Abstract
Staphylococcus argenteus, a novel staphylococcal species independent of S. aureus, causes a wide spectrum of infectious diseases. As detection of this species from humans and animals has been increasingly reported worldwide, its growing virulence and drug resistance via external genetic determinants has become concerning. In this study, the prevalence and genetic characteristics of virulence factors and drug resistance determinants were investigated for 82 S. argenteus clinical isolates in Hokkaido, Japan, for a one-year period starting in August 2019. These S. argenteus isolates corresponded to 0.66% of the total number of S. aureus isolates collected in the same period. The most prevalent genotype was sequence type (ST) 2250 and staphylocoagulase (coa) genotype XId (45.1%, n = 37), followed by ST1223-coa XV (30.5%, n = 25) and ST2198-coa XIV (24.4%, n = 20). Panton-Valentine leukocidin genes (lukS-PV-lukF-PV) were identified in a single ST2250 isolate. Only ST1223 isolates had the enterotoxin gene cluster (egc-2), seb, and selw (detection rate; 100%, 60%, and 84%, respectively), while sec, sey, sel26-sel27, tst-1 were only detected in ST2250 isolates (detection rate; 10.8%, 100%, 67.6%, and 10.8%, respectively). ST2198 isolates harbored selx at a significantly higher rate (60%) than isolates of other STs. Although most of S. argenteus isolates were susceptible to antimicrobials examined, ST2198 showed higher resistance rates to penicillin, macrolides, and aminoglycosides than other STs, and it harbored various resistance genes such as blaZ, erm(C), msr(A), lnuA, and aac(6′)-Ie-aph(2″)-Ia. Only one ST2250 isolate possessed SCCmec-IVc, showing resistance to oxacillin. blaZ was the most prevalent determinant of resistance in the three STs and belonged to two plasmid groups and a chromosomal group, suggesting its diverse origin. lnu(A) in ST2198 isolates was assigned to a major cluster with various staphylococcal species. The present study indicates that the prevalence of virulence factors and drug resistance profile/determinants differ depending on the lineage (ST) of S. argenteus.
1. Introduction
Staphylococcus argenteus is a novel coagulase-positive Staphylococcus species that is genetically closely related to Staphylococcus aureus and classified within the S. aureus complex (SAC) together with S. aureus, and S. schweitzeri [1]. S. aureus and S. argenteus are widely distributed in human and animals, while S. schweitzeri has been identified mostly in animals in Africa [2,3]. S. argenteus shows non-pigmented (creamy white) colonies on blood agar due to lack of the crtOPQMN operon responsible for production of carotenoid pigment, staphyloxanthin, which is essential for protection against oxidative stress and neutrophil killing [4]. Genetically divergent S. aureus of sequence type 75 (ST75) was first described in 2002 [5], and was later assigned to S. argenteus belonging to clonal complex 75 (CC75) for clinical isolates found in the Northern Territory of Australia during a period from 2004 to 2005 [6]. Thereafter, various STs and CCs have been identified for S. argenteus, including CC1223, CC2198, CC2250, CC2596 and CC2854 [7], and more than 60 STs have been currently assigned under several clonal complexes. ST2250 is considered to be the dominant clone with widespread geographical distribution including Europe [8,9,10,11,12], Southeast Asia [13,14,15,16], China [17], and Japan [18,19,20], while ST1223, the lineage related to CC75, is less prevalent and has been described as the pathogen responsible for outbreaks of food poisoning in Japan [21,22] as well as colonizing isolates in Cambodia and the Amazonian forest [23,24].
In recent years, S. argenteus has been increasingly reported worldwide as an emerging pathogen, causing a wide spectrum of infectious diseases such as skin and soft-tissue infections (SSTIs) [6,13], necrotizing fasciitis [4], chronic prosthetic-related infection [25,26,27], bacteremia and invasive endovascular infections [14,19,28] that may have high impact of mortality [29]. It has also been documented as a nasal colonizing isolate in healthy people [30], an environmental isolate in dental clinics [31], and has even been identified in retail foods [32]. The prevalence of S. argenteus in presumptive S. aureus varies depending on geographical area, specimen type and infection site. A high detection rate (71%) among presumptive community-acquired methicillin-resistant S. aureus (CA-MRSA) isolates was documented in Northern Australia [6]. Slightly higher prevalence was reported in Thailand (19% of community-onset isolates causing sepsis [7], 4.1% from invasive infection [33]), Laos (6% in SSTIs) [13] and Myanmar (3.5% in nasal isolates from food handlers [30] and 2.9% in clinical presumptive S. aureus isolates [15]). In contrast, low prevalence (<1%) was noted in European countries [9,10,11], eastern China (0.7%) [34], and Japan (1% in blood stream infections [19] and 0.55% in various clinical isolates [20]). Although S. argenteus has usually been detected by species-specific PCR or mass spectrometric analysis, the prevalence of S. argenteus has probably been underestimated worldwide because sequencing of specific genes is essential for identification of this species.
The majority of S. argenteus clinical isolates have been found to be methicillin-sensitive, whereas methicillin-resistant isolates were mostly identified in Australia, belonging to CC75 [2,6,35,36]. In contrast, methicillin-resistant isolates have rarely been detected in other lineages (ST1223, ST2250 and ST2793) in European countries [9,11,37] and Asia [13]. SCCmec of methicillin-resistant S. argenteus has been classified mostly as type IV, and rarely, as type V [2,9,13,37]. Previous reports have found that S. argenteus clinical isolates were susceptible to most of the antimicrobials tested [2,12,30,37], and the beta-lactamase gene blaZ has often been associated with them [9,13,15]. However, resistance to non-beta-lactam antimicrobials has been noted recently [29,30,37], furthermore, the occurrence of daptomycin resistance was reported for S. argenteus detected in a complex vascular graft infection in the USA [28]. Therefore, acquisition of drug resistance by S. argenteus is an issue of growing concern. A genomic study revealed that S. argenteus possesses most of the virulence factors of S. aureus, suggesting that S. argenteus has similar pathogenic potential to that of S. aureus [17]. Similarly, many reports have highlighted the pathogenicity and clinical importance of S. argenteus as an emerging infectious agent evidenced by the presence of various pathogenic genes [8,16,20,29,30,31].
In Japan, ST1223, ST2198, ST2250 S. argenteus has been reported to date: clinical isolates (these three STs) [20], food poisoning outbreaks [21,22] and keratoconjunctivitis due to ST1223 [38], purulent lymphadenitis [18], bacteremia [19] and mycotic aortic aneurysm [39] due to ST2250. Although various infection types are known for S. argenteus, information on the epidemiological trends and genetic characteristics of this species is still limited. The current study was conducted to elucidate the prevalence of virulence and drug resistance determinants in individual clones of S. argenteus clinical isolates collected for a one-year period in Hokkaido, the most northern of the main islands of Japan.
2. Results
2.1. Identification and Prevalence of S. argenteus
In a one-year period, we identified a total of eighty-two S. argenteus isolates from 82 patients, comprising 81 methicillin-susceptible and one single methicillin-resistant isolate. The isolation ratios of all S. argenteus to all S. aureus, methicillin-susceptible S. argenteus to MSSA, and methicillin-resistant S. argenteus to MRSA were 0.0066 (82/12,510), 0.01 (81/8,132), 0.0002 (1/4,378), respectively. This rate represents the very low prevalence of S. argenteus in Japan, as reported previously [19,20].
S. argenteus isolates were derived from diverse clinical specimens as listed in Table 1. The most common isolation source was the respiratory system (sputum (n = 16), nasal discharge (n = 11), pharynx (n = 6)), followed by stool (n = 12), ear discharge (n = 10), pus and ear pus (n = 9), urine (n = 7), and vaginal discharge (n = 4). The ratio of outpatients to inpatient was 2.2. The age range of the patients was 0–98 years (average age = 49 years), with a sex ratio (male to female) of 0.64.

Table 1.
Specimens and prevalence of virulence factors in different sequence types (STs) of S. argenteus isolates (n = 82).
2.2. Classification of ST and Coagulase Genotype
Among the eighty-two S. argenteus isolates, 45.1% (n = 37) were classified as ST2250, while 30.5% (n = 25) and 24.4% (n = 20) of isolates were assigned to ST1223 and ST2198, respectively (Table 1), according to multilocus sequence typing (MLST) of S. aureus. Coagulase genotypes, coa-XV, coa-XIV, and coa-XId were identified by sequencing of partial coa genes, and were assigned to ST1223, ST2198, and ST2250 isolates, respectively. These identified STs and coa-types were the same as those in our previous study [20]. Generally, four to nine S. argenteus isolates were identified in each month during the study period, while slightly higher prevalence of all the three STs was observed in October 2019 (Table S1). Three STs were almost uniformly detected in individual months (Table S1), and there was no significant difference in specimens of isolates depending on STs (Table 1) and also virulence factors and resistance determinants as described below. A single methicillin-resistant isolate, which belonged to ST2250 (isolate id: SG99) was detected in June 2020 (Table S2).
2.3. Prevalence of Virulence Factors
All the isolates harbored alpha-, beta-, and delta-hemolysin genes (hla, hlb, hld) whereas gamma-hemolysin gene (hlg) was identified in 86.6% of total isolates (Table 1 and Table S2). Only an ST2250 isolate from stool (SG38) had Panton-Valentine leukocidin (PVL) genes (lukS-PV-lukF-PV), which was assigned to haplotype H1, and its phage type was classified as ϕPVL. None of the isolates had ACME-arcA.
Only ST1223 isolates had the enterotoxin gene cluster (egc-2; seg-sei-sem-sen-seo-seu), seb, and selw (detection rate; 100%, 60%, and 84%, respectively), while sec, sey, sel26-sel27, tst-1 were only detected in ST2250 isolates (detection rate; 10.8%, 100%, 67.6%, and 10.8%, respectively). ST2198 isolates harbored selx at a significantly higher rate (60%) than isolates of other STs. The nucleotide sequences of egc-2 determined for five isolates (Table S3) were identical, and showed 98–100% identity to those of S. argenteus strains reported previously, while slightly lower identities were evident to egc-2 components of S. aureus strains (92–99%, seg, sei, sem, seo, seu; 88–90%, sen) (Table S3). Although most of the seb genes detected in ST1223 isolates were classified as seb3, this gene of a single isolate (SG63) was assigned to the new genetic variant seb6 as well as amino acid variant v4 (Figure S1) after the variant number reported previously [40]. sea (sea2) and sec (sec3) were only detected in an ST2198 isolate and four ST2250 isolates, respectively (Table S2). TSST-1 gene (tst-1) was found in four ST2250 isolates and its sequence was identical to others reported for S. aureus strains (e.g., N315). The prevalence of the components of immune evasion cluster (IEC) (sak, chp and scn) [41] was different depending on STs. Seven isolates of ST2198 harbored IEC, while IEC-A (sea2, sak, chp, scn) and IEC-B (sak, chp and scn) were detected in one and six isolates, respectively. Both sak and scn genes devoid of chp (IEC-E) were detected in ST2250 isolates (67.6%), while only scn (sak, chp-negative) was found in 28% of ST1223 isolates. Adhesin genes ebpS, fnbA, fnbB were detected in all the isolates, and clfB and eno showed high prevalence (80–95%) in all the three STs. Although sdrC, sdrD, sdrE were found in three STs, the prevalence of sdrC and sdrE was significantly high in ST1223 (72%) and ST2250 (54.1%), respectively.
2.4. Antimicrobial Susceptibility and Prevalence of Drug Resistance Genes
Among the 82 isolates studied, only an ST2250 isolate from blood (SG99) was mecA-positive and showed resistance to oxacillin and cefoxitin (Table 2 and Table S2), according to the determination of minimum inhibitory concentrations by broth microdilution tests. Sequence analysis of its whole SCCmec region, identified its genotype as type IV, subtype c (SCCmec-IVc), with highest identity (>99%) to that of MRSA strains TCH60 and NN1 belonging to ST30 [42,43]. Ampicillin resistance was found in 19.5% (16/82) of isolates, all of which harbored blaZ gene (Table 2 and Table S2). blaZ was detected in ST2198 at a significantly higher rate (60%, 12/20) while it was distributed in all three STs.

Table 2.
Prevalence of antimicrobial resistance and resistance genes in different STs of S. argenteus isolates (n = 82).
Resistance to macrolides and lincosamides was only found in ST2198 isolates. Seven ST2198 isolates showed resistance to erythromycin, clarithromycin, and azithromycin, and harbored one or two genes of msr(A), lnu(A), and erm(C), with msr(A) being the most common (5 isolates, 25% of ST2198). Four lnu(A)-positive isolates were resistant to lincomycin, and were susceptible to clindamycin. Only an isolate (SG70) had erm(C), together with lnu(A), and showed constitutive resistance to clindamycin, which was confirmed by the D-zone test. Resistance to tetracycline was detected in an ST2250 isolate with tet(K), which was sensitive to doxycycline and minocycline. An ST1223 isolate and five ST2198 isolates that harbored aac(6′)-Ie-aph(2″)-Ia were resistant to gentamicin with high MIC levels (≥512 μg/mL), while aph (3′)-IIIa was detected in two ST2250 isolates with kanamycin resistance.
2.5. Genetic Characterization of Drug Resistance Determinants
The nucleotide sequences of aminoglycoside, macrolide, and tetracycline resistance determinants aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa, erm(C), msr(A), and tet(K) of S. argenteus isolates were almost identical (99–100% identity) to those of S. aureus and coagulase-negative staphylococcal species (Table S4). aac(6′)-Ie-aph(2″)-Ia detected in six isolates was located between IS256 (IS256-flanking pattern A [44]). The erm(C) promoter region of S. argenteus isolate SG70 lacked a 107-bp sequence containing an ORF of leader peptide (19-amino acids), which is attributable to inducible expression of erm(C) [45] (Figure S2). Similar deletion in erm(C) regulator regions was identified in many sequences of S. aureus plasmid (>70) by BLAST search (data not shown), while S. argenteus strain M260_MSHR was found to have an intact leader peptide sequence (Figure S2).
Clear genetic diversity was revealed for blaZ and lnu(A). Based on origin, blaZ genes distributed to staphylococcal species have been classified by Olsen and coworkers into the plasmid (P) group, chromosomal (C) group, and intermediate (PC) group [46]. In the present study, phylogenetic analysis of blaZ was performed using the sequence data of the current 16 S. argenteus isolates, together with those of S. aureus, S. argenteus, coagulase-negative staphylococcus, and enterococcus (total 59 strains) obtained from the GenBank database. The phylogenetic dendrogram of blaZ (Figure 1a) indicated the presence of the three genetic groups, with the P-group being divided into two subgroups (P1, P2). blaZ of S. argenteus in the present study were mostly classified into the P-group (P1 and P2), while two isolates were assigned to the C-group. blaZ sequences of S. argenteus retrieved from the GenBank database clustered mostly in the P-group, with some strains being allocated to the C- or PC-groups. blaZ from ST2198 was assigned to the P1 group (5 isolates), P2 group (6 isolates) and C group (1 isolate). The sequence identity of blaZ between the P1- and P2-groups was 97.8–99.1%, while these groups had 94.2–95.4% identity to the C-group.
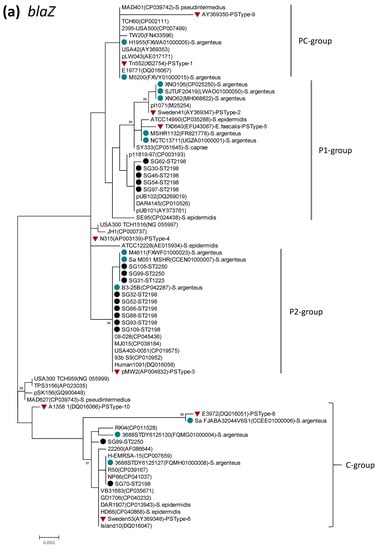
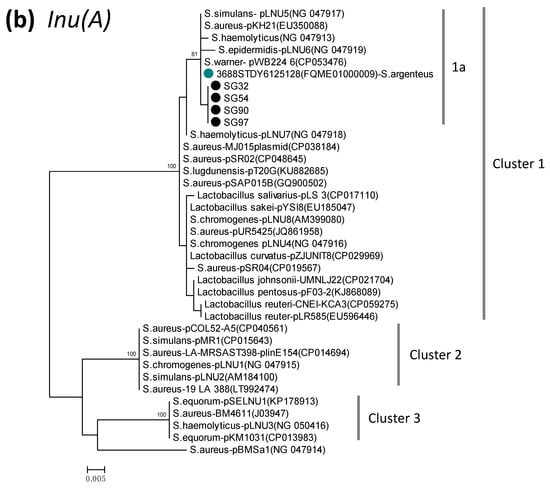
Figure 1.
Phylogenetic dendrogram of blaZ (a) and lnu(A) (b), constructed by the maximum likelihood method using MEGA X. The tree was statistically supported by bootstrapping with 1000 replicates, and genetic distances were calculated by the Kimura two-parameter model. Variation scale is provided at bottom. Percentage bootstrap support is indicated by values at each node (values <80 are omitted). Closed black and blue circles indicate S. argenteus isolates analyzed in the present study and strains available in the GenBank database, respectively. Inverted triangles denote genes of representative BlaZ protein sequence types (PS types) described previously [46], by which P (P1, P2)-, C-, and PC-groups were assigned as shown on the right (a). Cluster numbers 1–3 and subcluster 1a are shown on the right (b). The species name S. aureus was omitted (a), and GenBank accession numbers are shown in parenthesis followed by strain names.
A phylogenetic dendrogram of lnu(A) from S. argenteus and other bacterial species (Figure 1b) indicated the presence of three genetic groups (cluster 1–3). Cluster 1 was a major group including various species (98.6–99.8% identity within this cluster), and showed 92–93% identity to cluster 2 and 3. Four ST2198 S. argenteus isolates were assigned to cluster 1, and further to subcluster 1a along with only one sequence of S. argenteus strain available in GenBank (strain 3688STDY6125128, Thailand), S. aureus, and coagulase-negative staphylococcus. lnu(A) of S. aureus was dispersed in the three clusters.
Although S. argenteus isolates in the present study were susceptible to levofloxacin, four genes encoding targets of fluoroquinolone (gyrA, gyrB, grlA, grlB) were analyzed for their diversity from S. aureus. The sequence of these genes were determined for five isolates belonging to three STs, and were analyzed with the sequence data of S. argenteus strains, MSHR1132 and XNO106. Within the S. argenteus strains, the nucleotide sequence identity of the four genes was 99.6–100%, while S. argenteus showed 93.4% identity of gyrA/gyrB and 87.7% identity of grlA/grlB to those of S. aureus strains. Despite such diversity, deduced amino acid sequences of the quinolone-resistance determining region in the four proteins (GyrA, GyrB, ParC, ParE) were identical in S. argenteus and fluoroquinolone-susceptible S. aureus strains (N315, NCTC8325) (Figure S3) [47,48,49].
3. Discussion
In the present study, the prevalence and genetic characteristics of S. argenteus clinical isolates were investigated for a one-year period in northern Japan. The ratio of S. argenteus to all the S. aureus clinical isolates was 0.0066, which was comparable to that in our preceding study (0.0055, March–June, 2019) [20]. Among the three STs identified, ST2250 accounted for approximately half of the isolates, while the remaining was split into ST1223 (30.5%) and ST2189 (24.4%). A similar proportion of the three STs was also found previously at the same study site [20]. Moreover, S. argenteus of the three STs was isolated almost equally in each month throughout the year. In Japan, ST1223 and ST2250 S. argenteus have been reported in various types of infectious diseases, with ST1223 being mainly detected in food poisoning outbreaks [18,19,21,22,38,39]. While the predominance of ST2250 has been reported worldwide, ST1223 and ST2198 were less frequently detected in Asian countries [7,13,14,15,16,17,33]. In a recent study in Taiwan of blood isolates, ST1223 and ST2198 accounted for 11% and 2%, respectively [16]. Thus, our present and previous study [20] may indicate a distinct epidemiological feature of S. argenteus clones in northern Japan, i.e., relatively higher prevalence of ST1223 and ST2189. Higher mortality risk was reported for bacteremia due to S. argenteus compared to MSSA in Taiwan [29]. However, in our present study, most isolates were derived from specimens other than blood, and information about patients’ mortality rate was not available. Thus, the clinical impact of S. argenteus infection was not able to be evaluated.
It was notable in the present study that distribution of staphylococcal enterotoxin (SE)-like genes was distinctive depending on the three STs; seb, egc-2, and selw in ST1223, sey and sel26-sel27 in ST2250, and the dominance of selx in ST2198. Similar findings regarding the correlation of ST and toxin genes have been reported previously for S. argenteus isolates in Japan [19,20,21,22], Taiwan [16] and Myanmar [15]. Our study on the prevalence of newer SE(-like) genes in CA-MRSA revealed lower detection rates of selz (5.6%) and sel26-sel27 (0%) [50]. In contrast, S. argenteus isolates in the current study showed a higher prevalence (selz, 23.2%; sel26-sel27, 30.5%), indicating that these SE-like genes are more specifically distributed to S. argenteus. Differences in the prevalence of virulence factors may be related to pathogenicity and the clinical symptoms caused by infection with each ST. For example, the ST1223 strain has been reported as a pathogen of food poisoning, probably due to seb and egc-2 [21,22], and these genes were more commonly detected in ST1223 than other STs in isolates from retail food [32]. PVL genes in ΦPVL were detected in an ST2250 isolate from stool sample in our study, as first report in S. argenteus in Japan. Despite their very low prevalence in S. argenteus, PVL genes have been detected in ST2250 and ST2277 (related to ST2250) isolates from blood samples (sepsis) in France, in cases with an epidemiological link to Mayotte [8], and in 16% of S. argenteus isolates (all ST2250) from sepsis in Thailand [7]. Meanwhile, PVL genes were detected in S. argenteus isolates belonging to ST2250 from the nasal cavity of healthy humans in Myanmar [30] and the United Arab Emirates [31]. Although there is still little information, it is possible that the major clone ST2250 may rarely acquire PVL phage in regions where PVL-positive S. aureus is commonly distributed (e.g., Southeast Asia [51,52]), and spread locally via the movement of healthy carriers. Further evaluation may be necessary to investigate the clinical impact of PVL-positive S. argenteus, because it has been implicated in severe symptoms such as sepsis.
Although S. argenteus has been described as having the most virulence genes of S. aureus [17], there seems to be a distinct difference in prevalence of virulence factors depending on the lineages of these staphylococcal species. For example, sek, seq and speG were commonly found in PVL-positive ST8-SCCmecIVa (USA300 clone), and sec, sel, sep, tst-1, sasL were widely distributed among PVL-negative ST8-SCCmecIVl, while seb, tst-1 and egc-2 were associated with CC5 (ST5/ST764) MRSA isolates in both community and hospital settings [53,54,55]. In contrast, sec and tst-1 were rarely found, and sek, seq, sasL and speG were not identified in S. argenteus isolates [20,30].
Methicillin-resistant S. argenteus has been primarily identified in Australia as a common cause of community-onset skin infections, accounting for approximately 70% of presumptive MRSA isolates, and belongs to CC75 (ST75, ST1850), having mostly SCCmec-IV, rarely SCCmec-V [4,5,6,35]. mecA-positive S. argenteus strains were reported at very low frequency in European countries (UK, Belgium, Sweden) [1,2,9,11,37], revealing ST1223, ST2250, ST2793, and ST3240 (single-locus variant of ST2250) isolates with SCCmec-IV. In contrast, in Southeast Asia, methicillin-resistant S. argenteus has scarcely been detected despite prevalent areas of S. argenteus, following Australia, except for a single isolate of ST2250-SCCmec-IV in Lao PDR [13]. Although there had been no reports in Eastern Asia, in our study, methicillin-resistant S. argenteus isolate was first identified in Japan, and its genotype was clarified as ST2250/SCCmec-IV, the same trait as those of isolates in Belgium and Lao PDR [9,13]. Type IV SCCmec is known as a major type of community-acquired MRSA and is distributed to various clones including ST1, ST8, ST30, ST59, ST72, and ST80, among which ST30-SCCmec-IV was originally widely distributed to Asia, followed by spread to European countries [56,57]. In our study, SCCmec of the S. argenteus was assigned to subtype c (SCCmec-IVc) based on the J1 region of SCCmec [58], and the whole SCCmec sequence was almost identical to that of strains TCH60 (CC30, USA) [42] and NN1 (ST30, Japan) [43]. Actually, SCCmec-IVc was the most commonly found in ST30 isolates [59]. These findings suggested that the methicillin-resistant S. argenteus in our study in Japan may be close related to ST30 MRSA, which is locally distributed. In contrast, the presence of SCCmec-IVa was described for CC75 (ST1850) strain MSHR1132 in Australia [4], suggesting that the origin of SCCmec in ST1850 might be different from that of ST2250 S. argenteus.
Drug resistance rates in S. argenteus have been described as lower than those in S. aureus, while penicillin resistant isolates due to blaZ are common [2,7,12,13,15,29]. Although similar finding was observed in the present study, it was noted that ST2198 isolates exhibited significantly higher resistance rates than other STs to penicillin, macrolide-lincosamide, and aminoglycosides, associated with responsible resistance genes that have almost identical sequences to those known for S. aureus and other staphylococci. It is suggested that the higher prevalence of drug resistance is ascribable to either clonal spread of resistant strains among ST2198, or the unknown potential of ST2198 to acquire drug resistance genes via plasmid from other staphylococcal species.
Detection of blaZ was described in different clones (STs) of S. argenteus [12,13,14]. Phylogenetic analysis of blaZ in the present study indicated that blaZ in S. argenteus belongs mostly to the plasmid group, while some were assigned to the chromosomal group, irrespective of ST. Furthermore, blaZ of the plasmid group was discriminated into two distinct groups. These findings may suggest that blaZ of S. argenteus is of multiple origin, with most of them being derived from different plasmids.
Lincosamide resistance of S. argenteus in our study was considered to be mediated by lnu(A), which encodes lincosamide nucleotidyltransferase [60]. This gene has been rarely reported in S. argenteus, but was identified in four ST2198 isolates in the present study and was phylogenetically clustered with only one available S. argenteus gene in the GenBank database (the strain in Thailand), and also S. aureus and coagulase-negative staphylococci in a major cluster. Since lnu(A) in staphylococci is located in plasmid [60], it was suggested that plasmid carrying the major type of lnu(A) might have been transmitted to S. argenteus from other staphylococcal species. The erythromycin ribosomal methylase gene erm(C) is most widely distributed in staphylococcus via plasmid and is responsible for macrolide and lincosamide resistance [61], however, it has rarely been found in S. argenteus, except in strain M260_MSHR in Australia [1] and SG70 in the present study. Expression of erm(C) is inducible by the presence of macrolides when erm(C) gene is preceded by a short leader cistron (ermCL; ORF of 19 amino acid-peptide) [62]. S. argenteus strain M260_MSHR has the intact ermCL (Figure S2). However, mutations in the regulator region of erm(C), including deletion of ermCL, have been revealed to cause constitutive resistance to macrolides and lincosamides [45,62,63]. S. argenteus strain SG70 showed constitutive resistance to clindamycin, associated with deletion of 107-bp sequence containing ermCL in the erm(C) promoter region. Although this type of deletion mutation in erm(C) promoter has not been described in experimentally selected resistant clones, it might have already been widely distributed to S. aureus (e.g., USA300_FPR3757, Figure S2) as evidenced by the BLAST search in the present study.
In the present study, low rates of resistance were confirmed to tetracyclines and aminoglycosides due to efflux protein defined by tet(K) and aminoglycoside modifying enzymes encoded by aac(6′)-Ie-aph(2″)-Ia and aph(3′)-IIIa, respectively. Although similar findings were reported previously [7,20], no such resistance was observed for clinical isolates in other studies [7,12,19]. However, higher prevalence of tetracycline resistance [29] and tet(L)/aph(3′)-III [14] was described for blood isolates in Taiwan and Thailand, respectively. Notably, relatively high resistance rates to multiple antimicrobials including tetracycline/aminoglycoside were documented for S. argenteus from retail food (mostly meat and fish) [32]. Thus, the prevalence of tetracycline/aminoglycoside resistance in clinical S. argenteus of isolates should be carefully monitored, and attention should be given to animals and foodstuff as potential sources of drug resistance genes or resistant clones to humans. Fluoroquinolone resistance in S. argenteus clinical isolates has hardly been detected, while ciprofloxacin resistance was reported in isolates from retail food (meat) at very low rates [32]. In our present study, deduced QRDR amino acid sequences of DNA gyrase and topoisomerase IV subunits of fluoroquinolone-susceptible S. argenteus were confirmed to be identical to those of fluoroquinolone-susceptible S. aureus, despite evident genetic diversity (88–93% identity) of these proteins in these two species. This finding suggests that the target sites of fluoroquinolone in S. argenteus may be identical to those of S. aureus, and therefore, fluoroquinolone resistance in S. argenteus will probably be caused by mutation in QRDR as described for S. aureus [47,48,49].
In conclusion, here we have described the constant prevalence of three STs of S. argenteus clinical isolates, the distinctive distribution of virulence factors and drug resistance genes in the three STs based on a one-year surveillance study in northern Japan, and identified PVL-positive and SCCmec-IVc-positive isolates. Because the occurrence and potential spread of drug resistance/resistance genes in S. argenteus are of concern, further epidemiological study is necessary to monitor the phenotypic and genetic traits of this staphylococcal species.
4. Materials and Methods
4.1. Bacterial Isolates, Species Identification
S. argenteus were isolated from various clinical specimens that were submitted to the Sapporo Clinical Laboratory Inc., Sapporo, Japan, from medical facilities in Hokkaido for one year between August 2019 and July 2020. The clinical specimens were inoculated onto blood agar plates incubated aerobically at 37 °C for 24 h. Gram-positive, coagulase-positive isolates were collected for further study by bacteriological identification. Initial screening of S. argenteus was performed by MALDI-TOF mass spectrometry using MALDI Biotyper (BRUKER). Isolates assigned as S. argenteus were confirmed genetically, by PCR and sequencing of NRPS and thermostable nuclease gene (nuc) using previously reported primers and conditions [17,20]. During the same study period, the number of non-duplicate S. aureus isolates (single isolate per patient) was 12,510, which contained 8132 methicillin-susceptible S. aureus (MSSA) and 4378 MRSA isolates.
4.2. Antimicrobial Susceptibility Testing
Minimum inhibitory concentrations (MICs) within limited ranges were measured by broth microdilution tests against 18 antimicrobial agents (oxacillin, OXA; ampicillin, AMP; cefazolin, CFZ; cefmetazole, CMZ; flomoxef, FMX; imipenem, IPM; gentamicin, GEN; arbekacin, ABK; erythromycin, ERY; clindamycin, CLI; vancomycin, VAN; teicoplanin, TEC; linezolid, LZD; minocycline, MIN; Fosfomycin, FOF; levofloxacin, LVX; cefoxitin, FOX; trimethoprim/sulfamethoxazole, SXT) by using the Dry Plate ‘Eiken’ DP32 (Eiken Chemical, Tokyo, Japan). In addition, MIC was manually measured by broth microdilution for OXA, GEN, ERY, azithromycin (AZM), clarithromycin (CLA), doxycycline (DOX), kanamycin (KAN), lincomycin (LIN), quinupristin-dalfopristin (Q-D), tetracycline (TET), and virginiamycin/pristinamycin (V-G). Resistance or susceptibility was judged according to the breakpoints defined in the Clinical Laboratory Standards Institute (CLSI) guidelines [64] for most of the antimicrobial drugs examined. For fosfomycin and arbekacin, whose break points are not defined by CLSI guidelines, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint (FOF, 32 μg/mL, Staphylococcus spp.) [65] and a unique breakpoint (ABK, 4 μg/mL, which is higher than the 2 μg/mL defined by the Japanese Society of Chemotherapy for respiratory infection) [66] were used. For flomoxef, a breakpoint defined by the Japanese Society of Chemotherapy for urinary tract infection (16 μg/mL) [66] was applied.
4.3. Genetic Typing, Detection of Virulence Factors and Drug Resistance Genes
For all the isolates, the presence of staphylococcal 16s rRNA, nuc, mecA, PVL genes and ACME-arcA (arginine deiminase gene) was examined by multiplex PCR assay as described by Zhang et al. [67]. Sequence type (ST) was assigned according to multilocus sequence typing (MLST) https://pubmlst.org/ (accessed on 20 December 2020) [68]. Staphylocoagulase genotype (coa) was determined by partial sequencing of staphylocoagulase gene (D1, D2, and the central regions) and their highly similar staphylocoagulase sequences were searched by the Basic Local Alignment Search Tool (BLAST: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 December 2020)) as described previously [69].
The presence of 28 staphylococcal enterotoxin (SE) (-like) genes (sea-see, seg-selu, selx, sely, selw, selz, sel26 and sel27), the TSST-1 gene (tst-1) and exfoliative toxin genes (eta, etb and etd), leukocidins (lukDE and lukM), haemolysins (hla, hlb, hld and hlg), adhesin genes (eno, cna, sdrC, sdrD, sdrE, fib, clfA, clfB, fnbA, fnbB, icaA, icaD, ednA, ednB, bap and vWbp), and modulators of host defense (sak, chp and scn) were analyzed by multiplex or uniplex PCRs [15,20,30,50]. PVL-encoding phage (ϕ108, ϕPVL, ϕSa2958, ϕSa2MW, ϕSLT, ϕTCH60, ϕSa2usa, and ϕSa119) for a PVL-positive isolate was classified by multiplex or uniplex PCR as described previously [51], the full-length lukS-PV/lukF-PV sequences were determined by PCR and direct sequencing of PCR products. PVL haplotypes based on single-nucleotide polymorphism profiles were assigned according to the previously reported [51].
Genes conferring resistance to penicillin (blaZ), tetracycline (tet(K), tet(L), and tet(M)), macrolides-lincosamides-streptogramins (erm(A), erm(B), erm(C), erm(F), erm(T), erm(X), erm(Y), msr(A), lnu(A), lnu(B), vgaA, vgaB, vgaD, vgbA, vgbB, vatA, vatB, vatC, vatD, vatE, vatG, IsaA, IsaE, mefA/E), aminoglycoside (aminoglycoside modifying enzyme (AME) genes; aac(6′)-Im, aac(6′)-Ie-aph(2″)-Ia, ant(3″)-Ia, ant(4′)-Ia, ant(6)-Ia, ant(9)-Ia, ant(9)-Ib, aph(2″)-Ib, aph(2″)-Ic, aph(2″)-Id, and aph(3′)-IIIa), linezolid (optrA), chloramphenicol (cfr) were detected by uniplex or multiplex PCR using the primers previously reported [44,54,70,71] and newly designed in this study (Table S5). For isolates harboring erm gene, inducible macrolide-resistance phenotype was confirmed by the D-zone test, in which erythromycin and clindamycin disks are placed in an adjacent position 15 mm apart on Mueller-Hinton agar plate [64].
4.4. Sequencing and Phylogenetic Analysis of Drug Resistance Genes and Enterotoxin Genes
Nucleotide sequences of full-length ORF were determined for blaZ, lnu(A), erm(C), msr(A), AME genes, gyrA/B, parC/E genes by PCR with primers listed in Table S5, followed by Sanger sequencing using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA) on an automated DNA sequencer (ABI PRISM 3100). Entire ORF regions of individual genes were covered by PCR products. Accuracy of sequence data obtained from DNA sequencer was checked by referring to chromatogram data. Similarly, nucleotide sequences of the whole region of SCCmec, enterotoxin genes detected (sea, seb, sec, egc-2 cluster, selx, sey, selw, sel26-sel27), tst-1, and PVL genes were also determined. Phylogenetic dendrograms of drug resistance genes were constructed by the maximum likelihood method using the MEGA X software, together with sequence data of staphylococcal strains available in the GenBank database. The dendrograms were statistically supported by bootstrapping with 1000 replicates. Multiple alignment of nucleotide/amino acid sequences determined in the present study and those retrieved from the GenBank database was performed by the Clustal Omega program https://www.ebi.ac.uk/Tools/msa/clustalo/ (accessed on 20 December 2020), which was also used for calculation of sequence identity. All the sequence data of S. argenteus genes (lukS-PV/lukF-PV, sea, seb, sec, egc-2 cluster, selx, sey, selz, sel26, sel27, tst-1, blaZ, SCCmec, erm(C), msr(A), lnu(A), tet(K), aac(6′)-Ie-aph(2″)-Ia, gyrA/B, parC/E) determined in this study were deposited in the GenBank database under the accession numbers shown in Table S6.
4.5. Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics ver.26. The Chi-square test was used to analyze the differences in prevalence of bacterial attribute information among identified STs. A p-value < 0.05 was considered statistically significant.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/10/2/163/s1, Figure S1: Alignment of nucleotide (a) and amino acid (b) sequences of SEB, including nucleotide variants v1-v6 and amino acid variants v1-v4, Figure S2: Alignment of repL, erm(C) and its promoter region of S. aureus strain USA300_FPR3757 (plasmid pSA03) and USA300-SUR11 (plasmid pUSA05-1-SUR11), S. argenteus strain SaM260_MSHR and SG70 (present study), Figure S3: Alignment of partial GyrA, GyrB, ParC, and ParE amino acid sequences including quinolone-resistance determining regions (QRDR) of S. argenteus strains (SG95, SG99, SG103, XNO106, and MSHR1132) and S. aureus strains (N315 and NCTC8325) that are fluoroquinolone-susceptible. Table S1: Monthly incidence of three STs of S. argenteus, Table S2: Drug resistance profile and prevalence of virulence factors/drug resistance genes in 82 S. argenteus isolates in Hokkaido, Japan for one year-period (August 2019–July 2020), Table S3: Sequence identity of component genes (seg-sei-sem-sen-seo-seu) in egc-2 of S. argenteus SG48 in the present study to those of S. argenteus, S. aureus and S. schweitzeri isolates reported previously, Table S4: Nucleotide sequence identity of drug resistance genes detected in the present study to those of S.aureus and other staphylococcal species and enterococcus retrieved from GenBank database, Table S5: Primers used in this study, Table S6: GenBank accession numbers assigned to lukS-PV-lukF-PV, sea, seb, sec, egc-2, tst-1, blaZ, SCCmec complex, erm(C), msr(A), lnu(A), tet(K), aac(6′)-Ie-aph(2″)-Ia, gyrA/B, parC/E genes detected in the present study.
Author Contributions
Conceptualization, M.S.A. and N.K.; methodology, M.S.A. and N.K.; investigation, M.S.A., N.U., M.K. and M.H.; resources, M.I. (Miyo Ike) and M.I. (Masahiko Ito); data curation, M.S.A. and N.K.; writing—original draft preparation, M.S.A.; writing—review and editing, M.S.A. and N.K.; supervision, N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS (Japan Society for the Promotion of Science) KAKENHI Grant No. 20H03933.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tong, S.Y.; Schaumburg, F.; Ellington, M.J.; Corander, J.; Pichon, B.; Leendertz, F.; Bentley, S.D.; Parkhill, J.; Holt, D.C.; Peters, G.; et al. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: The non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Schaumburg, F.; Kearns, A.; Larsen, A.R.; Lindsay, J.A.; Skov, R.L.; Westh, H. Implications of identifying the recently defined members of the Staphylococcus aureus complex S. argenteus and S. schweitzeri: A position paper of members of the ESCMID Study Group for Staphylococci and Staphylococcal Diseases (ESGS). Clin. Microbiol. Infect. 2019, 25, 1064–1070. [Google Scholar] [CrossRef]
- Schuster, D.; Rickmeyer, J.; Gajdiss, M.; Thye, T.; Lorenzen, S.; Reif, M.; Josten, M.; Szekat, C.; Melo, L.D.R.; Schmithausen, R.M.; et al. Differentiation of Staphylococcus argenteus (formerly: Staphylococcus aureus clonal complex 75) by mass spectrometry from S. aureus using the first strain isolated from a wild African great ape. Int. J. Med. Microbiol. 2017, 307, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.C.; Holden, M.T.; Tong, S.Y.; Castillo-Ramirez, S.; Clarke, L.; Quail, M.A.; Currie, B.J.; Parkhill, J.; Bentley, S.D.; Feil, E.J.; et al. A very early-branching Staphylococcus aureus lineage lacking the carotenoid pigment staphyloxanthin. Genome Biol. Evol. 2011, 3, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Okuma, K.; Iwakawa, K.; Turnidge, J.D.; Grubb, W.B.; Bell, J.M.; O’Brien, F.G.; Coombs, G.W.; Pearman, J.W.; Tenover, F.C.; Kapi, M.; et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 2002, 40, 4289–4294. [Google Scholar] [CrossRef]
- McDonald, M.; Dougall, A.; Holt, D.; Huygens, F.; Oppedisano, F.; Giffard, P.M.; Inman-Bamber, J.; Stephens, A.J.; Towers, R.; Carapetis, J.R.; et al. Use of a single-nucleotide polymorphism genotyping system to demonstrate the unique epidemiology of methicillin-resistant Staphylococcus aureus in remote aboriginal communities. J. Clin. Microbiol. 2006, 44, 3720–3727. [Google Scholar] [CrossRef]
- Chantratita, N.; Wikraiphat, C.; Tandhavanant, S.; Wongsuvan, G.; Ariyaprasert, P.; Suntornsut, P.; Thaipadungpanit, J.; Teerawattanasook, N.; Jutrakul, Y.; Srisurat, N.; et al. Comparison of community-onset Staphylococcus argenteus and Staphylococcus aureus sepsis in Thailand: A prospective multicentre observational study. Clin. Microbiol. Infect. 2016, 22, 458.e11–458.e19. [Google Scholar] [CrossRef] [PubMed]
- Dupieux, C.; Blonde, R.; Bouchiat, C.; Meugnier, H.; Bes, M.; Laurent, S.; Vandenesch, F.; Laurent, F.; Tristan, A. Community-acquired infections due to Staphylococcus argenteus lineage isolates harboring the Panton-Valentine leucocidin, France, 2014. Eur. Surveill. 2015, 20, 21154. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Dodémont, M.; Vandendriessche, S.; Rottiers, S.; Tribes, C.; Roisin, S.; de Mendonça, R.; Nonhoff, C.; Deplano, A.; Denis, O. Low occurrence of the new species Staphylococcus argenteus in a Staphylococcus aureus collection of human isolates from Belgium. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1017–1022. [Google Scholar] [CrossRef]
- Hansen, T.A.; Bartels, M.D.; Høgh, S.V.; Dons, L.E.; Pedersen, M.; Jensen, T.G.; Kemp, M.; Skov, M.N.; Gumpert, H.; Worning, P.; et al. Whole genome sequencing of Danish Staphylococcus argenteus reveals a genetically diverse collection with clear separation from Staphylococcus aureus. Front. Microbiol. 2017, 8, 1512. [Google Scholar] [CrossRef]
- Tång Hallbäck, T.E.; Karami, N.; Adlerberth, I.; Cardew, S.; Ohlén, M. Methicillin-resistant Staphylococcus argenteus misidentified as methicillin-resistant Staphylococcus aureus emerging in western Sweden. J. Med. Microbiol. 2018, 67, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Alhussein, F.; Fürstenberg, J.; Gaupp, R.; Eisenbeis, J.; Last, K.; Becker, S.L.; Papan, C. Human infections caused by Staphylococcus argenteus in Germany: Genetic characterisation and clinical implications of novel species designation. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2461–2465. [Google Scholar] [CrossRef]
- Yeap, A.D.; Woods, K.; Dance, D.A.B.; Pichon, B.; Rattanavong, S.; Davong, V.; Phetsouvanh, R.; Newton, P.N.; Shetty, N.; Kearns, A.M. Molecular epidemiology of Staphylococcus aureus skin and soft tissue infections in the Lao People’s Democratic Republic. Am. J. Trop. Med. Hyg. 2017, 97, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Moradigaravand, D.; Jamrozy, D.; Mostowy, R.; Anderson, A.; Nickerson, E.K.; Thaipadungpanit, J.; Wuthiekanun, V.; Limmathurotsakul, D.; Tandhavanant, S.; Wikraiphat, C.; et al. Evolution of the Staphylococcus argenteus ST2250 clone in Northeastern Thailand is linked with the acquisition of livestock-associated staphylococcal genes. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; San, T.; San, N.; Oo, W.M.; Ko, P.M.; Thet, K.T.; Urushibara, N.; Kawaguchiya, M.; Sumi, A.; Kobayashi, N. Molecular characterization of Staphylococcus argenteus in Myanmar: Identification of novel genotypes/clusters in staphylocoagulase, protein Aalpha-haemolysin and other virulence factors. J. Med. Microbiol. 2019, 68, 95–104. [Google Scholar] [CrossRef]
- Hsu, J.C.; Wan, T.W.; Lee, H.; Wang, X.M.; Lin, Y.T.; Jung, C.J.; Lee, T.F.; Hsueh, P.R.; Teng, L.J. Heterogeneity of Molecular Characteristics among Staphylococcus argenteus Clinical Isolates (ST2250, ST2793, ST1223, and ST2198) in Northern Taiwan. Microorganisms 2020, 8, 1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-F.; Xu, X.; Song, Q.; Bai, Y.; Zhang, Y.; Song, M.; Shi, C.; Shi, X. Identification of Staphylococcus argenteus in Eastern China based on a nonribosomal peptide synthetase (NRPS) gene. Future Microbiol. 2016, 11, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Shinjoh, M.; Ohara, H.; Kawai, T.; Kamimaki, I.; Mizushima, R.; Kamada, K.; Itakura, Y.; Iguchi, S.; Uzawa, Y.; et al. Purulent lymphadenitis caused by Staphylococcus argenteus, representing the first Japanese case of Staphylococcus argenteus (multilocus sequence type 2250) infection in a 12-year-old boy. J. Infect. Chemother. 2018, 24, 925–927. [Google Scholar] [CrossRef]
- Kitagawa, H.; Ohge, H.; Hisatsune, J.; Masuda, K.; Aziz, F.; Hara, T.; Kuroo, Y.; Sugai, M. Low incidence of Staphylococcus argenteus bacteremia in Hiroshima, Japan. J. Infect. Chemother. 2019, 26, 140–143. [Google Scholar] [CrossRef]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Sumi, A.; Takahashi, S.; Ike, M.; Ito, M.; Habadera, S.; Kobayashi, N. Molecular Epidemiological Characterization of Staphylococcus argenteus Clinical Isolates in Japan: Identification of Three Clones (ST1223, ST2198, and ST2550) and a Novel Staphylocoagulase Genotype XV. Microorganisms 2019, 7, 389. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kubota, H.; Ono, H.K.; Kobayashi, M.; Murauchi, K.; Kato, R.; Hirai, A.; Sadamas, K. Food poisoning outbreak in Tokyo, Japan caused by Staphylococcus argenteus. Int. J. Food Microbiol. 2017, 262, 31–37. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Umeda, K.; Yonog, I.S.; Nakamura, H.; Yamamoto, K.; Kumeda, Y.; Kawatsu, K. Staphylococcal food poisoning caused by Staphylococcus argenteus harboring staphylococcal enterotoxin genes. Int. J. Food Microbiol. 2018, 265, 23–29. [Google Scholar] [CrossRef]
- Ruimy, R.; Armand-Lefevre, L.; Barbier, F.; Ruppe, E.; Cocojaru, R.; Mesli, Y.; Maiga, A.; Benkalfat, M.; Benchouk, S.; Hassaine, H.; et al. Comparisons between geographically diverse samples of carried Staphylococcus aureus. J. Bacteriol. 2009, 191, 5577–5583. [Google Scholar] [CrossRef]
- Ruimy, R.; Angebault, C.; Djossou, F.; Dupont, C.; Epelboin, L.; Jarraud, S.; Lefevre, L.A.; Bes, M.; Lixandru, B.E.; Bertine, M.; et al. Are host genetics the predominant determinant of persistent nasal Staphylococcus aureus carriage in humans? J. Infect. Dis. 2010, 202, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; You, B.; Tan, L.; Yu, S.; Li, H.; Bai, G.; Li, S.; Rao, X.; Xie, Z.; Shi, X.; et al. Clinical Staphylococcus argenteus Develops to Small Colony Variants to Promote Persistent Infection. Front. Microbiol. 2018, 9, 1347. [Google Scholar] [CrossRef]
- Diot, A.; Dyon-Tafani, V.; Bergot, M.; Tasse, J.; Martins-Simões, P.; Josse, J.; Valour, F.; Laurent, F. Investigation of a Staphylococcus argenteus Strain Involved in a Chronic Prosthetic-Joint Infection. Int. J. Mol. Sci. 2020, 21, 6245. [Google Scholar] [CrossRef] [PubMed]
- Söderquist, B.; Wildeman, P.; Stenmark, B.; Stegger, M. Staphylococcus argenteus as an etiological agent of prosthetic hip joint infection: A case presentation. Bone Jt. Infect. 2020, 5, 172–175. [Google Scholar] [CrossRef]
- Hao, S.; Abdelghany, M.; Lyden, A.; Sit, R.; Tan, M.; Tato, C.M.; DeRisi, J.L.; Miller, S.; Doernberg, S.B.; Langelier, C. Genomic Profiling of Evolving Daptomycin Resistance in a Patient with Recurrent Staphylococcus argenteus Sepsis. Antimicrob. Agents Chemother. 2020, 64, e00961-20. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lee, H.; Wang, X.M.; Lee, T.F.; Liao, C.H.; Teng, L.J.; Hsueh, P.R. High mortality impact of Staphylococcus argenteus on patients with community-onset staphylococcal bacteraemia. Int. J. Antimicrob. Agents 2018, 52, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; San, T.; Aye, M.M.; Mya, S.; Maw, W.W.; Zan, K.N.; Htut, W.H.W.; Kawaguchiya, M.; Urushibara, N.; Kobayashi, N. Prevalence and genetic characteristics of Staphylococcus aureus and Staphylococcus argenteus isolates harboring Panton-Valentine leukocidin, enterotoxins, and TSST-1 genes from food handlers in Myanmar. Toxins 2017, 9, 241. [Google Scholar] [CrossRef]
- Senok, A.; Nassar, R.; Kaklamanos, E.G.; Belhoul, K.; Fanas, S.A.; Nassar, M.; Azar, A.J.; Müller, E.; Reissig, A.; Gawlik, D.; et al. Molecular Characterization of Staphylococcus aureus Isolates Associated with Nasal Colonization and Environmental Contamination in Academic Dental Clinics. Microb. Drug Resist. 2020, 26, 661–669. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, F.; Dai, J.; Pang, R.; Zhang, J.; Zeng, H.; Gu, Q.; Zhang, S.; Zhang, Y.; et al. Staphylococcus argenteus isolated from retail foods in China: Incidence, antibiotic resistance, biofilm formation and toxin gene profile. Food Microbiol. 2020, 91, 103531. [Google Scholar] [CrossRef] [PubMed]
- Thaipadungpanit, J.; Amornchai, P.; Nickerson, E.K.; Wongsuvan, G.; Wuthiekanun, V.; Limmathurotsakul, D.; Peacock, S.J. Clinical and molecular epidemiology Staphylococcus argenteus infections in Thailand. J. Clin. Microbiol. 2015, 53, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Zhi, X.Y.; Zhang, J.; Paoli, G.C.; Cui, Y.; Shi, C.; Shi, X. Preliminary comparative genomics revealed pathogenic potential and international spread of Staphylococcus argenteus. BMC Genom. 2017, 18, 808. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Sharma-Kuinkel, B.K.; Thaden, J.T.; Whitney, A.R.; Yang, S.J.; Mishra, N.N.; Rude, T.; Lilliebridge, R.A.; Selim, M.A.; Ahn, S.H.; et al. Virulence of endemic nonpigmented northern Australian Staphylococcus aureus clone (clonal complex 75, S. argenteus) is not augmented by staphyloxanthin. J. Infect. Dis. 2013, 208, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.A.; Coombs, G.W.; Nimmo, G.R. Staphylococcus aureus ‘Down Under’: Contemporary epidemiology of S. aureus in Australia, New Zealand, and the South West Pacific. Clin. Microbiol. Infect. 2014, 20, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Giske, C.G.; Dyrkell, F.; Arnellos, D.; Vestberg, N.; Hermansson Panna, S.; Fröding, I.; Ullberg, M.; Fang, H. Transmission events and antimicrobial susceptibilities of methicillin-resistant Staphylococcus argenteus in Stockholm. Clin. Microbiol. Infect. 2019, 25, 1289.e5–1289.e8. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Sasaki, M.; Imai, W.; Kato, M.; Maehara, C.; Yasui, K.; Fukuzawa, S.; Murakami, H.; Kakisu, K.; Hori, Y.; et al. Bacterial keratoconjunctivitis caused by Staphylococcus argenteus belonging to sequence type 1223 isolated in Japan. J. Infect. Chemother. 2020, 26, 1002–1004. [Google Scholar] [CrossRef]
- Mitsutake, K.; Noriyuki Watanabe, N.; Haruka Karaushi, H.; Tarumoto, N.; Sachie Koyama, S.; Yasuhiro Ebihara, Y.; Yoshitake, A.; Hiroyuki Nakajima, H. Thoracic aortic mycotic aneurysm due to Staphylococcus argenteus: A case report. J. Infect. Chemother. 2020, 26, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Sihto, H.M.; Macori, G.; Stephan, R. Sequence Variability in Staphylococcal Enterotoxin Genes seb, sec, and sed. Toxins 2016, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Verkaik, N.J.; Benard, M.; Boelens, H.A.; de Vogel, C.P.; Nouwen, J.L.; Verbrugh, H.A.; Melles, D.C.; van Belkum, A.; van Wamel, W.J. Immune evasion cluster-positive bacteriophages are highly prevalent among human Staphylococcus aureus strains, but they are not essential in the first stages of nasal colonization. Clin. Microbiol. Infect. 2011, 17, 343–348. [Google Scholar] [CrossRef]
- McGavin, M.J.; Arsic, B.; Nickerson, N.N. Evolutionary blueprint for host- and niche-adaptation in Staphylococcus aureus clonal complex CC30. Front. Cell Infect. Microbiol. 2012, 2, 48. [Google Scholar] [CrossRef]
- Taneike, I.; Otsuka, T.; Dohmae, S.; Saito, K.; Ozaki, K.; Takano, M.; Higuchi, W.; Takano, T.; Yamamoto, T. Molecular nature of methicillin-resistant Staphylococcus aureus derived from explosive nosocomial outbreaks of the 1980s in Japan. FEBS Lett. 2006, 580, 2323–2334. [Google Scholar] [CrossRef][Green Version]
- Roy, S.; Aung, M.S.; Paul, S.K.; Ahmed, S.; Haque, N.; Khan, E.R.; Barman, T.K.; Islam, A.; Abedin, S.; Sultana, C.; et al. Drug Resistance Determinants in Clinical Isolates of Enterococcus faecalis in Bangladesh: Identification of Oxazolidinone Resistance Gene optrA in ST59 and ST902 Lineages. Microorganisms 2020, 8, 1240. [Google Scholar] [CrossRef]
- Lüthje, P.; Schwarz, S. Molecular analysis of constitutively expressed erm(C) genes selected in vitro in the presence of the non-inducers pirlimycin, spiramycin and tylosin. J. Antimicrob. Chemother. 2007, 59, 97–101. [Google Scholar] [CrossRef]
- Olsen, J.E.; Christensen, H.; Aarestrup, F.M. Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. J. Antimicrob. Chemother. 2006, 57, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J. Mechanisms of fluoroquinolone resistance: An update 1994–1998. Drugs 1999, 58, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Horii, T.; Suzuki, Y.; Monji, A.; Morita, M.; Muramatsu, H.; Kondo, Y.; Doi, M.; Takeshita, A.; Kanno, T.; Maekawa, M. Detection of mutations in quinolone resistance-determining regions in levofloxacin- and methicillin-resistant Staphylococcus aureus: Effects of the mutations on fluoroquinolone MICs. Diagn. Microbiol. Infect. Dis. 2003, 46, 139–145. [Google Scholar] [CrossRef]
- Sanfilippo, C.M.; Hesje, C.K.; Haas, W.; Morris, T.W. Topoisomerase mutations that are associated with high-level resistance to earlier fluoroquinolones in Staphylococcus aureus have less effect on the antibacterial activity of besifloxacin. Chemotherapy 2011, 57, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Ito, M.; Habadera, S.; Kobayashi, N. Prevalence and Genetic Diversity of Staphylococcal Enterotoxin (-Like) Genes sey, selw, selx, selz, sel26 and sel27 in Community-Acquired Methicillin-Resistant Staphylococcus aureus. Toxins 2020, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; San, T.; Urushibara, N.; San, N.; Oo, W.M.; Soe, P.E.; Kyaw, Y.; Ko, P.M.; Thu, P.P.; Hlaing, M.S.; et al. Molecular Characterization of Methicillin-Susceptible and -Resistant Staphylococcus aureus Harboring Panton-Valentine Leukocidin-Encoding Bacteriophages in a Tertiary Care Hospital in Myanmar. Microb. Drug Resist. 2020, 26, 360–367. [Google Scholar] [CrossRef]
- Severin, J.A.; Lestari, E.S.; Kuntaman, K.; Melles, D.C.; Pastink, M.; Peeters, J.K.; Snijders, S.V.; Hadi, U.; Duerink, D.O.; van Belkum, A.; et al. Unusually high prevalence of panton-valentine leukocidin genes among methicillin-sensitive Staphylococcus aureus strains carried in the Indonesian population. J. Clin. Microbiol. 2008, 46, 1989–1995. [Google Scholar] [CrossRef]
- Aung, M.S.; Kawaguchiya, M.; Urushibara, N.; Sumi, A.; Ito, M.; Kudo, K.; Morimoto, S.; Hosoya, S.; Kobayashi, N. Molecular Characterization of Methicillin-Resistant Staphylococcus aureus from Outpatients in Northern Japan: Increasing Tendency of ST5/ST764 MRSA-IIa with Arginine Catabolic Mobile Element. Microb. Drug Resist. 2017, 23, 616–625. [Google Scholar] [CrossRef]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Sumi, A.; Shinagawa, M.; Takahashi, S.; Kobayashi, N. Clonal Diversity and Genetic Characteristics of Methicillin-Resistant Staphylococcus aureus Isolates from a Tertiary Care Hospital in Japan. Microb. Drug Resist. 2019, 25, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Hirose, M.; Ito, M.; Habadera, S.; Kobayashi, N. Clonal diversity of methicillin-resistant Staphylococcus aureus (MRSA) from bloodstream infections in northern Japan: Identification of spermidine N-acetyltransferase gene (speG) in staphylococcal cassette chromosomes (SCCs) associated with type II and IV SCCmec. J. Glob. Antimicrob. Resist. 2021, 24, 207–214. [Google Scholar] [CrossRef]
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Huang, Y.C. New epidemiology of Staphylococcus aureus infection in Asia. Clin. Microbiol. Infect. 2014, 20, 605–623. [Google Scholar] [CrossRef]
- Kondo, Y.; Ito, T.; Ma, X.X.; Watanabe, S.; Kreiswirth, B.N.; Etienne, J.; Hiramatsu, K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 2007, 51, 264–274. [Google Scholar] [CrossRef]
- Ma, X.X.; Ito, T.; Chongtrakool, P.; Hiramatsu, K. Predominance of clones carrying Panton-Valentine leukocidin genes among methicillin-resistant Staphylococcus aureus strains isolated in Japanese hospitals from 1979 to 1985. J. Clin. Microbiol. 2006, 44, 4515–4527. [Google Scholar] [CrossRef]
- Feßler, A.T.; Wang, Y.; Wu, C.; Schwarz, S. Mobile lincosamide resistance genes in staphylococci. Plasmid 2018, 99, 22–31. [Google Scholar] [CrossRef]
- Feßler, A.T.; Wang, Y.; Wu, C.; Schwarz, S. Mobile macrolide resistance genes in staphylococci. Plasmid 2018, 99, 2–10. [Google Scholar] [CrossRef]
- Vazquez-Laslop, N.; Thum, C.; Mankin, A.S. Molecular mechanism of drug-dependent ribosome stalling. Mol. Cell. 2008, 30, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Wendlandt, S.; Kadlec, K.; Feßler, A.T.; van Duijkeren, E.; Schwarz, S. Two different erm(C)-carrying plasmids in the same methicillin-resistant Staphylococcus aureus CC398 isolate from a broiler farm. Vet. Microbiol. 2014, 171, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing; M100-S29; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2019.
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 9.0.; The European Committee on Antimicrobial Susceptibility Testing (EUCAST): Copenhagen, Denmark, 2019; pp. 25–28. [Google Scholar]
- Watanabe, A.; Yanagihara, K.; Matsumoto, T.; Kohno, S.; Aoki, N.; Oguri, T.; Sato, J.; Muratani, T.; Yagisawa, M.; Ogasawara, K.; et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the Surveillance Committee of Japanese Society of Chemotherapy, Japanese Association for Infectious Diseases, and Japanese Society for Clinical Microbiology in 2009: General view of the pathogens’ antibacterial susceptibility. J. Infect. Chemother. 2012, 18, 609–620. [Google Scholar] [PubMed]
- Zhang, K.; McClure, J.A.; Elsayed, S.; Louie, T.; Conly, J.M. Novel multiplex PCR assay for simultaneous identification of community-associated methicillin-resistant Staphylococcus aureus strains USA300 and USA400 and detection of mecA and panton-valentine leukocidin genes, with discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J. Clin. Microbiol. 2018, 46, 1118–1122. [Google Scholar]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kobayashi, N.; Nagashima, S.; Ishino, M.; Otokozawa, S.; Mise, K.; Sumi, A.; Tsutsumi, H.; Uehara, N.; Watanabe, N.; et al. Diversity of staphylocoagulase and identification of novel variants of staphylocoagulase gene in Staphylococcus aureus. Microbiol. Immunol. 2008, 52, 334–348. [Google Scholar] [CrossRef]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Aung, T.S.; Mya, S.; San, T.; Nwe, K.M.; Kobayashi, N. Virulence factors and genetic characteristics of methicillin-resistant and -susceptible Staphylococcus aureus isolates in Myanmar. Microb. Drug Resist. 2011, 17, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Isogai, N.; Urushibara, N.; Kawaguchiya, M.; Ghosh, S.; Suzaki, K.; Watanabe, N.; Quiñones, D.; Kobayashi, N. Characterization of Enterococcus faecium with macrolide resistance and reduced susceptibility to quinupristin/dalfopristin in a Japanese hospital: Detection of extensive diversity in erm(B)-regulator regions. Microb. Drug Resist. 2013, 19, 298–307. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).