Abstract
Lactobacillus strains with fine probiotic properties are continuously needed in the laying hen industry to improve the animals’ gut health and production performance. In this study, we isolated 57 Lactobacillus strains from the gut microbiota of 17 different chicken breeds in China. We characterized the probiotic features of these isolates, and evaluated the effects of a selected strain, Lactobacillus salivarius CML352, on the production performance and gut health of the late-phase laying hens. The results showed that the isolates varied much in probiotic properties, among which L. salivarius CML352 displayed high acid and bile salt tolerance, high hydrophobicity, auto-aggregation, and antibacterial activities. Whole genome sequencing analysis showed that CML352 was closely related to a strain isolated from human fecal samples, but had different functional potentials. Dietary supplementary of L. salivarius CML352 significantly reduced the Firmicutes to Bacteroidetes ratio, increased the expression of Muc-2, and decreased the expression of MyD88, IFN-γ, and TLR-4. Furthermore, strain CML352 reduced the birds’ abdominal fat deposition, and improved egg quality. Taken together, this study indicated that the newly isolated L. salivarius strain might be a worthy probiotic with positive impacts on the intestinal health and production performance of late-phase laying hens.
1. Introduction
The laying hen industry is one of the key animal husbandries worldwide. However, the late-phase of production (the egg production is below 90%) accounts for a large proportion of the whole period of layer production, during which the production performance and egg quality significantly declined, leading to a restricted economic benefit of layer production [1]. Thus, it is of great significance to improve the performance of laying hens during the late-phase of laying. One pivotal reason for the decreased productive performance of laying hens in the late phase of production could be the corresponding exhaustion of intestinal function [2].
It has been increasingly recognized that intestines play an important role in contributing to the overall health and production performance of layers, and even the poultry industry [3], for it not only allows absorption of nutrients but also acts as a barrier to prevent pathogens and toxins [4]. As a complex structure, a healthy chicken intestinal barrier is made up of four main components: the physical barrier, chemical barrier, immunological barrier, and the microbiological barrier; among them, microbiological barrier is an essential component [5], mainly formed by intestinal bacteria. Evidence has proved that microbiota in chicken intestine can improve production performance by regulating digestion and metabolism [6], and protect the host from pathogen colonization of the gut. A disorder of the gut microbiota promotes the development of diseases such as inflammatory bowel diseases (IBD) [7], metabolic disorders [8], and autoimmune diseases [9]. In recent years, probiotics have been recognized to have beneficial effects, and widely used in poultry husbandry to keep chicken intestinal homeostasis and improve the productivity through altering the composition and functions of gut microbiota in recent years [10,11].
One of the most prominent bacteria used as probiotics belong to the genera, Lactobacillus [12]. A wealth of information had been gathered over the past years regarding the beneficial health effects and possible mechanism of actions of Lactobacillus on poultry [13]. Lactobacillus species could colonize the epithelial tissues in chicken gut [14], and exhibit unique bactericidal activity through the production of organic acids, hydrogen peroxide and bacteriocins [15]. They are also widely recognized for their immunomodulatory effects on both humans and animals [16]. In broilers and layers, the role of Lactobacillus strains in affecting the microflora of digestive tract and increasing the growth and production performance of chickens has been revealed [17,18].
Different Lactobacillus strains have been proven to have discrepant beneficial effects in chicken, which might depend upon factors such as different bacterial strains, different concentrations, the form of probiotics, the ages of the hens, the appetites of the animals, and the hygiene conditions of the farm [19]. Among them, the strain’s probiotic activity is a very important precondition. Due to natural selection, Lactobacillus strains with different origins may evolve different features to adapt their regular living conditions. In recent years, the endogenous Lactobacillus from gastrointestinal tract, also known as autochthonous bacteria, is attracting more attention.
Depending on the relationship between the bacteria and the host, the gut microbiota is classified into autochthonous microbiota and allochthonous microbiota. Autochthonous microbiota is relatively safe and generally does not cause a secretion of antibodies in the host, or produces only low levels of antibodies, while allochthonous microbiota can usually trigger a strong immune response [20]. Meanwhile, it is emphasized that the ability of Lactobacillus to colonize the intestinal epithelial cells is host-specific [21]. An isolate from the host itself will be more adaptable to support a commensal relationship with the host as compared to bacteria from dissimilar organisms [22]. Thus, it is necessary to isolate and develop autochthonous Lactobacillus to be health-promoters for their original host species [23]. Previous studies have confirmed the positive effects of autochthonous Lactobacillus strains on animal growth performance, immunity, disease resistance, and gut microbiota balance [24,25].
Based on these findings, we hypothesized that healthy chickens’ digestive systems could be a potential source of Lactobacillus strains with fine probiotic properties and a resulting beneficial effect on laying hens. Therefore, in this study, we isolated and evaluated the probiotic properties of autochthonous Lactobacillus strains from Chinese local breed chickens. Then, we performed animal experiments to investigate the role of the selected strain in modulating gut microbiota, intestinal health, and egg quality in late-phase laying hens.
2. Materials and Methods
2.1. Sampling
Local breed chickens were locally sacrificed in different regions of China. The chicken intestines were separated and stored at −20 °C. The samples were then transported to our lab in dry ice, and the contents in duodenum, ileum, and cecum were collected in a super-clean table.
2.2. Isolation and Identification of Lactobacillus from Chicken Gut Contents
The procedure of isolation and identification was conducted according to Lee et al. [26] but with slight modifications. In addition, 0.1 g of chicken gut contents were suspended in 0.9 mL of sterile saline (0.85% w/v, pH 7.2–7.4) and homogenized with an electrical blender for 20 min. Serial decimal dilutions were prepared in the same diluent, and 0.1 mL was inoculated in triplicates by surface spreading on de Man, Rogosa, and Sharpe agar (MRS) containing 2% (w/v) CaCO3. Colonies that produced acid and formed clear halos were selected for further studies and streaked to purity on MRS Agar. For the identification of bacteria, colony PCR was performed with primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-ACGGCTACCTTGTTACGACTT-3′). The bacterial species was determined by BLAST searching the 16S rRNA gene sequences in the EZBioCloud database (https://www.ezbiocloud.net/) (accessed on 10 July 2021).
2.3. DNA Extraction
Each single colony was picked and cultured in normal MRS broth. For microbial genomic DNA extraction, 2 mL liquid cultures were centrifuged for 2 min at 8000× g. Total DNA from the pellets was extracted using a DNA extraction kit (CWBIO, Beijing, China) according to the manufacturer’s instructions.
2.4. In Vitro Study
2.4.1. Acid and Bile Salt Tolerance Assay
This assay was tested according to Leite et al. [27], with slight modifications. In addition, 0.1 mL of an overnight culture of Lactobacillus strains (108 CFU/mL) were inoculated into 5 mL of MRS broth previously adjusted to pH 3, or to MRS broth containing 0.3% or 0.5% (w/v) Oxgall (Solarbio, Beijing, China) to imitate gastric juice at 37 °C for 2 h. After incubation, the viability of cells was determined after 0, 2 h incubation by serial dilution and plating onto MRS agar. Isolates showing resistance more than 80% at pH 3 were considered acid tolerant strains. Survival rate (%) = Final (Log CFU/mL)/Initial (Log CFU/mL) × 100.
2.4.2. Auto and Co-Aggregation Assay
Auto-aggregation capacity was done according to Todorov et al. [28]. Tested Lactobacillus strains, grown aerobically in MRS broth for 24 h in 37 °C, were harvested by centrifugation (8000× g, 5 min), then washed twice, resuspended, and diluted in PBS. The absorbance OD600 of bacterial suspension was adjusted in the range of 0.25 ± 0.05. The initial absorbance value was recorded as A0. After a 2-h incubation at 37 °C, the OD600 value was measured again as At. Auto-aggregation was determined using the equation: auto-aggregation = [(A0 − At)/A0] × 100%.
For the evaluation of co-aggregation capacity, the Lactobacillus strains were similarly grown in MRS broth, harvested by centrifugation (8000× g, 5 min), washed, resuspended, and diluted. Each Lactobacillus suspension was mixed with the same volume of a suspension of the coaggregation partner such as Escherichia coli CAU0757 (E. coli), Salmonella typhimurium CMCC50115 (S. typhimurium), and Clostridium perfringens ATCC 13124 (C. perfringens), respectively, which were vortexed for 30 s. The determination method is the same as before.
2.4.3. Hydrophobicity Assay
The cell surface hydrophobicity of Lactobacillus strains, as a measure to evaluate adherence to hydrocarbons, was performed according to the method of Abbasiliasi et al. [29]. Three tubes, each containing 3 mL of each lactic acid bacteria (LAB) strain suspension in PBS (pH 7.2) at 108 CFU/mL, were mixed with the solvent n-hexadecane (1 mL) and vortexed for 1 min. The mixture was subsequently allowed to separate into 2 phases by standing for 5 to 10 min, after which the OD (at 600 nm) of the aqueous phase was measured with a spectrophotometer. Bacterial affinity to the solvent (hydrophobicity) was expressed using the formula (1 − A10min/A0min) × 100%, where A10min and A0min are the absorbance at 10 and 0 min, respectively.
2.4.4. Antibacterial Ability Test
Antimicrobial activity of the isolates against pathogenic bacteria viz. E. coli, S. typhimurium, and C. perfringens was determined by the Oxford Cup method in accordance with Muhammad et al. [30]. Briefly, 100 μL solution of pathogenic bacteria cultured overnight were plated onto LB agar. After the plate was dried, 4 Oxford cups were put onto the plate equidistantly with 200 μL test strains culture solution in each cup at 37 °C for 24 h. The diameter of the clear zones was scored as follows: less and equal 10 mm (weak, +), 10–15 mm (strong, ++), and more than 15 mm (very strong, +++). The Ampicillin and non-cultured MRS broth were used as positive and negative controls.
2.4.5. Hemolytic Activity
To be safe for use, probiotic strains must be nonhemolytic. All Lactobacillus strains tested were plated on Columbia agar, containing 5% (w/v) sheep blood, and incubated for 48 h at 37 °C. Blood agar plates were examined for signs of β-hemolysis (clear zones around colonies), α-hemolysis (green-hued zones around colonies), or γ-hemolysis (no zones around colonies) [31].
2.5. Genomic and Phylogenetic Analysis of the Selected Strain
The morphology of the strain was photographed and observed under scanning electron microscope. To evaluate the diversity of the selected Lactobacillus strain and the phylogenetic links to other Lactobacillus, sequences of the selected strain and 18 closed Lactobacillus strains were downloaded from the NCBI GenBank database (http://www.ncbi.nlm.nih.gov/genome/) (accessed on 19 December 2021); then, a phylogenetic tree was constructed using the iTOL (https://itol.embl.de/tree/120218717495301641453059) (accessed on 19 December 2021). Cluster of Orthologous Groups of proteins database (COG) was compared using BLASTP to identify the functional groups. BLAST Ring Image Generator (BRIG) was used to draw a genome map based on the JAVA platform. For comprehensive genetic analysis, an assembled genome was additionally annotated by RAST (https://rast.nmpdr.org/) (accessed on 21 December 2021). Database of Antimicrobial Activity and Structure of Peptides v3.0 (DBAASP, https://dbaasp.org/home (accessed on 1 March 2022) was used to predict the produced antimicrobial peptides (AMPs) by strain CML352.
2.6. Fermentation and Vacuum Lyophilization of Lactobacillus Powder
Lactobacillus fermentation and powder preparation were carried out in accordance with the method used by Chen et al. [32]. Lactobacillus strain was cultured in MRS broth at 37 °C for 24 h. After incubation, culture was centrifuged at 3000× g for 30 min at 4 °C, after which the pellets were washed with distilled water, in which 10% degreased milk powder was added as a protective agent. The mixture was then frozen dry and grinded into powder. In the end, the concentration of bacterial powder determined by serial dilution and plating onto MRS agar. The viability of the freeze-dried bacteria was nearly 1 × 1012 CFU/g and was stored at −80 °C until further use.
2.7. In Vivo Study
2.7.1. Animal Husbandry and Feeding Conditions
The experiment was carried out in accordance with the Chinese guidelines for animal welfare. In addition, 160 Hyline Brown laying hens at the age of 65 week were randomly divided into 2 dietary treatments according to dietary supplementation with 0 (Control), 1 × 108 CFU of the Lactobacillus salivarius CML 352 per kg of feed. The formula of basal diet and its nutrient contents are shown in Table 1. Each treatment consisted of 8 replicates with 10 laying hens (5 cages per replicate, 2 laying hens per cage). Before the start of the experiment, all hens were fed with experimental diets for 4 weeks. All birds were provided with ad libitum water and feed, as well as similar environmental and hygienic conditions throughout the experimental period. All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of China Agricultural University.

Table 1.
Composition and nutrient levels of the basal diet (air-dry basis, g/kg).
2.7.2. Sample Collection
At the end of the experiment, 16 birds in total randomly selected of each replicate group were fasted for 12 h and weighed. Five eggs per replicate were collected for egg quality determination. The ileum segments were carefully dissected and rinsed with sterilized saline and the cecal chyme was taken. All the samples were placed in liquid nitrogen immediately and stored at −80 °C till further analysis. Blood samples were collected (4 to 5 mL) after the hens were sacrificed. The fresh blood was kept standing for 60–120 min at 37 °C. Serum was obtained by centrifugation at 3000 rpm for 15 min and then stored at − 80 °C until use.
2.7.3. Laying Performance and Egg Quality
Egg production and egg weight were recorded daily by replicate and feed consumption for each replicate was weighed every week. Feed conversion ratio (FCR) was calculated as grams of feed consumption/egg weight for each replicate. As for egg quality measurement, the thickness of eggshells was measured by vernier caliper. Breaking strength, Haugh unit values, albumen height, and yolk color were determined by an Egg Analyzer (Israel Orka Food Technology Ltd., Ramat Hasharon, Israel).
2.7.4. The Analysis of Cecal Microbiota
Alpha diversity indices (including Chao1, Ace, Shannon, and Simpson index) were calculated to evaluate microbial species evenness on MicrobiomeAnalyst (https://www.microbiomeanalyst.ca/) (accessed on 25 December 2021). Beta diversity was evaluated by principal coordinate analysis (PCoA) based on the unweighted UniFrac distance [33]. Taxa abundances at the phylum and genus levels were statistically compared between the two groups. Linear discriminant analysis (LDA) combined effect size measurements (LEfSe) were used to identify the differences in microbial composition online in the Galaxy (http://huttenhower.sph.harvard.edu/galaxy/) (accessed on 25 December 2021) [34]. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUSt2) software was further used to predict the functional pathway from the 16S rRNA data [35]. STAMP version 2.1.1.0 was also used as a graphical tool.
2.7.5. Assay of Antioxidant Enzymes in Serum
The serum was used for the measurement of antioxidant indexes. Catalase (CAT), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), and the total antioxidant capacity (T-AOC) were measured using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
2.7.6. Quantitative RT-PCR Analysis of Gene Expression
Total RNA was extracted from ileum (50 mg) using Trizol reagent (Beyotime, Beijing, China) according to the manufacturer’s instructions. After extraction, complementary DNA (cDNA) was synthesized from 1 μg of total RNA using MMLV reverse transcriptase (TaKaRa, Dalian, China). Transcriptional changes were then identified by quantitative PCR, which was performed using the Premix Ex TaqTM with SYBR Green (TaKaRa, Dalian, China). Synthesized cDNA was stored at −20 °C until use. Expression levels of the following genes were analyzed by real-time quantitative PCR, which were listed in Table 2. The PCR conditions were as follows: pre-denaturation at 95 °C for 2 min for 1 cycle; followed by denaturation at 95 °C for 15 s and annealing at 60 °C for 15–30 s, repeat the above steps for a total of 40 cycles; with a melting curve at 95 °C for 15 s, 60 °C for 15 s and 95 °C for 15 s. Expressions of the genes were quantified with a BioRad CFX96 Sequence Detection System and TransStart Top Green qPCR SuperMix (TransGen Biotech, Beijing, China). mRNA was analyzed for relative expression of the genes mRNA contents after normalizing for β-actin.

Table 2.
Gene name, primer sequences.
2.8. Statistical Analysis
Statistical analysis was performed using SPSS Statistics 18.0 software. One-way (in vitro experiment) ANOVA and Student’s t-test (in vivo experiment) were used to calculate significance. The data were expressed as mean ± SEM. Differences were considered statistically significant if p ≤ 0.05.
3. Results
3.1. Isolation and Identification of Lactic Acid Bacteria from Chinese Local Breed Chickens
A total of 57 LAB strains, belonging to 18 different bacterial species, were isolated from fecal samples of Hy-line brown and 16 locally reared Chinese local chicken breeds (Table 3). In addition, 18, 6, and 6 strains were isolated from Hy-line brown, Yuqingxiang and Tuer chickens, respectively, which were the top three chicken breeds with the greatest number of LAB isolates. All the isolates were identified by aligning their 16S rRNA gene sequences with those in Ezbiocloud database, and these strains showed 99.71% to 100% 16S rRNA gene nucleotide identity with known LAB. L. salivarius were the most prevalent LAB in these chickens (15/57 isolates; 26%), followed by Ligilactobacillus agilis (9/57 isolates; 16%), probably suggesting that these two species were relatively dominant LAB in chicken gut. The following 11 Lactobacillus species were rare and were found only once in one chicken breed: Limosilactobacillus mucosae, Limosilactobacillus panis, Lactobacillus kitasatonis, Limosilactobacillus pontis, Lactobacillus aviarius subsp. Aviarius, Limosilactobacillus vaginalis, Limosilactobacillus coleohominis, Lactobacillus helveticus, Limosilactobacillus ingluviei, Limosilactobacillus oris, and Limosilactobacillus frumenti.

Table 3.
Information of 57 LAB isolates from the chicken gut.
We first tested the tolerance of the isolates to low pH and low bile salt concentrations, which are important indicators for the strains’ ability to survive in the host’s gastrointestinal tract. The results showed that less than 20 isolated Lactobacillus spp. displayed a satisfactory surviving rate of more than 80% after exposure to pH 3 for 2 h (Table 4). These were strains of the species L. saerimneri (CML344, CML398), L. salivarius (CML350, CML352, CML346, CML345, CML348), L. johnsonii (CML319, CML312, CML333), and L. agilis (CML313, CML104, CML341 CML323). Among them, eight strains were found to possess a remarkable surviving capacity of above 90%; CML319, CML313, CML350, CML352, and CML346 were the top five strains showing the highest pH tolerance. These 20 isolates were further evaluated their ability to survive in low bile salt concentrations (Table 5). We found that only 8 out of 20 isolates showed resistance to both 0.3% and 0.5% bile salts, in which CML319 (L. johnsonii) showed strongest tolerance compared to others under 0.3% bile salt condition, while CML352 (L. salivarius) performed better under 0.5% bile salt condition.

Table 4.
Survival rate of different Lactobacillus strains under pH = 3.

Table 5.
Survival rate of different LAB strains under different bile salt concentrations.
We then examined the auto-aggregation and co-aggregation abilities of six LAB strains selected based on their ability to tolerate pH and bile salts. The auto-aggregation ability of LAB stains makes an important contribution to the adhesion of intestinal cells and to avoid the colonization of pathogens, while the co-aggregation ability of bacteria eliminates gastrointestinal pathogens by preventing them from adhering to host tissues [36]. The auto-aggregation abilities of these isolates ranged from 78.55 to 84.70% with CML352 showing the highest (84.70%) (Table 6). The co-aggregation of the LAB strains against E. coli, S. typhimurium, and C. perfringens were between 47.12% and 48.49%, 47.92% and 61.72%, and 45.51% and 56.38%, respectively. CML319, CML350, and CML104 displayed the highest abilities against the three pathogens, respectively. A further investigation on the cell surface hydrophobicity activities of these strains indicated that CML352 had the highest resistance (57.66%), followed by CML350, CML344, CML398, CML319, and CML104 with 50.37, 49.08, 41.66, 41.13, and 21.21%, respectively (Table 6).

Table 6.
Auto and co-aggregation ability and Hydrophobicity of lactic acid bacteria strains.
Lastly, we evaluated the direct inhibitory effects of the six isolates on three microbial pathogens, and, for safety reasons, the strains’ hemolytic activities. The results showed that all the six strains inhibited the growth of C. perfringens, whereas their inhibitory abilities against E. coli and S. typhimurium were relatively lower (Table 7). The highest zone of inhibition (≥ 20 mm) against C. perfringens was observed with the isolates CML350, CML352, CML398, and CML344. CML319, CML350, CML352, and CML104 displayed slight α-type hemolysis, while CML344 and CML398 showed γ-type hemolysis. Taking all the probiotic characteristics of these isolates into account, we selected CML352 for further study because of its balanced probiotic features.

Table 7.
Antimicrobial activity and hemolysis of selected lactic acid bacteria strains.
3.2. Genomic and Phylogenetic Analysis of L. salivarius CML352
L. salivarius CML352 was isolated from Shanzhongxian chicken, a local breed in Anhui, China (Table 3). It is a short rod-shaped bacterium with a size of approximately 1.5 μm long by 0.6 μm wide (Figure 1A). We sequenced the genome of L. salivarius CML352, and a draft genome consisted of 172 contigs with an N50 value of 69,089 bp were obtained. The genome included 2,094,457 bp with a 32.6 % G + C content; 2121 coding sequences (CDSs) and 63 RNA genes were predicted (Figure 1B). A phylogenetic tree including strain CML352 and other 18 L. salivarius isolates in database was constructed based on the whole genome SNPs (Figure 1C). L. salivarius CML352 was found most closely related to L. salivarius JSWX5_1, a strain isolated from human fecal samples in China [37]. L. salivarius CML352 and JSWX5_1, together with two other strains, FHNXY73M9 and FGSYC2M4_2, clustered in the same clade in the phylogenetic tree.
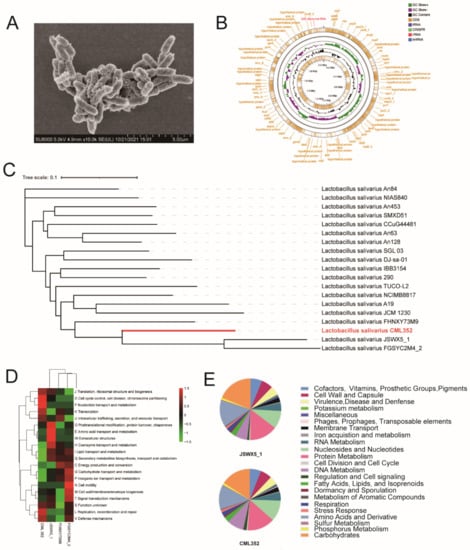
Figure 1.
(A) The SEM picture of CML 352, (B) the Genome map of CML 352, (C) phylogenetic tree of CML 352, (D) the clusters of orthologous groups (COG) repertoires, (E) the subsystem category distribution of JSWX5_1 and CML 352.
We then compared the COG functional categories of these four isolates (Figure 1D). The major COG categories detected in the four strains belonged to “Information storage and processing” (mainly ([J] Translation, ribosomal structure, and biogenesis, [K] Translation, [L] Replication, recombination and repair) and “Poorly characterized” (mainly ([S] Function unknown). The COG categories of [I] Lipid transport and metabolism, and [Q] Secondary metabolites biosynthesis, transport, and catabolism were less abundant in CML 352 compared with the others, while categories of “Cellular processes and signaling” ([K] Transcription, [J] post-translational modification, protein turnover, chaperones, and [L] Replication, recombination and repair), “Cellular processes and signaling” ([D] Cell cycle control, cell division, chromosome partitioning, [V] Defense mechanisms) and “Metabolism” ([F] Nucleotide transport and metabolism) were enriched in CML 352.
We further annotated the genomes of CML 352 and its close relative JSWX5_1 using a Rapid Annotation using Subsystem Technology (RAST) server. The results showed that nearly the same number of genes were assigned to SEED subsystems in these two strains (Figure 1E and Table S1). Compared with JSWX5_1, CML352 had more genes in Nucleosides and Nucleotides (84 vs. 73), Regulation and Cell signaling (13 vs. 8), and DNA Metabolism (68 vs. 50), but less genes in Virulence, Disease, and Defense (33 vs. 37), Protein Metabolism (111 vs. 114), Carbohydrates (121 vs. 146), and Amino Acids and Derivatives (97 vs. 108). Both two strains had no relevant genes in Photosynthesis, Motility and Chemotaxis, Secondary Metabolism, and Nitrogen Metabolism.
We next explored the specific genetic features of CML352 strain based on the functional roles in each subsystem (Table S1). CML352 had three genes that were relevant to phage packaging machinery and phage tail fiber, while no phage-related genes were found in JSWX5_1. In the subsystem of Nucleosides and Nucleotides, 49 genes related to purine metabolism existed in CML352, but only 38 such genes were present in JSWX5_1, indicating that CML352 may have more potent ability in metabolizing purine. Moreover, CML352 had more genes in DNA Metabolism than JSWX5_1. The most different category was CRISPRs (Clustered Regularly Interspaced Short Palindromic Repeats), in which CML352 had 13 related genes and JSWX5_1 only possessed two, which, to some extent, means that CML352 has a stronger immune function than JSWX5_1. Interestingly, three genes correlated to Sex pheromones in Enterococcus faecalis and other Firmicutes existed in CML352, but none was found in JSWX5_1. These genes seemed to be related to the synthesis of certain enzymes and proteins that could alter signaling, social behavior, and evolution of plasmids. Additionally, we found 41 predicted AMPs encoded by CML352, among which, nine, nine and two were active against E. coli, S. typhimurium and C. perfringens, respectively (Table S2), suggesting the antibacterial ability of CML352 were probably due to the specific AMPs.
We then added L. salivarius CML352 to the diet of late-phase laying hens and evaluated the effects on this strain on the bird’s gut microbiota, intestinal epithelial barrier, immunity, antioxidant activity, production performance, and egg quality.
3.3. L. salivarius CML352 Modulated the Gut Microbiota Composition and Function of Laying Hens
No significant differences of the alpha diversity of cecal microbiota, estimated by Shannon, Simpson, Ace, and Chao1 indices, were found between the two groups (p > 0.05). However, beta diversity results (PCoA analysis) indicated that the microbiota of L. salivarius CML352 group was clearly different from the control group (p < 0.05) (Figure 2A,B). Overall, the microbiota was dominated by the phylum Firmicutes and Bacteroidetes in the two groups. Dietary L. salivarius CML352 supplementation significantly decreased the relative abundance of phylum Firmicutes, while increasing the relative abundance of phylum Bacteroidetes (p < 0.05), thus leading to a significant decrease in Firmicutes to Bacteroidetes ratio (p < 0.05) (Figure 2D). The differentially abundant operational taxonomic units (OTUs) at the phylum and genus levels were further analyzed by LEfSe (LDA > 2.0; Figure 2E,F). As shown in Figure 2E, Bacteroidetes and Epsilonbacteraeota were enriched in L. salivarius CML352-treated group, while Firmicutes was the enriched phylum in the control group. At the genus level, Romboutsia, Ruminococcaceae UCG-013, Blautia, Ruminiclostridium 9, Ruminococcaceae UCG-004, Marvinbryantia, Ruminiclostridium 5, Lachnospiraceae UCG-010, CAG-56, and GCA-900066575 were enriched in L. salivarius CML352 group (Figure 2F).
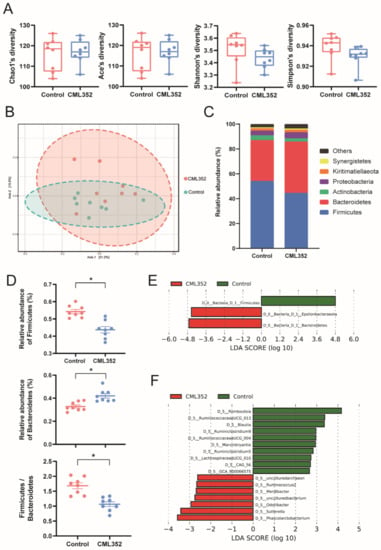
Figure 2.
Influence of dietary L. salivarius CML352 supplementation on the gut microbiota composition. (A) four different alpha diversity metrics, (B) PcoA 2D Graphics generated from MicrobiomeAnalyst, where red circles represent abundance in hens fed with L. salivarius CML352, blue circles for those of hens in the control group, (C) relative abundance of bacterial phyla detected in the samples. Those abundance below 1% were classified into “others”, (D) relative abundance of Firmicutes and Bacteroidetes phyla, and the ratio of Firmicutes to Bacteroides, asterisks indicated values significantly different (p < 0.05) between the groups, (E) the LEfSe analysis of the cecum microbiota at the phylum level, and (F) the LEfSe analysis of the cecum microbiota at the genus level.
The PICRUSt2 software was further used to predict the microbial functional categories and eight differentially abundant MetaCyc pathways were found between the two groups (q < 0.05, Figure 3). Among them, the microbial gene functions related to GDP-mannose biosynthesis were much higher in the fecal microbiome of the CML352 group, which has been recently proposed to play a vital role in the biosynthesis of Vitamin C as a valuable antioxidant [38]. In contrast, the abundance of pathways related to lipid synthesis were decreased in the CML352 group, such as phosphatidylglycerol biosynthesis II (non-plastidic), the super pathway of phospholipid biosynthesis I (bacteria), CDP-diacylglycerol biosynthesis I, and CDP-diacylglycerol biosynthesis II. In addition, the gene functions associated with methanogenesis from acetate in CML352 group were significantly lower than the control group, which has been presumed to reduce fat deposition and obesity [39].
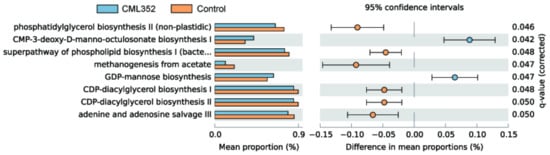
Figure 3.
PICRUSt analysis in the MetaCyc pathways; functional predictions for the fecal microbiome of the L. salivarius CML352 group and the control group. Significant MetaCyc pathways for the fecal microbiome of the 2 groups were identified by STAMP software.
3.4. Effects of L. salivarius CML352 on Intestinal Epithelial Barrier, Immunity, and Antioxidant Activity
No statistically significant differences (p > 0.05) were observed on the relative mRNA expression of zonula occludens-1 (ZO-1), Occludin, and Claudin-2 in ileal mucosa of laying hens in response to the addition of L. salivarius CML352 (Figure 4A). However, there was a significant increase (p < 0.05) in relative mRNA expression of mucin-2 (Muc-2) in the ileum. In addition, L. salivarius CML352 tended to decrease the relative mRNA expression of Claudin-2 (p < 0.1).
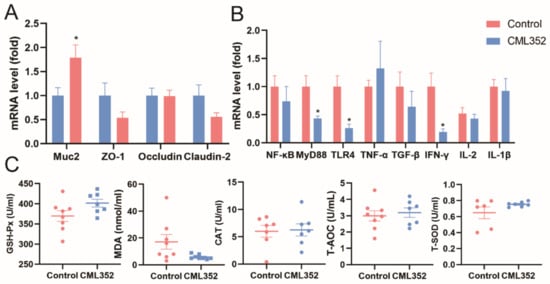
Figure 4.
(A) Effects of L. salivarius CML352 supplementation on intestinal epithelial barrier in ileum of laying hens. Total RNA was extracted and the expressions of Muc-2, ZO-1, Occludin and Claudin-2 and were measured by real-time PCR; (B) effects of L. salivarius CML352 supplementation on immunity in ileum of laying hens. Total RNA was extracted and the expressions of NF-κB, MyD88, TLR-4, TNF-α, TGF-α, IFN-γ, IL-2, and IL-1β were measured by real-time PCR; (C) effects of L. salivarius CML352 supplementation on serum antioxidant of laying hens. Data are expressed as mean ± SEM from three independent experiments. Asterisks indicated values significantly different (p < 0.05) between the groups.
Dietary supplementation with L. salivarius CML352 downregulated (p < 0.05) the relative mRNA expression of myeloid differentiation factor88 (MyD88), interferon-γ (IFN-γ) and toll-like receptor-4 (TLR-4) in the ileum, while having no obvious impact on tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-α), nuclear factor kappa-B (NF-κB), interleukin-2 (IL-2), and interleukin-1β (IL-1β) levels (Figure 4B).
Compared to the control group, L. salivarius CML352 supplementation tended to reduce the concentrations of MDA in serum (p < 0.1), whereas a slight but not significant increase in GSH-Px and T-SOD concentrations was observed in the treated group (p < 0.1). There was no difference in T-AOC and CAT activity (p > 0.05). Levels of serum T-AOC were similar to that in the control group. In addition, the values of CAT activity were not significantly affected by L. salivarius CML352 treatment (Figure 4C).
3.5. Effects of L. salivarius CML352 on Production Performance and Egg Quality
In comparison with the control, dietary L. salivarius CML352 supplementation had no obvious effect (p > 0.05) on egg production and feed conversion ratio of laying hens during the whole experiment (Table 8). However, the average egg weight was significantly increased (p < 0.05) during weeks 56–67, and a positive trend was observed during weeks 56–59 and 60–63 (p < 0.1). Meanwhile, L. salivarius CML352 addition significantly decreased average daily feed intake during weeks 64–67 (p < 0.05). For the entire experimental period, dietary supplementation with L. salivarius CML352 resulted in a slight reduction in the average weight of laying hens (p < 0.1), while the abdominal fat deposition was significantly reduced in the treated group (p < 0.05) (Table 9).

Table 8.
Effects of dietary supplementation with L. salivarius on laying performance 1.

Table 9.
Effects of dietary supplementation with L. salivarius on weight and abdominal fat deposition of laying hens.
With regard to egg quality, there was no apparent difference (p > 0.05) on yolk percentage, and albumen height at the end of the experiment, while a significant increase on eggshell strength and eggshell thickness (p < 0.05) and an increasing but not significant trend for Haugh units were observed (Table 10).

Table 10.
Effects of dietary supplementation with L. salivarius on egg quality of laying hens.
4. Discussion
4.1. Probiotic Properties Assessment
In this study, Lactobacillus strains were isolated from chicken fecal samples of Chinese local breed chickens in different regions, and the probiotic properties of selected strains were investigated. Even though the bacteria belonging to Lactobacillus species are common probiotics and are ‘generally recognized as safe’ (GRAS), the probiotic properties of LAB have been demonstrated to be strain-specific, and in vitro assessment of newly isolated strains is necessary [40]. Thus, 57 Lactobacillus isolates were tested for the simulated gastrointestinal tolerance, the antimicrobial effect, the co-aggregation ability and hydrophobicity, and the hemolysis activity.
Acid and bile salt tolerance are frequently considered during the selection of potential probiotic strains to guarantee their viability and functionality [41]. It is proposed that the degree of acid or bile salt tolerance of probiotics is highly variable and strain-dependent [42]. Plenty of studies have discussed the acid and bile salt environment tolerance of probiotics [43,44], and it has been confirmed that LABs could survive better in simulated intestinal environment than non-LAB strains [44]. Previous study showed that five out of twelve LAB isolates were detected to resist to acid pH value 3 and 0.45% (w/v) of bile salt, and the maximum resistance was 60.52% and 68.62%, respectively [45]. Similar results were observed in this study that only eight strains (one L. johnsonii, two L. agilis, three L. salivarius, and two L. saerimneri isolates) showed high tolerance to both the acid and bile salt environments, suggesting their potential ability to survive the harsh conditions in the real stomach and small intestinal tract. Adherence and colonization of the intestine are also important probiotic functions, which could suppress and exclude pathogenic microbes, and are essential for immunomodulatory activity [46]. The adhesive ability is closely related to the strain’s hydrophobicity and auto-aggregation capacity [47,48]. Additionally, the co-aggregation capability is demonstrated to prevent the GIT of the host from being colonized by pathogens [36]. All the tested strains in our study showed different degrees of adhesive properties, among which CML352 exhibited the highest auto-aggregation (84.75%) and hydrophobicity (57.66%) capabilities. Interestingly, CML352 also showed a strong inhibition against E. coli, S. typhimurium, and C. perfringens in vitro. As opposed to α-hemolysis, β-hemolytic activity is considered harmful. Strains exhibiting this capability are known to produce exotoxin, which lyses blood cells and therefore impacts the immune system [49]. Non-enterococcal LABs exhibiting α-hemolytic may harbor low virulence potential and have been regarded to be safe to use [26,50,51]. None of the strains in this study showed β-hemolysis, indicating that the selected strains were relatively safe to use. Collectively, we proposed that L. salivarius CML352 is a candidate probiotic that can survive the intestinal conditions, inhibit gut pathogens, and thus exert beneficial effects on laying hens. We should stress that, in the present study, we didn’t cover all probiotic features and safety concerns of a potential probiotics, such as resistance to lysozyme [52,53] and antibiotic susceptibility [54], which should be further considered and evaluated in the future work.
4.2. Effect of L. salivarius CML352 on Cecal Microbiota of Layers
Dietary supplementation of L. salivarius CML352 did not significantly influence the alpha diversity of the cecal microbiota of late-phase laying hens, but had a remarkable impact on the beta diversity. Studies have shown that Lactobacillus addition could increase cecum microbial diversity [55]. A significant reduction of Firmicutes and increase of Bacteroidetes were found after the CML352 treatment, thus inducing a sharply reduced ratio of Firmicutes to Bacteroidetes (F/B) in the chicken intestinal microbiota. Numerous human studies have consistently demonstrated that the F/B proportion is increased in obese people compared to lean people, and tend to decrease with weight loss [56]. In chicken, it is also demonstrated that the F/B ratio was positively correlated with the accumulation of chicken abdominal fat deposition [57]. This might be one of the reasons to explain the significant decrease of abdominal proportion and the positive trend of decreased average body weight in the CML352 group. The reduced abundance of Firmicutes phylum in the chicken gut may diminish the bacterial species with the ability to harvest energy from diet, thus leading to a large decrease in total body fat [58].
We further investigate the role of L. salivarius CML352 in chicken gut by analyzing the changes of microbial metabolic pathways. It was revealed that the most significantly reduced microbial pathways in the CML352 group were involved in the lipid synthesis. In addition, the gene functions associated with methanogenesis from acetate in CML352 group were significantly lower than the control group. Studies have demonstrated that methane could slow down the peristalsis of intestinal and increase the digestion time, thus improving the absorption of nutrients and inducing obesity [39]. Taken together, we suggested that dietary supplementation of L. salivarius CML352 in laying hens can modulate the gut microbiota by decreasing the F/B ratio, reducing lipid synthesis-related pathways and lowing methanogenesis from acetate, and thus reducing the abdominal fat deposition in layers.
In addition, the increase of Bacteroidetes may benefit the chicken gut health, as most species in Bacteroidetes produce short-chain fatty acids (SCFAs) as their final fermentation products [59]. The other CML352-enriched bacteria such as Phascolarctobacterium and Odoribacter were also SCFA-producers [60,61]. SCFAs play an important role in innate immunity as they have anti-inflammatory effects [62], which affects both nonimmune as well as immune intestinal cells and modulates intestinal homeostasis [63]. Additionally, SCFAs also serve as a vital energy source for the intestinal epithelial cells [64], and have important intestinal and immuno-modulatory function [65], and an influence on maintaining epithelial barrier function and suppressing proinflammatory cytokines [66].
4.3. Effect of L. salivarius CML352 on Intestinal Epithelial Barrier, Immunity, and Antioxidant Activity
The intestinal epithelium is an integral component of the intestinal barrier and provides the first line of defense against external stimuli [67], which could maintain intestinal homeostasis [68]. The integrity of the gut epithelium is maintained by the formation of tight junction protein complexes between cells. Studies in various animals have shown the beneficial effects of probiotics on the regulation of tight junction proteins, which could increase host epithelial barrier and protect against pathogen invasion into internal organs [69]. In this study, we observed a decreasing trend of mRNA expression of Claudin-2 (p < 0.1). Tight junction protein Claudin-2 is considered to be associated with the expression of cation-selective pores, which may increase paracellular permeability [70]. For example, an increase of Claudin-2 induced by S. typhimurium infection increased mucosal permeability, thus disrupting the intestinal barrier in chicks [71]. Mucins are protective, antimicrobial substances secreted by epithelial cells, among which Muc-2 is the primary gel-forming mucin in the mammalian gut [72], playing an essential role in preventing pathogenic bacteria from destroying enterocytes [73]. Our data indicated that dietary supplementation of L. salivarius CML352 significantly upregulated the expression of Muc-2. It is reported that microbial-derived SCFAs could boost the expression of tight junction proteins and repress paracellular permeability [74] through stimulating the goblet cells to secrete mucin, especially Muc-2. Thus, we suggest that the high expression of Muc-2 in L. salivarius CML352 group was probably due to the resulting enrichment of the SCFAs-producers. Taken together, dietary supplementation of L. salivarius CML352 decreased intestinal permeability and improved intestinal health of layers.
Studies have demonstrated that Lactobacillus or their cell components can improve immune function in animals [55]. For example, L. acidophilus supplementation enhanced the cellular and humoral immunity in E. coli challenged chickens [75]. In addition, probiotic Lactobacillus strains modulated the gut environment and stimulated the immune system in layer- and meat-type chickens [76]. This research demonstrated that Lactobacillus can increase host immunity with or without the challenge of pathogens. Here, we showed that supplementation of L. salivarius CML352 significantly reduced MyD88 and TLR4 expression, which were speculated to be associated with the underlying anti-inflammatory effect [77]. We also found that the level of mucosal pro-inflammatory cytokines IFN-γ was significantly reduced in the L. salivarius CML352-treated group, consistent with previous evaluation of the effects of L. salivarius on chickens [78]. These results indicated that the administration of L. salivarius CML352 decreased the inflammatory level and maintained immune homeostasis in layers.
It is reported that L. salivarius showed good anti-oxidative properties. For example, L. salivarius supplementation effectively alleviated the organ damage and enhanced anti-oxidative capacity in both acute heat stress and circular heat stress condition in chicken [79]. Malondialdehyde is a biomarker of lipid peroxidation and its level is directly proportionate to the extent of the cellular damage caused by free radicals [80]. We found that, when L. salivarius CML352 was added to the diet, a trend of reduction of MDA activity in the serum of layers occurred (p = 0. 079). Additionally, CML352 resulted in an increasing trend of GSH-Px (p = 0.075) and T-SOD (p = 0.090) in serum. These results suggested that L. salivarius CML352 can improve serum antioxidant status of layers. Similarly, the use of other Lactobacillus strains, i.e., L. plantarum JM113, also resulted in the greater SOD and GSH-Px activities and the decreased MDA activity in chickens [81]. It is proposed that the Nrf2-ARE signaling pathway was involved in the protective effect of L. plantarum and regulates the expression and activity of antioxidative enzymes [82]. Whether or not Nrf2-ARE signaling pathway mediated the anti-oxidative capacity of L. salivarius CML352 needs to be further investigated.
4.4. Effect of L. salivarius CML352 on the Production Performance of Layers
Our results showed that the average egg weight was significantly increased (p < 0.05) during the entire experiment, and a positive trend was observed during weeks 56–59 and 60–63 (p < 0.1). Meanwhile, L. salivarius CML352 addition significantly decreased average daily feed intake during weeks 64–67 (p < 0.05). A limited number of studies have investigated the effects of dietary supplementation with L. salivarius on the performance of laying hens. However, increased egg weight or improved feed efficiency in hens have been observed by using other Lactobacillus strains [83], and dietary administration of L. acidophilus at 2 × 109 and 3 × 109 CFU/kg significantly increased the average egg weight by 1.7 and 1.9 g, respectively, and, at the same time, reduced the daily feed consumption [17]. Similarly, various combinations of Lactobacillus species (L. rhamnosus, L. paracasei, and L. plantarum) significantly improve the egg production [84]. These beneficial effects may be attributed to the improvement of nutrient digestibility in the daily diet by Lactobacillus and thus increased the efficiency of food utilization and resulted in heavier eggs. Furthermore, a significant increase on eggshell strength and eggshell thickness (p < 0.05) were observed after the administration of L. salivarius CML352. This may be because Lactobacillus can produce a variety of vitamins in the intestinal tract of poultry and promote the absorption of vitamins and mineral elements [85]. Additionally, we observed an increasing but not significant trend for Haugh units (p < 0.1), a measurement of the internal quality of an egg, which is also supported by other studies using L. salivarius as a feed additive [86]. Collectively, our findings, together with results in previous studies, confirmed the role of L. salivarius in improving laying hens’ production performance and egg quality.
5. Conclusions
In summary, we isolated and characterized the probiotic properties of Lactobacillus strains from Chinese local breed chickens. We sequenced the genome of the newly isolated L. salivarius strain, CML352, and demonstrated the beneficial effects of this probiotic candidate on modulating the layers’ gut microbiota, abdominal fat deposition, immunity, antioxidant capacity, and production performance. Results gained so far indicated that our strain may have a potential effect on the laying hen industry, but a further investigation of the mechanisms involved is still needed to warrant a future application of this bacterium.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10040726/s1, Table S1: Basic information of two Lactobacillus salivarius strains in RAST server. Table S2: Predicted antimicrobial peptides produced by L. salivarius CML352 and the target pathogens.
Author Contributions
Conceptualization, C.X. and Y.H.; Methodology and formal analysis, C.X., F.W., and X.Y.; Data curation, C.X.; Writing—original draft preparation, C.X.; Writing—review and editing, Y.H. Software, Y.F.; Supervision, D.L. and Y.H.; Funding acquisition, D.L. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2021YFD1800400), the Open Project Program of CAS Key Laboratory of Pathogenic Microbiology and Immunology (CASPMI202102), the 2115 Talent Development Program of China Agricultural University, and the Chinese Universities Scientific Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The new nucleic acid sequences have been deposited at GenBank under the accession SRP361917. The version described in this paper is version SRP361917.
Acknowledgments
We would like to express our gratitude to the staff of the Zhuozhou Teaching Experimental Base of China Agricultural University for their selfless support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van den Brand, H.; Parmentier, H.K.; Kemp, B. Effects of housing system (outdoor vs cages) and age of laying hens on egg characteristics. Br. Poult. Sci. 2004, 45, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Rattanawut, J.; Pimpa, O.; Yamauchi, K.-E. Effects of dietary bamboo vinegar supplementation on performance, eggshell quality, ileal microflora composition, and intestinal villus morphology of laying hens in the late phase of production. Anim. Sci. J. Nihon Chikusan Gakkaiho 2018, 89, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef]
- Anderson, R.C.; Dalziel, J.E.; Gopal, P.K.; Bassett, S.; Ellis, A.; Roy, N.C. The Role of Intestinal Barrier Function in Early Life in the Development of Colitis. In Colitis; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef][Green Version]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2017, 53, 95–106. [Google Scholar] [CrossRef]
- Karlsson, F.; Tremaroli, V.; Nielsen, J.; Bäckhed, F. Assessing the Human Gut Microbiota in Metabolic Diseases. Diabetes 2013, 62, 3341–3349. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef]
- Zommiti, M.; Chikindas, M.L.; Ferchichi, M. Probiotics—Live Biotherapeutics: A Story of Success, Limitations, and Future Prospects—Not Only for Humans. Probiotics Antimicrob. Proteins 2020, 12, 1266–1289. [Google Scholar] [CrossRef]
- Zommiti, M.; Ferchichi, M. Probiotics and Prebiotics in Animal Feed; Academic Press: Cambridge, MA, USA, 2021; pp. 233–261. [Google Scholar] [CrossRef]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141, S15–S28. [Google Scholar] [CrossRef]
- Cherdyntseva, T.A.; Kotova, I.B.; Netrusov, A.I. The Isolation, Identification and Analyses of Lactobacillus Genus Bacteria with Probiotic Potential. Adv. Exp. Med. Biol. 2016, 897, 103–111. [Google Scholar] [CrossRef]
- Bouzaine, T.; Dauphin, R.D.; Thonart, P.; Urdaci, M.C.; Hamdi, M. Adherence and colonization properties of Lactobacillus rhamnosus TB1, a broiler chicken isolate. Lett. Appl. Microbiol. 2005, 40, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, C.; Donders, G.G.; Palmeira-De-Oliveira, R.; Queiroz, J.A.; Tomaz, C.; Martinez-De-Oliveira, J.; Palmeira-de-Oliveira, A. Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Express 2018, 8, 153. [Google Scholar] [CrossRef]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444s–450s. [Google Scholar] [CrossRef] [PubMed]
- Alaqil, A.A.; Abbas, A.O.; El-Beltagi, H.S.; El-Atty, H.K.A.; Mehaisen, G.M.K.; Moustafa, E.S. Dietary Supplementation of Probiotic Lactobacillus acidophilus Modulates Cholesterol Levels, Immune Response, and Productive Performance of Laying Hens. Animals 2020, 10, 1588. [Google Scholar] [CrossRef]
- Anjum, M.; Khan, G.; Afzal, A. Effect of dietary supplementation of multi-strain probiotic on broiler growth performance. Pak. Vet. J. 2005, 25, 25–29. [Google Scholar]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.E. Autochthonous microbiota, probiotics and prebiotics. Nutr. Hosp. 2015, 31 (Suppl. S1), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Nature of the Determinant Responsible for the Adhesion of Lactobacilli to Chicken Crop Epithelial Cells. J. Gen. Microbiol. 1975, 87, 245–250. [Google Scholar] [CrossRef]
- Lin, W.-C.; Ptak, C.P.; Chang, C.-Y.; Ian, M.-K.; Chia, M.-Y.; Chen, T.-H.; Kuo, C.-J. Autochthonous Lactic Acid Bacteria Isolated From Dairy Cow Feces Exhibiting Promising Probiotic Properties and In Vitro Antibacterial Activity Against Foodborne Pathogens in Cattle. Front. Vet. Sci. 2020, 7, 239. [Google Scholar] [CrossRef]
- Yamashita, M.M.; Ferrarezi, J.V.; Pereira, G.D.V.; Bandeira, G.; da Silva, B.C.; Pereira, S.A.; Martins, M.L.; Mouriño, J.L.P. Autochthonous vs allochthonous probiotic strains to Rhamdia quelen. Microb. Pathog. 2020, 139, 103897. [Google Scholar] [CrossRef] [PubMed]
- Yelnetty, A.; Ta, T.E. Indigenous Lactic Acid Bacteria Isolated from Spontaneously Fermented Goat Milk as Potential Probiotics. Pak. J. Biol. Sci. 2020, 23, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Varada, V.V.; Tyagi, A.K.; Banakar, P.S.; Das, A.; Tyagi, N.; Mallapa, R.H.; Kumar, S. Autochthonous Limosilactobacillus reuteri BFE7 and Ligilactobacillus salivarius BF17 probiotics consortium supplementation improves performance, immunity, and selected gut health indices in Murrah buffalo calves. Vet. Res. Commun. 2022, 46, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yun, H.S.; Cho, K.W.; Oh, S.; Kim, S.H.; Chun, T.; Kim, B.; Whang, K.Y. Evaluation of probiotic characteristics of newly isolated Lactobacillus spp.: Immune modulation and longevity. Int. J. Food Microbiol. 2011, 148, 80–86. [Google Scholar] [CrossRef]
- Leite, A.M.; Miguel, M.A.; Peixoto, R.S.; Ruas-Madiedo, P.; Paschoalin, V.M.; Mayo, B.; Delgado, S. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J. Dairy Sci. 2015, 98, 3622–3632. [Google Scholar] [CrossRef]
- Todorov, S.D.; Botes, M.; Guigas, C.; Schillinger, U.; Wiid, I.; Wachsman, M.B.; Holzapfel, W.H.; Dicks, L.M. Boza, a natural source of probiotic lactic acid bacteria. J. Appl. Microbiol. 2008, 104, 465–477. [Google Scholar] [CrossRef]
- Abbasiliasi, S.; Tan, J.S.; Bashokouh, F.; Ibrahim, T.A.T.; Mustafa, S.; Vakhshiteh, F.; Sivasamboo, S.; Ariff, A.B. In vitro assessment of Pediococcus acidilactici Kp10 for its potential use in the food industry. BMC Microbiol. 2017, 17, 121. [Google Scholar] [CrossRef]
- Muhammad, Z.; Ramzan, R.; Abdelazez, A.; Amjad, A.; Afzaal, M.; Zhang, S.; Pan, S. Assessment of the Antimicrobial Potentiality and Functionality of Lactobacillus plantarum Strains Isolated from the Conventional Inner Mongolian Fermented Cheese Against Foodborne Pathogens. Pathogens 2019, 8, 71. [Google Scholar] [CrossRef]
- Śliżewska, K.; Chlebicz-Wójcik, A.; Nowak, A. Probiotic Properties of New Lactobacillus Strains Intended to Be Used as Feed Additives for Monogastric Animals. Probiotics Antimicrob. Proteins 2021, 13, 146–162. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Li, C.; Shu, G. Response Surface Optimization of Lyoprotectant for Lactobacillus bulgaricus during Vacuum Freeze-Drying. Prep. Biochem. Biotechnol. 2015, 45, 463–475. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using Microbiome Analyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS ONE 2017, 12, e0188634. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Sun, C.; Yuan, J.; Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017, 7, 45308. [Google Scholar] [CrossRef] [PubMed]
- Armas, F.; Camperio, C.; Marianelli, C. In Vitro Assessment of the Probiotic Potential of Lactococcus lactis LMG 7930 against Ruminant Mastitis-Causing Pathogens. PLoS ONE 2017, 12, e0169543. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Shen, X.; Cen, S.; Zhang, C.; Tian, F.; Zhao, J.; Zhang, H.; Xue, Y.; Chen, W. Screening of Lactobacillus salivarius strains from the feces of Chinese populations and the evaluation of their effects against intestinal inflammation in mice. Food Funct. 2020, 11, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Wheeler, G.L. Ascorbic Acid in Plants: Biosynthesis and Function. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 291–314. [Google Scholar] [CrossRef]
- Samuel, B.S.; Gordon, J.I. A humanized gnotobiotic mouse model of host–archaeal–bacterial mutualism. Proc. Natl. Acad. Sci. USA 2006, 103, 10011–10016. [Google Scholar] [CrossRef]
- Bhat, M.I.; Singh, V.K.; Sharma, D.; Kapila, S.; Kapila, R. Adherence capability and safety assessment of an indigenous probiotic strain Lactobacillus rhamnosus MTCC-5897. Microb. Pathog. 2019, 130, 120–130. [Google Scholar] [CrossRef]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Muñoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013, 109 (Suppl. S2), S35–S50. [Google Scholar] [CrossRef]
- Fernández, S.; Fraga, M.; Silveyra, E.; Trombert, A.; Rabaza, A.; Pla, M.; Zunino, P. Probiotic properties of native Lactobacillus spp. strains for dairy calves. Benef. Microbes 2018, 9, 613–624. [Google Scholar] [CrossRef]
- Yamazaki, M.; Ohtsu, H.; Yakabe, Y.; Kishima, M.; Abe, H. In vitroscreening of lactobacilli isolated from chicken excreta to control Salmonella Enteritidis and Typhimurium. Br. Poult. Sci. 2012, 53, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Van Coillie, E.; Goris, J.; Cleenwerck, I.; Grijspeerdt, K.; Botteldoorn, N.; Van Immerseel, F.; De Buck, J.; Vancanneyt, M.; Swings, J.; Herman, L.; et al. Identification of lactobacilli isolated from the cloaca and vagina of laying hens and characterization for potential use as probiotics to control Salmonella Enteritidis. J. Appl. Microbiol. 2007, 102, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, D. Characterization of Lactobacillus isolated from dairy samples for probiotic properties. Anaerobe 2015, 33, 117–123. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, A.; de Llano, D.G.; Esteban-Fernández, A.; Requena, T.; Bartolomé, B.; Moreno-Arribas, M.V. Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol. 2014, 44, 220–225. [Google Scholar] [CrossRef]
- Ehrmann, M.A.; Kurzak, P.; Bauer, J.; Vogel, R.F. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 2002, 92, 966–975. [Google Scholar] [CrossRef]
- Suvarna, S.; Dsouza, J.; Ragavan, M.L.; Das, N. Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition. Food Sci. Biotechnol. 2018, 27, 745–753. [Google Scholar] [CrossRef]
- Adimpong, D.B.; Nielsen, D.S.; Sørensen, K.I.; Derkx, P.M.; Jespersen, L. Genotypic characterization and safety assessment of lactic acid bacteria from indigenous African fermented food products. BMC Microbiol. 2012, 12, 75. [Google Scholar] [CrossRef]
- Touret, T.; Oliveira, M.; Semedo-Lemsaddek, T. Putative probiotic lactic acid bacteria isolated from sauerkraut fermentations. PLoS ONE 2018, 13, e0203501. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.A.G.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef]
- Zommiti, M.; Connil, N.; Hamida, J.B.; Ferchichi, M. Probiotic Characteristics of Lactobacillus curvatus DN317, a Strain Isolated from Chicken Ceca. Probiotics Antimicrob. Proteins 2017, 9, 415–424. [Google Scholar] [CrossRef]
- Zommiti, M.; Almohammed, H.; Ferchichi, M. Purification and Characterization of a Novel Anti-Campylobacter Bacteriocin Produced by Lactobacillus curvatus DN317. Probiotics Antimicrob. Proteins 2016, 8, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Štšepetova, J.; Taelma, H.; Smidt, I.; Hütt, P.; Lapp, E.; Aotäht, E.; Mändar, R. Assessment of phenotypic and genotypic antibiotic susceptibility of vaginal Lactobacillus sp. J. Appl. Microbiol. 2017, 123, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, W.; Ci, W.; Zheng, Y.; Han, X.; Huang, J.; Zhu, J. Effects of Dietary Supplementation with Lactobacillus acidophilus and Bacillus subtilis on Mucosal Immunity and Intestinal Barrier Are Associated with Its Modulation of Gut Metabolites and Microbiota in Late-Phase Laying Hens. Probiotics Antimicrob. Proteins 2022, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Morton, J.M. The Human Gut Microbiome: A review of the effect of obesity and surgically induced weight loss. JAMA Surg. 2013, 148, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Gan, J.; Zeng, D.; Li, J.; Yu, H.; Zhao, H.; Yang, Y.; Tan, S.; Li, G.; Luo, C.; et al. Specific Microbial Taxa and Functional Capacity Contribute to Chicken Abdominal Fat Deposition. Front. Microbiol. 2021, 12, 643025. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017, 23, 2696–2704. [Google Scholar] [CrossRef]
- Li, N.; Yang, J.; Zhang, J.; Liang, C.; Wang, Y.; Chen, B.; Zhao, C.; Wang, J.; Zhang, G.; Zhao, D.; et al. Correlation of Gut Microbiome between ASD Children and Mothers and Potential Biomarkers for Risk Assessment. Genomics Proteom. Bioinform. 2019, 17, 26–38. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef]
- Goker, M.; Gronow, S.; Zeytun, A.; Nolan, M.; Lucas, S.; Lapidus, A.; Hammon, N.; Deshpande, S.; Cheng, J.F.; Pitluck, S.; et al. Complete genome sequence of Odoribacter splanchnicus type strain (1651/6). Stand. Genomic Sci. 2011, 4, 200–209. [Google Scholar] [CrossRef]
- Willemsen, L.E.M.; Koetsier, M.A.; Van Deventer, S.J.H.; Van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef]
- Kang, C.-H.; Kim, J.-S.; Park, H.M.; Kim, S.; Paek, N.-S. Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 Biotech. 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. In Proceedings of the Nutrition Society; Cambridge University Press (CUP): Cambridge, UK, 2021; Volume 80, pp. 37–49. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics to Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Lv, Y.; Chen, Q.; Feng, J.; Zhao, X. Lactobacillus plantarum Restores Intestinal Permeability Disrupted by Salmonella Infection in Newly-hatched Chicks. Sci. Rep. 2018, 8, 2229. [Google Scholar] [CrossRef]
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2011, 15, 57–62. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; Van Der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, K.; Zhang, A.; Chang, W.; Zheng, A.; Chen, Z.; Cai, H.; Liu, G. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult. Sci. 2021, 100, 101323. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.E.; Kramer, J.; Van Der Hulst, R.; Heres, L.; Jeurissen, S.H.M.; Boersma, W.J.A. Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. Br. Poult. Sci. 2004, 45, 355–366. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, S.; Lee, H.C.; Jung, S.K.; Kim, D.; Oh, K.B.; Yang, H.; Jo, Y.J.; Lee, H.S.; Lee, S.; Byun, S.J. Inclusion of Lactobacillus salivarius strain revealed a positive effect on improving growth performance, fecal microbiota and immunological responses in chicken. Arch. Microbiol. 2021, 203, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ishfaq, M.; Guo, Y.; Chen, C.; Li, J. Assessment of Probiotic Properties of Lactobacillus salivarius Isolated from Chickens as Feed Additives. Front. Vet. Sci. 2020, 7, 415. [Google Scholar] [CrossRef]
- Leng, Y.; Zhang, Y.; Zhang, Y.; Xue, X.; Wang, T.; Kang, Y. Ischemic post-conditioning attenuates the intestinal injury induced by limb ischemia/reperfusion in rats. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2011, 44, 411–417. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Duan, Y.; Yang, X. Antioxidant activity of Lactobacillus plantarum JM113 in vitro and its protective effect on broiler chickens challenged with deoxynivalenol. J. Anim. Sci. 2017, 95, 837–846. [Google Scholar] [CrossRef]
- Chooruk, A.; Piwat, S.; Teanpaisan, R. Antioxidant activity of various oral Lactobacillus strains. J. Appl. Microbiol. 2017, 123, 271–279. [Google Scholar] [CrossRef]
- Ramasamy, K.; Abdullah, N.; Jalaludin, S.; Wong, M.; Ho, Y.W. Effects of Lactobacillus cultures on performance of laying hens, and total cholesterol, lipid and fatty acid composition of egg yolk. J. Sci. Food Agric. 2009, 89, 482–486. [Google Scholar] [CrossRef]
- Naseem, S.; Willits, N.; King, A.J. Varying combinations of Lactobacillus species: Impact on laying hens’ performance, nitrogenous compounds in manure, serum profile, and uric acid in the liver. Transl. Anim. Sci. 2021, 5, txab018. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.; Kadirvel, R.; Natarajan, A.; Bhaskaran, M. Effect of probiotic supplementation on growth, nitrogen utilisation and serum cholesterol in broilers. Br. Poult. Sci. 1996, 37, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Guo, L.; Chen, B.; Hao, K.; Ma, H.; Liu, Y.; Min, Y. Effects of different probiotic fermented feeds on production performance and intestinal health of laying hens. Poult. Sci. 2022, 101, 101570. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).