Abstract
Post-COVID-19 is a chronic infection-related syndrome, including exacerbations of pre-existing or newly diagnosed conditions that have been established after the acute phase of COVID-19 and have demonstrated a wide range of systemic effects beyond the lungs. SARS-CoV-2 attaches to its receptor, angiotensin-converting enzyme 2 (ACE-2). Transmembrane serine protease 2 (TMPRSS2) facilitates viral entry and spread. ACE-2 receptors are detectable in several tissues, including the respiratory mucosa, digestive tract, heart, kidney, and brain. Several investigations have demonstrated an increase in digestive manifestations post-acute COVID-19, likely related to an alteration in the intestinal microbiota following infection. These changes can lead to a loss of species diversity, resulting in an overgrowth of opportunistic pathogens and deprivation of commensal bacteria. In this context, post-infection irritable bowel syndrome shows an increased incidence compared to controls. Growing evidence also suggests the enduring presence of SARS-CoV-2 in the gut tissue. Studies are ongoing to investigate antiviral agents that counteract prolonged COVID-19 symptoms. Therefore, the objectives of this review were to summarize the digestive manifestations, focusing on irritable bowel syndrome and therapeutic strategies. This review gives an overview of studies published in English in the last two years on the PubMed database.
1. Introduction
The World Health Organization defined post-COVID-19 as a syndrome characterized by a range of symptoms, which usually start within 3 months of the initial COVID-19 illness and last at least 2 months [1]. Therefore, post-COVID-19 is an infection-associated chronic condition, including exacerbations of preexisting or new-onset conditions, which develops after the acute phase of COVID-19, and demonstrates a broad range of systemic effects beyond the lungs [2]. Many of the studies were conducted 2 years after the pandemic. A recent systematic review and meta-analysis [3] highlights that COVID-19 survivors continue to experience symptoms and functional impairment even more than one year after the initial infection.
Globally, Al-Oraibi et al. [4] estimated the pooled prevalence and analyzed the most common post-COVID-19 symptoms among 6481 healthcare workers infected with acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The systematic review and meta-analysis found a pooled prevalence of long COVID-19 of 40%, with a median monitoring period of 22 weeks. The most frequent symptoms included fatigue (35%), neurological symptoms (25%), loss/decrease in smell and/or taste (25%), myalgia (22%), and shortness of breath (19%). Accordingly, a previous meta-analysis by Luo et al. [5] investigated the prevalence and determinants of prolonged symptoms after COVID-19, examining 211 studies with a total of 13,368,074 individuals. Within the first 3 months post-infection, the most reported symptoms were fatigue, post-traumatic stress disorder, mood disorders, and dyspnea. At 12 months and beyond, fatigue, dyspnea, psychiatric/neurological disorders, and joint pain were the frequently occurring manifestations. Determinants for prolonged symptoms included being a female, older, having severe COVID-19 during the acute phase, multiple medical conditions, longer hospital stays, and a higher basic metabolic rate.
SARS-CoV-2 attaches to its receptor ACE-2 (angiotensin-converting enzyme 2), and transmembrane serine protease 2 (TMPRSS2) promotes viral entry and spread [6]. The ACE-2 receptors are detected in the respiratory mucosa, gastrointestinal (GI) tract, heart, kidney, and brain [7]. The digestive manifestations may persist or newly develop following the acute phase of COVID-19 [8,9], affecting patients’ quality of life and complicating recovery.
Psychological disorders frequently occur after viral infections. The COVID-19 pandemic triggered a 25% increase in the prevalence of anxiety and depression worldwide [10]. Sperber et al. [11] reported a worldwide prevalence of irritable bowel syndrome (IBS) based on Roma IV criteria of 4.1% (range: 3.9–4.2%). In this context, the intestinal microbiota, which can produce 5-HT and is also modulated by 5-HT, could be crucial in the development of IBS and associated mood disorders through the microbiota–gut–brain axis [12].
Based on these premises, the objectives of this review were to summarize the digestive manifestations, with a particular focus on IBS and therapeutic strategies for the post-COVID-19 syndrome. To reach this objective, we conducted a literature search using the PubMed database for relevant studies published in English, particularly in the last two years.
2. Post-COVID-19 Digestive Manifestations
The alteration in the gut microbiota following COVID-19 has been the subject of close attention, and it can be hypothesized that the enduring dysbiosis following recovery may be related to long COVID-19 syndrome [13].
Li et al. [14] studied the gut microbiome profile among 13 asymptomatic infections, 24 post-acute COVID-19 syndrome patients, 31 discharged patients with SARS-CoV-2 re-positive, and 13 non-COVID-19 healthy controls. All the patients were unvaccinated. The asymptomatic and symptomatic patients presented different gut microbiome profiles. In particular, the microbiome profile of asymptomatic infected patients was enriched with short-chain fatty acids (SCFAs)-producing species Faecalibacterium prausnitzii, Blautia obeum, Roseburia hominis, and Gemmiger formicilis. Moreover, the microbiome profile of asymptomatic infected patients and patients who tested re-positive after discharge presented other SCFA-producing species, Bifidobacterium longum, Eubacterium ramulus, Phascolarctobacterium faecium, Butyricicoccus pullicaecorum, and Lactobacillus amylovorus; these were also inversely correlated with poor prognosis in COVID-19 cases.
Table 1 summarizes key differences in gut microbiota profiles between post-COVID-19 patients and controls.

Table 1.
Microbiota profile in post-COVID-19 syndrome.
Marasco et al. [19] conducted a multicenter study including 871 hospitalized patients (575 with COVID-19 and 296 without) to assess the prevalence of digestive symptoms. They found that nausea and diarrhea were more common in the COVID-19 group (59.7% of patients) compared to the non-COVID-19 group (43.2%). A month after admission, some patients continued to experience nausea. Accordingly, Ashktorab et al. [20], in 39 patients experiencing digestive symptoms, of whom 61.5% had nausea and vomiting, observed that patients experiencing vomiting during the acute infection were more likely to suffer from digestive manifestations associated with post-COVID-19.
A subsequent systematic review was conducted by Hawkings et al. [21] from early 2020 to mid-2023 in 28 different countries and included 45 studies, for 2,224,790 patients. An overall weighted prevalence of 10.8% of persistent digestive symptoms of any nature was found compared with 4.9% in healthy controls. Persistent symptoms of any duration showed a prevalence ranging from 0.2% to 24.1% in seven studies with a median monitoring period of 18 weeks. The incidence was evaluated from early 2022 to 2023, showing a high incidence of functional gastrointestinal disorders (FGIDs) after recovery from acute COVID-19, which highlighted an association between previous SARS-CoV-2 exposure and the occurrence of functional digestive disorders. Among the most incident frequent symptoms of post-acute COVID-19 across healthcare settings in seven countries [22], joint pain (from 1.6 to 14.3), abdominal pain (from 0.3 to 9.9), and other digestive manifestations (from 0.6 to 13.3), cough (from 0.3 to 9.1), and anxiety (from 0.8 to 11.4) were reported.
Baalbaki et al. [23] conducted a systematic literature review to evaluate whether omics-based analyses of different symptom-based phenotypes can contribute to understanding pathophysiological mechanisms and identify potential biomarkers and relevant traits in long-term COVID-19 patients compared to recovered individuals or healthy controls. In particular, studies on the microbiome using the technique of shotgun metagenomics have highlighted a relationship between altered microbiome composition, metabolic modifications, and the spectrum of neurological symptoms presented as a post-COVID-19 syndrome. Mohammed et al. [24] provided a systematic overview of 24 studies of digestive manifestations, excluding hepatic ones, and clinical implications in COVID-19 patients with abdominal symptoms, categorizing them into GI infections and inflammations, vascular disorders, structural abnormalities, other diagnosed abnormalities, and undiagnosed conditions. In the GI infections group, infections from Cryptosporidium species and Helicobacter pylori up to mucormycosis were reported, likely as a consequence of immunodeficiency or imbalance in microbiota composition. Among undiagnosed post-COVID-19 GI conditions, weight loss and FGID-like symptoms have been observed, highlighting the need for further research to understand persistent chronic manifestations after COVID-19 infection. Among inflammatory conditions, ulcerative colitis and acute pancreatitis have been reported in more severe cases, highlighting the impact of severe COVID-19 on the GI system and the need to monitor these types of patients recovering from COVID-19. Accordingly, a most recent study [25] conducted on 90 Inflammatory Bowel Disease (IBD) patients with a non-severe form of COVID-19, of whom 88.9% received at least standard two doses of vaccine against COVID-19, demonstrated that 19.30% of patients presented exacerbated digestive manifestations during COVID-19 and 38.1% of these developed post-COVID-19.
Choudhury et al. [26] analyzed the specific post-COVID-19 phenotype with digestive manifestations. In this systematic review and meta-analysis of studies reporting digestive manifestations in 12% of patients after COVID-19 and 22% as part of post-COVID syndrome, the most common symptoms include the following:
- -
- Loss of appetite
- -
- Loss of taste
- -
- Abdominal pain
- -
- Diarrhea
- -
- Nausea/vomiting
The reported pooled rate of IBS after COVID-19 was 0.17 (95% CI, 0.06–0.37, I2 = 96%) [26].
Regarding patients with FGID, Marasco et al. [27] in a meta-analysis highlighted that COVID-19 survivors were at risk of new onset of IBS compared to controls. A more recent post hoc analysis of a prospective multicenter cohort study [28] compared the psychological burden in patients with post-COVID-19 gut–brain interaction disorders with those with pre-existing IBS/functional dyspepsia (FD) and controls without gut–brain interaction disorders. Among patients with post-COVID-19 gut–brain interaction disorders, FD was the most prevalent. Irritable bowel syndrome was reported in 37.0% of patients. Furthermore, the study underlined that patients with post-COVID-19 gut–brain interaction disorders experienced a significant worsening.
All studies conducted so far underline the need to monitor patients for exacerbation of pre-existing gastroenterological conditions and evaluate a possible new onset in post-COVID-19 patients.
This review focuses specifically on IBS, as a high incidence of IBS has been reported among new-onset FGIDs in many studies, in addition to the significant impact on the quality of life of patients affected by this disorder. Furthermore, post-infectious (PI)-IBS can develop following acute infectious gastroenteritis. Given that gastrointestinal manifestations are commonly reported during the acute phase of COVID-19, a possible contributing factor to the increased incidence of IBS could be COVID-19-related gastroenteritis.
3. Post-COVID-19 Irritable Bowel Syndrome
Several pathophysiological mechanisms are implicated in the onset of IBS post-COVID-19. Pieces of evidence suggest that the microbiota profile plays a potentially more proactive role in the gut microbiome, where pre-existing or early-infection microbial profiles are involved in both the severity of COVID-19 and the development of post-COVID-19 sequelae [14]. The intestinal microbiota can affect motility and visceral perception via the brain–gut axis and the key role of integrative brain structures. Moreover, serotonin [12,29], neuropeptide Y [29,30], dopamine, γ-aminobutyric acid, and histamine [31] could be crucial in the modulation of gut–brain interactions [29,30]. The mechanism behind post-COVID-19 IBS resembles that of post-infectious IBS. An abnormal microbiota–gut-brain axis can lead to a dysregulation of intestinal neurotransmitters [31], resulting in visceral hypersensitivity and altered GI motility [32].
SARS-CoV-2 invades the intestine through ACE-2, which is highly present in the absorptive enterocytes of the ileum and large intestine, and through the TMPRSS2 receptor, which cleaves the coronavirus spike protein in the cell membrane, releases the fusion peptide into the membrane [33].
Edwinson et al. [34], for the first time, studied the role played by human microbiota in regulating the expression of ACE-2 receptors in the GI tract. They enrolled 12 patients (11 females) with Rome III IBS and 6 healthy volunteers who underwent sigmoid colon biopsies and pooled fecal samples for shotgun metagenomics to assess microbiota composition. IBS patients showed reduced microbial α-diversity and changes in microbiota composition. In particular, the phylum Methanobacteriota, the families Odoribacteraceae, Methanobacteriaceae, Odoribacteraceae, and Sutterellaceae, and the genus Methanobrevibacter decreased in IBS patients. The phylum Actinomycetota was enhanced in IBS patients compared with healthy controls. However, the study demonstrated a similar ACE-2 detection in colonic biopsies from IBS patients and healthy subjects. In the same study, a humanized mouse model demonstrated that healthy commensal microbiota and altered microbiota from IBS patients inhibited ACE-2 expression, suggesting the key role of commensal microbiota in regulating the expression of ACE-2 in the colon.
Apart from the involvement of ACE-2 receptors in the renin–angiotensin system, these receptors have a key function in the intestinal uptake of dietary amino acids, such as tryptophan [35], which regulates, via mTOR pathway activation, the secretion of antimicrobial peptides. These influence the composition of the intestinal microbiota and the susceptibility to inflammation of the large intestine [33]. Wong et al. [36], in patients experiencing persistent symptoms (3–22 months after acute infection) compared with 60 patients with acute COVID-19 and 30 individuals with symptom-free recovery from COVID-19, demonstrated a viral inflammation-driven serotonin depletion linked to a reduction in tryptophan absorption, thrombocytopenia, and increased MAO expression. The downregulation of ACE-2 receptors during the acute phase of COVID-19 in target cells contributes to SARS-CoV-2 pathogenesis, upregulating angiotensin II, which modulates the gene expression of several inflammatory cytokines via NF-κB signaling [37]. Cytokines and an altered microbial composition can induce systemic modulation of the brain structures and functions through the gut microbiota–immune system–brain axis [38,39].
Figure 1 summarizes the possible pathophysiological mechanisms underlying post-COVID-19 IBS.
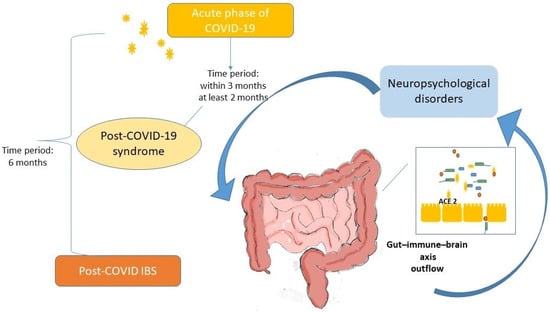
Figure 1.
Proposed pathophysiological mechanisms of post-COVID-19 irritable bowel syndrome. Legend: SARS-CoV-2 binds to ACE-2 receptors present in enterocytes of the ileum and large intestine, influencing the function of intestinal absorption of dietary amino acids, such as tryptophan, and the secretion of antimicrobial peptides. In the intestine, the virus alters the composition of the microbiota. Downregulation of receptors increases the gene expression of several inflammatory cytokines that induce epithelial and barrier damage. Viral persistence and immune and microbiota alterations can affect motility and visceral perception by neurotransmitters, leading to a systemic alteration in brain functions through the gut microbiota–immune–brain axis, resulting in post-COVID-19 irritable bowel syndrome-like symptoms among post-COVID-19 syndrome (within 3 months), confirming post-COVID-19 irritable bowel syndrome with a minimum of 6 months of symptom onset.
A Chinese study [15] developed a large single-site dataset covering different diseases, using a machine learning multi-class model to identify their fecal microbiome profiling. In this large cohort, the presence of Klebsiella pneumoniae, a well-characterized opportunistic pathogen, showed a positive association with Crohn’s disease, colorectal cancer, IBS-D, obesity, post-acute COVID-19 syndrome, and ulcerative colitis. The dysbiotic microbiome in post-COVID patients is characterised by a decrease in beneficial SCFA-producing bacteria and an increase in opportunistic bacteria [40].
Noviello et al. [41] demonstrated that loose stools, chronic fatigue, and somatization were increased 5 months after SARS-CoV-2 infection in comparison with control subjects. Ghoshal et al. [42], in a case–control study, using translated validated Rome Questionnaires, found that patients with previous COVID-19 had a higher probability of developing chronic bowel habit changes, dyspeptic symptoms, and their overlap during the first 3-month monitoring period and IBS (particularly IBS-D), uninvestigated dyspepsia (UD), and IBS–UD overlap during a 6-month monitoring period compared with the healthy controls. Moreover, the presence of digestive manifestations during COVID-19 was correlated with a higher occurrence of new onset of FGIDs in the follow-up. Accordingly, Marasco et al. [43] found that COVID-19 patients reported higher rates of IBS (3.2%), according to Rome IV criteria, than the control group (0.5%) at 12 months of follow-up. Table 2 summarizes the studies reporting post-COVID-19 IBS.

Table 2.
Studies reporting post-COVID-19 irritable bowel syndrome.
A single UK center case series [44] demonstrated the onset of chronic digestive symptoms, suggesting new post-COVID-19 digestive symptoms for 43.8% of the subjects involved in the study, 6 months after the acute phase of COVID-19. Accordingly to this suggestion, Austof et al. [45] studied the association between the occurrence of digestive symptoms during the acute phase and the development of post-COVID IBS according to Rome IV criteria in a cohort of 1475 subjects, of whom 33.8% developed digestive symptoms. The finding showed that patients with digestive symptoms during the acute phase of COVID-19 were slightly younger, more likely female, more prone to perceive stress, and with a more severe acute form than those without digestive symptoms. The incidence of post-COVID-19 reached 3.0%.
A Polish prospective, single-center study [46] evaluated, among 257 patients, the occurrence of digestive symptoms through the Rome IV Diagnostic Questionnaire for FGID in adults immediately after discharge and at 3 and 6 months post-COVID-19. In this study, the IBS-like symptoms were present after 3 months in 14 patients (5.4%), and IBS criteria were satisfied after 6 months in 15 patients (5.8%). A Turkish cross-sectional study [47] conducted from November 2020 to February 2021 on 233 eligible patients revealed a prevalence of 11.6% of IBS based on Rome IV criteria, 27.4% of depression, and 36.9% of anxiety assessed by the Hospital Anxiety and Depression Scale after COVID-19. Siyal et al. [48] evaluated the incidence of post-COVID-19 IBS based on Rome-IV criteria in 178 COVID-19 patients after discharge. IBS was found in 10.6% of patients, of whom 53.1% presented diarrhea-predominant, 31.2% constipation-predominant, and 15.6% mixed-type IBS. Risk factors were female sex, oxygen therapy, and high procalcitonin levels during the acute infection. A prospective study [49] highlighted a significantly higher number of new-onset disorders of gut–brain interaction compared with healthy controls at 3 and 6 months of the monitoring period. Accordingly, Zhang et al. [50] evaluated the incidence of post-COVID-19 FGID in 190 Chinese COVID-19 patients, finding an increment of new-onset post-COVID-19 FGID at 6 months compared with healthy controls, suggesting that digestive symptoms during the acute phase were an independent risk factor.
4. Therapeutic Strategies
Currently, the treatment for IBS patients follows the strategies provided by Italian guidelines [51]; however, some evidence suggests specific approaches, such as dietary interventions, probiotics, and antivirals.
COVID-19 causes changes in the gut microbiota, including a loss of species diversity in the gut, an overgrowth of opportunistic pathogens, and a deprivation of commensal bacteria. These changes may also affect the expression of ACE-2 receptors, which may influence the severity of COVID-19. Therefore, the therapeutic strategy for post-COVID-19 includes several targets, many of which are directed at modulating the intestinal microbiota, especially when symptoms are primarily GI.
5. Dietary, Prebiotics, Probiotics, Postbiotics, and Other Therapeutic Approaches
A single-center, double-blinded, and placebo-controlled randomized (1:1) trial was conducted by Calvani et al. [52] to investigate the effects of beetroot juice supplementation (200 mL) on physical function, gut microbiota, and inflammatory state in 31 adult patients affected by long COVID-19. The 14-day supplementation demonstrated changes in gut microbiota and inflammatory markers, as well as in fatigue resistance and the distance covered on the 6 min walk test, which increased from baseline. The microbiota significantly shifted toward bacteria with beneficial effects, such as Akkermansia, Oscillospira, Prevotella, Roseburia, Oscillospiraceae, and Turicibacter, in patients receiving beetroot juice supplementation compared with placebo.
A secondary analysis of this randomized controlled clinical trial by Marzetti et al. [53] investigated the effect of beetroot juice administration on circulating markers of mitochondrial quality and inflammatory markers and their relationship in 15 adult participants with long COVID-19 compared to 10 assigned to placebo. The study demonstrated that beetroot juice administration for 14 days reduced IL-1β, IL-8, and tumor necrosis factor-alpha serum levels. Still, mitochondrial quality markers did not show differences between the placebo and beetroot juice groups. An inverse correlation was found between vesicular markers of mitochondrial quality and performance on the 6 min walk test and flow-mediated dilation, regardless of group assignment, suggesting improved physical performance and endothelial function.
Ribeiro et al. [54] studied the association between food consumption and the onset of post-COVID syndrome in 1322 elderly Brazilians. They found that fruit consumption was associated with a lower incidence of this syndrome, while consumption of sugary drinks, cookies, sweets, and snacks was associated with a higher incidence. Accordingly, Barghchi et al. [55], in 246 recovered COVID-19 patients, found a relationship between good-quality food intake and psychological disorders during the post-infection period. Fruits, legumes, nuts, and whole grains were associated with a reduced risk.
Further insights will be available with the results of an ongoing trial involving the consumption of medical food, KetoCitra® [56], which provides exogenous beta-hydroxybutyrate to increase blood beta-hydroxybutyrate levels, inducing inflammatory inhibition and anti-inflammatory effects without a strict ketogenic diet.
Several lines of evidence suggest that prebiotics and probiotics contribute to mitigating neuroinflammation, with subsequent improvement of neuro-psychological state. Prebiotics, probiotics, and gut microbiota contribute to the formation of metabolites, including the neurotransmitters GABA and serotonin, and are crucial for maintaining psychological well-being. Alterations in their levels contribute to stress-related disorders. Given that digestive symptoms in post-COVID-19 IBS can be linked to viral persistence, gut microbiome, and immune alterations, affecting the gut–brain axis, evidence suggests the therapeutic effects of prebiotics and probiotics [57].
Giacosa et al. [58] explored the anti-inflammatory and intestinal antimicrobial modulating effects exerted by 30-day supplementation of Boswellia and Curcuma extracts combined with a low-FODMAP diet in 16 patients with long COVID-19 and IBS-like symptoms and 28 subjects with IBS without previous COVID-19 infection. Patients were recruited 60–120 days after the end of COVID-19 infection. The findings showed that both cohorts had a statistically significant decrease in bloating and relief of abdominal pain. However, enteral dysbiosis significantly decreased only in IBS patients, suggesting that patients with long COVID-19 and IBS-like symptoms may benefit from etiology-specific strategies for dysbiosis.
Horvath et al. [59] investigated the gut–lung axis to explore the effects of a probiotic in post-acute COVID-19 disease by a randomized, placebo-controlled trial to test the effects of a probiotic (Pro-Vi 5) for 6 months in 21 patients with a mild form of COVID-19. They found an improvement in tiredness, psychological state, microbiome composition, microbial-derived metabolites, lipoprotein levels, and markers of innate and adaptive immunity compared to controls. These findings suggest the treatment of post-acute COVID-19 syndrome during the monitoring period of 3–6 months.
Lau et al. [60] assessed a symbiotic (prebiotics and probiotics) preparation (SIM01) for the improvement of post-COVID-19 syndrome by a randomized, double-blinded, placebo-controlled trial in 463 patients with 14 post-COVID-19 symptoms, including digestive ones. At 6 months, significantly higher proportions of the SIM01 group had alleviation of fatigue, memory loss, difficulty in concentration, digestive symptoms, and general unwellness compared with the placebo group.
Ranisavljev et al. [61] conducted a randomized (1:1), placebo-controlled, double-blinded design clinical trial in people aged 18–65 years affected by post-COVID-19 syndrome, investigating the role played by the supplementation synbiotic mixture including Lactobacillus rhamnosus DSM32550, Humiome® Lactiplantibacillus plantarum DSM 34532, Bifidobacterium animalis subsp. lactis DSM 32269, Bifidobacterium longum DSM 32946, fructooligosaccharides, and zinc over 3 months. Findings demonstrated an amelioration of tissue metabolism and clinical features of post-COVID-19 fatigue syndrome.
The use of probiotics could be suggested for their potential multiple actions, including restoring intestinal microbiota, modulation of GI motility, visceral hypersensitivity, pain, and mucosal immune activation, which in turn improved gut–brain bidirectional interactions [51].
Postbiotics are diverse compounds, including bacteriocins, complex proteins, hydrogen peroxide, organic acids, exopolysaccharides, enzymes [62], SCFA, vitamins, and tryptophan [63], that have demonstrated immunomodulatory, anti-inflammatory, and antiviral effects. A wide range of postbiotics was secreted by probiotics with several beneficial effects on mental health.
With the premise that the anti-inflammatory effect of vitamins K2 and D3 attenuate the course of acute COVID-19 infection, Atieh et al. [64] investigated the role played by vitamins K2/D3 administration on long COVID-19 symptoms, gut and inflammatory markers in 151 long COVID-19 patients, demonstrating a significant improvement in the RECOVER Long COVID Index, the number of long COVID-19 symptoms, and several gut and inflammatory markers, such as markers of monocyte activation, high-sensitivity C-reactive protein, IL-6, inducible protein of 10 kDa, tumor necrosis factor receptors 1 and 2, and intercellular adhesion molecule-1. Accordingly, the supplementation with Pro Resolving lipid mediators (composed of 17-HAD, 14-HAD, and 18-HEPE) combined with telerehabilitation in ARACOV, a multicenter study, has been demonstrated to reduce the symptoms of patients with post-COVID-19.
Considering post-COVID-19 syndrome, as a consequence of alterations in the gut microbiome, Lau et al. [65] conducted a prospective, nonrandomized, open-label, interventional study, demonstrating that fecal microbiota transplantation improves insomnia and anxiety post-COVID-19-related by promoting the restoration of a “normal” gut microbiome.
7. Conclusions
Digestive symptoms are one of the main manifestations of post-COVID-19. To date, several studies conducted on large cohorts underscore the need for monitoring patients for exacerbation of pre-existing gastroenterological conditions and evaluating the new onset of brain–gut axis disorders in post-COVID-19 patients. Most studies conducted in various countries using the Rome IV criteria suggest that the incidence of post-COVID-19 IBS varies widely, ranging from 1.1 to 11.6%. Given its impact on quality of life, early recognition and a multidisciplinary approach are crucial for the management of post-COVID-19 IBS patients. Currently, several clinical trials are being conducted to test antiviral drugs that can act on the virus reservoir at any level where it is located. Some evidence suggests that these manifestations are due to the virus’s persistence in the GI tract. The results of the trials conducted so far do not appear to demonstrate a significant improvement in symptoms after antiviral treatment. However, these findings suggest that it is essential to await the results of studies conducted with longer-term antiviral treatment, or other antiviral agents, or a combination of these. The ongoing research results are crucial for understanding pathogenesis and targeted therapies. Emerging studies support the role of microbiome-targeted therapies.
Therefore, while the treatment of patients with IBS continues to follow the strategies provided by international guidelines, longitudinal studies are needed to thoroughly investigate the evolution of post-COVID-19 manifestations, especially for those manifestations, such as IBS, which have shown a higher incidence in the studies conducted so far, and the burden of long COVID-19 appears to be growing.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data reviewed in this manuscript are available online, particularly in PubMed.
Conflicts of Interest
The author declares no conflicts of interest.
References
- World Health Organization. Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/news-room/fact-sheets/detail/post-covid-19-condition-(long-covid) (accessed on 26 February 2025).
- Ely, E.W.; Brown, L.M.; Fineberg, H.V. National Academies of Sciences, Engineering, and Medicine Committee on Examining the Working Definition for Long COVID. Long COVID Defined. N. Engl. J. Med. 2024, 391, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.K.; Salzman, T.; Banal, A.; Morissette, K.; Domingo, F.R.; Cheung, A.M.; Cooper, C.L.; Boland, L.; Zuckermann, A.M.; Mullah, M.A.; et al. Global prevalence of post-COVID-19 condition: A systematic review and meta-analysis of prospective evidence. Health Promot. Chronic Dis. Prev. Can. Res. Policy Pract. 2025, 45, 112–138. [Google Scholar] [CrossRef] [PubMed]
- Al-Oraibi, A.; Woolf, K.; Naidu, J.; Nellums, L.B.; Pan, D.; Sze, S.; Tarrant, C.; Martin, C.A.; Gogoi, M.; Nazareth, J.; et al. Global prevalence of long COVID and its most common symptoms among healthcare workers: A systematic review and meta-analysis. BMJ Public Health 2025, 3, e000269. [Google Scholar] [CrossRef]
- Luo, D.; Mei, B.; Wang, P.; Li, X.; Chen, X.; Wei, G.; Kuang, F.; Li, B.; Su, S. Prevalence and risk factors for persistent symptoms after COVID-19: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2024, 30, 328–335. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018. [Google Scholar] [CrossRef]
- Ashraf, U.M.; Abokor, A.A.; Edwards, J.M.; Waigi, E.W.; Royfman, R.S.; Hasan, S.A.; Smedlund, K.B.; Hardy, A.M.G.; Chakravarti, R.; Koch, L.G. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol. Genom. 2021, 53, 51–60. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients with Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Europe (2022). COVID-19 Pandemic Triggers 25% Increase in Prevalence of Anxiety and Depression Worldwide. Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide#:~:text=In%20the%20first%20year%20of,Health%20Organization%20(WHO)%20today (accessed on 2 March 2022).
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Bellini, M.; Fornai, M.; Satta, P.U.; Bronzini, F.; Bassotti, G.; Blandizzi, C.; Colucci, R. The role of serotonin and its pathways in gastrointestinal disorders. In The Complex Interplay Between Gut-Brain, Gut-Liver, and Liver-Brain Axes; Stasi, C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 67–94. ISBN 9780128219270. [Google Scholar]
- Ancona, G.; Alagna, L.; Alteri, C.; Palomba, E.; Tonizzo, A.; Pastena, A.; Muscatello, A.; Gori, A.; Bandera, A. Gut and airway microbiota dysbiosis and their role in COVID-19 and long-COVID. Front. Immunol. 2023, 14, 1080043. [Google Scholar] [CrossRef]
- Lin, R.; Xiao, M.; Cao, S.; Sun, Y.; Zhao, L.; Mao, X.; Chen, P.; Tong, X.; Ou, Z.; Zhu, H.; et al. Distinct gut microbiota and health outcomes in asymptomatic infection, viral nucleic acid test re-positive, and convalescent COVID-19 cases. mLife 2022, 1, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, Q.; Lau, R.I.; Zhang, J.; Xu, Z.; Yeoh, Y.K.; Leung, T.W.H.; Tang, W.; Zhang, L.; Liang, J.Q.Y.; et al. Faecal microbiome-based machine learning for multi-class disease diagnosis. Nat. Commun. 2022, 13, 6818. [Google Scholar] [CrossRef] [PubMed]
- Comba, I.Y.; Mars, R.A.T.; Yang, L.; Dumais, M.; Chen, J.; Van Gorp, T.M.; Harrington, J.J.; Sinnwell, J.P.; Johnson, S.; Holland, L.A.; et al. Gut Microbiome Signatures During Acute Infection Predict Long COVID. bioRxiv 2024. bioRxiv:2024.12.10.626852. [Google Scholar] [CrossRef]
- Ferreira-Junior AFerreira-Junior, A.S.; Borgonovi, T.F.; De Salis, L.V.V.; Leite, A.Z.; Dantas, A.S.; De Salis, G.V.V.; Cruz, G.N.F.; De Oliveira, L.F.V.; Gomes, E.; Penna, A.L.B.; et al. Detection of Intestinal Dysbiosis in Post-COVID-19 Patients One to Eight Months after Acute Disease Resolution. Int. J. Environ. Res. Public Health 2022, 19, 10189. [Google Scholar] [CrossRef]
- Su, Q.; Lau, R.I.; Liu, Q.; Li, M.K.T.; Yan Mak, J.W.; Lu, W.; Lau, I.S.F.; Lau, L.H.S.; Yeung, G.T.Y.; Cheung, C.P.; et al. The gut microbiome associates with phenotypic manifestations of post-acute COVID-19 syndrome. Cell Host Microbe 2024, 32, 651–660.e4. [Google Scholar] [CrossRef]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Salvi, D.; Cacciari, G.; Kagramanova, A.; Bordin, D.; Drug, V.; Miftode, E.; Fusaroli, P.; et al. Prevalence of Gastrointestinal Symptoms in Severe Acute Respiratory Syndrome Coronavirus 2 Infection: Results of the Prospective Controlled Multinational GI-COVID-19 Study. Am. J. Gastroenterol. 2022, 117, 147–157. [Google Scholar] [CrossRef]
- Ashktorab, H.; Challa, S.R.; Singh, G.; Nanduri, S.; Ibrahim, M.; Martirosyan, Z.; Whitsell, P.; Chirumamilla, L.G.; Shayegh, N.; Watson, K.; et al. Gastrointestinal Manifestations and Their Association with Neurologic and Sleep Problems in Long COVID-19 Minority Patients: A Prospective Follow-Up Study. Dig. Dis. Sci. 2024, 69, 562–569. [Google Scholar] [CrossRef]
- Hawkings, M.J.; Vaselli, N.M.; Charalampopoulos, D.; Brierley, L.; Elliot, A.J.; Buchan, I.; Hungerford, D. A Systematic Review of the Prevalence of Persistent Gastrointestinal Symptoms and Incidence of New Gastrointestinal Illness after Acute SARS-CoV-2 Infection. Viruses 2023, 15, 1625. [Google Scholar] [CrossRef]
- Xie, J.; López-Güell, K.; Dedman, D.; Duarte-Salles, T.; Kolde, R.; López-Blasco, R.; Martínez, Á.; Mercier, G.; Abellan, A.; Arinze, J.T.; et al. Incidence of post-acute COVID-19 symptoms across healthcare settings in seven countries: An international retrospective cohort study using routinely-collected data. EClinicalMedicine. 2024, 77, 102903. [Google Scholar] [CrossRef]
- Baalbaki, N.; Slob, E.M.A.; Kazer, S.W.; IAbdel-Aziz, M.; Bogaard, H.J.; Golebski, K.; Maitland-van der Zee, A.H. The Omics Landscape of Long COVID-A Comprehensive Systematic Review to Advance Biomarker, Target and Drug Discovery. Allergy 2025, 80, 932–948. [Google Scholar] [CrossRef]
- Mohammed, I.; Podhala, S.; Zamir, F.; Shiyam, S.; Salameh, A.R.; Salahuddin, Z.; Salameh, H.; Kim, C.; Sinan, Z.; Kim, J.; et al. Gastrointestinal Sequelae of COVID-19: Investigating Post-Infection Complications-A Systematic Review. Viruses 2024, 16, 1516. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.Y.; Wu, J.F.; Weng, M.T.; Chuang, C.H.; Huang, T.Y.; Tai, W.C.; Tai, C.M.; Chung, C.S.; Chen, C.C.; Lin, C.P.; et al. Exacerbated gastrointestinal symptoms and long COVID in IBD patients with SARS-CoV-2 infection: A multi-center study from taiwan. J. Formos. Med. Assoc. 2024, 123, 866–874. [Google Scholar] [CrossRef]
- Choudhury, A.; Tariq, R.; Jena, A.; Vesely, E.K.; Singh, S.; Khanna, S.; Sharma, V. Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2022, 15, 17562848221118403. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Maida, M.; Cremon, C.; Barbaro, M.R.; Stanghellini, V.; Barbara, G. Meta-analysis: Post-COVID-19 functional dyspepsia and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2023, 58, 6–15. [Google Scholar] [CrossRef]
- Marasco, G.; Hod, K.; Colecchia, L.; Cremon, C.; Barbaro, M.R.; Cacciari, G.; Falangone, F.; Kagramanova, A.; Bordin, D.; Drug, V.; et al. Long-Term Impact of COVID-19 on Disorders of Gut-Brain Interaction: Incidence, Symptom Burden, and Psychological Comorbidities. United Eur. Gastroenterol. J. 2025. Advance online publication. [Google Scholar] [CrossRef]
- Stasi, C.; Bellini, M.; Costa, F.; Mumolo, M.G.; Ricchiuti, A.; Grosso, M.; Duranti, E.; Metelli, M.R.; Gambaccini, D.; Bianchi, L.; et al. Neuroendocrine markers and psychological features in patients with irritable bowel syndrome. Int. J. Color. Dis. 2013, 28, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Bellini, M.; Gambaccini, D.; Duranti, E.; de Bortoli, N.; Fani, B.; Albano, E.; Russo, S.; Sudano, I.; Laffi, G.; et al. Neuroendocrine Dysregulation in Irritable Bowel Syndrome Patients: A Pilot Study. J. Neurogastroenterol. Motil. 2017, 23, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ruan, G.; Chen, L.; Ying, S.; Li, G.; Xu, F.; Xiao, Z.; Tian, Y.; Lv, L.; Ping, Y.; et al. Neurotransmitter and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. Front. Endocrinol. 2022, 13, 817100. [Google Scholar] [CrossRef]
- Yu, L.C. Gastrointestinal pathophysiology in long COVID: Exploring roles of microbiota dysbiosis and serotonin dysregulation in post-infectious bowel symptoms. Life Sci. 2024, 358, 123153. [Google Scholar] [CrossRef]
- Perlot, T.; Penninger, J.M. ACE2—From the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013, 15, 866–873. [Google Scholar] [CrossRef]
- Edwinson, A.; Yang, L.; Chen, J.; Grover, M. Colonic expression of Ace2, the SARS-CoV-2 entry receptor, is suppressed by commensal human microbiota. Gut Microbes 2021, 13, 1984105. [Google Scholar] [CrossRef] [PubMed]
- Penninger, J.M.; Grant, M.B.; Sung, J.J.Y. The Role of Angiotensin Converting Enzyme 2 in Modulating Gut Microbiota, Intestinal Inflammation, and Coronavirus Infection. Gastroenterology 2021, 160, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalová, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867.e20. [Google Scholar] [CrossRef]
- Banu, N.; Panikar, S.S.; Leal, L.R.; Leal, A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: Therapeutic implications. Life Sci. 2020, 256, 117905. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Rosselli, M.; Bellini, M.; Laffi, G.; Milani, S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: The top-down and the bottom-up model. J. Gastroenterol. 2012, 47, 1177–1185. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Iqbal, N.T.; Khan, H.; Khalid, A.; Mahmood, S.F.; Nasir, N.; Khanum, I.; de Siqueira, I.; Van Voorhis, W. Chronic inflammation in post-acute sequelae of COVID-19 modulates gut microbiome: A review of literature on COVID-19 sequelae and gut dysbiosis. Mol. Med. 2025, 31, 22. [Google Scholar] [CrossRef]
- Noviello, D.; Costantino, A.; Muscatello, A.; Bandera, A.; Consonni, D.; Vecchi, M.; Basilisco, G. Functional gastrointestinal and somatoform symptoms five months after SARS-CoV-2 infection: A controlled cohort study. Neurogastroenterol. Motil. 2022, 34, e14187. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Ghoshal, U.; Rahman, M.M.; Mathur, A.; Rai, S.; Akhter, M.; Mostafa, T.; Islam, M.S.; Haque, S.A.; Pandey, A.; et al. Post-infection functional gastrointestinal disorders following coronavirus disease-19: A case-control study. J. Gastroenterol. Hepatol. 2022, 37, 489–498. [Google Scholar] [CrossRef]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Cacciari, G.; Falangone, F.; Kagramanova, A.; Bordin, D.; Drug, V.; Miftode, E.; Fusaroli, P.; et al. Post COVID-19 irritable bowel syndrome. Gut 2022. Advance online publication. [Google Scholar] [CrossRef]
- Cooney, J.; Appiahene, P.; Findlay, R.; Al-Hillawi, L.; Rafique, K.; Laband, W.; Shandro, B.; Poullis, A. COVID-19 infection causing residual gastrointestinal symptoms—A single UK centre case series. Clin. Med. 2022, 22, 181–183. [Google Scholar] [CrossRef]
- Austhof, E.; Bell, M.L.; Riddle, M.S.; Catalfamo, C.; McFadden, C.; Cooper, K.; Scallan Walter, E.; Jacobs, E.; Pogreba-Brown, K. Persisting gastrointestinal symptoms and post-infectious irritable bowel syndrome following SARS-CoV-2 infection: Results from the Arizona CoVHORT. Epidemiol. Infect. 2022, 150, e136. [Google Scholar] [CrossRef]
- Nazarewska, A.; Lewandowski, K.; Kaniewska, M.; Rosołowski, M.; Marlicz, W.; Rydzewska, G. Irritable bowel syndrome following COVID-19: An underestimated consequence of SARS-CoV-2 infection. Pol. Arch. Intern. Med. 2022, 132, 16323. [Google Scholar] [CrossRef]
- Farsi, F.; Zonooz, S.R.; Ebrahimi, Z.; Jebraili, H.; Morvaridi, M.; Azimi, T.; Sikaroudi, M.K.; Heshmati, J.; Khorrami, S.; Mokhtare, M.; et al. The Incidence of Post-infectious Irritable Bowel Syndrome, Anxiety, and Depression in Iranian Patients with Coronavirus Disease 2019 Pandemic: A Cross-Sectional Study. Turk. J. Gastroenterol. 2022, 33, 1033–1042. [Google Scholar] [CrossRef]
- Siyal, M.; Abbas, Z.; Ashraf, J.; Ali Qadeer, M.; Altaf, A. Incidence and predisposing factors for de novo post-COVID-19 irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2023, 35, 59–63. [Google Scholar] [CrossRef]
- Golla, R.; Vuyyuru, S.; Kante, B.; Kumar, P.; Thomas, D.M.; Makharia, G.; Kedia, S.; Ahuja, V. Long-term Gastrointestinal Sequelae Following COVID-19: A Prospective Follow-up Cohort Study. Clin. Gastroenterol. Hepatol. 2023, 21, 789–796.e1. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, C.; Xie, Y.; Zeng, F.; Chen, S.; Chen, R.; Zhang, X.; Huang, S.; Li, D.; Bai, F. Post-infection functional gastrointestinal disorders following coronavirus disease-19: A prospective follow-up cohort study. BMC Infect. Dis. 2023, 23, 422. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Di Nardo, G.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian guidelines for the management of irritable bowel syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig. Liver Dis. 2023, 55, 187–207. [Google Scholar] [CrossRef]
- Calvani, R.; Giampaoli, O.; Marini, F.; Del Chierico, F.; De Rosa, M.; Conta, G.; Sciubba, F.; Tosato, M.; Picca, A.; Ciciarello, F.; et al. Beetroot juice intake positively influenced gut microbiota and inflammation but failed to improve functional outcomes in adults with long COVID: A pilot randomized controlled trial. Clin. Nutr. 2024, 43, 344–358. [Google Scholar] [CrossRef]
- Marzetti, E.; Coelho-Júnior, H.J.; Calvani, R.; Girolimetti, G.; Di Corato, R.; Ciciarello, F.; Galluzzo, V.; Di Mario, C.; Tolusso, B.; Santoro, L.; et al. Mitochondria-Derived Vesicles and Inflammatory Profiles of Adults with Long COVID Supplemented with Red Beetroot Juice: Secondary Analysis of a Randomized Controlled Trial. Int. J. Mol. Sci. 2025, 26, 1224. [Google Scholar] [CrossRef]
- Ribeiro, G.J.S.; Morais, R.N.G.; Abimbola, O.G.; Dias, N.P.; Filgueiras, M.S.; Pinto, A.A.; Novaes, J.F. Unhealthy Food Consumption Is Associated with Post-Acute Sequelae of COVID-19 in Brazilian Elderly People. Infect. Dis. Rep. 2025, 17, 25. [Google Scholar] [CrossRef]
- Barghchi, H.; Araste, A.; Varasteh, N.; Dehnavi, Z.; Zare-Feyzabadi, R.; Vahedi Fard, M.; MohammadHasani, K.; Parirokh, J.; Khorasanchi, Z.; Mohammadi Bajgiran, M.; et al. Food Quality Is Associated with Depression, Anxiety, and Stress Among Recovered COVID-19 Patients: Finding From a Case-Control Study. Clin. Nutr. Res. 2025, 14, 17–29. [Google Scholar] [CrossRef]
- ClinicalTrials.gov ID:NCT05836402. Long COVID-19 Syndrome Lifestyle Intervention Study. Available online: https://clinicaltrials.gov/study/NCT05836402?cond=Post-COVID-19%20Syndrome&term=Gastrointestinal%20Diseases&intr=diet&rank=1 (accessed on 29 April 2024).
- Lim, H.X.; Khalid, K.; Abdullah, A.D.I.; Lee, L.H.; Raja Ali, R.A. Subphenotypes of Long COVID and the clinical applications of probiotics. Biomed. Pharmacother. 2025, 183, 117855. [Google Scholar] [CrossRef]
- Giacosa, A.; Barrile, G.C.; Gasparri, C.; Perna, S.; Rondanelli, M. Positive Effect of Lecithin-Based Delivery Form of Curcuma and Boswellia Extracts on Irritable Bowel Syndrome After COVID-19 Infection. Nutrients 2025, 17, 723. [Google Scholar] [CrossRef]
- Horvath, A.; Habisch, H.; Prietl, B.; Pfeifer, V.; Balazs, I.; Kovacs, G.; Foris, V.; John, N.; Kleinschek, D.; Feldbacher, N.; et al. Alteration of the Gut-Lung Axis After Severe COVID-19 Infection and Modulation Through Probiotics: A Randomized, Controlled Pilot Study. Nutrients 2024, 16, 3840. [Google Scholar] [CrossRef]
- Lau, R.I.; Su, Q.; Lau, I.S.F.; Ching, J.Y.L.; Wong, M.C.S.; Lau, L.H.S.; Tun, H.M.; Mok, C.K.P.; Chau, S.W.H.; Tse, Y.K.; et al. A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2024, 24, 256–265. [Google Scholar] [CrossRef]
- Ranisavljev, M.; Stajer, V.; Todorovic, N.; Ostojic, J.; Cvejic, J.H.; Steinert, R.E.; Ostojic, S.M. Correction: The effects of 3-month supplementation with synbiotic on patient-reported outcomes, exercise tolerance, and brain and muscle metabolism in adult patients with post-COVID-19 chronic fatigue syndrome (STOP-FATIGUE): A randomized Placebo-controlled clinical trial. Eur. J. Nutr. 2025, 64, 80. [Google Scholar] [CrossRef]
- Khani, N.; Abedi Soleimani, R.; Noorkhajavi, G.; Abedi Soleimani, A.; Abbasi, A.; Homayouni Rad, A. Postbiotics as potential promising tools for SARS-CoV-2 disease adjuvant therapy. J. Appl. Microbiol. 2022, 132, 4097–4111. [Google Scholar] [CrossRef]
- Kavita Om, H.; Chand, U.; Kushawaha, P.K. Postbiotics: An alternative and innovative intervention for the therapy of inflammatory bowel disease. Microbiol. Res. 2024, 279, 127550. [Google Scholar] [CrossRef] [PubMed]
- Atieh, O.; Daher, J.; Durieux, J.C.; Abboud, M.; Labbato, D.; Baissary, J.; Koberssy, Z.; Ailstock, K.; Cummings, M.; Funderburg, N.T.; et al. Vitamins K2 and D3 Improve Long COVID, Fungal Translocation, and Inflammation: Randomized Controlled Trial. Nutrients 2025, 17, 304. [Google Scholar] [CrossRef]
- Lau, R.I.; Su, Q.; Ching, J.Y.L.; Lui, R.N.; Chan, T.T.; Wong, M.T.L.; Lau, L.H.S.; Wing, Y.K.; Chan, R.N.Y.; Kwok, H.Y.H.; et al. Fecal Microbiota Transplantation for Sleep Disturbance in Post-acute COVID-19 Syndrome. Clin. Gastroenterol. Hepatol. 2024, 22, 2487–2496.e6. [Google Scholar] [CrossRef]
- Peluso, M.J.; Ryder, D.; Flavell, R.R.; Wang, Y.; Levi, J.; LaFranchi, B.H.; Deveau, T.M.; Buck, A.M.; Munter, S.E.; Asare, K.A.; et al. Tissue-based T cell activation and viral RNA persist for up to 2 years after SARS-CoV-2 infection. Sci. Transl. Med. 2024, 16, eadk3295. [Google Scholar] [CrossRef]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Rössler, A.; Kimpel, J.; Adolph, T.E.; Tilg, H. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology 2022, 163, 495–506.e8. [Google Scholar] [CrossRef]
- Ghafari, M.; Hall, M.; Golubchik, T.; Ayoubkhani, D.; House, T.; MacIntyre-Cockett, G.; Fryer, H.R.; Thomson, L.; Nurtay, A.; Kemp, S.A.; et al. Prevalence of persistent SARS-CoV-2 in a large community surveillance study. Nature 2024, 626, 1094–1101. [Google Scholar] [CrossRef]
- Zuo, W.; He, D.; Liang, C.; Du, S.; Hua, Z.; Nie, Q.; Zhou, X.; Yang, M.; Tan, H.; Xu, J.; et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: A cross-sectional cohort study in China. Lancet Infect. Dis. 2024, 24, 845–855. [Google Scholar] [CrossRef]
- Gostimirovic, M.; Rajkovic, J.; Bukarica, A.; Simanovic, J.; Gojkovic-Bukarica, L. Resveratrol and Gut Microbiota Synergy: Preventive and Therapeutic Effects. Int. J. Mol. Sci. 2023, 24, 17573. [Google Scholar] [CrossRef]
- Russo, C.; Valle, M.S.; Malaguarnera, L.; Romano, I.R.; Malaguarnera, L. Comparison of Vitamin D and Resveratrol Performances in COVID-19. Nutrients 2023, 15, 2639. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Berry, K.; Rajeevan, N.; Li, Y.; Mutalik, P.; Yan, L.; Bui, D.; Cunningham, F.; Hynes, D.M.; Rowneki, M.; et al. Effectiveness of Nirmatrelvir-Ritonavir Against the Development of Post-COVID-19 Conditions Among U.S. Veterans: A Target Trial Emulation. Ann. Intern. Med. 2023, 176, 1486–1497. [Google Scholar] [CrossRef]
- Krumholz, H.M.; Sawano, M.; Bhattacharjee, B.; Caraballo, C.; Khera, R.; Li, S.X.; Herrin, J.; Coppi, A.; Holub, J.; Henriquez, Y.; et al. The PAX LC Trial: A Decentralized, Phase 2, Randomized, Double-Blind Study of Nirmatrelvir/Ritonavir Compared with Placebo/Ritonavir for Long COVID. Am. J. Med. 2025, 138, 884–892.e4. [Google Scholar] [CrossRef]
- ClinicalTrials.gov ID NCT05668091. A Decentralized, Randomized Phase 2 Efficacy and Safety Study of Nirmatrelvir/Ritonavir in Adults with Long COVID. Available online: https://clinicaltrials.gov/study/NCT05668091?term=NCT05668091&rank=1 (accessed on 2 December 2024).
- Sawano, M.; Bhattacharjee, B.; Caraballo, C.; Khera, R.; Li, S.X.; Herrin, J.; Christian, D.; Coppi, A.; Warner, F.; Holub, J.; et al. Nirmatrelvir-ritonavir versus placebo-ritonavir in individuals with long COVID in the USA (PAX LC): A double-blind, randomised, placebo-controlled, phase 2, decentralised trial. Lancet Infect. Dis. 2025. Advance online publication. [Google Scholar] [CrossRef]
- Gragnani, L.; Lorini, S.; Marri, S.; Basile, U.; Santarlasci, V.; Monti, M.; Madia, F.; Petraccia, L.; Stasi, C.; Marello, N.; et al. Hematological and Genetic Markers in the Rational Approach to Patients with HCV Sustained Virological Response with or Without Persisting Cryoglobulinemic Vasculitis. Hepatology 2021, 74, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Mazzaro, C.; Quartuccio, L.; Adinolfi, L.E.; Roccatello, D.; Pozzato, G.; Nevola, R.; Tonizzo, M.; Gitto, S.; Andreone, P.; Gattei, V. A Review on Extrahepatic Manifestations of Chronic Hepatitis C Virus Infection and the Impact of Direct-Acting Antiviral Therapy. Viruses 2021, 13, 2249. [Google Scholar] [CrossRef] [PubMed]
- Paramythiotis, D.; Karlafti, E.; Didagelos, M.; Fafouti, M.; Veroplidou, K.; Protopapas, A.A.; Kaiafa, G.; Netta, S.; Michalopoulos, A.; Savopoulos, C. Post-COVID-19 and Irritable Bowel Syndrome: A Literature Review. Medicina 2023, 59, 1961. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov ID NCT06511063. Antiviral Clinical Trial for Long COVID-19. Available online: https://clinicaltrials.gov/study/NCT06511063?term=NCT06511063&rank=1 (accessed on 20 December 2024).
- ClinicalTrials.gov ID:NCT05595369. RECOVER-VITAL: Platform Protocol to Measure the Effects of Antiviral Therapies on Long COVID Symptoms (RECOVER-VITAL). Available online: https://clinicaltrials.gov/study/NCT05595369?term=NCT05595369&rank=1 (accessed on 18 April 2025).
- ClinicalTrials.gov ID:NCT05965726. RECOVER-VITAL: Platform Protocol, Appendix to Measure the Effects of Paxlovid on Long COVID Symptoms (RECOVER-VITAL). Available online: https://clinicaltrials.gov/study/NCT05965726?term=NCT05965726&rank=1 (accessed on 18 April 2025).
- ClinicalTrials.gov ID:NCT06441955. COVID-19 Long Haul Preventative and Health Promotion Care Clinical Trial Acceleration Program. Available online: https://clinicaltrials.gov/study/NCT06441955?term=NCT06441955&rank=1 (accessed on 27 November 2024).
- ClinicalTrials.gov ID:NCT05576662. Paxlovid for Treatment of Long COVID. Available online: https://clinicaltrials.gov/study/NCT05576662?term=NCT05576662&rank=1 (accessed on 25 September 2024).
- ClinicalTrials.gov ID:NCT05823896. ImPROving Quality of LIFe in the Long COVID Patient (PROLIFIC). Available online: https://clinicaltrials.gov/study/NCT05823896?term=NCT05823896&rank=1 (accessed on 4 December 2024).
- ClinicalTrials.gov ID:NCT05852873. PAxlovid loNg COVID-19 pRevention triAl with recruitMent in the Community in Norway. Available online: https://clinicaltrials.gov/study/NCT05852873?term=NCT05852873&rank=1 (accessed on 18 October 2024).
- ClinicalTrials.gov ID:NCT05999435. Study of LAU-7b for the Treatment of Long COVID in Adults (ESSOR). Available online: https://clinicaltrials.gov/study/NCT05999435?term=NCT05999435&rank=1 (accessed on 28 August 2024).
- ClinicalTrials.gov ID:NCT06161688. Ensitrelvir for Viral Persistence and Inflammation in People Experiencing Long COVID (PREVAIL-LC). Available online: https://clinicaltrials.gov/study/NCT06161688?term=NCT06161688&rank=1 (accessed on 10 February 2025).
- ClinicalTrials.gov ID:NCT04978259. SOLIDARITY Finland Long-COVID (Remdesivir Long-Term Follow-Up Study of COVID Patients). Available online: https://clinicaltrials.gov/study/NCT04978259?cond=NCT04978259.&rank=1 (accessed on 15 July 2022).
- ClinicalTrials.gov ID:NCT05911906. An Open-Label, Clinical Feasibility Study of the Efficacy of Remdesivir for Long-COVID. (ERASE-LC). Available online: https://clinicaltrials.gov/study/NCT05911906?term=NCT05911906&rank=1 (accessed on 24 March 2025).
- ClinicalTrials.gov ID:NCT06316843. Valacyclovir Plus Celecoxib for Post-Acute Sequelae of SARS-CoV-2 (PASC). Available online: https://clinicaltrials.gov/study/NCT06316843?term=NCT06316843&rank=1 (accessed on 22 January 2025).
- ClinicalTrials.gov ID:NCT06792214. Antiviral Strategies in the Prevention of Long-Term Cardiovascular Outcomes Following COVID-19: The paxloviD/Remdesivir Effectiveness for the prEvention of loNg COVID Clinical Trial (DEFEND). Available online: https://clinicaltrials.gov/study/NCT06792214?term=NCT06792214&rank=1 (accessed on 24 January 2025).
- Geng, L.N.; Bonilla, H.; Hedlin, H.; Jacobson, K.B.; Tian, L.; Jagannathan, P.; Yang, P.C.; Subramanian, A.K.; Liang, J.W.; Shen, S.; et al. Nirmatrelvir-Ritonavir and Symptoms in Adults with Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, O.P.O.; Horstia, S.; Laakkonen, S.; Rutanen, J.; Mustonen, J.M.J.; Kalliala, I.E.J.; Ansakorpi, H.; Kreivi, H.R.; Kuutti, P.; Paajanen, J.; et al. Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat. Commun. 2022, 13, 6152. [Google Scholar] [CrossRef]
- Cloherty, A.P.M.; Rader, A.G.; Patel, K.S.; Pérez-Vargas, J.; Thompson, C.A.H.; Ennis, S.; Niikura, M.; Wildenberg, M.E.; Muncan, V.; Schreurs, R.R.C.E.; et al. Berbamine suppresses intestinal SARS-CoV-2 infection via a BNIP3-dependent autophagy blockade. Emerg. Microbes Infect. 2023, 12, 2195020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).