Abstract
Cryptococcosis is the third most common cause of invasive fungal infection in solid organ transplant recipients and cryptococcal meningitis (CM) its main clinical presentation. CM outcomes, as well as its clinical features and radiological characteristics, have not yet been considered on a large scale in the context of kidney transplantation (KT). We performed a nationwide retrospective study of adult patients diagnosed with cryptococcosis after KT between 2002 and 2020 across 30 clinical centers in France. We sought to describe overall and graft survival based on whether KT patients with cryptococcosis developed CM or not. Clinical indicators of CNS involvement and brain radiological characteristics were assessed. Eighty-eight cases of cryptococcosis were diagnosed during the study period, with 61 (69.3%) cases of CM. Mortality was high (32.8%) at 12 months (M12) but not significantly different whether or not patients presented with CM. Baseline hyponatremia and at least one neurological symptom were independently associated with CM (p < 0.001). Positive serum cryptococcal antigen at diagnosis was also significantly associated with CM (p < 0.001). On magnetic resonance imaging (MRI), three patterns of brain injury were identified: parenchymal, meningeal, and vascular lesions. Although CM does not affect graft function directly, it entails a grim prognosis.
1. Introduction
Cryptococcus neoformans is an opportunistic pathogen ranking as the third most common cause of invasive fungal infection among solid organ transplant recipients [1]. The most frequent type of cryptococcosis manifestation is cryptococcal meningitis (CM), with a prevalence ranging from 25% to 72% across several studies in solid organ transplant recipients [2,3,4]. Taken as a whole, cryptococcosis indicates a grim prognosis with a mortality rate of about 10–25%, while patients with CM fare even worse, with an associated mortality of almost 40% [2,3,4]. The lack of specific symptoms and distinct clinical presentations, as well as an insidious onset, make diagnosis challenging, with significant delays in therapeutic management as a consequence.
Similar to invasive candidiasis—the most prevalent invasive mycosis in immunocompromised hosts—in that it affects both CNS and kidney function, cryptococcosis has not been in the scope of numerous studies [1,5,6]. For CM, studies dedicated to non-human immunodeficiency virus (HIV) patients have further highlighted some of the specific aspects of cryptococcosis in this population, including risk factors associated with neurological involvement [7], the role of plasma and/or cerebrospinal fluid (CSF) cryptococcal antigen (Ag) for prognostic evaluation [8], and the neurological complications of the disease [9]. However, CM outcomes—as well as its clinical features, radiological characteristics, and laboratory findings—have not been analyzed in a large cohort in this particular population [10,11,12].
We therefore conducted a multicentric retrospective study of a cohort of patients with cryptococcosis in 30 kidney transplantation (KT) centers in France.
Our primary objective was to describe the survival of patients with CM as compared to non-CM. Secondary objectives were the description of the radiological characteristics of CM in this specific population, with the aim of defining brain injury patterns and establishing their clinical correlations. We also sought to identify the clinical determinants of CM in KT recipients. Finally, we compared kidney graft survival in the group of patients with CM to patients with cryptococcosis without CM.
2. Materials and Methods
2.1. Design
This was a retrospective cohort study of adults diagnosed with cryptococcosis after KT between 2002 and 2020 in 30 French transplant centers.
2.2. Definitions
Proven CM was defined in compliance with the European Organization for Research and Treatment in Cancer and Mycoses Study Group (EORTC/MSG) definitions [13]: positive culture or ink smear of Cryptococcus from CSF or positive Ag in the CSF, or positive histopathological findings in brain tissue yielding 5–10 μm encapsulated yeasts. Patients with cryptococcosis not involving the central nervous system (CNS) were referred to as non-CM cryptococcosis. When lumbar punctures or anatomopathological tissues were not documented, the patient was considered non-CM. Hyponatremia was defined by a plasma sodium level below 135 mmol/L. Mortality rates and graft failure were evaluated 12 months after the diagnosis of cryptococcosis and analyzed throughout the follow-up.
Brain radiological abnormalities were defined as follows: (i) parenchymal lesions: lesions seated in the CNS generating inflammatory hypersignals in fluid-attenuated inversion recovery (FLAIR) imaging or associated with parenchymal enhancement on T1-weighted contrast, or the presence of hematoma; (ii) acute vascular lesions: all lesions displaying signs of acute ischemia on diffusion-weighted imaging and FLAIR imaging; (iii) meningeal lesions: all meningeal enhancement or ventriculitis on T1-weighted contrast; iv) hydrocephalus: increases in the volume of CSF and thus of the cerebral ventricles (ventriculomegaly) based on morphological MR sequences such as T2-weighted imaging.
2.3. Inclusion Criteria
Patients were included if they met the following study criteria: KT recipient (single or combined organ transplant) and proven diagnosis of cryptococcosis established between January 2002 and June 2020. Patients were excluded if they were under 18 years or had been on dialysis for more than three months before the diagnosis of cryptococcosis.
2.4. Clinical Symptoms and Laboratory Studies
Recipient characteristics, transplant characteristics, immunological parameters, and clinical and biological signs were included using a standardized case collection form. Brain magnetic resonance imaging (MRI) or computed tomography (CT) examinations were performed at the discretion of the physicians.
2.5. Statistical Analysis
For the description of continuous variables, the mean and the standard deviation (SD)—whenever they displayed a normal distribution—were used; otherwise, the median and interquartile range were used. The comparisons of the means and the proportions were carried out with the student’s t-test (or Mann–Whitney U test if appropriate) and the Chi2 test (or Fisher’s exact test if appropriate).
Logistical regression was used to compare patients with CM to their controls (cryptococcosis without meningeal involvement). The variables included in the final multivariate model were selected after a first univariate analysis if they had a degree of significance p less than or equal to 0.1.
Graft and patient survival were estimated using the Kaplan–Meier method and compared using the log rank test. The duration of follow-up was calculated from the date of the cryptococcosis diagnosis (starting point) and was followed through up to the date of graft failure or patient death, or at the end of the follow-up.
For patients who died with a functioning graft, graft survival was classified at the time of death as a functional graft.
All analyses were performed using R (version 4.0.0, R Foundation for Statistical Computing, Vienna, Austria) and STATA (StataCorp. 2017- Stata Statistical Software: Release 15. College Station, TX, USA). Values of p < 0.05 were considered significant, and all tests were two-tailed.
2.6. Ethics
This study was recorded in the Comity National Informatique and Liberty registry (registration number 2212716) in compliance with national policy (20 March 2019).
3. Results
Ninety-three patients were identified; three were excluded due to graft failure three months before diagnosis, and two were excluded due to an unspecified date of cryptococcosis diagnosis.
A total of 88 cases were included in the final analysis (Flowchart Figure S1). Of the 88 cases of cryptococcosis, 61 (69.3%) met the definition for CM.
3.1. General Characteristics of Patients with CM
3.1.1. Clinical Features
Of the 88 cases of cryptococcosis, the CNS ranked as the most common organ involved (n = 61, 69.3%). In 21.6% (n = 19) of the cases, the CNS was the only site of infection. The second most frequent organ involved was the respiratory tract (n = 28, 31.8%), followed by the skin (n = 19, 21.6%). The time between transplantation and cryptococcosis onset did not differ according to the presence or absence of CM (median 34.6 interquartile range (IQR) [1.6–72.4] vs. 42.9 [14.9–121] months, respectively (p = 0.581)). Regarding clinical symptoms (see details in Table 1b), patients with CM experienced more frequent vomiting (p < 0.001). Conversely, cough or dyspnea were less frequently reported (p = 0.049). Headaches (58.2%) and focal neurological signs (34.6%) were the most prevalent neurological signs in CM patients. Fever, present in 58% of cases, was found in a similar proportion whether in CM or non-CM subgroups (p = 0.348). Additionally, no difference was recorded when patients with CM were compared to other forms of cryptococcosis for the following conditions: age at diagnosis, post-transplant diabetes, history of other opportunistic fungal infections before cryptococcosis, positive HIV serostatus, induction therapy, or graft rejection before diagnosis (see details in Table 1a). We show a non-significant trend toward a greater prevalence of pre-transplant diabetes in the CM as compared to non-CM p = 0.378.

Table 1.
(a) Characteristics of CM and non-CM before diagnosis; (b) Characteristics of CM and non-CM at diagnosis and follow-up; (c) Microbiological characteristics of CM and non-CM at diagnosis.
3.1.2. Biological Characteristics
Hyponatremia was common at diagnosis of cryptococcosis, present in 61.8% of all cases (n = 42/68). Furthermore, plasma sodium levels were significantly lower in patients with CM, with a median IQR of 132 [130–134] mmol/L vs. 138 [135–139], respectively; p < 0.001. Among patients with CM, 25.5% (n = 13/51) had CSF white blood cell (WBC) counts within a normal range. CSF protein levels were increased in 76.5% (n = 39/51) of CM cases, with a median level of 0.9 [0.6–2.0] g/L. CSF glucose levels were lowered in 65.1% of CM cases (n = 28/43). Measurements of intracranial opening pressure were only recorded in 26.2% of CM cases (n = 16/61). When performed, pressure was found to be elevated in 93.7% of CM cases, with a median pressure of 30 cm H2O [16–32] (Figure S2).
Blood cultures tended to be more frequently positive for cryptococcosis in patients with CM (p = 0.055). Upon diagnosis, serum Ag at diagnosis was also more frequently positive in cases of CM (p < 0.001, Table 1c). When sampled, CSF antigen at diagnosis was positive in 100% (n = 33/33) of CM cases, the India ink test of the CSF yielded positive results for 41.2% (n = 21/51) of the patients, and CSF cultures were positive in 31.4% (n = 16/51) of cases (Table 1c). Different types of serological tests were used to detect Cryptococcus: enzyme-linked immunosorbent assay (ELISA) in 22% of cases (n = 11/50), Latex in 70% (n = 35/50), and immunochromatography in 8% (n = 4)
3.1.3. Immunosuppressive Regimen
The induction therapy and the immunosuppressive regimen were similar between the patients, irrespective of CM. Additionally, there was no difference in the type of calcineurin inhibitor (tacrolimus or ciclosporin) between patients with or without CM (p = 0.246).
3.1.4. Therapeutic Management
Among patients with CM, 77.8% (n = 42/54) received liposomal amphotericin B combined with flucytosine, as recommended by current guidelines.
The remaining patients received a single drug therapy or a combination of treatments that were not in line with recommendations. Of the patients who did not receive a treatment in keeping with guidelines, 9.6% (n = 5/54) died in the years following treatment, and in 80% (n = 4/5) of cases, death was directly ascribable to cryptococcosis (one patient relapsed 9 months after the onset of cryptococcosis; Table S1).
A total of 34.4% (n = 21/61) of patients required iterative lumbar punctures to manage intracranial hypertension. Seven patients required neurosurgical intervention for the placement of an external ventricular drain for the management of intracranial hypertension (11.5%, n = 7/61).
3.1.5. Determinants of CM
The determinants of CM were investigated based on the univariate analysis detailed in Table 2.

Table 2.
Determinants of CM in the univariate analysis.
Upon logistic regression analysis, the determinants independently associated with CM included the presence of at least one neurological symptom with an odds ratio (OR) of 60.71 (95% CI [9.12 to 404.20], p < 0.001) and natremia at the time of diagnosis (per 1 mmol/L increment) with an OR of 0.76 (95% CI [0.63 to 0.93], p = 0.008), as detailed in Table 3.

Table 3.
Determinants of CM in the multivariate analysis.
3.2. Prognosis of CM vs. Non-CM Cryptococcosis
3.2.1. Diagnostic Delay
There was a delay between the diagnosis of cryptococcosis and the first symptoms, with a median (IQR) of 23 days (7–52) for patients with cryptococcosis. Compared to patients with CM, the time interval did not differ in the subgroup of patients with non-CM, with a median of 25 days (9–58) vs. 21 days (5–34), p = 0.0823 (Figure 1). However, as shown on the density plot, some patients were diagnosed very late after the onset of CM (>120 days).
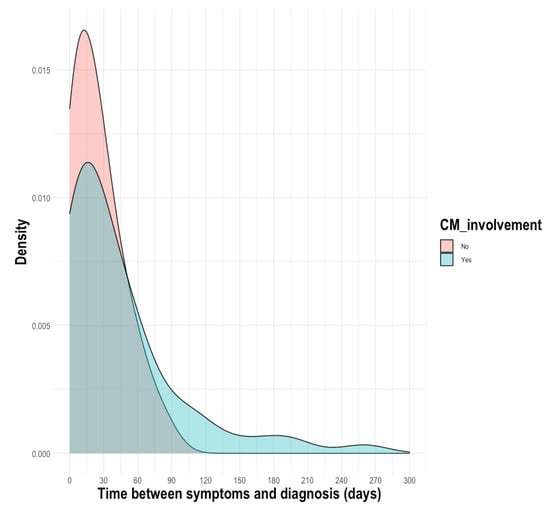
Figure 1.
Density plot of distribution of the time between the first symptoms and the diagnosis.
3.2.2. Patient Outcomes
At 12 months post-diagnosis, 32.8% of patients with CM died compared to 25.9% in the non-CM group (Table 4).

Table 4.
Outcomes after cryptococcosis comparing CM and non-CM.
At last follow-up, a total of 26 (42.6%) of patients with CM died at a median of 1.2 months (0.8–10.0) following diagnosis. However, CM did not confer worse patient survival compared with non-CM patients, (p = 0.42); see Figure 2a. Additionally, the presence of fungemia did not significantly impact survival (p = 0.27; Figure S3). Overall, six patients developed an immune reconstitution inflammatory syndrome (IRIS), including 83.3% (n = 5/6) in the CM group.
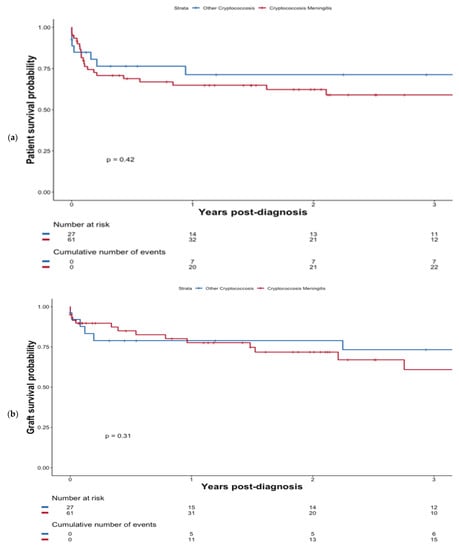
Figure 2.
Patient survival probability (a) and graft survival probability (b) after diagnosis of cryptococcosis depending on whether patients presented with CM or not.
3.2.3. Graft Survival
At the last follow-up, a total of 19 (31.1%) patients with CM experienced graft failure at a median of 9.4 months (0.2–33.0) post-diagnosis. Graft survival was not significantly altered in the case of CM (p = 0.31) see Figure 2b.
3.3. CM-Associated Features in Brain Imaging
3.3.1. Radiological Characteristics
Brain imaging (MRI and/or CT) was performed in 96.7% (n = 59/61) of cases with CM, using MRI (n = 41, 71.9%) or CT (n = 12, 21.0%) exclusively. When performed, CT scans did not yield positive findings, even in cases of confirmed CM, except for hydrocephalus (n = 1). There were significantly more abnormal MRIs than CT scans in the case of CM (p = 0.004). These results are summarized in Figure S4.
When performed, measurements of intracranial opening pressure were found to be elevated in 93.7% of CM cases. On brain imaging, the predominant injury patterns consisted of meningeal damage (n = 11, 18.6%), followed by parenchymal lesions (n = 10, 16.9%) and vascular injury (n = 10, 16.9%). Patients could display a combination of these patterns, as detailed in Figure 3. Cases of subtypes of brain lesions are shown in Figure S5.
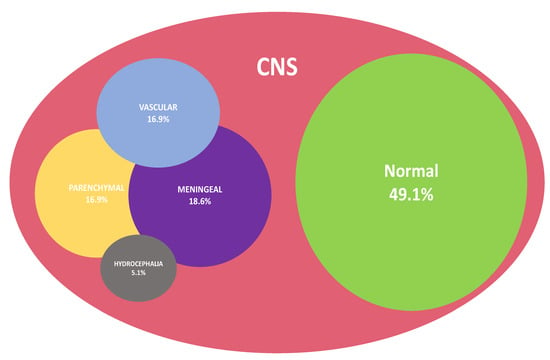
Figure 3.
Venn diagram representation summarizing brain injury patterns as displayed in imaging of patients with CM (n = 59/61).
3.3.2. Clinical Presentation According to Brain Injury Pattern
Symptoms did not differ depending on whether or not patients disclosed vascular injury in imaging. More specifically, patients with focal neurological signs were not found to be more frequent among patients with vascular injury on imaging; p = 0.256 (Table S2).
The pattern of symptoms was similar regardless of whether patients exhibited hydrocephalus in imaging. Specifically, patients with hydrocephalus did not have more headaches (p = 0.599; Table S3).
3.3.3. Outcomes According to the Type of Radiological Characteristics
Mortality tended to be worse if patients exhibited signs of brain parenchymal injury (n = 10); p = 0.051 (Figure 4).
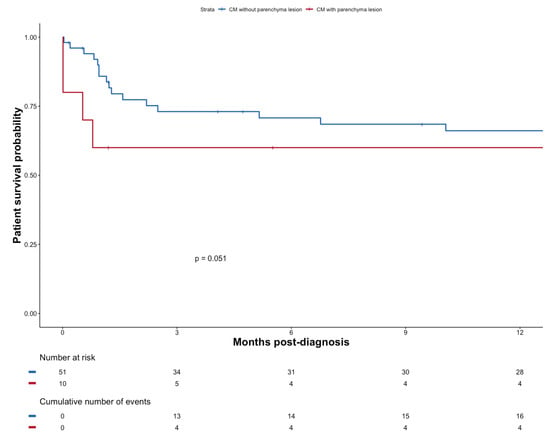
Figure 4.
Patient survival probability after diagnosis of CM with or without parenchyma lesion.
There was no significant survival difference after diagnosis of CM, whether patients presented evidence of vascular injury on the brain MRI (n = 10) or not (n = 49), p = 0.92 (see Figure 5). Evidence of vascular injury in imaging was not associated with reduced diagnostic delay (p = 0.9754).
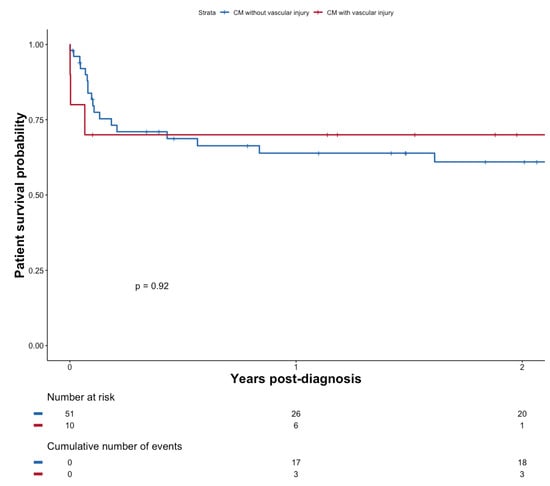
Figure 5.
Patient survival probability after diagnosis of CM with or without vascular injury.
4. Discussion
4.1. Clinical Features and Risk Factors
Neurotropism is one of the most prominent clinical features of pathogenic species of the genus Cryptococcus, explaining why CM is by far the most frequently identified clinical condition in cryptococcosis. Susceptibility to CNS infection is a universal trait of cryptococcosis, regardless of the underlying mechanism of immunosuppression (IS), but has been found to be even more pronounced in non-HIV-related IS compared to HIV-infected patients [7,8,9,14]. The clinical pattern of cryptococcosis-related CNS disease encompasses a broad range of neurological symptoms ranging from headaches to seizures. In our study, the presence of at least one neurological symptom was independently associated with CM. However, the predominant clinical picture is inconspicuous; in our study, a significant segment of our study population presented with non-specific signs (headaches, emesis, or dizziness), the neurological origin of which may easily have been discounted.
This is in line with previous studies in HIV-negative individuals where headaches appeared to be the leading manifestation, although with varying rates (24% to 100%) [15]. Bearing in mind that fever was only detected in 58% of patients, this makes for a misleading clinical picture that translates into significantly delayed diagnoses, which in turn exposes the patients to a heightened risk of mortality and debilitating neurological sequalae [9,14]. As a result, our patients with CM tend to experience prolonged diagnostic delays, compared to patients with non-CM cryptococcosis.
Consistent with previous studies, hyponatremia [16] was closely associated with the occurrence of CM. The underlying mechanism connecting hyponatremia with CM is unclear. Emesis and hyponatremia may well be interconnected, considering the pathophysiological interplay of emesis, hypovolemia, and arginine vasopressin release [16,17]. Nevertheless, clinicians should be alert to meaningful cues such as hyponatremia, together with a history of headaches and emesis.
Our study shows that a significant proportion of patients with proven CM display a normal count of WBC cells in the CSF (19.7%), a result akin to HIV patients with CM but hitherto unrecognized in transplant patients [18,19]. The urgent need for a timely diagnosis in the face of indefinite neurological-like symptoms, together with an unremarkable CSF examination, has spurred investigators to develop alternative strategies. The use of serum antigen is one such option.
Previous studies have shown that high titers of serum AG titer (>1/64) can accurately predict CM whenever patients present with clinical neurological manifestations [20,21]. Unfortunately, in our study, the serum AG titer could not be analyzed specifically due to an inordinately high level of missing data. However, in any case, serum testing is no substitute for clinician awareness: the success of this strategy presupposes increased vigilance and broad indications for serum AG titer testing.
In addition, high levels of serum AG titer do not obviate the need for the lumbar puncture required to document an elevated opening pressure (>25 mm Hg). This was demonstrated in a significant subset of patients in this study, higher than in other non-specific KT reports (22% [2] to 44% [14]).
Elevated opening pressure reflects raised intracranial pressure and carries its own implications in terms of survival, requiring repeat lumbar punctures or the placement of a permanent shunt [14]. It is thus meaningful that opening pressure was only documented in a fraction (26.5%) of the patient population—a deviation from current guidelines which has been reported in other settings. In our study, elevated intracranial pressure was detected in 83.3% of cases. Taken together, these results suggest further cases of intracranial hypertension may have been detected had lumbar punctures been performed more widely.
4.2. Outcome
In this cohort, cryptococcosis-associated mortality did not differ whether patients presented with CM or not at 12 months or at the last follow up (42% vs. 37%, p = 0.798). The fact that lumbar puncture was not carried out in a consistent fashion in our cohort may have obscured the significance of our results. Compared to the existing literature, the overall mortality when merging the CM and non-CM cryptococcosis subgroups was found to be higher. For instance, a large previous study, conducted in solid organ transplant patients, showed a mortality rate ranging between 15 and 20%—with the greatest rate (20%) observed in cases of CM [10].
Nevertheless, whenever KT patients are considered specifically, mortality rates have been shown to be comparable to our results; a recent study reported a mortality of 41% at 12-months follow-up in this subgroup [3]. Similarly, a series of 29 patients with cryptococcosis yielded a mortality rate of 34% at the last follow up [22]. Graft outcomes in our study were not significantly different for cases of CM.
4.3. Characteristics Based on CNS Imaging
According to our series, the pattern of injury of the CNS could be classified into three broad categories based on imaging—namely, vascular damage (n = 10), parenchymal lesions (n = 10) and meningeal (pachymeningeal and leptomeningeal) lesions (n = 11), with a near-even distribution.
Prior studies have produced conflicting results with respect to the type of CNS lesion upon imaging. In one study focusing on organ transplant recipients [10], cerebrovascular lesions were not described. Conversely, in recent reports [18,23] that included HIV negative patients, cerebral ischemic lesions were detected in 15–43% of these patients and their incidence was significantly higher in this segment of the population compared to HIV-positive patients. One explanation for these contrasting rates is partly due to the fact that Virchow–Robin spaces engulfed with fungus may closely mimic infarcts on diffusion restriction MRI, giving rise to differing interpretations. A recent retrospective analysis of 66 patients meeting the criteria for CM and investigated with appropriate brain imaging identified 20 patients (30.3%) with cerebral infarcts—all of which were of the lacunar type and often manifold (50%). Based on neuropathological reports and other forms of chronic meningitis recognized to involve vasculitis—first and foremost being tuberculous meningitis [24]—cerebrovascular injury is believed to arise through (i) dissemination of the inflammatory process to the vessels, originating either from basal exudates or meningeal infection and causing vascular strangulation and thrombosis; (ii) raised intracranial pressure hindering cerebral blood flow.
In our work, 38.7% of the patients with CM exhibited cerebrovascular involvement. In this subgroup of patients, symptoms related to an acute or sub-acute stroke may be partly veiled by other clinical features related to cryptococcosis and therefore go unrecognized. In keeping with this interpretation, clinical features did not differ whether brain imaging yielded evidence of vascular injury or not (p = 0.256). We failed to demonstrate that the type of CNS injury as characterized by imaging exerted a meaningful impact on survival. However, the association between brain parenchymal lesions and mortality verged on significance (p = 0.051), although the small cohort size means that the study may have been underpowered to detect any significant difference. Furthermore, the study was not devised to evaluate long-term persistent neurological impairment—a relevant issue given the prevalence of ischemic lesions.
5. Conclusions
Akin to other immunocompromised patients, this study confirms that the CNS is the most common organ affected when KT recipients develop cryptococcosis. Patient management is impaired by significantly protracted diagnostic delays. Non-specific symptoms may account for such delays. Our results suggest that clinicians should heed neurological manifestations, headaches, and hyponatremia, which hint at the occurrence of CM. Whenever cryptococcosis is confirmed, these signs should compel clinicians to perform lumbar punctures to both substantiate CM, assess opening pressure and, if need be, to remove CSF to control intracranial pressure. Our results also corroborate the emergence of a novel distinct radiological entity defined by vascular lesions. Meanwhile, patients exhibiting parenchymal lesions seem to be at a higher risk of more severe outcomes. Overall, compared to non-CM cryptococcosis patients, KT patients with CM do not fare worse with respect to patient and graft survival. Worryingly, in the setting of KT, the prognosis of patients with cryptococcosis remains dismal on both counts. In fact, the outcome of KT patients developing CM may be even more severe than in other immunocompromised patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11060699/s1, Figure S1: Flow Chart; Figure S2: Measurement of intracranial pressure in patients with cryptococcosis; Figure S3: Patient survival probability after diagnosis of Cryptococcosis with fungemia; Figure S4: Results of brain imaging by type for CM patients; Figure S5: MRI patient on magnetic resonance imaging; Table S1: Summary table of the clinical evolution of patients with induction treatment outside Guidelines; Table S2: Neurological symptoms based on the detection of vascular injury on brain imaging; Table S3: Neurological symptoms based on the detection of hydrocephalus on brain imaging.
Author Contributions
C.R. and F.L. supervised the study. L.T., G.D., F.L., O.L. and C.R. designed the study, analyzed and interpreted the data, wrote and edited the manuscript. L.T., G.D. (Gillian Divard), O.L., A.S., É.R., I.A., R.A., A.A. (Alexandre Alanio), A.A. (Adela Angoulvant), L.A., P.A., A.P.B., D.B., J.B., F.B., N.B., M.B., T.C. (Taieb Chouaki), T.C. (Thomas Crépin), M.-F.D., G.D. (Guillaume Desoubeaux), G.D. (Gary Doppelt), L.F., A.F., O.F., M.F., J.-P.G., C.G., L.H., C.H., X.I., N.K., H.K., R.K., L.L., C.L. (Christophe Legendre), M.L.Q.D., J.L., C.L. (Charlène Levi), M.M., D.M., J.M., V.M., F.M., N.M., M.N., P.P., M.-N.P., B.P., S.R., J.-P.R., B.S., R.S., J.T., M.V., C.V., O.V., L.M., F.L. and C.R. had access to the data and three authors (L.T., G.D. and C.R.) verified the data. C.R. was responsible for the decision to submit the manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the French Ethics Committee on the Treatment of Computerized Data in the Field of Medical Research (declaration number 2212716—20 March 2019).
Informed Consent Statement
The approval obtained from the CNIL (Comission Nationale Informatique et Libertés) grants us permission to collect and exploit patient data.
Data Availability Statement
The data and material are available upon request to the corresponding author.
Acknowledgments
The authors would like to thank Felicity Kay for their English language editing.
Ethics Approval
The study was recorded in the Comity National Informatique and Liberty registry (registration number 2212716) in compliance with national policy (20 March 2019).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pappas, P.G.; Alexander, B.D.; Andes, D.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive Fungal Infections among Organ Transplant Recipients: Results of the Transplant—Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, N.; Reyes, J.; Kusne, S.; Martin, M.; Fung, J. Cryptococcal meningitis after liver transplantation. Transplantation 1996, 61, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Wagener, M.M.; Singh, N. Cryptococcus neoformansInfection in Organ Transplant Recipients: Variables Influencing Clinical Characteristics and Outcome. Emerg. Infect. Dis. 2001, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- John, G.T.; Mathew, M.; Snehalatha, E.; Anandi, V.; Date, A.; Jacob, C.K.; Shastry, J.C.M. Cryptococcosis in renal allograft recipients. Transplantation 1994, 58, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. Campion EW, éditeur. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [Green Version]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Desnos-Ollivier, M.; Bretagne, S.; Dromer, F. The French Mycoses Study Group. The risk and clinical outcome of candidemia depending on underlying malignancy. Intensive Care Med. 2017, 43, 652–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osawa, R.; Alexander, B.D.; Lortholary, O.; Dromer, F.; Forrest, G.N.; Lyon, G.M.; Somani, J.; Gupta, K.L.; Del Busto, R.; Pruett, T.L.; et al. Identifying Predictors of Central Nervous System Disease in Solid Organ Transplant RecipientsWith Cryptococcosis. Transplantation 2010, 89, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Alexander, B.D.; Lortholary, O.; Dromer, F.; Gupta, K.L.; John, G.T.; del Busto, R.; Klintmalm, G.B.; Somani, J.; Lyon, G.M.; et al. Cryptococcus neoformans in Organ Transplant Recipients: Impact of Calcineurin—Inhibitor Agents on Mortality. J. Infect. Dis. 2007, 195, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Marr, K.A.; Sun, Y.; Spec, A.; Lu, N.; Panackal, A.; Bennett, J.; Pappas, P.; Ostrander, D.; Datta, K.; Zhang, S.X.; et al. A Multicenter, Longitudinal Cohort Study of Cryptococcosis in Human Immunodeficiency Virus–negative People in the United States. Clin. Infect. Dis. 2019, 70, 252–261. [Google Scholar] [CrossRef]
- Singh, N.; Dromer, F.; Perfect, J.R.; Lortholary, O. Immunocompromised Hosts: Cryptococcosis in Solid Organ Transplant Recipients: Current State of the Science. Clin. Infect. Dis. 2008, 47, 1321–1327. [Google Scholar] [CrossRef]
- Nelles, R.; Britton, S.; John, G.T.; Denaro, C. Parkinsonism and prolonged cognitive decline as a manifestation of cryptococcal meningitis in a renal transplant patient. BMJ Case Rep. 2022, 15, e245788. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Lee, W.-S.; Tsai, C.-A.; Fan, W.-C.; Wu, P.-F.; Wang, F.-D. Incidence of and risk factors for cryptococcosis in kidney transplant recipients in Taiwan—A nationwide population-based study. Int. J. Infect. Dis. 2019, 88, 154–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brizendine, K.D.; Baddley, J.W.; Pappas, P.G. Predictors of Mortality and Differences in Clinical Features among Patients with Cryptococcosis According to Immune Status. PLoS ONE 2013, 8, e60431. [Google Scholar] [CrossRef]
- Beardsley, J.; Sorrell, T.C.; Chen, S.C.-A. Central Nervous System Cryptococcal Infections in Non-HIV Infected Patients. J. Fungi 2019, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Momi, J.; Tang, C.; Abcar, A.C.; Kujubu, D.A.; Sim, J.J. Hyponatremia in a patient with cryptococcal meningitis: Syndrome of inappropriate antidiuretic hormone (SIADH) or cerebral salt wasting (CSW)? J. Hosp. Med. 2010, 5, 193–195. [Google Scholar] [CrossRef]
- Sahay, M.; Sahay, R. Hyponatremia: A practical approach. Indian J. Endocrinol. Metab. 2014, 18, 760–771. [Google Scholar] [CrossRef]
- Qu, J.; Zhou, T.; Zhong, C.; Deng, R.; Lü, X. Comparison of clinical features and prognostic factors in HIV-negative adults with cryptococcal meningitis and tuberculous meningitis: A retrospective study. BMC Infect. Dis. 2017, 17, 51. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Lortholary, O.; Dromer, F.; Alexander, B.D.; Gupta, K.L.; John, G.T.; Del Busto, R.; Klintmalm, G.B.; Somani, J.; Lyon, G.M.; et al. Central Nervous System Cryptococcosis in Solid Organ Transplant Recipients: Clinical Relevance of Abnormal Neuroimaging Findings. Transplantation 2008, 86, 647–651. [Google Scholar] [CrossRef]
- Hamadani, B.H.K.; Franco-Paredes, C.; McCollister, B.; Shapiro, L.; Beckham, J.D.; Henao-Martínez, A.F. Cryptococcosis and cryptococcal meningitis: New predictors and clinical outcomes at a United States academic medical centre. Mycoses 2017, 61, 314–320. [Google Scholar] [CrossRef]
- Huang, S.-H.; Chuang, Y.-C.; Lee, Y.-C.; Hung, C.-C.; Sheng, W.-H.; Su, J.-J.; Sun, H.-Y.; Chen, Y.-C.; Chang, S.-C. Lumbar puncture for non-HIV-infected non-transplant patients with cryptococcosis: Should it be mandatory for all? PLoS ONE 2019, 14, e0221657. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-L.; Chen, M.; Gu, J.-L.; Zhu, F.-Y.; Xu, X.-G.; Zhang, C.; Chen, J.-H.; Pan, W.-H.; Liao, W.-Q. Cryptococcosis in kidney transplant recipients in a Chinese university hospital and a review of published cases. Int. J. Infect. Dis. 2014, 26, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Zhou, Z.; Fang, X.; Peng, F.; Zhang, W. Magnetic resonance imaging study of cryptococcal neuroradiological lesions in HIV-negative cryptococcal meningitis. Eur. J. Clin. Microbiol. 2017, 36, 1367–1372. [Google Scholar] [CrossRef]
- Lan, S.-H.; Chang, W.; Lu, C.; Lui, C.; Chang, H. Cerebral infarction in chronic meningitis: A comparison of tuberculous meningitis and cryptococcal meningitis. QJM Int. J. Med. 2001, 94, 247–253. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).