1. Introduction
Streptococcus pneumoniae is a gram-positive, lancet-shaped bacterium that colonizes the human nasopharynx and causes pneumonia, bacteremia, meningitis, and other infections, mainly in children and older individuals [
1]. The capsule of
S. pneumoniae, composed of polysaccharides, is associated with virulence; based on the composition of the capsule,
S. pneumoniae is classified into more than 100 serotypes [
2]. The 7-valent pneumococcal conjugate vaccine (PCV7) developed for children showed a high coverage of pneumococcal serotypes of invasive pneumococcal diseases (IPD) in young children [
3]. In Japan, public funding of the PCV7 vaccination for children under five years of age began in November 2010 and the vaccine was incorporated into the routine vaccination schedule in April 2013. It was subsequently replaced with the 13-valent pneumococcal conjugate vaccine (PCV13) in November 2013. In adults aged 65 years and older, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) became a routine vaccination in November 2014.
Several studies have shown a significant decrease in vaccine serotypes (VT) in children as a result of routine vaccination [
4,
5,
6]. A reduction in VT in adults has also been reported [
7,
8,
9,
10]. With these serotype changes, the increase in antibiotic-resistant non-VT has become an issue. In particular, an increase in the meropenem-resistant serotype 15A has been reported in Japan [
11]. Whether the trends of these changes in serotype and drug susceptibility occur equivalently in remote areas with small populations or whether they have regional characteristics requires clarification. It would also be interesting to confirm the association with the serotypes contained in the 20-valent pneumococcal conjugate vaccine (PCV20), which was approved in the United States (U.S.) in 2021 [
12,
13]. This new vaccine, which has not yet been approved in Japan, contains seven serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F) not present in the PCV13. In this study, we investigated changes in
S. pneumoniae serotypes and antibiotic susceptibility between April 2012 and December 2016 in the Minamiaizu region of Fukushima Prefecture in Japan, which has a declining birthrate and aging population. These results may provide useful information for future pneumococcal vaccine policy.
3. Results
Of the total 281 isolates, 111 were from 2012–2013, 103 from 2014, and 67 from 2016; 56.2% were male. Most of the study population belonged to the 1–4 years age group (60.5%), while only 3.2% belonged to the 18–64 age group. It was noted that approximately 12.8% of the study participants were older than 65 years. Nasopharyngeal swabs accounted for most (82.9%) of the specimens, while otorrhea accounted for only 1.1%. Moreover, sputum was detected in 16.0% of the study population with all the individuals aged ≥18 and older, of whom the majority were older adults. Acute bronchitis was the most common diagnosis (37.7%), followed by upper respiratory infection (29.5%) and acute pneumonia (22.4%). Acute otitis media (10.3%) was the least common.
3.1. Distribution of Serotypes
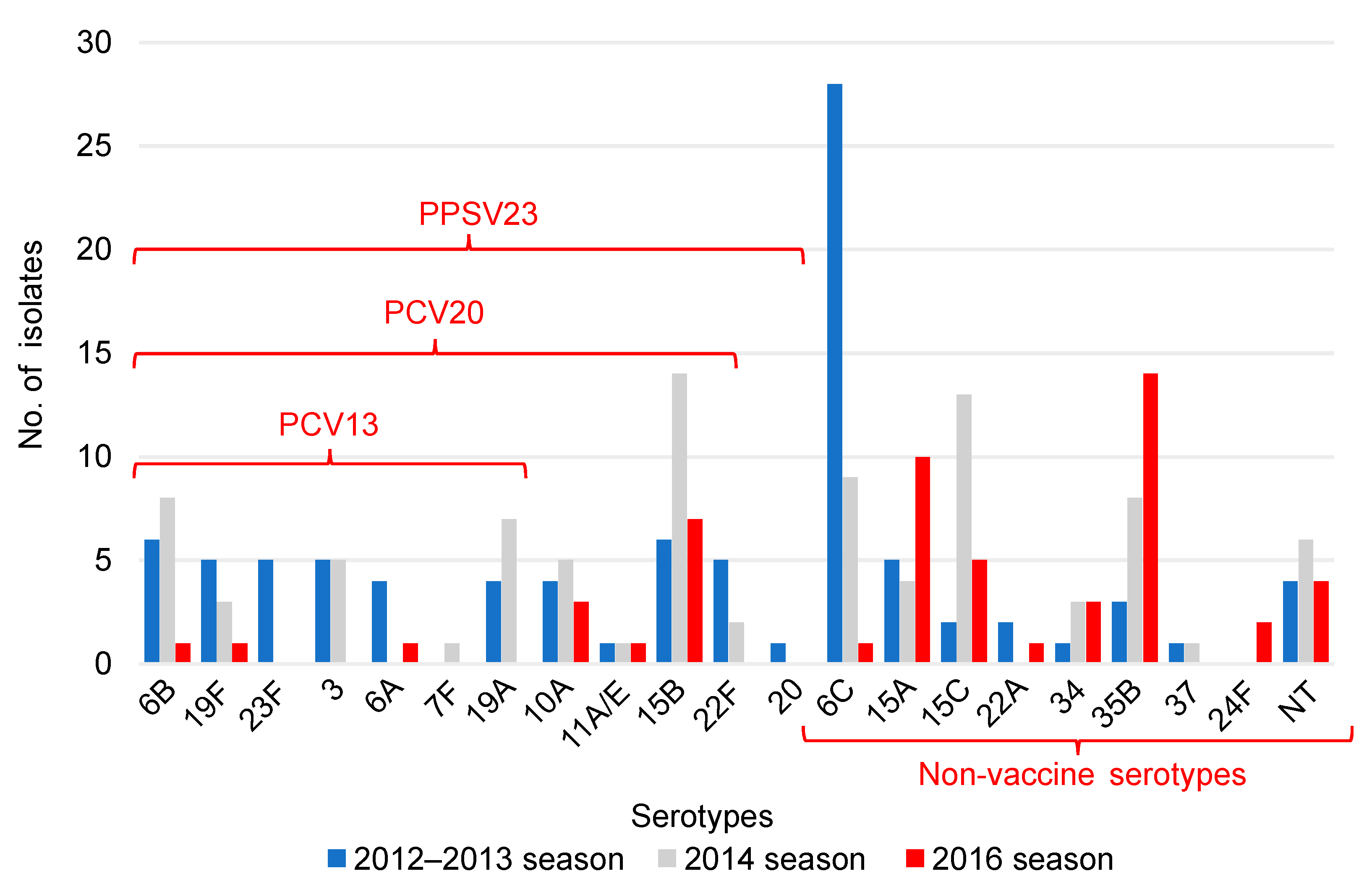
A significant decrease in the isolates of serotypes included in the PCV13 and an increase in the isolates of serotypes not included in the PCV13 were observed in children (
p ≤ 0.001). There was also a significant increase in all the isolates of serotypes not included in vaccines (
p ≤ 0.006) (
Table 2). In the 2012–2013 season, 6C was the serotype with the highest prevalence, whereas in the 2016 season, 35B was the most common serotype, followed by 15A (
Figure 1).
The PCV20 non-PCV13 serotypes cover seven serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F). The PPSV 23 non-PCV20 serotypes cover four serotypes (2, 9N, 17F, and 20). PCV13, 13-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
In adults, a significant increase in isolates with serotypes not included in any vaccine was observed (
p ≤ 0.026) (
Table 3); serotype 3 was the most common serotype detected in the 2012–2013 season, while 35B was the most detected in the 2016 season (
Figure 2).
The PCV20 non-PCV13 serotypes cover seven serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F). The PPSV 23 non-PCV20 serotypes cover four serotypes (2, 9N, 17F and 20). PCV13, 13-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
3.2. Antimicrobial Susceptibilities
In children, the penicillin and meropenem resistance rates decreased significantly during the 2014 season compared to those during the 2012–2013 and 2016 seasons. In adults, the penicillin resistance rate decreased significantly during the 2016 season compared to that during the 2012–2013 and 2014 seasons (
Table 4 and
Table 5).
Few resistant isolates of cefotaxime and levofloxacin were observed in children and adults in any of the seasons and more than 80% of isolates were resistant to erythromycin.
In this study, the most frequently detected serotype 6C drug resistance in children during the 2012–2013 season was penicillin resistance in 3.6% (1/28) and meropenem resistance in 0.0% (0/28). We detected a large number of serotype 15A (penicillin resistant: 90.0% [9/10]; meropenem resistant: 60.0% [6/10]) and 35B (penicillin resistant: 64.3% [9/14]; meropenem resistant: 50.0% [7/14]) cases resistant to penicillin and meropenem in children in the 2016 season (
Table 6). In adults, serotype 3, the most common in the 2012–2013 season, and serotype 35B, the most common in 2016, were both susceptible in penicillin and meropenem (
Table 7).
3.3. MLST
All isolates of serotype 15A (6, 6, and 12 isolates in the 2012–2013, 2014, and 2016 seasons, respectively) were classified as ST63.
4. Discussion
Streptococcus pneumoniae is a bacterium that is endemic to the respiratory tract. Although carrier status does not cause symptoms, the disease develops when the mucosal barrier fails and the bacteria invade the body and multiply. In addition to giving rise to IPD, such as meningitis and bacteremia, potentially life-threatening
S. pneumoniae is the most common cause of pneumonia and otitis media. Since pneumococcal infections are particularly prevalent in infants and young children, vaccination at an early age is imperative to prevent disease. In addition, since infants and toddlers often congregate in groups at daycare centers and other places of gathering, transmission of
S. pneumoniae may occur from one child to another, who then brings it home and exposes the adults living in the same household, thereby spreading the disease. IPD in adults and children decreased with the introduction of the PCV7 as a routine immunization in the U.S. in 2000 [
16]. In 2010, U.S. routine vaccination substituted the PCV7 with the PCV13 and affected serotype substitution of IPD in adults in 2012–2013 [
17]. In Japan, the PCV7 became a routine vaccination for infants in April 2013, and the PCV13 in November 2013; the prevalence of the PCV7 serotypes in adult IPD decreased from 43.3% to 23.8% during 2010–2013 after the introduction of the PCV7 in children [
7]. In addition, studies of serotypes in Japanese children and adults with IPD from 2010–2017 showed that the proportion of the PCV13 serotypes decreased from 89.0% to 12.1% in children and from 74.1% to 36.2% in adults [
9], indicating an indirect effect of pneumococcal vaccination in children and adults.
This study investigated serotypes and drug susceptibility of
S. pneumoniae isolates among children and older individuals at a single institution in a remote area of Japan with a low birthrate and an aging population before the introduction of the PCV13 in 2012–2013 and after its introduction in 2014 and 2016. The results showed a significant decrease in the PCV13 serotypes in children after the routine PCV13 vaccination (
Table 2) and a decreasing trend in adults, although no significant difference was observed (
Table 3). In children, serotype 6C was the most common serotype in the 2012–2013 season, but the serotypes 15A and 35B were detected in large numbers in the 2016 season (
Figure 1). In adults, serotype 3 was the most common serotype in the 2012–2013 season, while serotype 35B was the most frequently detected in the 2016 season (
Figure 2). Penicillin and meropenem drug resistance decreased significantly in children in the 2014 season, but resistance to both drugs increased in the 2016 season (
Table 4). In adults, resistance to penicillin significantly decreased in the 2016 season (
Table 5); penicillin and meropenem resistance rates were high for the serotypes 15A and 35B, which were detected in large numbers in children in the 2016 season (
Table 6).
Serotype 6C was differentiated from the classic serotype 6A in 2007 [
18]. Since the introduction of the PCV7, which contains a serotype 6B conjugate, serotype substitution has occurred, and a number of 6C isolates have been found in other countries [
19,
20,
21]. In Japan, serotype 6C was also detected in large numbers around 2012 [
22]. The PCV13, which contains a serotype 6A conjugate, may have cross-protection to serotype 6C [
23,
24,
25]. In this study, serotype 6C was the most common serotype in children in the 2012–2013 season but, in the 2016 season, serotype 6C was drastically reduced to one isolate (
Figure 1). This result may be due to the cross-protection of the PCV13. Most of the serotype 6C in children detected in the 2012–2013 season was susceptible to penicillin and meropenem (
Table 6). Serotype 6C was detected in a study of serotype trends in IPD and non-IPD in Japan, but no significant increase or drug resistance was observed in this isolate [
6,
9].
Serotype 3 differs from many other serotypes of
S. pneumoniae in that it forms mucoid colonies and has high capsule production, resulting in high virulence and resistance to vaccine-induced antibodies [
26]. A survey of serotype changes in adults with IPD in 2010–2013 in Japan showed that they accounted for 15.9% (114/715) of the cases and were most frequently detected among cases of acute pneumonia at 22.4% (84/375) [
7]. In a nationwide study of IPD in Japan in 2016–2018, serotype 3 was also common, with 15.3% (27/177), along with 12F at 16.4% (29/177) [
27]. Thus, the frequency has not decreased in Japan before or after the introduction of the PCV13. In this study, serotype 3 was the most common serotype during the 2012–2013 season in adults (
Figure 2). All the adult cases of serotype 3 were also acute pneumonia, although the number of adult specimens was small and most of the specimens were sputum. The nationwide surveillance of pediatric patients in Japan after the introduction of the PCV13 showed a low prevalence rate of serotype 3 among IPD and non-IPD patients in 2014, at 0.8% and 8.5%, respectively [
5]. In this study, the proportion of serotype 3 was also low in children in all seasons (
Figure 1). Serotype 3 remains one of the predominant serotypes worldwide, even after the introduction of the vaccine, and is more important in older adults [
28].
After the introduction of the PCV13, a rapid increase in serotypes 15A and 35B was observed in IPD cases in both adults and children in several countries worldwide [
29,
30,
31,
32,
33]. Studies in the U.S., U.K., Italy, and other countries have shown that 15A and 35B are endemic in the nasopharynx as well as in IPD cases and are highly resistant to antibiotics [
34,
35,
36,
37,
38]. In Japan, a significant increase in IPD cases of serotypes 15A and 35B in both adults and children was also observed after the introduction of the PCV7 and the PCV13 [
9]. In a nationwide survey of children and adults with and without IPD in Japan between 2015 and 2017, serotype 15A was most frequently detected in non-IPD isolates, followed by 35B. Drug resistance was significantly higher in non-IPD isolates, and serotypes 15A and 35B showed a trend toward multidrug resistance [
6]. In a survey of individuals without IPD in Japan in 2018–2019, serotypes 15A and 35B were most frequently detected in children, indicating multidrug resistance [
39].
In this study, we observed an increase in serotypes 15A and 35B isolates which were resistant to penicillin and meropenem in children during the 2016 season (
Table 6); however, in adults, two serotype 15A and four serotype 35B isolates were detected and susceptible to both penicillin and meropenem in the 2016 season (
Table 7). The causes for these observations are unclear, but we may not have detected any resistant isolates in adults due to the limited sample size. As described above, the increase in serotype 15A and 35B drug-resistant
S. pneumoniae is a problem throughout the world, including Japan. However, the increase in multidrug-resistant
S. pneumoniae 15A has become a problem in Japan in particular. Nakano S et al. reported an increase in pneumococcal infections caused by meropenem-resistant serotype 15A-ST63 isolates in Japan after the introduction of the PCV13 [
11]. In this study, all isolates of serotype 15A were susceptible to cefotaxime and MLST analysis showed all isolates to be ST63. In Japan, serotype 15A ST9084 isolates were detected and resistant to cefotaxime and these isolates were derived from clones of serotype 15A ST63 and generated by a recombination event in the pbp2x region [
40]. The future trends of the spread of these isolates should be monitored carefully. In the U.S., the PCV20 was approved for individuals aged 18 and older in June 2021 [
12,
13]. This new vaccine has not yet been approved in Japan. Seven serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F) have been added to the PCV20 in addition to the PCV13 serotypes, but serotypes 15A and 35B are not included and there is concern that these serotypes may increase among pneumococcal infections, including IPD.
A limitation of this study is that it is a retrospective study conducted at a single institution. The sample size was small and there were insufficient adult participants to confirm significant differences. Specimens obtained from children may have contained
S. pneumoniae in a carrier state. Sputum specimens were obtained from all adults, whereas nasopharyngeal swab specimens were obtained from most of the children. Furthermore, the carriage rates of
S. pneumoniae are particularly high in children compared to those in older adults [
38,
41,
42]. Although vaccination history could not be confirmed, the PCV13 immunization coverage among Japanese children has remained above 95% throughout the study period [
9] and the rate was expected to be similar in the regions studied in this study. This study was stopped after 2016 because the physician in charge of this study was transferred to another hospital and after 2019, due to the outbreak of a new coronavirus infection, it became difficult to collect specimens in outpatients and detection of
S. pneumoniae decreased dramatically. Although we were unable to confirm the serotypes of recent pneumococcal specimens, this study provides important data to Japanese public health officials and bacterial researchers which could help confirm
S. pneumoniae serotype changes before and after the PCV13.