Abstract
Bumblebees (Bombus spp.) are an essential element of the ecosystem and the global economy. They are valued pollinators in many countries around the word. Unfortunately, there has been a decline in the bumblebee population, which is attributed to, among others, pathogens and reduced access to food due to the loss of natural nesting sites. Lotmaria passim and Crithidia mellificae, protozoan pathogens of the family Trypanosomatidae, commonly infect bumblebees, including in Poland. In this study, a Polish population of bumblebees was screened for L. passim and C. mellificae. The experiment was performed on 13 adult bumblebees belonging to 4 species: B. lapidarius, B. lucorum, B. pascuorum, and B. terrestris. Protozoa of the family Trypanosomatidae were identified by PCR. Only L. passim was identified in one B. pascuorum individual. Further research is needed to confirm the effect of concurrent pathogens on the decline of bumblebee populations.
1. Introduction
Bumblebees (Bombus spp.) are an essential element of the ecosystem and the global economy. They are valued pollinators in many countries of the word [1,2,3]. There are around 300 species of Bombus spp. in the world, 37 of which have been identified in Poland [4]. The most common species are Bombus lapidaries, B. lucorum, B. pascuorum, and B. terrestris. Bumblebee species are protected, but their populations continue to decline [5]. The exact cause of bumblebee mortality remains unknown, but it is believed that an increase in the number of pathogens may contribute to the decline of bumblebee populations [6,7,8]. Bumblebees are infected by protozoa of the family Trypanosomatidae, including Lotmaria passim and Crithidia mellificae. Trypanosomatids belong to the phylum Euglenozoa, class Kinetoplastea, subclass Metakinetoplastina, order Trypanosomatida, family Trypanosomatidae [9,10]. These protozoa were first detected in vertebrates and the honey bee [11,12]. Protozoa of the family Trypanosomatidae colonize the digestive system of honey bees [13,14]. They were also found in insects of the order Hymenoptera, bumblebees and mosquitoes [9,10,15,16,17,18]. Research has demonstrated that these pathogens are transmitted by the oral-fecal route [19]. There is evidence to indicate that trypanosomatids influence the health of insects by changing their lifestyle, lifespan, physiology, and immune response [20,21,22]. However, L. passim is currently considered the dominant protozoan globally [19,23,24,25,26]. On the other hand, the influence of L. passim and C. mellificae pathogens has not been thoroughly investigated to date. Currently, L. passim is most frequently detected in honey bees [19], whereas C. mellificae is identified sporadically [23]. The interest in trypanosomatids has significantly increased of late due to their possible role in bee mortality [27,28], high prevalence [23,25,29] and possible implication in honeybee colony losses [24,28,30]. Currently, L. passim is being studied due to its negative influence on the behavior, physiology, and immune response of bees [14,29,31]. In the authors’ previous research, L. passim and C. mellificae were detected in honey bees living in tree trunks, in bees kept in apiaries, and in the brood [26,32].
Bumblebees are highly effective pollinators, therefore their health should be closely monitored. Arismendi et al. [33] analyzed the presence of Apicystis bombi, C. bombi, and Nosema bombi viruses in bumblebees in southern Chile. They detected A. bombi and C. bombi (>78%) in Bombus dahlbomii, Bombus terrestris, and Bombus ruderatus. Multiple infections with N. bombi, A. bombi, and C. bombi were also reported in B. terrestris and B. ruderatus. The prevalence of L. passim was low (<6%) in bumblebee species.
Gamboa et al. [34] also studied various pathogens in bumblebees. They analyzed the prevalence of ABPV, BQCV, DWV, LSV, SBV, N. ceranae, C. bombi, Apicystis bombi, and Spiroplasma apis in B. stratus. Nosema ceranae was omnipresent in the studied samples; A. bombi was detected in 12 out of 19 samples, and LSV was identified in 13 samples. Nosema ceranae was frequently detected in the tested samples, whereas A. bombi was noted in 12 out of 19 samples, and LSV - in 13 samples. In addition, Plischuk et al. [35] investigated the pathogens associated with Bombus spp. in Bolivia and Peru. They identified the AmFV virus, N. ceranae, L. passim, C. bombi, and five species of mites (of the genera Parasitellus Willmann, Pneumolaelaps Berlese, Proctolaelaps Berlese, Tyrophagus Oudemans, and Kuzinia Zachvatkinother) in bumblebees. Crithidia bombi was detected in 11 bumblebees, L. passim was observed in 7 out of 23 insects, and V. ceranae was identified in 4 bumblebees. They also detected various pathogens in pollinators.
Pislak-Ocepek et al. [36] examined clinically healthy bumblebee (Bombus spp.) and honeybee workers to determine the incidence and prevalence of pathogens and to identify possible relationships between infections in bumblebees and honeybees. Insects were sourced from flowers at four different locations in Slovenia. Bumblebees were infected by ABPV (8.8%), BQCV (58.5%), DWV (6.8%), SBV (24.5%), LSV (15.6%), N. bombi (16.3%), N. ceranae (8.2%), A. bombi (15%), and Crithidia bombi (17%), whereas N. apis and L. passim infections were not observed. Their study confirmed that bumblebees can be simultaneously infected with several pathogens, many of which are shared with honeybees. In the present study L. passim, the most prevalent honeybee trypanosomatid, was identified in bumblebees. Similar observations were made by Bartolome et al. [37] who confirmed the simultaneous presence of L. passim and C. mellificae in two B. terrestris individuals.
Averill et al. [38] collected 1205 bumblebees from different flowering plants and found that all parasites were less prevalent in Bombus impatiens (69.2% parasite-free) than in B. bimaculatus, B. perplexus, and B. vagans (43.4% parasite-free). They also examined the prevalence of selected pathogens, including L. passim, in Bombus spp. Interestingly, trypanosomatid infections were significantly less prevalent in B. impatiens (11.0%) than in B. vagans (32.2%), B. bimaculatus (48.5%), or B. perplexus (49.5%).
The aim of this study was to determine whether Polish bumblebee populations are infected with L. passim and C. mellificae. Little is known about the mechanisms responsible for the transmission of trypanosomes between different hosts, and the present study was undertaken to fill in this knowledge gap.
2. Results
A total of 13 bumblebees (samples) were analyzed. Lotmaria passim was identified in only one B. pascuorum individual. Crithidia mellificae was not found in any of the tested samples. Detailed results are presented in Table 1 and Figure 1.

Table 1.
Presence of protozoa of the family Trypanosomatidae in bumblebee species.
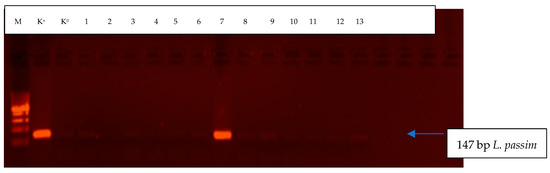
Figure 1.
Electrophoretic separation of amplification products (L. passim) in bumblebee samples. M-GeneRulerTM 100 bp DNA Ladder Plus volume marker (Fermentas); K+–positive control, includes DNA isolated from L. passim (University of Belgrade, Faculty of Veterinary Medicine, Department of Biology); K0–”zero” control, DNA was replaced with water; Paths 1 to 13–bumblebee samples.
3. Discussion
The latest molecular testing methods facilitate the search for and analysis of insect pathogens, including pathogens that cause asymptomatic infections. Crithidia bombi is most often detected in bumblebees [39], but the presence of C. mellificae has not been investigated to date. Therefore, the present study was undertaken to screen Bombus spp. for the presence of, among others, C. mellificae. The prevalence of protozoa of the family Trypanosomatidae, L. passim and C. mellificae, was low in 13 bumblebees from the Polish population. Only one sample tested positive for L. passim. Crithidia mellificae was not identified in any of the analyzed bumblebees.
In the past, many pathogenic isolates had been identified as C. mellificae and were later reclassified to L. passim. At present, this species is considered to be the main pathogen of the honey bee [19]. Only L. passim was detected in the present study, which could be attributed to the fact that L. passim is much more prevalent in insects than C. mellificae. The prevalence of L. passim and C. mellificae in bees colonizing tree trunks was examined in our previous study. Twenty-six bees were sampled from tree trunks, and none of them showed clinical symptoms of parasitic infection. Lotmaria passim was identified in fourteen samples [26]. Bee larvae of different ages were also screened for parasites. Lotmaria passim was found in 21 colonies, but C. mellificae was not detected in any of the studied samples. Moreover, significant differences (p < 0.05) in the prevalence of L. passim were noted between 10-day-old larvae vs. 4-, 8-, 12-, 16- and 21- day-old bee brood [34], which suggests that these parasites are ubiquitous. Honeybees are often a source of infection for wild pollinators. According to Gajger et al. [40], honeybee viruses such as BQCV and DWV infect wild bumblebees in Croatia. Similar results were reported by Toplak et al. 2020 [41] who observed that honeybee viruses probably spill over from managed honeybee colonies to wild bumblebees that visit the same flowers. These observations were confirmed by Agler et al. [42] who found that the prevalence of DWV was higher in bumblebees collected near apiaries and when neighboring bees were highly infected. The prevalence of BQCV was also higher in bumblebees collected near apiaries. Lotmaria passim and C. mellificae could be transmitted between pollinators through flowers. In the present study, parasites were detected in bumblebees, and further research is needed to confirm that protozoa of the family Trypanosomatidae can be transmitted from honeybees to wild bumblebees, and that flowers can be an important route of transmission.
Further research is needed to determine the influence of L. passim and C. mellificae infections on the decline of bumblebee populations. The role played by these parasites in bumblebee populations should be investigated. In the present study, L. passim was also identified in pollinators.
4. Materials and Methods
Thirteen adult bumblebees belonging to 4 species (B. lapidarius, B. lucorum, B. pascuorum, and B. terrestris) were collected for the study. Bumblebees are protected in Poland, and the researchers received permission to catch only two bumblebees of each species (authorization from the Minister of the Environment, decision No. DLP-III-4102-327/2359/14/MD). Dead bumblebees (that died of natural causes without any disease symptoms) were harvested in 2015 in southern Poland. The insects were collected from a field in the Medicinal Herb Garden in Wrocław, Botanical Garden in Wroclaw, and the following landscape parks in Lower Silesia: Bystrzyca River Valley, Barycza River Valley, Jezierzyca River Valley, and Ślęża Mountain. Genomic DNA was isolated from whole bumblebees. Each insect was homogenized in a mortar under sterile conditions. The Genomic Mini kit (A&A Biotechnology, Gdynia, Polska) was used to isolate DNA. DNA was isolated according to the manufacturer’s instructions. The samples were stored in a freezer at −20 ℃ until further analysis.
The DNA isolated from L. passim and C. mellificae was identified with the use of HotStarTaq Plus Polymerase (Qiagen, Hilden, Germany) and the HotStarTaq Plus Master Mix Kit (Qiagen). The PCR mixture of 20 μL consisted of 10 μL of 2× HotStarTaq Plus Master Mix, 2 µL of CoralLoad Concentrate 10×, 0.1 μL of each primer, and 1 to 3 µL of DNA (around 1200 ng/reaction), supplemented with RNase-Free Water to 20 µL. The primers for the synthesis of L. passim and C. mellificae DNA [18] were developed by Genomed (Warsaw, Poland). The following primers were used: for L. passim–LpCytb_F2 5′-AGTaTGAGCaGTaGGtTTTaTTATa-3′ and LpCytb_R 5′-gcCAaAcACCaATaACtGGtACt-3; for C. mellificae –CmCytb_F 5′-AGTtTGAgCtGTtGGaTTTgTt-3 and CmCytb_R 5′-AACCtATtACaGGcACaGTTGC-3′ [18].
Lotmaria passim and C. mellificae were identified by single PCR under the following conditions: initial denaturation at 94 °C for 45 s, followed by 35 cycles at 94 °C for 45 s, primer annealing at 55 °C for 45 s, and elongation at 72 °C for 1 min. The final extension step was conducted at 72 °C for 10 min. Every PCR reaction had two controls: one positive control with L. passim or C. mellificae DNA (University of Belgrade, Faculty of Veterinary Medicine, Department of Biology) and one “zero” control where DNA was replaced with water. PCR products were separated by electrophoresis in 2% agarose gel containing 6 µL of the Midori green stain for visualizing the DNA fragments of L. passim and C. mellificae. Product size was compared with the GeneRulerTM 100bp 36 Ladder Plus (Fermentas) molecular weight standard. Electrophoresis results were archived using the GelDoc (Bio-Rad, Hercules, CA, USA) imaging system.
Author Contributions
Conceptualization, M.M.; Methodology, M.M.; Investigation, M.M.; Data Analysis, M.M.; Writing—Original Draft Preparation, M.M.; Supervision, M.M.; Supervision, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland, grant No. 2018/02/X/NZ/01070, and the project was financially supported by the Minister of Science and Higher Education under the program entitled “Regional Initiative of Excellence” for the years 2019-2023, Project No. 010/RID/2018/19, amount of funding: PLN 12 000 000.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Winter, K.; Adams, L.; Thorp, R.; Inouye, D.; Day, L.; Ascher Buchmann, S. Importation of Non-Native Bumble Bees into North America: Potential Consequences of Using Bombus Terrestris and Other Non-Native Bumble Bees for the Greenhouse Crop Pollination in Canada, Mexico, and the United States; North American Pollinator Protection Campaign: San Francisco, CA, USA, 2006. [Google Scholar]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–882. [Google Scholar] [CrossRef]
- Mobley, M.W.; Gegear, R.J. Immune-cognitive system connectivity reduces bumblebee foraging success in complex multisensory floral environments. Sci. Rep. 2018, 8, 5953. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowski, T.; Pawlikowski, K. Trzmielowate Polski (Hymenoptera: Apidae: Bombini); Wydawnictwo Naukowe Uniwersytetu Mikołaja Kopernika: Toruń, Poland, 2012. [Google Scholar]
- Mobley, M.W.; Gegear, R.J. One size does not fit all: Caste and sex differences in the response of bumblebees (Bombus impatiens) to chronic oral neonicotinoid exposure. PLoS ONE 2018, 13, e0200041. [Google Scholar] [CrossRef] [PubMed]
- Colla, S.R.; Otterstatter, M.C.; Gegear, R.J.; Thomson, J.D. Plight of the bumble bee: Pathogen spillover from commercial to wild populations. Biol. Conserv. 2006, 129, 461–467. [Google Scholar] [CrossRef]
- Goulson, D.; Hughes, W.O.H. Mitigating the anthropogenic spread of bee parasites to protect wild pollinators. Biol. Conserv. 2015, 191, 10–19. [Google Scholar] [CrossRef]
- Sokol, R.; Michalczyk, M.; Micholap, P. Preliminary studies on the occurrence of honeybee pathogens in the national bumblebee population. Ann. Parasitol. 2018, 64, 385–390. [Google Scholar] [PubMed]
- Kostygov, A.Y.; Karnkowska, A.; Votýpka, J.; Tashyreva, D.; Maciszewski, K.; Yurchenko, Y.; Lukeš, J. Euglenozoa: Taxonomy, diversity and ecology, symbioses and viruses. Open Biol. 2021, 11, 200407. [Google Scholar] [CrossRef] [PubMed]
- Maslov, D.A.; Votýpka, J.; Yurchenko, V.; Lukeš, J. Diversity and phylogeny of insect trypanosomatids: All that is hidden shall be revealed. Trends Parasitol. 2013, 29, 43–52. [Google Scholar] [CrossRef]
- Fantham, H.B.; Porter, A. Note on certain protozoa found in bees. Suppl. J. Board. Agricul. 1912, 19, 138. [Google Scholar]
- Giavarini, I. Sui flagellati dell’ intestino tenue dell’ape domestica. Bolletino Zool. 1950, 17, 603–608. [Google Scholar] [CrossRef]
- Arismendi, N.; Caro, S.; Castro, M.P.; Vargas, M.; Riveros, G.; Venegas, T. Impact of Mixed Infections of Gut Parasites Lotmaria passim and Nosema ceranae on the Lifespan and Immune-related Biomarkers in Apis mellifera. Insects 2020, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Buendía-Abad, M.; García-Palencia, P.; de Pablos, L.M.; Alunda, J.M.; Osuna, A.; Martín-Hernández, R.; Higes, M. First description of Lotmaria passim and Crithidia mellificae haptomonad stages in the honeybee hindgut. Int. J. Parasitol. 2022, 52, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Kamhawi, S. Molecular Aspects of Parasite-Vector and Vector-Host Interactions in Leishmaniasis. Annu. Rev. Microbiol. 2001, 55, 453–483. [Google Scholar] [CrossRef]
- Schmid-Hempel, R.; Tognazzo, M. Molecular divergence defines two distinct lineages of Crithidia bombi (Trypanosomatidae), parasites of bumblebees. J. Eukaryot. Microbiol. 2010, 57, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Graystock, P.; Goulson, D.; Hughes, W.O.H. The relationship between managed bees and the prevalence of parasites in bumblebees. Peerj 2014, 2, e522. [Google Scholar] [CrossRef]
- Strobl, V.; Yañez, O.; Straub, L.; Albrecht, M.; Neumann, P. Trypanosomatid parasites infecting managed honeybees and wild solitary bees. Int. J. Parasitol. 2019, 49, 605–613. [Google Scholar] [CrossRef]
- Schwarz, R.S.; Bauchan, G.R.; Murphy, C.A.; Ravoet, J.; De Graaf, D.C.; Evans, J.D. Characterization of two species of trypanosomatidae from the Honey Bee Apis mellifera: Crithidia mellificae Langridge and McGhee, and Lotmaria passim n. gen., n. sp. J. Eukaryot. Microbiol. 2015, 62, 567–583. [Google Scholar] [CrossRef]
- Runckel, C.; Flenniken, M.L.; Engel, J.C.; Ruby, J.G.; Ganem, D.; Andino, R.; DeRisi, J.L. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS ONE 2011, 6, e20656. [Google Scholar] [CrossRef]
- Schwarz, R.S.; Evans, J.D. Single and mixed-species trypanosome andmicrosporidia infections elicit distinct, ephemeral cellular and humoral immune responses in honey bees. Dev. Comp. Immunol. 2013, 40, 300–310. [Google Scholar] [CrossRef]
- Lukeš, J.; Wheeler, R.; Jirsová, D.; David, V.; Archibald, J.M. Massive mitochondrial DNA content in diplonemid and kinetoplastid protists. IUBMB Life 2018, 70, 1267–1274. [Google Scholar] [CrossRef]
- Arismendi, N.; Bruna, A.; Zapata, N.; Vargas, M. PCR-specific detection of recently described Lotmaria passim (Trypanosomatidae) in Chilean apiaries. J. Invertebr. Pathol. 2016, 134, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Castelli, L.; Branchiccela, B.; Invernizzi, C.; Tomasco, I.; Basualdo, M.; Rodriguez, M.; Zunino, P.; Antúnez, K. Detection of Lotmaria passim in Africanized and European honey bees from Uruguay, Argentina and Chile. J. Invertebr. Pathol. 2019, 160, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Vejnovic, B.; Stevanovic, J.; Schwarz, R.S.; Aleksic, N.; Mirilovic, M.; Jovanovic, N.M.; Stanimirovic, Z. Quantitative PCR assessment of Lotmaria passim in Apis mellifera colonies co-infected naturally with Nosema ceranae. J. Invertebr. Pathol. 2018, 151, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Sokół, R.; Bancerz-Kisiel, A. Presence of Lotmaria passim, Crithidia mellificae and Nosema spp. in differently aged Apis mellifera brood. J. Apic. Res. 2022. [Google Scholar] [CrossRef]
- Ravoet, J.; Maharramov, J.; Meeus, I.; de Smet, L.; Wenseleers, T.; Smagghe, G.; de Graaf, D.C. Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLoS ONE 2013, 8, e72443. [Google Scholar] [CrossRef] [PubMed]
- Cepero, A.; Ravoet, J.; Gómez-Moracho, T.; Bernal, J.; Del Nozal, M.J.; Bartolomé, C.; Maside, X.; Meana, A.; González-Porto, A.V.; de Graaf, D.C.; et al. Holistic screening of collapsing honey bee colonies in Spain: A case study, BMC Res. Notes 2014, 7, 649. [Google Scholar] [CrossRef]
- Bartolomé, C.; Buendía-Abad, M.; Benito, M.; Sobrino, B.; Amigo, J.; Carracedo, A.; Martín-Hernández, R.; Higes, M.; Maside, X. Longitudinal analysis on parasite diversity in honeybee colonies: New taxa, high frequency of mixed infections and seasonal patterns of variation. Sci. Rep. 2020, 10, 10454. [Google Scholar] [CrossRef]
- D’Alvise, P.; Seeburger, V.; Gihring, K.; Kieboom, M.; Hasselmann, M. Seasonaldynamics and co-occurence patterns of honey bee pathogens revealed by high-throughput RT- qPCR. Ecol. Evol. 2019, 9, 10241–10252. [Google Scholar] [CrossRef]
- Nanetti, A.; Ellis, J.D.; Cardaio, I.; Cilia, G. Detection of Lotmaria passim, Crithidia mellificae and Replicative Forms of Deformed Wing Virus and Kashmir Bee Virus in the Small Hive Beetle (Aethina tumida). Pathogens 2021, 10, 372. [Google Scholar] [CrossRef]
- Michalczyk, M.; Bancerz-Kisiel, A.; Sokół, R. Lotmaria passim as Third Parasite Gastrointestinal Tract of Honey Bees Living in Tree Trunk. J. Apic. Sci. 2020, 64, 143–151. [Google Scholar] [CrossRef]
- Arismendi, N.; Riveros, G.; Zapata, N.; Smagghe, G.; González, C.; Vargas, M. Occurrence of bee viruses and pathogens associated with emerging infectious diseases in native and non-native bumble bees in southern Chile. Biol. Invasions 2021, 23, 1175–1189. [Google Scholar] [CrossRef]
- Gamboa, V.; Ravoet, J.; Brunain, M.; Smagghe, G.; Meeus, I.; Figueroa, J.; de Graaf, D. Bee pathogens found in Bombus atratus from Colombia: A case study. J. Invertebr. Pathol. 2015, 129, 36–39. [Google Scholar] [CrossRef]
- Plischuk, S.; de Landa, G.F.; Revainera, P.; Quintana, S.; Pocco, M.E.; Cigliano, M.M.; Lange, C.E. Parasites and pathogens associated with native bumble bees (Hymenoptera: Apidae: Bombus spp.) from highlands in Bolivia and Peru. Stud. Neotrop. Fauna Environ. 2021, 56, 93–98. [Google Scholar] [CrossRef]
- Pislak Ocepek, M.; Toplak, I.; Zajc, U.; Bevk, D. The Pathogens Spillover and Incidence Correlation in Bumblebees and Honeybees in Slovenia. Pathogens 2021, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, C.; Buendía-Abad, M.; Ornosa, C.; De la Rúa, P.; Martín-Hernández, R.; Higes, M.; Maside, X. Bee Trypanosomatids: First Steps in the Analysis of the Genetic Variation and Population Structure of Lotmaria passim, Crithidia bombi and Crithidia mellificae. Microb. Ecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Averill, A.L.; Couto, A.V.; Andersen, J.C.; Elkinton, J.S. Parasite Prevalence May Drive the Biotic Impoverishment of New England (USA) Bumble Bee Communities. Insects 2021, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Schlüns, H.; Sadd, B.M.; Schmid-Hempel, P.; Crozier, R.H. Infection with the trypanosome Crithidia bombi and expression of immune-related genes in the bumblebee Bombus terrestris. Dev. Comp. Immunol. 2010, 34, 705–709. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Šimenc, L.; Toplak, I. The First Detection and Genetic Characterization of Four Different Honeybee Viruses in Wild Bumblebees from Croatia. Pathogens 2021, 10, 808. [Google Scholar] [CrossRef]
- Toplak, I.; Šimenc, L.; Pislak Ocepek, M.; Bevk, D. Determination of Genetically Identical Strains of Four Honeybee Viruses in Bumblebee Positive Samples. Viruses 2020, 12, 1131. [Google Scholar] [CrossRef]
- Alger, S.A.; Burnham, P.A.; Boncristiani, H.F.; Brody, A.K. RNA virus spillover from managed honeybees (Apis mellifera) to wild bumblebees (Bombus spp.). PLoS ONE 2019, 14, e0217822. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).