of myxobacteria in the genus Corallococcus, and demonstrates its potential to produce novel secondary metabolites.
2. Materials and Methods
2.1. Isolation and Maintenance
Strain ZKHCc1 1396T was isolated at the Helmholtz Centre for Infection Research (HZI) in autumn 2017 from a soil sample collected in October 2015 along the Chalus Road between Tehran and Mazandaran 36°39′18.00″ N, 51°25′13.44″ E, Iran. Chalus Road is a mountainous area connecting Tehran to several northern cities. Its climate is classified as temperate and warm, with more rain in the winter (www.piniran.com, accessed on 4 March 2019). The average annual temperature is 15.7 °C, while the average yearly rainfall is 1081 mm. Its highest temperature (ca. 25.6 °C) peaks in August, while its lowest (ca. 7.2 °C) occurs in February.
The isolation of myxobacteria was based on the standard bacterial baiting technique [3,4,5]. The soil sample was poured to water agar with streaks of living E. coli K-12 DSM 498 bait. The strain was purified by repeated transfers of the swarm edge onto new mineral salt agar [3,4,5]. Axenic culture was maintained on standard VY/2 agar [3,4] and CY–H medium (w/v: 50% CY medium (0.3% Casitone (Difco, Franklin Lakes, NJ, USA), 0.1% yeast extract (Difco), 0.1% CaCl2·2H2O); 50% H medium (0.2% soy meal flour (Hensel, Magstadt, Germany), 0.2% glucose (Sigma-Aldrich, St. Louis, MO, USA), 0.8% soluble starch (Carl Roth, Karlsruhe, Germany), 0.2% yeast extract (Difco), 0.1% CaCl2, 0.1% MgSO4), 50 mM HEPES, and 8 mg Fe-EDTA; adjusted to pH 7.4 with KOH before autoclaving), supplemented with 500 µg ml−1 vitamin B12. Culture in CY–H broth was rotary shaken at 160 r.p.m. for seven days. Both the VY/2 agar plate culture and CY–H broth were incubated at 30 °C. Sample for long-term storage in a −80 °C freezer were prepared from an actively growing CY–H culture and preserved using 20–25% (v/v) glycerol as a cryoprotectant [4].
2.2. Physiology and Chemotaxonomy
Growth morphology characterization was performed on standard nutrient-lean media, including VY/2 and water agar baited with E. coli K-12, and in standard Casitone-containing CY [23] and CY–H media. All solid media in this study contained 1.6% (w/v) Bacto agar. Fruiting bodies and myxospores were observed from previously described nutrient-lean agar media, while the vegetative cells were studied after cultivation in CY–H broth after six days of shaking (160 r.p.m., 30 °C). Swarming was observed on lean media and as well as on Casitone-containing agars. The fruiting bodies and swarm colonies were examined using an Olympus SZX12 stereomicroscope, while the vegetative cells and myxospores were studied using a Zeiss AX10 phase-contrast microscope, photographed using a Zeiss Axiocam MRC camera, and analyzed using AxioVision LE software.
Gram-staining, oxidase, and catalase tests were based on previously described methods [18,28]. The API ZYM® (bioMérieux) and API® Coryne reactions were conducted following the manufacturer’s instructions. Temperature tolerance of the novel strain was determined at 18, 25, 30, 35, 37, and 40 °C, while pH tolerance was tested at pH 5.0–9.0 with intervals of pH 0.5. Both temperature and pH determination were performed in VY/2 agar and were assessed based on the colony growth.
Antibiotic resistance of the novel isolate was tested on VY/2 agar with 50 µg ml−1 antibiotic concentration. The tested antibiotics were ampicillin, amikacin, cefotaxime (Carl Roth), ceftazidime, imipenem, gentamicin, and trimethoprim-sulfamethoxazole (Sigma-Aldrich). All antibiotics were filter-sterilized before being added to the autoclaved agar, which was cooled down to 55 °C before plating.
Microbial predation of the novel myxobacterium was tested using Bacillus subtilis DSM 10T, Micrococcus luteus DSM 1790, Escherichia coli DSM 1116, and Wickerhamomyces anomalus DSM 6766T. Predation was evaluated for clearing of the baited strain, which indicated cell lysis. Degradation of cellulose and chitin was determined based on previously described methods [17], while agar degradation was determined in all solid media, using Bacto agar (1.6% w/v) as a solidifying agent.
The fatty acid extraction was performed using the fatty acid methyl ester method (FAME) [29,30]. The strain was cultivated in 50 mL myxovirescin medium (w/v: 1% soy peptone, 0.025% MgSO4, 0.005% CaCl2, 1 mg/L CoCl2, 100 mM HEPES; adjusted to pH 7.0 with KOH before autoclaving) under shaking conditions (160 r.p.m., 30 °C, six days) before it was harvested by centrifugation (21,000× g, 10 min, 4 °C) and extracted for fatty acids. Analysis and identification of fatty acids was performed by GC–MS based on the standard method for myxobacteria [30].
2.3. Genome and Phylogenetic Analysis
For genomic DNA isolation, the cells were obtained from an actively growing CY–H culture and the DNA was extracted following the standard method for Gram-negative bacteria using the Puregene Core Kit A from Qiagen. The amplification of the 16S rRNA gene was performed using the universal primers F27 (5′-GAGTTTGATCCTGGCTCAGGA-3′) and R1525 (5′-AAGGAGGTGATCCAGCCGCA-3′) [17]. The amplified PCR products were purified using a Macherey Nagel NucleoSpin Kit, separated by gel electrophoresis (0.8% (w/v) agarose, at 70 V, for 45 min), and subsequently sequenced using primers F27 [18], R1525, R518, F1100, and R1100 [19]. The 16S rRNA gene sequence was aligned using the Cap contig assembly of the BioEdit Sequence Alignment Editor software version 7.0.5 [31].
The 16S rRNA gene sequence phylogenetic analysis was conducted using the GGDC web server (http://ggdc.dsmz.de/, accessed on 25 January 2022) [32]. Pairwise sequence similarities were calculated according to the method of Meier-Kolthoff et al. [33]. Phylogenies were inferred using the phylogenomics pipeline developed by DSMZ [34] adapted to single genes, and the sequence alignment was performed using MUSCLE [35]. Maximum likelihood (ML) and maximum parsimony (MP) trees were constructed using RAxML [36] and TNT [37], respectively. For ML, the autoMRE bootstrapping criterion [38], and a subsequent search for the best tree, was employed. In MP, 1000 replicates from bootstrapping were used in conjunction with tree bisection and reconnection branch swapping, and ten random sequence additional replicates. The sequences were evaluated for a compositional bias using the Χ2 test implemented in PAUP* [39].
The genome sequencing of strain ZKHCc1 1396T was carried out using next-generation sequencing technology (Illumina) with MiSeq 600 cycle v3. De novo genome assembly was performed using a Unicycler [40]. Predicted genes, tRNA genes, rRNA genes, and other characteristics of the genome were annotated using PROKKA [41]. In addition, the annotated data from the Prokaryotic Genome Annotation Pipeline (PGAP) of NCBI [42] were also used for genomic comparisons of all Corallococcus type strain genomes. The possible contamination of the genomic data was evaluated using the ContEst16S algorithm to analyze the 16S rRNA gene fragments (https://www.ezbiocloud.net/tools/contest16s, accessed on 10 March 2020) [43]. The complete 16S rRNA gene sequence of the novel strain was extracted from its genome, and this was used for the phylogenetic analysis and percentage similarity comparisons with the closest type strains. The percentage DNA G + C content was determined based on the strain’s genome sequence.
The genomic sequence data of strain ZKHCc1 1396T was uploaded in the Type Strain Genome Server (TYGS) (https://tygs.dsmz.de, accessed on 25 January 2022) for a whole-genome-based taxonomic analysis [44] with the recently introduced methodological updates and features [32]. Information on nomenclature, synonymy, and associated taxonomic literature was provided by the List of Prokaryotic names with Standing Nomenclature (LPSN, available at https://lpsn.dsmz.de, accessed on 25 January 2022) [45].
The uploaded genome was compared against all type strain genomes in the TYGS database using the MASH algorithm [46]. Additionally, the genome data of Corallococcus silvisoli c25j21T (JAAAPJ000000000) was also added separately from the NCBI website (https://www.ncbi.nlm.nih.gov/, accessed on 26 January 2022) because it was not yet listed in the TYGS database. The closest type strains were chosen based on the smallest MASH distances and the 16S rRNA gene sequences. Extraction of 16S rRNA gene sequences from the genome was completed using RNAmmer [47], and was subsequently BLASTed [48] against the type strain’s 16S rRNA gene sequences available in the TYGS database. This was used as a proxy to search for the top 50 matching type strains (according to the bitscore) and to calculate the distances using the Genome BLAST Distance Phylogeny approach (GBDP) under the “coverage” and distance formula d5 algorithm [49]. These distances were used in determining the closest type strain genomes.
Phylogenomic inference was performed using the GBDP. Intergenomic distances were inferred using “trimming” and distance formula d5 algorithm [49] with 100 distance replicates. The digital DDH values (dDDH) and confidence intervals were calculated using the GGDC 3.0 recommended settings [46,49]. The resulting intergenomic distances were used to infer a balanced minimum evolution tree with FASTME 2.1.6.1 branch support, and include SPR post-processing [50]. Branch support was calculated from 100 pseudo-bootstrap replicates each. The phylogenetic trees were rooted at the midpoint [51], and was visualized using a PhyD3 program [52].
The genome sequences of the 11 closely related type strains were retrieved from the NCBI database (https://www.ncbi.nlm.nih.gov/, accessed on 26 January 2022). Average nucleotide identity (ANI) was calculated using the algorithm OrthoANIu (OrthoANI using USEARCH) [53], while the digital DNA–DNA hybridization (dDDH) was determined using the Genome-to-Genome Distance Calculator (GGDC) version 2.1, http://ggdc.dsmz.de (accessed on 26 January 2022) [49].
BiG-SCAPE Analysis
Genome data for all of the type strains of Corallococcus species were downloaded from the NCBI database. The genome data were analyzed using AntiSMASH version 6.0.0 (available at https://antismash.secondarymetabolites.org/, accessed on 2 February 2022) to identify the secondary metabolite gene clusters using the “relaxed” strictness setting [54,55]. All of the predicted biosynthetic gene clusters (BGCs) were then analyzed using the BiG-SCAPE program (version 1.1.2 (3 June 2021)), with the MiBIG database (version 2.1) as a reference [56,57], and Pfam database version 34.0 [58]. Some parameters that were used include a distance cut-off score of 0.3, and the search terms “hybrid” and “mix”. Generated networks were visualized with Cytoscape (version 3.8.2) [59].
2.4. Extract Production, Antimicrobial Assay, and Extract Analysis
The strain ZKHCc1 1396T pre-culture was grown in a 100 mL flask containing 20 mL CY–H broth and incubated on a rotary shaker (160 r.p.m.) for 7–14 days at 30 °C. To screen for secondary metabolites, the resultant cultures (20 mL) were transferred in 250 mL flasks containing 100 mL of the production media, including E medium, CY medium, P medium, POL medium, S medium, M medium, and myxovirescin medium (Table S2), and supplemented with 2% (v/v) XAD-16 adsorber resin. After 14 days of incubation at 30 °C, the resins and cells were collected together by filtering through a fine metal mesh. They were extracted with 70 mL acetone for one hour, filtered with filter paper, and concentrated in vacuo at 40 °C using a rotary evaporator (Heidolph, Schwabach, Germany). The dried extract was resolved in 1 mL methanol and was used (20 µL/strain) in the antimicrobial assay against E. coli DSM 1116, E. coli TolC, S. aureus Newman, C. albicans DSM 1665, Pseudomonas aeruginosa DSM 19882, B. subtilis DSM 10T, Micrococcus luteus DSM 1790, M. smegmatis ATCC 700084, Chromobacterium violaceum DSM 30191T, M. hiemalis DSM 2656T, and Wickerhamomyces anomalus DSM 6766T. These assay strains were obtained from the Microbial Strain Collection Group (MISG) of Helmholtz Centre for Infection Research (HZI) in Braunschweig, Germany. These bacteria were cultivated in Mueller–Hinton broth (Merck) to obtain OD600 of 0.01. Yeast was cultivated in Mycosel broth [60] to obtain OD600 of 0.05.
The crude extract, which showed high and interesting biological activity, was chosen for further analysis using HPLC-DAD Agilent 1260 series coupled with a MaXis ESI–TOF mass spectrometer (Bruker Daltonics, Bremen, Germany). Column C18 Acquity UPLC BEH (Ultra Performance Liquid Chromatography Ethylene-Bridged Hybrid, Waters) and two solvents (solvent A: H2O + 0.1% formic acid; solvent B: ACN + 0.1% formic acid) were used in the HPLC system. The compound separation was performed with a flow rate of 0.6 mL/min, column temperature of 40 °C, and the gradient condition was as follows: 5% B in 0–0.5 min, 5–100% B in 0.5–20 min, and 100% B in 20–25 min [61,62]. Chromatogram and spectrum analysis was conducted using Compass DataAnalysis Version 4.4 (Bruker Daltonics, Billerica, MA, USA). Fractions were selected based on retention time every two minutes in the range of 1.8–20 min from the base peak chromatogram (BPC). For compound prediction, the detected accurate mass (with ±0.01 Da) was manually searched for using the database of Dictionary of Natural Products version 30.1.
3. Results and Discussion
3.1. Taxonomic Identification
Strain ZKHCc1 1396T showed the characteristic features of myxobacteria, which include swarming, fruiting, and myxospore formation (Figure 1). In all solid agar media, the colony produced a coherent swarm after inoculation at the center of the Petri dish (Figure 1a,b). On VY/2 agar, the colony spread fast and formed dense orange fruiting bodies, while a halo was formed after clearing the Baker’s yeast cells (Figure 1a). In contrast, the growth on CY agar was slower and the colony appeared a darker orange due to swarming and some cell aggregates (Figure 1b). The strain produced a thin and transparent swarm with ripples and a flare-shaped pattern at the colony edges in VY/2 agar (Figure 1c), while veins were commonly produced in Casitone-containing CY agar (Figure 1d). Swarms remained on the surface of the agar with no diffusing pigment. Yellowish-to-orange, hard, coral- or horn-shaped fruiting bodies were observed in standard, nutrient-lean VY/2 and water agars, and measured 250–543.5 µm, commonly visible to the naked eye (Figure 1e,f). These unique fruiting body structures were not observed in CY and CY–H agars, but instead were replaced by orange cell aggregates. Fruiting bodies contained tightly packed, rounded, and optically refractile myxospores with a thick coat, and measured 1.3–2.2 µm in diameter (Figure 1g). In contrast, the vegetative cells were non-motile, phase-dark, flexuous, and nearly spindle-shaped rods that measured 4.0–7.6 µm (Figure 1h). All these growth stage characteristics fit within the genus Corallococcus [23].
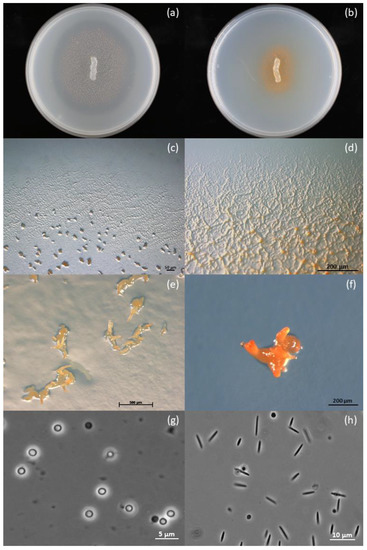
Figure 1.
Growth morphologies of strain ZKHCc1 1396T. (a) Colony on VY/2 agar showing dense orange fruiting bodies around the agar inoculum and lysis of Baker’s yeast cells, as indicated by a halo around it. (b) Colony on CY agar showing darker orange color produced by the swarming cells. (c) Thin and transparent swarm on VY/2 agar with characteristic ripples and flares along the colony edges and fruiting bodies. (d) Swarm on CY agar with pronounced veins and some cell mounds. (e) Coral- or horn-shaped fruiting bodies produced on VY/2 agar. (f) Fruiting body produced on water agar baited with E. coli K-12 bait. (g) Optically refractile and rounded myxospores from a fruiting body produced on water agar. (h) Flexuous and\or slightly tapering vegetative rod cells obtained from CY–H broth. Petri dish diameter is 15 mm (a,b). Stereophotomicrograph (c–f). Phase-contrast photomicrograph (g,h).
Strain ZKHCc1 1396T was catalase-positive, oxidase-negative, and stained Gram-negative. It showed positive API ZYM reactions (+3 to +5) to alkaline phosphatase, C8 esterase lipase, C14 lipase, trypsin, and acid phosphatase, and weak positive reactions (+1 to +2) to C4 esterase, leucine arylamidase, valine arylamidase, cysteine arylamidase, α-chymotrypsin, and naphthol-AS-BI-phosphohydrolase. API ZYM reactions were negative (0) to α- and β-galactosidase, β-glucuronidase, α- and β-glucosidase, N-acetyl-β-glucosaminidase, α-mannosidase, and α-fucosidase. In the API® Coryne, only the gelatin hydrolysis and alkaline phosphatase exhibited positive reactions, which were both similar to those from the same tests using the API ZYM. All these differentiating characteristics with Corallococcus type strains are summarized in Table 1.

Table 1.
Differentiating characteristics of strain ZKHCc1 1396T and genomic information compared with type strains of Corallococcus.
Strain ZKHCc1 1396T exhibited colony growth at 18–35 °C but not at the higher temperatures tested (37 and 40 °C). The optimal growth of the strain was observed at 35 °C; this differs from most of the Corallococcus type strains except for AB050AT, which shows nearly the same pattern. The pH range of the isolate was determined between pH 5.5–10 and was optimal at pH 6.0–8.5, which is in the bracket for most Corallococcus type strains (Table 1).
Strain ZKHCc1 1396T was susceptible to amikacin, cefotaxime, and ceftazidime, but not ampicillin, gentamicin, imipenem, or trimethoprim. The susceptibility to both cefotaxime and ceftazidime hallmarks the difference in the antibiotic profiles among Corallococcus (Table 1).
Cellulose powder, filter paper, chitin, and agar were not degraded or digested, suggesting that the new strain lacks cellulolytic, chitinolytic, and agarolytic activity, respectively. The lysis of the tested bacteria and yeast is not surprising, as it is a common characteristic of the genus Corallococcus and many other myxobacteria in the family Myxococcaceae.
The major cellular fatty acids of strain ZKHCc1 1396T were iso-C17:0 2-OH (31.0%), iso-C15:0 (15.8%), iso-C17:1 (11.7%), and iso-C17:0 (9.4%) (Table 2). The remarkably high amount of branched-chain fatty acids (94.2%) over the straight-chain type agrees with previous myxobacterial fatty acid studies on the genus Corallococcus, and thus differentiates it from the related genera Myxococcus, Pyxidicoccus (Garcia et al., 2011), and Simulacricoccus (Garcia et al. 2018). Among the branched-chain fatty acids, iso-C15:0, iso-C16:0, iso-C17:0, and iso-C17:1 were found to be the most abundant, accounting to 15.8%, 5.6%, 9.4%, and 11.7%, respectively. Moreover, iso-C15:0 was found in all Corallococcus type species (Table S1), and seemed to be one of the major fatty acids in this genus, and as well as in the whole Myxococcaceae family [29]. The overall fatty acid patterns and their major types indicates that strain ZKHCc1 1396T belongs to the genus Corallococcus, but differs from other type species in their fatty acid quantities.

Table 2.
Cellular fatty acid profile of strain ZKHCc1 1396T.
The amplified, almost complete 16S rRNA gene sequence of strain ZKHCc1 1396T was about 1501 bp, while the complete sequence obtained from the genome was 1536 bp, and was determined as identical. Based on the complete 16S rRNA gene sequence, the closest type strain similarities were Corallococcus terminator CA054AT (99.67%) and C. praedator CA031BT (99.17%). Phylogenetic analyses revealed that strain ZKHCc1 1396T clustered within the Corallococcus clade, but formed a separate branch with the closest type strains (Figure 2).
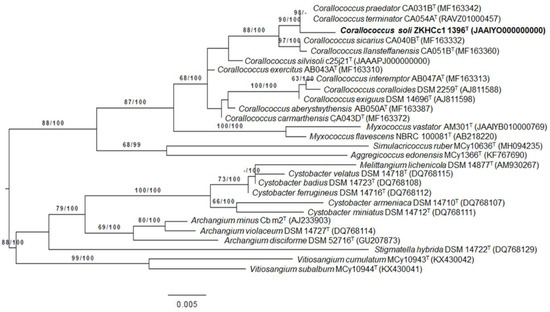
Figure 2.
Phylogenetic tree based on 16S rRNA gene sequence of strain ZKHCc1 1396T and related type strains. ML tree inferred under the GTR + GAMMA model and rooted by midpoint rooting. The branches are scaled in terms of the expected number of substitutions per site. The numbers above the branches are support values when larger than 60% from ML (left) and MP (right) bootstrapping. The ML bootstrapping did not converge; hence, 1000 replicates were conducted, and the average support was 70.64%. The MP bootstrapping average support was 84.24%.
According to the PROKKA annotation, the assembled draft genome of strain ZKHCc1 1396T (GenBank accession No. JAAIYO000000000) consisted of 9,437,609 bp and was characterized by 69.8 %mol G + C content. The genome was predicted to contain 7535 genes comprising 7453 protein-coding genes, 66 tRNA genes, three rRNA genes, and one copy each of the 5S rRNA, 16S rRNA, and 23S rRNA gene. No signs of contamination were found in the genome based on one copy of the 16S rRNA gene. In contrast, the number of genes, proteins, and RNAs varied in number based on the NCBI PGAP annotation pipeline. For comparison with the type strains, the PGAP annotation was used since all data are available in NCBI (Table 1). Strain ZKHCc1 1396T differs among type strains of other Corallococcus type species by having the smallest genome size and the least number of genes, proteins, and tRNAs (Table 1).
The phylogenomic tree supports the novelty of strain ZKHCc1 1396T as it forms a novel branch in the Corallococcus clade. The closest species type strain appears to be Corallococcus praedator CA031BT and C. terminator CA054AT (Figure 3). Furthermore, the difference of the isolated myxobacterium is indicated by the ANI and dDDH values (Table 3). All type strains compared have ANI values lower than the species cut-off (95%), and with dDDH scores below the 70% threshold value.
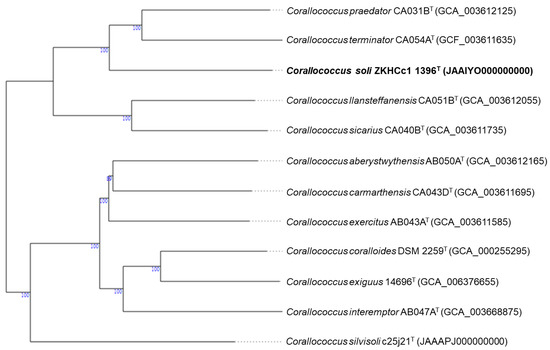
Figure 3.
Genome BLAST Distance Phylogeny (GBDP) shows Corallococcus soli ZKHCc1 1396T and the closely related Corallococcus type strain genomes curated in the genome server (TYGS) database. The numbers at the node represent > 60% GBDP pseudo-bootstrap confidence support (based on 100 replications and, on average, 98.8% branch support).

Table 3.
The similarity of strain ZKHCc1 1396T to Corallococcus type strains based on ANI and dDDH.
3.2. Comparison and Networking of the Secondary Metabolite Biosynthetic Gene Clusters (BGCs)
All of the eleven Corallococcus strains show a 100% similarity score for the geosmin gene cluster (Table 4), while nine strains exhibited 100% for the rhizomide A/rhizomide B/rhizomide C gene cluster. However, based on AntiSMASH evaluation of all the genomes of the Corallococcus type strains, none of them contained the corallopyronin BGC, including the type strain of Corallococcus coralloides DSM 2259T. High similarity scores (≥60%) were found in all strains for the BGC of VEPE/AEPE/TG-1, and nine strains for a carotenoid and myxochelin A/myxochelin B. Our results are in accordance with a previous study by Ahearne et al. [64], which suggested that all of the myxobacterial strains from the Myxococcaceae family contained the BGC of geosmin, VEPE/AEPE/TG-1, and a carotenoid. Strain ZKHCc1 1396T appears to be closely similar in the BGC pattern of Corallococcus terminator CA054AT, but lacks the BGC for icosalide A/icosalide B.

Table 4.
Percentage similarity of the predicted biosynthetic gene cluster (BGC) of strain ZKHCc1 1396T and Corallococcus type strains.
In the nonribosomal peptide synthetase (NRPS) gene cluster, one BGC of strain ZKHCc1 1396T formed a cluster with numerous edges with the BGCs of other Corallococcus type strains together with the BGC of VEPE/AEPE/TG-1 from the MIBiG (minimum information about a biosynthetic gene cluster) database (Figure 4). Two NRPS gene clusters of strain ZKHCc1 1396T were found to have one edge. In the type I PKS (polyketide synthases) gene cluster, one BGC of strain ZKHCc1 1396T had two edges, while in the other PKS gene cluster, it possessed three edges. Strain ZKHCc1 1396T formed seven gene cluster families (GCFs) with the other strains in the NRPS–PKS hybrid gene clusters, whereas five GCFs were created in the RiPPs (ribosomally synthesized and post-translationally modified peptides) gene cluster that contained BGC of strain ZKHCc1 1396T. One GCF in the terpene gene cluster comprised one of the BGCs of strain ZKHCc1 1396T, which was connected with the some BGCs of other Corallococcus type strains, and a carotenoid gene cluster from MIBiG database. For the other BGCs, there were two GCFs containing the BGC of strain ZKHCc1 1396T. Overall, from the analysis of gene cluster network using the BiG-SCAPE platform, the BGCs of strain ZKHCc1 1396T could form one or more GCFs to the other type strains of Corallococcus species in various types of BGCs.
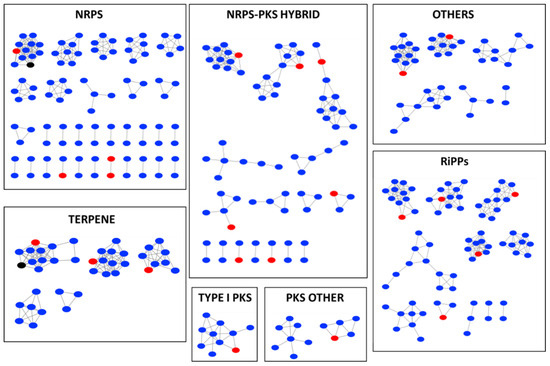
Figure 4.
BiG-SCAPE BGC sequence similarity networks among myxobacteria in the genus Corallococcus and the related sequences from MIBIG database. Strain ZKHCc1 1396T (red), MIBIG database related sequence (black), and all type strains of Corallococcus species (blue): Corallococcus exercitus AB043AT, C. interemptor AB047AT, C. aberystwythensis AB050AT, C. praedator CA031BT, C. sicarius CA040BT, C. carmarthensis CA043DT, C. llansteffanensis CA051BT, C. terminator CA054AT, C. coralloides DSM 2259T, C. exiguus DSM 14696 T, and C. silvisoli c25j21T. Singletons were removed from the analysis.
From the various secondary metabolite production media, the cultivation in P medium was shown to have the most bioactive crude extract against Gram-positive bacteria (Figure 5). The BPC chromatogram of strain ZKHCc1 1396T obtained from cultivation and extraction in P medium produced more than twenty high peaks (above the 90% relative intensity compared to the highest peak) in the range of 1.8–20 min. (Figure 6). The detected ion masses ranged from 209.1645 Da to 1371.9401 Da (Table 5), suggesting the presence of diverse compounds produced by strain ZKHCc1 1396T. Interestingly, no hit compound from DNP was found from the genus Corallococcus. Two hits were detected with similar masses to myxobacterial species Chondromyces crocatus. Analysis of fraction 19 showed no hits in the DNP database, suggesting the possibility of discovering a novel compound from strain ZKHCc1 1396T. Further study is needed to confirm the compounds produced by strain ZKHCc1 1396T, which can be conducted by the isolation and structure elucidation of the compounds.
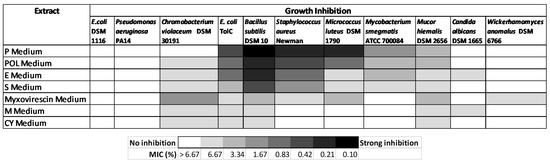
Figure 5.
Heat map of antimicrobial activity of the extract of strain ZKHCc1 1396T.
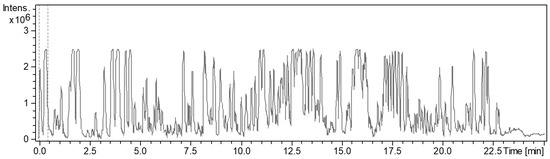
Figure 6.
HPLC chromatogram of strain ZKHCc1 1396T crude extract obtained after cultivation in P medium.

Table 5.
Fraction analysis of strain ZKHCc1 1396T crude extract obtained after cultivation and extraction in P medium.
3.3. Description of Corallococcus soli sp. Nov.
Corallococcus soli (so’li. L. gen. n. soli, of soil, referring to a myxobacterium isolated from a soil sample collected in Iran).
Vegetative cells are phase-dark and flexuous rods (4.0–7.6 µm) in CY–H medium, and move by gliding on agar. Swarm colonies are transparent in VY/2 and water agars, with a rippling pattern. Bright orange colonies with veins are evident in CY and CY–H agars. Fruiting bodies in VY/2 and water agars appear orange and very large (250–543.5 µm) forming cartilaginous, hard horns and coral-shaped structures. Myxospores packed in the fruiting bodies are refractile, spherical-to-ellipsoidal (1.3–2.2 µm diameter) with a distinct spore coat. Other characteristics include aerobic, mesophilic (18–35 °C, optimum at 35 °C), neutrophilic (pH 5.5–10, optimum at pH 6.0–8.5), Gram-negative, catalase-positive, and oxidase-negative. We found that agar, chitin, cellulose are not degraded by this strain. Moreover, the strain exhibits positive API ZYM reactions to alkaline phosphatase, C8 esterase lipase, C14 lipase, trypsin, and acid phosphatase, weak positive reactions to C4 esterase, leucine arylamidase, valine arylamidase, cysteine arylamidase, α-chymotrypsin, and naphthol-AS-BI-phosphohydrolase, and negative API ZYM reactions to α- and β-galactosidase, β-glucuronidase, α- and β-glucosidase, N-acetyl-β-glucosaminidase, α-mannosidase, and α-fucosidase. It can hydrolyze gelatin but unable to ferment glucose, ribose, xylose, mannitol, maltose, lactose, sucrose, or glycogen. Furthermore, it exhibits a negative API® Coryne reaction to nitrate, pyrazinamidase, pyrrolidonyl arylamidase, and urease, and is resistant to ampicillin, gentamicin, imipenem, and trimethoprim, but sensitive to amikacin, cefotaxime, and ceftazidime. The major cellular fatty acids of this strain are iso-C17:0 2-OH, iso-C15:0, iso-C17:1, and iso-C17:0. The DNA G + C content is 69.7 mol%, and the genome size of the type strain is 9,437,609 bp. The draft genome sequence is available under DDBJ/ENA/GenBank accession number JAAIYO000000000. The type strain is ZKHCc1 1396T (=NCCB 100659T = CIP 111634T), isolated in 2017 from a soil sample collected along Chalus Road between Tehran and Mazandaran in Iran
4. Conclusions
Based on polyphasic characterizations, which include morphological, physiochemical, and genomic studies, strain ZKHCc1 1396T appears to represent a novel species of Corallococcus, for which we propose the name Corallococcus soli sp. nov. (type strain ZKHCc1 1396T (=NCCB 100659T = CIP 111634T)).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10071262/s1. Two supplementary tables are available with the online version of this article. Table S1: Differentiating fatty acid profile of strain ZKHCc1 1396T against all Corallococcus species. Table S2: Media and ingredients used in this study.
Author Contributions
Conceptualization, J.W., G.H.E., R.G., Z.K.B.; Genomic analysis, C.R., R.G.; Funding acquisition, R.G., R.M.; Investigation, Z.K.B., R.G.; Methodology, Z.K.B., P.K., R.G., M.J.; Supervision, R.G.; R.M.; Validation, J.W.; Writing, review, & editing, Z.K.B., R.G., All authors have read and agreed to the published version of the manuscript.
Funding
R.G. and R.M. were supported by the German Centre for Infection Research (Deutsches Zentrum for Infektionsforschung) under grant no. DZIF TTU09.811.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Detailed data concerning this study is available upon request.
Acknowledgments
We gratefully thank Fabienne Hennessen, Katja Gemperlein, Wera Collisi, Birte Trunkwalter, Steffi Schulz, and Klaus Peter Conrad for their help and excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Waite, D.W.; Chuvochina, M.; Pelikan, C.; Parks, D.H.; Yilmaz, P.; Wagner, M.; Loy, A.; Naganuma, T.; Nakai, R.; Whitman, W.B.; et al. Proposal to reclassify the proteobacterial classes Deltaproteobacteria and Oligoflexia, and the phylum Thermodesulfobacteria into four phyla reflecting major functional capabilities. Int. J. Syst. Evol. Microbiol. 2020, 70, 5972–6016. [Google Scholar] [CrossRef] [PubMed]
- Dawid, W. Biology and global distribution of myxobacteria in soils. FEMS Microbiol. Rev. 2000, 24, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, H.; Dworkin, M. The myxobacteria. In The Prokaryotes, 2nd ed.; Balows, A., Trüper, H.G., Dworkin, M., Harder, W., Schleifer, K.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 3416–3487. [Google Scholar]
- Shimkets, L.J.; Dworkin, M.; Reichenbach, H. The myxobacteria. In The Prokaryotes: A Handbook on the Biology of Bacteria; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 7, pp. 31–115. [Google Scholar]
- Garcia, R.; Müller, R. The family Polyangiaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 247–279. [Google Scholar]
- Iizuka, T.; Jojima, Y.; Fudou, R.; Yamanaka, S. Isolation of myxobacteria from the marine environment. FEMS Microbiol. Lett. 1998, 169, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; La Clair, J.; Müller, R. Future directions of marine myxobacterial natural product discovery inferred from metagenomics. Mar. Drugs 2018, 16, 303. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.; Müller, R. The Family Haliangiaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 173–181. [Google Scholar]
- Garcia, R.; Müller, R. The Family Nannocystaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 213–229. [Google Scholar]
- Gerth, K.; Pradella, S.; Perlova, O.; Beyer, S.; Müller, R. Myxobacteria: Proficient producers of novel natural products with various biological activities–past and future biotechnological aspects with the focus on the genus Sorangium. J. Biotechnol. 2003, 106, 233–253. [Google Scholar] [CrossRef]
- Garcia, R.O.; Krug, D.; Müller, R. Chapter 3. Discovering natural products from myxobacteria with emphasis on rare producer strains in combination with improved analytical methods. In Methods in Enzymology; Hopwood, D., Ed.; Academic Press: Burlington, NJ, USA, 2009; Volume 458, pp. 59–91. [Google Scholar]
- Plaza, A.; Müller, R. Chapter 6 Myxobacteria: Chemical diversity and screening strategies. In Natural Products: Discourse, Diversity, and Design, 1st ed.; Osbourn, A., Goss, R.J., Carter, G.T., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2014; pp. 103–124. [Google Scholar]
- Weissman, K.J.; Müller, R. Myxobacterial secondary metabolites: Bioactivities and modes-of-action. Nat. Prod. Rep. 2010, 27, 1276–1295. [Google Scholar] [CrossRef]
- Shimkets, L.; Woese, C.R. A phylogenetic analysis of the myxobacteria: Basis for their classification. Proc. Natl. Acad. Sci. USA 1992, 89, 9459–9463. [Google Scholar] [CrossRef] [Green Version]
- Spröer, C.; Reichenbach, H.; Stackebrandt, E. The correlation between morphological and phylogenetic classification of myxobacteria. Int. J. Syst. Bacteriol. 1999, 49, 1255–1262. [Google Scholar] [CrossRef]
- Garcia, R.; Gerth, K.; Stadler, M.; Dogma, I.J.; Müller, R. Expanded phylogeny of myxobacteria and evidence for cultivation of the ‘unculturables’. Mol. Phylogenet. Evol. 2010, 57, 878–887. [Google Scholar] [CrossRef]
- Garcia, R.; Gemperlein, K.; Müller, R. Minicystis rosea gen. nov., sp. nov., a polyunsaturated fatty acid-rich and steroid-producing soil myxobacterium. Int. J. Syst. Evol. Microbiol. 2014, 64, 3733–3742. [Google Scholar] [CrossRef]
- Garcia, R.; Stadler, M.; Gemperlein, K.; Müller, R. Aetherobacter fasciculatus gen. nov., sp. nov. and Aetherobacter rufus sp. nov., novel myxobacteria with promising biotechnological applications. Int. J. Syst. Evol. Microbiol. 2016, 66, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Müller, R. Simulacricoccus ruber gen. nov., sp. nov., a microaerotolerant, non-fruiting, myxospores forming soil myxobacterium and emended description of the family Myxococcaceae. Int. J. Syst. Evol. Microbiol. 2018, 68, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Reichenbach, H.; Ring, M.W.; Müller, R. Phaselicystis flava gen. nov., sp. nov., an arachidonic acid- containing soil myxobacterium, and the description of Phaselicystidaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 1524–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, K.I.; Garcia, R.O.; Gerth, K.; Irschik, H.; Müller, R. Sandaracinus amylolyticus gen. nov., sp. nov., a starch-degrading soil myxobacterium, and description of Sandaracinaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, P.G.; Ingleby, O.; Girdwood, S.; Cookson, A.R.; Morphew, R.M.; Whitworth, D.E. Predatory organisms with untapped biosynthetic potential: Descriptions of novel Corallococcus species C. aberystwythensis sp. nov., C. carmarthensis sp. nov., C. exercitus sp. nov., C. interemptor sp. nov., C. llansteffanensis sp. nov., C. praedator sp. nov., C. sicarius sp. nov., and C. terminator sp. nov. Appl. Environ. Microbiol. 2020, 86, e01931-19. [Google Scholar]
- Garcia, R.; Müller, R. The family Myxococcaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 191–212. [Google Scholar]
- Jansen, R.; Höfle, G.; Irschik, H.; Reichenbach, H. Antibiotika aus Gleitenden Bakterien, XXIV. Corallopyronin A, B und C–drei neue Antibiotika aus Corallococcus coralloides Cc c127 (Myxobacterales). Liebigs Ann. Chem. 1985, 4, 822–836. [Google Scholar] [CrossRef]
- Schmitz, A.; Kehraus, S.; Schäberle, T.F.; Neu, E.; Almeida, C.; Roth, M.; König, G.M. Corallorazines from the myxobacterium Corallococcus coralloides. J. Nat. Prod. 2014, 77, 159–163. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.J.; Kim, G.W.; Cho, K.; Takahashi, S.; Koshino, H.; Kim, W.G. Isolation of coralmycins A and B, potent anti-Gram negative compounds from the myxobacteria Corallococcus coralloides M23. J. Nat. Prod. 2016, 79, 2223–2228. [Google Scholar] [CrossRef]
- Erol, Ö.; Schäberle, T.F.; Schmitz, A.; Rachid, S.; Gurgui, C.; El Omari, M.; Lohr, F.; Kehraus, S.; Piel, J.; Müller, R.; et al. Biosynthesis of the myxobacterial antibiotic corallopyronin A. ChemBioChem 2010, 11, 1253–1265. [Google Scholar] [CrossRef]
- Gerhardt, P.; Murray, R.G.E.; Costilow, R.N.; Nester, E.W.; Wood, W.A.; Krieg, N.R.; Phillips, G.E. (Eds.) Manual of Methods for General Bacteriology; American Society for Microbiology: Washington, DC, USA, 1981; pp. 415–416. [Google Scholar]
- Garcia, R.; Pistorius, D.; Stadler, M.; Müller, R. Fatty acid-related phylogeny of myxobacteria as an approach to discover polyunsaturated omega-3/6 fatty acids. J. Bacteriol. 2011, 193, 1930–1942. [Google Scholar] [CrossRef] [Green Version]
- Gemperlein, K.; Rachid, S.; Garcia, R.O.; Wenzel, S.C.; Müller, R. Polyunsaturated fatty acid biosynthesis inmyxobacteria: Different PUFA synthases and their product diversity. Chem. Sci. 2014, 5, 1733–1741. [Google Scholar] [CrossRef]
- Hall, T.A. BIOEDIT: A user-friendly biological sequence alignment editor and analysis program from windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2021, 50, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M.; Spröer, C.; Klenk, H.-P. When should a DDH experiment be mandatory in microbialtaxonomy? Arch. Microbiol. 2013, 195, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Hahnke, R.L.; Petersen, J.; Scheuner, C.; Michael, V.; Fiebig, A.; Rohde, C.; Rohde, M.; Fartmann, B.; Goodwin, L.A.; et al. Complete genome sequence of DSM 30083T, the type strain (U5/41T) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand. Genom. Sci. 2014, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Pattengale, N.D.; Alipour, M.; Bininda-Emonds, O.R.P.; Moret, B.M.E.; Stamatakis, A. How many bootstrap replicates are necessary? J. Comput. Biol. 2010, 17, 337–354. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4.0 b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; O’Neill, K.R.; Haft, D.H.; DiCuccio, M.; Chetvernin, V.; Badretdin, A.; Coulouris, E.; Chitsaz, F.; Derbyshire, M.K.; Durkin, A.S.; et al. RefSeq: Expanding the Prokaryotic Genome Annotation Pipeline reach with protein family model curation. Nucleic Acids Res. 2021, 49, D1020–D1028. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Chalita, M.; Ha, S.M.; Na, S.I.; Yoon, S.H.; Chun, J. ContEst16S: An algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2017, 67, 2053–2057. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C. LPSN–List of Prokaryotic names with Standing in Nomenclature (bacterio. net), 20 years on. Int. J. Syst. Evol. Microbiol. 2018, 68, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef] [Green Version]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Staerfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef] [Green Version]
- Farris, J.S. Estimating phylogenetic trees from distance matrices. Am. Nat. 1972, 106, 645–667. [Google Scholar] [CrossRef]
- Kreft, L.; Botzki, A.; Coppens, F.; Vandepoele, K.; Van Bel, M. PhyD3: A phylogenetic tree viewer with extended phyloXML support for functional genomics data visualization. Bioinformatics 2017, 33, 2946–2947. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Lim, J.M.; Kwon, S.J.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. AntiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Muñoz, J.C.; Terlouw, B.R.; Van Der Hooft, J.J.J.; Van Santen, J.A.; Tracanna, V.; Duran, H.G.S.; Andreu, V.P.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020, 48, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Muñoz, J.C.; Selem-Mojica, N.; Mullowney, M.W.; Kautsar, S.A.; Tryon, J.H.; Parkinson, E.I.; De Los Santos, E.L.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; et al. A computational framework to explore large-scale biosynthetic diversity. Nat. Chem. Biol. 2020, 16, 60–68. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families’ database in 2021. Nucleic Acids Res. 2021, 49, 412–419. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2013, 13, 2498–2504. [Google Scholar] [CrossRef]
- Cazin, J., Jr.; Wiemer, D.F.; Howard, J.J. Isolation, growth characteristics, and long-term storage of fungi cultivated by attine ants. Appl. Environ. Microbial. 1989, 55, 1346–1350. [Google Scholar] [CrossRef] [Green Version]
- Khosravi Babadi, Z.; Ebrahimipour, G.; Wink, J.; Narmani, A.; Risdian, C. Isolation and identification of Streptomyces sp. Act4Zk, a good producer of Staurosporine and some derivatives. Lett. Appl. Microbiol. 2021, 72, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Primahana, G.; Risdian, C.; Mozef, T.; Wink, J.; Surup, F.; Stadler, M. Amycolatomycins A and B, Cyclic Hexapeptides Isolated from an Amycolatopsis sp. 195334CR. Antibiotics 2021, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Feng, G.D.; Liu, Y.; Zhou, Y.; Deng, X.; Yao, Q.; Zhu, H. Corallococcus silvisoli sp. nov., a novel myxobacterium isolated from subtropical forest soil. Arch. Microbiol. 2022, 204, 141. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, A.; Albataineh, H.; Dowd, S.E.; Stevens, D.C. Assessment of Evolutionary Relationships for Prioritization of Myxobacteria for Natural Product Discovery. Microorganisms 2021, 9, 1376. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).