Abstract
Tick-borne bacterial pathogens (TBBPs) show a worldwide distribution and represent a great impact on public health. The brown dog tick (Rhipicephalus sanguineus) is a vector of several pathogens that affect dogs and sometimes humans as well. In addition, TBBPs represent a diagnostic challenge and imply financial resources and medical treatment for long periods of time. In the present study, R. sanguineus s. l. was identified as the main tick species naturally parasitizing dogs that inhabit. Juárez City, Chihuahua, in the Paso del Norte region, Mexico–US Border, representing 99.8% of the cases. Additionally, an end-point PCR was performed to search for whether pathogens in R. sanguineus s. l. can transmit in DNA extracted from ticks and dog blood samples. This is the first molecular detection of Rickettsia rickettsi infecting domestic dogs in Mexico; however, other pathogens were also identified, such as Ehrlichia canis and Anaplasma platys in both ticks and dog blood samples, while Anaplasma phagocytophilum was identified only in dog blood samples. Moreover, co-detection in tick pools and co-infection in the analyzed dog blood samples could be found. Similarly, this research showed that dogs were found mostly parasitized by adult female ticks, increasing the possibility of transmission of E. canis.
1. Introduction
Tick-borne bacterial pathogens (TBBPs) such as ehrlichiosis, borreliosis, anaplasmosis, and rickettsiosis are well-known diseases of veterinary and medical importance [1]. The etiological agents of TBBPs are zoonotic bacteria that significantly impact public health, such as Ehrlichia canis, Borrelia spp., Rickettsia rickettsii, and Anaplasma spp., among others [2,3,4]. Ixodidae (hard ticks) and Argasidae (soft ticks) families (Acari order) can transmit TBBPs [5,6]. Both groups of ticks are obligate ectoparasites of vertebrates, including birds, mammals, reptiles, and amphibians, with humans as incidental hosts [7,8]. Several epidemiological studies using domestic animals as sentinels have been utilized to monitor infectious diseases, including TBBPs [9]. Those epidemiological methods can provide substantial surveillance information to identify dynamics in the infection and health status of the animal and human populations, determine patterns in a pathogen mix [10], provide evidence of disease absence, or estimate the prevalence of a given pathogen [11]. Moreover, they are essential to controlling and preventing diseases timely in a relatively inexpensive manner. Thus, dogs can play a valuable role as sentinel hosts for monitoring diseases [12] since they live in close contact with humans and livestock, and are susceptible to many emerging or re-emerging human TBBPs [13]. Therefore, they represent an essential human epidemiological surveillance tool to explore the variability in the TBBPs [9].Conversely, ticks can be used to monitor TBBPs. The use of hematophagous arthropods to survey vertebrates for the presence of infectious disease agents is called xenosurveillance and is well documented [14,15]. Hence, knowledge of the infestation with ticks can be useful to estimate associations between tick density and the prevalence of TBBPs, and to understand the ecology of pathogen transmission [16].
Currently, there is no epidemiological information about the infestation with ticks that naturally parasitize dogs and the prevalence of TBBPs in Juarez City, in the Paso del Norte border region. Therefore, to expand our knowledge about the epidemiology of ticks in this binational region, this study has the following objectives: (1) to estimate tick infestation load and species of ticks in both free-ranging dogs (FRD) and home dogs and (2) to identify the association among the prevalence of TBBPs with tick infestation and other risk factors of dogs in Juarez City, Chihuahua, on the Mexico–U.S. border.
2. Results
2.1. Morphological and Molecular Identification of Ticks
Out of the total ticks collected in this study, 99.82% (n = 1688) were morphologically identified as Rhipicephalus sanguineus s.l. (Figure 1, Figure 2, Figure 3 and Figure 4), whereas the remaining 0.18% (n = 3) were identified as Otobius megnini (Figure 5). Morphological identification was corroborated using DNA barcode analyses. The DNA barcodes (not shown) corresponded to those reported in genomic databases [17,18].
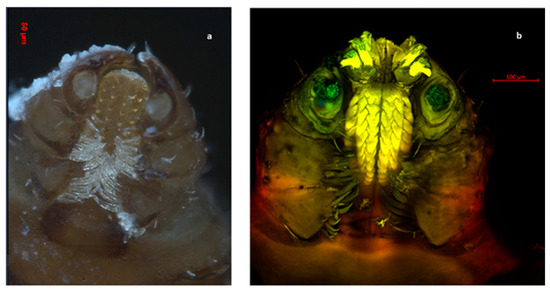
Figure 1.
Ventral view of the oral apparatus of an R. sanguineus s. l. tick. The hypostome shows four rows of teeth as well as the depression of the four joints present in the pedipalp. (a) Photography was carried out with an Axio Zoom V6 microscope. (b) Photography was carried outobtained with an LSM 700 confocal scanning microscope.
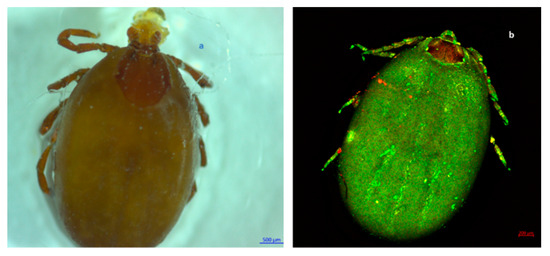
Figure 2.
Dorsal view of a fully blood-engorged female of R. sanguineus s. l. female. Observe the basis of the hexagonal-shaped gnathostome and the scutum in the first third of the body oridiosoma. In addition, ocelli are present on the sides of the coat of arms (rudimentary eyes). (a) Photography obtained with a Stemi 200C microscope. (b) Photography obtained with an LSM 700 confocal scanning microscope.
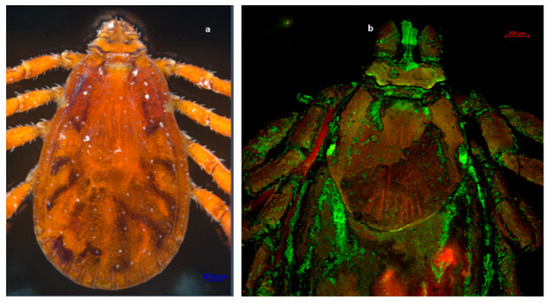
Figure 3.
Dorsal view of a male tick of R. sanguineus s. l. Observe the basis of the hexagonal-shaped gnathostome, with a complete scutum and the ocelli featuring at each end of the scutum. (a) R. sanguineus s. l. male. Photography was carried out with an Axio Zoom V6 microscope. (b) R. sanguineus s. l. female. Photography obtained with an LSM 700 confocal scanning microscope.
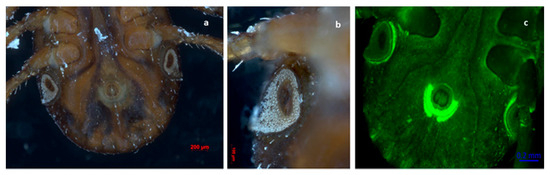
Figure 4.
Rear ventral view of a semi-fully engorged female tick of R. sanguineus s. l. Observe the inverted U-shaped genital aperture and the spiracles below the fourth pair of legs. Festoons on the back side of the specimen can be seen. (a,b) Photography obtained with an Axio Zoom V6 microscope. (c) Photography obtained with a laser scanning confocal 700 (LSM 700).

Figure 5.
Dorsal view of Otobius megnini nymphs. Characterized by the spines contained throughout the body, specimen in (b) shows the spines-like processes are in the first half of the idiosoma. (a) Photography obtained with a Stemi 200C microscope. (b) Photography obtained with an Axio Zoom V6 microscope.
2.2. Tick Infestation between 2013 and 2014
In the first 2-year period of the study (2013–2014), 979 ticks corresponding to R. sanguineus s. l., were collected (548 and 431 ticks in 2013 and 2014, respectively) from 237 dogs inspected (137 FRD and 100 home dogs: designated as two categories). Table 1 show the infestation load of each life stage of R. sanguineous s. l. by year both of the FRD and home dogs. The LSM of total ticks infesting FRD was significantly higher than that of home dogs. A similar situation was seen in females, males, blood-fed females, and unfed females. However, in nymphs and larvae, no statistical differences were present. Table 2 show the Linear Square mean (LSM) ± Standard error (SE) for each life metamorphic stage of ticks per year and both dog categories (FRD/home dogs), including the p-values from T-student and goodness of fit of chi-square/df.

Table 1.
Incidence of Ripicephalus sanguineus s. l. in 237 dogs examined in Juarez City, Chihuahua, Mexico in 2013–2014: Feeding status of females plus number of males and immature stages found in 137 FRD and 100 home dogs explored in both years.

Table 2.
Linear square mean (LSM) Standard error (SE) and Mean for each life metamorphic stage of ticks per year and both dog categories (FRD/home dogs), including the p-values from T-student and goodness of fit of chi-square/df.
2.3. Prevalence of Tick-Borne Pathogens in Dogs
In the 2015 study, infection with E. canis was detected in 53.60% (104/194), A. platys in 24.74% (48/194), A. phagocytophilum in 12.88% (25/194), and R. rickettsii in 5.67% (11/194) of dogs. Co-infections of two to three pathogens were detected in 21.64% (42/194) of dogs, where E. canis and A. platys were 9.27% (18/194), A. platys and A. phagocytophilum were 3.6% (7/194), E. canis and R. rickettsii were 1.54% (3/194), E. canis and A. phagocytophilum were 1.03% (2/194), E. canis, A. platys, and A. phagocytophilum were 4.64% (9/194), E. canis, A. phagocytophilum, and R. rickettsii were 1.03% (2/194), and for E. canis, A. platys, and R. rickettsii were 0.52% (1/194). Finally, B. burgdorferi s. l. was not detected by PCR in dogs.
2.4. Prevalence of Tick-Borne Pathogens in Ticks
In the case of TBBPs detection in the tick pools, 72% (43/60) were positive for some of the pathogens analyzed, and 28% (17/60) were negative by PCR molecular detection. The highest prevalence estimated was for E. canis, with 66.6% (40/60) of the positive samples, followed by A. platys with 8.3% (5/60) and R. rickettsii with 5% (3/60). All tick pools were PCR negative for A. phagocytophilum and B. burgdorferi s. l. Pathogen co-detection was also observed in tick pools, with the most common ones being E. canis and A. platys with 6.6% (4/60) and E. canis with R. rickettsii in 1.6% of the pools (1/60).
Access numbers of sequences are available at NCBI as follows: E. canis MK386937, MK386938, and MK386938.1; A. platys MK386768; and finally R. rickettsii MK350322 and MK350322.1. A. phagocytophilum could not be sequenced.
2.5. Association of the Prevalence TBBPs among Dog Risk Factors
Since E. canis infection showed more than 50% prevalence in dogs (n = 104), only this pathogen was used to understand the association between TBBPs and risk factors (breed, tick infestation sites on dogs, age, sex, total ticks, females, males, blood-fed females, unfed females, nymphs, and larvae) (Supplementary Table S2). The results of GLIMMIX for multivariate logistic regression showed a good fit for the model (Pearson’s Chi-square/degrees of freedom = 149.47/138 = 0.96). The unique predictor variable that showed a trend of E. canis prevalence was represented by female ticks (α = 0.09; using a critical level of α = 0.10 as selection criteria) (Figure 6); the remaining explanatory variables were associated with alpha levels higher than 0.10. This relationship was detected when the number of total ticks varied significantly as a response variable on male ticks per dog. Therefore, the association indicates that for each female tick, the probability of E. canis increased (Figure 7).
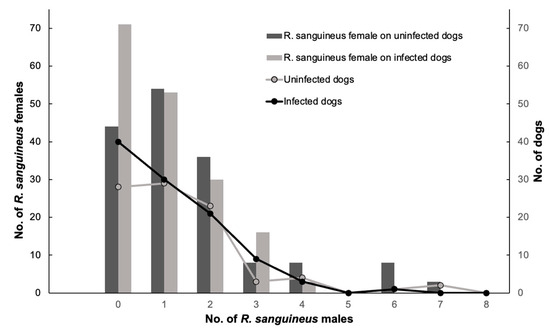
Figure 6.
Abundance of R. sanguineus s. l. female ticks on dogs infected and uninfected for E. canis and its association to the dogs infested by a variable number of R. sanguineus s.l. males.
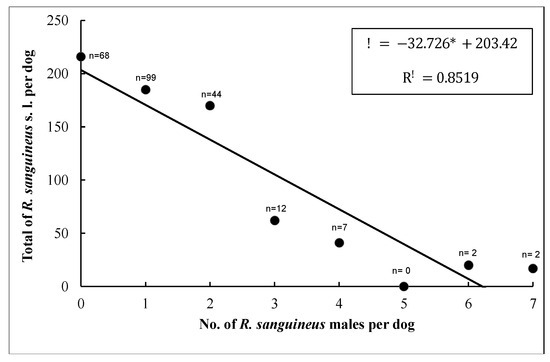
Figure 7.
Relationship between the number of total ticks and number of male ticks per dog. Dots denote the number of dogs recorded for each class of number of male ticks, from zero up to seven. n = number of dogs.
3. Discussion
In previous studies in Mexico, the seroprevalence of TBBPs detected in both ticks and animals was common [19,20,21,22,23], and surveys on the diversity of ticks were carried out [21,24]. However, this research represents the first systematic study performed at the Mexico–US border, where the co-infestation with R. sanguineus s. l. and O. megnini, naturally parasitizing domestic dogs and FRD, the prevalence of TBBPs, and the association among the infestation with R. sanguineus s. l. males and E. canis infection in dogs were identified. Likewise, this is the first report in this region of active infection for R. rickettsii in dogs and co-infection of other TBBPs. It is essential to highlight that this study, like others of global relevance, reflects the importance of carrying out epidemiological studies in dogs due to the high probability of R. sanguineus s. l. representing a vector to transmit TBBPs to humans [3,12].
In the present study, we observed two species of ticks naturally parasitizing dogs. According to taxonomic characteristics, these species were identified as R. sanguineus s. l. and O. megnini (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5). The DNA barcodes corresponded to those reported in genomic databases [17,18].
Different studies were performed in Mexico to identify ticks that parasitize dogs. For example, Cruz-Vázquez et al. (1998) [25] and Tinoco-Gracia et al. (2009) [26] only reported R. sanguineus, while it was the most predominant species in studies conducted by Galaviz-Silva et al. (2013) [27] and Ojeda-Chi et al. (2019) [28].
Although R. sanguineus is native to Africa, it has a cosmopolitan distribution due to the migratory movements of humans and their dogs. Global studies showed that R. sanguineus is a species well adapted to urban areas, where it is the most common [3,29,30]. This is in accordance with our results, i.e., the high prevalence of this tick is in the urban area of Juarez. In addition, based on these studies, we confirm that R. sanguineus s. l. is well adapted to the arid region of northern Mexico, better than other species of ticks [31,32,33].
According to the results shown in Table 2, there were no significant differences between sexes, developmental stages, or feeding degrees of R. sanguineus s. l. ticks, most likely because this study was performed in the seasons with a higher incidence of ticks and the diseases they transmit [3,34]. Regarding the finding of O. megnini parasitizing one of the dogs included, a similar case was already described in a dog from the north-central zone of Mexico [35].
In this research, the TBBP with the highest prevalence was E. canis, with 53.6% of positive dogs. This prevalence is superior in comparison with other studies using the PCR assay, even in Caribbean countries with a tropical climate, such as Grenada, with 24.7% of positive dogs to E. canis that were 100% parasitized by R. sanguineus [36]. Furthermore, the prevalence is also high when compared with other studies in Mexico; for example, Almazan et al. (2016) reported a prevalence of 10% [33]. In another study, where the prevalence was determined twice, there was a prevalence of 29.26% (72/246) for E. canis at the first sampling event, whereas at the second sampling event, it was 38.46% (20/52) [37]. Finally, Pat-Nah et al. (2015) determined a 36% prevalence of this disease in Yucatan, Mexico [38]. Similarly, in the neighboring state of Texas, USA, the prevalence of this disease was 1.62% (19/1171) [39], indicating that the prevalence of E. canis in our study is the highest reported so far.
The second-prevalent pathogen of TBBPs was A. platys, the etiologic agent of canine infectious cyclic thrombocytopenia, with 24.74% of cases (48/194). Studies on this pathogen in this region are also uncommon: the first molecular report of A. platys in Mexico was published in 2013 [40] and confirmed in 2015 [33]; likewise, a study performed in Texas, a state bordering Juarez, Mexico, found a prevalence of this pathogen in 0.17% of dogs (2/1171) [39] and in areas relatively close to the region of this study. It was also reported in white-tailed deer (0.4%) [41]. However, this species was described in several countries of the American continent, along with other tick-borne pathogens [42,43,44].
The etiologic agent of human granulocytic anaplasmosis, A. phagocytophilum, was detected in 12.88% of the dogs (25/194). In a study performed in southern Mexico, a prevalence of 27% was found [45], which is higher than that of the present study. In addition, other studies conducted in the same area as the present study and nearby places showed varying results. For example, Modarelli et al. (2019) did not detect this pathogen in dogs [39]. Furthermore, A. phagocytophilum was reported in horses (0.8% prevalence) from the same geographic area [46] and in ticks from dogs in Chihuahua state [47] which is the same state that the area where the present study was performed. These differences could be attributed to the conditions in which each investigation was carried out. Each analysis varied in sample size, sampling time, and species included, which may further support the observed disparities.
The lowest prevalence of tick-borne pathogens found in the present research was observed for R. rickettsii (5.67%) (11/194), the etiologic agent of Rocky Mountain Spotted Fever (RMSF). Although it was present in a low proportion, the presence of this pathogen is critical since rickettsiosis is a zoonotic disease and has caused death in humans in North America [48,49,50]. In fact, the state of Chihuahua is now the first national place in the presentation of RMSF in humans in Mexico [51]. Additionally, RMSF is considered the most important rickettsiosis in America, with high mortality [52]. In Mexico, cases of human rickettsiosis have increased in recent years and include affections in children. These data are also applicable to the state of Chihuahua, where this disease had a mortality of 35.92% (334/120) during the 2013–2019 period [51].
In dogs, RMSF was detected by the Indirect Fluorescent Antibody test in Arizona (prevalence of 53.4 to 66.4%) [53] and Oklahoma (54.6% to 88.5%) [54]. However, in these investigations, the pathogen was not detected by PCR. On the other hand, PCR studies were carried out on dogs in Texas, but this pathogen was not identified in blood samples [39]. In another study carried out in Carolina, R. rickettsii was only detected by PCR in 1.01% of the dogs (1/99), whereas via serology, it was detected in 21.21% (21/99) [55]. In Yucatán, Mexico, Ojeda-Chi et al. (2019) carried out an investigation that included blood samples from 246 dogs, in which they could not find the DNA of this rickettsia [28]. Nevertheless, in Mexico, the presence of R. rickettsii was identified by PCR in humans [56] and ticks [22,57,58]. This is the first study performed in Mexico where R. rickettsii is identified in the blood of dogs by PCR, indicating an active infection.
Finally, it was impossible to detect B. burgdorferil s. l., the etiological agent of Lyme disease, which was neither found in dogs nor ticks analyzed in this study. This finding could perhaps be attributed to the particular characteristics of B. burgdorferi, which is highly transitory in blood. Lyme disease is one of the most important zoonoses in the US, with a presentation of around 300,000 cases per year in humans [59], and was also described in dogs and ticks at the US–Mexico border [27,60,61,62]. The absence of this pathogen in our study might be explained by the fact that the primary vector of Borrelia burgdorferi is another tick, Ixodes spp. not R. sanguineus s. l. or O. megnini, which are the ticks that were identified in this area.
The co-detection of tick-borne diseases are frequent in small animal practice and are described in both animals and ticks via PCR [19,22,33,39,53]. In some cases, they are detected in dogs using other diagnostic techniques, such as ELISA [63]. Similarly, in other domestic species in the Juárez region (Mexico–US Border), it was possible to detect co-infection in horses and ticks by PCR [46]. Additionally, co-infection of tick-borne pathogens was also observed in wild species in nearby areas [41,45]. The co-infection with the highest prevalence in the analyzed dogs was that of E. canis and A. platys (9.27%; 18/194), most likely because both pathogens share the tick vector and their primary host is the dog; this co-infection was even reported in areas close to where the present study was conducted [39]. The other observed co-infections in dogs were present at a lower percentage, and in most of these cases, E. canis was involved (18.04%; 34/194). This could be because more than 99% of the identified ticks in this study were R. sanguineus s. l., which has the dog as its primary host and is the main vector of E. canis, although it is also associated with the presence of different rickettsia. Additionally, only dogs with tick infestation at the time of inspection were included in this study, increasing the possibility of finding active infections.
The detection of E. canis in ticks in this study was only performed in pools of adult ticks. However, we noted that for each female found in dogs, the possibility of transmission of E. canis increased. These results differ from those described by other researchers who observed that high infestation with males ticks could influence the prevalence of E. canis because of the transstadial transmission of E. canis to dogs by ticks that feed on them during the immature stages; the male R. sanguineus may be able to transmit E. canis in the absence of female ticks [63]. During the different stages of its life cycle, this tick ingests blood; larvae and nymphs of R. sanguineus do it to molt. In contrast, adults feed on blood for reproduction purposes. On the other hand, females need large amounts of blood to produce eggs, increasing their body weight up to 100 times after feeding [64]. In contrast to what happens with male ticks, adult males only consume blood for spermatogenesis and to complete the reproductive process [65]. The fact that the present study showed an increase in the possibility of transmission of E. canis in dogs parasitized by female ticks might be due to the blood-feeding period of females being longer than males. However, more research on this point is needed.
Additionally, in a study by Ipek et al. (2018), E. canis was detected by PCR in 3 out of 12 (25%) male pools and 6 of 14 (42.8%) female pools obtained from dogs infected with E. canis [66]. Similarly, in another investigation in Colombia, the pools of male R. sanguineus presented a lower percentage of positives to E. canis than the pools of female ticks: 11.8% in males versus 16.3% in female ticks [67].
4. Materials and Methods
4.1. Ethical Approval and Informed Consent
This study was reviewed and approved by the Ethical and Bioethical Committee of the Autonomous University of Juarez (CIBE-2016-1-16), Mexico, and was performed in compliance with Mexican and American guidelines for research animals (Guide for the Care and Use of Laboratory Animals in National Resource Council, 2011). All owners of the sampled dogs signed an informed consent form as authorization for the collection of the ticks and blood samples. Molecular analysis was carried out at the Laboratory of Veterinary Clinical Pathology and Molecular Biology of the Autonomous University of Juárez and the Babesia Laboratory Unit at the National Center for Disciplinary Research in Animal Health and Safety (CENID-SAI) of the National Institute of Forestry, Agricultural, and Livestock Research (INIFAP), Mexico.
4.2. Study Area and Samples
This study was conducted in Juarez, in the Northwest of Mexico (31°43′59″ N; 106°28′59″; at 1120 m above sea level) in the Chihuahua Desert [68]. Juárez City has about one and a half million inhabitants (INEGI, 2020). The climate is very dry, extreme, and semi-desertic, with highs of 40 °C during summer and −19 °C in winter. Juarez is a binational metropolitan area connected with El Paso County, Texas, and Donna Ana county, New Mexico, US. These three cities work together to promote commerce, tourism, and industry throughout the region by signing a sister cities agreement, where the migration of people accompanied by domestic animals (pets) is frequent [69].
4.2.1. Dog Recruitment and Sampling
The only inclusion criterion for the study was that dogs presented ticks at the time of inspection. This is in order to identify the tick species present in the locality. Between January 2013 and December 2014, 137 free-roaming dogs (FRD) were recruited from the municipal anti-rabies center (MARC). Additionally, 100 randomized home dogs were selected from various veterinary clinics (VC). In both cases, when dogs arrived at MARC or VC, a general inspection was performed, and it was at that moment that the ticks were captured. It was not considered whether dogs showed any signs of TBBPs-associated disease or any other, only the presence of ticks. In 2015, 195 home dogs were recruited only from VC. Figure 8 show the location of the MARC and the distribution of the neighborhoods, including the VCs, in Juarez. A questionnaire was filled in by all dog holders only in 2015 to record information about the household address, breed, age, sex, and clinical history. Blood samples (only for dogs in 2015) were collected from cephalic or jugular venopunction into BD Vacutainer® EDTA K2 tubes; the cell package was separated prior to freezing at −20 °C for further analyses. To detect the TBBPs in ticks and correlate these results with the bacteria found in dogs, ticks from each dog were collected using cotton impregnated with ethanol and entomological forceps. Ticks were stored in plastic bottles with 90% ethanol and frozen (−20 °C) until their identification and/or analysis at the laboratory.
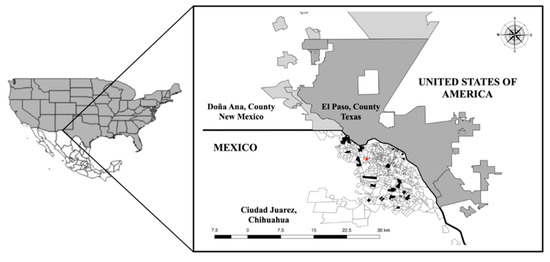
Figure 8.
Geographic location of the binational transboundary metropolitan area between Mexico (in white with divisions: Juarez City, Chihuahua) and the United States of America (in dark gray: El Paso, County, Texas; in soft gray: Doña Ana, County, New Mexico). In Juarez City, the red star indicates the MARC, while the black areas show the locations of the neighborhoods where the VCs were located.
4.2.2. Association among the Prevalence of TBBPs and Tick Stage Risk Factors
To estimate which risk factors influenced the probability of TBBD prevalence (Y = 1, infected dogs; Y = 0, uninfected dogs), GLIMMIX with Multiple Logistic Regression was constructed. Breed, tick infestation sites on dogs (head, ears, neck, body trunk, chest, abdomen, armpits, and legs), age (puppy, adult, and geriatric), sex (male and female), total ticks, females, males, blood-fed females, unfed females, nymphs, and larvae were included as predictors.
4.2.3. Tick Sampling and Identification
From March 2014 to February 2015, ticks were removed from dogs and morphologically identified and grouped according to their metamorphic stages (females, males, blood-feed females, unfed females, nymphs, and larvae), preserved in 70% ethanol, and kept at −20 °C until identification. The identification of tick species was performed using the taxonomic keys established by Martínez-Ibañez (2015) [70]. The DNA barcoding protocols were used to support morphological identification; a 98% sequence similarity between our specimens and those of GenBank and BOLD was considered for correct identification [71,72,73].
The tick counts from both years were found to conform to a negative binomial distribution and were thus examined using a GLIMMIX model. Here, separate sets of GLIMMIXs were run according to each metamorphic stage. The general model in each analysis was as follows: ticks = year + treat + error (where ticks = metamorphic stages, year = 2013/2014; treat = FRD/home), which fit the data well with no evidence of over-dispersion [74].
4.3. DNA Extraction from Tick Pools and Blood Dog Samples
The DNA extraction from dog blood samples was performed individually, while DNA extraction from ticks was performed by making 60 pools of 5 ticks each taken randomly from dogs with a positive result to any of the pathogens studied. To form the pools, female and male ticks, both adults, were included, as it was the most observed stage. Tick pools were homogenized to perform DNA extraction. In both cases, tick pools and dog blood samples, the extraction was performed using the UltraClean BloodSpin DNA Isolation Kit® (MoBio Laboratories Inc., Carlsbad, CA, USA; Cat. No. 12200-S) following the manufacturer’s protocol.
4.4. PCR Amplification and Sequencing
To determine infection by Ehrlichia canis, Anaplasma phagocytophilum, Anaplasma platys, Rickettsia rickettsii, and Borrelia burgdorferi s.l. in tick pools and dog blood samples, species-specific PCRs were performed targeting 16S ribosomal RNA genes and the gene ompA coding the outer membrane protein A of R. rickettsii, as described elsewhere [75,76,77,78,79]. Table 3 show the oligonucleotide sequences and the PCR conditions. Each 25 µL PCR reaction contained GoTaq Green Master mix (PROMEGA®, No, Cat M7123., Fitchburg, WI, USA) 12.5 µL, DNA extracted from tick pools or dog blood samples 5 µL, forward oligonucleotide (10 pmol) 1 µL, reverse oligonucleotide (10 pmol) 1 µL, and nuclease-free water 5.5 µL. The DNA positive controls of B. burgdorferi s. l. and R. rickettsii were donated by Dr Luis Tinoco-Gracia. The DNA positive controls of E. canis, A. platys and A. phagocytophilum were provided by the Babesia Laboratory Unit of CENID-SAI of the National Institute of Forest, Agricultural, and Livestock Research, Mexico. As negative controls for PCR reactions, we used nuclease-free water along with uninfected dog genomic DNA.

Table 3.
Sequences of primer sets and protocols used for PCR detection.
The PCR was performed in a C1000 Touch™ Thermal Cycler (Biorad Laboratories, Hercules, CA, USA); PCR products were analyzed by agarose gel electrophoresis stained with 1% ethidium bromide and examined by a gel documentation system (BioDoc-it, 220; UVP Imaging System; Upland, CA, USA). The PCR products amplified from dog blood or tick pools were purified using the Wizard® SV Gel and PCR Clean-Up System (No. Cat A9281, Madison WI, USA). To validate the identity of the TBBPs molecularly detected, three randomly selected positive samples from each pathogen were purified and sent to Eurofins Genomics Laboratory (Louisville, KY, USA) for bi-directional sequencing. The obtained sequences were edited using the MEGA software v. 7.0 and subsequently aligned with all other sequences from the GenBank database in nBLAST to obtain the percentage of identity; a sequence identity of 98% was considered for correct identification [80].
4.5. Statistical Analysis
All statistical analyses were performed using the Statistical Analysis System (SAS 9.4, 2013) OnDemand for the Academics online version. The total count of each life metamorphic stage of ticks was used to estimate the tick infestation load per dog. The generalized linear mixed model (under the GLIMMIX procedure) was used to fit the infestation with each life metamorphic stage of ticks collected to a negative binomial distribution; the least square mean (LSM) of each life metamorphic stage of ticks was then calculated as a response variable (RV) in a negative binomial regression and subsequently compared between FRD and home dogs [74]. The statistical significance of collection among FRD and home dogs was determined using the Student t-test for independent samples.
5. Conclusions
In this study, we identified R. sanguineus s. l. as the main tick that naturally parasitizes both home dogs and free-ranging dogs in Juarez City at the Mexico–US border. Likewise, it was possible to demonstrate the presence of some tick-borne pathogens such as E. canis and A. platys DNA in ticks and home dogs and A. phagocytophilum only in home dogs. It is also important to highlight the presence of co-infections in just over 20% of the patients studied as well as co-detection in the tick pools analyzed. Moreover, this is the first report in Mexico that demonstrates the presence of R. rickettsii in the blood of dogs, highlighting the importance of monitoring these findings as this disease is zoonotic. Additionally, it was possible to establish the association between infestation with R. sanguineus s. l. females and E. canis infection in dogs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11050552/s1, Figure S1: Distance genetic analyses tree R. sanguineus; Figure S2: Evolutionary relationships of taxa; Table S1: Blast diagnostic seq. R. sanguineus; Table S2 Regression analysis.
Author Contributions
Conceptualization, D.M.B.-R., J.A.G.-H., J.V.F.-M., and C.A.R.-A.; methodology, J.J.L.-A., S.O.-L., O.R.C.-L., A.F.-L., and L.M.H.-T.; validation, J.J.L.-A., S.V.L.-T., L.M.H.-T., and F.M.-I.; formal analysis, J.A.G.-H. and A.Q.-C.; investigation, D.M.B.-R., J.A.G.-H., and J.V.F.-M.; resources, D.M.B.-R., J.V.F.-M., and C.A.R.-A.; data curation, J.A.G.-H. and R.R.-B.; writing—original draft preparation, D.M.B.-R. and J.A.G.-H.; writing—review and editing, J.V.F.-M., J.J.L.-A., A.Q.-C., B.A.-R., R.R.-B., and C.A.R.-A.; visualization, B.A.-R. and R.R.-B.; supervision, D.M.B.-R., B.A.-R., and R.R.-B.; project administration, D.M.B.-R.; funding acquisition, D.M.B.-R. and C.A.R.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by PROMEP/103.5/11/4377, CONACYT-INFR-2014-01-224673, and CONACYT-INFRA-2012-01-187983.
Institutional Review Board Statement
The authors confirm that the ethical policies of the journal were followed. This work was reviewed and approved by the Ethical and Bioethical Committee of the Autonomous University of Juarez (CIBE-2016-1-16), Mexico, and was performed in compliance with Mexican and American guidelines for research on animals (Guide for the Care and Use of Laboratory Animals in National Resource Council, 2011).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
The authors wish to express their gratitude to Luis Tinoco Gracia, at Laboratorio de Salud Pública Veterinaria Instituto de Investigaciones en Ciencias Veterinarias, Universidad Autónoma de Baja California for providing positive control samples and Filiberto Reyes-Villanueva for the final revision in the statistical analysis. They also express their gratitude to Alejandro Martínez-Martínez (UACJ), Naún Lobo-Galo (UACJ), and José Manuel Martínez-López (Division of Microscopy at Química Tech S. A. de C. V.) for the facilities and assistance with the confocal microscope. Luis M. Hernández-Triana would like to thank the Department of the Environment, Food, and Rural Affairs (DEFRA) for project SV3045.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129 (Suppl. 1), S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Barandika, J.F.; Hurtado, A.; García-Esteban, C.; Gil, H.; Escudero, R.; Barral, M.; Jado, I.; Juste, R.A.; Anda, P.; García-Pérez, A.L. Tick-borne zoonotic bacteria in wild and domestic small mammals in northern Spain. Appl. Environ. Microbiol. 2007, 73, 6166–6171. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Kernif, T.; Leulmi, H.; Raoult, D.; Parola, P. Emerging Tick-Borne Bacterial Pathogens. Microbiol. Spectr. 2016, 4, 4.3.27. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Nava, S. Names for Ixodidae (Acari: Ixodoidea): Valid, synonyms, incertae sedis, nomina dubia, nomina nuda, lapsus, incorrect and suppressed names—with notes on confusions and misidentifications. Zootaxa 2014, 3767, 1–256. [Google Scholar] [CrossRef]
- Estrada-Peña, A. Ticks as vectors: Taxonomy, biology and ecology. Rev. Sci. Tech. 2015, 34, 53–65. [Google Scholar] [CrossRef]
- Guzmán-Cornejo, C.; Robbins, R.G.; Guglielmone, A.A.; Montiel-Parra, G.; Rivas, G.; Pérez, T.M. The Dermacentor (Acari, Ixodida, Ixodidae) of Mexico: Hosts, geographical distribution and new records. Zookeys 2016, 569, 1–22. [Google Scholar] [CrossRef]
- Lydecker, H.W.; Banks, P.B.; Hochuli, D.F. Counting ticks (Acari: Ixodida) on hosts is complex: A review and comparison of methods. J. Med. Entomol. 2019, 56, 1527–1533. [Google Scholar] [CrossRef]
- Halliday, J.E.; Meredith, A.L.; Knobel, D.L.; Shaw, D.J.; Bronsvoort, B.M.D.C.; Cleaveland, S. A framework for evaluating animals as sentinels for infectious disease surveillance. J. R. Soc. Interface 2007, 4, 973–984. [Google Scholar] [CrossRef]
- Walter, K.S.; Carpi, G.; Evans, B.R.; Caccone, A.; Diuk-Wasser, M.A. Vectors as epidemiological sentinels: Patterns of within-tick Borrelia burgdorferi diversity. PLoS Pathog. 2016, 12, e1005759. [Google Scholar] [CrossRef]
- Salman, M.D. Animal Disease Surveillance and Survey Systems: Methods and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2003. [Google Scholar]
- Cleaveland, S.; Meslin, F.X.; Breiman, R. Dogs can play useful role as sentinel hosts for disease. Nature 2006, 440, 605. [Google Scholar] [CrossRef] [PubMed]
- de Paiva Diniz, P.P.V.; Schwartz, D.S.; De Morais, H.S.A.; Breitschwerdt, E.B. Surveillance for zoonotic vector-borne infections using sick dogs from southeastern Brazil. Vector-Borne Zoon. Dis. 2007, 7, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Grubaugh, N.D.; Sharma, S.; Krajacich, B.J.; Fakoli, L.S., III; Bolay, F.K.; Diclaro, J.W., II; Johnson, W.E.; Ebel, G.D.; Foy, B.D.; Brackney, D.E. Xenosurveillance: A novel mosquito-based approach for examining the human-pathogen landscape. PLoS Negl. Trop. Dis. 2015, 9, e0003628. [Google Scholar] [CrossRef] [PubMed]
- Fauver, J.R.; Gendernalik, A.; Weger-Lucarelli, J.; Grubaugh, N.D.; Brackney, D.E.; Foy, B.D.; Ebel, G.D. The use of xenosurveillance to detect human bacteria, parasites, and viruses in mosquito bloodmeals. Am. J. Trop. Med. Hyg. 2017, 97, 324. [Google Scholar] [CrossRef]
- Jennett, A.L.; Smith, F.D.; Wall, R. Tick infestation risk for dogs in a peri-urban park. parasites Vectors 2013, 6, 358. [Google Scholar] [CrossRef]
- Lv, J.; Wu, S.; Zhang, Y.; Zhang, T.; Feng, C.; Jia, G.; Lin, X. Development of a DNA barcoding system for the Ixodida (Acari: Ixodida). Mitochondrial DNA 2014, 25, 142–149. [Google Scholar] [CrossRef]
- Ondrejicka, D.A.; Morey, K.C.; Hanner, R.H. DNA barcodes identify medically important tick species in Canada. Genome 2017, 60, 74–84. [Google Scholar] [CrossRef]
- Eremeeva, M.E.; Zambrano, M.L.; Anaya, L.; Beati, L.; Karpathy, S.E.; Santos-Silva, M.M.; Salceda, B.; MacBeth, D.; Olguin, H.; Dasch, G.A.; et al. Rickettsia rickettsii in Rhipicephalus ticks, Mexicali, Mexico. J. Med. Entomol. 2011, 48, 418–421. [Google Scholar] [CrossRef]
- Martínez-Vega, P.P.; Bolio-Gonzalez, M.E.; Rodríguez-Vivas, R.I.; Gutierrez-Blanco, E.; Pérez-Osorio, C.; Villegas-Perez, S.L.; Sauri-Arceo, C.H. Associated factors to seroprevalence of Ehrlichia spp. in dogs of Quintana Roo, Mexico. J. Trop. Med. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.I.; Apanaskevich, D.A.; Ojeda-Chi, M.M.; Trinidad-Martínez, I.; Reyes-Novelo, E.; Esteve-Gassent, M.D.; de León, A.P. Ticks collected from humans, domestic animals, and wildlife in Yucatan, Mexico. Vet. Parasitol. 2016, 215, 106–113. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Nava-Reyna, E.; Ávila-Rodríguez, V.; González-Álvarez, V.H.; Castillo-Martínez, A.; Siller-Rodríguez, Q.K.; Cabezas-Cruz, A.; Dantas-Torres, F.; Almazán, C. Detection of Rickettsia spp. in Rhipicephalus sanguineus (sensu lato) collected from free-roaming dogs in Coahuila state, northern Mexico. Parasites Vectors 2019, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Pieracci, E.G.; De La Rosa, J.D.P.; Rubio, D.L.; Perales, M.E.S.; Contreras, M.V.; Drexler, N.A.; Nicholson, W.L.; De La Rosa, J.J.P.; Chung, I.H.; Kato, C.; et al. Seroprevalence of spotted fever group rickettsiae in canines along the United States–Mexico border. Zoon. Public Health 2019, 66, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Estrada, I.; Balagot, C.; Fierro, M.; Kriner, P.; Iniguez-Stevens, E.; Kjemtrup, A.; Foley, J. Spotted fever group rickettsiae canine serosurveillance near the US–Mexico border in California. Zoon. Public Health 2020, 67, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Cruz Vazquez, C.; García Vázquez, Z.; Morales Soto, M. Prevalence of Rhipicephalus sanguineus infestation in dogs in Cuernavaca, Morelos, Mexico. Parasitol. Al Día 1998, 22, 29–32. [Google Scholar] [CrossRef]
- Tinoco-Gracia, L.; Quiroz-Romero, H.; Quintero-Martínez, M.T.; Rentería-Evangelista, T.B.; González-Medina, Y.; Barreras-Serrano, A.; Hori-Oshima, S.; Moro, M.H.; Vinasco, J. Prevalence of Rhipicephalus sanguineus ticks on dogs in a region on the Mexico-USA border. Vet. Rec. 2009, 164, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Galaviz-Silva, L.; Pérez-Treviño, K.C.; Molina-Garza, Z.J. Distribution of ixodid ticks on dogs in Nuevo León, Mexico, and their association with Borrelia burgdorferi sensu lato. Exp. Appl. Acarol. 2013, 61, 491–501. [Google Scholar] [CrossRef]
- Ojeda-Chi, M.M.; Rodriguez-Vivas, R.I.; Esteve-Gasent, M.D.; Pérez de León, A.A.; Modarelli, J.J.; Villegas-Perez, S.L. Ticks infesting dogs in rural communities of Yucatan, Mexico and molecular diagnosis of rickettsial infection. Transbound. Emerg. Dis. 2019, 66, 102–110. [Google Scholar] [CrossRef]
- Muñoz, L.; Casanueva, M.E. Garrapatas (Acari: Ixodidae) en perros de la ciudad de Concepción, Chile. Archiv. Med. Vet. 2002, 34, 131–134. [Google Scholar] [CrossRef]
- Iwakami, S.; Ichikawa, Y.; Inokuma, H. A nationwide survey of ixodid tick species recovered from domestic dogs and cats in Japan in 2011. Ticks Tick-Borne Dis. 2014, 5, 771–779. [Google Scholar] [CrossRef]
- Yoder, J.A.; Benoit, J.B.; Rellinger, E.J.; Tank, J.L. Developmental profiles in tick water balance with a focus on the new Rocky Mountain spotted fever vector, Rhipicephalus sanguineus. Med. Vet. Entomol. 2006, 20, 365–372. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Figueredo, L.A.; Otranto, D. Seasonal variation in the effect of climate on the biology of Rhipicephalus sanguineus in southern Europe. Parasitology 2011, 138, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Almazán, C.; González-Álvarez, V.H.; de Mera, I.G.F.; Cabezas-Cruz, A.; Rodríguez-Martínez, R.; de la Fuente, J. Molecular identification and characterization of Anaplasma platys and Ehrlichia canis in dogs in Mexico. Ticks Tick-Borne Dis. 2016, 7, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806)(Acari: Ixodidae): From taxonomy to control. Vet. Parasitol. 2008, 152, 173–185. [Google Scholar] [CrossRef] [PubMed]
- González-Álvarez, V.H.; Almazán-García, C.; Siller-Rodríguez, Q.K.; Sánchez-Ramos, F.J.; Valdés-Perezgasga, M.T.; Ortega-Morales, A.I. Otobius megnini (Dugès) on a dog from the North-Central part of Mexico. Southwest Entomol. 2018, 43, 267–270. [Google Scholar] [CrossRef]
- Yabsley, M.J.; McKibben, J.; Macpherson, C.N.; Cattan, P.F.; Cherry, N.A.; Hegarty, B.C.; Breitschwerdt, E.B.; O’Connor, T.; Chandrashekar, R.; Paterson, T.; et al. Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp. in dogs from Grenada. Vet. Parasitol. 2008, 151, 279–285. [Google Scholar] [CrossRef]
- Ojeda-Chi, M.M.; Rodriguez-Vivas, R.I.; Esteve-Gasent, M.D.; de León, A.A.P.; Modarelli, J.J.; Villegas-Perez, S.L. Ehrlichia canis in dogs of Mexico: Prevalence, incidence, co–infection and factors associated. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101351. [Google Scholar] [CrossRef]
- Pat-Nah, H.; Rodriguez-Vivas, R.I.; Bolio-Gonzalez, M.E.; Villegas-Perez, S.L.; Reyes-Novelo, E. Molecular diagnosis of Ehrlichia canis in dogs and ticks Rhipicephalus sanguineus (Acari: Ixodidae) in Yucatan, Mexico. J. Med. Entomol. 2015, 52, 101–104. [Google Scholar] [CrossRef]
- Modarelli, J.J.; Tomeček, J.M.; Piccione, J.; Ferro, P.J.; Esteve-Gasent, M.D. Molecular prevalence and ecoregion distribution of select tick-borne pathogens in Texas dogs. Transbound. Emerging. Dis. 2019, 66, 1291–1300. [Google Scholar] [CrossRef]
- Lira-Amaya, J.J.; Comas-González, A.G.; Álvarez-Martínez, J.A.; Rojas-Martínez, C.; Figueroa-Millán, J.V. Detección molecular en perros de co-infección múltiple con patógenos transmitidos por garrapatas. Primer reporte en México. Actual. Med. Vet. Zootec. Méx. 2013, 5, 30–35. [Google Scholar]
- Yu, S.; Modarelli, J.; Tomeček, J.M.; French, J.T.; Hilton, C.; Esteve-Gasent, M.D. Prevalence of common tick-borne pathogens in white-tailed deer and coyotes in south Texas. Int. J. Parasitol. Parasites Wildl. 2020, 11, 129–135. [Google Scholar] [CrossRef]
- Ramos, R.; Ramos, C.; Araújo, F.; Oliveira, R.; Souza, I.; Pimentel, D.; Galindo, M.; Santana, M.; Rosas, E.; Faustino, M.; et al. Molecular survey and genetic characterization of tick-borne pathogens in dogs in metropolitan Recife (north-eastern Brazil). Parasitol. Res. 2010, 107, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Lasta, C.S.; dos Santos, A.P.; Messick, J.B.; Oliveira, S.T.; Biondo, A.W.; Vieira, R.F.C.; Dalmolin, M.L.; González, F.H.D. Molecular detection of Ehrlichia canis and Anaplasma platys in dogs in Southern Brazil. Rev. Bras. Parasitol. Vet. 2013, 22, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Hernandez, G.; André, M.R.; Cendales, D.M.; de Sousa, K.C.M.; Gonçalves, L.R.; Rondelli, M.C.H.; Machado, R.Z.; Tinucci-Costa, M. Detecção molecular de espécies de Anaplasma em cães na Colômbia. Rev. Bras. Parasitol. Vet. 2016, 25, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Rojero-Vazquez, E.; Gordillo-Pérez, G.; Weber, M. Infection of Anaplasma phagocytophilum and Ehrlichia spp. in opossums and dogs in Campeche, Mexico: The role of tick infestation. Front. Ecol. Evol. 2017, 5, 161. [Google Scholar] [CrossRef]
- Medrano-Bugarini, R.A.; Figueroa-Millán, J.V.; Rivera-Chavira, B.E.; Lira-Amaya, J.J.; Rodríguez-Alarcón, C.A.; Beristain-Ruiz, D.M.; Adame-Gallegos, J.R. Detection of Theileria equi, Babesia caballi, and Anaplasma phagocytophilum DNA in soft ticks and horses at Ciudad Juarez, Mexico. Southwest Entomol. 2019, 44, 647–658. [Google Scholar] [CrossRef]
- Prado-Ávila, S.R.; Rascón-Cruz, Q.; Beristain-Ruiz, D.M.; Adame-Gallegos, J.R. Identificación del agente etiológico de la anaplasmosis granulocítica humana en la garrapata café de perro en Chihuahua, México. Salud Pública Mex. 2018, 60, 377. [Google Scholar] [CrossRef]
- Parola, P.; Socolovschi, C.; Jeanjean, L.; Bitam, I.; Fournier, P.E.; Sotto, A.; Labauge, P.; Raoult, D. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl. Trop. Dis. 2008, 2, e338. [Google Scholar] [CrossRef]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States: A practical guide for health care and public health professionals. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef]
- Drexler, N.A.; Dahlgren, F.S.; Heitman, K.N.; Massung, R.F.; Paddock, C.D.; Behravesh, C.B. National surveillance of spotted fever group rickettsioses in the United States, 2008–2012. Am. J. Trop. Med. Hyg. 2016, 94, 26. [Google Scholar] [CrossRef]
- Sistema Nacional de Vigilancia Epidemiológica. Available online: www.gob.mx (accessed on 28 February 2022).
- Eremeeva, M.E.; Dasch, G.A. Challenges posed by tick-borne rickettsiae: Eco-epidemiology and public health implications. Front. Public Health 2015, 3, 55. [Google Scholar] [CrossRef]
- Diniz, P.P.; Beall, M.J.; Omark, K.; Chandrashekar, R.; Daniluk, D.A.; Cyr, K.E.; Koterski, J.F.; Robbins, R.G.; Lalo, P.G.; Hegarty, B.C.; et al. High prevalence of tick-borne pathogens in dogs from an Indian reservation in northeastern Arizona. Vector-Borne Zoon. Dis. 2010, 10, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.W.; Little, S.E. Vector-borne infections in tornado-displaced and owner-relinquished dogs in Oklahoma, USA. Vector-Borne Zoon. Dis. 2016, 16, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Birkenheuer, A.J.; Hegarty, B.C.; Bradley, J.M.; Levy, M.G.; Breitschwerdt, E.B. Comparison of serological and molecular panels for diagnosis of vector-borne diseases in dogs. Parasites Vectors 2014, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Tinoco-Gracia, L.; Lomelí, M.R.; Hori-Oshima, S.; Stephenson, N.; Foley, J. Molecular confirmation of Rocky Mountain spotted fever epidemic agent in Mexicali, Mexico. Emerg. Infect. Dis. 2018, 24, 1723. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Rosado, K.; Peniche-Lara, G.; Tello-Martín, R.; Zavala-Velázquez, J.; de Campos Pacheco, R.; Labruna, M.B.; Sánchez, E.C.; Zavala-Castro, J. Rickettsia rickettsii isolation from naturally infected Amblyomma parvum ticks by centrifugation in a 24-well culture plate technique. Open Vet. J. 2013, 3, 101–105. [Google Scholar] [CrossRef]
- Sosa-Gutiérrez, C.G.; Vargas-Sandoval, M.; Torres, J.; Gordillo-Pérez, G. Tick-borne rickettsial pathogens in questing ticks, removed from humans and animals in Mexico. J. Vet. Sci. 2016, 17, 353–360. [Google Scholar] [CrossRef]
- Hinckley, A.F.; Connally, N.P.; Meek, J.I.; Johnson, B.J.; Kemperman, M.M.; Feldman, K.A.; White, J.L.; Mead, P.S. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect. Dis. 2014, 59, 676–681. [Google Scholar] [CrossRef]
- Feria-Arroyo, T.P.; Castro-Arellano, I.; Gordillo-Pérez, G.; Cavazos, A.L.; Vargas-Sandoval, M.; Grover, A.; Torres, J.; Medina, R.F.; de León, A.A.; Esteve-Gassent, M.D. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region. Parasites Vectors 2014, 7, 199. [Google Scholar] [CrossRef]
- Solís-Hernández, A.; Rodríguez-Vivas, R.I.; Esteve-Gasent, M.D.; Villegas-Pérez, S.L. Detección de Borrelia burgdorferi sensu lato en perros y sus garrapatas en comunidades rurales de Yucatán, México. Rev. Biol. Trop. 2018, 66, 428–437. [Google Scholar] [CrossRef][Green Version]
- Fesler, M.C.; Shah, J.S.; Middelveen, M.J.; Du Cruz, I.; Burrascano, J.J.; Stricker, R.B. Lyme Disease: Diversity of Borrelia species in California and Mexico detected using a novel immunoblot assay. Healthcare 2020, 8, 97. [Google Scholar] [CrossRef]
- Bremer, W.G.; Schaefer, J.J.; Wagner, E.R.; Ewing, S.A.; Rikihisa, Y.; Needham, G.R.; Jittapalapong, S.; Moore, D.L.; Stich, R.W. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet. Parasitol. 2005, 131, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.P.; Mangold, A.J.; João, C.F.; Bechara, G.H.; Guglielmone, A.A. Biological and DNA evidence of two dissimilar populations of the Rhipicephalus sanguineus tick group (Acari: Ixodidae) in South America. Vet. Parasitol. 2005, 130, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sanches, G.S.; de Oliveira, P.R.; André, M.R.; Machado, R.Z.; Bechara, G.H.; Camargo-Mathias, M.I. Copulation is necessary for the completion of a gonotrophic cycle in the tick Rhipicephalus sanguineus (Latreille, 1806)(Acari: Ixodidae). J. Insect. Physiol. 2012, 58, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Ipek, N.D.S.; Özübek, S.; Aktas, M. Molecular evidence for transstadial transmission of Ehrlichia canis by Rhipicephalus sanguineus sensu lato under field conditions. J. Med. Entomol. 2018, 55, 440–444. [Google Scholar] [CrossRef]
- Arroyave, E.; Cornwell, E.R.; McBride, J.W.; Díaz, C.A.; Labruna, M.B.; Rodas, J.D. Detection of tick-borne rickettsial pathogens in naturally infected dogs and dog-associated ticks in Medellin, Colombia. Rev. Brasil. Parasitol. Vet. 2020, 29. [Google Scholar] [CrossRef]
- Baddock, M.C.; Gill, T.E.; Bullard, J.E.; Acosta, M.D.; Rivera Rivera, N.I. Geomorphology of the Chihuahuan Desert based on potential dust emissions. J. Maps 2011, 7, 249–259. [Google Scholar] [CrossRef]
- PHAO. Salud en las Américas Edición de 2012. Panorama Regional y Perfiles de País. Available online: https://iris.paho.org/handle/10665.2/3272 (accessed on 4 October 2021).
- Martínez, I.F. Garrapatas de importancia veterinaria. Capítulo 9. Técnicas Para el Diagnóstico de Parásitos con Importancia en Salud Pública o Veterinaria, 1st ed.; Conasa Ampave: Mexico D. F., Mexico, 2015; pp. 258–303. [Google Scholar]
- Hebert, P.D.; Gregory, T.R. The promise of DNA barcoding for taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Chaverri, L.G.; Rodriguez-Perez, M.A.; Prosser, S.W.; Hebert, P.D.; Gregory, T.R.; Johnson, N. DNA barcoding of Neotropical black flies (Diptera: Simuliidae): Species identification and discovery of cryptic diversity in Mesoamerica. Zootaxa 2015, 3936, 93–114. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Montes de Oca, F.; Prosser, S.W.; Hebert, P.D.; Gregory, T.R.; McMurtrie, S. DNA barcoding as an aid for species identification in austral black flies (Insecta: Diptera: Simuliidae). Genome 2017, 60, 348–357. [Google Scholar] [CrossRef]
- Stroup, W.W. Living with Generalized Linear Mixed Models. 2011. Available online: https://support.sas.com/resources/papers/proceedings11/349-2011.pdf (accessed on 4 October 2021).
- Wen, B.; Rikihisa, Y.; Mott, J.M.; Greene, R.; Kim, H.-Y.; Zhi, N.; Couto, G.C.; Unver, A.; Bartsch, R. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J. Clin. Microbiol. 1997, 35, 1852–1855. [Google Scholar] [CrossRef]
- Martin, A.R.; Brown, G.K.; Dunstan, R.H.; Roberts, T.K. Anaplasma platys: An improved PCR for its detection in dogs. Experim. Parasitol. 2005, 109, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Adelson, M.E.; Rao, R.V.S.; Tilton, R.C.; Cabets, K.; Eskow, E.; Fein, L.; Occi, J.L.; Mordechai, E. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in Northern New Jersey. J. Clin. Microbiol. 2004, 42, 2799–2801. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tse, H.; Yuen, K.Y. Then and now: Use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect. 2008, 14, 908–934. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).