Suppression of Grape White Rot Caused by Coniella vitis Using the Potential Biocontrol Agent Bacillus velezensis GSBZ09
Abstract
:1. Introduction
2. Results
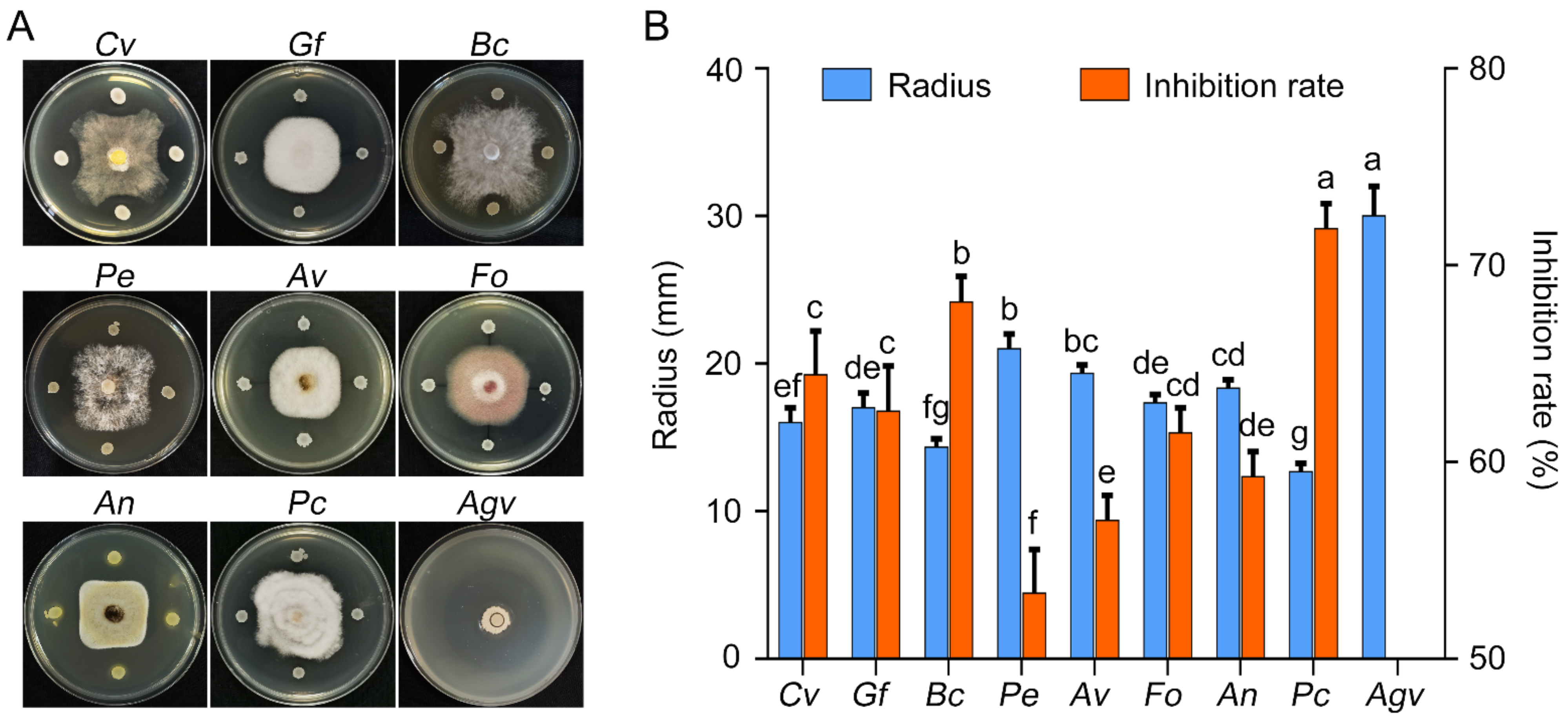
2.1. Biocontrol Effect of B. velezensis GSBZ09 against Plant Pathogens
2.2. GSBZ09 Was Identified as Bacillus velezensis
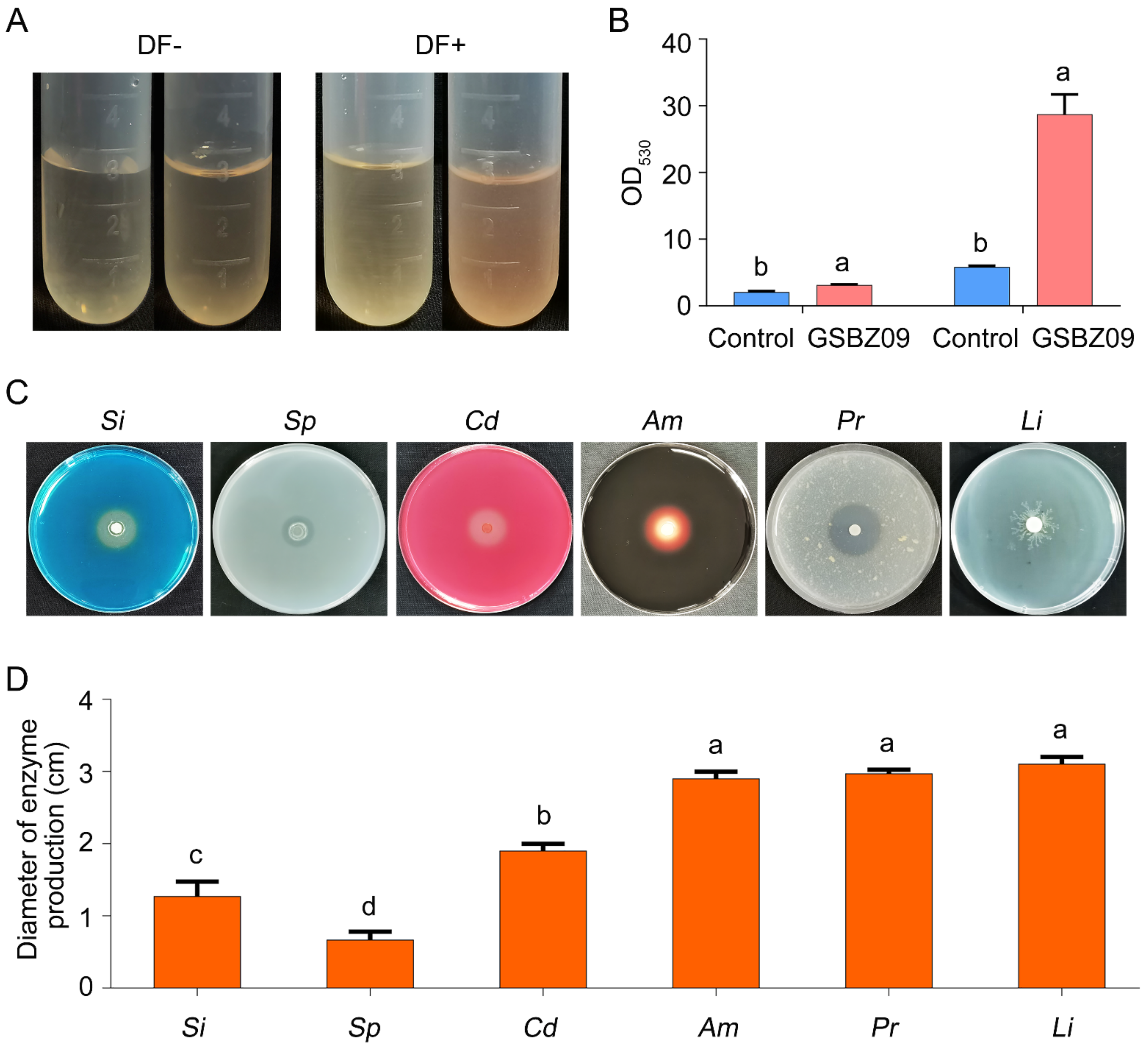
2.3. Detection of Extracellular Enzyme Production and Growth-Promoting Traits of Strain GSBZ09
2.4. Antibiotic Resistance and Hemolysis Assay
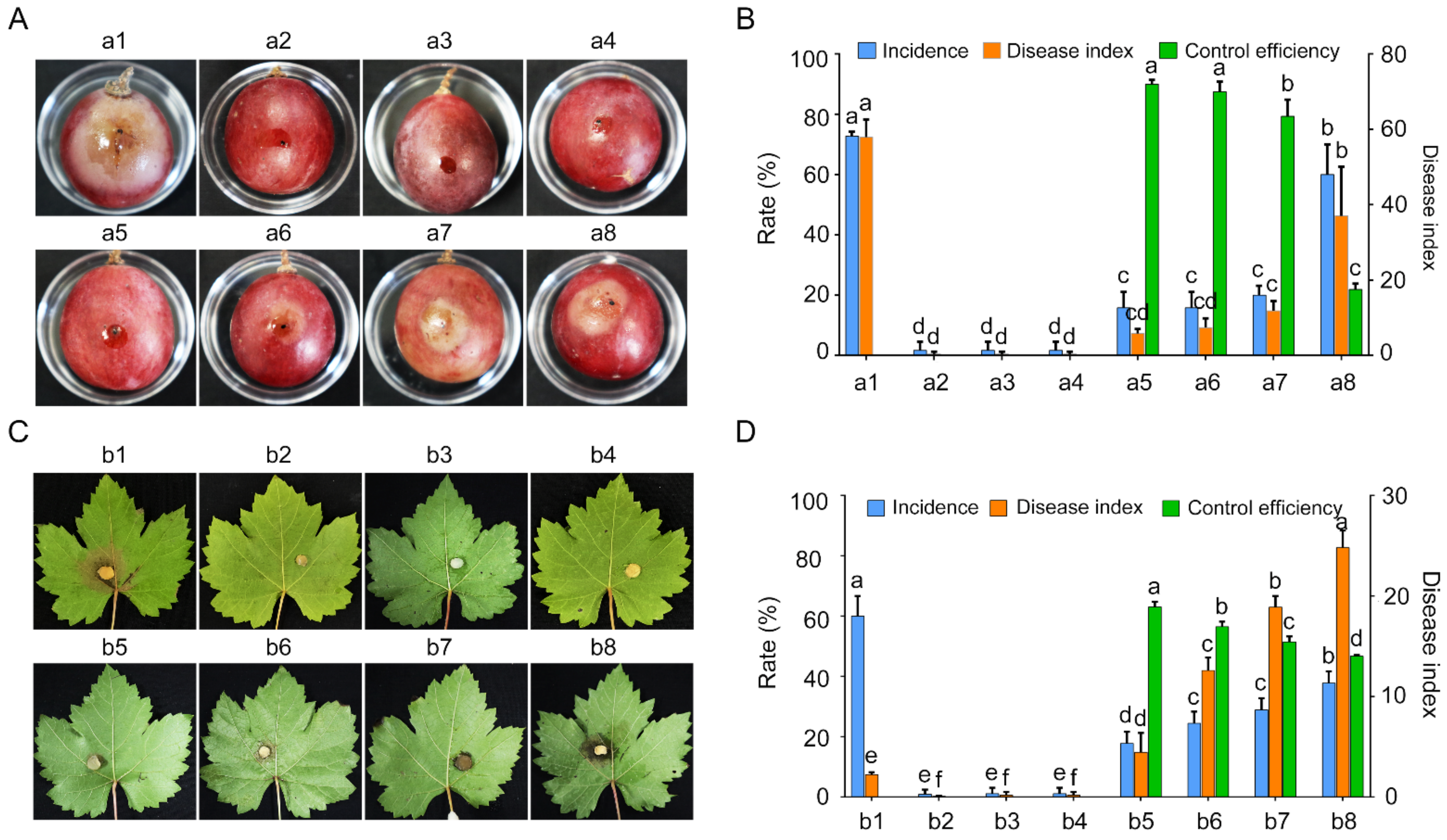
2.5. GSBZ09 Has High Biocontrol Efficiency on Grape White Rots Caused by C. vitis
2.6. Effects of GSBZ09 and the Culture Filtrate on Antioxidant Activity and Plant Growth Promotion
3. Discussion
4. Materials and Methods
4.1. Isolation of Bacillus Strains and Antagonism Assays
4.2. Identification of Strain GSBZ09
4.2.1. Genomic DNA Extraction and Phylogenetic Analysis
4.2.2. Morphological, Physiological and Biochemical Tests
4.3. Measurement of Extracellular Enzyme Production
4.3.1. Protease Production
4.3.2. Cellulose Degradation
4.3.3. Amylase Production
4.3.4. Lipase Production
4.4. Measurement of IAA Production, Siderophores and Mineral Phosphate Solubilization
4.5. Antibiotic Resistance and Hemolysis Assay
4.6. Assessment of Biocontrol Activity and Plant Growth Promotion
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, T.; Languasco, L.; Li, M.; Rossi, V. Effects of Temperature and Wetness Duration on Infection by Coniella diplodiella, the Fungus Causing White Rot of Grape Berries. Plants 2021, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.; Li, P.; Sun, L.; Jiang, J.; Fan, X.; Liu, C.; Zhang, Y. Genome Assembly and Transcriptome Analysis of the Fungus Coniella diplodiella During Infection on Grapevine (Vitis vinifera L.). Front. Microbiol. 2021, 11, 599150. [Google Scholar] [CrossRef] [PubMed]
- Chethana, K.W.T.; Zhou, Y.; Zhang, W.; Liu, M.; Xing, Q.K.; Li, X.H.; Yan, J.Y.; Hyde, K.D. Coniella vitis sp. nov. Is the Common Pathogen of White Rot in Chinese Vineyards. Plant Dis. 2017, 101, 2123–2136. [Google Scholar] [CrossRef] [PubMed]
- Bisiach, M. White Rot. In Compendium of Grape Disease; Pearson, R.C., Goheen, A.C., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1988; pp. 22–23. [Google Scholar]
- He, Z.; Cui, C.; Jiang, J.X. First Report of White Rot of Grape Caused by Pilidiella castaneicola in China. Plant Dis. 2017, 101, 1673. [Google Scholar] [CrossRef]
- Zhou, S.; Li, B. Genome Sequence Resource of Coniella vitis, a Fungal Pathogen Causing Grape White Rot Disease. Mol. Plant-Microbe Interact. 2020, 33, 787–789. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Wang, L.-T.; Lee, F.-L.; Tai, C.-J.; Kuo, H.-P. Bacillus velezensis is a later heterotypic synonym of Bacillus amyloliquefaciens. Int. J. Syst. Evol. Microbiol. 2008, 58, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Xie, X.; Zhao, Y.; Shi, Y.; Chai, A.; Li, L.; Li, B. Whole-genome analysis of Bacillus velezensis ZF2, a biocontrol agent that protects Cucumis Sativus against Corynespora leaf spot diseases. 3 Biotech 2020, 10, 186. [Google Scholar] [CrossRef]
- Shao, J.-H.; Li, Y.-C.; Li, Z.-F.; Xu, Z.-H.; Xun, W.-B.; Zhang, N.; Feng, H.-C.; Miao, Y.-Z.; Shen, Q.-R.; Zhang, R.-F. Participating mechanism of a major contributing gene ysnE for auxin biosynthesis in Bacillus amyloliquefaciens SQR9. J. Basic. Microbiol. 2021, 61, 569–575. [Google Scholar] [CrossRef]
- Cai, X.-C.; Xi, H.; Liang, L.; Liu, J.-D.; Liu, C.-H.; Xue, Y.-R.; Yu, X.-Y. Rifampicin-Resistance Mutations in the rpoB Gene in Bacillus velezensis CC09 have Pleiotropic Effects. Front. Microbiol. 2017, 8, 178. [Google Scholar] [CrossRef] [Green Version]
- Agersø, Y.; Stuer-Lauridsen, B.; Bjerre, K.; Jensen, M.G.; Johansen, E.; Bennedsen, M.; Brockmann, E.; Nielsen, B. Antimicrobial Susceptibility Testing and Tentative Epidemiological Cutoff Values for Five Bacillus Species Relevant for Use as Animal Feed Additives or for Plant Protection. Appl. Environ. Microbiol. 2018, 84, 01108–01118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leveau, J.H.J.; Gerards, S. Discovery of a bacterial gene cluster for catabolism of the plant hormone indole 3-acetic acid. FEMS Microbiol. Ecol. 2008, 65, 238–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [Green Version]
- Choub, V.; Ajuna, H.B.; Won, S.-J.; Moon, J.-H.; Choi, S.-I.; Maung, C.E.H.; Kim, C.-W.; Ahn, Y.S. Antifungal Activity of Bacillus velezensis CE 100 against Anthracnose Disease (Colletotrichum gloeosporioides) and Growth Promotion of Walnut (Juglans regia L.) Trees. Int. J. Mol. Sci. 2021, 22, 10438. [Google Scholar] [CrossRef]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-I.; Teaumroong, N. Co-Inoculation of Bacillus velezensis Strain S141 and Bradyrhizobium Strains Promotes Nodule Growth and Nitrogen Fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef]
- Pajčin, I.; Vlajkov, V.; Frohme, M.; Grebinyk, S.; Grahovac, M.; Mojićević, M.; Grahovac, J. Pepper Bacterial Spot Control by Bacillus velezensis: Bioprocess Solution. Microorganism 2020, 8, 1463. [Google Scholar] [CrossRef]
- Khalid, F.; Khalid, A.; Fu, Y.; Hu, Q.; Zheng, Y.; Khan, S.; Wang, Z. Potential of Bacillus velezensis as a probiotic in animal feed: A review. J. Microbiol. 2021, 59, 627–633. [Google Scholar] [CrossRef]
- Zeng, Q.; Xie, J.; Li, Y.; Gao, T.; Zhang, X.; Wang, Q. Comprehensive Genomic Analysis of the Endophytic Bacillus altitudinis Strain GLB197, a Potential Biocontrol Agent of Grape Downy Mildew. Front. Genet. 2021, 12, 729603. [Google Scholar] [CrossRef]
- Zeng, Q.; Xie, J.; Zhang, X.; Li, Y.; Wang, Q. Complete genome sequence data of Bacillus pumilus GLB197, an effective antagonist of grape downy mildew. Data Brief 2020, 30, 105423. [Google Scholar] [CrossRef]
- Aoki, T.; Aoki, Y.; Ishiai, S.; Otoguro, M.; Suzuki, S. Impact of Bacillus cereus NRKT on grape ripe rot disease through resveratrol synthesis in berry skin. Pest Manag. Sci. 2017, 73, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, C.; Béjar, V.; Martínez-Checa, F.; Llamas, I.; Quesada, E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 191–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Gu, W.; Xu, H.-Y.; Yang, G.-L.; Shan, X.-F.; Chen, G.; Wang, C.-F.; Qian, A.-D. Complete genome sequence of Bacillus velezensis 157 isolated from Eucommia ulmoides with pathogenic bacteria inhibiting and lignocellulolytic enzymes production by SSF. 3 Biotech 2018, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, K.; Aoki, Y.; Suzuki, S. Isolation and Characterization of Endophyte Bacillus velezensis KOF112 from Grapevine Shoot Xylem as Biological Control Agent for Fungal Diseases. Plants 2021, 10, 1815. [Google Scholar] [CrossRef]
- Nifakos, K.; Tsalgatidou, P.C.; Thomloudi, E.-E.; Skagia, A.; Kotopoulis, D.; Baira, E.; Delis, C.; Papadimitriou, K.; Markellou, E.; Venieraki, A.; et al. Genomic Analysis and Secondary Metabolites Production of the Endophytic Bacillus velezensis Bvel1: A Biocontrol Agent against Botrytis cinerea Causing Bunch Rot in Post-Harvest Table Grapes. Plants 2021, 10, 1716. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S.U.; Khan, W.U.; Saleh, T.A.; Khan, M.H.U.; Ullah, S.; Ali, A.; Ikram, M. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. Comptes Rendus. Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Antifungal Activity of Lipopeptides from Bacillus XT1 CECT 8661 Against Botrytis cinerea. Front. Microbiol. 2018, 9, 1315. [Google Scholar] [CrossRef]
- Batista, B.D.; Dourado, M.N.; Figueredo, E.F.; Hortencio, R.O.; Marques, J.P.R.; Piotto, F.A.; Bonatelli, M.L.; Settles, M.L.; Azevedo, J.L.; Quecine, M.C. The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato (Solanum lycopersicum cv. Micro-Tom). Arch. Microbiol. 2021, 203, 3869–3882. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus Solubilization by Bacillus Species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef] [Green Version]
- Chuljerm, H.; Deeudom, M.; Fucharoen, S.; Mazzacuva, F.; Hider, R.C.; Srichairatanakool, S.; Cilibrizzi, A. Characterization of two siderophores produced by Bacillus megaterium: A preliminary investigation into their potential as therapeutic agents. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129670. [Google Scholar] [CrossRef]
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Glauser, M.P.; Bernard, J.P.; Moreillon, P.; Francioli, P. Successful Single-Dose Amoxicillin Prophylaxis Against Experimental Streptococcal Endocarditis: Evidence for Two Mechanisms of Protection. J. Infect. Dis. 1983, 147, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Hsieh, S.P.; Kuo, P.A.; Jane, W.N.; Tu, J.; Wang, Y.N.; Ko, C.H. Impact of disinfectant and nutrient concentration on growth and bioflm formation for a Pseudomonas strain and the mixed cultures from a fine papermachine system. Int. Biodeter. Biodegr. 2009, 63, 998–1007. [Google Scholar] [CrossRef]
- Yuan, L.; Li, L.; Zheng, F.; Shi, Y.; Xie, X.; Chai, A.; Li, B. The complete genome sequence of Rahnella aquatilis ZF7 reveals potential beneficial properties and stress tolerance capabilities. Arch. Microbiol. 2020, 202, 483–499. [Google Scholar] [CrossRef]
- Yi, Y.; Shan, Y.; Liu, S.; Yang, Y.; Liu, Y.; Yin, Y.; Hou, Z.; Luan, P.; Li, R. Antagonistic Strain Bacillus amyloliquefaciens XZ34-1 for Controlling Bipolaris sorokiniana and Promoting Growth in Wheat. Pathogens 2021, 10, 1526. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Durrant, W.; Dong, X. Systemic Acquired Resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Kumari, A.; Goyal, M.; Mittal, A.; Kumar, R. Defensive capabilities of contrasting sorghum genotypes against Atherigona soccata (Rondani) infestation. Protoplasma 2021, 1–14. [Google Scholar] [CrossRef]
- Masmoudi, F.; Tounsi, S.; Dunlap, C.A.; Trigui, M. Halotolerant Bacillus spizizenii FMH45 promoting growth, physiological, and antioxidant parameters of tomato plants exposed to salt stress. Plant Cell Rep. 2021, 40, 1199–1213. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods–A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Jaschke, P.R.; Dotson, G.A.; Hung, K.S.; Liu, D.; Endy, D. Definitive demonstration by synthesis of genome annotation completeness. Proc. Natl. Acad. Sci. USA 2019, 116, 24206–24213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Faruqu, F.N.; Liam-Or, R.; Abu Abed, O.; Li, D.; Venner, K.; Errington, R.J.; Summers, H.; Wang, J.T.; Al-Jamal, K.T. Design of experiment (DoE)-driven in vitro and in vivo uptake studies of exosomes for pancreatic cancer delivery enabled by copper-free click chemistry-based labelling. J. Extracell. Vesicles 2020, 9, 1779458. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, X.; Liu, H.; Guo, L.; Lin, Y.; Liu, X.; Xiong, Y.; Ni, K.; Yang, F. Effects of Lactic Acid Bacteria on Microbial Metabolic Functions of Paper Mulberry Silage: A BIOLOG ECO Microplates Approach. Front. Microbiol. 2021, 12, 689174. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Wang, H.T.; Nie, Y.F.; Wang, Z.C.; Huang, D.Y.; Qiu, X.Y.; Chen, J.C. Effect of inoculating flower stalks and vegetable waste with ligno-cellulolytic microorganisms on the composting process. J. Environ. Sci. Health B 2004, 39, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Ghodsalavi, B.; Ahmadzadeh, M.; Soleimani, M.; Madloo, P.B.; Taghizad Farid, R. Isolation and characterization of rhizobacteria and their effects on root extracts of Valeriana officinalis. Aust. J. Crop. Sci. 2013, 7, 338–344. [Google Scholar]
- Kotasthane, A.; Agrawal, T.; Kushwah, R.; Rahatkar, O.V. In-vitro antagonism of Trichoderma spp. against Sclerotium rolfsii and Rhizoctonia solani and their response towards growth of cucumber, bottle gourd and bitter gourd. Eur. J. Plant Pathol. 2015, 141, 523–543. [Google Scholar] [CrossRef]
- Yuan, C.L.; Mou, C.X.; Wu, W.L.; Guo, Y.B. Efect of diferent fertilization treatments on indole-3-acetic acid producing bacteria in soil. J. Soils Sediments 2011, 11, 322–329. [Google Scholar] [CrossRef]
- Kelel, M.; Abera, G.; Yisma, A.; Molla, B.; Gebre, N.; Adugna, T.; Wesse, G. Isolation of phosphate solubilizing bacteria from acacia tree rhizophere soil. J. Microbiol. Biotech. Res. 2014, 4, 9–13. [Google Scholar]
- Keneni, A.; Assefa, F.; Prabu, P.C. Isolation of phosphate solubilizing bacteria from the rhizosphere of faba bean of Ethiopia and their abilities on solubilizing insoluble phosphates. J. Agric. Sci. Technol. 2010, 12, 79–89. [Google Scholar]
- Himpsl, S.D.; Mobley, H.L.T. Siderophore Detection Using Chrome Azurol S and Cross-Feeding Assays. Methods Mol. Biol. 2019, 2021, 97–108. [Google Scholar] [CrossRef]
- Rahal, E.A.; Kazzi, N.; Kanbar, A.; Abdelnoor, A.M.; Matar, G.M. Role of rifampicin in limiting Escherichia coli O157:H7 Shiga-like toxin expression and enhancement of survival of infected BALB/c mice. Int. J. Antimicrob. Agents 2011, 37, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Brillard, J.; Ribeiro, C.; Boemare, N.; Brehélin, M.; Givaudan, A. Two Distinct Hemolytic Activities in Xenorhabdus nematophila Are Active against Immunocompetent Insect Cells. Appl. Environ. Microbiol. 2001, 67, 2515–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-L.; Zhang, R.; Anand, P.; Stomberski, C.; Qian, Z.; Hausladen, A.; Wang, L.; Rhee, E.P.; Parikh, S.M.; Karumanchi, S.A.; et al. Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury. Nature 2019, 565, 96–100. [Google Scholar] [CrossRef]
- Varoquaux, N.; Cole, B.; Gao, C.; Pierroz, G.; Baker, C.R.; Patel, D.; Madera, M.; Jeffers, T.; Hollingsworth, J.; Sievert, J.; et al. Transcriptomic analysis of field-droughted sorghum from seedling to maturity reveals biotic and metabolic responses. Proc. Natl. Acad. Sci. USA 2019, 116, 27124–27132. [Google Scholar] [CrossRef] [Green Version]
- Buitrago, L.; Langdon, W.Y.; Sanjay, A.; Kunapuli, S.P. Tyrosine phosphorylated c-Cbl regulates platelet functional responses mediated by outside-in signaling. Blood 2011, 118, 5631–5640. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Li, T.; Jiang, X.; Tang, X.; Zhang, J.; Yuan, L.; Wei, Y. Suppression of Grape White Rot Caused by Coniella vitis Using the Potential Biocontrol Agent Bacillus velezensis GSBZ09. Pathogens 2022, 11, 248. https://doi.org/10.3390/pathogens11020248
Yin X, Li T, Jiang X, Tang X, Zhang J, Yuan L, Wei Y. Suppression of Grape White Rot Caused by Coniella vitis Using the Potential Biocontrol Agent Bacillus velezensis GSBZ09. Pathogens. 2022; 11(2):248. https://doi.org/10.3390/pathogens11020248
Chicago/Turabian StyleYin, Xiangtian, Tinggang Li, Xilong Jiang, Xiaoning Tang, Jiakui Zhang, Lifang Yuan, and Yanfeng Wei. 2022. "Suppression of Grape White Rot Caused by Coniella vitis Using the Potential Biocontrol Agent Bacillus velezensis GSBZ09" Pathogens 11, no. 2: 248. https://doi.org/10.3390/pathogens11020248
APA StyleYin, X., Li, T., Jiang, X., Tang, X., Zhang, J., Yuan, L., & Wei, Y. (2022). Suppression of Grape White Rot Caused by Coniella vitis Using the Potential Biocontrol Agent Bacillus velezensis GSBZ09. Pathogens, 11(2), 248. https://doi.org/10.3390/pathogens11020248




