Abstract
Trypanosoma cruzi is the causative agent of Chagas disease, which affects 6–7 million people worldwide. Although the possibility of oral transmission was first scientifically suggested in 1913, it was not until 1968 that the first confirmed cases of human infection via food consumption were reported. This long gap contributed to the widespread perception that oral transmission was a rare or incidental event. Over the past two decades, significant advances have been made in understanding the biological and clinical aspects of oral transmission, including the molecular mechanisms by which metacyclic trypomastigotes establish infection via the digestive route. Experimental studies in murine models have further deepened our knowledge of the biology and pathogenesis of oral infection. Concurrently, multiple outbreaks of T. cruzi infection through contaminated food and beverages have been reported across Latin America, providing valuable insights into the molecular epidemiology and clinical characteristics of this transmission route. Moreover, experimental evidence has shown that the consumption of meat from animals infected during the acute phase can also lead to T. cruzi infection, highlighting carnivory as a potential alternative transmission mechanism. This review aims to comprehensively analyze oral infection by T. cruzi, considering clinical and epidemiological data, parasite biology, and findings from murine experimental models. Strategies for controlling foodborne transmission of Chagas disease are also discussed.
1. Introduction
Chagas disease is a Neglected Tropical Disease first described by Carlos Chagas in 1909 [1]. It is endemic in Latin America, present in 22 countries across the Americas [2,3]. However, because of population migrations, carriers of infection or disease are now found throughout the world, which is why Chagas is considered a global public health problem [4]. Chagas disease is caused by a flagellated protozoan called Trypanosoma cruzi (Kinetoplastida, Trypanosomatidae) [5]. According to the World Health Organization (WHO), there are 6 to 7 million people infected with T. cruzi worldwide [6]. Approximately 75 million people are at risk of acquiring the infection, and more than 10.000 people die each year from the disease [6].
When transmission occurs via the vector route or oral route (ingestion of food contaminated with triatomine droppings), the infective form is the metacyclic trypomastigote (MT). This form invades nucleated cells and differentiates into amastigotes, which divide by binary fission. The amastigotes subsequently differentiate into blood trypomastigotes (BT), which leave the cell to enter the bloodstream, spread to other sites in the organism, and can eventually be ingested by triatomines when they feed on a T. cruzi-infected host [7,8].
When infection occurs by post-transfusion mechanisms, by the transplacental route, transplantation of organs from infected persons, or consumption of meat or blood from infected animals, the infective form is the BT. This stage, like the MT, is able to invade nucleated cells, differentiate within them into the amastigote stage, proliferate intracellularly, and then differentiate into the BT stage. The latter exit the cell, disseminate via the bloodstream, and can also be infective for triatomines when they feed on T. cruzi-infected hosts [8] (Figure 1).
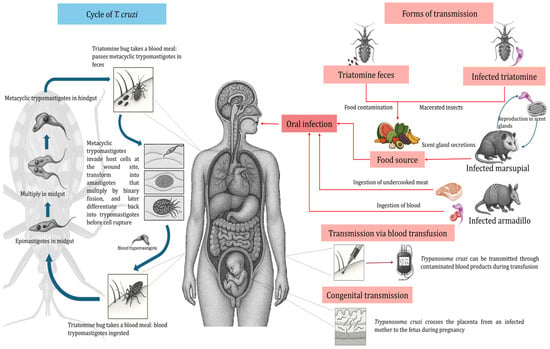
Figure 1.
Life cycle of Trypanosoma cruzi, including different transmission mechanisms.
In recent decades, however, oral transmission has become an increasingly important mechanism in the epidemiology of Chagas disease [9,10,11]. Notably, 70% of the ~1000 acute Chagas disease cases documented in the first ten years of this century were the result of oral transmission [12,13]. In fact, oral infection with T. cruzi is currently the most important transmission route in Brazil. Several outbreaks have been documented in the Amazon region and in some states of the northeast and south of Brazil, and this trend has been on the rise in recent years [14]. Approximately 3.060 cases of acute Chagas disease were reported in Brazil between 2007 and 2019, with a predominance in the north region (94.4%). Pará state had the major percentage of cases (74.54%), most attributed to the ingestion of contaminated food [15]. This trend may be explained by several factors. One important factor is based on human activities: through predation, invasion, and deforestation of jungles and forests, humans have disrupted the ecological balance. This has reduced the diversity of wild mammals that serve as food sources for triatomines. Consequently, triatomines, which are typically wild or peridomestic, have started to invade homes in search of new food sources (blood meals). This increases the chances of food contamination, especially of homemade fruit juices, which have been the most frequent source of infection in the majority of documented oral transmission outbreaks [10]. Therefore, although oral transmission of T. cruzi infection is not a global problem, it is a situation of high epidemiological risk in Brazil and countries in northern South America where domestic or peridomestic vectors still exist. On the other hand, the growing export and widespread consumption of açaí pulp in different parts of the world raise the possibility of a more widespread epidemiological risk for this route of infection of the parasite.
The objective of this review is to comprehensively analyze oral infection by T. cruzi. Then, we will describe the parasite evolutionary context, proposing oral infection as an ancestral mechanism of infection and analyzing the main clinical and epidemiological findings, as well as parasitic biology and experimental oral infection in the murine model. The present and future challenges in the perspective of strategies for controlling oral infection by T. cruzi will also be analyzed.
2. The Beginning of Oral Transmission by T. cruzi
T. cruzi exhibits a high degree of genetic heterogeneity that has been associated with the observed variability in clinical manifestations, geographical distribution, and preferential parasite–vector interactions. The parasite is classified into at least seven discrete typification units (DTUs): TcI–TcVI and TcBat [16]. Some DTUs have been tentatively associated with particular geographic distributions, transmission cycles, or clinical outcomes, but these patterns are not yet fully understood [16,17,18,19,20]. It has been suggested that the infective form of the parasite (e.g., BT vs. MT) and the route of infection (oral vs. other routes) can differentially modulate the course of T. cruzi infection in mice [20,21].
Trypanosoma cruzi is an ancient parasite. The origin of T. cruzi, as well as other kinetoplastid species, has been inferred primarily through molecular phylogenetic analyses based on ribosomal RNA gene sequences. In fact, phylogenetic analysis of 18S rRNA sequences indicates that the Trypanosoma brucei clade diverged from the T. cruzi clade approximately 100 million years ago, following the separation of Africa, South America, and Euramerica [22].
A Bayesian coalescent analysis has estimated that TcBat could have emerged about 688,000 years ago, whereas TcI emerged around 614,000 years ago. These dates suggest that the current diversity of T. cruzi DTUs is relatively recent in evolutionary terms, occurring during the late Pleistocene, and long after the initial radiation of the genus Trypanosoma [23].
These temporal estimates support a scenario in which ancient divergence events at the genus level were followed much later by more recent diversification within the T. cruzi lineage, likely associated with ecological shifts and host colonization events.
The southern supercontinent hypothesis proposes that the T. cruzi lineage originated in association with South American marsupials, particularly opossums, around the time when South America separated from Gondwana [24]. This model suggests a long-term co-evolution between T. cruzi and terrestrial mammals of South American origin [24]. In contrast, the bat seeding hypothesis argues that the common ancestor of the T. cruzi clade, including T. rangeli, was originally a bat trypanosome. According to this model, trypanosomes diversified within chiropteran hosts and later underwent multiple independent host-switching events from bats to terrestrial mammals, one of which gave rise to modern T. cruzi. Maximum likelihood phylogenetic reconstructions support this hypothesis by clustering TcBat as basal to all other T. cruzi DTUs [25].
Further support for the bat seeding model comes from the detection of T. cruzi in the salivary glands of the hematophagous bat Diaemus youngi, suggesting that bats may participate actively in sylvatic transmission cycles. This opens the possibility that bats acted not only as ancestral hosts, but also as ecological bridges facilitating the parasite’s spread to other mammalian groups [26].
In summary, all these data indicate that T. cruzi emerged long before Homo sapiens (~200,000 years ago in Africa) [27]. By the time humans migrated to the New World around 30,000 years ago, they encountered a genetically diverse array of T. cruzi strains carried by arthropod vectors, in addition to infected terrestrial mammals. These early humans were thus exposed to T. cruzi infection first (and only much later to Chagas disease as a pathology) [25]. Initially, the humans who migrated into what is now South America were hunter-gatherers [28] participating in the sylvatic cycle of T. cruzi either by consuming meat from animals infected with the parasite (such as camelids or small rodents) or by inadvertently consuming the infected arthropod vectors themselves [29,30,31]. This is supported by evidence of very high T. cruzi infection rates in skeletal remains from various pre-Colombian civilizations of Latin America, such as Incas, Chinchorro, and others [29,30,31].
Notably, through PCR amplification of kinetoplastid DNA sequences, T. cruzi infection has been identified in ancient human remains, underscoring the long-standing role of oral transmission. Aufderheide et al. [32] examined 283 mummified bodies from southern Peru and northern Chile, finding a Chagas disease prevalence of approximately 41% over the past 9000 years. They suggested that oral transmission might have occurred through eating infected, undercooked meat (e.g., guinea pigs, Cavia porcellus). Likewise, TcI DNA was detected in Brazilian mummies dated to 4500–7000 years before present [33], and a mummy from the same region showing evidence of megacolon (a classic chronic Chagas pathology) was dated to around 560 ± 40 years A.P. (after present) [34].
PCR analyses of American ancient tissue samples have confirmed these findings. For example, tissues from six mummies (2000 BC–1400 AD) in the Archaeological Museum of San Pedro de Atacama (northern Chile) were tested, and four of the six (66.6%) had amplifiable T. cruzi DNA [28]. Similarly, a mummy ~1150 years BP from Lower Pecos, Texas (USA), showed evidence of T. cruzi infection, the oldest known infection in North America [35,36], and this individual also had pathological signs consistent with megacolon [30]. Analysis of coprolites (preserved feces) from that mummy suggests a remarkably diverse diet, including rodents, bats, and arthropods [30]. Such broad dietary habits raise the possibility that triatomines or even infected bats might have been eaten by these prehistoric populations of hunter-gatherers [35,36]. Additionally, an extremely high percentage (84%) of mummies from the Lower Pecos region exhibited dental abscesses, a condition that could have facilitated oral transmission of T. cruzi via open sores in the mouth [29].
Available evidence suggests that humans became involved in the T. cruzi transmission cycle on a larger scale once they settled into sedentary communities, domesticated animals, and began practicing agriculture (which in the Andean regions of South America occurred between 6000 and 8000 years ago) [22,31,37]. The presence of domesticated animals inside human dwellings, combined with the cultivation and storage of grains like wheat and maize, attracted rodent populations as well as triatomines. In human habitations, triatomine bugs found not only a variety of food sources but also shelter, which led to the establishment of a domestic T. cruzi transmission cycle.
Historical accounts suggest that oral transmission of Chagas disease may have occurred in prehistoric and early historic times. For example, chronicles from around the year 1330 describe an epidemic in Coyoacán (central Mexico). Following a disruption of the fish supply, inhabitants reportedly suffered high morbidity and mortality with symptoms including edema of the eyelids, face, and feet, and hemorrhagic diarrhea. Many people died, mainly the young and the elderly. This clinical picture may suggest a possible case of foodborne outbreak of Chagas disease, possibly acquired after hunting and consuming infected opossums (Didelphis spp.), which are natural reservoirs of T. cruzi [38].
In the modern scientific literature, T. cruzi human oral infection was observed first in animal models in 1913 [39]. Subsequently, experimental infections in mice using blood contaminated with trypomastigotes, urine, and feces from triatomines infected with T. cruzi confirmed this potential mode of transmission [40]. The first human cases of orally acquired Chagas disease were reported by Silva et al. in 1968 [9,41]. Subsequently, Yaeger in 1971 documented the transmission of T. cruzi to opossums via the oral route, suggesting that wild animals could become infected by ingesting food or water contaminated with metacyclic trypomastigotes (e.g., via anal gland secretions of infected opossums) [42]. In fact, some reported human oral infection cases have been attributed to contact with opossum secretions [42].
Oral transmission was initially studied in animal reservoirs and wild vectors by Barretto et al. (in 1978) [43]. It is considered the ancestral mode of parasite transmission, as it is the most common form of infection between animals and vectors and has allowed T. cruzi to be maintained in nature over millennia [44]. In the earliest stages, Chagas disease was an endemo-enzootic infection, with T. cruzi cycling in wild environments largely through oral transmission, when different animals ingested infected vectors or through carnivory in predator–prey interactions [11,45].
All this evidence together suggests that oral transmission was especially relevant in the early peopling and settlement of the Americas by indigenous cultures.
3. The Current Role of the Oral Route in Human T. cruzi Infection
Currently, oral transmission of T. cruzi occurs as a result of ingesting food and beverages contaminated with the parasite. This typically happens either by blending triatomine insects (or their feces) into food products. For example, contamination can occur in fruit juices (such as açaí or sugar cane juice) when triatomines or their droppings are ground up with the fruit, or via tainted foods. A concept map of oral transmission is shown in Figure 2.
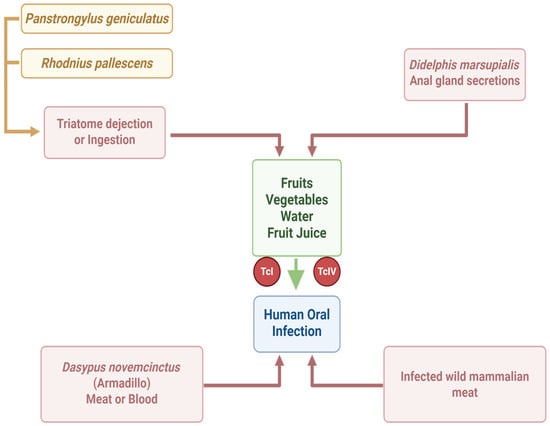
Figure 2.
Concept map of human oral infection by Trypanosoma cruzi (created in https://BioRender.com).
Outbreaks of orally acquired Chagas disease have been described in several other South American countries as well, including Venezuela, Colombia, Bolivia, Argentina, French Guiana, and Ecuador [12,46,47,48,49,50,51,52]. These outbreaks have been associated with consuming a variety of contaminated foods and drinks, such as wild game meat (e.g., armadillo), fruits or vegetables, sugar cane juice (“guarapo”), açaí palm fruit pulp, guava juice, bacaba (palm fruit) juice, babaçu coconut, and even wine made from palm fruit [12,13,53,54,55]. From 1965 to 2000, about 50% of acute Chagas cases in the Amazon region were attributed to oral transmission [12,13], and between 2000 and 2010, this proportion reached 70%. Venezuela experienced the largest documented outbreaks: two urban school-based outbreaks affecting 103 and 88 people, respectively (including both children and adults) [10,18]. The total number of cases and outbreaks of oral T. cruzi infection described to date, indicating, where possible, the source of infection and the DTU involved, is shown in Table 1.

Table 1.
Reported oral Chagas disease outbreaks in South América.
Importantly, orally acquired infections tend to be more severe. The mortality rate in orally infected patients ranges from 0 up to 44%, which is significantly higher than the <5–10% mortality seen in vector-transmitted (bite-associated) acute Chagas cases [48]. In summary, currently, the oral route is the most frequent and significant mode of T. cruzi transmission in many areas [9]. In some regions of the Americas, oral transmission of T. cruzi has not yet been reported, but the risk of such outbreaks remains real. Human cases of oral infection could occur mainly in two scenarios:
- Tourist or non-local populations visiting tropical areas of countries like Brazil, Colombia, or Venezuela, who may consume local foods, juices, or fruit pulps that are contaminated with infected triatomine feces, or who eat raw/undercooked “exotic” meats (such as armadillo).
- Communities that consume meat from free-ranging domestic or wild animals (e.g., rabbits, hares, or goats) in areas where wild triatomines are present.
For example, in Chile, the consumption of undercooked meat from goats or rabbits could pose an oral transmission risk. Indeed, infections in Chilean rabbits and goats have been reported [98,99]. Additionally, climate change may expand the range of wild triatomine vectors like Mepraia gajardoi (Reduviidae, Triatominae) and Mepraia spinolai (Reduviidae, Triatominae) (the main wild vectors in Chile) [100]. On the other hand, various traditionally domestic animals such as pigs [101], sheep, and horses have also been found infected with T. cruzi in different places in Latin America [102,103].
All these factors make oral transmission of T. cruzi, particularly through carnivory (eating infected meat), an emerging challenge for Chagas disease control. It is necessary to deepen our understanding of the biological and molecular determinants that enable BT or tissue culture-derived trypomastigotes (TCT) to establish infection via the oral route, using appropriate animal models for both acute and chronic infection.
Thus, in all cases, a critical event is preventing infection, where laboratory studies have provided useful information in this direction. Protective measures include covering and sealing food containers to prevent contact with insects, pasteurizing fruit juices (as is now mandated for açaí pulp in some regions), and freezing wild game meat before consumption to kill parasites. This is particularly relevant, considering that experimental studies have shown T. cruzi survival and virulence in açaí pulp are unaffected by prior incubation at room temperature for 24 h, at 4 °C for 144 h, or at –20 °C for 26 h. These results indicate that T. cruzi can survive and remain virulent in açaí pulp under various conditions, and that cooling or brief freezing are not adequate methods for preventing T. cruzi infection via oral consumption [104]. On the other hand, heating açaí pulp above 43 °C for 20 min can prevent foodborne acute Chagas disease [105]. In vitro experiments have demonstrated a loss of T. cruzi trypomastigote viability, which was only complete after 120 h at –20 °C and 144 h at +2 °C, whereas parasites remained alive after 168 h at −80 °C [106]. On the other hand, the parasite can survive in sugarcane juice for 4 to 12 h [106]. A summary of the main preventive measures useful for people living in endemic areas with vector transmission and for tourists is shown in Table 2.

Table 2.
Sources of infection and strategies for the prevention of oral Chagas disease outbreaks in South América.
4. Clinical Aspects of Orally Acquired Chagas Disease
The clinical course of Chagas disease is commonly divided into acute, indeterminate (asymptomatic), and chronic phases [107]. If symptoms develop during acute infection, they are usually mild and nonspecific. After the acute phase, patients enter an indeterminate (asymptomatic) phase, during which no clinical symptoms are present (although infection can be serologically detected for life). Approximately 30% of infected individuals eventually progress from the indeterminate phase to the chronic phase of Chagas disease. The most significant clinical manifestation of chronic Chagas is cardiomyopathy, which develops in about 20–30% of infected people. Chagas can also affect the gastrointestinal tract (GI) and the nervous system; the most common GI manifestations are megaesophagus and megacolon [17]. The drugs currently used to treat Chagas disease are nifurtimox and benznidazole. These are considered effective for treating acute and early chronic infection in children under 15 years of age. However, their efficacy and therapeutic value in chronic infection is controversial [108].
Unlike vector-borne acute Chagas infections (which are often asymptomatic or very mild), acute Chagas disease acquired through the oral route is usually symptomatic and presents with more severe manifestations, mainly affecting children and young adults (<18 years old). However, the clinical manifestations can vary greatly from patient to patient [18], likely depending on the size of the inoculum and the host’s immune response. After an incubation period of roughly 3 to 22 days following ingestion of T. cruzi, the first sign is often an unexplained, persistent high fever. This is typically accompanied by symptoms such as headache, profound weakness, and edema of the face and extremities. Acute myocarditis evidenced by arrhythmias, pericardial effusion, and cardiomegaly is another prominent feature of orally acquired infection [107]. Mortality in outbreaks of orally transmitted Chagas disease can reach as high as one-third of those infected (though it varies, reported between 5% and 33%) [109,110]. Higher acute mortality has been linked to several factors: T. cruzi strain involved [111], the patient’s age, the parasite load in the infected host, and delays in diagnosis and treatment [13]. Similarly, the infection appears to be more severe in children, the elderly, and immunocompromised individuals [6,13].
A study by Dos Santos et al. [112] examined changes in liver function and coagulation factors in 102 patients with acute Chagas disease acquired orally in the Brazilian Amazon. The most frequent symptoms were fever (98% of cases), asthenia (83.3%), edema of the face and limbs (80.4%), headache (74.5%), and myalgia (72.5%). Levels of liver enzyme alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were higher in 30 of these acute patients compared to uninfected controls. Moreover, patients showed elevated plasma levels of activated protein C and reduced levels of factor VII (a clotting factor). The study concluded that oral T. cruzi infection is associated with disseminated intravascular coagulation and liver function impairment [112]. Thus, the clinical presentation of acute Chagas disease differs markedly depending on the route of infection. Vector- borne infection tends to be mild or asymptomatic, whereas oral infection often leads to severe, symptomatic disease. These differences are illustrated in Figure 3.
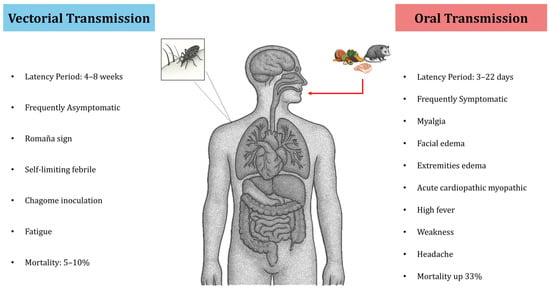
Figure 3.
Main differences in the clinical presentation of Chagas disease according to the mechanism of infection.
Clinical and Laboratory Diagnostics
Laboratory diagnosis presents some difficulties, such as the fact that no single technique is capable of detecting 100% of cases. In addition, in some cases, it is necessary to take more than one blood sample. Furthermore, reference laboratories are generally located far from the site of the outbreak, which delays the arrival of samples and, therefore, diagnosis. However, the detection of parasites using direct methods, such as fresh blood observation and microhematocrit, is a rapid method that can be performed without sophisticated equipment and has good sensitivity in the acute phase of infection [113].
Serological diagnosis of acute infection cases due to contaminated food consumption has traditionally been based on the detection of IgG and IgM using ELISA and immunofluorescence. However, the most appropriate procedure is to request three blood samples: one in a tube without anticoagulant for serology; one collected in a tube with heparin for culture, microhematocrit, and inoculation in animals [113,114]; and a third sample collected in a tube with ethylenediaminetetraacetic acid for PCR [115].
However, sometimes, the clinical manifestations may be nonspecific, which makes diagnosis a real challenge for clinicians, given that oral acute Chagas disease can be confused with other acute febrile illnesses, such as leptospirosis, visceral leishmaniasis, malaria, enteric fever, rickettsioses, and some arboviruses [116]. Then, the following criteria must be used in confirming acute Chagas disease cases: (1) exposure to the probable source of contamination; (2) manifestation or absence of symptoms; and (3) confirmation of laboratory diagnosis by parasitological tests, among them thick blood smear, hemoculture, xenodiagnoses, PCR, and serological tests [83,115].
5. Oral Infection by T. cruzi in Animal Models
In laboratory animal models, oral T. cruzi infection can be established either by direct inoculation into the oral cavity (oral infection, OI) or by gastric intubation/gavage (gastric infection, GI). These two methods represent slightly different routes of entry, yet both produce clear parasitemia and heart tissue infection during the acute phase. Barreto-de-Albuquerque et al. [21] provided conclusive evidence that the initial site of parasite entry dramatically affects the host’s immune response and disease outcome. In their study, mice infected via OI showed higher parasitemia levels and greater mortality than mice infected via GI, even when the same parasite strain and dose were used. Interestingly, heart inflammation was more extensive in GI-infected mice, whereas liver lesions were more severe in OI-infected animals, accompanied by higher serum ALT and AST levels in the OI group [21]. This indicates that the entry route alters the tissue tropism and pathology of infection.
Another study compared oral inoculation to intraperitoneal (IP) inoculation in mice, using different DTUs and inoculum sizes. Overall, mice infected orally showed lower parasitemia, infectivity, and mortality rates than those infected via the IP route, regardless of the parasite strain (DTU) or dose [117]. Within this study, mice infected with a TcII strain had higher parasitemia and mortality rates than those infected with a TcI strain. A larger volume of inoculum caused a higher infection rate when administered orally. BT challenges were more virulent than MT challenges for both oral and IP routes. However, even for a virulent BT strain, orally inoculated mice had lower parasitemia and mortality than mice inoculated IP. In contrast, oral inoculation with culture-derived MTs produced infection levels similar to IP, and those MT-inoculated mice showed more histopathological changes than BT-inoculated mice (for both routes) [117]. These findings imply that T. cruzi virulence and pathology depend on a combination of the parasite form, the dose, and the route of entry.
Silva-dos-Santos et al. [118] used bioluminescence imaging and quantitative PCR to study oral T. cruzi infection in mice. They infected mice with a bioluminescent strain of T. cruzi (Dm28c-luc, a TcI strain) via the oral route. In vivo imaging showed that the nasomaxillary region (around the mouth and nose) is the initial site of parasite invasion. By 7–21 days post-infection, the bioluminescence signal intensified in the thorax, abdomen, and genital area, reflecting dissemination of the parasite to different tissues. Similarly, using a fluorescent strain (Dm28c-GFP), nests of T. cruzi amastigotes were detected in the nasal cavity by day 6 post-infection. Quantitative PCR at days 7 and 21 post-infection showed that the parasite was predominantly found in the nasal cavity and had expanded there. These results clearly demonstrate that the oral cavity and adjacent structures (like the nasal passages) are the primary target regions in oral T. cruzi infection, leading to early parasite multiplication in the nasal mucosa.
A characteristic feature of oral-route T. cruzi infection is prominent facial and hind limb edema. In some cases, hemorrhagic manifestations and risk of thromboembolism are also observed. In one investigation, BALB/c mice were orally infected with MT of the Tulahuén strain, and researchers analyzed the cytokine response and hemostatic changes during acute infection. Orally infected mice, compared to uninfected controls, showed markedly elevated serum levels of proinflammatory cytokines (TNF-α, IFN-γ, IL-6), as well as leukocytosis and thrombocytopenia. These hematologic changes were accompanied by prolonged activated partial thromboplastin time, consumption of factor VIII, and increased D-dimer levels—a pattern indicative of disseminated intravascular coagulation [119].
Another study evaluated the digestive tract pathology in OI mice with the T. cruzi Berenice-78 (Be-78) strain, compared to mice infected via the intraperitoneal route. Both groups exhibited a similar peak in parasitemia, but OI mice had a higher mortality rate than IP-infected mice. When examining immune cell populations, the OI group showed a prolonged presence of CD4+ T-lymphocytes (lasting longer than in the IP group). Histopathologically, OI mice had greater parasite loads and inflammatory infiltrates in the stomach, duodenum, and colon at 28 days post-infection. These data suggest that OI induces a different profile of parasitological and immune responses compared to IP infection, with oral infection being more virulent and producing greater tissue parasitism in gastric organs during acute T. cruzi infection [120].
When BALB/c mice were infected with the Colombian strain (TcI) via different routes (IP, OI, GI, ocular, and cutaneous), and using either culture-derived MT or BT, distinct patterns were observed [20]. Parasitemia remained intermittent and low in mice inoculated with MTs (for all routes), whereas mice inoculated with BTs developed high parasitemia. A muscle tropism was noted in oral or GI infections with BT forms, whereas MT infections led to a wider distribution of the parasite in various tissues. Interestingly, tissue inflammation was more intense in oral or GI infections with BT. Mice inoculated with BT via the GI route had a similar IFN-γ level, but lower IL-10 levels compared to mice infected with MTs via GI. Additionally, TNF-α levels were higher in BT-infected mice, which could explain the more severe heart inflammation seen in those animals [20].
It has also been reported that in mice, oral T. cruzi infection leads to diffuse parasitism in bone marrow cells, targeting perivascular and intravascular regions and areas close to bone. Extramedullary hematopoiesis observed in the spleen during oral infection may be a compensatory mechanism to maintain blood cell production during the acute phase of T. cruzi infection [121].
The basis of T. cruzi virulence via the oral route has been investigated using a particularly virulent isolate. One study analyzed a T. cruzi isolate called “SC” obtained from a patient with severe acute Chagas disease acquired orally, using a mouse model. It was observed that when mice were inoculated orally with the metacyclic forms of the SC isolate (which express high levels of the surface glycoprotein gp90), the mice developed high parasitemia and high mortality, despite the SC isolate showing reduced infectivity in vitro [122]. Upon recovering parasites from the mouse stomach 1 h after oral inoculation, the gp90 molecule on the parasites was found to be completely degraded, and the parasite’s ability to invade cultured cells (HeLa and Caco-2) was markedly increased [122]. In contrast, the parasite’s gp82 surface molecule was more resistant to digestive enzymes in gastric juice, and the presence of gastric mucin further enhanced host cell invasion by the MT of the SC strain. Notably, reference strains CL and G, which express gp90 isoforms that are resistant to gastric degradation, did not show changes in infectivity after exposure to gastric juice. Taken together, these findings suggest that an exacerbation of T. cruzi infectivity after interaction with host stomach components may underlie the severity of acute Chagas disease in human oral outbreaks [122]. In the case of the SC isolate, the loss of gp90, due to its digestion, presumably allowed the parasites to invade host tissues more effectively, contributing to severe disease. The main characteristics of the experimental murine infection, depending on whether it occurs orally or gastric, are shown in Figure 4.
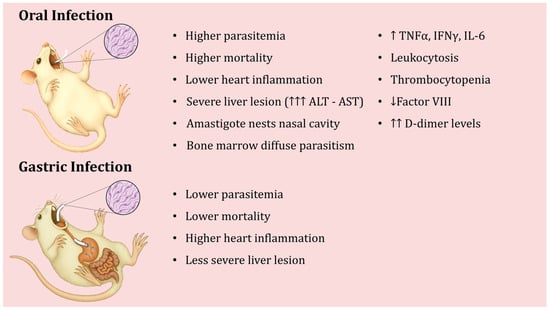
Figure 4.
Differences in the course of experimental oral infection with Trypanosoma cruzi depending on whether inoculation is oral or gastric.
6. Animals as Reservoirs and Spreading Factors
Mathematical models have shown that oral transmission among domestic animals can substantially contribute to the spread of Chagas disease [123]. Oral transmission provides an alternative route to vector bites for infecting domestic mammals, which are key reservoir hosts in the infection cycle. This means that even if triatomine bugs do not frequently bite certain domestic animals, those animals might still get infected (and subsequently infect vectors) by orally ingesting insects or contaminated food. Such a dynamic can lead to high infection rates in domestic mammals and ultimately maintain high infection pressure in humans [122].
In nature, over 180 species across 25 mammalian families have been described as potential reservoirs of T. cruzi [9]. In documented oral transmission outbreaks, about 70% of implicated reservoir hosts are various species of marsupials didelphid (opossums), such as Didelphis marsupialis, D. aurita, and D. albiventris, as well as other opossum-related species like Marmosa spp., Monodelphis domestica, and Caluromys lanatus. These animals are often synanthropic and can act as both reservoirs and, indirectly, vectors (because predators or scavengers may ingest them). The next most common reservoirs, each accounting for ~10% in those outbreaks, are dogs (Canis lupus familiaris), black rats (Rattus rattus), and certain wild rodents such as Trichomys laurentius (São Lourenço punaré) and Cavia aperea (Brazilian guinea pig). Dogs, in particular, were identified as domestic reservoir hosts as early as 1969 [44]. Dogs not only serve as a blood source for triatomines but can also ingest insects or food contaminated with triatomine feces, thus participating in both vectorial and oral transmission routes. Because dogs live in such close contact with people, they are excellent sentinels for parasite circulation in domestic environments [124]. In urban settings, rats are often the main urban reservoir of T. cruzi, followed by dogs and cats, in maintaining a peridomestic cycle [125]. Other naturally infected reservoirs include various wild rodents. In Venezuela, for example, species like Dasyprocta agouti (agouti) and Trichomys apereoides have been found infected [125]. In Brazil and other regions, wild rodents such as Echimys dasythrix and species of Akodon have also been noted in the wild cycle [126].
In Chile, the epidemiological scenario appears slightly different due to the absence or low presence of some typical vector species in domestic settings. However, very high infection rates have been observed in wild rodents there [126]. This could be explained by oral transmission in the wild: rodents might be ingesting infected triatomines (Mepraia species) [127] or food contaminated with triatomine feces. Mepraia spinolai (Reduviidae, Triatominae), a wild triatomine in Chile, has infection rates ranging from ~40% up to 76% in some studies [128,129]. PCR surveys have found T. cruzi DNA in 25–41% of tested individuals of the wild rodent Phyllotis darwini, 13–70% of Octodon degus (degu rodents), and ~27% of Rattus rattus in certain sylvatic areas of Chile [130]. Separately, a study in rural Chilean homes found 83.1% of captured rodents (commensal rodents near houses) were infected; among these, P. darwini had 100% infection, R. rattus 83.6%, Mus musculus 83.3%, and O. degus 50% [127]. There is even evidence of T. cruzi infecting reptiles; for instance, in one study from Chile, T. cruzi DNA was detected in 11 of 13 Microlophus (lizard) blood samples and in all tested individuals of certain lizard species (18 Liolaemus platei “Plate’s lizards”, 3 Liolaemus nigroviridis “Dark lizards”, and 10 Homonota penai “marked geckos”) [131]. Such findings lead to the hypothesis that carnivorous or omnivorous animals (like cats or pigs) could become infected by eating smaller infected animals (e.g., rodents or lizards). For example, cats or feral pigs might prey on or scavenge infected rodents, thus acquiring T. cruzi orally.
In Chile’s central region, PCR-based studies have shown T. cruzi infection in 17–57% of dogs [130,132,133], and about 11% of dogs in the north were seropositive [133]. In central Chile, Mepraia spinolai is the prevalent vector, but this insect has a long defecation delay after biting (on average ~8 min) [134], meaning it often defecates after leaving the host, reducing the efficiency of classical transmission. This raises the question: how are so many dogs and wild rodents infected in those areas? A plausible explanation is oral transmission—for instance, rodents (or other animals) might ingest insects or food contaminated with M. spinolai feces. This would support a significant role for the oral route of infection in such rural or semi-wild environments, even in countries like Chile where no human oral cases have been officially reported yet.
7. What We Know About T. cruzi DTUs and Mechanisms Involved in Oral Infections
In northern South America and the Amazon region, where most oral Chagas outbreaks have occurred, the predominant T. cruzi genetic group is DTU TcI [135,136,137]. TcI was the genotype identified in parasites isolated from patients in orally transmitted outbreaks in Venezuela and Colombia [50,126]. TcI also predominates in Mexico [138,139]. T. cruzi strains used in oral infection research to date have included isolates from Guatemala, Venezuela, and various regions of Brazil [82,122,140,141]. Interestingly, two orally transmitted outbreaks in northern Brazil involved T. cruzi strains of DTUs TcI and TcIV [82].
According to Barbosa et al. [142], metacyclic forms of Mexican T. cruzi strains (TcI) are poorly infectious by the oral route and invade cultured cells inefficiently. Consistently, those Mexican TcI strains caused either undetectable or very low parasitemia when mice were inoculated orally. This outcome is similar to what has been observed with other Latin American TcI strains in oral models [82]. It contrasts with findings for some TcII and TcVI strains, which have produced patent parasitemia in mice and efficiently invaded host cells in vitro via the oral route [117,141,143,144]. Despite the genetic diversity of T. cruzi, a common theme from these studies is the reduced oral infectivity of TcI (and, to a lesser extent, TcIV) strains. In other words, MT of TcI parasites seem less capable of migrating through the gastric mucus, invading target epithelial cells in the stomach, and establishing infection, compared to strains from DTUs like TcII or TcVI [117,141,143,145].
Furthermore, a study by Lewis et al. [145] using highly sensitive bioluminescence imaging and quantitative histopathology shed light on oral infections in mice with another perspective. They found that both MT and BT could establish infection via the oral cavity, but only MT forms led to consistent infections when delivered directly to the stomach by gavage. In their experiments, they used MT and BT forms from the CL Brener strain (TcVI), and noted that while both forms were orally infective, the metacyclic forms were more efficient at establishing infection through the gastric route.
Most existing knowledge about oral T. cruzi infection mechanisms comes from studies on MT [146]. In vitro-generated MT forms (analogous to the stage transmitted by insect vectors) have been studied to identify factors important for oral infection. One key surface molecule is gp82, which plays an essential role in host cell invasion and the establishment of infection via the oral route. Gp82 has the ability to bind gastric mucin and induce host cell signaling cascades that result in actin cytoskeleton rearrangement and lysosome exocytosis events that facilitate parasite internalization [147]. Gp82 is also relatively resistant to pepsin digestion at acidic pH, allowing it to retain its function in the stomach environment. Conversely, another surface molecule, gp90, is a stage-specific glycoprotein that negatively T. cruzi strains expressing high levels of gp90 typically invade host cells poorly in vitro. However, their infectivity by the oral route can vary because different gp90 isoforms have different susceptibilities to pepsin. Parasites with gp90 that are easily degraded by gastric juice become highly invasive once that inhibition is lifted [140]. Parasites expressing gp90 isoforms resistant to digestion do not gain such an advantage in the stomach [140].
An example of DTU-related differences involves a bat-associated strain. MTs from the BAT strain (a strain isolated from bats) were shown to migrate through an artificial gastric mucin layer and infect HeLa cells in vitro. However, MTs of the BAT strain were less effective than those of CL or G strains at infecting mice via oral or IP routes [148]. Meanwhile, multiple outbreaks of acute orally acquired Chagas in the Brazilian Amazon have been linked to TcIV strains, which appear quite infective by the oral route in mice. In one comparative study, four TcIV strains from Amazonian outbreaks were tested in mice: orally inoculated mice had higher peak parasitemia, higher positivity rates by PCR, and higher parasite loads in tissues than mice inoculated with the same strains via IP injection. This indicates a higher intensity of infection when these strains are acquired orally (perhaps because the oral route targets different initial tissues or evades some immune responses) [144].
While the molecular basis of MT interactions with the gastric environment is fairly well characterized [146], much less is known about BT in oral infection. Earlier studies suggested that BTs are inefficient at causing infection via the oral route in mice [149] and some even proposed that carnivory might not be a significant transmission mode [150]. Nevertheless, many observations, old and new, suggest that carnivory (predator–prey transmission) can play a central role in T. cruzi spread, especially in wild cycles. Predators consuming infected prey could become infected through their mucosal membranes while chewing or digesting the meat [45,55]. Only recently have there been experimental data to directly support this route. In a recent paper, Torres et al. [151] have reported that consuming meat from T. cruzi-infected animals in the acute phase can indeed transmit the infection to other animals, providing the first direct experimental evidence that carnivory is a viable transmission mechanism for Chagas disease. In these experiments, trypomastigotes and even intracellular amastigotes from a TcI T. cruzi clone (H510 C8C3) were capable of infecting mice when administered orally (either by feeding infected tissue to mice or by gavage) [151]. In all cases, 60–100% of mice that consumed meat from acutely infected donors (or were fed trypomastigote/amastigote suspensions) developed high parasitemia, and up to 80% succumbed to infection [151]. These observations also shed light on why bloodstream forms are less infective orally: according to these authors, trypomastigote motility in the presence of gastric mucin was reduced by ~30%. When both mucin and pepsin (at pH 3.5) were present (simulating stomach conditions), only about 6% of trypomastigotes managed to migrate across a filter in vitro. Similarly, the ability of TCT to infect human gastric epithelial cells (AGS cell line) in the presence of mucin was reduced by ~20%. The available evidence suggests that MTs belonging to the DTU TcI would be less infectious than other DTUs. However, this is the first reported evidence of oral infectivity of TCT from this DTU and therefore raises some questions. Are TcI TCT more infective than their MT counterparts? Are there differences in the infectivity of different strains belonging to TcI? Only experimental trials can answer this question, but it has been suggested that TcI has shown great genetic diversity, indicating plausible subdivisions needed for this group [152]. This report also identified potential parasite proteins involved in overcoming these barriers: an in vitro adhesion assay showed that cruzipain (Czp), a major cysteine protease of T. cruzi, can bind to AGS cells. Additionally, LC-MS/MS proteomic analysis suggested that T. cruzi trans-sialidase (TS), other cysteine proteinases (CPs), and gp63 metalloprotease could all interact with proteins of the human stomach mucosa, implicating them in attachment or invasion processes. Notably, several human gastric mucins have cleavage sites for cysteine proteases like cruzipain [151]. All these data allowed us to propose a model of interaction between orally introduced T. cruzi (especially TCT) and the gastric mucosa, and compare this with what we know about the interaction of MTs with the gastric mucosa. A model of trypomastigote interaction with the gastric mucosa is shown in Figure 5.
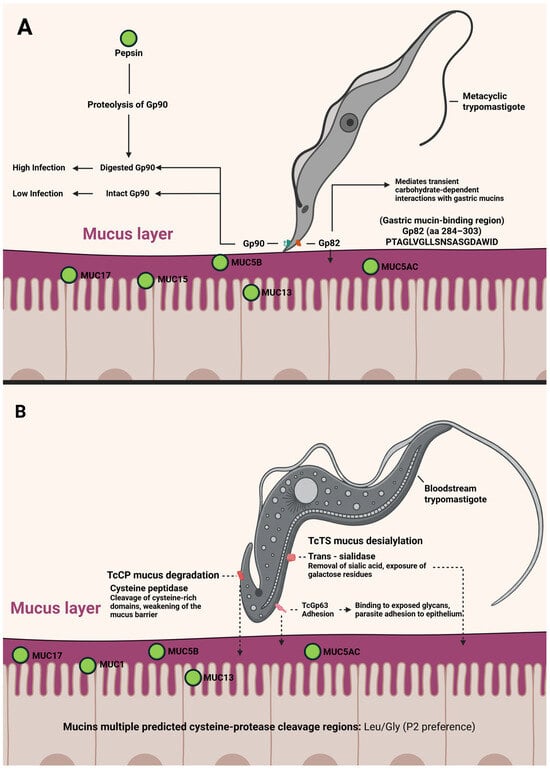
Figure 5.
A model of metacyclic trypomastigote (A) and tissue culture derived trypomastigote or blood trypomastigote (B) interaction with the mammalian gastric mucosa. In all cases, the solid line corresponds to experimentally verified events, while the dotted line corresponds to events based on indirect evidence or bioinformatic analysis. (Created in https://BioRender.com).
In summary, consuming meat from animals infected in the acute phase can transmit T. cruzi infection orally. However, it remains to be investigated whether oral transmission is possible from animals in the indeterminate or chronic phase of infection. It is conceivable that in later stages, the success of oral transmission would depend on the parasite load in specific organs/tissues and on the parasite’s genotype (DTU), which might determine its ability to resist stomach acidity, penetrate mucus, and invade gastric cells. Thus, it is reasonable to hypothesize that the particular T. cruzi DTUs involved in food contamination could be critical determinants of oral transmission success. Indeed, earlier studies by Hoft et al. [149] noted poor oral infectivity of blood trypomastigotes from the Tulahuén strain (TcVI), and Cortéz et al. [143] observed that trypomastigotes of the Y strain (TcII) were also poorly infective orally. It is also likely that the intrinsic virulence of each strain or isolate (for example, higher expression of virulence factors such as cruzipain, trans-sialidases, and gp63) plays a role [153]. Furthermore, recent research suggests that the upregulation of certain metabolic and biosynthetic pathways in the parasite might contribute to virulence [154]. Finally, the genetic background of the host is another important factor. A parasite strain highly infective in one host species might be much less so in another species.
It is also necessary to consider that the infectious inocula used in laboratory models may differ from those that a host might receive in nature. For example, in food contaminated by the presence of a complete triatomine, all existing trypomastigotes are present in its intestinal contents, which in T. infestans can reach 684,000 trypomastigotes [155], theoretically enough to infect more than a hundred people. However, in the fecal matter of a triatomine, it is estimated to be between 3000 and 4000 metacyclic trypomastigotes per microliter of feces [155]. Although there is insufficient information regarding the parasitemia that wild animals could develop, one report mentions high parasite levels in wild pigs [156]. Studies with goats experimentally infected with T. cruzi showed peak parasitemia levels of 1.43 × 106 parasites by mL−1 on day 12 post-infection. However, the parasites were not detected 22 days post-infection [157]. Nevertheless, some of these animals tested positive in xenodiagnosis seven months after inoculation. Similarly, goats naturally infected with T. cruzi have shown low levels of parasitemia, suggestive of chronic forms of T. cruzi infection [157].
In summary, the findings reported by Torres et al. [151], although interesting, should be taken with caution, as the conditions of the experimental models may not always reflect what actually occurs in nature. On the one hand, genetic background could be a determining factor. Pioneering work by Pizzi showed that not all mouse strains have the same susceptibility to T.cruzi infection [158]. Therefore, there could be species in nature that are refractory to infection or that, even in the acute phase, have low parasitemia. It is known that some strains of T. cruzi such as G [159] or C8C3lvir [154] in laboratory models give subpatent parasitemia. On the other hand, studies in wild animals have shown seropositivity for T. cruzi in the presence of negative blood culture and PCR results. These cryptic infections are indicative of a subclinical infection at the time of blood collection. However, given that the transmission systems of multi-host parasites are dynamic over time, animals without parasitemia could present high parasitemia during periods of transient immunosuppression due to malnutrition, concurrent infections, pregnancy, and lactation [160].
8. Concluding Remarks and Future Perspectives
Acute Chagas disease resulting from the consumption of food or meat contaminated with T. cruzi from infected animals has emerged as the primary mode of transmission in areas of Latin America where wild or peridomestic triatomine vectors persist. This epidemiological shift presents new challenges in multiple domains.
Implementation of Control and Prevention Strategies: The encroachment of triatomine vectors and T. cruzi reservoir hosts into urban and periurban areas increases the risk of oral transmission. Control strategies must therefore adapt to these changing conditions. This includes not only traditional vector control measures (insecticide spraying and housing improvements to exclude insect vectors) but also enhanced food safety interventions. Reducing poverty and improving housing in rural and peripheral urban regions will decrease human contact with vectors. Education is crucial: communities must be informed about the risk of consuming food contaminated with triatomine feces or infected meat. Only when people recognize these risks will they adopt proper food hygiene practices. In most Brazilian states and countries where oral transmission has been reported, with the exception of açaí treatment, these prevention programs do not exist.
For widely distributed commercial products like açaí pulp, routine screening for T. cruzi contamination (for example, by PCR) should be considered. Tourists visiting endemic areas should also be cautioned against consuming uncooked foods or beverages (such as fresh palm juice) that might be contaminated. Such public health messaging, including school-based programs, could help prevent future outbreaks. Therefore, safe food production programs must be urgently implemented, aligned with improved diagnostic and therapeutic tools to combat foodborne Chagas [55].
Diagnosis and Treatment of Orally Acquired Chagas Disease: Outbreaks of oral T. cruzi transmission often involve many patients with fulminant acute disease, which can overwhelm local health services. Early diagnosis and treatment are vital, as the prognosis improves significantly with prompt antiparasitic therapy during the acute infection.
Medical personnel in endemic and at-risk areas must be trained to recognize the signs of acute Chagas disease, especially in scenarios suggestive of foodborne transmission (for example, multiple patients presenting with fever and edema after sharing a common meal).
Strengthening laboratory diagnostic capacity for acute Chagas (serology, PCR, microscopy) is also important. Furthermore, continuous medical education programs should incorporate information on orally transmitted Chagas disease so that physicians, laboratory technicians, and public health workers can respond quickly and effectively when an outbreak is suspected.
Advancing Knowledge of T. cruzi Biology in Oral Transmission: Despite recent progress, there remain many unknowns about how T. cruzi establishes infection via the oral route. Key questions include whether the success of oral infection depends on the parasite’s life stage or genotype. Our experimental evidence showed that a clone of T. cruzi (TcI, clone C8C3hvir) was able to infect BALB/c mice via oral inoculation (either by gavage or by feeding the mice infected meat). However, it is not yet known whether all T. cruzi discrete typing units (DTUs) are equally capable of oral infectivity, or if some strains are naturally attenuated in their ability to establish infection via this route.
It is also unclear whether meat from animals in the chronic or indeterminate phase of infection (which carry lower parasite loads) can cause oral infections notably; all documented oral Chagas outbreaks in humans have been traced to acutely infected sources (e.g., freshly infected game animals or heavily infected vector droppings). Similarly, it remains unknown whether all common laboratory animals (and by extension, all potential reservoir host species) are equally susceptible to oral infection by all T. cruzi strains, or whether certain host–parasite combinations are less permissive to infection. Another critical area of inquiry is the mechanism of mucosal traversal: what parasite factors enable T. cruzi to survive and penetrate the host’s gastric environment?
Oral transmission of Chagas disease has thus shifted from being considered a rare or unusual event to being recognized as a significant epidemiological route, especially in certain regions. Addressing this form of transmission will require interdisciplinary efforts—from improving food safety and public awareness to deepening our scientific understanding of how T. cruzi behaves in the host digestive system. Future research and public health initiatives must confront these challenges in order to reduce the incidence of orally acquired Chagas disease.
Author Contributions
Conceptualization, J.G., S.Z. and K.M.; methodology, B.G., J.S.F., A.A. and J.L.V.; software, K.M.; investigation, A.A., S.Z., K.M., J.S.F., B.G. and J.L.V.; writing—original draft preparation, J.G.; writing—review and editing, J.G., S.Z. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Abril, M.; Abad, F.; Altcheh, J.; Angeleri, P.; Batista, C.; Albajar, P.; Bassani, R.; Bern, C.; Espinal, C.; Chuit, R.; et al. Enfermedad de Chagas en las Américas: Una revisión de la situación actual de salud pública y su visión para el futuro; Organización Mundial de La Salud: Geneva, Switzerland, 2018. [Google Scholar]
- Beatty, N.L.; Hamer, G.L.; Moreno-Peniche, B.; Mayes, B.; Hamer, S.A. Chagas disease, an endemic disease in the United States. Emerg. Infect. Dis. 2025, 31, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R.; Vĩas, P.A. Chagas disease: A new worldwide challenge. Nature 2010, 465, S6–S7. [Google Scholar] [CrossRef]
- Bern, C. Chagas’ disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef]
- Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 29 May 2025).
- Tyler, K.M.; Engman, D.M. The life cycle of Trypanosoma cruzi revisited. Int. J. Parasitol. 2011, 31, 472–481. [Google Scholar] [CrossRef]
- Tomasina, R.; González, F.C.; Cabrera, A.; Basmadjián, Y.; Robello, C. From Trypomastigotes to Trypomastigotes: Analyzing the one-way intracellular journey of Trypanosoma cruzi by ultrastructure expansion microscopy. Pathogens 2024, 13, 866. [Google Scholar] [CrossRef]
- Coura, J.R. The main sceneries of chagas disease transmission. The vectors, blood and oral transmissions—A comprehensive review. Mem. Inst. Oswaldo Cruz 2015, 110, 277–282. [Google Scholar] [CrossRef]
- de Noya, B.A.; González, O.N. An ecological overview on the factors that drives to Trypanosoma cruzi oral transmission. Acta Trop. 2015, 151, 95–102. [Google Scholar] [CrossRef]
- Coura, J.R. Transmission of chagasic infection by oral route in the natural history of Chagas disease. Rev. Soc. Bras. Med. Trop. 2006, 39, 113–117. [Google Scholar]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral Transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Bruneto, E.G.; Fernandes-Silva, M.M.; Toledo-Cornell, C.; Martins, S.; Ferreira, J.M.B.; Corrêa, V.R.; da Costa, J.M.; Pinto, A.Y.D.N.; de Sousa, D.S.M.; Pinto, M.C.G.; et al. Case-fatality from orally transmitted acute Chagas disease: A systematic review and meta-analysis. Clin. Infect. Dis. 2021, 72, 1084–1092. [Google Scholar] [CrossRef]
- Doença de Chagas Aguda—Casos Confirmados Notificados no Sistema de Informação de Agravos de Notificação-Brasil. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/chagasbr.def (accessed on 29 May 2025).
- Ano. Secretaria de Vigilância em Saúde. Ministério da Saúde Doença de Chaga, n.d. Available online: https://www.saude.gov.br/saude-de-a-z/d/doenca-de-chagas (accessed on 25 December 2025).
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci. Data 2022, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.M.; Miller, S.M.; Evora, P.R.B. The chronic gastrointestinal manifestations of Chagas disease. Clinics 2009, 64, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- de Noya, B.A.; Díaz-Bello, Z.; Colmenares, C.; Ruiz-Guevara, R.; Mauriello, L.; Muñoz-Calderón, A.; Noya, O. Update on oral Chagas disease outbreaks in Venezuela: Epidemiological, clinical and diagnostic approaches. Mem. Inst. Oswaldo Cruz 2015, 110, 377–386. [Google Scholar] [CrossRef]
- Benítez, J.A.; Araujo, B.; Contreras, K.; Rivas, M.; Ramírez, P.; Guerra, W.; Calderon, N.; Terren, C.A.; Barrera, R.; Rodriguez-Morales, A.J. Urban outbreak of acute orally acquired Chagas disease in Táchira, Venezuela. J. Infect. Dev. Ctries. 2013, 7, 638–641. [Google Scholar] [CrossRef]
- Gonçalves, K.R.; Mazzeti, A.L.; da Silva Nascimento, A.F.; Castro–Lacerda, J.M.; Nogueira-Paiva, N.C.; Mathias, F.A.S.; Reis, A.B.; Caldas, S.; Bahia, M.T. The entrance route: Oral, mucous, cutaneous, or systemic has a marked influence on the outcome of Trypanosoma cruzi experimental infection. Acta Trop. 2022, 234, 106581. [Google Scholar] [CrossRef]
- Barreto-de-Albuquerque, J.; Silva-dos-Santos, D.; Pérez, A.R.; Berbert, L.R.; de Santana-van-Vliet, E.; Farias-de-Oliveira, D.A.; Moreira, O.T.; Roggero, E.; Carvalho-Pinto, C.A.; Jurberg, J.; et al. Trypanosoma cruzi infection through the oral route promotes a severe infection in mice: New disease form from an old infection? PLoS Negl. Trop. Dis. 2015, 9, e0003849. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.R.; Gibson, W.C. The evolution of pathogenic trypanosomes. Cad. Saúde Pública. 1999, 15, 673–684. [Google Scholar] [CrossRef]
- Guhl, F.; Auderheide, A.; Ramírez, J.D. From ancient to contemporary molecular eco-epidemiology of Chagas disease in the Americas. Int. J. Parasitol. 2014, 44, 605–612. [Google Scholar] [CrossRef]
- Briones, M.R.S.; Souto, R.P.; Stolf, B.S.; Zingales, B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol. Biochem. Parasitol. 1999, 104, 219–232. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Teixeira, M.M.G.; Stevens, J.R. The evolution of Trypanosoma cruzi: The “bat seeding” hypothesis. Trends Parasitol. 2012, 28, 136–141. [Google Scholar] [CrossRef]
- Villena, F.E.; Gomez-Puerta, L.A.; Jhonston, E.J.; Del Alcazar, O.M.; Maguiña, J.L.; Albujar, C.; Laguna-Torres, V.A.; Recuenco, S.E.; Ballard, S.B.; Ampuero, J.S. First report of Trypanosoma cruzi infection in salivary gland of bats from the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2018, 99, 723–728. [Google Scholar] [CrossRef]
- Henry, J.P. Genetics and origin of Homo sapiens. Medecine/Sciences 2019, 35, 39–45. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Britto, C.; Cardoso, M.A.; Fernandes, O.; Reinhard, K.; Araújo, A. Paleoparasitology of Chagas disease revaled by infected tissues from Chilean mummies. Acta Trop. 2000, 75, 79–84. [Google Scholar] [CrossRef]
- Araújo, A.; Jansen, A.M.; Reinhard, K.; Ferreira, L.F. Paleoparasitology of Chagas disease—A review. Mem. Inst. Oswaldo Cruz 2009, 104, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, K.J.; Fink, T.M.; Skiles, J. A case of megacolon in Rio Grande Valley as a possible case of Chagas disease. Mem. Inst. Oswaldo Cruz 2003, 98, 165–172. [Google Scholar] [CrossRef]
- Rothhammer, F.; Allison, M.J.; Núñez, L.; Standen, V.; Arriaza, B. Chagas’ disease in pre-Columbian South America. Am. J. Phys. Anthropol. 1985, 68, 495–498. [Google Scholar] [CrossRef]
- Aufderheide, A.C.; Salo, W.; Madden, M.; Streitz, J.; Buikstra, J.; Guhl, F.; Arriaza, B.; Renier, C.; Wittmers, L.E., Jr.; Fornaciari, G.; et al. A 9,000-year record of Chagas’ disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.S.; Iniguez, A.M.; Otsuki, K.; Ferreira, L.F.; Araújo, A.; Vicente, A.C.P.; Jansen, A.M. Chagas disease in ancient Hunter-Gatherer population, Brazil. Emerg. Infect. Dis. 2008, 14, 1001–1002. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Iñiguez, A.M.; Lima, V.S.; Mendonça De Souza, S.M.F.; Ferreira, L.F.; Vicente, A.C.P.; Jansen, A.M. Pre-Columbian Chagas disease in Brazil: Trypanosoma cruzi I in the archaeological remains of a human in Peruaçu Valley, Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 2008, 103, 514–516. [Google Scholar] [CrossRef]
- Reinhard, K.J. Archaeoparasitology in North America. Am. J. Phys. Anthropol. 1990, 82, 145–163. [Google Scholar] [CrossRef]
- Reinhard, K.J.; Geib, P.R.; Callahan, M.M.; Hevly, R.H. Discovery of colon contents in a skeletonized burial: Soil sampling for dietary remains. J. Archaeol. Sci. 1992, 19, 697–705. [Google Scholar] [CrossRef]
- Coimbra, C.E.A. Human settlements, demographic pattern, and epidemiology in lowland Amazonia: The case of Chagas’s disease. Am. Anthropol. 1988, 90, 82–97. [Google Scholar] [CrossRef]
- Reperant, L.A. Putative 14th century outbreak of foodborne Chagas disease, Mexico. Vector-Borne Zoonotic Dis. 2023, 23, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Brumpt, E. Precis de parasitology. J. Parasitol. 1928, 14, 277. [Google Scholar] [CrossRef]
- Mayer, H. Infeccion experimental con Trypanosoma cruzi por via digestiva. An. Inst. Med. Reg. 1961, 5, 43–48. [Google Scholar]
- Da Silva, N.N.; Clausell, D.T.; Nólibos, H.; De Mello, A.L.; Ossanai, J.; Rapone, T.; Snell, T. Epidemic outbreak of Chagas disease probably due to oral contamination. Rev. Inst. Med. Trop. Sao Paulo 1968, 10, 265–276. [Google Scholar]
- Yaeger, R.G. Transmission of Trypanosoma cruzi infection to opossums via the oral route. J. Parasitol. 1971, 57, 1375–1376. [Google Scholar] [CrossRef]
- Barretto, M.P.; Ribeiro, R.D.; Belda Neto, F.M. Reservoirs and wild vectors of Trypanosoma cruzi: Infection of mammals by oral route. Rev. Bras. Biol. 1978, 38, 455–459. [Google Scholar]
- López-García, A.; Gilabert, J.A. Oral transmission of Chagas disease from a One Health approach: A systematic review. Trop. Med. Int. Health 2023, 28, 689–698. [Google Scholar] [CrossRef]
- Coura, J.R.; Junqueira, A.C.V.; Fernandes, O.; Valente, S.A.S.; Miles, M.A. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 2002, 18, 171–176. [Google Scholar] [CrossRef]
- Sánchez, L.V.; Ramírez, J.D. Congenital and oral transmission of American trypanosomiasis: An overview of physiopathogenic aspects. Parasitology 2013, 140, 147–159. [Google Scholar] [CrossRef]
- Alarcón de Noya, B.; Colmenares, C.; Díaz-Bello, Z.; Ruiz-Guevara, R.; Medina, K.; Muñoz-Calderón, A.; Mauriello, L.; Cabrera, E.; Montiel, L.; Losada, S. Orally-transmitted Chagas disease: Epidemiological, clinical, serological and molecular outcomes of a school microepidemic in Chichiriviche de la Costa, Venezuela. Parasite Epidemiol. Control. 2016, 1, 188–198. [Google Scholar] [CrossRef]
- Benchimol Barbosa, P.R. The oral transmission of Chagas’ disease: An acute form of infection responsible for regional outbreaks. Int. J. Cardiol. 2006, 112, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.P.; Bastos, C.; Araújo, E.; Mascarenhas, A.V.; Netto, E.M.; Grassi, F.; Silva, M.; Tatto, E.; Mendoça, L.; Araujo, R.F.; et al. Acute Chagas disease outbreak associated with oral transmission. Rev. Soc. Bras. Med. Trop. 2008, 41, 296–300. [Google Scholar] [CrossRef]
- Ramírez, J.D.; Montilla, M.; Cucunubá, Z.M.; Floréz, A.C.; Zambrano, P.; Guhl, F. Molecular epidemiology of human oral Chagas disease outbreaks in Colombia. PLoS Negl. Trop. Dis. 2013, 7, e2041. [Google Scholar] [CrossRef]
- Blanchet, D.; Brenière, S.F.; Schijman, A.G.; Bisio, M.; Simon, S.; Véron, V.; Mayence, C.; Demar-Pierre, M.; Djossou, F.; Aznar, C. First report of a family outbreak of Chagas disease in French Guiana and posttreatment follow-up. Infect. Gen. Evol. 2014, 28, 245–250. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Martinho, P.C.; Neto, V.A.; Cruz, R.R.B. Pasteurization of human milk to prevent transmission of Chagas disease. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.S.; Schmidt, F.L.; Guaraldo, A.M.A.; Franco, R.M.B.; Dias, V.L.; Passos, L.A.C. Chagas’ disease as a foodborne illness. J. Food Prot. 2009, 72, 441–446. [Google Scholar] [CrossRef]
- Barbosa-Ferreira, J.M.; Guerra, J.A.D.O.; De Santana Filho, F.S.; Magalhães, B.M.L.; Coelho, L.I.A.R.C.; Barbosa, M.D.G.V. Cardiac involvement in acute Chagas’ disease cases in the Amazon region. Arq. Bras. Cardiol. 2010, 94, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Simões-Neto, E.A.; Santos, D.W.d.C.L.; Bomfim, M.R.Q.; Costa, J.M.L.; Simões, A.F.; Vasconcelos, L.D.; Sodré, D.C.; Costa, A.C.M.; Dumont, S.V.R.; de Melo, B.O.; et al. Oral Chagas disease outbreak by bacaba juice ingestion: A century after Carlos Chagas’ discovery, the disease is still hard to manage. PLoS Negl. Trop. Dis. 2024, 18, e0012225. [Google Scholar] [CrossRef]
- Santalla Vargas, J.; Carrasco, P.O.; Espinoza, E.; Rios, T.; Brutus, L. First reported outbreak of Chagas disease in the Bolivian Amazonean zone: A report of 14 cases of oral transmission of acute Trypanosoma cruzi in Guayaramerín, Beni-Bolivia. Biofarbo 2011, 19, 52–58. [Google Scholar]
- Neves da Silva, N.; Clausell, D.T.; Nólib, H.; Leite de Mello, A.; Üssanai, J.; Rapone, T.; Snell, T. Surto epidêmico de doença de Chagas com provável contaminação oral. Rev. Inst. Med. Trop. Sao Paulo 1968, 10, 265–276. [Google Scholar]
- Belém, E.M.; Shaw, J.; Lainson, R. Considerações sôbre a epidemiología dos primeiros casos autóctones de doença de Chagas registrados em Belém, Pará, Brasil. Rev. Saude Publica 1969, 3, 153–157. [Google Scholar]
- Rodrigues, I.D.C.; Souza, A.A.D.; Terceros, R.; Valente, S. Doença de Chagas na amazónia: I. Registro de oito casos autóctones em macapá. Rev. Soc. Bras. Med. Trop. 1988, 21, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Shikanai-Yasuda, M.A.; Marcondes, C.B.; Guedes, L.A.; Siqueira, G.S.; Barone, A.A.; Dias, J.C.; Neto, V.A.; Tolezano, J.E.; Peres, B.A.; Arrauda, E.R., Jr.; et al. Possible oral transmission of acute Chagas disease in Brazil. Rev. Inst. Med. Trop. São Paulo 1991, 33, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Viana, S.; Farias, E.; Lima, F.; Batista, L.; Vieira, A.; Silva, L.; Lobato, C.; Nascimento, S.; Chalub, S. Doença de Chagas no Estado do Acre, registro de 3 casos de miocardiopatia chagásica aguda autóctone no município de Rio Branco, 1993. Rev. Soc. Bras. Med. Trop. 1994, 27, 77. [Google Scholar]
- Valente, S.A.d.S.; da Costa Valente, V.; das Neves Pinto, A.Y.; de Jesus Barbosa César, M.; dos Santos, M.P.; Miranda, C.O.S.; Cuervo, P.; Fernandes, O. Analysis of an acute Chagas disease outbreak in the Brazilian Amazon: Human cases, triatomines, reservoir mammals and parasites. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 291–297. [Google Scholar] [CrossRef]
- Pinto, A.Y.; Harada, G.S.; Valente Vd Abud, J.E.; Gomes Fd Souza, G.C.; Valente, S.A. Cardiac attacks in patients with acute Chagas disease in a family micro-outbreak, in Abaetetuba, Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2001, 34, 413–419. [Google Scholar] [CrossRef]
- Pinto, A.Y.d.N.; Valente, S.A.d.S.; Valente, V.d.C. Emerging acute Chagas disease in Amazonian Brazil: Case reports with serious cardiac involvement. Braz. J. Infect. Dis. 2004, 8, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.Y.; Farias, J.R.; Marcal, A.S.; Galucio, A.L.; Costi, R.R.; Valente, V.C.; Silva Valente, S.A. Doença de Chagas Aguda grave autóctone na Amazonia brasileira. Rev. Para. Med. 2007, 21, 7–12. [Google Scholar]
- De Medeiros, M.B.; Guerra, J.A.D.O.; De Lacerda, M.V.G. Meningoencephalitis in a patient with acute Chagas disease in the Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2008, 41, 520–521. [Google Scholar] [CrossRef]
- Pinto, A.Y.D.N.; Valente, S.A.; Valente, V.D.C.; Ferreira, A.G.; Coura, J.R. Acute phase of Chagas disease in the Brazilian Amazon region: Study of 233 cases from Pará, Amapá and Maranhão observed between 1988 and 2005. Rev. Soc. Bras. Med. Trop. 2008, 41, 602–614. [Google Scholar] [CrossRef]
- Borborema, M.; Guerra, J.; Malheiros, R.; Fé, N.; Lacerda, M.; Coelho, L.D. Doença de Chagas urbana em Tefé-AM: Relato de nove casos com suspeita de transmissão oral. Rev. Soc. Bras. Med. Trop. 2005, 38, 491. [Google Scholar]
- Steindel, M.; Kramer Pacheco, L.; Scholl, D.; Soares, M.; de Moraes, M.H.; Eger, I.; Kosmann, C.; Sincero, T.C.M.; Stoco, P.H.; Murta, S.M.F.; et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn. Microbiol. Infect. Dis. 2008, 60, 25–32. [Google Scholar] [CrossRef]
- Nóbrega, A.A.; Garcia, M.H.; Tatto, E.; Obara, M.T.; Costa, E.; Sobel, J.; Aruajo, W.N. Oral transmission of Chagas disease by consumption of açaí palm fruit, Brazil. Emerg. Infect. Dis. 2009, 15, 653–655. [Google Scholar] [CrossRef] [PubMed]
- de Góes Cavalcanti, L.P.; Rolim, D.A.; Pires Neto, J.A.; Feitosa Vilar, D.C.L. Microepidemia de doença de Chagas aguda por transmissão oral no Ceará. Cad. Saúd Colet. 2009, 17, 911–927. [Google Scholar]
- Bastos, C.J.C.; Aras, R.; Mota, G.; Reis, F.; Dias, J.P.; de Jesus, R.S.; Freire, M.S.; Araújo, E.G.; Prazeres, J.; Grassi, M.F.R. Clinical outcomes of thirteen patients with acute Chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Negl Trop. Dis. 2010, 4, e711. [Google Scholar] [CrossRef]
- de Barros Moreira Beltrão, H.; de Paula Cerroni, M.; de Freitas, D.R.C.; Das Neves Pinto, A.Y.; da Costa Valente, V.; Valente, S.A.; Costa, E.G.; Sobel, J. Investigation of two outbreaks of suspected oral transmission of acute Chagas disease in the Amazon region, Para State, Brazil, in 2007. Trop. Dr. 2009, 39, 231–232. [Google Scholar] [CrossRef]
- Monteiro, W.M.; Magalhães, L.K.; Santana Filho, F.S.; Borborema, M.; Silveira, H.; Barbosa, M.D.G.V. Trypanosoma cruzi TcIII/Z3 genotype as agent of an outbreak of Chagas disease in the Brazilian Western Amazonia. Trop. Med. Int. Health 2010, 15, 1049–1051. [Google Scholar] [CrossRef]
- Da Costa Valente, V.; das Neves Pinto, A.Y. Doença de Chagas congênita por infecção aguda maternal por Trypanosoma cruzi transmitida via oral. Rev. Pan-Amaz. Saude 2011, 2, 89–94. [Google Scholar]
- Borba, C.C.; Maués Pereira Moia, J.; de Souza, C.B.; de Barros Barreto, J. Doença de Chagas aguda grave: Relato de caso. Rev. Para. Med. 2010, 24, 39–44. [Google Scholar]
- de Souza-Lima, R.d.C.; Barbosa, M.d.G.V.; Coura, J.R.; Arcanjo, A.R.L.; Nascimento, A.d.S.; Ferreira, J.M.B.B.; Magalhães, L.K.; Alburquerque, B.C.; Araújo, G.A.N.; Guerra, J.A.O. Outbreak of acute Chagas disease associated with oral transmission in the Rio Negro region, Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2013, 46, 510–514. [Google Scholar] [CrossRef]
- Barbosa, M.d.G.V.; Ferreira, J.M.B.B.; Arcanjo, A.R.L.; Santana, R.A.G.; Magalhães, L.K.C.; Magalhães, L.K.C.; Mota, D.T.; Fé, N.F.; Monteiro, W.M.; Silveira, H.; et al. Chagas disease in the State of Amazonas: History, epidemiological evolution, risks of endemicity and future perspectives. Rev. Soc. Bras. Med. Trop. 2015, 48, 27–33. [Google Scholar] [CrossRef]
- de Góes Costa, E.; dos Santos, S.O.; Sojo-Milano, M.; Amador, E.C.C.; Tatto, E.; Souza, D.S.M.; Costa, F.; Póvoa, R.M.S. Acute Chagas Disease in the Brazilian Amazon: Epidemiological and clinical features. Int. J. Cardiol. 2017, 235, 176–178. [Google Scholar] [CrossRef]
- Vargas, A.; Malta, J.M.A.S.; da Costa, V.M.; Cláudio, L.D.G.; Alves, R.V.; Cordeiro, G.D.S.; Aguiar, L.M.A.; Percio, J. Investigation of an outbreak of acute Chagas disease outside the Amazon Region, in Rio Grande do Norte State, Brazil, 2016. Cad. Saude Publica 2018, 34, e00006517. [Google Scholar] [PubMed]
- de Souza, D.d.S.M.; Araujo, M.T.F.; Garcez, P.d.S.; Furtado, J.C.B.; Figueiredo, M.T.S.; Povoa, R.M.S. Anatomopathological aspects of acute Chagas myocarditis by oral transmission. Arq. Bras. Cardiol. 2016, 107, 77–80. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, V.L.T.; Piotto, M.R.; Esper, H.R.; Nakanishi, E.Y.S.; Fonseca, C.d.A.; Assy, J.G.P.L.; Berreta, O.C.P.; França, F.O.S.; Lopes, M.H. Detection of Trypanosoma cruzi DTUs TcI and TcIV in two outbreaks of orally-transmitted Chagas disease in the Northern region of Brazil. Rev. Inst. Med. Trop. Sao Paulo 2023, 65, e7. [Google Scholar] [CrossRef]
- de Sousa, D.R.T.; de Oliveira Guerra, J.A.; Ortiz, J.V.; do Nascimento Couceiro, K.; da Silva e Silva, M.R.H.; Jorge Brandão, A.R.; Guevara, E.; Arcanjo, A.R.L.; de Oliveira Júnior, E.F.; Smith-Doria, S.; et al. Acute Chagas disease associated with ingestion of contaminated food in Brazilian western Amazon. Trop. Med. Int. Health 2023, 28, 541–550. [Google Scholar] [CrossRef]
- de Brito, A.K.S.B.; de Sousa, D.R.T.; da Silva Junior, E.F.; da Silva Ruiz, H.J.; Arcanjo, A.R.L.; Ortiz, J.V.; de Brito, S.S.; Jesus, D.V.; de Lima, J.R.C.; Couceiro, K.N.; et al. Acute micro-outbreak of Chagas disease in the southeastern Amazon: A report of five cases. Rev. Soc. Bras. Med. Trop. 2022, 55, e0687-2021. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Ramirez, A.N.; Cucunubá, Z.; Bact, P.Z. Brote de Chagas agudo en Lebrija, Santander 2008. Rev. Obs. Salud Pública Santander 2009, 4, 28–36. [Google Scholar]
- Zambrano, P.; Cucunubá, Z.; Montilla, M.; Flórez, C.; Parra, E.; Cortés, L. Brotes de síndrome febril asociado a miocarditis aguda chagásica de posible transmisión oral en el departamento de Santander, diciembre de 2008 a mayo de 2009. Inf. Quinc. Epidemiol. Nac. 2010, 15, 17–32. [Google Scholar]
- Rios, J.F.; Arboleda, M.; Montoya, A.N.; Alarcon, E.P.; Parra-Henao, G.J. Probable brote de transmisión oral de enfermedad de Chagas en Turbo, Antioquia. Biomedica 2011, 31, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Soto, H.; Tibaduiza, T.; Montilla, M.; Triana, O.; Suárez, D.C.; Torres, M.T.; Arias, M.T.; Lugo, L. Investigación de vectores y reservorios en brote de Chagas agudo por posible transmisión oral en Aguachica, Cesar, Colombia. Cad. Saude Publica 2014, 30, 746–756. [Google Scholar] [CrossRef]
- Zuleta-Dueñas, L.P.; López-Quiroga, Á.J.; Torres-Torres, F.; Castañeda-Porras, O. Possible oral transmission of Chagas disease among hydrocarbons sector workers in Casanare, Colombia, 2014. Biomedica 2017, 37, 218–232. [Google Scholar] [PubMed]
- Segura-Alba, M.L.; Hernandez, C.; Guerra, A.P.; Luna, N.; Cortes, L.J.; Acevedo, C.R.; Ballesteros, N.; Ayala, M.S.; Vera, M.J.; Diaz, R.A.C.; et al. Acute Chagas disease outbreaks in Colombia in 2019. IJID Reg. 2024, 12, 100410. [Google Scholar] [CrossRef] [PubMed]
- Vergara, H.D.; Gómez, C.H.; Faccini-Martínez, Á.A.; Herrera, A.C.; López, M.J.; Camacho, C.; Muñoz, L.; Cruz.Saavedra, L.; Hernández, C.; Ramírez, J.D. Acute Chagas Disease outbreak among military personnel, Colombia, 2021. Emerg. Infect. Dis. 2023, 29, 1882. [Google Scholar] [CrossRef]
- Gutierrez, S.A.; Jaimes-Dueñez, J.; Cruz-Saavedra, L.; Hernandez, C.; Cantillo-Barraza, O.; Alvarez, F.; Blanco, M.; Leal, B.; Marínez, L.; Medina, M.; et al. An Outbreak of acute Chagas disease possibly spread through oral transmission involving animal reservoirs in eastern Colombia. Am. J. Trop. Med. Hyg. 2023, 110, 36. [Google Scholar] [CrossRef]
- Benítez, J.Z.; Díaz, D.C.; Colorado, L.A.; Murillo, L.M.; Orozco, M.T.; Vallecilla, S.; Padilla, J.C.; Olivera, M.J. First report of an acute case of Chagas disease in the municipality of Miraflores, Guaviare, Colombia. Rev. Peru. Med. Exp. Salud Publica 2024, 41, 203. [Google Scholar] [CrossRef]
- Martín, A.; Alarcón de Noya, B.; Montero, R.; Rojas, C.; Garrido, E.; Ruiz-Guevara, R.; Diaz-Bello, Z. Archivos venezolanos de puericultura y pediatría. Arch. Venez. Pueric. Pediatr. 1939, 72, 97–100. [Google Scholar]
- De Noya, B.A.; Díaz-Bello, Z.; Colmenares, C.; Ruiz-Guevara, R.; Mauriello, L.; Zavala-Jaspe, R.; Suarez, J.A.; Abate, T.; Naranjo, L.; Paiva, M.; et al. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J. Infect. Dis. 2010, 201, 1308–1315. [Google Scholar] [CrossRef]
- de Noya, B.; Martinez, J. Transmision oral de la enfermedad de Chagas en Venezuela: Un segundo brote escolar. Rev. Fac. Cienc. Salud 2009, 13, 9. [Google Scholar]
- Añez, N.; Crisante, G.; Rojas, A.; Dávila, D. Boletín de malariología y salud ambiental. Bol. Malariol. Salud Ambient. 2003, 53, 01–10. [Google Scholar]
- Alcaíno, T.; Lorca-Herrera, M.; Issotta, A.; Gorman, T. Chagas’ disease in goats from the metropolitan region, Chile: Seroepidemiological survey and experimental infection. Parasitol. Día 1995, 19, 30–36. [Google Scholar]
- Ulloa, M.; Traslaviña, M.; Alcaíno, H.; Apt, W.; Sandoval, J. Enfermedad de Chagas en caninos y caprinos sinantrópicos de la provincia del Choapa (IV Región), Chile. Parasitol. Día 1989, 13, 120–124. [Google Scholar]
- Garrido, R.; Bacigalupo, A.; Peña-Gómez, F.; Bustamante, R.O.; Cattan, P.E.; Gorla, D.E.; Botto-Mahan, C. Potential impact of climate change on the geographical distribution of two wild vectors of Chagas disease in Chile: Mepraia spinolai and Mepraia gajardoi. Parasit. Vectors 2019, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.M.; de Góes Cavalcanti, L.P.; de Souza, R.d.C.M.; Barbosa, S.E.; Xavier, S.C.d.C.; Jansen, A.M.; Ramalho, R.D.; Diotaiut, L. Domestic, Peridomestic and wild hosts in the transmission of Trypanosoma cruzi in the Caatinga area colonised by Triatoma brasiliensis. Mem. Inst. Oswaldo Cruz 2014, 109, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.M.; Wisnivesky-Coli, C.; Gürtler, R.; Lazzari, J.; Bujas, M.A.; Segura, E.L. Trypanosoma cruzi infection in humans, dogs and goats in rural areas of the province of Córdoba. Medicina 1985, 45, 539–546. [Google Scholar]
- de Araújo-Neto, V.T.; Barbosa-Silva, A.N.; Medeiros Honorato, N.R.; Sales, L.M.L.; de Cassia Pires, R.; do Nascimento Brito, C.R.; da Matta Guedes, P.M.; da Cunha Galvão, L.M.; da Camara, A.C.J. Molecular identification of Trypanosoma cruzi in domestic animals in municipalities of the State of Rio Grande do Norte, Brazil. Parasitol. Res. 2023, 122, 207–215. [Google Scholar] [CrossRef]
- Barbosa, R.L.; Dias, V.L.; Pereira, K.S.; Schmidt, F.L.; Franco, R.M.B.; Guaraldo, A.M.A.; Alves, D.P.; Passos, L.A.C. Survival in vitro and virulence of Trypanosoma cruzi in açaí pulp in experimental acute Chagas disease. J. Food Prot. 2012, 75, 601–606. [Google Scholar] [CrossRef]
- Barbosa, R.L.; Pereira, K.S.; Dias, V.L.; Schmidt, F.L.; Alves, D.P.; Guaraldo, A.M.A.; Passos, L.A.C. Virulence of Trypanosoma cruzi in açai (Euterpe oleraceae Martius) pulp following mild heat treatment. J. Food Prot. 2016, 79, 1807–1812. [Google Scholar] [CrossRef]
- Suzuki, A.F.; de Souza, E.R.; Spadella, M.A.; Chagas, E.F.B.; Hataka, A.; Martins, L.P.A. Oral infection and survival of Trypanosoma cruzi in sugarcane juice conditioned at different temperatures. Acta Parasitol. 2024, 69, 251–259. [Google Scholar] [CrossRef]
- de Sousa, A.S.; Vermeij, D.; Ramos, A.N.; Luquetti, A.O. Chagas disease. Lancet 2024, 403, 203–218. [Google Scholar] [CrossRef]
- Cordeiro da Silva, A.; Calogeropoulou, T.; Costi, M.; Alunda, J. Drugs for vector-borne protozoal diseases in a one health scenario. A european perspective. ACS Infect. Dis. 2024, 10, 3715–3720. [Google Scholar] [CrossRef] [PubMed]
- Filigheddu, M.T.; Górgolas, M.; Ramos, J.M. Orally-transmitted Chagas disease. Med. Clin. 2017, 148, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Añez, N.; Crisante, G.; Rojas, A.; Dávila, D. Acute Chagas disease outbreak of possible oral transmission in Merida, Venezuela[Brote de enfermedad de Chagas agudo de posible transmisión oral en Mérida, Venezuela]. Bol. Malariol. Salud Ambient. 2013, 53, 1–11. [Google Scholar]
- Pittella, J.E.H. Central nervous system involvement in Chagas disease: A hundred-year-old history. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 973–978. [Google Scholar] [CrossRef]
- Dos Santos, V.R.C.; Antunes, D.; de Souza, D.D.S.M.; Moreira, O.C.; Lima, I.C.d.A.; Farias-De-oliveira, D.A.; Lobo, J.P.; de Meis, E.; Coura, J.R.; Svino, W.; et al. Human acute Chagas disease: Changes in factor VII, activated protein C and hepatic enzymes from patients of oral outbreaks in Pará state (Brazilian Amazon). Mem. Inst. Oswaldo Cruz 2020, 115, e190364. [Google Scholar] [CrossRef]
- Feilij, H.; Muller, L.; Gonzalez Cappa, S.M. Direct micromethod for diagnosis of acute and congenital Chagas’ disease. J. Clin. Microbiol. 1983, 18, 327–330. [Google Scholar] [CrossRef]
- Luquetti, A.O.; Rassi, A. Diagnóstico laboratorial da infecção pelo Trypanosoma cruzi. In Trypanosoma cruzi e Doença de Chagas, 2nd. ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2000; pp. 344–378. [Google Scholar]
- Schijman, A.G.; Bisio, M.; Orellana, L.; Sued, M.; Duffy, T.; Mejia-Jaramillo, A.M.; Cura, C.; Auter, F.; Veron, V.; Qvarnstrom, Y.; et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl. Trop. Dis. 2011, 5, e931. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Villamil-Gómez, W.E.; Schultz, J.; Henao-Martínez, A.F.; Parra-Henao, G.; Rassi, A.; Rodríguez-Morales, A.J.; Suarez, J.A. A deadly feast: Elucidating the burden of orally acquired acute Chagas disease in Latin America—Public health and travel medicine importance. Travel. Med. Infect. Dis. 2020, 36, 101565. [Google Scholar] [CrossRef]
- Dias, G.B.M.; Gruendling, A.P.; Araújo, S.M.; Gomes, M.Ô.L.; Toledo, M.J.D.O. Evolution of infection in mice inoculated by the oral route with different developmental forms of Trypanosoma cruzi I and II. Exp. Parasitol. 2013, 135, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Silva-dos-Santos, D.; Barreto-de-Albuquerque, J.; Guerra, B.; Moreira, O.C.; Berbert, L.R.; Ramos, M.T.; Mascarenhas, B.A.S.; Britto, C.; Morrot, A.; Villa-Verde, D.M.S.; et al. Unraveling Chagas disease transmission through the oral route: Gateways to Trypanosoma cruzi infection and target tissues. PLoS Negl. Trop. Dis. 2017, 11, e0005507. [Google Scholar] [CrossRef] [PubMed]
- Antunes, D.; Marins-Dos-Santos, A.; Ramos, M.T.; Mascarenhas, B.A.S.; De Carvalho Moreira, C.J.; Farias-De-Oliveira, D.A.; Savino, W.; Monteiro, R.Q.; de Meis, J. Oral route driven acute Trypanosoma cruzi infection unravels an IL-6 dependent hemostatic derangement. Front. Immunol. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Carvalho, L.M.; de Carvalho, T.V.; Ferraz, A.T.; Marques, F.d.S.; Roatt, B.M.; Fonseca, K.D.S.; Reis, L.E.S.; Carneiro, C.M.; de Abreu Vieira, P.M. Histopathological changes in the gastrointestinal tract and systemic alterations triggered by experimental oral infection with Trypanosoma cruzi. Exp. Parasitol. 2020, 218, 108012. [Google Scholar] [CrossRef]
- Marins-Dos-Santos, A.; Ayres-Silva, J.d.P.; Antunes, D.; Moreira, C.J.d.C.; Pelajo-Machado, M.; Alfaro, D.; Zapata, A.G.; Bonomo, A.C.; Savino, W.; de Meis, J.; et al. Oral Trypanosoma cruzi acute infection in mice targets primary lymphoid organs and triggers extramedullary hematopoiesis. Front. Cell Infect. Microbiol. 2022, 12, 800395. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, C.; Cortez, M.; Ferreira, D.; Yoshida, N. Interaction with host factors exacerbates Trypanosoma cruzi cell invasion capacity upon oral infection. Int. J. Parasitol. 2007, 37, 1609–1616. [Google Scholar] [CrossRef]
- Coffield, D.J.; Spagnuolo, A.M.; Shillor, M.; Mema, E.; Pell, B.; Pruzinsky, A.; Zetye, A. A Model for Chagas disease with oral and congenital transmission. PLoS ONE 2013, 8, e67267. [Google Scholar] [CrossRef]
- Guarner, J. Chagas disease as example of a reemerging parasite. Semin. Diagn. Pathol. 2019, 36, 164–169. [Google Scholar] [CrossRef]
- Muñoz-Calderón, A.; Díaz-Bello, Z.; Valladares, B.; Noya, O.; López, M.C.; Alarcón de Noya, B.; Thomas, M.C. Oral transmission of Chagas disease: Typing of Trypanosoma cruzi from five outbreaks occurred in Venezuela shows multiclonal and common infections in patients, vectors and reservoirs. Infect. Genet. Evol. 2013, 17, 113–122. [Google Scholar] [CrossRef]
- Yefi-Quinteros, E.; Muñoz-San Martín, C.; Bacigalupo, A.; Correa, J.P.; Cattan, P.E. Trypanosoma cruzi load in synanthropic rodents from rural areas in Chile. Parasit. Vectors 2018, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Coronado, X.; Rozas, M.; Botto-Mahan, C.; Ortíz, S.; Cattan, P.E.; Solari, A. Molecular epidemiology of Chagas disease in the wild transmission cycle: The evaluation in the sylvatic vector Mepraia spinolai from an endemic area of Chile. Am. J. Trop. Med. Hyg. 2009, 81, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Ihle-Soto, C.; Costoya, E.; Correa, J.P.; Bacigalupo, A.; Cornejo-Villar, B.; Estadella, V.; Solari, A.; Ortiz, S.; Hernández, H.J.; Botto-Mahan, C.; et al. Spatio-temporal characterization of Trypanosoma cruzi infection and discrete typing units infecting hosts and vectors from non-domestic foci of Chile. PLoS Negl. Trop. Dis. 2019, 13, e0007170. [Google Scholar] [CrossRef] [PubMed]
- Galuppo, S.; Bacigalupo, A.; García, A.; Ortiz, S.; Coronado, X.; Cattan, P.E.; Solari, A. Predominance of Trypanosoma cruzi genotypes in two reservoirs infected by sylvatic Triatoma infestans of an endemic area of Chile. Acta Trop. 2009, 111, 90–93. [Google Scholar] [CrossRef]
- Rozas, M.; Botto-Mahan, C.; Coronado, X.; Ortiz, S.; Cattan, P.E.; Solari, A. Short report: Trypanosoma cruzi infection in wild mammals from a chagasic area of Chile. Am. J. Tropi Med. Hyg. 2005, 73, 517–519. [Google Scholar] [CrossRef]
- Botto-Mahan, C.; Correa, J.P.; Araya-Donoso, R.; Farías, F.; Juan, E.S.; Quiroga, N.; Campos-Soto, R.; Reyes-Olivares, C.; González-Acuña, D. Lizards as Silent Hosts of Trypanosoma cruzi. Emerg. Infect. Dis. 2022, 28, 1250–1253. [Google Scholar] [CrossRef]
- Cevidanes, A.; Di Cataldo, S.; Muñoz-San Martín, C.; Latrofa, M.S.; Hernández, C.; Cattan, P.E.; Otranto, D.; Millán, J. Co-infection patterns of vector-borne zoonotic pathogens in owned free-ranging dogs in central Chile. Vet. Res. Commun. 2023, 47, 575–588. [Google Scholar] [CrossRef]
- Reyes, C.; González, C.R.; Alvarado, S.; Flores, L.; Martin, C.; Oyarce, A.; Aylwin, M.P.; Canals, M.; Parra, A.; Valderrama, L. Chagas disease in northern Chile: Detection of Trypanosoma cruzi in children, dogs and triatomine bugs. Acta Trop. 2022, 235, 106631. [Google Scholar] [CrossRef]
- Botto-Mahan, C.; Cattan, P.E.; Medel, R. Chagas disease parasite induces behavioural changes in the kissing bug Mepraia spinolai. Acta Trop. 2006, 98, 219–223. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; Miles, M.A.; Carrasco, H.J.; Lewis, M.D.; Yeo, M.; Vargas, J.; Torrico, F.; Diosque, P.; Valente, V.; Valente, S.A.; et al. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathog. 2009, 5, e1000410. [Google Scholar] [CrossRef]
- Miles, M.A.; Llewellyn, M.S.; Lewis, M.D.; Yeo, M.; Baleela, R.; Fitzpatrick, S.; Gaunt, M.W.; Mauricio, I.L. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: Looking back and to the future. Parasitology 2009, 136, 1509–1528. [Google Scholar] [CrossRef]
- O’Connor, O.; Bosseno, M.F.; Barnabé, C.; Douzery, E.J.P.; Brenière, S.F. Genetic clustering of Trypanosoma cruzi I lineage evidenced by intergenic miniexon gene sequencing. Infect. Genet. Evol. 2007, 7, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Bosseno, M.F.; Barnabé, C.; Sierra, M.J.R.; Kengne, P.; Guerrero, S.; Lozano, F.; Ezequiel, K.; Gastélum, M.; Breniere, S.F. Short report: Wild ecotopes and food habits of Triatoma longipennis infected by Trypanosoma cruzi lineages I and II in Mexico. Am. J. Trop. Med. Hyg. 2009, 80, 98–991. [Google Scholar] [CrossRef]
- Cortez, M.; Neira, I.; Ferreira, D.; Luquetti, A.O.; Rassi, A.; Atayde, V.D.; Yoshida, N. Infection by Trypanosoma cruzi metacyclic forms deficient in gp82 but expressing a related surface molecule, gp30. Infect. Immun. 2003, 71, 6184–6191. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.; Silva, M.R.; Neira, I.; Ferreira, D.; Sasso, G.R.S.; Luquetti, A.O.; Rassi, A.; Yoshida, N. Trypanosoma cruzi surface molecule gp90 downregulates invasion of gastric mucosal epithelium in orally infected mice. Microbes Infect. 2006, 8, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Maeda, F.Y.; Clemente, T.M.; Macedo, S.; Cortez, C.; Yoshida, N. Host cell invasion and oral infection by Trypanosoma cruzi strains of genetic groups TcI and TcIV from chagasic patients. Parasit. Vectors 2016, 9, 189. [Google Scholar] [CrossRef]
- Barbosa, C.G.; Gómez-Hernández, C.; Rezende-Oliveira, K.; Da Silva, M.V.; Ferreira Rodrigues, J.P.; Tiburcio, M.G.S.; Ferreira, T.B.; Rodrigues, V.; Yoshida, N.; Ramirez, L.E. Oral infection of mice and host cell invasion by Trypanosoma cruzi strains from Mexico. Parasitol. Res. 2019, 118, 1493–1500. [Google Scholar] [CrossRef]
- Cortez, C.; Martins, R.M.; Alves, R.M.; Silva, R.C.; Bilches, L.C.; Macedo, S.; Atayde, V.D.; Kawashita, S.Y.; Briones, M.R.S.; Yoshida, N. Differential infectivity by the oral route of Trypanosoma cruzi lineages derived from Y strain. PLoS Negl. Trop. Dis. 2012, 6, e1804. [Google Scholar] [CrossRef]
- Margioto Teston, A.P.; de Abreu, A.P.; Abegg, C.P.; Gomes, M.L.; de Ornelas Toledo, M.J. Outcome of oral infection in mice inoculated with Trypanosoma cruzi IV of the Western Brazilian Amazon. Acta Trop. 2017, 166, 212–217. [Google Scholar] [CrossRef]
- Lewis, M.D.; Francisco, A.F.; Jayawardhana, S.; Langston, H.; Taylor, M.C.; Kelly, J.M. Imaging the development of chronic Chagas disease after oral transmission. Sci. Rep. 2018, 8, 11292. [Google Scholar] [CrossRef]
- Yoshida, N. Molecular basis of mammalian cell invasion by Trypanosoma cruzi. An. Acad. Bras. Cienc. 2006, 78, 87–111. [Google Scholar] [CrossRef]
- Staquicini, D.I.; Martins, R.M.; Macedo, S.; Sasso, G.R.S.; Atayde, V.D.; Juliano, M.A.; Yoshida, N. Role of GP82 in the selective binding to gastric mucin during oral infection with Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2010, 4, e613. [Google Scholar] [CrossRef]
- Maeda, F.Y.; Alves, R.M.; Cortez, C.; Lima, F.M.; Yoshida, N. Characterization of the infective properties of a new genetic group of Trypanosoma cruzi associated with bats. Acta Trop. 2011, 120, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hoft, D.F. Differential mucosal infectivity of different life stages of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 1996, 55, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Roellig, D.M.; Ellis, A.E.; Yabsley, M.J. Oral transmission of Trypanosoma cruzi with opposing evidence for the theory of carnivory. J. Parasitol. 2009, 95, 360–364. [Google Scholar] [CrossRef]
- Torres, V.; Contreras, V.; Gutiérrez, B.; San Francisco, J.; Catalán, A.; Vega, J.L.; Moon, K.M.; Foster, L.J.; de Almeida, R.F.; Kalergis, A.M.; et al. Oral infectivity through carnivorism in murine model of Trypanosoma cruzi infection. Front. Cell Infect. Microbiol. 2024, 14, 1297099. [Google Scholar] [CrossRef]
- Guhl, F.; Ramírez, J.D. Trypanosoma cruzi I diversity: Towards the need of genetic subdivision? Acta Trop. 2011, 119, 1–4. [Google Scholar] [CrossRef]
- San Francisco, J.; Barría, I.; Gutiérrez, B.; Neira, I.; Muñoz, C.; Sagua, H.; Araya, J.E.; Andrade, J.C.; Zailberger, A.; Catalán, A.; et al. Decreased cruzipain and gp85/trans-sialidase family protein expression contributes to loss of Trypanosoma cruzi trypomastigote virulence. Microbes Infect. 2017, 19, 55–61. [Google Scholar] [CrossRef]
- San Francisco, J.; Astudillo, C.; Vega, J.L.; Catalán, A.; Gutiérrez, B.; Araya, J.E.; Zailberger, A.; Marina, A.; García, C.; Sanchez, N.; et al. Trypanosoma cruzi pathogenicity involves virulence factor expression and upregulation of bioenergetic and biosynthetic pathways. Virulence 2022, 13, 1827–1848. [Google Scholar] [CrossRef]
- Schaub, G.A. Trypanosoma cruzi: Quantitative studies of development of two strains in small intestine and rectum of the vector Triatoma infestans. Exp. Parasitol. 1989, 68, 260–273. [Google Scholar] [CrossRef]
- Herrera, H.M.; Abreu, U.G.P.; Keuroghlian, A.; Freitas, T.P.; Jansen, A.M. The role played by sympatric collared peccary (Tayassu tajacu), white-lipped peccary (Tayassu pecari), and feral pig (Sus scrofa) as maintenance hosts for Trypanosoma evansi and Trypanosoma cruzi in a sylvatic area of Brazil. Parasitol. Res. 2008, 103, 619–624. [Google Scholar] [CrossRef]
- Muñoz-San Martín, C.; Campo Verde Arbocco, F.; Saavedra, M.; Actis, E.A.; Ríos, T.A.; Abba, A.M.; Morales, M.E.; Cattan, P.E.; Jahn, G.A.; Superina, M. High rates of Trypanosoma cruzi infection in goats from Mendoza province, Argentina: Parasite loads in blood and seasonal variation. Acta Trop. 2020, 208, 105493. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, T. Inmunología de la Enfermedad de Chagas; Editorial Universidad de Chile: Santiago, Chile, 1957. [Google Scholar]
- Yoshida, N. Surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi. Infect. Immun. 1983, 40, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Barreto, W.T.G.; de Macedo, G.C.; Barros, J.H.d.S.; Xavier, S.C.d.C.; Garcia, C.M.; Mourão, G.; de Oliveira, J.; Rimoldi, A.R.; de Oliveira Porfírio, G.E.; et al. The reservoir system for Trypanosoma (Kinetoplastida, Trypanosomatidae) species in large neotropical wetland. Acta Trop. 2019, 199, 105098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.