Abstract
Globally, Klebsiella pneumoniae (K. pneumoniae) has been identified as a serious source of infections. The objectives of our study were to investigate the prevalence of multidrug-resistant (MDR) K. pneumoniae in Tanta University Hospitals, Gharbia Governorate, Egypt; characterize their carbapenem resistance profiles; and identify their different capsular serotypes. We identified and isolated 160 (32%) K. pneumoniae from 500 different clinical samples, performed antimicrobial susceptibility testing, and then used multiplex PCR to detect carbapenemase genes and capsular serotypes K1, K2, K3, K5, K20, K54, and K57. We detected phenotypic carbapenem resistance in 31.3% (50/160) of the isolates; however, molecular assays revealed that 38.75% (62/160) of isolates were carrying carbapenemase-encoding genes. Generally, blaOXA-48 was the prevalent gene (15.5%), followed by blaVIM (15%), blaIMP (7.5%), blaKPC (4%), and blaNDM (3.8%). BlaVIM and blaOXA-48 correlated with phenotypic resistance in 91.67% and 88% of the isolates that harbored them, respectively. Capsular typing showed that the most prevalent pathotype was K1 (30.6%), followed by K57 (24.2%), K54 (19.35%), K20 (9.67%), and K2 (6.45%). A critical risk to community health is posed by the high incidence of multidrug-resistant (MDR) virulent K. pneumoniae isolates from our hospital, and our study examines this pathogen’s public health and epidemiological risks.
Keywords:
Klebsiella pneumoniae; carbapenem resistance; capsular serotypes; blaOXA-48; blaVIM; blaKPC; blaNDM; blaIMP 1. Introduction
One of the biggest pressures on healthcare systems around the world is the rising prevalence of antibiotics-resistant clinical bacterial isolates [1,2]. Understanding the genetic factors of antibiotic resistance is essential to stop the spread of MDR bacteria [3].
Among these MDR bacteria, K. pneumoniae is regarded as one of the top six factors contributing to healthcare-associated infections and drug resistance [4]. As an opportunistic pathogen, K. pneumoniae consists of Gram-negative bacilli and is a member of the enterobacterales family that primarily affects people who are immunocompromised or are admitted to hospitals. Numerous ailments, such as sepsis, bacteremia, pneumonia, and urinary tract infections, are attributed to K. pneumoniae [5].
A sizeable portion of illnesses brought on by Klebsiella spp. is a result of two significant pathotypes, notably the MDR and hypervirulent (hv), which eventually produce convergent genetic copies, termed multidrug-resistant and hypervirulent (MDRhv) Klebsiella spp. [6].
New antimicrobial-resistance genes were initially found in K. pneumoniae, and they later spread to further pathogens: carbapenem-resistant K. pneumoniae (CRKP) genes (blaKPC, blaOXA-48 and blaNDM-1) are examples [7]. The essential pathogenic component, known as the capsule, an extracellular polysaccharide structure that hinders the host immune response and shields the invading pathogens from phagocytosis, is responsible for the increasing death and morbidity rates linked to K. pneumoniae infections [8].
Klebsiella has at least 79 different capsular varieties, with each depicting the capsular polysaccharide’s (CPS; the K antigen) molecular structure differently. These types have been connected to the severity of the sickness and the type of infection [9]. Several capsular (K) types, mainly K1, K2, K5, K20, K54, and K57, are correlated to invasive septicemia obtained in the community, pneumonia, and liver abscesses [10]. Furthermore, K3 is attributed to rhinoscleroma [11].
Information about capsule serotypes can be quickly retrieved from whole-genome sequence (WGS) data by typing the relevant capsule (K) biosynthesis loci [12]. A chromosomal region of 10–30 kbp and 10–30 genes make up the K locus. The preserved genes for the export and synthesis of capsules are found in the 5′-(galF, cpsACP, wzi, wza, wzb, wzc) and 3′-(ugd) most areas, and they surround the genes that code for the synthesis of capsule sugar, namely Wzy repeat-unit polymerase and Wzx capsule-specific flippase [13].
Molecular capsular typing is the main technique used to categorize K. pneumoniae isolates, and it has outstanding consistency and can distinguish between clinical isolates [14]. Multiplex PCRs have been successfully used to identify the capsule repeat-unit polymerase Wzy genes [15].
Few studies on MDR K. pneumonia capsular typing have been conducted in Egypt [16,17]. Consequently, we assessed the prevalence of nosocomial MDR K. pneumoniae infections in our tertiary care hospitals and characterized their carbapenem resistance profiles.
2. Materials and Methods
2.1. Study Design
We carried out our cross-sectional study in the Tanta University Hospitals’ Clinical Pathology and Medical Microbiology and Immunology Department over the course of a year, from June 2021 to June 2022. The hospitals have a combined capacity of 2040 beds, including 130 ICU beds, and serve over 190,000 patients annually. Our study received permission from Tanta University’s Institutional Review Board for the Faculty of Medicine in Egypt (Approval code 35789/9/22).
2.2. Study Subjects
A total of 500 patients from Tanta University hospital’s Pediatrics, Chest, Medicine, and Intensive Care Unit (ICU) departments were enrolled in this study. The included patients had hospital-acquired infections (HAIS). We studied 160 clinical isolates of Klebsiella from 500 samples from different body sites (blood, CSF, urine, wound, and sputum) of 500 patients.
2.3. Identification of Bacterial Isolates
We gathered blood, CSF, urine, wounds, and sputum samples from different infection sites and quickly sent them to the Microbiology Department laboratory for additional processing. First, we codified the samples, and then we cultivated aerobically at 37 °C on blood agar, nutrient agar, chocolate agar, and MacConkey agar plates (Oxoid, UK) for 24–48 h. We predominantly used routine microbiological methods for the phenotypic detection of isolated pathogens [18]. Thereafter, we further processed only K. pneumonia. We verified K. pneumonia using the Vitek-2 automated system (Biomérieux, Marcy-LÉtoile, Paris, France) in accordance with the manufacturer’s recommendations. We kept all K. pneumoniae isolates at −80 °C in brain–heart infusion broth (20% glycerol; Oxoid, UK) until they were needed.
2.4. Antimicrobial Susceptibility Testing and Phenotypic Detection of Carbapenemases
We performed the modified Kirby–Bauer disc diffusion method to assess the antibiotic susceptibility of all identified K. pneumoniae isolates on Muller–Hinton agar (Oxoid, UK) plates. The antibiotics used were amoxicillin/ clavulanic acid (AMO) 20/10 μg, ciprofloxacin (CIP) 5 μg, cefuroxime (CXM) 30 μg, piperacillin–tazobactam (TPZ) 110 μg, cefoxitin (FOX) 30 μg, cefipime (FEP) 30 μg, ceftriaxone (CRO) 30 μg, ceftazidime (CAZ) 30 μg, cefotaxime (CTX) 30 μg, trimethoprim–sulfamethoxazole (SXT) 25 µg, imipenem (IMI) 10 μg, ertapenem (ERT) 10 μg, and meropenem (MEM) 10 μg (Oxoid, UK). We used the modified Hodge test (MHT) to check for carbapenemase production in isolates, which showed intermediate or resistant zones for ertapenem according to CLSI guidelines [19]. We used E. coli ATCC 25922 as a susceptible strain and K. pneumoniae ATCC BAA-1705 as a positive control. We interpreted data generated by the susceptibility assay using the CLSI 2021 guidelines [19]. The multiple antibiotic resistance (MAR) index of each isolate was estimated according to Tambekar et al.’s method [20].
2.5. Multiplex PCR for Capsular Typing of K. pneumoniae and Detection of Carbapenemases-Encoding Genes
We used two distinct multiplex PCR assays to carry out the molecular characterization of the carbapenem resistance genes and capsular typing of K. pneumoniae. The K1, K2, K5, K20, K54, K57, and K3 capsular antigens were the targets of the first multiplex PCR typing [21] (Table 1). We utilized primer sets for the carbapenemases-encoding genes blaVIM, blaIMP, blaKPC, blaOXA-48, and blaNDM in the second multiplex PCR [22]. (Table 1)

Table 1.
Primer sequences used in molecular detection of capsular genes and carbapenem resistance genes of K. pneumoniae [23].
We obtained total genomic DNA using Qiagen DNA extraction kits (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. Then, we kept the extraction at −20 °C until the following stage.
We used Dream Taq TM Green PCR Master Mix (Fermentas, Waltham, MA, USA) to amplify the tested gene as per the manufacturer’s directions using a Bio-Rad PTC-200 Thermal Cycler (Bio-Rad, Hercules, CA, USA). We created the PCR conditions for capsular and carbapenemase genes molecular typing according to Ssekatawa et al.’s method [23]. We electrophoresed PCR products on a 1.5% agarose gel stained with ethidium bromide and photographed with UV illumination. We used a 100-2000 base-pairs standard DNA ladder (Biomatik, Wilmington, DE, USA) for sizing the PCR products.
2.6. Statistical Analysis
We analyzed the data with IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp, New York, NY, USA, 2017). We utilized numbers and percentages to present qualitative data. We used a p-value of ≤0.05 to determine statistical significance.
3. Results
3.1. Distribution of Isolated K. pneumoniae in Clinical Samples
We separated K. pneumoniae from distinct types of specimens collected from patients admitted at Tanta university tertiary hospital. We collected 500 samples; however, only 160 specimens yielded K. pneumoniae, while the remaining specimens either yielded different organisms or provided no growth. Regarding the 160 samples, 80 were isolated from urine, 40 from pus swabs, 20 from sputum, 10 from tracheal aspirates, and 10 from blood (Table 2).

Table 2.
Prevalence of Klebsiella pneumoniae isolated from various clinical specimens.
3.2. Antibiotic Susceptibility Patterns and Phenotypic Detection of Carbapenemases
Based on the disc diffusion assay, the majority of the isolated K. pneumoniae showed significant levels of resistance to used antibiotics. Overall, 99.4% of the isolates exhibited resistance to cefotaxime, while 99% showed resistance to amoxicillin–clavulanic acid and ceftazidime. Furthermore, 98.1% of the isolates exhibited resistance to each of cefuroxime and ceftriaxone, whereas 95% and 94.4% were resistant to trimethoprim–sulfamethoxazole and cefepime, respectively. We observed resistance to piperacillin–tazobactam and ciprofloxacin as the next highest among 81.8% of the isolates, followed by cefoxitin (60%). We found the lowest resistance rate corresponding to imipenem and ertapenem (31.3%), followed by meropenem (30%). All carbapenem-resistant isolates (100%) were MHT positive. The MAR index ranged from 0.69 to 1.0.
3.3. Carbapenemase-Encoding Genes Distribution
Based on the results obtained by Multiplex PCR assay, out of 160 K. pneumoniae isolates, 38.75% (62/160) contained single or mixed carbapenemase genes (Table 3 and Table 4). Of those, blaOXA-48 was the most predominant, with a prevalence of (15.5%) (25/160), followed by blaVIM (24/160 = 15%), blaIMP (12/160 = 7.5%), blaKPC (7/160 = 4%), and blaNDM (6/160 = 3.8%) (Figure 1).

Table 3.
Prevalence of carbapenemase-encoding genes in total Klebsiella pneumoniae isolates.

Table 4.
Distribution of single and mixed carbapenemase genes among the genotypically resistant isolate.
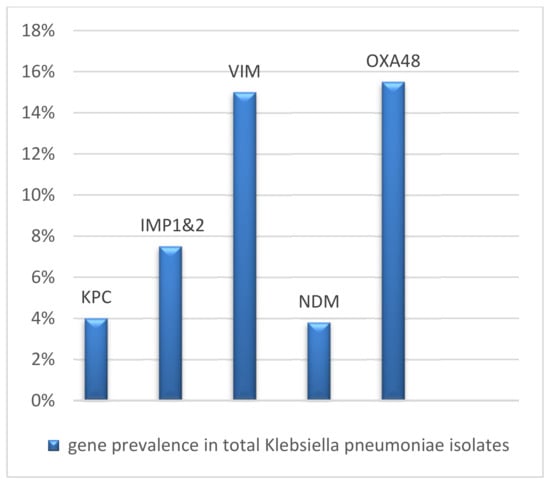
Figure 1.
Gene prevalence in Klebsiella pneumoniae isolates.
3.4. Correlation between Genotypic and Phenotypic Assays
We detected variations between the genotypic and phenotypic resistance of the isolates. A total of 24 isolates harbored the VIM gene, and 22 (91.67%) showed phenotypic carbapenem resistance. This was followed by OXA-48, which showed phenotypic resistance in 22 (88%) of the isolates, then Kpc in 5 (71.43%), IMP-1&2 in 9 (75%), and NDM in 4 (66.67%) (Table 5).

Table 5.
Correlation between genotypic and phenotypic resistance.
3.5. Prevalence of Capsular Types in Isolates Harboring Carbapenemases-Encoding Genes
Our multiplex PCR assay results showed that out of 62 carbapenem-resistant isolates, 19 (30.6%) harbored capsular gene K1, followed by the K57 (15; 24.2%), K54 (12; 19.35%), K20 (6; 9.67%), and K2 genes (4; 6.45%). However, we did not detect the K3 and K5 genes in any of the collected isolates (Figure 2).
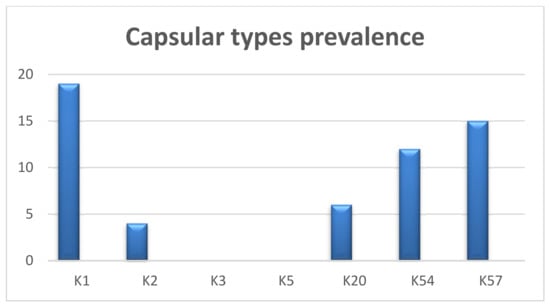
Figure 2.
Prevalence of capsular types in carbapenem genotypically resistant isolates.
3.6. Correlation between Source, Antimicrobial Resistance Pattern, Multiple Antibiotic Resistance (MAR) Index, Distribution of Carbapenemase-Encoding Genes, and Capsular Types
The comprehensive correlation between an isolate’s source, antimicrobial resistance pattern, MAR index, carbapenemases genes, and capsular serotypes is displayed in Table 6. We found no significant relations when correlating the different carbapenemase genes detected during our study with capsular serotypes (Table 7).

Table 6.
Correlation between source of samples, antimicrobial resistance pattern, MAR index, carbapenemase genes, and capsular genes.

Table 7.
Correlation between carbapenemases and capsular genes.
4. Discussion
K. pneumoniae has been identified as one of the most popular causes of infections developed in hospitals and the community [24]. The appearance of, MDR and hvKP strains, as well as their rapid clinical propagation, is particularly concerning [25] because their resistance propagation is associated with mobile genetic components, which may additionally hold virulence factors, such as the capsule, siderophores, fimbriae, and lipopolysaccharides (LPS) [26]. Therefore, when highly pathogenic bacteria develop antibiotic resistance, the situation deteriorates [23].
Therefore, we analyzed the frequency of carbapenem-resistant pathogenic K. pneumoniae in our tertiary care hospitals to better understand its dangers. Our survey findings show that 50% of K. pneumoniae isolates were found in urine, 25% in pus swabs, 20% in sputum, and 6.25% in both blood and tracheal aspirates. Our results are similar to those of a study conducted at Al-Azhar University, Egypt [27]. Additionally, further research carried out in Uganda concluded that most K. pneumoniae isolates were obtained from urine, pus, and blood [23].
However, a study in New York conducted by Parrott et al. [28] confirmed that most K. pneumoniae isolates were recovered from blood culture, followed by wound swabs. Additionally, Palmeiro et al. [29] found that blood specimens yielded the highest number of isolates. Furthermore, Sedighi P et al. [30] found that throat, urine, and tracheal swabs were the most prevalent samples, while wound, blood, sputum, and abscess cultures showed the least amounts of isolates.
This variation in results may be explained by variations in sample type and case count, sampling conditions, sampling times, sampling locations, sampling countries, and patient general health.
We determined that the isolates we detected in our study were MDR because of their resistance to several types of antibiotics. Meropenem had a 30% resistance rate, whereas imipenem and ertapenem both had a 31.3% resistance rate. This outcome was consistent with the research conducted by Farhadi et al. [31], who observed that 33% of the K. pneumoniae isolates were resistant to imipenem. Furthermore, Pereira et al. [32] found that 73 Klebsiella isolates found in samples of a urinary tract infection were extremely resistant to IMP.
Moreover, Moghadas et al. [33] found that only 7.5% of their isolates were resistant to IMP, and their survey of North and West Africa highlighted a noticeably increased phenotypic resistance to carbapenems (>50%) [34,35,36,37]. Additionally, a bigger study that examined the South African provinces of Gauteng, KwaZulu-Natal, Western Cape, and Free State found that imipenem, meropenem, and doripenem had overwhelmingly high phenotypic resistance rates of between 47 and 50%, while ertapenem had rates between 84% and 89%.
The disparity in sensitivity patterns between the aforementioned studies may be attributed to various antibiotic policies, the emergence of resistant strains because of indiscriminate antimicrobial therapy, the patient’s immune status, various infection control strategies, or frequent hospitalization.
We must determine whether the K. pneumoniae isolate produces carbapenemase in order to conduct epidemiological research and choose the best course of treatment for infections [38]. Regarding the PCR-based carbapenemase gene identification, blaOXA-48 was the most prevalent, with a genotypic frequency of (15.5%), followed by blaVIM type (15%), blaIMP (7.5%), blaKPC (4%), and blaNDM (3.8%). Our findings were consistent with another Egyptian study conducted by Raheel et al. [39], who demonstrated that the blaOXA-48 gene (96.2%) was the most frequently present gene, while the blaKPC gene (7.5%) was the least common. Additionally, our result is consistent with recent research that identified the OXA-48 gene and its variations as the most popular gene [35,40,41,42].
OXA- 48 was initially discovered in a K. pneumoniae strain from Turkey in 2003. OXA-48 intermittently reached neighboring nations in the southern and eastern Mediterranean Sea, as well as North Africa [43]. This explains why OXA-48 is more common in Tunisia and Egypt than anywhere else [35,41].
Nevertheless, Lopes et al. and Hussein et al. [44,45] found that carbapenem-resistant K. pneumoniae isolates had a higher level of blaKPC expression. Furthermore, El-Monir et al. [46] reported that both blaVIM and blaNDM-1 were the most prevalent genes detected in Egypt. Additionally, further studies showed that the most abundant genes in East Africa were VIM and IMP [40,47], whereas NDM was the most common in South Africa [47,48,49,50].
We recovered more than one resistance gene in 12 K. pneumoniae isolates, which is in accordance with many previously published studies that demonstrated that A. baumannii and K. pneumoniae carry several genes, increasing their likelihood of being multi- or pan-drug resistant [49,51,52,53,54]. However, this can be contested because of the possibility of resistance spreading and the restricted accessibility of antibiotics useful for therapy, as well as the diminishing effectiveness of older antibiotics, such as colistin [55,56].
Our study found that genotypic resistance was generally higher than overall phenotypic resistance. For example, 25 isolates harbored the OXA-48 gene, and 22 (88%) of them showed phenotypic carbapenem resistance. This can be explained by many reports that described OXA-48 and its variant genes’ oxacillinases as having limited hydrolyzing activity for carbapenems [43,57,58].
The capsule is a key element affecting K. pneumoniae’s pathogenicity. Numerous investigations revealed that the virulence of infections generated by K. pneumoniae is influenced by the capsular forms [59,60]. In several strains of Klebsiella spp., the gene cluster architecture responsible for producing capsular polysaccharide (CPS) has been previously analyzed [61]. The Wzy and Wzx genes, which generate the proteins necessary for the polymerization and assembly of the various CPS subunits, are situated in a variable region in the center of the CPS locus. As a result, the foundation of PCR capsular typing assays is the significant sequence diversity of the Wzy gene among the various capsular types [62]. Considering this, we identified and characterized the K. pneumoniae capsular serotypes that were most clinically relevant using the Wzy gene.
Our results revealed that (30.6%) of K. pneumoniae isolates harbored capsular gene K1, followed by the K57 (24.2%), K54 (19.35%), K20 (9.67%), and K2 genes (6.45%); however, we did not detect the K3 and K5 genes in the collected isolates.
Ssekatawa et al. [23] found that K1, K2, K3, K5, and K20 made up 46.7% of the K. pneumoniae isolates; according to capsular typing by heptaplex PCR, while none of the isolates had K54 or K57.
These findings correspond to research conducted by Fung et al. and Chuang et al. [60,63], who concluded that the greatest virulent capsular forms of K. pneumoniae K1 and K2 were responsible for septicemia and liver abscesses. Furthermore, according to two surveys conducted in Taiwan by Fang et al. and Lin et al. [59,62], the K1, K2, K3, K5, and K20 genes were the most common capsular types in pneumonic and liver abscess patients. Moreover, Paczosa and Mecsas [64] reported that among the 519 invasive strains they investigated, K2 isolates were found in the largest numbers. In addition, Choi et al. [65] found that K24 was the most prevalent capsule type.
We evaluated the correlation between capsular serotypes and the presence of carbapenemase genes. Our results revealed that carbapenemases genes could not be related to any capsular serotypes (data were statistically not significant). Nonetheless, Soltani et al. [66] found a correlation between blaOXA-48 and K20 in a study conducted in Iran.
5. Conclusions
Our research highlighted high incidence rates for carbapenem-resistant K. pneumoniae in our tertiary care hospital. Although our study did not seek to identify other virulence determinants, the considerable prevalence of carbapenem resistance among capsular serotypes that we found raises the possibility of carbapenem-resistant hypervirulent K. pneumoniae, which must be assessed in further studies.
Author Contributions
Conceptualization, M.S.T. and S.Y.M.; data curation, Y.A.Z.; formal analysis, M.M.H., Y.A.Z. and R.M.E.; investigation, M.A.A.; methodology, M.S.T., M.A.A. and S.Y.M.; resources, M.S.T. and M.M.S.; software, M.M.S., Y.A.Z. and R.M.E.; supervision, M.S.T.; validation, M.M.H., M.M.S., Y.A.Z. and R.M.E.; visualization, M.M.H.; writing—original draft, M.S.T. and S.Y.M.; writing—review and editing, M.M.H., M.M.S. and M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Institutional Review Board of Tanta University Faculty of Medicine in Egypt gave the study its approval (approval code 35789/9/22). All techniques were conducted in accordance with the ethical recommendations of the relevant committee on human experimental research (institutional and national), as well as the principles outlined in the Helsinki Declaration (1975), as updated in (2013).
Informed Consent Statement
All participants or their parents (in the case of pediatric patients) provided written informed permission.
Data Availability Statement
Data are accessible upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. EU Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2013. EFSA J. 2015, 13, 4036. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Carnelutti, A.; Graziano, E.; Russo, A. Multidrug-resistant Klebsiella pneumoniae: Challenges for treatment, prevention and infection control. Expert Rev. Anti-Infect. Ther. 2018, 16, 749–761 . [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gormanm, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Sa Pessoa, J. Klebsiella pneumoniae infection biology: Living to counteract host defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef]
- Chen, L.; Kreiswirth, B.N. Convergence of carbapenem-resistance and hypervirulence in Klebsiella pneumoniae. Lancet Infect. Dis. 2018, 18, 2–3. [Google Scholar] [CrossRef]
- Wyres, K.; Holt, K.E. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr. Opin. Microbiol. 2018, 45, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Tian, P.; Tan, T. Research advances in the virulence factors of Klebsiella pneumonia—A review. Acta Microbiol. Sin 2015, 55, 1245–1252. [Google Scholar]
- Cortés, G.; Borrell, N.; de Astorza, B.; Gómez, C.; Sauleda, J.; Albertí, S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect. Immun. 2002, 70, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Siri, G.P.; Sithebe, N.P.; Ateba, C.N. Identification of Klebsiella species isolate from Modimola dam (Mafikeng) North West Province South Africa. J. Afr. J. Microbiol. Res. 2011, 5, 3958–3963. [Google Scholar]
- Fevre, C.; Passet, V.; Deletoile, A.; Barbe, V.; Frangeul, L.; Almeida, A.S.; Brisse, S. PCR-based identification of Klebsiella pneumoniae subsp. rhinoscleromatis, the agent of rhinoscleroma. PLoS Negl. Trop. Dis. 2011, 5, e1052. [Google Scholar] [CrossRef]
- Wyres, K.L.; Wick, R.R.; Gorrie, C.; Jenney, A.; Follador, R.; Thomson, N.R.; Holt, K.E. Identification of Klebsiella capsule synthesis loci from whole genome data. Microb. Genom. 2016, 2, e000102. [Google Scholar] [CrossRef]
- Pan, Y.J.; Lin, T.L.; Chen, C.T.; Chen, Y.Y.; Hsieh, P.F.; Hsu, C.R.; Wang, J.T. Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Sci. Rep. 2015, 5, 15573. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.K.; Fung, C.P.; Chang, F.Y.; Lee, N.; Yeh, K.M.; Koh, T.H.; Ip, M. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J. Clin. Microbiol. 2011, 49, 3761–3765. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Cao, X.L.; Shen, H.; Zhang, Z.F.; Ning, M.Z.; Zhou, W.Q. Investigations on the virulence, serotypes and genotyping of Klebsiella pneumoniae producing KPC-2. Chin. J. Clin. Lab. Sci. 2015, 33, 591–595. [Google Scholar]
- Wasfi, R.; Elkhatib, F.W.; Ashour, M.H. Molecular typing and virulence analysis of multidrug resistant Klebsiella pneumoniae clinical isolates recovered from Egyptian hospitals. Sci. Rep. 2016, 6, 38929. [Google Scholar] [CrossRef]
- Mohamed, E.R.; Ali, M.Y.; Waly, N.G.F.M.; Halby, H.M.; El-Baky, R.M.A. The Inc FII Plasmid and its Contribution in the Transmission of blaNDM-1 and blaKPC-2 in Klebsiella pneumoniae in Egypt. Antibiotics 2019, 8, 266. [Google Scholar] [CrossRef]
- Forbes, B.A.; Sahm, D.F.; Weissfeld, A.S. Study Guide for Bailey and Scott’s Diagnostic Microbiology-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Clinical and Laboratory Standards Institute [CLSI]. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; Supplement M100, Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2021. [Google Scholar]
- Tambekar, D.; Dhanorkar, D.; Gulhane, S.; Khandelwal, V.; Dudhane, M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr. J. Biotechnol. 2006, 5, 1562–1565. [Google Scholar]
- Zhang, S.; Yang, G.; Ye, Q.; Wu, Q.; Zhang, J.; Huang, Y. Phenotypic and Genotypic Characterization of Klebsiella pneumoniae Isolated From Retail Foods in China. Front. Microbiol. 2018, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Dallenne, C.; Costa, A.D.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Nakavuma, J.L.; Kato, C.D.; Ejobi, F.; Tweyongyere, R.; Eddie, W.M. Prevalence of pathogenic Klebsiella pneumoniae based on PCR capsular typing harbouring carbapenemases encoding genes in Uganda tertiary hospitals. Antimicrob. Resist. Infect. Control 2021, 10, 1–10. [Google Scholar] [CrossRef]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Thomson, N.R. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef] [PubMed]
- Twenhafel, N.A.; Whitehouse, C.A.; Stevens, E.L.; Hottel, H.E.; Foster, C.D.; Gamble, S.; Steele, K.E. Multisystemic abscesses in African green monkeys (Chlorocebus aethiops) with invasive Klebsiella pneumoniae—Identification of the hypermucoviscosity phenotype. Vet. Pathol. 2008, 45, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Gaiarsa, S.; Comandatore, F.; Gaibani, P.; Corbella, M.; Dalla Valle, C.; Epis, S.; Sassera, D. Genomic epidemiology of Klebsiella pneumoniae in Italy and novel insights into the origin and global evolution of its resistance to carbapenem antibiotics. Antimicrob. Agents Chemother. 2015, 59, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Abo Samra, M.A.A.; Ali, N.K.; El-Madboly, A.A.E. Detection of Multi-Drug Resistant Klebsiella pneumoniae in Al-Zahraa University Hospital. Egypt. J. Hosp. Med. 2019, 75, 3006–3012. [Google Scholar] [CrossRef]
- Parrott, A.M.; Shi, J.; Aaron, J.; Green, D.A.; Whittier, S.; Wu, F. Detection of multiple hypervirulent Klebsiella pneumoniae strains in a New York City hospital through screening of virulence genes. Clin. Microbiol. Infect. 2021, 27, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Palmeiro, J.K.; De Souza, R.F.; Schörner, M.A.; Passarelli-Araujo, H.; Grazziotin, A.L.; Vidal, N.M.; Dalla-Costa, L.M. Molecular epidemiology of multidrug-resistant Klebsiella pneumoniae isolates in a Brazilian tertiary hospital. Front. Microbiol. 2019, 10, 1669. [Google Scholar] [CrossRef]
- Sedighi, P.; Zarei, O.; Karimi, K.; Taheri, M.; Karami, P.; Shokoohizadeh, L. Molecular typing of Klebsiella pneumoniae clinical isolates by Enterobacterial repetitive intergenic consensus polymerase chain reaction. Int. J. Microbiol. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Farhadi, M.; Ahanjan, M.; Goli, H.R.; Haghshenas, M.R.; Gholami, M. High frequency of multidrug-resistant (MDR) Klebsiella pneumoniae harboring several β-lactamase and integron genes collected from several hospitals in the north of Iran. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 1–9. [Google Scholar] [CrossRef]
- Pereira, A.; Petrucci, T.; Simões, M.J. Klebsiella pneumoniae do Serotipo K1 e do Clone Hipervirulento ST23: Primeiro Caso Documentado em Portuga. Acta Med. Port. 2017, 30. [Google Scholar] [CrossRef]
- Moghadas, A.J.; Kalantari, F.; Sarfi, M.; Shahhoseini, S.; Mirkalantari, S. Evaluation of virulence factors and antibiotic resistance patterns in clinical urine isolates of Klebsiella pneumoniae in Semnan, Iran. Jundishapur J. Microbiol. 2018, 11, e63637. [Google Scholar] [CrossRef]
- Kotb, S.; Lyman, M.; Ismail, G.; Abd El Fattah, M.; Girgis, S.A.; Etman, A.; Talaat, M. Epidemiology of carbapenem-resistant Enterobacteriaceae in Egyptian intensive care units using National Healthcare–associated Infections Surveillance Data, 2011–2017. Antimicrob. Resist. Infect. Control 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- ElMahallawy, H.A.; Zafer, M.M.; Amin, M.A.; Ragab, M.M.; Al-Agamy, M.H. Spread of carbapenem resistant Enterobacteriaceae at tertiary care cancer hospital in Egypt. Infect. Dis. 2018, 50, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Ogbolu, D.O.; Webber, M.A. High-level and novel mechanisms of carbapenem resistance in Gram-negative bacteria from tertiary hospitals in Nigeria. Int. J. Antimicrob. Agents 2014, 43, 412–417. [Google Scholar] [CrossRef]
- Elramalli, A.; Almshawt, N.; Ahmed, M.O. Current problematic and emergence of carbapenemase-producing bacteria: A brief report from a Libyan hospital. Pan Afr. Med. J. 2017, 26, 180. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.M.; Wang, S.; Chiu, H.C.; Kao, C.Y.; Wen, L.L. Combination of modified carbapenem inactivation method (mCIM) and EDTA-CIM (eCIM) for phenotypic detection of carbapenemase-producing Enterobacteriaceae. BMC Microbiol. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Raheel, A.; Azab, H.; Hessam, W.; Abbadi, S.; Ezzat, A. Detection of carbapenemase enzymes and genes among carbapenem-resistant Enterobacteriaceae isolates in Suez Canal University Hospitals in Ismailia, Egypt. Microbes Infect. Dis. 2020, 1, 24–33. [Google Scholar] [CrossRef]
- Perovic, O.; Ismail, H.; Quan, V.; Bamford, C.; Nana, T.; Chibabhai, V.; Singh-Moodley, A. Carbapenem-resistant Enterobacteriaceae in patients with bacteraemia at tertiary hospitals in South Africa, 2015 to 2018. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Kollenda, H.; Frickmann, H.; Helal, R.B.; Wiemer, D.F.; Naija, H.; El Asli, M.S.; Moussa, M.B. Screening for carbapenemases in ertapenem-resistant Enterobacteriaceae collected at a Tunisian hospital between 2014 and 2018. Eur. J. Microbiol. Immunol. 2019, 9, 9–13. [Google Scholar] [CrossRef]
- Mahrach, Y.; Mourabit, N.; Arakrak, A.; Bakkali, M.; Laglaoui, A. Phenotypic and molecular study of carbapenemase-producing Enterobacteriaceae in a regional hospital in northern Morocco. J. Clin. Med. Sci. 2019, 3, 113. [Google Scholar]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791. [Google Scholar] [CrossRef]
- Lopes, E.; Saavedra, M.J.; Costa, E.; de Lencastre, H.; Poirel, L.; Aires-de-Sousa, M. Epidemiology of carbapenemase-producing Klebsiella pneumoniae in northern Portugal: Predominance of KPC-2 and OXA-48. J. Glob. Antimicrob. Resist. 2020, 22, 349–353. [Google Scholar] [CrossRef]
- Hussein, N.H.; Hussein AL-Kakei, S.N.; Taha, B.M. The predominance of Klebsiella pneumoniae carbapenemase (KPC-type) gene among high-level carbapenem-resistant Klebsiella pneumoniae isolates in Baghdad, Iraq. Mol. Biol. Rep. 2022, 49, 4653–4658. [Google Scholar] [CrossRef]
- Elmonir, W.; Abd El-Aziz, N.K.; Tartor, Y.H.; Moustafa, S.M.; Abo Remela, E.M.; Eissa, R.; Saad, H.A.; Tawab, A.A. Emergence of Colistin and Carbapenem Resistance in Extended-Spectrum β-Lactamase Producing Klebsiella pneumoniae Isolated from Chickens and Humans in Egypt. Biology 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Singh-Moodley, A.; Perovic, O. Antimicrobial susceptibility testing in predicting the presence of carbapenemase genes in Enterobacteriaceae in South Africa. BMC Infect. Dis. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kateete, D.P.; Nakanjako, R.; Namugenyi, J.; Erume, J.; Joloba, M.L.; Najjuka, C.F. Carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii at Mulago hospital in Kampala, Uganda (2007–2009). Springerplus 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Okoche, D.; Asiimwe, B.B.; Katabazi, F.A.; Kato, L.; Najjuka, C.F. Prevalence and characterization of carbapenem-resistant Enterobacteriaceae isolated from Mulago National Referral Hospital, Uganda. PLoS ONE 2015, 10, e0135745. [Google Scholar] [CrossRef] [PubMed]
- Ampaire, L.M.; Katawera, V.; Nyehangane, D.; Boum, Y.; Bazira, J. Epidemiology of carbapenem resistance among multi-drug resistant Enterobacteriaceae in Uganda. Br. Microbiol. Res. J. 2015, 8, 418. [Google Scholar] [CrossRef]
- Wade, D.M.; Hankins, M.; Smyth, D.A.; Rhone, E.E.; Mythen, M.G.; Howell, D.C.; Weinman, J.A. Detecting acute distress and risk of future psychological morbidity in critically ill patients: Validation of the intensive care psychological assessment tool. Crit. Care 2014, 18, 1–9. [Google Scholar] [CrossRef]
- Masseron, A.; Poirel, L.; Ali, B.J.; Syed, M.A.; Nordmann, P. Molecular characterization of multidrug-resistance in Gram-negative bacteria from the Peshawar teaching hospital, Pakistan. New Microbes New Infect. 2019, 32, 100605. [Google Scholar] [CrossRef]
- Sadeghi, M.R.; Ghotaslou, R.; Akhi, M.T.; Asgharzadeh, M.; Hasani, A. Molecular characterization of extended-spectrum β-lactamase, plasmid-mediated AmpC cephalosporinase and carbapenemase genes among Enterobacteriaceae isolates in five medical centres of East and West Azerbaijan, Iran. J. Med. Microbiol. 2016, 65, 1322–1331. [Google Scholar] [CrossRef]
- Haji, S.H.; Aka, S.T.H.; Ali, F.A. Prevalence and characterisation of carbapenemase encoding genes in multidrug-resistant Gram-negative bacilli. PLoS ONE 2021, 16, e0259005. [Google Scholar] [CrossRef]
- Solgi, H.; Badmasti, F.; Aminzadeh, Z.; Giske, C.G.; Pourahmad, M.; Vaziri, F.; Shahcheraghi, F. Molecular characterization of intestinal carriage of carbapenem-resistant Enterobacteriaceae among inpatients at two Iranian university hospitals: First report of co-production of bla NDM-7 and bla OXA-48. Eur. J. Clin. Microbiol. Infect Dis. 2017, 36, 2127–2135. [Google Scholar] [CrossRef]
- Di Tella, D.; Tamburro, M.; Guerrizio, G.; Fanelli, I.; Sammarco, M.L.; Ripabelli, G. Molecular Epidemiological Insights into Colistin-Resistant and Carbapenemases-Producing Clinical Klebsiella pneumoniae Isolates. Infect Drug Resist. 2019, 12, 3783–3795. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! J. Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef]
- Codjoe, F.S.; Donkor, E.S. Carbapenem resistance: A review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.T.; Lai, S.Y.; Yi, W.C.; Hsueh, P.R.; Liu, K.L.; Chang, S.C. Klebsiella pneumoniae genotype K1: An emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin. Infect Dis. 2007, 45, 284–293. [Google Scholar] [CrossRef]
- Fung, C.P.; Chang, F.Y.; Lee, S.C.; Hu, B.S.; Kuo, B.I.; Liu, C.Y.; Siu, L.K. A global emerging disease of Klebsiella pneumoniae liver abscess: Is serotype K1 an important factor for complicated endophthalmitis? Gut 2002, 50, 420–424. [Google Scholar] [CrossRef]
- Pan, Y.J.; Fang, H.C.; Yang, H.C.; Lin, T.L.; Hsieh, P.F.; Tsai, F.C.; Wang, J.T. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J. Clin. Microbiol. 2008, 46, 2231–2240. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, Y.P.; Wang, F.D.; Fung, C.P. Community-onset Klebsiella pneumoniae pneumonia in Taiwan: Clinical featurof the disease and associated microbiological characteristics of isolates from pneumonia and nasopharynx. Front. Microbiol. 2015, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.P.; Fang, C.T.; Lai, S.Y.; Chang, S.C.; Wang, J.T. Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J. Infect Dis. 2006, 193, 645–654. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Hegerle, N.; Nkeze, J.; Sen, S.; Jamindar, S.; Nasrin, S.; Sen, S.; Permala-Booth, J.; Sinclair, J.; Tapia, M.D.; et al. The Diversity of Lipopolysaccharide (O) and Capsular Polysaccharide (K) Antigens of Invasive Klebsiella pneumoniae in a Multi-Country Collection. Front. Microbiol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Soltani, E.; Hasani, A.; Rezaee, M.A.; Nahandi, M.; Hasani, A.; Gholizadeh, P. An Alliance of Carbapenem-Resistant Klebsiella pneumoniae with Precise Capsular Serotypes and Clinical Determinants: A Disquietude in Hospital Setting. Can. J. Infect Dis. Med. Microbiol. 2022, 21, 6086979. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).