Mobile Colistin Resistance (mcr) Genes in Cats and Dogs and Their Zoonotic Transmission Risks
Abstract
:1. Introduction
2. Material and Methods
2.1. Design and Collection of Articles
2.2. Inclusion and Exclusion Criteria
2.3. Extraction of the Dataset
3. Results and Discussion
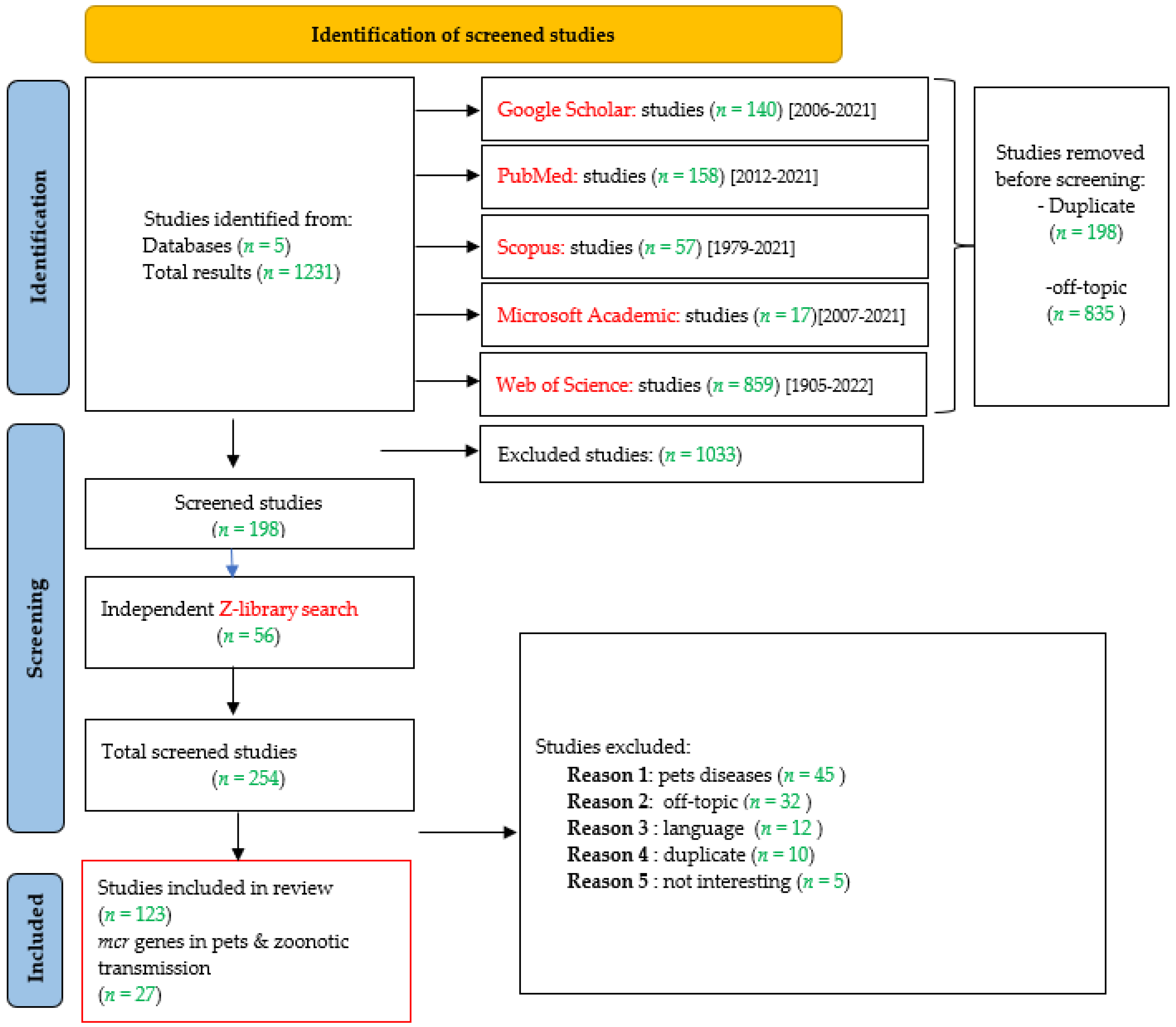
3.1. Bibliographic Research
3.2. Colistin Resistance Mechanisms
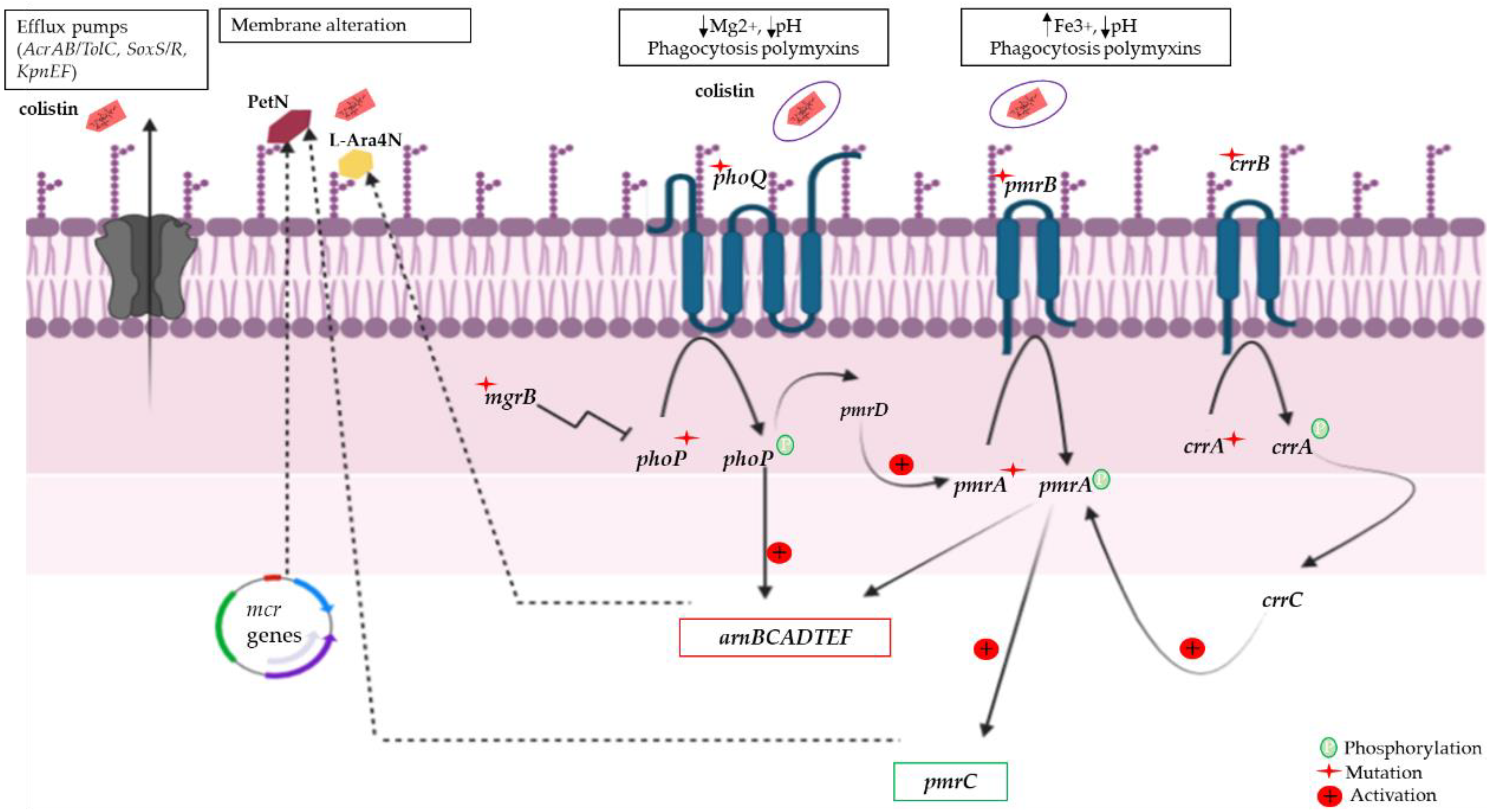
3.2.1. Chromosomic Colistin Resistance
3.2.2. Plasmid-Mediated Colistin Resistance
3.3. Antimicrobial Uses
3.3.1. Antimicrobials and Colistin Treatment for Pets
3.3.2. Antimicrobial Use and Antimicrobial Resistance
3.3.3. Approaches Aimed at Reducing Antimicrobial Use
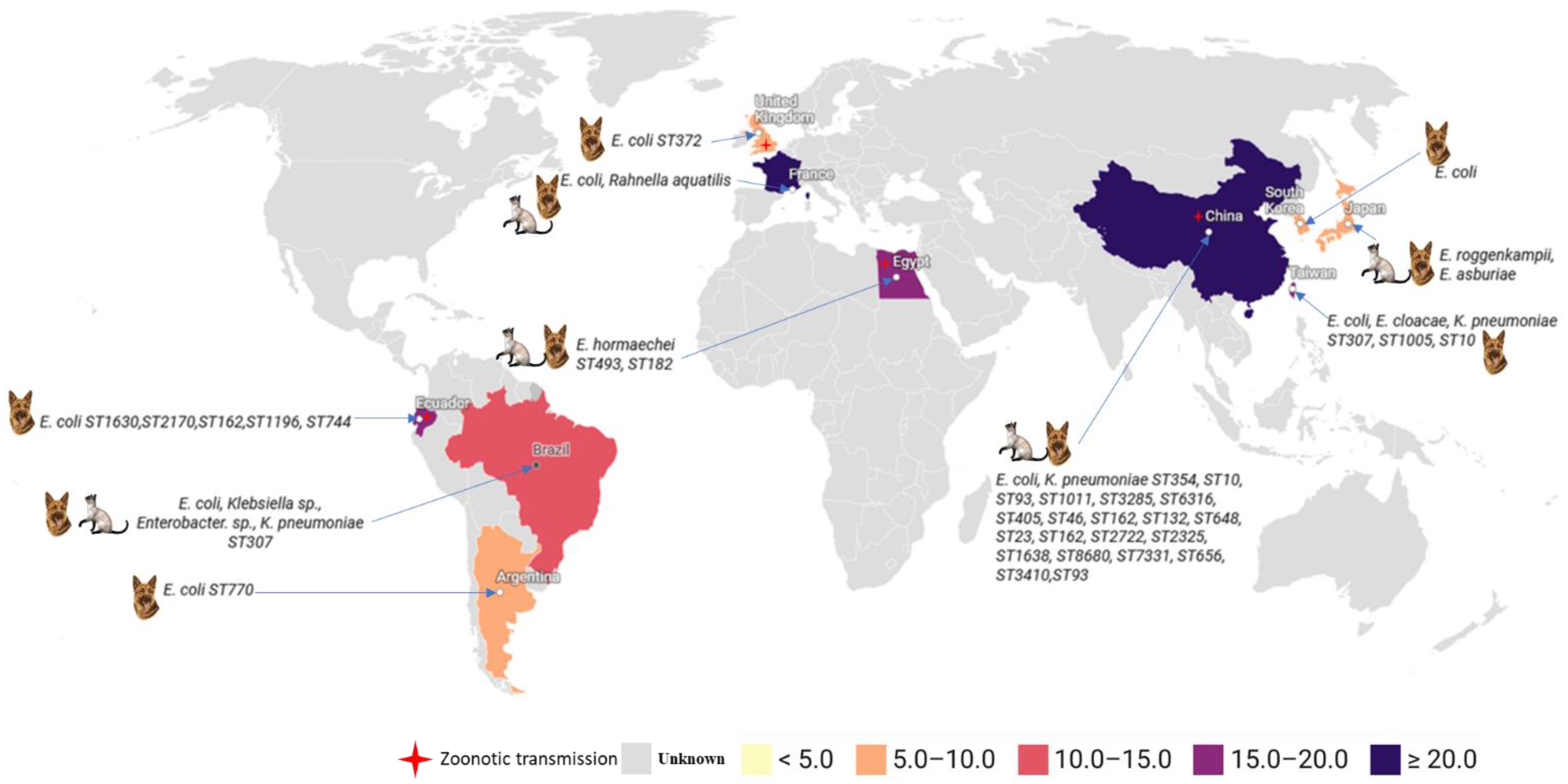
3.4. mcr Genes in Companion Animals
3.4.1. mcr Genes in Dogs
3.4.2. mcr Genes in Cats
3.4.3. Source of mcr Genes in Pets
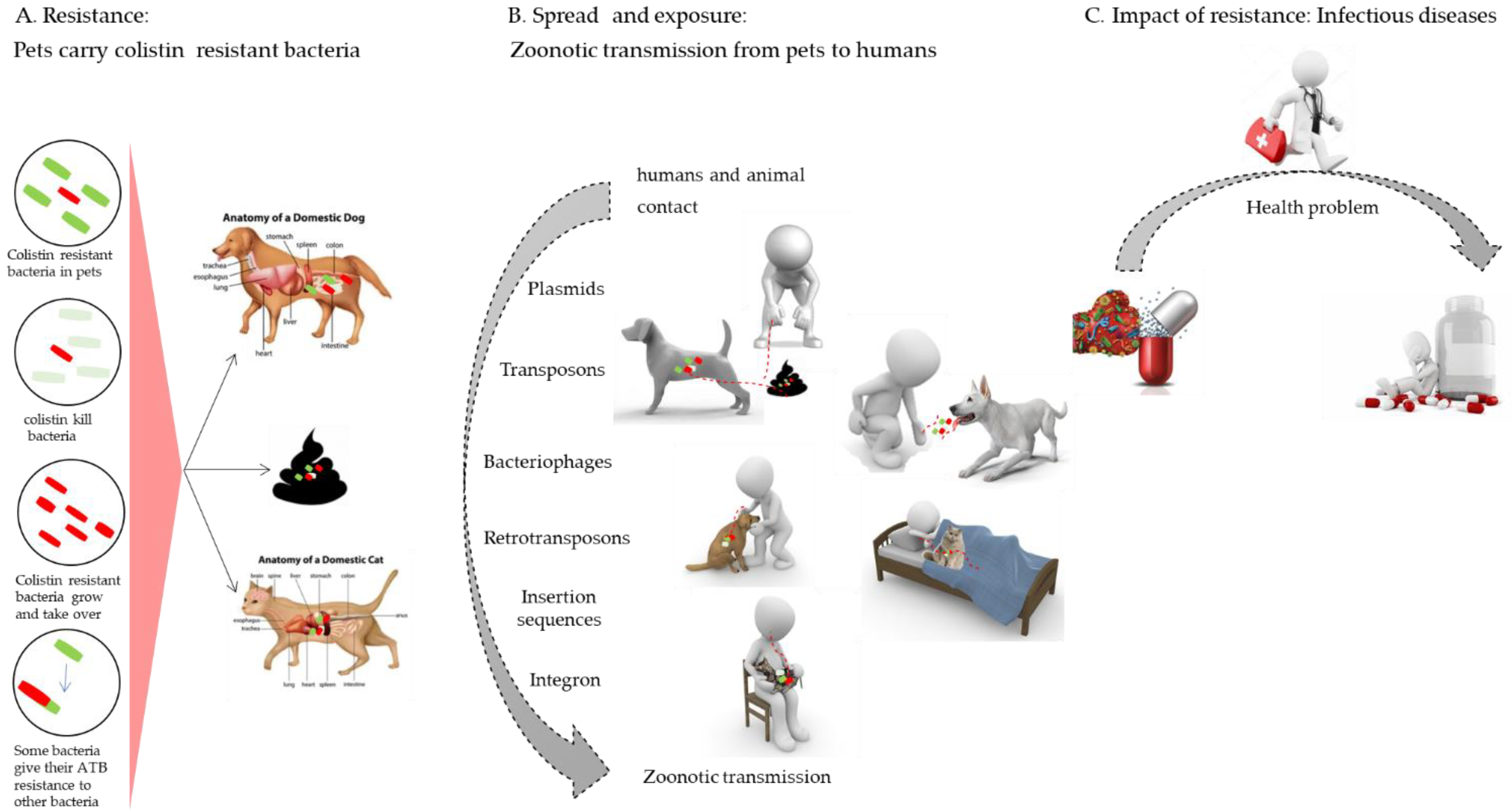
3.5. Zoonotic Transmission
3.5.1. Zoonotic Transmission of mcr Genes between Pets and Humans
3.5.2. Health Risks Associated with Colistin-Resistant Bacteria in Pets
3.5.3. Strategies to Control the Zoonotic Transmission of Colistin Resistance from Pets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beetz, A.; Uvnäs-Moberg, K.; Julius, H.; Kotrschal, K. Psychosocial and psychophysiological effects of human-animal interactions: The possible role of oxytocin. Front. Psychol. 2012, 3, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- New Perspectives on Human-Animal Interactions: Theory, Policy, and Research—Google Livres. Available online: https://books.google.fr/books?hl=fr&lr=&id=EHkMjpMFlWIC&oi=fnd&pg=PA523&dq=human+provides+tenderness+towards+certain+pets+&ots=q4WKWf_1wb&sig=2ZmeUxJtwQnwgPK8kjQ_nOLTwkU&redir_esc=y#v=onepage&q=humanprovidestendernesstowardscertainpets&f=false (accessed on 17 January 2022).
- Szwabe, K.; Błaszkowska, J. Stray dogs and cats as potential sources of soil contamination with zoonotic parasites. Ann. Agric. Environ. Med. 2017, 24, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Belas, A. Extended-spectrum–beta-lactamases, cephalosporinases, and carbapenemase-producing Escherichia coli in the human-dog interface. Ph.D. Thesis, Universidade de Lisboa, Lisboa, Portugal, 2021. [Google Scholar]
- DeVincent, S.J.; Viola, C. Introduction to animal antimicrobial use data collection in the United States: Methodological options. Prev. Vet. Med. 2006, 73, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Unmet medical needs in antibacterial therapy. Biochem. Pharmacol. 2006, 71, 991–995. [Google Scholar] [CrossRef]
- Berlana, D.; Llop, J.M.; Fort, E.; Badia, M.B.; Jódar, R. Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am. J. Health Pharm. 2005, 62, 39–47. [Google Scholar] [CrossRef]
- Nation, R.L.; Li, J. Colistin in the 21st Century. Curr. Opin. Infect. Dis. 2009, 22, 535. [Google Scholar] [CrossRef]
- Rozenberg-Arska, M.; Dekker, A.W.; Verhoef, J. Colistin and trimethoprim-sulfamethoxazole for the prevention of infection in patients with acute non-lymphocytic leukaemia. Decrease in the emergence of resistant bacteria. Infection 1983, 11, 167–169. [Google Scholar] [CrossRef]
- Aghapour, Z.; Gholizadeh, P.; Ganbarov, K.; Bialvaei, A.Z.; Mahmood, S.S.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Yousefi, B.; Kafil, H.S. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect. Drug Resist. 2019, 12, 965. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Malhotra-Kumar, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a novel plasmid-mediated colistinresistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Eurosurveillance 2016, 21, 30280. [Google Scholar] [CrossRef]
- Eichhorn, I.; Feudi, C.; Wang, Y.; Kaspar, H.; Feßler, A.T.; Lübke-Becker, A.; Michael, G.B.; Shen, J.; Schwarz, S. Identification of novel variants of the colistin resistance gene mcr-3 in Aeromonas spp. from the national resistance monitoring programme GERM-Vet and from diagnostic submissions. J. Antimicrob. Chemother. 2018, 73, 1217–1221. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance 2017, 22, 30589. [Google Scholar] [CrossRef] [Green Version]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. Enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017, 72, 3317–3324. [Google Scholar] [CrossRef] [Green Version]
- AbuOun, M.; Stubberfield, E.J.; Duggett, N.A.; Kirchner, M.; Dormer, L.; Nunez-Garcia, J.; Randall, L.P.; Lemma, F.; Crook, D.W.; Teale, C.; et al. mcr-1 and mcr-2 (mcr-6.1) variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J. Antimicrob. Chemother. 2017, 72, 2745–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.Q.; Li, Y.X.; Lei, C.W.; Zhang, A.Y.; Wang, H.N. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2018, 73, 1791–1795. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Zhou, Y.; Wang, Z.; Wang, Y.; Zhang, S.; Shen, Z. Emergence of colistin resistance gene mcr-8 and its variant in Raoultella ornithinolytica. Front. Microbiol. 2019, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Carroll, L.M.; Gaballa, A.; Guldimann, C.; Sullivan, G.; Henderson, L.O.; Wiedmann, M. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype typhimurium isolate. MBio 2019, 10, e00853-19. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Feng, Y.; Liu, L.; Wei, L.; Kang, M.; Zong, Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 2020, 9, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Jayol, A.; Poirel, L.; Dortet, L.; Nordmann, P. National survey of colistin resistance among carbapenemase-producing Enterobacteriaceae and outbreak caused by colistin-resistant blaOXA-48-producing Klebsiella pneumoniae, France, 2014. Eurosurveillance 2016, 21, 30339. [Google Scholar] [CrossRef] [Green Version]
- Dandachi, I.; Chabou, S.; Daoud, Z.; Rolain, J.M. Prevalence and emergence of extended-spectrum cephalosporin-, carbapenem- and colistin-resistant gram negative bacteria of animal origin in the Mediterranean basin. Front. Microbiol. 2018, 9, 2299. [Google Scholar] [CrossRef]
- Grami, R.; Mansour, W.; Mehri, W.; Bouallègue, O.; Boujaâfar, N.; Madec, J.; Haenni, M. Impact of food animal trade on the spread of mcr-1-mediated colistin resistance, tunisia, July 2015. Eurosurveillance 2016, 21, 30144. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, I.; Fayad, E.; Sleiman, A.; Daoud, Z.; Rolain, J.M. Dissemination of Multidrug-Resistant and mcr-1 Gram-Negative Bacilli in Broilers, Farm Workers, and the Surrounding Environment in Lebanon. Microb. Drug Resist. 2020, 26, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.S.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect. Drug Resist. 2020, 13, 3255. [Google Scholar] [CrossRef]
- Ben Khedher, M.; Baron, S.A.; Riziki, T.; Ruimy, R.; Raoult, D.; Diene, S.M.; Rolain, J.M. Massive analysis of 64,628 bacterial genomes to decipher water reservoir and origin of mobile colistin resistance genes: Is there another role for these enzymes? Sci. Rep. 2020, 10, 5970. [Google Scholar] [CrossRef] [Green Version]
- Elbediwi, M.; Li, Y.; Paudyal, N.; Pan, H.; Li, X.; Xie, S.; Rajkovic, A.; Feng, Y.; Fang, W.; Rankin, S.C.; et al. Global Burden of Colistin-Resistant Bacteria: Mobilized Colistin Resistance Genes Study (1980–2018). Microorganisms 2019, 7, 461. [Google Scholar] [CrossRef] [Green Version]
- Bhat, A.H. Bacterial zoonoses transmitted by household pets and as reservoirs of antimicrobial resistant bacteria. Microb. Pathog. 2021, 155, 104891. [Google Scholar] [CrossRef]
- Skarżyńska, M.; Zaja̧c, M.; Bomba, A.; Bocian, Ł.; Kozdruń, W.; Polak, M.; Wia̧cek, J.; Wasyl, D. Antimicrobial Resistance Glides in the Sky—Free-Living Birds as a Reservoir of Resistant Escherichia coli With Zoonotic Potential. Front. Microbiol. 2021, 12, 673. [Google Scholar] [CrossRef]
- Ahmed, Z.S.; Elshafiee, E.A.; Khalefa, H.S.; Kadry, M.; Hamza, D.A. Evidence of colistin resistance genes (mcr-1 and mcr-2) in wild birds and its public health implication in Egypt. Antimicrob. Resist. Infect. Control 2019, 8, 197. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of Polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef] [Green Version]
- Dénervaud Tendon, V.; Poirel, L.; Nordmann, P. Transferability of the mcr-1 Colistin Resistance Gene. Microb. Drug Resist. 2017, 23, 813–814. [Google Scholar] [CrossRef] [Green Version]
- Kaye, K.S.; Pogue, J.M.; Tran, T.B.; Nation, R.L.; Li, J. Agents of Last Resort: Polymyxin Resistance. Infect. Dis. Clin. 2016, 30, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Zhu, B.; Gao, G.F. Metagenomic data screening reveals the distribution of mobilized resistance genes tet(X), mcr and carbapenemase in animals and humans. J. Infect. 2020, 80, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, X.-Y.; Xia, Y.-B.; Guo, Z.-W.; Ma, Z.-B.; Yi, M.-Y.; Lv, L.-C.; Lu, P.-L.; Yan, J.-C.; Huang, J.-W.; et al. Clonal Spread of Escherichia coli ST93 Carrying mcr-1-Harboring IncN1-IncHI2/ST3 Plasmid Among Companion Animals, China. Front. Microbiol. 2018, 9, 2989. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Raoult, D. Colistin: An Antimicrobial for the 21st Century? Clin. Infect. Dis. 2002, 35, 901–902. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.S.; Barone, A.A.; Penço, J.; Santos, M.V.; Marinho, I.S.; Arruda, E.A.G.; Manrique, E.I.; Costa, S.F. Intravenous Colistin as Therapy for Nosocomial Infections Caused by Multidrug-Resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin. Infect. Dis. 1999, 28, 1008–1011. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.M.; Ly, N.; Anderson, D.; Yang, J.C.; Macander, L.; Jarkowski, A.; Forrest, A.; Bulitta, J.B.; Tsuji, B.T. Resurgence of Colistin: A review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 1279–1291. [Google Scholar] [CrossRef]
- Kempf, I.; Fleury, M.A.; Drider, D.; Bruneau, M.; Sanders, P.; Chauvin, C.; Madec, J.Y.; Jouy, E. What do we know about resistance to colistin in Enterobacteriaceae in avian and pig production in Europe? Int. J. Antimicrob. Agents 2013, 42, 379–383. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [Green Version]
- Publish or Perish. Available online: https://harzing.com/resources/publish-or-perish (accessed on 17 January 2022).
- Z-Library. The World’s Largest Ebook Library. Available online: https://z-lib.org/ (accessed on 17 January 2022).
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.F.; Vinogradov, E.; Seemann, T.; Henry, R.; Crane, B.; St. Michael, F.; Cox, A.D.; et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 2010, 54, 4971–4977. [Google Scholar] [CrossRef] [Green Version]
- Hussein, A.H.M.; Ghanem, I.A.I.; Eid, A.A.M.; Ali, M.A.; Sherwood, J.S.; Li, G.; Nolan, L.K.; Logue, C.M. Molecular and phenotypic characterization of Escherichia coli isolated from broiler chicken flocks in Egypt. Avian Dis. 2013, 57, 602–611. [Google Scholar] [CrossRef]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti-Infect. Ther. 2014, 10, 917–934. [Google Scholar] [CrossRef]
- Sun, S.; Negrea, A.; Rhen, M.; Andersson, D.I. Genetic analysis of colistin resistance in Salmonella enterica serovar typhimurium. Antimicrob. Agents Chemother. 2009, 53, 2298–2305. [Google Scholar] [CrossRef] [Green Version]
- Gunn, J.S. Bacterial modification of LPS and resistance to antimicrobial peptides. J. Endotoxin Res. 2001, 7, 57–62. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A Modification Systems in Gram-Negative Bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef] [Green Version]
- Kox, L.F.F.; Wösten, M.M.S.M.; Groisman, E.A. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 2000, 19, 1861–1872. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Latifi, T.; Groisman, E.A. Closing the loop: The PmrA/PmrB two-component system negatively controls expression of its posttranscriptional activator PmrD. Proc. Natl. Acad. Sci. USA 2003, 100, 4706–4711. [Google Scholar] [CrossRef] [Green Version]
- Mitrophanov, A.Y.; Jewett, M.W.; Hadley, T.J.; Groisman, E.A. Evolution and Dynamics of Regulatory Architectures Controlling Polymyxin B Resistance in Enteric Bacteria. PLOS Genet. 2008, 4, e1000233. [Google Scholar] [CrossRef] [Green Version]
- Trent, M.S.; Pabich, W.; Raetz, C.R.H.; Miller, S.I. A PhoP/PhoQ-induced Lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J. Biol. Chem. 2001, 276, 9083–9092. [Google Scholar] [CrossRef] [Green Version]
- Falagas, M.E.; Rafailidis, P.I.; Matthaiou, D.K. Resistance to Polymyxins: Mechanisms, frequency and treatment options. Drug Resist. Updat. 2010, 13, 132–138. [Google Scholar] [CrossRef]
- Lacour, S.; Doublet, P.; Obadia, B.; Cozzone, A.J.; Grangeasse, C. A novel role for protein-tyrosine kinase Etk from Escherichia coli K-12 related to Polymyxin resistance. Res. Microbiol. 2006, 157, 637–641. [Google Scholar] [CrossRef]
- Moon, K.; Gottesman, S. A PhoQ/P-regulated small RNA regulates sensitivity of Escherichia coli to antimicrobial peptides. Mol. Microbiol. 2009, 74, 1314–1330. [Google Scholar] [CrossRef] [Green Version]
- Baron, S.; Hadjadj, L.; Rolain, J.M.; Olaitan, A.O. Molecular mechanisms of Polymyxin resistance: Knowns and unknowns. Int. J. Antimicrob. Agents 2016, 48, 583–591. [Google Scholar] [CrossRef]
- Skov, R.L.; Monnet, D.L. Plasmid-mediated colistin resistance (mcr-1 gene): Three months later, the story unfolds. Eurosurveillance 2016, 21, 30155. [Google Scholar] [CrossRef] [Green Version]
- Ling, Z.; Yin, W.; Shen, Z.; Wang, Y.; Shen, J.; Walsh, T.R. Epidemiology of mobile colistin resistance genes mcr-1 to mcr-9. J. Antimicrob. Chemother. 2020, 75, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Guo, J.; Cheng, Q.; Yang, Z.; Chan, E.W.C.; Chen, S.; Hao, Q. Crystal Structure of Escherichia coli originated mcr-1, a phosphoethanolamine transferase for Colistin resistance. Sci. Rep. 2016, 6, 38793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.; Urmi, U.L.; Rana, M.; Sultana, F.; Jahan, N.; Hossain, B.; Iqbal, S.; Hossain, M.M.; Mosaddek, A.S.M.; Nahar, S. High abundance of the colistin resistance gene mcr-1 in chicken gut-bacteria in Bangladesh. Sci. Rep. 2020, 10, 17292. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Dong, X.; Wu, J.; Rao, D.; Zhang, L.; Faraj, Y.; Yang, K. Metadata Analysis of mcr-1-Bearing plasmids inspired by the sequencing evidence for horizontal transfer of antibiotic resistance genes between polluted river and wild birds. Front. Microbiol. 2020, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Snesrud, E.; He, S.; Chandler, M.; Dekker, J.P.; Hickman, A.B.; McGann, P.; Dyda, F. A model for transposition of the colistin resistance gene mcr-1 by ISApl1. Antimicrob. Agents Chemother. 2016, 60, 6973–6976. [Google Scholar] [CrossRef] [Green Version]
- Nang, S.C.; Li, J.; Velkov, T. The rise and spread of mcr plasmid-mediated Polymyxin resistance. Crit. Rev. Microbiol. 2019, 45, 131–161. [Google Scholar] [CrossRef]
- Liu, G.; Ali, T.; Gao, J.; Ur Rahman, S.; Yu, D.; Barkema, H.W.; Huo, W.; Xu, S.; Shi, Y.; Kastelic, J.P.; et al. Co-Occurrence of plasmid-mediated colistin resistance (mcr-1) and extended-spectrum β-lactamase encoding genes in Escherichia coli from Bovine Mastitic Milk in China. Microb. Drug Resist. 2020, 26, 685–696. [Google Scholar] [CrossRef]
- Hamame, A.; Davoust, B.; Rolain, J.-M.; Diene, S.M. Genomic characterisation of an mcr-1 and mcr-3-producing Escherichia coli strain isolated from pigs in France. J. Glob. Antimicrob. Resist. 2022, 28, 174–179. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Borriello, S.P.; Pokludová, L. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, J.; Corcoran, C.; Prentice, E.; Moodley, M.; Mendelson, M.; Poirel, L.; Nordmann, P.; Brink, A.J. Emergence of plasmid-mediated colistin resistance (mcr-1) among Escherichia coli isolated from South African patients. S. Afr. Med. J. 2016, 106, 449–450. [Google Scholar] [CrossRef]
- Prescott, J.F. Antimicrobial use in food and companion animals. Anim. Health Res. Rev. 2008, 9, 127–133. [Google Scholar] [CrossRef]
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial usage and resistance in companion animals: A cross-sectional study in three european countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Schnepf, A.; Kramer, S.; Wagels, R.; Volk, H.A.; Kreienbrock, L. Evaluation of Antimicrobial Usage in Dogs and Cats at a Veterinary Teaching Hospital in Germany in 2017 and 2018. Front. Vet. Sci. 2021, 8, 689018. [Google Scholar] [CrossRef]
- Hur, B.; Hardefeldt, L.Y.; Verspoor, K.; Baldwin, T.; Gilkerson, J.R. Using natural language processing and VetCompass to understand antimicrobial usage patterns in Australia. Aust. Vet. J. 2019, 97, 298–300. [Google Scholar] [CrossRef]
- Ebrahim, M.; Gravel, D.; Thabet, C.; Abdesselam, K.; Paramalingam, S.; Hyson, C. Anitmicrobial Resistance (AMR): Antimicrobial use and antimicrobial resistance trends in Canada: 2014. Canada Commun. Dis. Rep. 2016, 42, 227. [Google Scholar] [CrossRef]
- Kumazawa, J.; Yagisawa, M. The history of antibiotics: The Japanese story. J. Infect. Chemother. 2002, 8, 125–133. [Google Scholar] [CrossRef]
- Makita, K.; Sugahara, N.; Nakamura, K.; Matsuoka, T.; Sakai, M.; Tamura, Y. Current status of antimicrobial drug use in japanese companion animal clinics and the factors associated with their use. Front. Vet. Sci. 2021, 8, 1119. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Silley, P. Antimicrobial resistance in zoonotic bacteria: Lessons learned from host-specific pathogens. Anim. Health Res. Rev. 2008, 9, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kempf, I.; Jouy, E.; Chauvin, C. Colistin use and colistin resistance in bacteria from animals. Int. J. Antimicrob. Agents 2016, 48, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.; Casewell, M.; Cox, T.; De Groot, B.; Friis, C.; Jones, R.; Nightingale, C.; Preston, R.; Waddell, J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004, 53, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Buckland, E.L.; O’Neill, D.; Summers, J.; Mateus, A.; Church, D.; Redmond, L.; Brodbelt, D. Characterisation of antimicrobial usage in cats and dogs attending UK primary care companion animal veterinary practices. Vet. Rec. 2016, 179, 489. [Google Scholar] [CrossRef] [Green Version]
- Lhermie, G.; La Ragione, R.M.; Weese, J.S.; Olsen, J.E.; Christensen, J.P.; Guardabassi, L. Indications for the use of highest priority critically important antimicrobials in the veterinary sector. J. Antimicrob. Chemother. 2020, 75, 1671–1680. [Google Scholar] [CrossRef]
- Maruve, S.A. Knowledge, Attitudes and Practices of Veterinarians on Antibiotic Use, Resistance and Its Containment in South Africa. Available online: https://researchspace.ukzn.ac.za/handle/10413/19519. (accessed on 10 February 2022).
- Armengol, E.; Domenech, O.; Fusté, E.; Pérez-Guillén, I.; Borrell, J.H.; Sierra, J.M.; Vinas, M. Efficacy of combinations of colistin with other antimicrobials involves membrane fluidity and efflux machinery. Infect. Drug Resist. 2019, 12, 2031. [Google Scholar] [CrossRef] [Green Version]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [Green Version]
- Catry, B.; Cavaleri, M.; Baptiste, K.; Grave, K.; Grein, K.; Holm, A.; Jukes, H.; Liebana, E.; Navas, A.L.; Mackay, D.; et al. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): Development of resistance in animals and possible impact on human and animal health. Int. J. Antimicrob. Agents 2015, 46, 297–306. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Doi, Y.; Huang, X.; Li, H.-Y.; Zhong, L.-L.; Zeng, K.-J.; Zhang, Y.-F.; Patil, S.; Tian, G.-B. Possible Transmission of mcr-1–Harboring Escherichia coli between Companion Animals and Human. Emerg. Infect. Dis. 2016, 22, 1679. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, R.; Li, J.; Wu, Z.; Yin, W.; Schwarz, S.; Tyrrell, J.M.; Zheng, Y.; Wang, S.; Shen, Z.; et al. Comprehensive resistome analysis reveals the prevalence of NDM and mcr-1 in Chinese poultry production. Nat. Microbiol. 2017 24 2017, 2, 16260. [Google Scholar] [CrossRef]
- Guenther, S.; Falgenhauer, L.; Semmler, T.; Imirzalioglu, C.; Chakraborty, T.; Roesler, U.; Roschanski, N. Environmental emission of multiresistant Escherichia coli carrying the colistin resistance gene mcr-1 from German swine farms. J. Antimicrob. Chemother. 2017, 72, 1289–1292. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Wang, Y.; Schwarz, S.; Walsh, T.R.; Ou, Y.; Wu, Y.; Li, M.; Shen, Z. mcr-1 in Enterobacteriaceae from Companion Animals, Beijing, China, 2012–2016. Emerg. Infect. Dis. 2017, 23, 710. [Google Scholar] [CrossRef]
- Rumi, M.V.; Mas, J.; Elena, A.; Cerdeira, L.; Muñoz, M.E.; Lincopan, N.; Gentilini, É.R.; Di Conza, J.; Gutkind, G. Co-occurrence of clinically relevant β-lactamases and mcr-1 encoding genes in Escherichia coli from companion animals in Argentina. Vet. Microbiol. 2019, 230, 228–234. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Zhang, Y.; Zhang, Z.; Lei, L.; Xia, Z. Increasing Prevalence of ESBL-Producing Multidrug Resistance Escherichia coli From Diseased Pets in Beijing, China From 2012 to 2017. Front. Microbiol. 2019, 10, 2852. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Wang, X.; Bai, X.; Ma, J.; Dang, R.; Xiong, Y.; Fanning, S.; Bai, L.; Yang, Z. Characterization of five Escherichia coli isolates co-expressing ESBL and mcr-1 resistance mechanisms from different origins in China. Front. Microbiol. 2019, 10, 1994. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Paredes, D.; Haro, M.; Leoro-Garzón, P.; Barba, P.; Loaiza, K.; Mora, F.; Fors, M.; Vinueza-Burgos, C.; Fernández-Moreira, E. Multidrug-resistant Escherichia coli isolated from canine faeces in a public park in Quito, Ecuador. J. Glob. Antimicrob. Resist. 2019, 18, 263–268. [Google Scholar] [CrossRef]
- Chang, M.-H.; Chen, G.-J.; Lo, D.-Y. CHROMOSOMAL LOCATIONS OF mcr-1 IN Klebsiella pneumoniae AND Enterobacter cloacae FROM DOGS. Taiwan Veter. J. 2019, 45, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Kobs, V.C.; Valdez, R.E.; de Medeiros, F.; Fernandes, P.P.; Deglmann, R.C.; Gern, R.M.M.; França, P.H.C. mcr-1-carrying Enterobacteriaceae isolated from companion animals in Brazil. Pesqui. Veterinária Bras. 2020, 40, 690–695. [Google Scholar] [CrossRef]
- Du, C.; Feng, Y.; Wang, G.; Zhang, Z.; Hu, H.; Yu, Y.; Liu, J.; Qiu, L.; Liu, H.; Guo, Z.; et al. Co-occurrence of the mcr-1.1 and mcr-3.7 genes in a multidrug-resistant Escherichia coli isolate from China. Infect. Drug Resist. 2020, 13, 3649–3655. [Google Scholar] [CrossRef]
- Loayza-Villa, F.; Salinas, L.; Tijet, N.; Villavicencio, F.; Tamayo, R.; Salas, S.; Rivera, R.; Villacis, J.; Satan, C.; Ushiña, L.; et al. Diverse Escherichia coli lineages from domestic animals carrying colistin resistance gene mcr-1 in an Ecuadorian household. J. Glob. Antimicrob. Resist. 2020, 22, 63–67. [Google Scholar] [CrossRef]
- Albán, M.V.; Núñez, E.J.; Zurita, J.; Villacís, J.E.; Tamayo, R.; Sevillano, G.; Villavicencio, F.X.; Calero-Cáceres, W. Canines with different pathologies as carriers of diverse lineages of Escherichia coli harboring mcr-1 and clinically relevant β-lactamases in central Ecuador. J. Glob. Antimicrob. Resist. 2020, 22, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.C.; Mechesso, A.F.; Kang, H.Y.; Kim, S.-J.; Choi, J.-H.; Kim, M.H.; Song, H.-J.; Yoon, S.-S.; Lim, S.-K. First Report of an Escherichia coli Strain Carrying the Colistin Resistance Determinant mcr-1 from a Dog in South Korea. Antibiotics 2020, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wang, Y.Y.; He, J.; Cai, C.; Liu, Q.; Yang, D.; Zou, Z.; Shi, L.; Jia, J.; Wang, Y.Y.; et al. Prevalence and risk analysis of mobile colistin resistance and extended-spectrum β-lactamase genes carriage in pet dogs and their owners: A population based cross-sectional study. Emerg. Microbes Infect. 2021, 10, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lei, L.; Zhang, H.; Dai, H.; Song, Y.; Li, L.; Wang, Y.; Xia, Z. Molecular Investigation of Klebsiella pneumoniae from Clinical Companion Animals in Beijing, China, 2017–2019. Pathogens 2021, 10, 271. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Feng, Y.; Zhang, Z.; Hu, H.; Liu, J.; Qiu, L.; Guo, Z.; Huang, J.; Qiu, J.; et al. Colistin-resistance mcr genes in Klebsiella pneumoniae from companion animals. J. Glob. Antimicrob. Resist. 2021, 25, 35–36. [Google Scholar] [CrossRef]
- Nittayasut, N.; Yindee, J.; Boonkham, P.; Yata, T.; Suanpairintr, N.; Chanchaithong, P. Multiple and High-Risk Clones of Extended-Spectrum Cephalosporin-Resistant and blaNDM-5-Harboring Uropathogenic Escherichia coli from cats and dogs in Thailand. Antibiotics 2021, 10, 1374. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Oreiby, A.F.; El-Hafeez, A.A.A.; Okanda, T.; Haque, A.; Anwar, K.S.; Tanaka, M.; Miyako, K.; Tsuji, S.; Kato, Y.; et al. First report of multidrug-resistant carbapenemase-producing bacteria coharboring mcr-9 associated with respiratory disease complex in pets: Potential of animal-human transmission. Antimicrob. Agents Chemother. 2021, 65, 633. [Google Scholar] [CrossRef]
- Singleton, D.A.; Pongchaikul, P.; Smith, S.; Bengtsson, R.J.; Baker, K.; Timofte, D.; Steen, S.; Jones, M.; Roberts, L.; Sánchez-Vizcaíno, F.; et al. Temporal, Spatial, and Genomic Analysis of Enterobacteriaceae Clinical Antimicrobial Resistance in Companion Animals Reveals Phenotypes and Genotypes of One Health Concern. Front. Microbiol. 2021, 12, 2160. [Google Scholar] [CrossRef]
- Sato, T.; Usui, M.; Harada, K.; Fukushima, Y.; Nakajima, C.; Suzuki, Y.; Yokota, S. Complete Genome Sequence of an mcr-10-Possessing Enterobacter roggenkampii Strain Isolated from a dog in Japan. Microbiol. Resour. Announc. 2021, 10, e0042621. [Google Scholar] [CrossRef]
- Hamame, A.; Davoust, B.; Rolain, J.M.; Diene, S.M. Screening of colistin-resistant bacteria in domestic pets from France. Animals 2022, 12, 633. [Google Scholar] [CrossRef]
- Hayakawa Ito de Sousa, A.T.; dos Santos Costa, M.T.; Makino, H.; Cândido, S.L.; de Godoy Menezes, I.; Lincopan, N.; Nakazato, L.; Dutra, V. Multidrug-resistant mcr-1 gene-positive Klebsiella pneumoniae ST307 causing urinary tract infection in a cat. Braz. J. Microbiol. 2021, 52, 1043–1046. [Google Scholar] [CrossRef]
- Sato, T.; Usui, M.; Harada, K.; Fukushima, Y.; Nakajima, C.; Suzuki, Y.; Yokota, S. Complete Genome Sequence of an mcr-9-Possessing Enterobacter asburiae Strain Isolated from a Cat in Japan. Microbiol. Resour. Announc. 2021, 10, e0028121. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Che, J.; Xiong, Y.; Xu, Y.; Zhang, L.; Lan, R.; Xia, L.; Walsh, T.R.; Xu, J.; et al. Detection and dissemination of the colistin resistance gene, mcr-1, from isolates and Fecal samples in China. J. Med. Microbiol. 2017, 66, 119–125. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X.; Li, W.; Li, L.; Liao, X.; Xing, S. Abundance, diversity and diffusion of antibiotic resistance genes in cat feces and dog feces. Environ. Pollut. 2022, 292, 118364. [Google Scholar] [CrossRef]
- Saraf, A. Epidemiology of Colistin Resistance in Enterobacteriaceae Isolates from Perianal Region of Pet Dogs. Master’s Thesis, ICAR-Indian Veterinary Research Institute, Uttar Pradesh, India, July 2017. Available online: https://doi.org/10.13140/RG.2.2.29497.06243 (accessed on 15 February 2022).
- Fukuda, A.; Usui, M.; Okubo, T.; Tagaki, C.; Sukpanyatham, N.; Tamura, Y. Co-harboring of cephalosporin (bla)/colistin (mcr) resistance genes among Enterobacteriaceae from flies in Thailand. FEMS Microbiol. Lett. 2018, 365, 178. [Google Scholar] [CrossRef]
- dos Santos, L.D.R.; Furlan, J.P.R.; Ramos, M.S.; Gallo, I.F.L.; de Freitas, L.V.P.; Stehling, E.G. Co-occurrence of mcr-1, mcr-3, mcr-7 and clinically relevant antimicrobial resistance genes in environmental and fecal samples. Arch. Microbiol. 2020, 202, 1795–1800. [Google Scholar] [CrossRef]
- Tolosi, R.; Apostolakos, I.; Laconi, A.; Carraro, L.; Grilli, G.; Cagnardi, P.; Piccirillo, A. Rapid detection and quantification of plasmid-mediated colistin resistance genes (mcr-1 to mcr-5) by real-time PCR in bacterial and environmental samples. J. Appl. Microbiol. 2020, 129, 1523–1529. [Google Scholar] [CrossRef]
- Vu, T.M.H.; Kasuga, I. Prevalence of plasmid-mediated colistin resistance gene mcr-1 in domestic wastewater. IOP Conf. Ser. Earth Environ. Sci. 2020, 496, 012015. [Google Scholar] [CrossRef]
- Hadjadj, L.; Riziki, T.; Zhu, Y.; Li, J.; Diene, S.M.; Rolain, J.M. Study of mcr-1 Gene-Mediated Colistin Resistance in Enterobacteriaceae Isolated from humans and animals in different countries. Genes 2017, 8, 394. [Google Scholar] [CrossRef] [Green Version]
- Teo, J.W.P.; Chew, K.L.; Lin, R.T.P. Transmissible colistin resistance encoded by mcr-1 detected in clinical Enterobacteriaceae isolates in Singapore. Emerg. Microbes Infect. 2016, 5, e87. [Google Scholar] [CrossRef]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M.; et al. Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef]
- Wipler, J.; Čermáková, Z.; Hanzálek, T.; Horáková, H.; Žemličková, H. [Sharing bacterial microbiota between owners and their pets (dogs, cats)]. Klin. Mikrobiol. Infekc. Lek. 2017, 23, 48–57. [Google Scholar]
- Faires, M.C.; Tater, K.C.; Weese, J.S. An investigation of methicillin-resistant Staphylococcus aureus colonization in people and pets in the same household with an infected person or infected pet. J. Am. Vet. Med. Assoc. 2009, 235, 540–543. [Google Scholar] [CrossRef] [Green Version]
- Effelsberg, N.; Kobusch, I.; Linnemann, S.; Hofmann, F.; Schollenbruch, H.; Mellmann, A.; Boelhauve, M.; Köck, R.; Cuny, C. Prevalence and zoonotic transmission of colistin-resistant and carbapenemase-producing Enterobacterales on German pig farms. One Health 2021, 13, 100354. [Google Scholar] [CrossRef]
- Trung, N.V.; Matamoros, S.; Carrique-Mas, J.J.; Nghia, N.H.; Nhung, N.T.; Chieu, T.T.B.; Mai, H.H.; van Rooijen, W.; Campbell, J.; Wagenaar, J.A.; et al. Zoonotic transmission of mcr-1 colistin resistance gene from small-scale poultry farms, Vietnam. Emerg. Infect. Dis. 2017, 23, 529. [Google Scholar] [CrossRef] [Green Version]
- Pomba, C.; Belas, A.; Menezes, J.; Marques, C. The public health risk of companion animal to human transmission of antimicrobial resistance during different types of animal infection. Adv. Anim. Health Med. Prod. 2020, 265–278. [Google Scholar] [CrossRef]
- Werner, G.; Coque, T.M.; Franz, C.M.A.P.; Grohmann, E.; Hegstad, K.; Jensen, L.; van Schaik, W.; Weaver, K. Antibiotic resistant enterococci—Tales of a drug resistance gene trafficker. Int. J. Med. Microbiol. 2013, 303, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Flament-simon, S.C.; de Toro, M.; García, V.; Blanco, J.E.; Blanco, M.; Alonso, M.P.; Goicoa, A.; Díaz-gonzález, J.; Nicolas-chanoine, M.H.; Blanco, J. Molecular Characteristics of Extraintestinal Pathogenic E. coli (ExPEC), Uropathogenic E. coli (UPEC), and Multidrug Resistant E. coli Isolated from Healthy Dogs in Spain. Whole Genome Sequencing of Canine ST372 Isolates and Comparison with Human Isolates Causing Extraintestinal Infections. Microorganisms 2020, 8, 1712. [Google Scholar] [CrossRef]
- Pomba, C.; Endimiani, A.; Rossano, A.; Saial, D.; Couto, N.; Perreten, V. First report of blaOXA-23-mediated carbapenem resistance in sequence type 2 multidrug-resistant Acinetobacter baumannii associated with urinary tract infection in a cat. Antimicrob. Agents Chemother. 2014, 58, 1267–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, A.; Pottenger, S.; Timofte, D.; Moore, M.; Wright, L.; Kukavica-Ibrulj, I.; Jeukens, J.; Levesque, R.C.; Freschi, L.; Pinchbeck, G.L.; et al. Reservoirs of resistance: Polymyxin resistance in veterinary-associated companion animal isolates of Pseudomonas aeruginosa. Vet. Rec. 2019, 185, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharaibeh, M.H.; Shatnawi, S.Q. An overview of colistin resistance, mobilized colistin resistance genes dissemination, global responses, and the alternatives to colistin: A review. Veter. World 2019, 12, 1735–1746. [Google Scholar] [CrossRef] [Green Version]
- Ngbede, E.O.; Poudel, A.; Kalalah, A.; Yang, Y.; Adekanmbi, F.; Adikwu, A.A.; Adamu, A.M.; Mamfe, L.M.; Daniel, S.T.; Useh, N.M.; et al. Identification of mobile colistin resistance genes (mcr-1.1, mcr-5 and mcr-8.1) in Enterobacteriaceae and Alcaligenes fecalis of human and animal origin, Nigeria. Int. J. Antimicrob. Agents 2020, 56, 106108. [Google Scholar] [CrossRef]
- Rapoport, M.; Faccone, D.; Pasteran, F.; Ceriana, P.; Albornoz, E.; Petroni, A.; Group, the M.; Corso, A. First Description of mcr-1-Mediated Colistin Resistance in Human Infections Caused by Escherichia coli in Latin America. Antimicrob. Agents Chemother. 2016, 60, 4412–4413. [Google Scholar] [CrossRef] [Green Version]
- Stoesser, N.; Mathers, A.J.; Moore, C.E.; Day, N.P.J.; Crook, D.W. Colistin resistance gene mcr-1 and pHNSHP45 plasmid in human isolates of Escherichia coli and Klebsiella pneumoniae. Lancet Infect. Dis. 2016, 16, 285–286. [Google Scholar] [CrossRef] [Green Version]
- Payne, M.; Croxen, M.A.; Lee, T.D.; Mayson, B.; Champagne, S.; Leung, V.; Bariso, S.; Hoang, L.; Lowe, C. mcr-1–Positive Colistin-Resistant Escherichia coli in Traveler Returning to Canada from China—Volume 22, Number 9—September 2016—Emerging Infectious Diseases journal—CDC. Emerg. Infect. Dis. 2016, 22, 1673–1675. [Google Scholar] [CrossRef] [Green Version]
- Hasman, H.; Hammerum, A.M.; Hansen, F.; Hendriksen, R.S.; Olesen, B.; Agersø, Y.; Zankari, E.; Leekitcharoenphon, P.; Stegger, M.; Kaas, R.S.; et al. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, denmark 2015. Eurosurveillance 2015, 20, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Papa-Ezdra, R.; Grill Diaz, F.; Vieytes, M.; García-Fulgueiras, V.; Caiata, L.; Ávila, P.; Brasesco, M.; Christophersen, I.; Cordeiro, N.F.; Algorta, G.; et al. First three Escherichia coli isolates harboring mcr-1 in Uruguay. J. Glob. Antimicrob. Resist. 2020, 20, 187–190. [Google Scholar] [CrossRef]
- Pathak, A.; Singh, S.; Kumar, A.; Prasad, K.N. Emergence of chromosome borne colistin resistance gene, mcr-1 in clinical isolates of Pseudomonas aeruginosa. Int. J. Infect. Dis. 2020, 101, 22. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Mataseje, L.F.; Robertson, J.; Nash, J.H.E.; Boerlin, P.; Toye, B.; Irwin, R.; Melano, R.G. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect. Dis. 2016, 16, 289–290. [Google Scholar] [CrossRef] [Green Version]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Dafale, N.A.; Srivastava, S.; Purohit, H.J. Zoonosis: An Emerging Link to Antibiotic Resistance Under “One Health Approach”. Indian J. Microbiol. 2020, 60, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Zhuo, A.; Govendir, M.; Rowbotham, S.J.; Labbate, M.; Degeling, C.; Gilbert, G.L.; Dominey-Howes, D.; Ward, M.P. Factors influencing the behaviour and perceptions of Australian veterinarians towards antibiotic use and antimicrobial resistance. PLoS ONE 2019, 14, e0223534. [Google Scholar] [CrossRef]
- Davies, M.; Stewart, P.R. Transferable drug resistance in man and animals: Genetic relationship between r-plasmids in enteric bacteria from man and domestic pets. Aust. Vet. J. 1978, 54, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Laxminarayan, R.; Mendelson, M. How should we respond to the emergence of plasmid-mediated colistin resistance in humans and animals? Int. J. Infect. Dis. 2017, 54, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.; Cheng, G.; Iqbal, Z.; Ai, X.; Hussain, H.I.; Huang, L.; Dai, M.; Wang, Y.; Liu, Z.; Yuan, Z. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014, 5, 288. [Google Scholar] [CrossRef] [Green Version]
- Damborg, P.; Broens, E.M.; Chomel, B.B.; Guenther, S.; Pasmans, F.; Wagenaar, J.A.; Weese, J.S.; Wieler, L.H.; Windahl, U.; Vanrompay, D.; et al. Bacterial Zoonoses Transmitted by Household Pets: State-of-the-Art and Future Perspectives for Targeted Research and Policy Actions. J. Comp. Pathol. 2016, 155, S27–S40. [Google Scholar] [CrossRef] [Green Version]
- Larson, E. Community Factors in the Development of Antibiotic Resistance. Annu. Rev. Public Health 2007, 28, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Wall, S. Prevention of antibiotic resistance—An epidemiological scoping review to identify research categories and knowledge gaps. Glob. Health Action 2020, 12, 1756191. [Google Scholar] [CrossRef]
- Lipsitch, M.; Siber, G.R. How can vaccines contribute to solving the antimicrobial resistance problem? MBio 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Bloomfield, S.F. Home hygiene: A risk approach. Int. J. Hyg. Environ. Health 2003, 206, 1–8. [Google Scholar] [CrossRef]
- Umber, J.K.; Bender, J.B. Pets and Antimicrobial Resistance. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 279–292. [Google Scholar] [CrossRef]
- Hembach, N.; Schmid, F.; Alexander, J.; Hiller, C.; Rogall, E.T.; Schwartz, T. Occurrence of the mcr-1 colistin resistance gene and other clinically relevant antibiotic resistance genes in microbial populations at different municipal wastewater treatment plants in Germany. Front. Microbiol. 2017, 8, 1282. [Google Scholar] [CrossRef]
- Hille, K.; Möbius, N.; Akmatov, M.K.; Verspohl, J.; Rabold, D.; Hartmann, M.; Günther, K.; Obi, N.; Kreienbrock, L. Zoonoses research in the German National Cohort: Feasibility of parallel sampling of pets and owners. Bundesgesundheitsblatt. Gesundheitsforsch. Gesundheitsschutz 2014, 57, 1277–1282. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
| Source | Papers | H Index | G Index | AWCR | E Index | H Coverage | G Coverage | Year First | Year Last |
|---|---|---|---|---|---|---|---|---|---|
| Google Scholar | 140 | 30 | 71 | 1217 | 60.87 | 91.2 | 99.9 | 2006 | 2021 |
| Web of Science | 859 | 39 | 67 | 2319 | 46.97 | 36.73 | 53.34 | 1905 | 2022 |
| PubMed | 158 | 10 | 9.29 | 15 | 9.56 | 9.76 | 9.56 | 2012 | 2022 |
| Scopus | 57 | 16 | 23 | 213 | 14.04 | 71.3 | 83.6 | 1979 | 2022 |
| Microsoft Academic | 17 | 4 | 8 | 41 | 6.86 | 79.7 | 96.2 | 2007 | 2022 |
| mcr Genes | Bacterial Species | Sequence Type | Number of Isolates | Isolation Source | Year | Country | Reference |
|---|---|---|---|---|---|---|---|
| mcr-1 | E. coli | ST354 | 4 | Fecal sample | 2016 | China | [84] |
| mcr-1 | E. coli | / | 5 | Fecal sample | 2017 | China | [85] |
| mcr-1 | E. coli | ST10 | 1 | Fecal sample | 2017 | China | [86] |
| mcr-1 | E. coli | / | 45 | Nasal and rectal swabs | 2017 | China | [87] |
| K. pneumoniae | / | 2 | |||||
| mcr-1 | E. coli | ST93 | 4 | Urine, nasal secretion, fecal sample, diarrhea | 2018 | China | [35] |
| ST1011 | 1 | ||||||
| ST3285 | 1 | ||||||
| New ST | 1 | ||||||
| mcr-1 | E. coli | ST770 | 1 | Urinary tract infection | 2019 | Argentina | [88] |
| mcr-1 | E. coli | ST6316 | 1 | Uterus | 2019 | China | [89] |
| ST405 | 1 | ||||||
| ST46 | 1 | Urine | |||||
| mcr-1 | E. coli | ST162 | 1 | Clinical sample | 2019 | China | [90] |
| mcr-1 | E. coli | / | 1 | Fecal sample | 2019 | Ecuador | [91] |
| mcr-1 | K. pneumoniae | ST307 | 2 | Urine, pyometra | 2019 | Taiwan | [92] |
| E. cloacae | ST1005 | 2 | Urine | ||||
| mcr-1 | E. coli | / | 1 | Urine | 2020 | Brazil | [93] |
| Klebsiella sp. | / | 1 | Abdominal seroma | ||||
| Enterobacter. sp. | / | 1 | Nasal secretion | ||||
| mcr-1/mcr-3.7 | E. coli | ST132 | 1 | Fecal sample | 2020 | China | [94] |
| mcr-1 | E. coli | ST1630 | 1 | Rectal swabs | 2020 | Ecuador | [95] |
| ST2170 | 1 | ||||||
| mcr-1 | E. coli | ST162 | 1 | Fecal sample | 2020 | Ecuador | [96] |
| ST1196 | 1 | ||||||
| ST744 | 1 | ||||||
| mcr-1 | E. coli | ST162 | 1 | Diarrhea | 2020 | South Korea | [97] |
| mcr-1 | K. pneumoniae | / | 149 | Fecal sample | 2021 | China | [100] |
| mcr-1 | E. coli | ST648 | 3 | Rectal swabs | 2021 | China | [98] |
| ST23 | 1 | ||||||
| ST162 | 1 | ||||||
| ST2722 | 1 | ||||||
| ST2325 | 1 | ||||||
| ST1638 | 1 | ||||||
| ST8680 | 1 | ||||||
| ST7331 | 1 | ||||||
| mcr-1 | K. pneumoniae | ST656 | 1 | Urine | 2021 | China | [99] |
| mcr-2 | K. pneumoniae | / | 11 | Fecal sample | 2021 | China | [100] |
| mcr-3 | K. pneumoniae | / | 15 | Fecal sample | 2021 | China | [100] |
| mcr-3 | E. coli | ST10 | 1 | Clinical sample | 2021 | Taiwan | [101] |
| mcr-4 | K. pneumoniae | / | 6 | Fecal sample | 2021 | China | [100] |
| mcr-5 | K. pneumoniae | / | 18 | Fecal sample | 2021 | China | [100] |
| mcr-8 | K. pneumoniae | ST3410 | 1 | Nasal swabs | 2021 | China | [99] |
| mcr-9 | K. pneumoniae | / | 5 | Fecal sample | 2021 | China | [100] |
| mcr-9 | E. hormaechei | ST493 | 2 | Clinical sample | 2021 | Egypt | [102] |
| mcr-9 | E. coli | ST372 | 1 | Clinical sample | 2021 | United Kingdom | [103] |
| mcr-10 | K. pneumoniae | / | 4 | Fecal sample | 2021 | China | [100] |
| mcr-10 | E. roggenkampii | / | 1 | Pus | 2021 | Japan | [104] |
| mcr_1 | E. coli | 10 | Fecal sample | 2020 | France | [105] |
| mcr Genes | Species | ST | Number of Isolates | Source | Year | Country | Reference |
|---|---|---|---|---|---|---|---|
| mcr-1 | E. coli | ST93 | 1 | Fecal sample | 2016 | China | [84] |
| New ST | 1 | ||||||
| mcr-1 | E. coli | / | 1 | Nasal and rectal swabs | 2017 | China | [87] |
| mcr-1 | E. coli | ST93 | 1 | Diarrhea | 2018 | China | [35] |
| mcr-1 | K. pneumoniae | ST307 | 1 | Urinary tract infection | 2021 | Brazil | [106] |
| mcr-9 | E. hormaechei | ST493 | 1 | Clinical samples | 2021 | Egypt | [102] |
| ST182 | 2 | ||||||
| mcr-9 | E. asburiae | / | 1 | Nasal swab | 2021 | Japan | [107] |
| mcr-1 | E. coli | 4 | Fecal sample | 2020 | France | [105] | |
| mcr-1 | Rahnella aquatili | 1 | Fecal sample | 2020 | France | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamame, A.; Davoust, B.; Cherak, Z.; Rolain, J.-M.; Diene, S.M. Mobile Colistin Resistance (mcr) Genes in Cats and Dogs and Their Zoonotic Transmission Risks. Pathogens 2022, 11, 698. https://doi.org/10.3390/pathogens11060698
Hamame A, Davoust B, Cherak Z, Rolain J-M, Diene SM. Mobile Colistin Resistance (mcr) Genes in Cats and Dogs and Their Zoonotic Transmission Risks. Pathogens. 2022; 11(6):698. https://doi.org/10.3390/pathogens11060698
Chicago/Turabian StyleHamame, Afaf, Bernard Davoust, Zineb Cherak, Jean-Marc Rolain, and Seydina M. Diene. 2022. "Mobile Colistin Resistance (mcr) Genes in Cats and Dogs and Their Zoonotic Transmission Risks" Pathogens 11, no. 6: 698. https://doi.org/10.3390/pathogens11060698
APA StyleHamame, A., Davoust, B., Cherak, Z., Rolain, J.-M., & Diene, S. M. (2022). Mobile Colistin Resistance (mcr) Genes in Cats and Dogs and Their Zoonotic Transmission Risks. Pathogens, 11(6), 698. https://doi.org/10.3390/pathogens11060698






