Abstract
Globally, there has been an increase in squamates (particularly lizards and snakes) being kept as pets. Additionally, urban spread has resulted in greater human encroachment and interaction with the natural habitat of wild squamates. A potential consequence of increasing human interaction with squamates is the increased potential for disease transfer. This review collates the literature describing clinical salmonellosis cases that were definitively linked to a squamate through testing of the animal and population-based studies which investigate the risk of salmonellosis linked to pet squamates. It was demonstrated that although squamate-acquired salmonellosis accounted for a small percentage of total cases, children under five were at greatest risk, with the clinical manifestations tending to be more severe. In many cases, it was noted that the patient was unaware of the risks associated with keeping squamates and did not practice proper hand hygiene after handling the animals or cleaning cages. This highlights the need for more education focused on informing the general public of ways to reduce the risk of salmonellosis from pet squamates. There is also the need for future research into the role of wild squamates in the spread of human salmonellosis, both directly and indirectly through cross contamination.
1. Introduction
Salmonella is a significant cause of disease, affecting both humans and animals [1]. It is the causative agent of salmonellosis, a gastrointestinal disease of public health significance [2]. Globally, it is estimated that there are 93.8 million cases of salmonellosis each year [3]. Infrequently, Salmonella is also responsible for more invasive diseases, such as bacteraemia with or without metastatic disease, skin and bone infections, urinary tract infections, meningitis and splenic abscess [4,5,6,7,8]. Primarily, Salmonella is considered a foodborne pathogen, with contaminated food attributed to 80 million cases of salmonellosis annually [3,9]. However, in the USA it has been estimated the 6% of sporadic salmonellosis cases and 11% of cases in people aged under 21 are caused by reptile and amphibian contact [10]. This estimate was supported by a literature review conducted by Sauteur et al. [11] that examined published studies from 1965 to 2012 describing reptile associated salmonellosis in children aged less than 18 years. A total of 182 cases were identified. The primary reptile associated with gastrointestinal salmonellosis was turtles; however, exposure to iguanas was significantly more prevalent in children with invasive Salmonella diseases (septicaemia or meningitis).
Exotic pets, including lizards and snakes, have become increasingly popular [1,12,13]. Salmonella is often detected from captive reptiles and reports of salmonellosis linked to reptile pets are increasing [14]. A study in Malaysia demonstrated that 83.3% of captive lizards (Iguanidae, Agamidae, Scincidae, Gekkonidae, Varanidae) and 25% of wild lizards (Agamidae, Scincidae, Gekkonidae) were positive for Salmonella [15]. The significantly higher (p < 0.05) carriage rate in captive lizards compared with wild lizards is supported by a similar study conducted in Germany by Geue and Löschner [16] and could be attributed to horizontal transmission of Salmonella from humans and other animals to the captive lizards. In Japan, 66% (47/71) of lizards and 100% (23/23) of snakes from a pet store were positive for Salmonella [17]. In Croatia, it was found that 48.4% of captive lizards, and 8.9% of captive snakes belonging to a private owner or the Zagreb Zoo, were positive for Salmonella [18]. Thirty nine percent (58/149) of lizards and 29% (31/106) of snakes, housed in zoos and with private keepers in Poland, tested positive for Salmonella [19]. Additionally, in Canada, 51% of pet snakes and 48% of pet lizards submitted for autopsy were found to be positive for Salmonella and salmonellosis was identified as the cause of death in about one third of the Salmonella-positive animals [20]. The incidence of Salmonella in the pet trade is likely underreported as few studies have explicitly examined the incidence of this bacterium, despite the increase of snakes and lizards as pets worldwide.
One of the implications of the increasing demand for exotic pets is the potential for international importation of reptiles resulting in disease globalization [21]. In 1996, Sweden no longer required a certificate stating that an animal was Salmonella-free prior to importation into the country; consequently, an increase in the incidence of reptile-associated salmonellosis was observed in 1997 [13]. Additionally, a study in the USA found that 80% (88/110) of wild-caught Indonesian Tokay geckos (Gekko gecko) imported into the USA were positive for Salmonella. This included 14 different serogroups and 17 unique serotypes, several of which demonstrated antibiotic resistance [13].
The presence of Salmonella in wild lizards and snakes (squamates) may also be playing a role in human salmonellosis [13]. One consequence of urbanisation resulting in increasing human encroachment into natural ecosystems is a greater potential for interaction between humans and wild animals, leading to greater potential for the transfer of zoonotic pathogens [22]. There have been several studies which have investigated the presence of Salmonella in wild lizards and snakes [23,24,25,26]. This includes a study in the Galápagos, Ecuador, that found 62/63 (98%) of land iguanas (C. subcristatus) were positive for Salmonella [23]. Another study of wild snakes in Poland found that 14/16 (86%) of dead wild European grass snakes (Natrix natrix) and smooth snakes (Coronella austriaca) were positive for Salmonella [25]. In Spain, a study during the spring and summer period, 49% of wild lizards and snakes tested were positive for Salmonella [26]. Another study from Australia found that Salmonella enterica was present in 83% (50/60) of wild Australian sleepy lizards (Tiliqua rugosa). Analysis of the distribution of Salmonella genotypes suggested that the bacteria was spread from host to host within the lizards’ social network, rather than exposure to the same environmental source [24].
The increase in human interaction with lizards and snakes suggests that they may play an increasingly significant role in the spread of human salmonellosis, particularly with regard to the more invasive infections observed in younger children [10,11,13]. This article examines the current evidence linking human salmonellosis to lizards and snakes. Studies from the last 20 years which confirm a squamate as the source of a human Salmonella infection, and population-based studies which examine the likelihood of human Salmonella infections as a consequence of exposure to lizards and snakes, are analysed. Trends in Salmonella species, squamate species, exposure routes, and patient outcomes are discussed.
2. Results
The population-based studies that examined the likelihood of notified salmonellosis cases to be linked to lizards and snakes are presented in Table 1. The studies are limited to the UK [27], USA [28,29,30,31], and Germany [32]. The most recent study is from the UK, which identifies that over a quarter of salmonellosis cases in children under five years of age are linked to reptile exposure; however, the species of reptiles involved were not examined [27]. The focus on exposure of children is supported by a study from the USA that demonstrated the median age for reptile associated salmonellosis was 11 years old. This study also demonstrated that reptile exposure was attributed to 3.5% of all salmonellosis cases and of these lizards and snakes were attributed to 47% and 20%, respectively [28]. Additionally another study from the USA investigated the source of notified S. Marina cases, specifically, and found 81% of patients were infants (less than one year of age) and 88% of these cases reported exposure to iguanas [29].

Table 1.
Salmonellosis population-based studies investigating the risks associated with snakes and lizards.
The case reports from the last twenty years which identify and confirm snakes or lizards as a source of human Salmonella infection are presented in Table 2. Studies were from Switzerland [33], Germany [34], USA [6,35,36,37,38], Australia [39], UK [7,40,41,42,43], France [44], The Netherlands [8], and Canada [13]. The most commonly-identified lizard or snakes were bearded dragons [34,35,40,45] and iguanas [6,13,36,37,42,43]; although other species included corn snakes [37,44], a water dragon [41], a boa constrictor [38], and a gecko [7]. The cases described in Table 2 demonstrate a range of clinical presentations including gastrointestinal, sinus, blood, brain, bone, urinary tract, and spleen infections and primarily involved young children and immunocompromised patients. In several cases observed in children less than six months of age the Salmonella infection was fatal [36,37,41,42]. The most commonly-identified infectious agents were Salmonella Marina [6,13,37] and Salmonella Poona [13,36,42]. Interestingly, not all patients described direct contact with the lizard or snake, despite an identical isolate from both animal and patient [35,37,40,45], suggesting that indirect contact may play a role in the spread of lizard- and snake-associated salmonellosis. Indirect exposure could also apply to the cases involving young children less than six months of age who are unlikely to have had direct contact with the snake or lizard [7,36,37,41,42,43,45]. This transmission route was speculated by Glick and Sherman [46] who suggested that the mother or another family member acted as the vector transferring the Salmonella from the Gecko to the four day old baby. The role of indirect exposure is illustrated by the outbreak involving a potluck dinner which was prepared in the cook’s home and the Salmonella serotype responsible for the outbreak was found in her vacuum cleaner and from one of her pet bearded dragons which were kept in an adjacent room [35]. Similarly, in a case from Australia, the patient had no direct contact with the bearded dragon, but the vacuum cleaner contained the same Salmonella serotype, suggesting that this could be the route of exposure [45]. A common observation made in the case reports was that patients and the parents of patients were not aware of the risk associated with handling lizards and reported poor hand hygiene practices after handling of snakes and lizards and cleaning of enclosures. It was noted in a case involving a 29 years old male from Switzerland that the patient did no wash or disinfect his hands after handling snakes, feeding them, or cleaning their terrarium [33].

Table 2.
Studies describing human Salmonella infection confirmed to be caused by squamate exposure.
3. Discussion
Increasing interaction with snakes and lizards, both as captive pets and through encroachment of their natural ecosystem, may result in an increase in the transmission of salmonellosis [13]. This study demonstrates that snake- and lizard-associated salmonellosis is being reported across the globe. It also highlights the diversity of clinical presentation of Salmonella infections associated with snakes and lizards and their potential severity. Children were the primary demographic identified in squamate-associated salmonellosis cases and the clinical manifestations were typically more severe than other cases of salmonellosis. These findings support the US Centres for Disease Control and Prevention (CDC) recommendation that children under the age of five should avoid contact with reptiles and that these animals should not be kept in childcare centres [47].
The lack of the general public’s knowledge regarding the dangers associated with lizards and snakes was also highlighted and is supported by the finding of previous studies as one of the main risks for reptile associated salmonellosis [13,37]. In 2003, the US CDC found that only 4/49 US state health departments interviewed required pet store owners to provide information regarding risk of salmonellosis with the purchase of a turtle and no state health department required salmonellosis information to be provided to a person purchasing a lizard of snake [48]. This demonstrates the need for more education aimed at informing snakes and lizards handlers of the associated risk of salmonellosis and the importance of good hand hygiene practices [37]. Good hygiene is particularly important in cases where persons handling snakes and lizards were identified as the potential vector transmitting Salmonella to children. Additionally, in cases where there was no direct contact with the lizard or snakes, vacuum cleaners were identified as a potential source. The mechanical disturbance and agitation of settled dust containing microorganisms by a vacuum cleaner can provide a mechanism for the dispersal of bioaerosols [49]. It has been demonstrated vacuum cleaners can disperse bacteria emissions at concentrations as high at 105 bacteria per minute [50].
No studies which definitively identified a case of human salmonellosis caused by wild lizards and snakes were identified. However, previous studies have demonstrated the potential for wild lizards and snakes to carry Salmonella and, as such, they could potentially be playing a role in human cases [23,24,25,26]. The presence of Salmonella in wild lizards and snakes also presents other issues relating to biosecurity and animal health. It has been demonstrated that Salmonella is an opportunistic pathogen of lizards and snakes with weakened immune systems [51] and can cause bone infection in snakes [52,53]. There is also the potential for lizards and snakes to transfer Salmonella to animal production facilities, such as poultry farms. This could have significant consequences, particularly with the spread of antibiotic-resistant strains between flocks and after disinfection processes [54,55].
4. Materials and Methods
The databases Scopus and Web of Science were searched for articles written in English over the last twenty years containing the keywords (Salmonella OR salmonellosis) AND (lizards OR lizard OR Squamata OR Squamate OR snake OR snakes OR Amphisbaenia). Figure 1 presents the systematic approach to article inclusion or exclusion. Articles were screened by reading titles and abstracts and initially excluded if they did not refer to human salmonellosis or if they were review articles. Articles were then read in full and excluded if they described a clinical case of Salmonella infection linked to a squamate which was not confirmed through testing of the animal. Articles were included if they were population studies investigating squamates as a potential risk for salmonellosis and clinical cases that were definitively linked to a squamate through testing of the animal and comparison of animal and human Salmonella isolates.
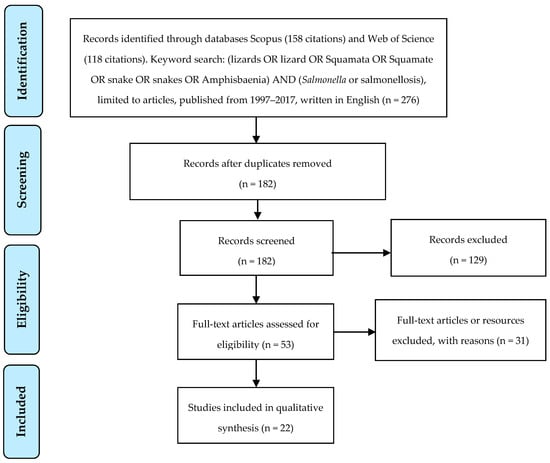
Figure 1.
Overview of search methods and articles’ inclusion and exclusion criteria.
5. Conclusions
This review demonstrates that salmonellosis associated with lizards and snakes is an emerging global issue of public health concern. Although it only accounts for a small proportion of all salmonellosis cases, the evidence suggests that it predominately affects children under five years of age and the clinical manifestations can be severe. There is a need for greater education aimed at informing people who keep lizards and snakes as pets of the potential risks and the best ways to protect themselves.
Author Contributions
H.W. prepared first draft, K.W. and M.G.G. edited first draft and provided academic input, and all authors read and approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Jong, B.; Andersson, Y.; Ekdahl, K. Effect of regulation and education on reptile-associated salmonellosis. Emerg. Infect. Dis. 2005, 11, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.T.; Feasey, N.A.; Gordon, M.A.; Keddy, K.H.; Angulo, F.J.; Crump, J.A. Global burden of invasive nontyphoidal Salmonella disease, 2010. Emerg. Infect. Dis. 2015, 21, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; Studies, I.C.o.E.D.B.o.I. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.B.; Rubin, R.H. The spectrum of Salmonella infection. Infect. Dis. Clin. N. Am. 1988, 2, 571–598. [Google Scholar]
- Nowinski, R.J.; Albert, M.C. Salmonella osteomyelitis secondary to iguana exposure: A case report. Clin. Orthop. Relat. Res. 2000, 372, 250–253. [Google Scholar] [CrossRef]
- Embil, J.M.; Nicolle, L.E. Salmonella urinary tract infections associated with exposure to pet iguanas. Clin. Infect. Dis. 1997, 25, 172. [Google Scholar] [CrossRef] [PubMed]
- Cyriac, J.; Wozniak, E. Infantile Salmonella meningitis associated with gecko-keeping. Commun. Dis. Public Health 2000, 3, 66–67. [Google Scholar] [PubMed]
- Berendes, T.; Keijman, J.; te Velde, L.; Oostenbroek, R. Splenic abscesses caused by a reptile-associated Salmonella infection. Dig. Surg. 2007, 24, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Crim, S.M.; Griffin, P.M.; Tauxe, R.; Marder, E.P.; Gilliss, D.; Cronquist, A.B.; Cartter, M.; Tobin-D’Angelo, M.; Blythe, D.; Smith, K. Preliminary incidence and trends of infection with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 us sites, 2006–2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 495–499. [Google Scholar] [PubMed]
- Mermin, J.; Hutwagner, L.; Vugia, D.; Shallow, S.; Daily, P.; Bender, J.; Koehler, J.; Marcus, R.; Angulo, F.J. Emerging infections program foodnet working group. Reptiles, amphibians, and human Salmonella infection: A population-based, case-control study. Clin. Infect. Dis. 2004, 38, S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Sauteur, P.M.M.; Relly, C.; Hug, M.; Wittenbrink, M.M.; Berger, C. Risk factors for invasive reptile-associated salmonellosis in children. Vector-Borne Zoonotic Dis. 2013, 13, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Ward, L. Salmonella perils of pet reptiles. Commun. Dis. Public Health 2000, 3, 2. [Google Scholar] [PubMed]
- Woodward, D.L.; Khakhria, R.; Johnson, W.M. Human salmonellosis associated with exotic pets. J. Clin. Microbiol. 1997, 35, 2786–2790. [Google Scholar] [PubMed]
- Mitchell, M.A.; Shane, S.M. Preliminary findings of Salmonella spp. In captive green iguanas (iguana iguana) and their environment. Prev. Vet. Med. 2000, 45, 297–304. [Google Scholar] [CrossRef]
- Cheng, B.Y.; Wong, S.P.; Dykes, G.A. Salmonella associated with captive and wild lizards in malaysia. Herpetol. Notes 2014, 7, 145–147. [Google Scholar]
- Geue, L.; Löschner, U. Salmonella enterica in reptiles of german and austrian origin. Vet. Microbiol. 2002, 84, 79–91. [Google Scholar] [CrossRef]
- Nakadai, A.; Kuroki, T.; Kato, Y.; Suzuki, R.; Yamai, S.; Yaginuma, C.; Shiotani, R.; Yamanouchi, A.; Hayashidani, H. Prevalence of Salmonella spp. In pet reptiles in japan. J. Vet. Med. Sci. 2005, 67, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Lukac, M.; Pedersen, K.; Prukner-Radovcic, E. Prevalence of Salmonella in captive reptiles from croatia. J. Zoo Wildl. Med. 2015, 46, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, T.; Chrząstek, K.; Wieliczko, A. Salmonella serovar spectrum associated with reptiles in poland. Acta Vet. Brno 2014, 83, 287–294. [Google Scholar] [CrossRef]
- Onderka, D.K.; Finlayson, M.C. Salmonellae and salmonellosis in captive reptiles. Can. J. Comp. Med. 1985, 49, 268–270. [Google Scholar] [PubMed]
- Mihalca, A.D. Ticks imported to europe with exotic reptiles. Vet. Parasitol. 2015, 213, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Paparini, A.; Jian, F.; Robertson, I.; Ryan, U. Public health significance of zoonotic cryptosporidium species in wildlife: Critical insights into better drinking water management. Int. J. Parasitol. Parasites Wildl. 2015, 5, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Hendriksen, R.S.; Lorenzetti, S.; Onorati, R.; Gentile, G.; Dell’Omo, G.; Aarestrup, F.M.; Battisti, A. Characterization of Salmonella occurring at high prevalence in conolophus subcristatus in galapagos islands, ecuador. PLoS ONE 2011, 6, e23147. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Godfrey, S.; Gordon, D. Social networks and the spread of Salmonella in a sleepy lizard population. Mol. Ecol. 2012, 21, 4386–4392. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Wasyl, D.; Różycki, M.; Bilska-Zając, E.; Fafiński, Z.; Iwaniak, W.; Krajewska, M.; Hoszowski, A.; Konieczna, O.; Fafińska, P. Free-living snakes as a source and possible vector of Salmonella spp. And parasites. Eur. J. Wildl. Res. 2016, 62, 161–166. [Google Scholar] [CrossRef]
- Briones, V.; Téllez, S.; Goyache, J.; Ballesteros, C.; del Pilar Lanzarot, M.; Domínguez, L.; Fernández-Garayzábal, J.F. Salmonella diversity associated with wild reptiles and amphibians in spain. Environ. Microbiol. 2004, 6, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; Oshin, F. Reptile-associated salmonellosis in children aged under 5 years in south west england. Arch. Dis. Child. 2015, 100, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Whitten, T.; Bender, J.; Smith, K.; Leano, F.; Scheftel, J. Reptile-associated salmonellosis in minnesota, 1996–2011. Zoonoses Public Health 2015, 62, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Mermin, J.; Hoar, B.; Angulo, F.J. Iguanas and Salmonella marina infection in children: A reflection of the increasing incidence of reptile-associated salmonellosis in the united states. Pediatrics 1997, 99, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.; Yartel, A.; Moriarty, K.; Nathan, L.; Salehi, E.; Tengelsen, L.; Patel, N.; Lynch, M. Salmonella kingabwa infections and lizard contact, united states, 2005. Emerg. Infect. Dis. 2007, 13, 661. [Google Scholar] [CrossRef] [PubMed]
- Varma, J.K.; Marcus, R.; Stenzel, S.A.; Hanna, S.S.; Gettner, S.; Anderson, B.J.; Hayes, T.; Shiferaw, B.; Crume, T.L.; Joyce, K. Highly resistant Salmonella newport-mdrampc transmitted through the domestic us food supply: A foodnet case-control study of sporadic Salmonella newport infections, 2002–2003. J. Infect. Dis. 2006, 194, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Rabsch, W.; Prager, R.; Tietze, E.; Koch, J.; Mutschmann, F.; Roggentin, P.; Frank, C. Babies and bearded dragons: Sudden increase in reptile-associated Salmonella enterica serovar tennessee infections, germany 2008. Vector-Borne Zoonotic Dis. 2011, 11, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Horvath, L.; Kraft, M.; Fostiropoulos, K.; Falkowski, A.; Tarr, P.E. Salmonella enterica subspecies diarizonae maxillary sinusitis in a snake handler: First report. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2016; p. ofw066. [Google Scholar]
- Pees, M.; Rabsch, W.; Plenz, B.; Fruth, A.; Prager, R.; Simon, S.; Schmidt, V.; Münch, S.; Braun, P. Evidence for the transmission of Salmonella from reptiles to children in germany, july 2010 to october 2011. Euro Surveill. 2013, 18. [Google Scholar] [CrossRef]
- Lowther, S.A.; Medus, C.; Scheftel, J.; Leano, F.; Jawahir, S.; Smith, K. Foodborne outbreak of Salmonella subspecies iv infections associated with contamination from bearded dragons. Zoonoses Public Health 2011, 58, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; De Gortari, M.; Lin, T.; Barrett, B. Ribotyping of Salmonella poona in iguana-associated zoonotic salmonellosis. J. Vet. Diagn. Investig. 1998, 10, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control Prevention. Reptile-associated salmonellosis—Selected states, 1996–1998. MMWR Morb. Mortal. Wkly. Rep. 1999, 48, 1009. [Google Scholar]
- Jafari, M.; Forsberg, J.; Gilcher, R.O.; Smith, J.W.; Crutcher, J.M.; McDermott, M.; Brown, B.R.; George, J.N. Salmonella sepsis caused by a platelet transfusion from a donor with a pet snake. N. Engl. J. Med. 2002, 347, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Moffatt, C.R.; Dyda, A.; Hundy, R.; Kaye, A.L.; Krsteski, R.; Rockliff, S.; Kampen, R.; Kelly, P.M.; O’Brien, E.D. An outbreak of gastroenteritis due to Salmonella typhimurium phage type 170 associated with consumption of a dessert containing raw egg. Commun. Dis. Intell. Q. Rep. 2010, 34, 329. [Google Scholar] [PubMed]
- Cooke, F.J.; De Pinna, E.; Maguire, C.; Guha, S.; Pickard, D.J.; Farrington, M.; Threlfall, E.J. First report of human infection with Salmonella enterica serovar apapa resulting from exposure to a pet lizard. J. Clin. Microbiol. 2009, 47, 2672–2674. [Google Scholar] [CrossRef] [PubMed]
- Communicable Disease Surviellance Centre. Fatal neonatal Salmonella meningitis linked to pet reptile. Commun. Dis. Rep. CDR Wkly. 2000, 10, 52. [Google Scholar]
- Communicable Disease Surviellance Centre. Baby dies of Salmonella poona infection linked to pet reptile. Commun. Dis. Rep. CDR Wkly. 2000, 10, 161. [Google Scholar]
- Sanyal, D.; Douglas, T.; Roberts, R. Salmonella infection acquired from reptilian pets. Arch. Dis. Child. 1997, 77, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Ehlinger, M.; Stanchina, C.; Giacomelli, M.C.; Gicquel, P.; Karger, C.; Clavert, J.M. Salmonella enterica subsp. Arizonae bone and joints sepsis. A case report and literature review. Orthopaedics Traumatol. Surg. Res. 2009, 95, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.; Lafferty, A.R.; Khan, S.; Krsteski, R.; Valcanis, M.; Powling, J.; Veitch, M. Salmonella rubislaw gastroenteritis linked to a pet lizard. Med. J. Aust. 2010, 193, 54–55. [Google Scholar] [PubMed]
- Glick, S.R.; Alter, S.J. Salmonella enterica serotype enteritidis meningitis in a 4-day-old neonate: A case report. Infect. Dis. Clin. Pract. 2014, 22, 46–48. [Google Scholar] [CrossRef]
- Vora, N.M.; Smith, K.M.; Machalaba, C.C.; Karesh, W.B. Reptile-and amphibian-associated salmonellosis in childcare centers, united states. Emerg. Infect. Dis. 2012, 18, 2092–2094. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Reptile-associated salmonellosis--selected states, 1998–2002. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 1206. [Google Scholar]
- Veillette, M.; Knibbs, L.D.; Pelletier, A.; Charlebois, R.; Blais Lecours, P.; He, C.; Morawska, L.; Duchaine, C. Microbial contents of vacuum cleaner bag dust and emitted bioaerosols and their implications for human exposure indoors. Appl. Environ. Microbiol. 2013, 79, 6331–6336. [Google Scholar] [CrossRef] [PubMed]
- Knibbs, L.D.; He, C.; Duchaine, C.; Morawska, L. Vacuum cleaner emissions as a source of indoor exposure to airborne particles and bacteria. Environ. Sci. Technol. 2012, 46, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Sting, R.; Ackermann, D.; Blazey, B.; Rabsch, W.; Szabo, I. Salmonella infections in reptiles—Prevalence, serovar spectrum and impact on animal health. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 202–208. [Google Scholar] [PubMed]
- Isaza, R.; Garner, M.; Jacobson, E. Proliferative osteoarthritis and osteoarthrosis in 15 snakes. J. Zoo Wildl. Med. 2000, 31, 20–27. [Google Scholar] [PubMed]
- Ramsay, E.C.; Daniel, G.B.; Tryon, B.W.; Merryman, J.I.; Morris, P.J.; Bemis, D.A. Osteomyelitis associated with Salmonella enterica ss arizonae in a colony of ridgenose rattlesnakes (crotalus willardi). J. Zoo Wildl. Med. 2002, 33, 301–310. [Google Scholar] [PubMed]
- Ogunleye, A.O.; Carlson, S. Characterization of a multidrug resistant Salmonella enterica give isolated from a lizard captured in a poultry house in nigeria. Afr. J. Biomed. Res. 2017, 20, 53–58. [Google Scholar]
- Ogunleye, A.; Ajuwape, A.; Alaka, O.; Adetosoye, A. Characterization of a Salmonella enterica serotype pullorum isolated from a lizard co-habitating with poultry. Afr. J. Microbiol. Res. 2013, 7, 1215–1221. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).