Digestive Manifestations of Post-COVID-19: A Focus on Therapeutic Strategies
Abstract
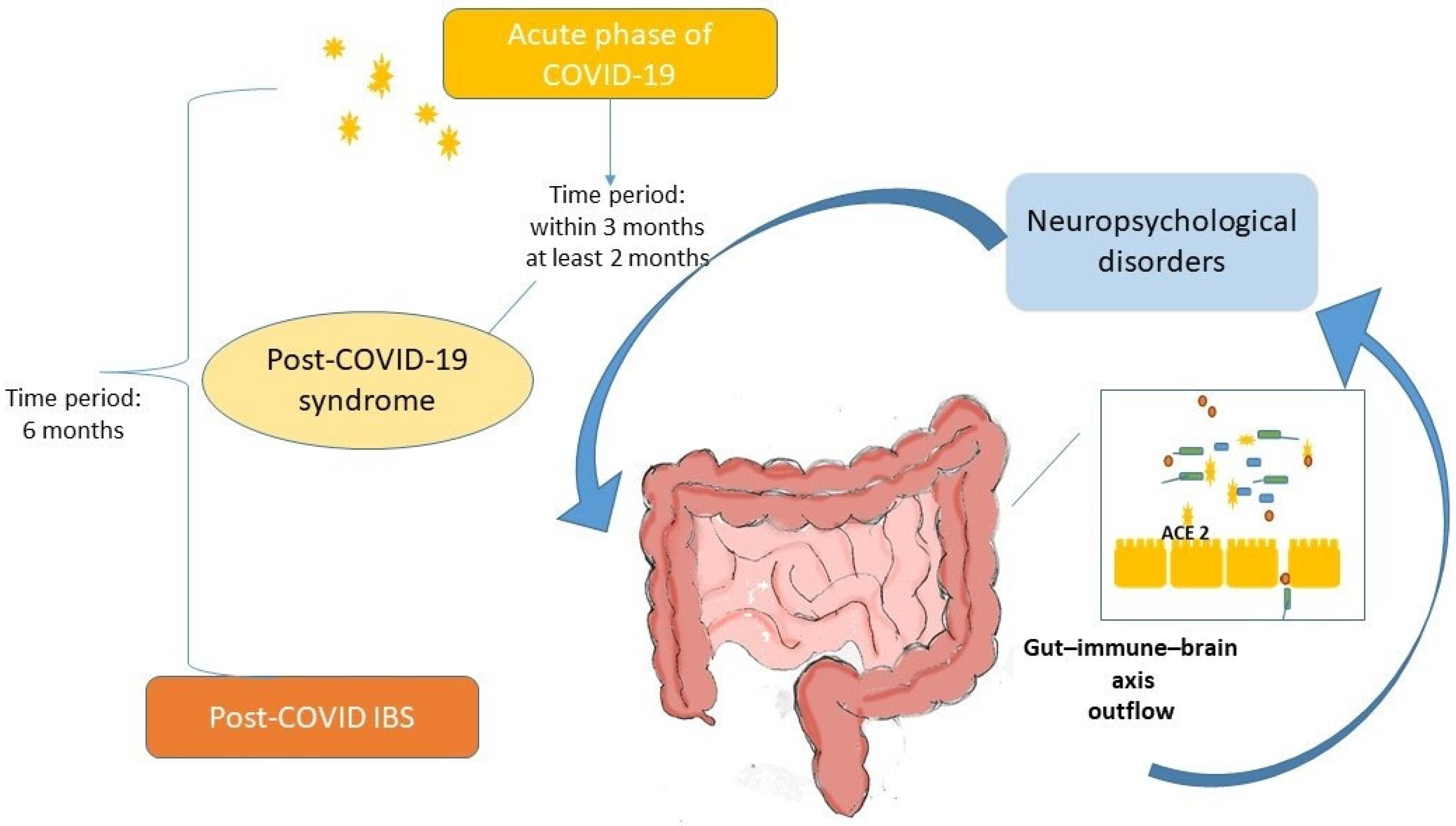
1. Introduction
2. Post-COVID-19 Digestive Manifestations
- -
- Loss of appetite
- -
- Loss of taste
- -
- Abdominal pain
- -
- Diarrhea
- -
- Nausea/vomiting
3. Post-COVID-19 Irritable Bowel Syndrome
4. Therapeutic Strategies
5. Dietary, Prebiotics, Probiotics, Postbiotics, and Other Therapeutic Approaches
6. Antiviral Approach
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/news-room/fact-sheets/detail/post-covid-19-condition-(long-covid) (accessed on 26 February 2025).
- Ely, E.W.; Brown, L.M.; Fineberg, H.V. National Academies of Sciences, Engineering, and Medicine Committee on Examining the Working Definition for Long COVID. Long COVID Defined. N. Engl. J. Med. 2024, 391, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.K.; Salzman, T.; Banal, A.; Morissette, K.; Domingo, F.R.; Cheung, A.M.; Cooper, C.L.; Boland, L.; Zuckermann, A.M.; Mullah, M.A.; et al. Global prevalence of post-COVID-19 condition: A systematic review and meta-analysis of prospective evidence. Health Promot. Chronic Dis. Prev. Can. Res. Policy Pract. 2025, 45, 112–138. [Google Scholar] [CrossRef] [PubMed]
- Al-Oraibi, A.; Woolf, K.; Naidu, J.; Nellums, L.B.; Pan, D.; Sze, S.; Tarrant, C.; Martin, C.A.; Gogoi, M.; Nazareth, J.; et al. Global prevalence of long COVID and its most common symptoms among healthcare workers: A systematic review and meta-analysis. BMJ Public Health 2025, 3, e000269. [Google Scholar] [CrossRef]
- Luo, D.; Mei, B.; Wang, P.; Li, X.; Chen, X.; Wei, G.; Kuang, F.; Li, B.; Su, S. Prevalence and risk factors for persistent symptoms after COVID-19: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2024, 30, 328–335. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018. [Google Scholar] [CrossRef]
- Ashraf, U.M.; Abokor, A.A.; Edwards, J.M.; Waigi, E.W.; Royfman, R.S.; Hasan, S.A.; Smedlund, K.B.; Hardy, A.M.G.; Chakravarti, R.; Koch, L.G. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol. Genom. 2021, 53, 51–60. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients with Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Europe (2022). COVID-19 Pandemic Triggers 25% Increase in Prevalence of Anxiety and Depression Worldwide. Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide#:~:text=In%20the%20first%20year%20of,Health%20Organization%20(WHO)%20today (accessed on 2 March 2022).
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Bellini, M.; Fornai, M.; Satta, P.U.; Bronzini, F.; Bassotti, G.; Blandizzi, C.; Colucci, R. The role of serotonin and its pathways in gastrointestinal disorders. In The Complex Interplay Between Gut-Brain, Gut-Liver, and Liver-Brain Axes; Stasi, C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 67–94. ISBN 9780128219270. [Google Scholar]
- Ancona, G.; Alagna, L.; Alteri, C.; Palomba, E.; Tonizzo, A.; Pastena, A.; Muscatello, A.; Gori, A.; Bandera, A. Gut and airway microbiota dysbiosis and their role in COVID-19 and long-COVID. Front. Immunol. 2023, 14, 1080043. [Google Scholar] [CrossRef]
- Lin, R.; Xiao, M.; Cao, S.; Sun, Y.; Zhao, L.; Mao, X.; Chen, P.; Tong, X.; Ou, Z.; Zhu, H.; et al. Distinct gut microbiota and health outcomes in asymptomatic infection, viral nucleic acid test re-positive, and convalescent COVID-19 cases. mLife 2022, 1, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, Q.; Lau, R.I.; Zhang, J.; Xu, Z.; Yeoh, Y.K.; Leung, T.W.H.; Tang, W.; Zhang, L.; Liang, J.Q.Y.; et al. Faecal microbiome-based machine learning for multi-class disease diagnosis. Nat. Commun. 2022, 13, 6818. [Google Scholar] [CrossRef] [PubMed]
- Comba, I.Y.; Mars, R.A.T.; Yang, L.; Dumais, M.; Chen, J.; Van Gorp, T.M.; Harrington, J.J.; Sinnwell, J.P.; Johnson, S.; Holland, L.A.; et al. Gut Microbiome Signatures During Acute Infection Predict Long COVID. bioRxiv 2024. bioRxiv:2024.12.10.626852. [Google Scholar] [CrossRef]
- Ferreira-Junior AFerreira-Junior, A.S.; Borgonovi, T.F.; De Salis, L.V.V.; Leite, A.Z.; Dantas, A.S.; De Salis, G.V.V.; Cruz, G.N.F.; De Oliveira, L.F.V.; Gomes, E.; Penna, A.L.B.; et al. Detection of Intestinal Dysbiosis in Post-COVID-19 Patients One to Eight Months after Acute Disease Resolution. Int. J. Environ. Res. Public Health 2022, 19, 10189. [Google Scholar] [CrossRef]
- Su, Q.; Lau, R.I.; Liu, Q.; Li, M.K.T.; Yan Mak, J.W.; Lu, W.; Lau, I.S.F.; Lau, L.H.S.; Yeung, G.T.Y.; Cheung, C.P.; et al. The gut microbiome associates with phenotypic manifestations of post-acute COVID-19 syndrome. Cell Host Microbe 2024, 32, 651–660.e4. [Google Scholar] [CrossRef]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Salvi, D.; Cacciari, G.; Kagramanova, A.; Bordin, D.; Drug, V.; Miftode, E.; Fusaroli, P.; et al. Prevalence of Gastrointestinal Symptoms in Severe Acute Respiratory Syndrome Coronavirus 2 Infection: Results of the Prospective Controlled Multinational GI-COVID-19 Study. Am. J. Gastroenterol. 2022, 117, 147–157. [Google Scholar] [CrossRef]
- Ashktorab, H.; Challa, S.R.; Singh, G.; Nanduri, S.; Ibrahim, M.; Martirosyan, Z.; Whitsell, P.; Chirumamilla, L.G.; Shayegh, N.; Watson, K.; et al. Gastrointestinal Manifestations and Their Association with Neurologic and Sleep Problems in Long COVID-19 Minority Patients: A Prospective Follow-Up Study. Dig. Dis. Sci. 2024, 69, 562–569. [Google Scholar] [CrossRef]
- Hawkings, M.J.; Vaselli, N.M.; Charalampopoulos, D.; Brierley, L.; Elliot, A.J.; Buchan, I.; Hungerford, D. A Systematic Review of the Prevalence of Persistent Gastrointestinal Symptoms and Incidence of New Gastrointestinal Illness after Acute SARS-CoV-2 Infection. Viruses 2023, 15, 1625. [Google Scholar] [CrossRef]
- Xie, J.; López-Güell, K.; Dedman, D.; Duarte-Salles, T.; Kolde, R.; López-Blasco, R.; Martínez, Á.; Mercier, G.; Abellan, A.; Arinze, J.T.; et al. Incidence of post-acute COVID-19 symptoms across healthcare settings in seven countries: An international retrospective cohort study using routinely-collected data. EClinicalMedicine. 2024, 77, 102903. [Google Scholar] [CrossRef]
- Baalbaki, N.; Slob, E.M.A.; Kazer, S.W.; IAbdel-Aziz, M.; Bogaard, H.J.; Golebski, K.; Maitland-van der Zee, A.H. The Omics Landscape of Long COVID-A Comprehensive Systematic Review to Advance Biomarker, Target and Drug Discovery. Allergy 2025, 80, 932–948. [Google Scholar] [CrossRef]
- Mohammed, I.; Podhala, S.; Zamir, F.; Shiyam, S.; Salameh, A.R.; Salahuddin, Z.; Salameh, H.; Kim, C.; Sinan, Z.; Kim, J.; et al. Gastrointestinal Sequelae of COVID-19: Investigating Post-Infection Complications-A Systematic Review. Viruses 2024, 16, 1516. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.Y.; Wu, J.F.; Weng, M.T.; Chuang, C.H.; Huang, T.Y.; Tai, W.C.; Tai, C.M.; Chung, C.S.; Chen, C.C.; Lin, C.P.; et al. Exacerbated gastrointestinal symptoms and long COVID in IBD patients with SARS-CoV-2 infection: A multi-center study from taiwan. J. Formos. Med. Assoc. 2024, 123, 866–874. [Google Scholar] [CrossRef]
- Choudhury, A.; Tariq, R.; Jena, A.; Vesely, E.K.; Singh, S.; Khanna, S.; Sharma, V. Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2022, 15, 17562848221118403. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Maida, M.; Cremon, C.; Barbaro, M.R.; Stanghellini, V.; Barbara, G. Meta-analysis: Post-COVID-19 functional dyspepsia and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2023, 58, 6–15. [Google Scholar] [CrossRef]
- Marasco, G.; Hod, K.; Colecchia, L.; Cremon, C.; Barbaro, M.R.; Cacciari, G.; Falangone, F.; Kagramanova, A.; Bordin, D.; Drug, V.; et al. Long-Term Impact of COVID-19 on Disorders of Gut-Brain Interaction: Incidence, Symptom Burden, and Psychological Comorbidities. United Eur. Gastroenterol. J. 2025. Advance online publication. [Google Scholar] [CrossRef]
- Stasi, C.; Bellini, M.; Costa, F.; Mumolo, M.G.; Ricchiuti, A.; Grosso, M.; Duranti, E.; Metelli, M.R.; Gambaccini, D.; Bianchi, L.; et al. Neuroendocrine markers and psychological features in patients with irritable bowel syndrome. Int. J. Color. Dis. 2013, 28, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Bellini, M.; Gambaccini, D.; Duranti, E.; de Bortoli, N.; Fani, B.; Albano, E.; Russo, S.; Sudano, I.; Laffi, G.; et al. Neuroendocrine Dysregulation in Irritable Bowel Syndrome Patients: A Pilot Study. J. Neurogastroenterol. Motil. 2017, 23, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ruan, G.; Chen, L.; Ying, S.; Li, G.; Xu, F.; Xiao, Z.; Tian, Y.; Lv, L.; Ping, Y.; et al. Neurotransmitter and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. Front. Endocrinol. 2022, 13, 817100. [Google Scholar] [CrossRef]
- Yu, L.C. Gastrointestinal pathophysiology in long COVID: Exploring roles of microbiota dysbiosis and serotonin dysregulation in post-infectious bowel symptoms. Life Sci. 2024, 358, 123153. [Google Scholar] [CrossRef]
- Perlot, T.; Penninger, J.M. ACE2—From the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013, 15, 866–873. [Google Scholar] [CrossRef]
- Edwinson, A.; Yang, L.; Chen, J.; Grover, M. Colonic expression of Ace2, the SARS-CoV-2 entry receptor, is suppressed by commensal human microbiota. Gut Microbes 2021, 13, 1984105. [Google Scholar] [CrossRef] [PubMed]
- Penninger, J.M.; Grant, M.B.; Sung, J.J.Y. The Role of Angiotensin Converting Enzyme 2 in Modulating Gut Microbiota, Intestinal Inflammation, and Coronavirus Infection. Gastroenterology 2021, 160, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalová, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867.e20. [Google Scholar] [CrossRef]
- Banu, N.; Panikar, S.S.; Leal, L.R.; Leal, A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: Therapeutic implications. Life Sci. 2020, 256, 117905. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Rosselli, M.; Bellini, M.; Laffi, G.; Milani, S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: The top-down and the bottom-up model. J. Gastroenterol. 2012, 47, 1177–1185. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Iqbal, N.T.; Khan, H.; Khalid, A.; Mahmood, S.F.; Nasir, N.; Khanum, I.; de Siqueira, I.; Van Voorhis, W. Chronic inflammation in post-acute sequelae of COVID-19 modulates gut microbiome: A review of literature on COVID-19 sequelae and gut dysbiosis. Mol. Med. 2025, 31, 22. [Google Scholar] [CrossRef]
- Noviello, D.; Costantino, A.; Muscatello, A.; Bandera, A.; Consonni, D.; Vecchi, M.; Basilisco, G. Functional gastrointestinal and somatoform symptoms five months after SARS-CoV-2 infection: A controlled cohort study. Neurogastroenterol. Motil. 2022, 34, e14187. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Ghoshal, U.; Rahman, M.M.; Mathur, A.; Rai, S.; Akhter, M.; Mostafa, T.; Islam, M.S.; Haque, S.A.; Pandey, A.; et al. Post-infection functional gastrointestinal disorders following coronavirus disease-19: A case-control study. J. Gastroenterol. Hepatol. 2022, 37, 489–498. [Google Scholar] [CrossRef]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Cacciari, G.; Falangone, F.; Kagramanova, A.; Bordin, D.; Drug, V.; Miftode, E.; Fusaroli, P.; et al. Post COVID-19 irritable bowel syndrome. Gut 2022. Advance online publication. [Google Scholar] [CrossRef]
- Cooney, J.; Appiahene, P.; Findlay, R.; Al-Hillawi, L.; Rafique, K.; Laband, W.; Shandro, B.; Poullis, A. COVID-19 infection causing residual gastrointestinal symptoms—A single UK centre case series. Clin. Med. 2022, 22, 181–183. [Google Scholar] [CrossRef]
- Austhof, E.; Bell, M.L.; Riddle, M.S.; Catalfamo, C.; McFadden, C.; Cooper, K.; Scallan Walter, E.; Jacobs, E.; Pogreba-Brown, K. Persisting gastrointestinal symptoms and post-infectious irritable bowel syndrome following SARS-CoV-2 infection: Results from the Arizona CoVHORT. Epidemiol. Infect. 2022, 150, e136. [Google Scholar] [CrossRef]
- Nazarewska, A.; Lewandowski, K.; Kaniewska, M.; Rosołowski, M.; Marlicz, W.; Rydzewska, G. Irritable bowel syndrome following COVID-19: An underestimated consequence of SARS-CoV-2 infection. Pol. Arch. Intern. Med. 2022, 132, 16323. [Google Scholar] [CrossRef]
- Farsi, F.; Zonooz, S.R.; Ebrahimi, Z.; Jebraili, H.; Morvaridi, M.; Azimi, T.; Sikaroudi, M.K.; Heshmati, J.; Khorrami, S.; Mokhtare, M.; et al. The Incidence of Post-infectious Irritable Bowel Syndrome, Anxiety, and Depression in Iranian Patients with Coronavirus Disease 2019 Pandemic: A Cross-Sectional Study. Turk. J. Gastroenterol. 2022, 33, 1033–1042. [Google Scholar] [CrossRef]
- Siyal, M.; Abbas, Z.; Ashraf, J.; Ali Qadeer, M.; Altaf, A. Incidence and predisposing factors for de novo post-COVID-19 irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2023, 35, 59–63. [Google Scholar] [CrossRef]
- Golla, R.; Vuyyuru, S.; Kante, B.; Kumar, P.; Thomas, D.M.; Makharia, G.; Kedia, S.; Ahuja, V. Long-term Gastrointestinal Sequelae Following COVID-19: A Prospective Follow-up Cohort Study. Clin. Gastroenterol. Hepatol. 2023, 21, 789–796.e1. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, C.; Xie, Y.; Zeng, F.; Chen, S.; Chen, R.; Zhang, X.; Huang, S.; Li, D.; Bai, F. Post-infection functional gastrointestinal disorders following coronavirus disease-19: A prospective follow-up cohort study. BMC Infect. Dis. 2023, 23, 422. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Di Nardo, G.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian guidelines for the management of irritable bowel syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig. Liver Dis. 2023, 55, 187–207. [Google Scholar] [CrossRef]
- Calvani, R.; Giampaoli, O.; Marini, F.; Del Chierico, F.; De Rosa, M.; Conta, G.; Sciubba, F.; Tosato, M.; Picca, A.; Ciciarello, F.; et al. Beetroot juice intake positively influenced gut microbiota and inflammation but failed to improve functional outcomes in adults with long COVID: A pilot randomized controlled trial. Clin. Nutr. 2024, 43, 344–358. [Google Scholar] [CrossRef]
- Marzetti, E.; Coelho-Júnior, H.J.; Calvani, R.; Girolimetti, G.; Di Corato, R.; Ciciarello, F.; Galluzzo, V.; Di Mario, C.; Tolusso, B.; Santoro, L.; et al. Mitochondria-Derived Vesicles and Inflammatory Profiles of Adults with Long COVID Supplemented with Red Beetroot Juice: Secondary Analysis of a Randomized Controlled Trial. Int. J. Mol. Sci. 2025, 26, 1224. [Google Scholar] [CrossRef]
- Ribeiro, G.J.S.; Morais, R.N.G.; Abimbola, O.G.; Dias, N.P.; Filgueiras, M.S.; Pinto, A.A.; Novaes, J.F. Unhealthy Food Consumption Is Associated with Post-Acute Sequelae of COVID-19 in Brazilian Elderly People. Infect. Dis. Rep. 2025, 17, 25. [Google Scholar] [CrossRef]
- Barghchi, H.; Araste, A.; Varasteh, N.; Dehnavi, Z.; Zare-Feyzabadi, R.; Vahedi Fard, M.; MohammadHasani, K.; Parirokh, J.; Khorasanchi, Z.; Mohammadi Bajgiran, M.; et al. Food Quality Is Associated with Depression, Anxiety, and Stress Among Recovered COVID-19 Patients: Finding From a Case-Control Study. Clin. Nutr. Res. 2025, 14, 17–29. [Google Scholar] [CrossRef]
- ClinicalTrials.gov ID:NCT05836402. Long COVID-19 Syndrome Lifestyle Intervention Study. Available online: https://clinicaltrials.gov/study/NCT05836402?cond=Post-COVID-19%20Syndrome&term=Gastrointestinal%20Diseases&intr=diet&rank=1 (accessed on 29 April 2024).
- Lim, H.X.; Khalid, K.; Abdullah, A.D.I.; Lee, L.H.; Raja Ali, R.A. Subphenotypes of Long COVID and the clinical applications of probiotics. Biomed. Pharmacother. 2025, 183, 117855. [Google Scholar] [CrossRef]
- Giacosa, A.; Barrile, G.C.; Gasparri, C.; Perna, S.; Rondanelli, M. Positive Effect of Lecithin-Based Delivery Form of Curcuma and Boswellia Extracts on Irritable Bowel Syndrome After COVID-19 Infection. Nutrients 2025, 17, 723. [Google Scholar] [CrossRef]
- Horvath, A.; Habisch, H.; Prietl, B.; Pfeifer, V.; Balazs, I.; Kovacs, G.; Foris, V.; John, N.; Kleinschek, D.; Feldbacher, N.; et al. Alteration of the Gut-Lung Axis After Severe COVID-19 Infection and Modulation Through Probiotics: A Randomized, Controlled Pilot Study. Nutrients 2024, 16, 3840. [Google Scholar] [CrossRef]
- Lau, R.I.; Su, Q.; Lau, I.S.F.; Ching, J.Y.L.; Wong, M.C.S.; Lau, L.H.S.; Tun, H.M.; Mok, C.K.P.; Chau, S.W.H.; Tse, Y.K.; et al. A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2024, 24, 256–265. [Google Scholar] [CrossRef]
- Ranisavljev, M.; Stajer, V.; Todorovic, N.; Ostojic, J.; Cvejic, J.H.; Steinert, R.E.; Ostojic, S.M. Correction: The effects of 3-month supplementation with synbiotic on patient-reported outcomes, exercise tolerance, and brain and muscle metabolism in adult patients with post-COVID-19 chronic fatigue syndrome (STOP-FATIGUE): A randomized Placebo-controlled clinical trial. Eur. J. Nutr. 2025, 64, 80. [Google Scholar] [CrossRef]
- Khani, N.; Abedi Soleimani, R.; Noorkhajavi, G.; Abedi Soleimani, A.; Abbasi, A.; Homayouni Rad, A. Postbiotics as potential promising tools for SARS-CoV-2 disease adjuvant therapy. J. Appl. Microbiol. 2022, 132, 4097–4111. [Google Scholar] [CrossRef]
- Kavita Om, H.; Chand, U.; Kushawaha, P.K. Postbiotics: An alternative and innovative intervention for the therapy of inflammatory bowel disease. Microbiol. Res. 2024, 279, 127550. [Google Scholar] [CrossRef] [PubMed]
- Atieh, O.; Daher, J.; Durieux, J.C.; Abboud, M.; Labbato, D.; Baissary, J.; Koberssy, Z.; Ailstock, K.; Cummings, M.; Funderburg, N.T.; et al. Vitamins K2 and D3 Improve Long COVID, Fungal Translocation, and Inflammation: Randomized Controlled Trial. Nutrients 2025, 17, 304. [Google Scholar] [CrossRef]
- Lau, R.I.; Su, Q.; Ching, J.Y.L.; Lui, R.N.; Chan, T.T.; Wong, M.T.L.; Lau, L.H.S.; Wing, Y.K.; Chan, R.N.Y.; Kwok, H.Y.H.; et al. Fecal Microbiota Transplantation for Sleep Disturbance in Post-acute COVID-19 Syndrome. Clin. Gastroenterol. Hepatol. 2024, 22, 2487–2496.e6. [Google Scholar] [CrossRef]
- Peluso, M.J.; Ryder, D.; Flavell, R.R.; Wang, Y.; Levi, J.; LaFranchi, B.H.; Deveau, T.M.; Buck, A.M.; Munter, S.E.; Asare, K.A.; et al. Tissue-based T cell activation and viral RNA persist for up to 2 years after SARS-CoV-2 infection. Sci. Transl. Med. 2024, 16, eadk3295. [Google Scholar] [CrossRef]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Rössler, A.; Kimpel, J.; Adolph, T.E.; Tilg, H. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology 2022, 163, 495–506.e8. [Google Scholar] [CrossRef]
- Ghafari, M.; Hall, M.; Golubchik, T.; Ayoubkhani, D.; House, T.; MacIntyre-Cockett, G.; Fryer, H.R.; Thomson, L.; Nurtay, A.; Kemp, S.A.; et al. Prevalence of persistent SARS-CoV-2 in a large community surveillance study. Nature 2024, 626, 1094–1101. [Google Scholar] [CrossRef]
- Zuo, W.; He, D.; Liang, C.; Du, S.; Hua, Z.; Nie, Q.; Zhou, X.; Yang, M.; Tan, H.; Xu, J.; et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: A cross-sectional cohort study in China. Lancet Infect. Dis. 2024, 24, 845–855. [Google Scholar] [CrossRef]
- Gostimirovic, M.; Rajkovic, J.; Bukarica, A.; Simanovic, J.; Gojkovic-Bukarica, L. Resveratrol and Gut Microbiota Synergy: Preventive and Therapeutic Effects. Int. J. Mol. Sci. 2023, 24, 17573. [Google Scholar] [CrossRef]
- Russo, C.; Valle, M.S.; Malaguarnera, L.; Romano, I.R.; Malaguarnera, L. Comparison of Vitamin D and Resveratrol Performances in COVID-19. Nutrients 2023, 15, 2639. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Berry, K.; Rajeevan, N.; Li, Y.; Mutalik, P.; Yan, L.; Bui, D.; Cunningham, F.; Hynes, D.M.; Rowneki, M.; et al. Effectiveness of Nirmatrelvir-Ritonavir Against the Development of Post-COVID-19 Conditions Among U.S. Veterans: A Target Trial Emulation. Ann. Intern. Med. 2023, 176, 1486–1497. [Google Scholar] [CrossRef]
- Krumholz, H.M.; Sawano, M.; Bhattacharjee, B.; Caraballo, C.; Khera, R.; Li, S.X.; Herrin, J.; Coppi, A.; Holub, J.; Henriquez, Y.; et al. The PAX LC Trial: A Decentralized, Phase 2, Randomized, Double-Blind Study of Nirmatrelvir/Ritonavir Compared with Placebo/Ritonavir for Long COVID. Am. J. Med. 2025, 138, 884–892.e4. [Google Scholar] [CrossRef]
- ClinicalTrials.gov ID NCT05668091. A Decentralized, Randomized Phase 2 Efficacy and Safety Study of Nirmatrelvir/Ritonavir in Adults with Long COVID. Available online: https://clinicaltrials.gov/study/NCT05668091?term=NCT05668091&rank=1 (accessed on 2 December 2024).
- Sawano, M.; Bhattacharjee, B.; Caraballo, C.; Khera, R.; Li, S.X.; Herrin, J.; Christian, D.; Coppi, A.; Warner, F.; Holub, J.; et al. Nirmatrelvir-ritonavir versus placebo-ritonavir in individuals with long COVID in the USA (PAX LC): A double-blind, randomised, placebo-controlled, phase 2, decentralised trial. Lancet Infect. Dis. 2025. Advance online publication. [Google Scholar] [CrossRef]
- Gragnani, L.; Lorini, S.; Marri, S.; Basile, U.; Santarlasci, V.; Monti, M.; Madia, F.; Petraccia, L.; Stasi, C.; Marello, N.; et al. Hematological and Genetic Markers in the Rational Approach to Patients with HCV Sustained Virological Response with or Without Persisting Cryoglobulinemic Vasculitis. Hepatology 2021, 74, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Mazzaro, C.; Quartuccio, L.; Adinolfi, L.E.; Roccatello, D.; Pozzato, G.; Nevola, R.; Tonizzo, M.; Gitto, S.; Andreone, P.; Gattei, V. A Review on Extrahepatic Manifestations of Chronic Hepatitis C Virus Infection and the Impact of Direct-Acting Antiviral Therapy. Viruses 2021, 13, 2249. [Google Scholar] [CrossRef] [PubMed]
- Paramythiotis, D.; Karlafti, E.; Didagelos, M.; Fafouti, M.; Veroplidou, K.; Protopapas, A.A.; Kaiafa, G.; Netta, S.; Michalopoulos, A.; Savopoulos, C. Post-COVID-19 and Irritable Bowel Syndrome: A Literature Review. Medicina 2023, 59, 1961. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov ID NCT06511063. Antiviral Clinical Trial for Long COVID-19. Available online: https://clinicaltrials.gov/study/NCT06511063?term=NCT06511063&rank=1 (accessed on 20 December 2024).
- ClinicalTrials.gov ID:NCT05595369. RECOVER-VITAL: Platform Protocol to Measure the Effects of Antiviral Therapies on Long COVID Symptoms (RECOVER-VITAL). Available online: https://clinicaltrials.gov/study/NCT05595369?term=NCT05595369&rank=1 (accessed on 18 April 2025).
- ClinicalTrials.gov ID:NCT05965726. RECOVER-VITAL: Platform Protocol, Appendix to Measure the Effects of Paxlovid on Long COVID Symptoms (RECOVER-VITAL). Available online: https://clinicaltrials.gov/study/NCT05965726?term=NCT05965726&rank=1 (accessed on 18 April 2025).
- ClinicalTrials.gov ID:NCT06441955. COVID-19 Long Haul Preventative and Health Promotion Care Clinical Trial Acceleration Program. Available online: https://clinicaltrials.gov/study/NCT06441955?term=NCT06441955&rank=1 (accessed on 27 November 2024).
- ClinicalTrials.gov ID:NCT05576662. Paxlovid for Treatment of Long COVID. Available online: https://clinicaltrials.gov/study/NCT05576662?term=NCT05576662&rank=1 (accessed on 25 September 2024).
- ClinicalTrials.gov ID:NCT05823896. ImPROving Quality of LIFe in the Long COVID Patient (PROLIFIC). Available online: https://clinicaltrials.gov/study/NCT05823896?term=NCT05823896&rank=1 (accessed on 4 December 2024).
- ClinicalTrials.gov ID:NCT05852873. PAxlovid loNg COVID-19 pRevention triAl with recruitMent in the Community in Norway. Available online: https://clinicaltrials.gov/study/NCT05852873?term=NCT05852873&rank=1 (accessed on 18 October 2024).
- ClinicalTrials.gov ID:NCT05999435. Study of LAU-7b for the Treatment of Long COVID in Adults (ESSOR). Available online: https://clinicaltrials.gov/study/NCT05999435?term=NCT05999435&rank=1 (accessed on 28 August 2024).
- ClinicalTrials.gov ID:NCT06161688. Ensitrelvir for Viral Persistence and Inflammation in People Experiencing Long COVID (PREVAIL-LC). Available online: https://clinicaltrials.gov/study/NCT06161688?term=NCT06161688&rank=1 (accessed on 10 February 2025).
- ClinicalTrials.gov ID:NCT04978259. SOLIDARITY Finland Long-COVID (Remdesivir Long-Term Follow-Up Study of COVID Patients). Available online: https://clinicaltrials.gov/study/NCT04978259?cond=NCT04978259.&rank=1 (accessed on 15 July 2022).
- ClinicalTrials.gov ID:NCT05911906. An Open-Label, Clinical Feasibility Study of the Efficacy of Remdesivir for Long-COVID. (ERASE-LC). Available online: https://clinicaltrials.gov/study/NCT05911906?term=NCT05911906&rank=1 (accessed on 24 March 2025).
- ClinicalTrials.gov ID:NCT06316843. Valacyclovir Plus Celecoxib for Post-Acute Sequelae of SARS-CoV-2 (PASC). Available online: https://clinicaltrials.gov/study/NCT06316843?term=NCT06316843&rank=1 (accessed on 22 January 2025).
- ClinicalTrials.gov ID:NCT06792214. Antiviral Strategies in the Prevention of Long-Term Cardiovascular Outcomes Following COVID-19: The paxloviD/Remdesivir Effectiveness for the prEvention of loNg COVID Clinical Trial (DEFEND). Available online: https://clinicaltrials.gov/study/NCT06792214?term=NCT06792214&rank=1 (accessed on 24 January 2025).
- Geng, L.N.; Bonilla, H.; Hedlin, H.; Jacobson, K.B.; Tian, L.; Jagannathan, P.; Yang, P.C.; Subramanian, A.K.; Liang, J.W.; Shen, S.; et al. Nirmatrelvir-Ritonavir and Symptoms in Adults with Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, O.P.O.; Horstia, S.; Laakkonen, S.; Rutanen, J.; Mustonen, J.M.J.; Kalliala, I.E.J.; Ansakorpi, H.; Kreivi, H.R.; Kuutti, P.; Paajanen, J.; et al. Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat. Commun. 2022, 13, 6152. [Google Scholar] [CrossRef]
- Cloherty, A.P.M.; Rader, A.G.; Patel, K.S.; Pérez-Vargas, J.; Thompson, C.A.H.; Ennis, S.; Niikura, M.; Wildenberg, M.E.; Muncan, V.; Schreurs, R.R.C.E.; et al. Berbamine suppresses intestinal SARS-CoV-2 infection via a BNIP3-dependent autophagy blockade. Emerg. Microbes Infect. 2023, 12, 2195020. [Google Scholar] [CrossRef]
| Authors | Study Design | Country | Study Population | Microbiota Profile in Controls | Microbiota Profile in Post-COVID-19 Syndrome |
|---|---|---|---|---|---|
| Li et al. [14] | Longitudinal study | China | 13 asymptomatic infections, 24 post-acute COVID-19 syndrome patients, 31 discharged patients with SARS-CoV-2 re-positive, and 13 non-COVID-19 healthy controls | Asymptomatic infected patients and patients without symptoms after discharge had significantly higher microbial diversity than patients with adverse outcomes. A relative abundance of Bacteroides was found in non-COVID-19 healthy subjects | Post-acute COVID-19 syndrome patients were enriched with opportunistic pathogens (Escherichia coli, Clostridium ramosum, Klebsiella ornithinolytica, and Hungatella hathewayi) |
| Su et al. [15] | Machine-learning methods | China | 2320 individuals with different characterized phenotypes (174 colorectal cancer; 168 colorectal adenomas; 200 Crohn’s disease; 147 ulcerative colitis; 145 irritable bowel syndrome—diarrhea subtype; 148 obesity; 143 cardiovascular disease; 302 post-acute COVID-19 syndrome and 893 healthy controls | Compared to controls, almost all disease states were associated with a decreased abundance of Bacillota or Actinomycetota and an increase in Bacteroidota | Post-acute COVID-19 syndrome and other different phenotypes (Crohn’s disease, colorectal cancer, irritable bowel syndrome—diarrhea subtype, obesity, ulcerative colitis) were positively associated with Klebsiella pneumonia and negatively correlated with Roseburia intestinalis Post-acute COVID-19 syndrome showed a significant increase in abundance of Phocaeicola vulgatus and Bacteroides xylanisolvens, while those with UC were enriched in Bacteroides ovatus |
| Comba et al. [16] | Prospective | US | 799 subjects: 380 positive and 419 negative for SARS-CoV-2. Within the 1-year follow-up, 80 positive patients for SARS-CoV-2 developed long COVID-19 | SARS-CoV-2-negative subjects had higher α-diversity based on the Chao1 metric but comparable based on the Shannon index | The presence of some specific species during the acute phase, such as Prevotella species, Leuconostoc species, and members of the Lactobacillaceae family like Eubacterium species and Agathobacter species predict long COVID-19 Changes in Lachnospiraceae were associated with the development of digestive symptoms |
| Ferreira-Junior et al. [17] | Longitudinal study | Brazil | 149 patients, months after having acute COVID-19, of whom approximately 39% developed clinical manifestations after the acute phase; 71 controls | Compared with controls, differences in the microbiota diversity in post-COVID-19 patients | Possible association between post-COVID-19 dysbiosis and some genera, including Desulfovibrio, Haemophilus, Dialister, and Prevotella, in addition to decreased beneficial microbes, associated with antibiotic-induced dysbiosis, such as Bifidobacterium and Akkermansia |
| Su et al. [18] | Cross-sectional and longitudinal cohorts | China | 1207 with post-COVID-19 (n = 1011 in two cross-sectional cohorts and n = 196 longitudinal cohort) A cohort of 201 previous COVID-19 subjects without post-COVID-19 syndrome and a cohort of 653 healthy subjects without COVID-19 exposure were employed as non-Post-COVID-19 controls | The diversity (Shannon) and richness (observed number of species) of the gut microbiome in the post-COVID-19 syndrome were significantly lower than control group |
Enrichment of opportunistic pathogens, such as Klebsiella quasipneumoniae and Mediterraneibacter gnavus, in subjects with post-COVID-19 syndrome Coprobacillus cateniformis being positively associated with most digestive symptoms |
| Authors | Study Design | Country | Study Population | Methods | Time Interval Between COVID-19 and Post-COVID-19 IBS |
|---|---|---|---|---|---|
| Ghoshal et al. [42] | Case–control study | India Bangladesh | 280 COVID-19 patients and 264 controls | Follow up at 1, 3, and 6 months using translated validated Rome Questionnaires | At 6 months 15 (5.3%) developed IBS and 5 (1.8%) IBS–UD overlap |
| Marasco et al. [43] | Prospective, multicenter, controlled study | Italy | 883 hospitalized patients without digestive symptoms (614 COVID-19 patients and 269 controls) | Follow up at 1, 6, and 12 months post-hospitalization by Rome IV criteria | At 6 months 0.5% of COVID-19 patients developed IBS versus 3.2% in controls |
| Cooney et al. [44] | Single-center case series | UK | 122 COVID-19 patients, of whom 48 completed the follow-up survey | Weblink to a symptom survey at the point of their acute COVID-19 illness, and 6 months later, a follow-up survey | At 6 months new digestive symptoms affecting 21 patients (43.8 %); hypothesis of a post-COVID-19 IBS |
| Austhof et al. [45] | Population-based COVID-19 cohort | USA | 1475 COVID-19 patients (N = 976 no digestive symptoms at baseline; N = 499 digestive symptoms) | Follow-up at 1.5, 3, 4.5, and 6 months Post-COVID-19 IBS diagnosed by Rome IV criteria | Average 6.2 months (175 days, S.D.: 61.6) IBS occurred in 3.0% (n = 15) of participants |
| Nazarewska et al. [46] | Prospective, single-center evaluation | Poland | 257 COVID-19 patients | Follow-up at 3 and 6 months by Rome IV Diagnostic Questionnaire | After 3 and 6 months of follow-up IBS-like symptoms in 14 (5.4%) and IBS in 15 individuals (5.8%) |
| Farsi et al. [47] | Cross-sectional study | Iran | 233 COVID-19 patients | Follow-up at 6 months by Rome IV criteria questionnaire | At 6 months 27 (11.6%) patients developed IBS |
| Siyal et al. [48] | Prospective | Pakistan | 303 hospitalized COVID-19 patients without a prior history of IBS | Rome-IV criteria | IBS symptoms in 32 (10.6%) patients, of whom 17 (53.13%) diarrhea-predominant, 10 (31.25%) constipation-predominant, and 5 (15.62%) mixed-type IBS |
| Golla et al. [49] | Prospective follow-up cohort study | India | 320 COVID-19 patients, 2 control groups (320 healthy spouses/family controls and 280 healthy COVID-19-negative controls) | Follow up at 1, 3, and 6 months by the Rome IV criteria | At 3 months, 8 (2.5%) had IBS-like symptoms |
| Zhang et al. [50] | Prospective | China | 190 COVID-19 patients and 160 healthy controls | Follow-up for 1, 3, and 6 months by Rome III and Rome IV questionnaires | At 6 months 7 (3.7%) COVID-19 patients developed IBS |
| ClinicalTrials.gov ID | Phase | State | Official Title | Interventions/Treatments |
|---|---|---|---|---|
| NCT05668091 [74] | Phase 2 | Completed | An Interventional Decentralized Phase 2, Randomised, Double-Blind, 2-Arm Study to Investigate the Efficacy and Safety of Orally Administered Nirmatrelvir/Ritonavir Compared with Placebo/Ritonavir in Participants with Long COVID | Drug: Nirmatrelvir Drug: Ritonavir Drug: Placebo |
| NCT06511063 [79] | Phase 2 | Recruiting | Investigating the Feasibility of Repurposing HIV Antivirals in Adults with Long COVID | Truvada (tenofovir disoproxil/emtricitabine, TDF/FTC, Group 1) or Selzentry (Group 2), or a placebo (pill) (Group 3), taken daily for 90 days |
| NCT05595369 [80] | Phase 2 | Completed | RECOVER-VITAL: A Platform Protocol for Evaluation of Interventions for Viral Persistence, Viral Reactivation, and Immune Dysregulation in Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) | Experimental: Paxlovid 25-day dosing Experimental: Paxlovid 15-day dosing Placebo Comparator: Control |
| NCT05965726 [81] | Phase 2 | Completed | RECOVER-VITAL: A Platform Protocol for Evaluation of Interventions for Viral Persistence, Viral Reactivation, and Immune Dysregulation in Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) | Drug: Paxlovid 25-day dosing Drug: Paxlovid 15-day dosing Drug: Control |
| NCT06441955 [82] | Phase 4 | Recruiting | COVID-19 Long Haul Syndrome: Undiagnosed Disorder Post COVID-19 Alternative Treatment Study. | Drug: Ritonavir-Boosted Nirmatrelvir (Paxlovid) Diagnostic Test: Physiological Evaluation Biological: Moderna COVID-19 Vaccine Behavioral: Biopsychological Behavioral: Behavioral (e.g., Psychotherapy, Lifestyle Counseling) Genetic: Genetic (including gene transfer, stem cell, and recombinant DNA) Combination Product: Multidisciplinary approach |
| NCT05576662 [83] | Phase 2 | Completed | Selective Trial Of Paxlovid for PASC (STOP-PASC): Randomised Double-Blind Placebo-Controlled Pilot Trial of Paxlovid for the Treatment of PASC | Drug: Nirmatrelvir Drug: Placebo Drug: Ritonavir |
| NCT05823896 [84] | Phase 2 | Completed | An Interventional, Double-Blinded, 2-Arm Study to Investigate the Efficacy of Orally Administered Nirmatrelvir/Ritonavir Compared with Placebo/Ritonavir in Non-hospitalized Adult Participants Suffering from Post-COVID | Drug: Nirmatrelvir/ritonavir Drug: Placebo/ritonavir |
| NCT05852873 [85] | Phase 3 | Recruiting | PAxlovid loNg COVID-19 pRevention triAl With recruitMent In the Community in Norway | Drug: Nirmatrelvir/ritonavir Drug: Placebo |
| NCT05999435 [86] | Phase 2 Phase 3 | Active, not recruiting | A Double-Blind, Randomised, Placebo-Controlled, Adaptive Phase 2/3 Study of the Efficacy of LAU-7b in the treatment of Adults with Long COVID and Moderate to Severe Symptoms | Drug: LAU-7b for 3 cycles Drug: LAU-7b for 1 cycle, then placebo Other: Placebo for 3 cycles |
| NCT06161688 [87] | Phase 2 | Active, not recruiting | Placebo-Controlled, Randomised Trial of Ensitrelvir (S-217622) for Viral Persistence and Inflammation in People Experiencing Long COVID (PREVAIL-LC) | Drug: Ensitrelvir Other: Placebo |
| NCT04978259 [88] | Phase 4 | Unknown | Long-term Follow-up of a Randomised Multicenter Trial on Impact of Long-COVID in Hospitalized COVID-19 Patients | Drug: Remdesivir |
| NCT05911906 [89] | Phase 4 | Recruiting | An Open-label, Clinical Feasibility Study of the Efficacy of Remdesivir for Long-COVID. | Drug: Remdesivir |
| NCT06316843 [90] | Phase 2 | Completed | A Randomised, Double-Blinded, Placebo-Controlled, Pilot Study of the Combination of Valacyclovir + Celecoxib (IMC-2) for the Treatment of Post-Acute Sequelae of SARS-CoV-2 Infection in Adults | Drug: Valacyclovir/celecoxib dose 1 Drug: Valacyclovir/celecoxib dose 2 Drug: Placebo |
| NCT06792214 [91] | Phase 4 | Recruiting | Antiviral Strategies in the Prevention of Long-term Cardiovascular Outcomes Following COVID-19: The paxloviD/Remdesivir Effectiveness For the prEvention of loNg coviD (DEFEND) Clinical Trial | Drug: Nirmatrelvir/ritonavir Drug: Remdesivir |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasi, C.; Bellini, M. Digestive Manifestations of Post-COVID-19: A Focus on Therapeutic Strategies. Pathogens 2025, 14, 555. https://doi.org/10.3390/pathogens14060555
Stasi C, Bellini M. Digestive Manifestations of Post-COVID-19: A Focus on Therapeutic Strategies. Pathogens. 2025; 14(6):555. https://doi.org/10.3390/pathogens14060555
Chicago/Turabian StyleStasi, Cristina, and Massimo Bellini. 2025. "Digestive Manifestations of Post-COVID-19: A Focus on Therapeutic Strategies" Pathogens 14, no. 6: 555. https://doi.org/10.3390/pathogens14060555
APA StyleStasi, C., & Bellini, M. (2025). Digestive Manifestations of Post-COVID-19: A Focus on Therapeutic Strategies. Pathogens, 14(6), 555. https://doi.org/10.3390/pathogens14060555









