Abstract
Background: Recommendations for managing patients with cerebral cryptococcomas are scarce across multiple clinical guidelines. Due to the deficiency of high-quality data coupled with an increasing number of at-risk patients, the purpose of this review is to describe the demographic characteristics, causative pathogen, intracranial imaging, surgical and/or pharmacological interventions, as well as outcomes of patients with cerebral cryptococcomas to improve recognition and management. Methods: We conducted a scoping review in accordance with the PRISMA guidelines using PubMed and Web of Science. Reports were included if the following details were presented: (1) site of infection; (2) treatment details which at least include the specific antifungal therapy administered, if applicable; and (3) patient outcome. Results: A total of 40 records representing 47 individual patients were included, of which the median age was 48.5 years, 75% were male, and 60% reported a significant past medical, surgical, or social history. C. neoformans was isolated more often than C. gattii (74% vs. 26%, respectively). Patients most often presented with headache, altered mental status and/or confusion, and vomiting occurring over a median of 30 days; though few were noted to have significant findings on physical examination. More than 50% of patients had a single cerebral cryptococcoma lesion, whereas perilesional edema was present in 73% of cases. Surgical intervention occurred in 49% of patients. An amphotericin B-based formulation was administered as “induction” therapy to 91% of patients, but combined with flucytosine or fluconazole in only 58%, for an overall median of 42 days. Fifty two percent of patients received “maintenance” therapy for a median of 126 days, in which fluconazole was most often used. Corticosteroids were administered to approximately 30% of patients for a median of 31.5 days. Overall, mortality was 34%. Conclusion: Based on our findings, management should include antifungal therapy for a minimum of 6 months with considerations for concomitant corticosteroids in the setting of perilesional edema, as well as surgical intervention. Emphasis should be placed on providing well-documented treatment details in future case reports and series to allow for the development of more concise evidence-based recommendations.
1. Background
Despite a decreasing incidence among persons living with HIV due to antiretroviral therapy (ART), rates of cryptococcosis have been increasing in patients who have undergone solid organ transplant or hematopoietic cell transplant [1]. In addition, cryptococcosis is occurring more frequently in non-HIV-infected, non-transplant patients, including those with malignancies, autoimmune diseases, diabetes mellitus, cirrhosis, as well as those receiving immunosuppressive medications [2,3,4,5,6,7,8]. Though most patients have one or more risk factors for cryptococcosis, approximately 30% do not [8].
Disseminated Cryptococcus spp. infection most often results in meningoencephalitis (CM), but may lead to development of a focal parenchymal brain mass known as cerebral cryptococcoma [9]. After accumulating and infecting the perivascular and subarachnoid spaces, Cryptococcus spp. may then invade the brain parenchyma [10,11]. Though the exact mechanism and overall incidence are unclear, cases of cerebral cryptococcomas have been reported in both immunocompetent and immunodeficient patients [12]. Recommendations for managing patients with cerebral cryptococcomas are scarce across multiple clinical guidelines [13,14,15]. Most data comprise a select few patients with cerebral cryptococcomas enrolled in larger studies of patients with CM, studies conducted prior to introduction of azole antifungals and ART, in addition to case reports and expert opinion. Due to the deficiency of high-quality data coupled with an increasing number of at-risk patients, the purpose of this review is to describe the demographic characteristics, causative pathogen, intracranial imaging, surgical and/or pharmacological interventions, as well as outcomes of patients with cerebral cryptococcomas to improve recognition and management.
2. Methods
2.1. Search Strategy
This scoping review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [16]. To identify and select cases for inclusion [17], a systematic literature search using PubMed and Web of Science was performed from 1 January 2010 through 25 May 2021. The literature search only included articles published since 2010, as the practice guidelines for the management of cryptococcal disease were last updated by the Infectious Diseases Society of America using data through December 2009 [14]. The following search terms were used: “cryptococcoma”, “neurocryptococcosis”, “intracranial cryptococcosis”, and “cerebral cryptococcosis”. References within articles of interest were scanned to capture additional sources.
2.2. Inclusion and Exclusion Criteria
English language publications were considered, and results were limited to studies, case reports, and case series involving human subjects at least 18 years of age with evidence of cerebral cryptococcoma. To be included, the report must have provided the following details: (1) site of infection; (2) treatment details which at least include the specific antifungal therapy administered, if applicable; and (3) patient outcome. Articles that did not provide disease-specific treatment details or outcomes, as well as conference abstracts, editorials, and review articles were excluded.
2.3. Data Extraction Process
Articles were screened by title and abstract for possible inclusion by three reviewers (DBC, AR, and AY). Full text was then sought for articles that met the inclusion criteria. Data abstracted included demographic characteristics; medical, surgical, and social history; causative pathogens; clinical manifestations; site and description of lesion(s); treatment details, including the specific antifungal therapy, dose, route, and treatment duration; and surgical and/or adjunctive therapies, if applicable, as well as outcome.
3. Results
A total of 269 records were identified. After removing duplicates and exclusions, 74 full-text records were screened for eligibility, of which 49 were excluded (Supplementary Figure S1) [16]. An additional 15 records, some of which were published prior to 2010, were identified from references within articles of interest for a total of 40 records [9,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] representing 47 individual patients included in the systematic scoping review (Table 1).

Table 1.
Reported cases of cerebral cryptococcomas.
3.1. Demographic Characteristics
Patient ages ranged from 19 to 75 years (median 48.5 years), and 75% (n = 35) were male. Though 40% (n = 19) of the patients did not report a past medical, surgical, or social history, of the 60% (n = 28) who did, 36% (n = 10) were HIV-infected, 21% (n = 6) had hypertension, and 18% (n = 5) had diabetes mellitus. One patient was in her second trimester of pregnancy with no other significant history [39], one patient had a history of polycythemia vera and monoclonal gammopathy of unknown significance [24], whereas two patients reported close contact with pigeons [41,46]. Of those with HIV, absolute CD4 count ranged from 0 to 157 cells/μL [9,28,36,42,43,50], whereas only three patients were noted to be on ART [36,42]. Six patients, all of whom were HIV-infected, were previously treated for CM which occurred from 2 to 22 months prior to their diagnosis of cerebral cryptococcoma [9,36,42,53].
3.2. Causative Pathogens
C. neoformans was responsible for 74% of the 34 cases in which an organism was isolated and speciated [19,22,23,24,26,27,28,29,30,32,34,36,37,39,41,44,45,46,47,50,52,53,54]. Among the nine cases which implicated C. gattii as the infectious etiology, only four provided explicit details as to the method for identifying the organism, either via isolation from a clinical specimen or genotypic testing [18,31,40,48]. Of the remaining 13 cases, Cryptococcus spp. was identified via histopathologic examination following biopsies or surgical resection of the cerebral lesions [20,25,33,38,42,55,56], except for 4 cases from a single case series published in 1990 where Cryptococcus spp. was isolated in culture but not speciated [43].
3.3. Clinical Manifestations
Patients with cerebral cryptococcomas most often presented with headache (58%, n = 26), altered mental status and/or confusion (38%, n = 17), and vomiting (31%, n = 14). Less common manifestations included fever (18%, n = 8), drowsiness or fatigue (16%, n = 7), seizures (13%, n = 6), and visual disturbances or blurry vision (11%, n = 5). On physical examination, 18% (n = 8) were noted to have papilledema, whereas 16% (n = 7) and 11% (n = 5) were observed to have upper and lower extremity weakness, respectively. Time from symptom onset to presentation ranged from 1 day to 365 days (median 30 days).
3.4. Site and Description of Lesion(s)
In 66% (n = 31) of cases, magnetic resonance imaging (MRI) was used to identify cerebral cryptococcomas, of which 74% (n = 24) utilized gadolinium. A single cerebral cryptococcoma lesion was present in 53% (n = 25) of patients, whereas multiple lesions were identified in 28% (n = 13) of cases. Cryptococcomas were most often detected in the frontal lobe (21%, n = 9), basal ganglia (21%, n = 9), and parietal lobe (19%, n = 8). One patient also had evidence of a concomitant T11-12 cryptococcoma [18]. Perilesional edema was present in 73% (n = 30) of patients, whereas hydrocephalus was identified in 38% (n = 9).
3.5. Treatment Details
Treatment details were provided for 96% (n = 45) of patients, as two patients died prior to surgical or pharmacological intervention [36,39]. Forty-nine percent (n = 22) of patients underwent surgical resection of one or more lesions, in which one patient underwent complete surgical resection of the lesion and antifungal therapy was not administered post-operatively [20]. Notably, surgical intervention occurred more often in those with lesions measuring 3 or more centimeters. Alternatively, surgical intervention was performed less often in patients with more than one lesion (38%) despite high rates of perilesional edema (71%). Management of intracranial pressure (ICP) was described in only a few cases, but of those, ventricular shunts were placed in three patients [18,22,43], whereas another two patients underwent external ventricular drain (EVD) implantation [42,52] as a result of failure to control ICP with serial lumbar punctures (LPs) [26], clinical deterioration [30], or development of hydrocephalus [50].
Of the 44 patients who received antifungal therapy, “induction” regimens most often consisted of an amphotericin B-based formulation (91%, n = 40) combined with flucytosine or fluconazole in 45% (n = 18) or 13% (n = 5), respectively. The duration of “induction” antifungal therapy varied widely from 7 to 180 days (median 42 days). The specific amphotericin B formulation utilized was included in 70% (n = 28) of cases, whereas specific doses, including mg/kg/day or total mg/dose, were reported in 53% (n = 21). Among patients who received an amphotericin B-based formulation, the median duration of therapy was 38 days (range 7–180 days), but details were limited as to why some treatment durations were abbreviated or prolonged. However, in one patient, amphotericin B was discontinued after the development of hyperkalemia, hypomagnesemia, and a pruritic rash, all of which improved after the initiation of fluconazole [33]. Fluconazole was exclusively administered to patients who did not receive an amphotericin B-based formulation as “induction” therapy for a median duration of 56 days (range 32 to 84 days).
Antifungal therapy was continued as “maintenance” therapy in 52% (n = 23) of patients who received “induction” therapy. Fluconazole was most often administered (83%, n = 19), followed by voriconazole (17%, n = 4). Fluconazole doses ranged from 200 to 1200 mg/day [25,27,28], whereas voriconazole 200 to 300 mg twice daily was most often used [18]. One patient received, every other day, amphotericin B deoxycholate (AmB-d) as “maintenance” therapy [19]. The duration of “maintenance” antifungal therapy ranged from 60 to 510 days (median 126 days), with one patient receiving lifelong fluconazole [50]. Two patients received localized therapy whereby one patient received intrathecal amphotericin B [43], whereas AmB-d was directly administered into the abscess cavity in the other [21].
Corticosteroids were administered to 27% (n = 12) of the 44 patients who received “induction” antifungal therapy for a median of 31.5 days (range 1–60 days). The specific corticosteroid and dose utilized was often not mentioned, but where described, dexamethasone 12 to 28 mg/day in three to four divided doses were most frequently administered [18,42,50]. The rationale for starting corticosteroids were provided in very few cases, and included initiation during the early phases of antifungal therapy, as well as later on in the treatment course in response to complications, such as increased ICP despite serial LPs [42], presence of hydrocephalus on repeat imaging [42], spinal cord edema, and paradoxical immune reconstitution inflammatory syndrome (IRIS) during week 8 of antifungal therapy [18]. Adjunctive medications, besides corticosteroids, were not widely used. One patient was treated with interferon (INF)-γ three times per week after 3 weeks of antifungal therapy for refractory disease [18], whereas another was started on subcutaneous adalimumab every 2 weeks due to clinical deterioration despite antifungal therapy [9].
3.6. Outcomes
Mortality was 31% (n = 14) among the 45 patients who underwent surgical or pharmacological intervention. Causes of death included refractory hydrocephalus [22], Pneumocystis jirovecii pneumonia [43], sepsis or septic shock [32,55], post-operative cardiovascular complications [45], as well as torsades de pointes secondary to fluconazole [35]. Time to death ranged from 2 to 420 days (median 25.5 days).
Patients who died were more likely to report a significant past medical or surgical history (64% vs. 40%), be infected with C. neoformans (69% vs. 45%), present with altered mental status or confusion (53% vs. 30%), nuchal rigidity (20% vs. 0%), as well as upper or lower extremity weakness (27% and 27% vs. 3% and 10%, respectively) compared to survivors. The presence of multiple lesions and perilesional edema were similar between groups. However, fewer patients that died underwent surgery (40% vs. 53%). Though an amphotericin B-based formulation was commonly administered as “induction” therapy in both groups (80% vs. 90%), those who died received a shorter duration of “induction” therapy (median, 21 days (range, 10–42 days) vs. 42 days (range, 7–180 days)). In addition, the duration of “maintenance” therapy was much shorter in those who died (median, 60 days (range, 6–76 days) vs. 180 days (range, 60–730 days)), despite 90% of patients in each group receiving fluconazole or voriconazole. Approximately 40% of patients in each group received corticosteroids, but the median duration of therapy was 4.5 days (range, 1–8 days) in patients who died compared to 49 days (range, 21 to 60 days) in those who survived.
Of the 31 patients who survived, 94% (n = 29) were described as having symptomatic, clinical, and/or radiographic improvement during follow-up. Median time to follow-up was 255 days (range, 7 to 4380 days). Though most patients were asymptomatic at follow-up, one described residual neuromotor symptoms on day 1460 [25], whereas another patient experienced improvement in symptoms initially, but later required readmission due to worsening neurologic symptoms [48].
Few cases described recurrent disease during the follow-up period [22,28,29,30,41,42,48], with even fewer detailing subsequent surgical or pharmacological intervention. An HIV-infected patient was asymptomatic with near complete radiographic resolution after 7 months of antifungal therapy and 5 months of ART, but then experienced a recurrence with worsening basal ganglia lesions on MRI, prompting initiation of combination antifungal therapy and corticosteroids for 6 weeks, followed by long-term fluconazole [28]. Radiographic improvement was noted after 6 months of treatment, but the patient experienced another recurrence 4 months later despite remaining on fluconazole and ART. The patient was treated with combination antifungal therapy for 6 weeks, then transitioned to long-term voriconazole, instead of fluconazole, without the development of new symptoms after 10 months. In another case, a patient who underwent surgical resection of left frontal lobe and left temporal lobe cryptococcomas followed by antifungal therapy, presented after 6 months due to non-adherence with new onset headaches and upper extremity weakness with the identification of two right parietal lobe lesions [29,30]. The patient underwent surgical resection, and was then started on oral fluconazole with reported symptom improvement after 8 weeks.
4. Discussion
In this systematic scoping review, we present a thorough analysis of the data, describing the demographic characteristics, diagnostic findings, treatment modalities, as well as outcomes for 47 individual patients with cerebral cryptococcomas. Though data presented across all 40 records were quite heterogeneous, as all but three were case reports, this review provides valuable insight into an understudied, and perhaps under recognized, area with limited guideline recommendations due to low quality evidence [13,14,15] despite an increasing frequency of at-risk patients [2,3,4,5,6,7,8]. As a result, findings from this systematic scoping review may lead to improvements in patient care and better clinical outcomes.
Though previous data suggest cerebral cryptococcomas are most often caused by C. gattii [14], C. neoformans was isolated more often than C. gattii in our analysis (Table 2). However, in 27% (n = 13) of cases, the cryptococcal isolate was not speciated, largely due to identification via histopathologic examination, which cannot distinguish between C. neoformans and C. gattii. Furthermore, C. gattii was initially recognized as a variant of C. neoformans, and later recognized as an independent species [57]. C. gattii was recently reclassified as a species complex composed of four individual species. Improved methods to differentiate C. gattii from C. neoformans over the recent years have led to greater recognition of the global environmental distribution of C. gattii. Notably, all the cases where C. gattii was detected were published between 2005 and 2017 [18,21,31,35,40,48,49,51,52]. Due to resource limitations to perform species identification in many laboratories, it is likely that C. gattii may be responsible for some of the cases attributed to C. neoformans presented in this review.

Table 2.
Comparisons between cerebral cryptococcomas caused by C. neoformans and C. gattii.
Cerebral cryptococcomas are challenging to diagnose, and are often missed on initial evaluation due to their highly variable clinical presentation in patients with and without comorbidities or risk factor for cryptococcosis. Similar to epidemiologic findings in patients with CM [58], males comprised 75% of the cases included in our analyses. Almost 80% were at least 30 years of age, in which 14% of patients were aged 30 to 39 years, 29% were 40 to 49, 29% were 50 to 59, 14% were 60 to 69, and 14% were over 70 years. Overall, most patients reported a past medical or surgical history including known risk factors for cryptococcosis, such as HIV-infection, diabetes, or idiopathic CD4 lymphocytopenia [59]. Patients with C. gattii were less likely to report a significant past medical or surgical history, whereas those with C. neoformans were more likely to be HIV-infected, which is similar to findings from previous data [58,60]. A few patients reported social histories with established ecologic risk factors, including living in or traveling to Australia in the case of C. gattii [18,49], and exposure to birds and bird droppings in the case of C. neoformans [41,46]. Patients with cerebral cryptococcomas presented with a wide range of often non-specific symptoms and physical exam findings, from headache and altered mental status to vomiting, giddiness, word-finding difficulties, and cerebellar signs, which were more common among patients 50 years and older. Clinical manifestations differed between patients with C. neoformans and those with C. gattii (Table 2).
All 47 individual patients included in our review underwent neuroimaging, of which MRI was utilized more often than CT (66% vs. 34%, respectively). Though CTs are often preferentially performed due to availability and timeliness [61], previous data suggest increased sensitivity for detecting cryptococcoma lesions using gadolinium-enhanced MRI compared to standard MRI or CT [10,54,61]. MRI findings vary, ranging from hyper-intense on T2-weighted images, to non-enhancing on postcontrast T1-weighted images (Figure 1). Due to the rarity of cerebral cryptococcomas and challenges associated with diagnosis, it is common for them to be mistaken for neoplasms, as well as pyogenic or tubercular abscesses, prior to surgery [52]. Less often, cerebral cryptococcomas have been misdiagnosed as neurocysticercosis [39], or even a tumefactive demyelinating lesion, a rare focal demyelinating disease [62]. Cryptococcomas are sometimes confused in neuroimaging with dilated perivascular spaces (Virchow–Robin spaces) that coalesce to form gelatinous pseudocysts [63]. However, cryptococcomas resulting from invasion of Cryptococcus spp. into the brain parenchyma may develop in a variety of locations throughout the brain [10,11,52]. The frontal and parietal lobes (21% and 19%, respectively), as well as the basal ganglia (21%) were most often involved, whereas the thalamus (5%) or the pons (2%) were rarely involved among the 47 individual patients included in our analysis. The median number of lesions identified was one, but ranged from one to three among reports that provided specified details. Forty-seven percent of patients were noted to have more than one or multiple lesions throughout the brain parenchyma, which was more common amongst patients with C. gattii (Table 2). Characteristics of the lesions were not universally reported, but the size of the cryptococcomas varied substantially from less than 1 cm to 5 or 6 cm. C. neoformans was identified more often among cases where measurements were provided [23,24,34,44,50]. Perilesional edema and hydrocephalus were slightly more common among patients with C. neoformans than those with C. gattii (Table 2). Intracranial imaging is a valuable tool to determine the extent of disease severity in patients with disseminated cryptococcosis, but is insufficient to establish a diagnosis of cerebral cryptococcoma.
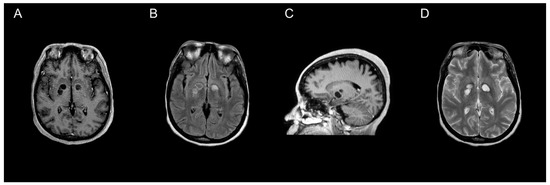
Figure 1.
MRI characterization of cryptococcomas. MRI of the brain showing a nonenhancing cryptococcoma (axial plane T1-weighted post contrast (A), axial plane T2-FLAIR (B), post-contrast parasagittal (C), and axial plane T2-weighted (D)).
Amphotericin B and flucytosine followed by long-term fluconazole remains the mainstay of treatment in CM [13,14,15]. Treatment strategies for patients with CM have largely been extrapolated to patients with cerebral cryptococcomas, as no prospective studies have been performed. Though guidelines suggest initial therapy for patients with cerebral cryptococcoma should include lipid-associated formulation of amphotericin B, in lieu of AmB-d when available, in combination with oral flucytosine for at least 42 days followed by oral fluconazole 400 mg to 800 mg per day for 180 to 540 days [14,15], regimen selection and duration were inconsistent across all most cases. More than 90% of patients received an amphotericin B-based formulation, of which 58% received combination therapy, whereas only 52% received “maintenance” therapy. Details about the specific formulation and/or dose utilized and associated rationale for “induction” and “maintenance” antifungal therapies were rarely provided.
Approximately 25% of all patients received corticosteroids, of which only 50% had perilesional edema. Though limited details regarding the specific corticosteroid and/or dose administered were reported, the median duration of corticosteroid therapy was 21 days (range, 1–60 days) among patients with perilesional edema. In addition, details regarding corticosteroid tapers were also not provided. Although guidelines mention corticosteroids in the setting of cerebral cryptococcomas with surrounding edema, no recommendations for which specific corticosteroid and/or dose are available [14,15].
Of the 47 individual patients included in this analysis, 34% died prior to undergoing surgical or pharmacological intervention, discharge, or during the follow-up period, similar to that observed with CM [1]. Fewer patients who underwent surgical intervention died, which may necessitate greater emphasis on the role of surgery as a component of the overall management for cerebral cryptococcomas in the guidelines [13,14,15]. In addition, prolonged durations of “induction” and “maintenance” therapy were reported more often in patients who survived, and should continue to be considered the standard of care. However, the guidelines recommend a “gradual reduction” in corticosteroids [14,15], but the vague recommendations should be modified to emphasize the importance that the tapering should occur over at least a 3-to-6-week period.
5. Limitations
Limitations of this systematic scoping review must be acknowledged. First, we chose to complete a scoping systematic review due to the lack of an available comprehensive review about cerebral cryptococcomas. Second, our analysis only included 47 individual patients derived from 40 reports. In order to abstract consistent data each report, we had to exclude publications that did not provide information on the site of infection; treatment regimen at least including the specific antifungal therapy administered, if applicable; and patient outcome. Third, specific details about the pharmacological treatments administered, such as the route, dose, and/or duration, were often not reported, which limited our abilities to provide clear treatment recommendations, but may be due to the paucity of recommendations provided in the guidelines [13,14,15]. Additionally, few reports clearly delineated “induction” therapy versus “maintenance” therapy. Fourth, the duration of “induction”, “maintenance”, and corticosteroid therapy was longer in patients who survived than those who died, which could represent survival-related selection bias. Lastly, due to the inherent difficulties of diagnosing cerebral cryptococcomas, many additional cases may be unrecognized and, therefore, not reported.
6. Conclusions
Despite guideline recommendations, diagnosis and treatment of cerebral cryptococcomas is very heterogeneous due to the lack of high-quality data. In our analysis, C. neoformans was identified more often than C. gattii as the causative pathogen in cases where an organism was isolated and speciated, which may be due to difficulties differentiating C. neoformans from C. gattii, and underreporting. Furthermore, cerebral cryptococcomas are most often identified as a single lesion with surrounding edema on gadolinium-enhanced MRI of the brain, and occur more often in male patients over 30 years of age who frequently report a significant past medical, surgical, or social history, and present with non-specific symptoms and physical exam findings. Though the most efficacious treatment remains undefined, a multipronged approach should be utilized that includes corticosteroids in the setting of perilesional edema tapered over 3 to 6 weeks; considerations for surgical intervention, if feasible; as well as a prolonged duration of antifungal therapy. Emphasis should be placed on providing species identification and well-documented treatment details in future reports to allow for the development of more concise evidence-based recommendations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11020205/s1. Figure S1: Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram.
Author Contributions
D.B.C.: conceptualization, data curation, formal analysis, methodology, visualization, writing—original draft, writing—review and editing. A.R.: conceptualization, data curation, formal analysis, methodology, visualization, writing—original draft, writing—review and editing. A.Y.: conceptualization, data curation, formal analysis, methodology, visualization, writing—original draft, writing—review and editing. A.F.H.-M.: conceptualization, supervision, writing—original draft, writing—review and editing. T.B.: writing—review and editing. C.F.-P.: conceptualization, supervision, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global Burden of Disease of HIV-Associated Cryptococcal Meningitis: An Updated Analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- George, I.A.; Spec, A.; Powderly, W.G.; Santos, C.A.Q. Comparative Epidemiology and Outcomes of Human Immunodeficiency Virus (HIV), Non-HIV Non-Transplant, and Solid Organ Transplant Associated Cryptococcosis: A Population-Based Study. Clin. Infect. Dis. 2018, 66, 608–611. [Google Scholar] [CrossRef]
- Chamilos, G.; Lionakis, M.S.; Kontoyiannis, D.P. Call for Action: Invasive Fungal Infections Associated with Ibrutinib and Other Small Molecule Kinase Inhibitors Targeting Immune Signaling Pathways. Clin. Infect. Dis. 2017, 66, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Hevey, M.A.; George, I.A.; Raval, K.; Powderly, W.G.; Spec, A. Presentation and Mortality of Cryptococcal Infection Varies by Predisposing Illness: A Retrospective Cohort Study. Am. J. Med. 2019, 132, 977–983.e1. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.A.; Felsen, U.; Wang, T.; Pirofski, L.-A. Cryptococcus Neoformans Infection in Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Patients at an Inner-City Tertiary Care Hospital in the Bronx. Med. Mycol. 2019, 58, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Sun, Y.; Spec, A.; Lu, N.; Panackal, A.; Bennett, J.; Pappas, P.; Ostrander, D.; Datta, K.; Zhang, S.X.; et al. A Multicenter, Longitudinal Cohort Study of Cryptococcosis in Human Immunodeficiency Virus–Negative People in the United States. Clin. Infect. Dis. 2019, 70, 252–261. [Google Scholar] [CrossRef]
- Rosen, L.B.; Freeman, A.F.; Yang, L.M.; Jutivorakool, K.; Olivier, K.N.; Angkasekwinai, N.; Suputtamongkol, Y.; Bennett, J.E.; Pyrgos, V.; Williamson, P.R.; et al. Anti–GM-CSF Autoantibodies in Patients with Cryptococcal Meningitis. J. Immunol. 2013, 190, 3959–3966. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Perfect, J.R.; Cloud, G.A.; Larsen, R.A.; Pankey, G.A.; Lancaster, D.J.; Henderson, H.; Kauffman, C.A.; Haas, D.W.; Saccente, M.; et al. Cryptococcosis in Human Immunodeficiency Virus–Negative Patients in the Era of Effective Azole Therapy. Clin. Infect. Dis. 2001, 33, 690–699. [Google Scholar] [CrossRef] [Green Version]
- Sitapati, A.M.; Kao, C.L.; Cachay, E.R.; Masoumi, H.; Wallis, R.S.; Mathews, W.C. Treatment of HIV-Related Inflammatory Cerebral Cryptococcoma with Adalimumab. Clin. Infect. Dis. 2010, 50, e7–e10. [Google Scholar] [CrossRef] [Green Version]
- Tien, R.D.; Chu, P.K.; Hesselink, J.R.; Duberg, A.; Wiley, C. Intracranial Cryptococcosis in Immunocompromised Patients: CT and MR Findings in 29 Cases. Am. J. Neuroradiol. 1991, 12, 283–289. [Google Scholar]
- Colombo, A.C.; Rodrigues, M.L. Fungal Colonization of the Brain: Anatomopathological Aspects of Neurological Cryptococcosis. An. Acad. Bras. Ciências 2015, 87, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Franco-Paredes, C.; Chastain, D.B.; Rodriguez-Morales, A.J.; Marcos, L.A. Cryptococcal Meningoencephalitis in HIV/AIDS: When to Start Antiretroviral Therapy? Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DHHS. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Adult_OI.pdf (accessed on 11 January 2021).
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [Green Version]
- >World Health Organization Guidelines Approved by the Guidelines Review Committee. In Guidelines for The Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; World Health Organization: Geneva, Switzerland, 2018.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for Conducting Systematic Scoping Reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Amburgy, J.W.; Miller, J.H.; Ditty, B.J.; Lune, P.V.; Muhammad, S.; Fisher, W.S. Cryptococcus Gattiiin an Immunocompetent Patient in the Southeastern United States. Case Rep. Infect. Dis. 2016, 2016, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayardelle, P.; Giard, N.; Maltais, R.; Delorme, J.; Brazeau, M. Success with Amphotericin B and 5-Fluorocytosine in Treating Cerebral Cryptococcoma Accompanying Cryptococcal Meningitis. Can. Med. Assoc. J. 1982, 127, 732–733. [Google Scholar]
- Brunasso, L.; Costanzo, R.; Cascio, A.; Florena, A.; Sparacia, G.; Iacopino, D.G.; Grasso, G. Seizure in Isolated Brain Cryptococcoma: Case Report and Review of the Literature. Surg. Neurol. Int. 2021, 12, 153. [Google Scholar] [CrossRef]
- Colom, M.F.; Frasés, S.; Ferrer, C.; Jover, A.; Andreu, M.; Reus, S.; Sánchez, M.; Torres-Rodríguez, J.M. First Case of Human Cryptococcosis due to Cryptococcus Neoformans Var. Gattii in Spain. J. Clin. Microbiol. 2005, 43, 3548–3550. [Google Scholar] [CrossRef] [Green Version]
- Coppens, Y.; Kalala, J.-P.; Van Roost, D.; Broecke, C.V.D.; Vogelaers, D. Cryptococcoma Unresponsive to Antifungal Treatment in a 63-Year-Old Non-Hiv-Infected Male. Acta Clin. Belg. 2006, 61, 359–362. [Google Scholar] [CrossRef]
- Guha, A.; Merchen, L. Cerebral Cryptococcoma-Why? J. Kuwait Med. Assoc. 2015, 47, 346–347. [Google Scholar]
- Guhjjar, M.K.; Ghazanfar, H.; Ashraf, S.; Gaddam, M.; Matela, A. Disseminated Cryptococcal Disease in a Patient with Monoclonal Gammopathy of Undetermined Significance and Polycythemia Vera: A Case Report and Review of the Literature. Cureus 2021, 13, e1245. [Google Scholar] [CrossRef]
- Hagan, J.E.; Dias, J.S.; Villasboas-Bisneto, J.C.; Falcão, M.B.; Ko, A.I.; Ribeiro, G.S. Puerperal Brain Cryptococcoma in an HIV-Negative Woman Successfully Treated with Fluconazole: A Case Report. Rev. Soc. Bras. Med. Trop. 2014, 47, 254–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraga, A.; Yatomi, M.; Ozaki, D.; Kamitsukasa, I.; Kuwabara, S. Cryptococcosis Mimicking Lung Cancer with Brain Metastasis. Clin. Neurol. Neurosurg. 2015, 135, 93–95. [Google Scholar] [CrossRef]
- Ho, T.; Lee, H.; Lee, K.; Chen, W. Diffusion-Weighted and Conventional Magnetic Resonance Imaging in Cerebral Cryptococcoma. Acta Radiol. 2005, 46, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wei, H.; Meng, F.; Xu, C.; Cheng, C.; Yang, Y. Recurrent Cryptococcal Immune Reconstitution Inflammatory Syndrome in an HIV-Infected Patient after Anti-Retroviral Therapy: A Case Report. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, A.; Mpanza, P.; Lekgwara, P.; Otto, D.; Otto, D. Multicentric Cryptococcomas Mimicking Neoplasia in Immunocompetent Patient. World Neurosurg. 2018, 118, 5–8. [Google Scholar] [CrossRef]
- Kelly, A.; Lekgwara, P.; Otto, D. Recurring Multicentric Granulomatous Cryptococcomas in the Contralateral Cerebral Hemisphere in an Adult Immunocompetent Patient with Known Previous Disease. World Neurosurg. 2020, 140, 79–88. [Google Scholar] [CrossRef]
- King, V.S.S.; Winder, M.J. Multiple Cerebral Cryptococcomas in an Immunocompetent Man: An Unlikely Diagnosis. ANZ J. Surg. 2013, 84, 588–590. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Corbett, A. Intracranial and Dermatological Cryptococcal Infection in an Immunocompetent Man. J. Clin. Neurosci. 2004, 11, 765–767. [Google Scholar] [CrossRef]
- Kumar, M.; Bajaj, A.; Tewari, M.K.; Singh, P.; Das Radotra, B. Cerebellar Cryptococcoma in an Immunocompetent Adult: A Rare Occurrence Report of a Case and Review of Literature. Indian J. Neurosurg. 2020, 9, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; You, C.; Liu, Q.; Liu, Y. Central Nervous System Cryptococcoma in Immunocompetent Patients: A Short Review Illustrated by a New Case. Acta Neurochir. 2009, 152, 129–136. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.H.; Grayson, M.L. Torsades de Pointes in a Patient Receiving Fluconazole for Cerebral Cryptococcosis. Am. J. Health-Syst. Pharm. 2008, 65, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Musubire, A.K.; Boulware, D.R.; Meya, D.; Rhein, J. Diagnosis and Management of Cryptococcal Relapse. J. AIDS Clin. Res. 2013, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nadkarni, T.; Menon, R.K.; Desai, K.I.; Goel, A. A Solitary Cryptococcal Granuloma in an Immunocompetent Host. Neurol. India 2005, 53, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Nakwan, N.; Songjamrat, A.; Tungsinmonkong, K.; Nakwan, N. Cerebellar Cryptococcoma in an Immunocompetent Adult Patient. Southeast Asian J. Trop. Med. Public Health 2009, 40, 1034–1037. [Google Scholar]
- Nucci, A.; Maciel, J.A., Jr.; Queiroz, L.D.S.; Montenegro, M.A.; De Carvalho, R.B. Pseudocystic Form of Neurocryptococcosis in Pregnancy: Case Report. Arq. Neuro-Psiquiatr. 1999, 57, 678–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, F.D.M.; Severo, C.B.; Guazzelli, L.S.; Severo, L.C. Cryptococcus Gattii Fungemia: Report of a Case with Lung and Brain Lesions Mimicking Radiological Features of Malignancy. Rev. Inst. Med. Trop. São Paulo 2007, 49, 263–265. [Google Scholar] [CrossRef]
- Paiva, A.L.C.; De Aguiar, G.B.; Lovato, R.M.; Zanetti, A.V.D.; Panagopoulos, A.T.; Veiga, J.C.E. Cryptococcoma Mimicking a Brain Tumor in an Immunocompetent Patient: Case Report of an Extremely Rare Presentation. São Paulo Med. J. 2017, 136, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, K.D.; Pappas, P.G.; Chin-Hong, P.; Baxi, S.M. A Paradoxical Decline: Intracranial Lesions in Two HIV-Positive Patients Recovering from Cryptococcal Meningitis. BMJ Case Rep. 2015, 2015, bcr2015212108. [Google Scholar] [CrossRef]
- Popovich, M.J.; Arthur, R.H.; Helmer, E. CT of Intracranial Cryptococcosis. Am. J. Roentgenol. 1990, 154, 603–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, S.; Marak, R.; Jain, S.; Dhole, T. Posterior Fossa Midline Cryptococcoma in a Patient with Idiopathic CD4 Lymphocytopenia. Indian J. Med. Microbiol. 2012, 30, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Sabbatani, S.; Manfredi, R.; Pavoni, M.; Consales, A.; Chiodo, F. Voriconazole Proves Effective in Long-Term Treatment of a Cerebral Cryptococcoma in a Chronic Nephropathic HIV-Negative Patient, after Fluconazole Failure. Mycopathologia 2004, 158, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Saigal, G.; Post, M.J.; Lolayekar, S.; Murtaza, A. Unusual Presentation of Central Nervous System Cryptococcal Infection in an Immunocompetent Patient. AJNR Am. J. Neuroradiol. 2005, 26, 2522–2526. [Google Scholar]
- Santander, X.A.; Gutiérrez-González, R.; Cotúa, C.; Tejerina, E.; Rodríguez, G.-B. Intraventricular Cryptococcoma Mimicking a Neoplastic Lesion in an Immunocompetent Patient with Hydrocephalus: A Case Report. Surg. Neurol. Int. 2019, 10, 115. [Google Scholar] [CrossRef]
- Sellers, B.; Hall, P.; Cine-Gowdie, S.; Hays, A.L.; Patel, K.; Lockhart, S.R.; Franco-Paredes, C. Cryptococcus Gattii: An Emerging Fungal Pathogen in the Southeastern United States. Am. J. Med. Sci. 2012, 343, 510–511. [Google Scholar] [CrossRef]
- Solis, W.G.; Hansen, M. Fluorescence in a Cryptococcoma Following Administration of 5-Aminolevulinic Acid Hydrochloride (Gliolan). BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef]
- Troncoso, A.; Fumagalli, J.; Shinzato, R.; Gulotta, H.; Toller, M.; Bava, J. CNS Cryptococcoma in an HIV-Positive Patient. J. Int. Assoc. Physicians AIDS Care 2002, 1, 131–133. [Google Scholar] [CrossRef]
- Ulett, K.B.; Cockburn, J.W.J.; Jeffree, R.; Woods, M.L. Cerebral Cryptococcoma Mimicking Glioblastoma. BMJ Case Rep. 2017, 2017, bcr2016218824. [Google Scholar] [CrossRef]
- Uppar, A.; Raj, A.P.; Konar, S.; Kandregula, S.; Shukla, D.; Somanna, S.; Devi, B.; Yasha, C.; Chandrashekar, N. Intracranial Cryptococcoma—Clinicopathologic Correlation and Surgical Outcome: A Single-Institution Experience. World Neurosurg. 2018, 115, e349–e359. [Google Scholar] [CrossRef]
- Velamakanni, S.S.; Bahr, N.C.; Musubire, A.K.; Boulware, D.R.; Rhein, J.; Nabeta, H.W. Central Nervous System Cryptococcoma in a Ugandan Patient with Human Immunodeficiency Virus. Med. Mycol. Case Rep. 2014, 6, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, X.-Y.; Zhang, Y. Central Nervous System Cryptococcoma Mimicking Demyelinating Disease: A Case Report. BMC Neurol. 2020, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-H.; Lin, S.-F.M.; Chiu, M.-C.; Kuo, C.-L.; Huang, H.-T.; Shoung, H.-M. Cerebral Cryptococcoma in an HIV-Negative Patient: Experience Learned from a Case. J. Neuropsychiatry Clin. Neurosci. 2014, 26, E34–E35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.X.; De Zhi, K. Multiple Cerebellar Abscess and Pneumonia Caused by Cryptococcus in an Immunocompetent Adult Patient. Pak. J. Med. Sci. 2011, 27, 448–450. [Google Scholar]
- Hagen, F.; Khayhan, K.; Theelen, B.; Kolecka, A.; Polacheck, I.; Sionov, E.; Falk, R.; Parnmen, S.; Lumbsch, T.; Boekhout, T. Recognition of Seven Species in the Cryptococcus Gattii/Cryptococcus Neoformans Species Complex. Fungal Genet. Biol. 2015, 78, 16–48. [Google Scholar] [CrossRef] [Green Version]
- Pyrgos, V.; Seitz, A.E.; Steiner, C.A.; Prevots, D.R.; Williamson, P.R. Epidemiology of Cryptococcal Meningitis in the US: 1997–2009. PLoS ONE 2013, 8, e56269. [Google Scholar] [CrossRef] [Green Version]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. North Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.-A.; Slavin, M.A.; Heath, C.H.; Playford, E.G.; Byth, K.; Marriott, D.; Kidd, S.E.; Bak, N.; Currie, B.; Hajkowicz, K.; et al. Clinical Manifestations of Cryptococcus Gattii Infection: Determinants of Neurological Sequelae and Death. Clin. Infect. Dis. 2012, 55, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Charlier, C.; Dromer, F.; Lévêque, C.; Chartier, L.; Cordoliani, Y.-S.; Fontanet, A.; Launay, O.; Lortholary, O.; for the French Cryptococcosis Study Group. Cryptococcal Neuroradiological Lesions Correlate with Severity during Cryptococcal Meningoencephalitis in HIV-Positive Patients in the HAART Era. PLoS ONE 2008, 3, e1950. [Google Scholar] [CrossRef]
- Gologorsky, Y.; DeLaMora, P.; Souweidane, M.M.; Greenfield, J. Cerebellar Cryptococcoma in an Immunocompetent Child. J. Neurosurg. Pediatr. 2007, 107, 314–317. [Google Scholar] [CrossRef]
- Hospenthal, D.R.; Bennett, J.E. Persistence of Cryptococcomas on Neuroimaging. Clin. Infect. Dis. 2000, 31, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).