Abstract
The growth of microorganisms and lower plants on building walls may respond the central principle of the biophilic design: sustained engagement with nature. As such, bioreceptive concrete has great potential to increase the biodiversity in our cities. In addition, by actively participating in the carbon and nitrogen cycles, biologically active, bioreceptive concrete has the potential to reduce the building’s environmental impact considerably. In the present study, we analyze the biological growth on concrete and critically review the current research approaches in the bioreceptivity evaluation. The uncontrolled and unaesthetic growth of fungal colonies, poor long-term survivability of the laboratory-developed biofilms, and a lack of field applications were identified among the major factors that hinder the practical application of bioreceptive concrete in the building envelope. Our ongoing field tests have shown that concrete’s controlled and aesthetically pleasant greening may be achieved in several years. We argue that such nature-integrated solutions would emphasize the beauty of the aging buildings while offering clear, practical benefits.
1. Introduction
The operation of buildings is an extremely energy-consuming process, constituting around 40% of the world-produced energy [1]. Most of the operational energy demand (around 75%) is influenced by the thermal performance of the building envelope., i.e., summer heat gain and winter heat loss [2]. The fully glazed façades, in this respect, neither meet the requirement of energy efficiency nor sustainability [3]. Studies [4,5] have shown that fully glazed façades consume up to 60% more energy for heating, cooling, and lighting than buildings with the optimal window-to-wall ratio.
A vertical greening systems (VGS), including green walls and living walls, offers a more sustainable and energy-efficient solution for the building envelope. While VGS represent a small segment of the global façade market (the estimated share is 1–2%), their growth trajectory is strongly driven by Sustainable Development Goals [6]. When designed with the optimal window-to-wall ratio (generally from 0.3 to 0.5 [7]), VGS may offer an attractive biophilic building’s skin while also bringing natural light to the interior [8]. Several studies have demonstrated that the VGS may not only increase the energy efficiency of buildings [9,10] but also sequester carbon dioxide [11], act as an air filter capturing small particulate matter [12], and contribute to the physiological health and well-being of residents [13].
Unfortunately, the existing VGS (e.g., hydroponic felt-based or pre-vegetated modular panel systems) are extremely expensive, with average installation costs of 750 EUR/m2 [14]. As a result, practical applications of living walls are scarce, except for specific luxury projects. To simplify the installation of VGS and reduce maintenance costs, an alternative idea has been introduced: growing microorganisms and lowering plants directly on a concrete surface [15,16]. Such biological growth on concrete is analogous to the organisms found in the cryptogramic ground covers (CGC) and cryptogramic plant covers (CPC): bacteria, fungi, algae, lichens, and non-vascular plants (bryophytes; liverworts, hornworts, mosses) [17]. These photoautotrophic communities can fix atmospheric CO2 and N2, producing carbon and nitrogen-containing organic compounds. Cryptogamic covers were estimated to correspond to 7% of net primary carbon and almost 50% of nitrogen uptake by terrestrial vegetation [18]. The annual carbon sequestration of cryptogamic covers varies between 17 g·C/m2 and 103 g·C/m2, depending on the climatic region [18]. It was estimated that globally, CGC may support 6.43 Gt more carbon in the soil layer than bare soils [19]. Such sequestration ability is lower in comparison to VGS with flowering plants (410–950 g·C/m2) and similar to the carbon sequestration performance of grass species (50–120 g·C/m2) [20,21]. Nevertheless, the major advantage of cryptogamic covers is their extreme resilience and ability to regrow after prolonged drought periods [17]. Thus, the extension of cryptogamic covers on the building envelope would transform the city landscape into a more natural environment and contribute to global carbon and nitrogen sequestration.
The life-sustaining concrete must possess a specific aptitude to be colonized by living organisms without undergoing bio-deterioration, also called bioreceptivity [22]. Most natural building materials, such as bricks, stones, timber, or concrete, possess certain primary or secondary bioreceptivity [23,24]. The primary bioreceptivity is characteristic of the surface with the initial mechanical, physical, and chemical properties. The exposed surface is affected by environmental and biological actions and gradually acquires secondary bioreceptivity [25]. While most of the new concrete structures show imperceptible biological growth, the weathered, carbonated, and leached concrete may host life for bacteria and even lower plants [26]. Despite the numerous benefits and potential impact on the sustainability of buildings, only a few studies have explored the practical applicability of bioreceptive concrete in façades [27,28,29,30]. Most concrete bioreceptivity studies were limited only to laboratory tests using single-species model microorganisms [31,32,33,34,35,36,37,38,39,40,41,42]. Although the accelerated laboratory tests may give a quick tentative bioreceptivity property of the material, the real-world biological colonization, driven by the volatile environmental and functional interplay between multiple species, may be significantly different [43,44]. A few field studies did not prove that the bioreceptive concrete can form attractive, visible growth patterns, either with the quick degradation of laboratory-developed biofilm [28] or no visible growth of microorganisms [45].
The Present study aims to analyze the biological growth on concrete in the terrestrial environment, considering different scales bioreceptivity evaluation (Figure 1). First, we analyze the biological growth on concrete from the microscopic scale. Next, we highlight the limitations of the currently performed laboratory-scale research. Finally, we discuss the challenges and opportunities of using bioreceptive concrete on a building scale.
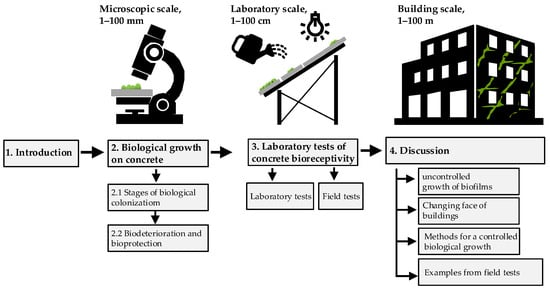
Figure 1.
Structure of the study.
We also report interim field test results on long-term biological colonization of Layered Living Concrete (LLC) panels. By demonstrating concrete’s relatively quick and controlled greening, we advocate the broader use of bioreceptive materials in the building envelope. Microorganisms and lower plants growing on concrete have the inherent ability to adapt to seasonal and local climate changes, thus reflecting the connection between the building and the surrounding environment. Such nature-integrated solutions would emphasize the aging buildings’ beauty while offering clear, practical benefits and aesthetically pleasant looks.
2. Biological Growth on Concrete
Externally exposed concrete surfaces offer an extremely abiotic terrestrial environment with minimal water and nutrients, quick water evaporation, and intense UV radiation. In addition, subaerial mineral substrates are constantly subjected to rapid temperature, moisture level, and relative humidity changes. These factors drive microbes to adopt specialized survival strategies, often centered around stress-resilient metabolic adaptations [46]. For example, some microbes synthesize UV-absorbing compounds like mycosporine-like amino acids and carotenoid pigments. These pigments act like a microscopic sunscreen, quenching free radicals before they damage cellular components [47]. To survive prolonged dry periods, many microorganisms are capable of forming spores and remain in a dormant state until favorable environmental conditions. To minimize the effects of UV radiation and scarcity of water, microbes preferentially colonize microcracks and pores, which offer shade, moisture retention, and protection from surface-level stressors [48]. The formation of biofilm is another common strategy that enhances the survivability of microorganisms on concrete. The mechanisms associated with biofilm formation will be more broadly discussed in Section 2.2. In general, only specific stress-tolerant microorganisms can colonize mineral-based substrates by scavenging nutrients from the atmosphere and rainwater, utilizing plant residues, dust, or waste products from other microbes [49].
Historically, the biological growth on concrete and cultural heritage stone surfaces was associated with accelerated deterioration caused by the metabolic activity of living organisms [50]. The process of biogenic deterioration was supported by widespread evidence of durability and mechanical integrity issues of biologically affected structures, specifically sewer systems, subsea pipelines, bridge piers, and off-shore platforms [51,52,53,54]. On the other hand, lithobionic communities have also been seen as enhancing biodiversity [55], sequestering atmospheric carbon and nitrogen [18], bringing positive aesthetical characteristics [27], or even acting as bioprotective layers [56,57,58]. The next subsections give an overview of the biological growth on concrete and discuss the possible biodeteriorative or bioprotective mechanisms.
2.1. Stages of Biological Colonization
Typically, the phototrophic carbon and nitrogen-fixing species (such as Cyanobacteria and Green algae) are pioneering microorganisms that begin the colonization of concrete surfaces [59,60]. As biological growth progresses, various representatives of fungal and archaeal communities, as well as many other bacterial species, colonize concrete and create a complete ecosystem [61].
Before the first pioneering microorganisms attach to the surface of concrete, the spontaneous process of biogenic conditioning alters substratum properties such as surface roughness, surface charge, chemical composition, and surface hydrophobicity/hydrophilicity [62]. Conditioning films are passive, non-living surface layers that ease the further adhesion of living microorganisms. In the marine environment, the formation of such conditioning film is driven by the adsorption of abiotic (proteins, lipids, polysaccharides, and other organic molecules from the environment) and biotic components (fragments of microbial cells, enzymes, or extracellular substances) [63,64,65]. The conditioning film occurs within seconds or minutes of immersion. In the terrestrial environment, the biogenic conditioning is a much slower process and is mostly driven by the airborne organic deposition, such as micromycetes and bacterial spores [66,67]. By balancing negative charges, the conditioning film provides receptor sites and eases further bacterial adhesion [68].
Later on, gravity- or advection-driven cells attach to the hard surface forming a monolayer and initiating the first stage biofilm formation [61] (Figure 2A). At first, these air or rain-driven microorganisms have a relatively weak bond with the substrate and may be easily moved by shear forces [69]. Under favorable conditions, the attached cells form clusters and microcolonies. Subsequently, it begins to produce and secrete into the surrounding environment extracellular polymeric substances (EPS), mostly composed of microbial biopolymers such as proteins, exopolysaccharides, nucleic acids, and lipids [70]. These autotrophic microorganisms are capable of converting inorganic carbon (atmospheric CO2) into organic compounds that can be used by others [71]. Similarly, some species can fix molecular dinitrogen (atmospheric N2) and convert it into ammonia (NH3) [72].
EPS promotes further microbial colonization, cell clustering via hydrogen bonding as well as cell entrapment, and eventually the mature biofilm is established [73,74]. Typical EPS concentration on concrete surfaces colonized by biofilms ranges from 50 to 500 µg/cm2 of surface area [75]. As a fundamental component of a biofilm, EPS plays a major role in both structural organization and functionality. In addition, it acts as an antimicrobial agent and protects the microbial community from the external environment (e.g., bactericides). Moreover, EPS serves as energy and carbon supply for the cells and acts as a water retention and communication tool [76].
The single species may initiate the formation of biofilm. Due to the relative simplicity and ease of control, single-species biofilms are mostly studied under laboratory conditions [72,73,77]. Contrarily, in the natural environment, self-sustaining microbial communities form a complex patchy biofilm containing different autotrophic and heterotrophic bacteria, algae, fungi, and lichens [59,78]. These microorganisms commonly form mutualistic interactions between autotrophs and heterotrophs, allowing for an efficient exchange of nutrients, functional interplay, and increased survival capability. As such, phototrophic microorganisms are commonly distributed at the upper biofilm layers, collecting the sun’s energy and shielding the heterotrophic microorganisms at the bottom layers [79]. The neighboring heterotrophic species recycle the photorespiration byproducts, thus effectively increasing the biomass of the phototroph-heterotroph consortium [70].
A complex interplay between the different bacterial species was demonstrated in [80]. A full nitrogen cycle was observed on the relatively small-scale stone-based subaerial biofilm (SAB) with nitrogen-fixing cyanobacteria (that convert molecular nitrogen N2 into ammonia NH3), nitrifying bacteria (converting ammonia NH3 into the nitrite NO2− and then to nitrate NO3−) as well as denitrifying bacteria, that close the nitrogen cycle (turning nitrogen oxides back to nitrogen gas N2). Lichens, a symbiosis of phototrophic algae (or cyanobacteria), heterotrophic fungi, and bacteria are another prominent example of mutualistic interactions [60]. The phototropic partner in such symbiosis produces the organic energy via carbon dioxide fixation, whereas the fungal partner offers the sheltering structures and sustains the moisture level [81]. Generally, the naturally growing biofilms form complex, highly stratified, self-organized structures where collectively organized species improve the ability to acquire nutrients from the environment and increase survivability under extreme conditions [70]. Subaerial biofilms seldom face antagonism because the extreme environmental conditions impose such high metabolic demands that energy-intensive processes like antibiosis are no longer viable [82].
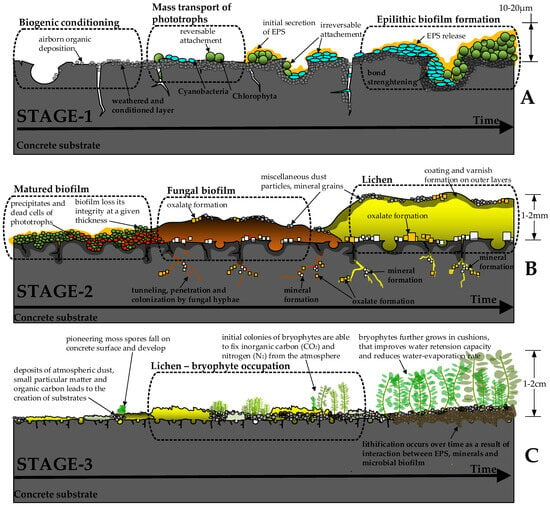
Figure 2.
Representation of possible mechanisms involved in biological colonization of concrete surface. At Stage 1 (A), gravity-driven phototrophic bacteria start to form initial biofilms and irreversibly attach to the concrete surface. At favorable environmental conditions (Stage 2), the fungal biofilms start to develop, which may also be accompanied by the growth of lichens (B). At Stage 3, the accumulated deposits of atmospheric dust, small particulate matter, and organic carbon from the dead microorganism cells allow for the growth of the first plants, typically bryophytes (C). Adopted from [49,60,66,67,79,81,83]. Not to scale.
Villa et al. [59] suggested a trait-based approach for the life-history analysis of sub-aerial biofilms (SABs) in analogy to vascular plants’ survival and colonization strategies. The underlying idea was to separate microorganisms into three groups according to their metabolic activity rate, survival strategy, and response to disturbance: (1) competitors, (2) stress tolerators, and (3) ruderals. Competitors are adapted to quickly consume available resources and engage in long-term site occupation. Hyphomycetes and Microcolonial fungi are typical competitors in the subaerial biofilms. Stress tolerators, such as Green algae and Cyanobacteria, have high resource-use efficiency and can survive in a harsh environment (temperature fluctuations, UV radiation, low moisture levels). Finally, ruderals can produce a copious amount of low-cost biomass and quickly colonize a new surface after a major disturbance (such as fire, drought, or storm). Actinobacteria was classified as a typical ruderal microorganism in a subaerial biofilm.
The microbiological composition of the SAB strongly depends on the dominant bacteria’s life strategy (competition, stress-toleration, or ruderal colonization), which, in turn, is governed by environmental conditions. For example, slow-growing, stress-tolerant species such as Cyanobacteria and Green algae will dominate in areas likely to face prolonged stresses (constant UV radiation with limited precipitation). The predominance of algal biofilms is a typical scenario in hot and warm-summer Mediterranean climate zones (Csa and Csb, according to Köppen climate classification), where green bio-patina is commonly found on building façades and stone monuments [77,78]. Contrarily, competitor microorganisms dominate with the extensive development of fungi and lichens in humid continental and tropical climate zones [84,85].
As shown in Figure 2B, the development of fungi-dominated biofilms denotes the second stage of biological colonization. At this stage, fungal hyphae penetrate microcracks and open concrete pores, commonly forming stable layers of mineral crust (mostly carbonates, phosphates, oxides, and oxalates [49]). In favorable environmental conditions, the growth of fungal biofilms may be accompanied by the development of lichens. Similarly to bacterial phototroph-heterotroph consortiums, lichens may store nutrients from the surrounding environment and resist extreme environmental conditions [60] (prolonged periods of drought, intense UV radiation, and temperature fluctuations). Lichens actively affect the concrete substrate by penetrating the open pores and surface cracks, producing organic carboxylic acids, and initiating various bio-mineralization pathways [49,60]. As a result, a stable layer of biological crust may form on the concrete surface that may have both bio-deteriorative and bio-protective effects [55,86].
The mature bacterial-fungi-lichen crust accumulates atmospheric dust, small particulate matter, decaying plants, and animal feces. EPS plays a major role in this process by enhancing the adhesion and binding together of different small particles to the surface [87]. The mineral dissolution, caused by the metabolic activity of bacteria and fungi, physically disrupts the concrete matrix. As minerals dissolve, essential elements like calcium, magnesium, and trace metals become bioavailable. These nutrients support microbial communities and indirectly benefit bryophytes once they establish [88]. In addition, dead cells of bacteria and fungi enrich the crust with organic carbon, nitrogen, and phosphorus [85]. These deposits serve as an initial substrate suitable for the first pioneer plants [60], thus starting the third stage of biological colonization (Figure 2C). Most commonly, nonvascular spore-forming bryophytes (typically mosses) are the first plants that can survive on the concrete surface [89]. Mosses do not have roots and attach themselves to the surface using rhizoids. Remarkably, mosses are poikilohydric plants, meaning water status completely depends on their environment [90]. As such, mosses can tolerate desiccation and resume their metabolism after rewetting. Additionally, some moss species can transport atmospheric nitrogen to the soil through a mutualistic relationship with nitrogen-fixing cyanobacteria [91,92].
The recent study by Ramakrishnan et al. [93] has highlighted that mosses represent a currently untapped source of microbial biodiversity. It was found that mosses harbored almost twice as much microbial richness and diversity as vascular plants [93]. Such moss-bacteria symbiosis is vital in boreal forests and arctic tundra, where it comprises almost 50% of total nitrogen input [94]. In addition, moss-associated microbes oxidize methane and contribute to the decomposition of organic matter [95]. The superior ability of mosses to host the bacterial communities can be explained by their morphological and physiological traits: mosses lack complex vasculature and a protective cuticle, so their rhizoids, caulids, and phyllids are open to colonization by a broader environmental pool [93]. In addition, mosses absorb water and nutrients directly through their surfaces, creating a moist microenvironment ideal for microbial colonization. Dense moss mats trap water, forming thin films where bacteria can attach and form biofilms [95]. Such unique features make mosses a promising plant for bioreceptive concrete walls [96,97,98,99,100]. A broader perspective on the potentiality of mosses in urban ecosystems will be discussed in Section 3.
2.2. Biodeterioration and Bioprotection
Concrete-surface-attached microorganisms not only encase themselves in the self-produced EPS matrix but also actively alter the surrounding microenvironment by affecting the porosity, density, water content, sorption properties, and mechanical stability of the surface [76]. As a result, the formation of biofilm is a three-dimensional process, where the third dimension (penetration depth) may vary between several micrometers and several millimeters [58]. Such biofilm-substrate interaction is crucial for the functionality of microorganisms; however, it may also compromise the durability of the building material [101].
The biological activity of microorganisms and plants causes two major disruptive processes: biogeophysical and biogeochemical [55]. Biogeophysical deterioration of substrate is a result of the hyphal, rhizine, or root penetration of growing plants [86], thermal stress caused by surface discoloration [55], or expansion and contraction of cells during wetting and drying cycles [56]. Biogeochemical deterioration is caused by the metabolic activity of microorganisms that secrete corrosive metabolites such as organic acids [50]. A prominent and widespread example of biogeochemical deterioration is the biocorrosion of concrete used in sewer systems [52,53]. The wastewaters contain an abundant amount of sulfate ions (SO4−), which are reduced to hydrogen sulfide (H2S) by sulfate-reducing microorganisms [53]. In the H2S-rich environment, part of the hydrogen sulfide reacts with oxygen, forming elemental sulfur, sulfite, and thiosulfate, which are further transformed to sulfuric acid (H2SO4) by sulfur-oxidizing bacteria [52]. Sulfuric acid reacts with concrete components (mostly free lime Ca(OH)2), forming low-strength expansive products such as gypsum or ettringite [50,53]. It was estimated that deterioration of concrete in the sewer systems may reach several millimeters per year [54], making sulfate-rich environments extremely vulnerable to a biological attack. Similarly, an intense degradation of concrete may occur in an ammonia-rich environment, where specific bacterial strains can oxidize ammonia into nitric acid. The reaction of nitric acids with concrete results in the formation of highly water-soluble calcium nitrate salt, which may quickly damage concrete up to 20 mm in depth [50]. Contrary to bacteria-driven sulfate and nitric acid concrete corrosion, fungal-induced concrete deterioration is much less understood [102]. Generally, fungal-induced concrete deterioration involves several biochemical pathways, primarily driven by the production of organic acids, such as oxalic, citric, lactic or acetic. These organic acids react with calcium compounds in concrete (calcium hydroxide and calcium silicate hydrates), forming water-soluble salts like calcium acetate or calcium oxalate. These salts form solubilization zones and act like crack initiators [102].
A biocorrosion of concrete was also observed in a marine environment, where biologically induced concrete deterioration occurs simultaneously with chemical corrosion [103]. It was argued that the penetration of an EPS matrix inside the concrete and, subsequently, biophysical erosion is accelerated by the chemical reaction of seawater salts and cement matrix that increases the concrete’s porosity and surface roughness [56]. Nevertheless, concrete structures in the terrestrial environment seem to undergo less severe biological deterioration. Commonly, terrestrial lithobionic communities do not directly contribute to the macro-scale cracking or spalling of concrete but rather reduce the alkalinity of the concrete surface, which prompts the corrosion of the steel reinforcement [50]. The penetration depth of terrestrial biofilm on the aged stonework was found in the range of 100–500 μm for fungal biofilms [104] and 1–2 mm in the case of endolithic lichens [58]. When dried or removed, biofilms result only in fine-scale material loss [56]. In some cases, the biological growth on concrete may even have a bioprotective effect, mostly explained by the local shielding effect [86]. The biofilm or biological crust on the surface of a mineral substrate may: (1) act as a physical barrier from aggressive weathering conditions [55]; (2) mitigate the surface temperature fluctuations and thermal stress [57]; (3) protect from short-term wetting and drying cycles [105]; (4) bond the loose surface particles by forming a biological crust [56]; or (5) densify the porous structure of the substrate [58]. The formation of biological crust on a concrete surface is basically driven by microbially induced calcium carbonate precipitation (MICP). The process of mineral formation is a result of metabolic activities of microorganisms, such as urea hydrolysis, denitrification, or sulfate reduction [106]. Some bacterial species use MICP to stabilize their living environment, contributing to both survival and structural integrity. The formation of biological crust may also protect concrete surfaces from colonization by more destructive microorganisms, such as nitrifying or sulfate-reducing bacteria [106]. When corrosively harmless microbes colonize a concrete surface first, they consume available resources, leaving little for harmful microbes like sulfur-oxidizing or sulfate-reducing bacteria that cause corrosion. The biofilm’s deteriorative or protective function on concrete is difficult to determine due to the complexity of the substrate-biofilm-atmosphere ecosystem. Generally, stress-tolerant species are likely to be less deteriorative because they do not disrupt the surface. Contrarily, ruderal species, occurring in the areas with climatic disturbances, are more prone to biodeteriorative mechanisms [59]. Depending on the environmental conditions, the same biofilm can shift towards more bioprotective or biodeteriorative dominated mechanisms [82].
To summarize, severe biological deterioration is mostly characteristic of concrete in aggressive, sulfate- or ammonia-rich environments. In such conditions, sulfuric or nitric acids may be biologically synthesized. The reaction of these acids with concrete components results in the formation of low-strength or soluble products, leading to the quick degradation of concrete’s mechanical properties. On the other hand, the biological deterioration of concrete in the terrestrial environment is a much slower process that requires decades to form a biological crust. In some cases, this biological crust may even have a bioprotective effect.
3. Laboratory and Field Testing of Concrete Bioreceptivity
An overview of the laboratory studies related to the bioreceptivity testing of concrete is presented in Table 1. Field studies on concrete bioreceptivity are summarized in Table 2. Due to concrete’s complex and relatively slow biological colonization, simplified and accelerated laboratory tests are commonly employed for bioreceptivity evaluation [101]. Generally, biological growth is stimulated by inoculating a single species of microorganisms [31,32,33,34,35,36,37,38,39,40,41]. Using this approach, biological growth may be strictly controlled by applying suitable nutrients and imposing the required temperature, humidity, and UV radiation [78]. Due to the limitations in time, accelerated laboratory tests are generally performed at intervals of 1–20 weeks, using fast-growing algal microorganisms [33,36,38,39,40,41]. Typically, the biofilm may reach thickness of 20–40 µm over 1–2 weeks under controlled laboratory conditions [107]. Figure 3 illustrates the current trends in the bioreceptivity analysis, by summarizing data presented in Table 1 and Table 2. It is evident that short-term laboratory studies using single organisms are currently the dominant approach for bioreceptivity evaluation. Nevertheless, studying the growth of individual community members lacks ecological succession and is in sharp contrast to the formation of natural SAB. The natural formation of multiple species biofilms is driven by the external environment, which provides nutrients, moisture, space, and physical and chemical stressors [72]. As such, complex and highly stratified biological structures form on the surface of concrete, where different species enhance the survivability rate through mutualistic relationships and functional interplay.

Table 1.
Laboratory studies of concrete bioreceptivity * in the terrestrial environment.

Table 2.
Field studies of concrete bioreceptivity.
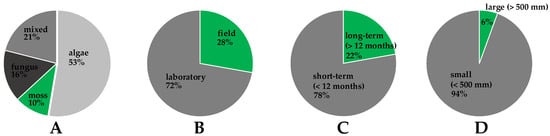
Figure 3.
The parameters and conditions used in concrete bioreceptivity studies: (A) the type of biological agent; (B) the environmental conditions; (C) the duration of the tests; (D) the size of the specimens.
The laboratory studies of single-species biofilm formation have another inherent deficiency: they may instead show the ability of the studied microorganism to colonize the concrete surface rather than the aptitude of concrete to be colonized [78]. For example, a broad experimental program on concrete bioreceptivity using Chlorella vulgaris model organisms was reported in [33]. The accelerated laboratory algal fouling tests have shown that specimens produced with magnesium phosphate cement (MPC) had a noticeably higher percentage of fouling area than ordinary Portland cement specimens (OPC). It was concluded that due to lower pH and more suitable chemical composition, MPC-based specimens had a higher bioreceptivity [33,43]. Nevertheless, when the specimens of the same concrete mix composition were tested in the natural environment, no visible growth was found on any specimen type [45]. The previously used Chlorella vulgaris model organism was not even detected on the concrete surface. Similarly, Tran et al. [44] argued that the experimental conditions of accelerated tests are far different from the real ones. It was shown that concrete’s initial surface pH, porosity, and roughness have much less correlation with biological colonization in field exposure in comparison to the accelerated laboratory tests [44]. These examples support the statement that the ability of the specific microorganism to grow under laboratory conditions does not necessarily imply that the material has the aptitude to be colonized in the natural environment.
After experimenting with the bioreceptive façade panels, Veeger et al. [28] highlighted another issue with the laboratory-developed biofilm. The algal biofilm showed a stable development and quick greening of horizontally placed façade panels under optimal growing conditions: panels were kept in water with the waterline just below the concrete surface, at constant room temperature, 90% humidity, and 12 h cycle artificial lighting. However, biofilm completely lost its integrity after the real-world exposure, with only slight recovery signs after 5 months of field testing. The authors argued that such quick disruption of laboratory-developed biofilm may be attributed to the lack of EPS, which helps to mediate the extreme environmental actions. As the laboratory specimens were kept under constant and favorable conditions, the cell energy was directed to the production of biomass rather than EPS [28]. The discussed example implies that the test setup may predetermine the growth of dominant microorganisms and bias the bioreceptivity properties of the tested material. In general, two main test methods to study biological growth on concrete may be distinguished: (1) static and (2) dynamic [77]. In the former case, the development of biofilm is studied on a horizontal surface, providing water by spraying, condensation, or capillary suction. To prevent the inoculated organisms from being washed-out, the flow of water is restricted in the static conditions [28,31,32]. Contrarily, dynamic conditions simulate the flow of rainwater over the mineral substrate, creating shear stresses on microorganisms [39,41,108]. Growing organisms must overcome the flow forces and firmly anchor themselves to the substrate [77].
An overview of the test setups used to stimulate biological growth on concrete is presented in Figure 4. The examples of static and dynamic test conditions are presented in Figure 4A–C, and Figure 4D–F, respectively.
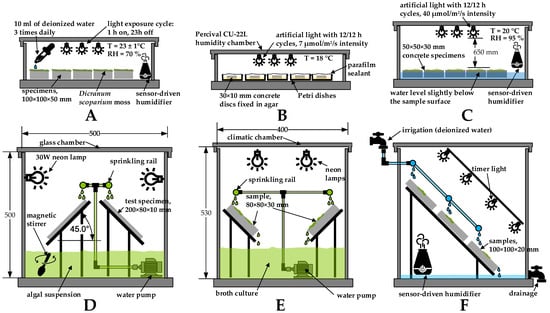
Figure 4.
Test arrangements used for concrete bioreceptivity evaluation: (A) adopted from [32]; (B) adopted from [31]; (C) adopted from [28]; (D) adopted from [41]; (E) adopted from [108]; (F) adopted from [39].
Although dynamic tests introduce the shear stress factor for the microorganism growth, the temperature, humidity, and UV radiation are generally maintained constant inside the growth chambers [33,36,39,41,108]. As a result, the biofilms developed in such conditions may lack resistance and resilience to environmental stress factors. To overcome this deficiency, laboratory tests using multiple-species microorganisms under variable conditions (drying periods, fluctuations in temperature, humidity, and UV radiation) should be designed and standardized in future studies of concrete bioreceptivity. Alternatively, specific cultivation-adaptation regimes may be employed to stimulate the growth of resistant microbiological colonies. Such tests would provoke the natural competition between microorganisms and the development of more resistant and resilient biofilm. It would also serve as a transitional tool between the ideal laboratory conditions and field applications. To summarize, accelerated laboratory tests may give the material a quick tentative bioreceptivity property. The real-world biological colonization, driven by the volatile environmental and functional interplay between multiple species, may differ significantly. There is an urgent need for a standardized, tiered testing protocol that transitions from simple, static, single-species lab tests to complex, multi-species, dynamic field trials. Such a framework could help unify research efforts and make comparative studies more consistent and meaningful.
We also argue that the long-term field testing of structural-scale bioreceptive concrete panels is indispensable to evaluate the real-time concrete colonization, changes in aesthetics, and durability. Such a testing technique is also compatible with ecological succession, allowing for natural primary colonization of concrete surfaces. Even though field tests are generally labor-intensive, prolonged, and hardly controllable, the gathered knowledge on biofilm formation may provide invaluable data on the long-term survivability and the dominating species of bio-colonized concrete. The next section provides insights into the natural concrete colonization of existing buildings and reports on the current status of our long-term test results on the development of bio-receptive façade panels.
4. Discussion: Potentiality of Bioreceptive Concrete in Building Façades
The natural colonization of concrete surfaces is a long-term process. The aesthetic appearance of a SAB on a building façade is controlled by the material intrinsic parameters (bioreceptivity) and the environmental conditions. North-faced, commonly wet façades with rough texture show rapid signs of colonization, whereas south-facing smooth concrete façades may show no signs of biological fouling for decades. As was discussed in Section 2, colonization starts with the irreversible attachment of autotrophic bacteria. The initial biological activity of the autotrophic bacteria commonly does not result in the development of significant biomass and discoloration of the surface [45]. Only at colonization stage 2, with the more intense development of fungi or algae, the first signs of visible discoloration occur [37]. In practice, in a favorable growth condition, it takes approximately one year to see the first visible growth and 2–5 years to develop an intense algal cover [35,44]. Depending on the dominating microorganisms, the building façades are typically tinted with green (as a result of Cyanobacteria and Green-algae dominated biofilms), black-gray (as a result of fungal domination), or red stains (caused by Red microalgae) [109,110]. An example of the typical discoloration pattern of 9 years north-facing concrete wall in the humid continental climate zone (Vilnius, Lithuania) is presented in Figure 5. The available moisture principally controls the biological growth on this wall: the top part of the wall collects the water from the horizontal surface, whereas the bottom part primarily obtains the moisture by capillary suction. As a result, well-visible fungal and algal stains have developed, proceeding to the second stage of biological colonization (Figure 5). The same 9-year concrete wall also shows initial signs of the third colonization stage (Figure 6). Although mosses can grow on vertical concrete surfaces, their growth pattern is irregular and mostly concentrated at surface indentations, cracks, and other irregularities. Similarly to algal and fungal biofilms, moss growth can only be observed in water-available regions and can hardly attach to the smooth vertical concrete surface (Figure 6). Such uncontrolled growth of mosses, algal, and fungal biofilms, however, may negatively impact the visual aesthetics of the façade and provoke a special chemical or mechanical treatment (such as the use of biocides or high-pressure cleaners) [85,111,112]. The discussed example of biological growth on an existing concrete wall highlights two main problems of naturally developed biofilms: (1) the biological growth concentrates only in shaded, moisture-available regions, and (2) uncontrolled growth, dominated by fungal and algal biofilms, compromises the initial aesthetics of the concrete wall.
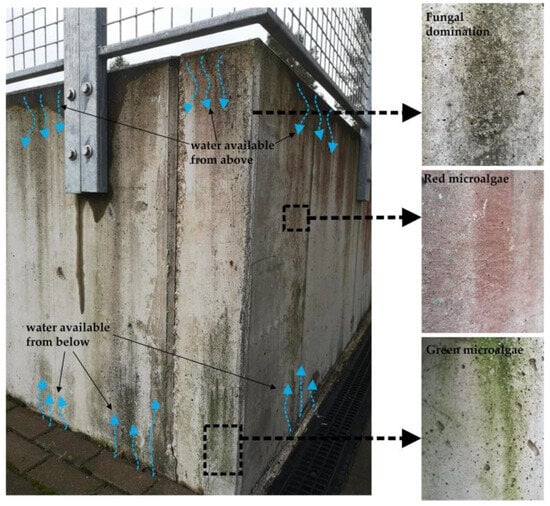
Figure 5.
Biological stains on a 9-year-old concrete wall. Black, green, and red discolorations are caused by the biological activity of microalgal and fungal species.

Figure 6.
The signs of the third stage of biological colonization on a 9-year-old concrete wall: growth of lichens and mosses starts at the corners, cracks, and surface irregularities.
Contrarily, the growth of lichens and bryophytes on the walls is generally tolerated and even considered pleasant. A broad visitor opinion survey on the perception of biological growth on historic buildings has been carried out in Oxfordshire [105]. In total, 143 completed surveys have been analyzed of a wide-range visitor demographics. The results have indicated that most of the public and experts felt positive about the look of moss growth on historic walls (90% of experts and 77% of visitors agreed with the statement “I enjoy the look of mosses on historic buildings”). The survey has shown that the growth of lower plants on historic buildings was tolerated and associated with age and authenticity. A broader discussion and more methodological details of the performed survey are presented in [105].
Bioreceptive concrete risks being colonized only by fungal and algal biofilms. Such biological growth commonly results in gray-black and green-black discolorations that may negatively impact the aesthetics of the façade. Only at the third concrete colonization stage (Figure 2C), i.e., the lower-plant-dominated bioreceptive concrete may become tolerable and acceptable by the general public. To mitigate the uncontrolled and unaesthetic growth of fungal colonies on concrete surfaces, specific regions with distinct growing conditions should be designed. In regions with favorable conditions, the biological growth should be stimulated by providing water, shading, and porous substrate. Contrarily, in regions where discoloration is not acceptable, a smooth, durable, and low-porous concrete should be used. An example of such a system, named Layered Living Concrete (LLC) façade panel, was previously designed by the Author [30] and is briefly presented next.
The underlying idea under the development of LLC was to shorten the time required for the natural growth of mosses from several decades to several years. The LLC panel consists of three main elements: (1) high-performance synthetic fiber reinforced concrete (HPFRC) that ensures the durability (the determined resistance to more than 1000 freeze–thaw cycles [113]) and structural integrity of the panel; (2) light-weight pervious concrete (LWPC), that provides a rough, bio-receptive, and highly permeable surface; (3) biological booster (BB), that directly serves as a growing substrate at the initial colonization stages (Figure 7). Principally, LLC panels may be used in the usual ventilated façades systems as an alternative to the stone, concrete, or fiber cement cladding [114,115].
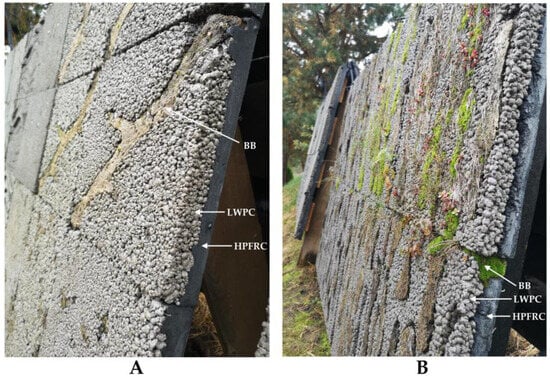
Figure 7.
The composition and biological growth on the Layered Living Concrete (LLC) façade panels: the biological growth on the 5-month-old LLC panels is hardly noticeable (A), whereas well-developed moss can be seen on 19-month-old panels (B).
The rational composition of all materials, as well as the optimal distribution of biological boosters, are still the subjects of ongoing investigations. Our initial results have shown [30,116] that the rationally dimensioned thin-walled panels may be produced when the compressive and residual tensile strength of the HPFRC layer exceeds 100 MPa and 1.5 MPa, respectively. For the uniform distribution of irrigation water, the infiltration rate of the LWPC layer should exceed 2.5 cm/s. The biological booster, composed of recycled paper pulp and a soil crust, should have at least 50% of the active component (soil crust). Water retaining capacity tests have indicated that the composition of LWPC and distribution of BB within the panel may result in a 300% difference in the stored water.
Several long-term experimental campaigns examining the biological growth on the LLC panels are ongoing under natural environmental conditions (Figure 7). The first test series began in March 2021, with the installation of four 400 × 600 mm differently faced (East–West, North–South) panels. The second series of LLC panel field tests began in April 2023 with 12 additional 400 × 600 mm panels. Finally, the third experimental series started in July 2024, examining the biological growth on 48 LLC panels (300 × 300 mm). Several important insights were gathered from the long-term field observations:
- The biological booster allows for a significantly faster greening of concrete surfaces: colonization stage 3 starts several months after panel installation with germination of mosses and fungi (Figure 8A). We recommend the production of biological boosters from the local soil crust, as it naturally contains indigenous bacteria, spores of mosses, fungi, and lichens. Those species are adapted to the local climate and require only several months of latency.
 Figure 8. Biological growth on the LLC: after three months of field exposure the initial growth of fungi and mosses starts to be visible on panels of test Series 3 (A) and growth evolution in 6–18 months period on panels of test Series 2 (B).
Figure 8. Biological growth on the LLC: after three months of field exposure the initial growth of fungi and mosses starts to be visible on panels of test Series 3 (A) and growth evolution in 6–18 months period on panels of test Series 2 (B). - After two years of field exposure, no visible signs of biological colonization by algal or fungal microorganisms were observed on the pervious concrete surface. This confirms that the natural colonization of concrete is a long-term process, taking several years to several decades [35]. Regarding the application of bioreceptice concrete in building façades, such a lag between the installation and the visible biological growth may be unacceptable. Thus, a BB may solve this problem by offering a relatively quick growth of mosses (Figure 8B).
- Irrigation water should be supplied for quicker biological growth. In all tested LLC panels, the BB was kept wet, providing water from the drip irrigation system. Although such a living wall may not be considered a self-sustained system, supplied water not only accelerated the growth of mosses but also initiated the development of some vascular plants (mostly sedums). The supplied irrigation water also resulted in a more pleasant appearance of LLC during prolonged drought periods.
- During field tests, we attempted to grow some drought-resistance vascular plants (Sedum acre, Sedum spurium, Saxifraga arendsii, Festuca rubra commutata, Festuca trichophytic). Although some vascular plants (Festuca rubra commutata, Festuca trichophytic) showed a quick initial greening, most of them did not survive the first winter (Figure 9). After several years of field exposure, domination of mosses on all panels was observed. The long-term (10–20 years) aesthetics and practical benefits (air filtration, noise-reduction, carbon sequestration, etc.) of such bioreceptive concrete panels are subjects of future investigations.
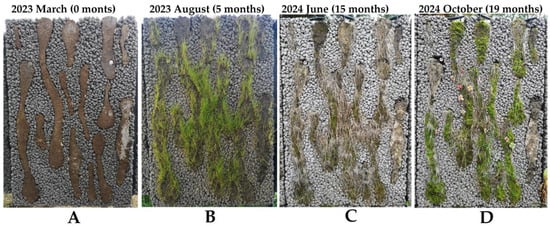 Figure 9. Growth evolution on a LLC panel of test Series 2: (A) initial view just after panel installation; (B) germination and growth of grasses (Festuca rubra commutata, Festuca trichophylla) five months after panel exposure; (C) desiccation of grasses after the first winter; (D) moss domination 19 months after panel exposure.
Figure 9. Growth evolution on a LLC panel of test Series 2: (A) initial view just after panel installation; (B) germination and growth of grasses (Festuca rubra commutata, Festuca trichophylla) five months after panel exposure; (C) desiccation of grasses after the first winter; (D) moss domination 19 months after panel exposure. - The BB allows for quicker biological colonization and control of the aesthetic appearance of the façade. As such, the BB may be distributed in rhythmic, repetitive, or flowing patterns for both aesthetics and the optimized water retention capacity.
- The shape and distribution of BB within the LLC panel are crucial for both anchoring ability and water retention capacity. Figure 9D shows an example of poor water distribution on the panel: the desiccated and shrank BB at the side edges of the panel could not support the moss growth. Based on the water retention tests and a long-term field observations, continuous diagonal shape of BB was proposed for optimal greening performance [116].
- The bioreceptive concrete panels adapt to the seasonal changes with the fast growth of vascular plants (mostly sedums and several indigenous grass species) in spring and mosses in autumn. The biological growth almost stops in winter. Such a nature-controlled aesthetic appearance of the façade suits the principles of biophilic design: constant engagement with nature emphasizing the flow of time and seasonal changes. The naturally weathered and lichen-moss overgrown bioreceptive concrete panels may create a sense of maturity and additional value [117]. Nevertheless, the long-term aesthetics as well as the durability of LLC panels are subjects of ongoing investigations.
- All LLC panels were installed on the testing frame immediately after demolding from the formwork. However, the process of surface conditioning before panel installation may favor a quicker colonization by pioneering microorganisms. As discussed in Section 2.1, the conditioning film forms within several minutes in the aqueous environment. Thereby, initial immersion of panels into a solution rich in biomolecules may create a conditioning film and accelerate the further colonization of concrete panels. Future research should address this hypothesis, assessing the impact of pre-conditioning on biological colonization.
The ongoing field tests allow us to examine the ability of concrete to host biological growth, changes in the aesthetical appearance, and the durability of concrete façade panels in real-time. Although field tests were previously highly prioritized [44,45], to the Author’s knowledge, this is one of few ongoing experimental programs on long-term concrete bioreceptivity. By implementing broad experimental programs (currently, 64 LLC panels are being tested), we advocate the broader use of bioreceptive concrete in the building’s envelope. The bioreceptive and living concrete may not only offer a natural-looking cryptogramic cover for buildings but also sequester carbon dioxide, filter air, reduce urban noise, and mitigate the effect of the heat islands. Future research should be performed to quantify these ecological, heat and noise-reduction parameters of bioreceptive concrete panels. The real-world demonstrative projects with a large-scale application of bioreceptive concrete panels for a building’s envelope are also essential for the further development and optimization of this façade system.
We believe that bioreceptive concrete-dominated façades (optimally covering 50–70% of the external walls [7,118] would be a more economical, and ecological alternative to fully glazed buildings. Optimizing window placement for daylight, views, and ventilation may provide a connection with the environment while maintaining the low energy demands for heating and cooling [119].
Bioreceptive façades also meet the general public’s need for a larger share of vegetation in the urban environment [120]. Concrete panels covered with microorganisms, lichens, and lower plants may enrich the biodiversity offering a direct experience of aging nature. When properly designed, bioreceptive concrete façade may fulfill several attributes of biophilic design (presence of plants, natural ecosystems, natural colors, and the patina of time) [121].
5. Concluding Remarks
Incorporating cryptogamic covers for a building envelope renders the central principle of biophilic design: sustained engagement with nature. As such, bioreceptive concrete has great potential to increase biodiversity in our cities. In addition, by actively participating in the carbon and nitrogen cycles, biologically active cryptogamic covers have the potential to considerably reduce the buildings’ environmental impact. By hosting the natural ecosystem of microorganisms and lower plants, featuring environmentally controlled aesthetics and colors, the bioreceptive concrete has a great potential for application in the biophilic design practice.
The current research on concrete bioreceptivity was mainly performed under laboratory conditions, simulating the accelerated colonization of concrete by single-species microorganisms. Such tests may give the material a quick tentative bioreceptivity property; however, the real-world biological colonization, driven by the volatile environmental and functional interplay between multiple species, may be significantly different. Laboratory tests, performed under ideal conditions, may commonly show the ability of the selected microorganism to colonize the concrete rather than the intrinsic property of concrete to be colonized by different microorganisms. Future work would benefit from developing hybrid protocols that bridge controlled laboratory conditions and dynamic field exposure. Surface conditioning of bioreceptive concrete panels with the specifically developed biologically active solutions is another field for future investigations.
Our ongoing long-term field tests of LLC panels have shown that concrete’s visible, controlled greening may be achieved in several years. In contrast, several decades may be required to form the natural biological crust on hard surfaces, like stone monuments or concrete walls. Still, such uncontrolled natural growth may result in irregular discolorations and compromise the structure’s aesthetics. By incorporating the biologically active regions in the LLC wall, we stimulate the quick germination and growth of lower plants, control the aesthetic of the wall panel, and facilitate the further colonization of the wall panel by microorganisms.
We urgently need more field studies in different climatic regions. The bioreceptive concrete exposed to natural environmental conditions would allow us to examine the long-term survivability of microorganisms and document the changes in the aesthetics of aging concrete. The refined look of naturally developed biofilms may encourage developers and architects to consider the cryptogamic covers for the building envelope.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Azari, R. Life cycle energy consumption of buildings; embodied+ operational. In Sustainable Construction Technologies; Butterworth-Heinemann: Oxford, UK, 2019; pp. 123–144. [Google Scholar]
- Venkatraj, V.; Dixit, M.K.; Yan, W.; Lavy, S. Evaluating the impact of operating energy reduction measures on embodied energy. Energy Build. 2020, 226, 110340. [Google Scholar] [CrossRef]
- Butera, F.M. Glass architecture: Is it sustainable. In Proceedings of the Passive and Low Energy Cooling for the Built Environment, Santorini, Greece, 19–21 May 2005. [Google Scholar]
- Aksamija, A.; Peters, T. Heat transfer in facade systems and energy use: Comparative study of different exterior wall types. J. Archit. Eng. 2017, 23, C5016002. [Google Scholar] [CrossRef]
- Giordano, R.; Giovanardi, M.; Guglielmo, G.; Micono, C. Embodied energy and operational energy evaluation in tall buildings according to different typologies of façade. Energy Procedia 2017, 134, 224–233. [Google Scholar] [CrossRef]
- Fei, W.; Opoku, A.; Agyekum, K.; Oppon, J.A.; Ahmed, V.; Chen, C.; Lok, K.L. The critical role of the construction industry in achieving the sustainable development goals (SDGs): Delivering projects for the common good. Sustainability 2021, 13, 9112. [Google Scholar] [CrossRef]
- Goia, F. Search for the optimal window-to-wall ratio in office buildings in different European climates and the implications on total energy saving potential. Sol. Energy 2016, 132, 467–492. [Google Scholar] [CrossRef]
- Wijesooriya, N.; Brambilla, A. Bridging biophilic design and environmentally sustainable design: A critical review. J. Clean. Prod. 2021, 283, 124591. [Google Scholar] [CrossRef]
- Li, J.; Zheng, B.; Shen, W.; Xiang, Y.; Chen, X.; Qi, Z. Cooling and energy-saving performance of different green wall design: A simulation study of a block. Energies 2019, 12, 2912. [Google Scholar] [CrossRef]
- Susca, T.; Zanghirella, F.; Colasuonno, L.; Del Fatto, V. Effect of green wall installation on urban heat island and building energy use: A climate-informed systematic literature review. Renew. Sustain. Energy Rev. 2022, 159, 112100. [Google Scholar] [CrossRef]
- Jozay, M.; Zarei, H.; Khorasaninejad, S.; Miri, T. Maximising CO2 Sequestration in the City: The Role of Green Walls in Sustainable Urban Development. Pollutants 2024, 4, 91–116. [Google Scholar] [CrossRef]
- Srbinovska, M.; Andova, V.; Mateska, A.K.; Krstevska, M.C. The effect of small green walls on reduction of particulate matter concentration in open areas. J. Clean. Prod. 2021, 279, 123306. [Google Scholar] [CrossRef]
- Cardinali, M.; Balderrama, A.; Arztmann, D.; Pottgiesser, U. Green walls and health: An umbrella review. Nat. Based Solut. 2023, 3, 100070. [Google Scholar] [CrossRef]
- Manso, M.; Teotónio, I.; Silva, C.M.; Cruz, C.O. Green roof and green wall benefits and costs: A review of the quantitative evidence. Renew. Sustain. Energy Rev. 2021, 135, 110111. [Google Scholar] [CrossRef]
- Manso, S.; Aguado, A. The use of bio-receptive concrete as a new typology of living wall systems. Matériaux Tech. 2016, 104, 502. [Google Scholar]
- Cruz, M.; Beckett, R. Bioreceptive design: A novel approach to biodigital materiality. Arq Archit. Res. Q. 2016, 20, 51–64. [Google Scholar] [CrossRef]
- Mitchell, R.L.; Strullu-Derrien, C.; Sykes, D.; Pressel, S.; Duckett, J.G.; Kenrick, P. Cryptogamic ground covers as analogues for early terrestrial biospheres: Initiation and evolution of biologically mediated proto-soils. Geobiology 2021, 19, 292–306. [Google Scholar]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Büdel, B.; Andreae, M.O.; Pöschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Guirado, E.; Reich, P.B.; Ochoa-Hueso, R.; Berdugo, M.; Sáez-Sandino, T.; Blanco-Pastor, J.L.; Tedersoo, L.; Plaza, C.; Ding, J.; et al. The global contribution of soil mosses to ecosystem services. Nat. Geosci. 2023, 16, 430–438. [Google Scholar] [CrossRef]
- Charoenkit, S.; Yiemwattana, S.; Rachapradit, N. Plant characteristics and the potential for living walls to reduce temperatures and sequester carbon. Energy Build. 2020, 225, 110286. [Google Scholar] [CrossRef]
- Charoenkit, S.; Yiemwattana, S. Living walls and their contribution to improved thermal comfort and carbon emission reduction: A review. Build. Environ. 2016, 105, 82–94. [Google Scholar] [CrossRef]
- Guillitte, O. Bioreceptivity: A new concept for building ecology studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Sáiz-Jiménez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of building stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef]
- Perini, K.; Castellari, P.; Giachetta, A.; Turcato, C.; Roccotiello, E. Experiencing innovative biomaterials for buildings: Potentialities of mosses. Build. Environ. 2020, 172, 106708. [Google Scholar] [CrossRef]
- Sanmartín, P.; Miller, A.Z.; Prieto, B.; Viles, H.A. Revisiting and reanalysing the concept of bioreceptivity 25 years on. Sci. Total Environ. 2021, 770, 145314. [Google Scholar] [CrossRef]
- Veeger, M.; Nabbe, A.; Jonkers, H.; Ottelé, M. Bioreceptive concrete: State of the art and potential benefits. Heron 2023, 68, 47–76. [Google Scholar]
- Cruz, M. Design for Ageing Buildings: An Applied Research of Poikilohydric Living Walls. In The Routledge Companion to Contemporary Architectural History; Routledge: London, UK, 2021; pp. 452–468. [Google Scholar]
- Veeger, M.; Prieto, A.; Ottelé, M. Exploring the possibility of using bioreceptive concrete in building façades. J. Facade Des. Eng. 2021, 9, 73–86. [Google Scholar]
- Mustafa, K.F.; Prieto, A.; Ottele, M. The role of geometry on a self-sustaining bio-receptive concrete panel for facade application. Sustainability 2021, 13, 7453. [Google Scholar] [CrossRef]
- Jakubovskis, R.; Malaiškienė, J.; Gribniak, V. Bio-colonization layered concrete panel for greening vertical surfaces: A field study. Case Stud. Constr. Mater. 2023, 19, e02394. [Google Scholar] [CrossRef]
- Cook, J.; Tonon, C.; Stohl, L.; von Werder, J. Assessment of concrete bioreceptivity and model organism performance for use in algal biofilm green facade systems. In BAM-Beiträge Zum Forschungskolloquium Green Intelligent Building, Proceedings of the 11. Jahrestagung des DAfStb mit 63. Forschungskolloquium, Berlin, Germany, 16–17 October 2024; Bundesanstalt für Materialforschung und-prüfung (BAM): Berlin, Germany, 2024; pp. 159–166. [Google Scholar]
- Elgaali, H.H.; Lopez-Arias, M.; Velay-Lizancos, M. Accelerated CO2 exposure treatment to enhance bio-receptivity properties of mortars with natural and recycled concrete aggregate. Constr. Build. Mater. 2024, 449, 138423. [Google Scholar] [CrossRef]
- Manso, S.; De Muynck, W.; Segura, I.; Aguado, A.; Steppe, K.; Boon, N.; De Belie, N. Bioreceptivity evaluation of cementitious materials designed to stimulate biological growth. Sci. Total Environ. 2014, 481, 232–241. [Google Scholar] [CrossRef]
- Veeger, M.; Ottelé, M.; Prieto, A. Making bioreceptive concrete: Formulation and testing of bioreceptive concrete mixtures. J. Build. Eng. 2021, 44, 102545. [Google Scholar] [CrossRef]
- Escadeillas, G.; Bertron, A.; Blanc, P.; Dubosc, A. Accelerated testing of biological stain growth on external concrete walls. Part 1: Development of the growth tests. Mater. Struct. 2007, 40, 1061–1071. [Google Scholar] [CrossRef]
- Escadeillas, G.; Bertron, A.; Ringot, E.; Blanc, P.J.; Dubosc, A. Accelerated testing of biological stain growth on external concrete walls. Part 2: Quantification of growths. Mater. Struct. 2009, 42, 937–945. [Google Scholar] [CrossRef]
- Giannantonio, D.J.; Kurth, J.C.; Kurtis, K.E.; Sobecky, P.A. Effects of concrete properties and nutrients on fungal colonization and fouling. Int. Biodeterior. Biodegrad. 2009, 63, 252–259. [Google Scholar] [CrossRef]
- De Muynck, W.; Ramirez, A.M.; De Belie, N.; Verstraete, W. Evaluation of strategies to prevent algal fouling on white architectural and cellular concrete. Int. Biodeterior. Biodegrad. 2009, 63, 679–689. [Google Scholar] [CrossRef]
- Stohl, L.; Cook, J.; von Werder, J. Bioreceptive Concrete Surfaces: Understanding Material-Biology Interactions for Façade Greening. In Proceedings of the 11. Jahrestagung des DAfStb mit 63. Forschungskolloquium der BAM, Berlin, Germany, 16–17 October 2024; pp. 148–153. [Google Scholar]
- Tzortzi, J.N.; Hasbini, R.A.; Ballottari, M.; Bellamoli, F. The living concrete experiment: Cultivation of photosynthetically active microalgal on concrete finish blocks. Sustainability 2024, 16, 2147. [Google Scholar] [CrossRef]
- Grosseau, P.; Dalod, E.; Govin, A.; Lors, C.; Guyonnet, R.; Damidot, D. Effect of the chemical composition of building materials on algal biofouling. In Concrete 2015/RILEM Week-27th Biennial National Conference of the Concrete Institute of Australia in Conjunction with the 69th RILEM Week; The Concrete Institute of Australia: Sydney, Australia, 2015; pp. 735–744. [Google Scholar]
- Wiktor, V.; Grosseau, P.; Guyonnet, R.; Garcia-Diaz, E.; Lors, C. Accelerated weathering of cementitious matrix for the development of an accelerated laboratory test of biodeterioration. Mater. Struct. 2011, 44, 623–640. [Google Scholar] [CrossRef][Green Version]
- Blanco, S.M.; de Cea, A.A.; Pérez, I.S.; De Belie, N. Bioreceptivity Optimisation of Concrete Substratum to Stimulate Biological Colonisation. Ph.D. Dissertation, Universitat Politècnica de Catalunya, Catalonia, Spain, 2014. [Google Scholar]
- Tran, T.H.; Govin, A.; Guyonnet, R.; Grosseau, P.; Lors, C.; Damidot, D.; Deves, O.; Ruot, B. Influence of the intrinsic characteristics of mortars on their biofouling by pigmented organisms: Comparison between laboratory and field-scale experiments. Int. Biodeterior. Biodegrad. 2014, 86, 334–342. [Google Scholar] [CrossRef]
- Manso, S.; Calvo-Torras, M.Á.; De Belie, N.; Segura, I.; Aguado, A. Evaluation of natural colonisation of cementitious materials: Effect of bioreceptivity and environmental conditions. Sci. Total Environ. 2015, 512, 444–453. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- de Carvalho, C.C. Biofilms: Microbial strategies for surviving UV exposure. In Ultraviolet Light in Human Health, Diseases and Environment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 233–239. [Google Scholar]
- Hofbauer, W.K.; Gärtner, G. Microbial Life on Façades; Springer Spektrum: Berlin/Heidelberg, Germany, 2021; pp. 29–191. [Google Scholar]
- Gadd, G.M.; Dyer, T.D. Bioprotection of the built environment and cultural heritage. Microb. Biotechnol. 2017, 10, 1152–1156. [Google Scholar] [CrossRef]
- Noeiaghaei, T.; Mukherjee, A.; Dhami, N.; Chae, S.R. Biogenic deterioration of concrete and its mitigation technologies. Constr. Build. Mater. 2017, 149, 575–586. [Google Scholar] [CrossRef]
- Yuan, H.; Dangla, P.; Chatellier, P.; Chaussadent, T. Degradation modeling of concrete submitted to biogenic acid attack. Cem. Concr. Res. 2015, 70, 29–38. [Google Scholar] [CrossRef]
- Soleimani, S.; Isgor, O.B.; Ormeci, B. Resistance of biofilm-covered mortars to microbiologically influenced deterioration simulated by sulfuric acid exposure. Cem. Concr. Res. 2013, 53, 229–238. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Bhuiyan, M.; Robert, D.; Roychand, R.; Gao, L.; Cole, I.; Pramanik, B.K. Bio-corrosion in concrete sewer systems: Mechanisms and mitigation strategies. Sci. Total Environ. 2024, 921, 171231. [Google Scholar]
- Madraszewski, S.; Dehn, F.; Gerlach, J.; Stephan, D. Experimentally driven evaluation methods of concrete sewers biodeterioration on laboratory-scale: A critical review. Constr. Build. Mater. 2022, 320, 126236. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Viles, H.A. A review of the nature, role and control of lithobionts on stone cultural heritage: Weighing-up and managing biodeterioration and bioprotection. World J. Microbiol. Biotechnol. 2020, 36, 100. [Google Scholar] [CrossRef]
- Bone, J.R.; Stafford, R.; Hall, A.E.; Herbert, R.J. Biodeterioration and bioprotection of concrete assets in the coastal environment. Int. Biodeterior. Biodegrad. 2022, 175, 105507. [Google Scholar] [CrossRef]
- Carter, N.E.A.; Viles, H.A. Experimental investigations into the interactions between moisture, rock surface temperatures and an epilithic lichen cover in the bioprotection of limestone. Build. Environ. 2003, 38, 1225–1234. [Google Scholar] [CrossRef]
- Concha-Lozano, N.; Gaudon, P.; Pages, J.; De Billerbeck, G.; Lafon, D.; Eterradossi, O. Protective effect of endolithic fungal hyphae on oolitic limestone buildings. J. Cult. Herit. 2012, 13, 120–127. [Google Scholar] [CrossRef]
- Villa, F.; Stewart, P.S.; Klapper, I.; Jacob, J.M.; Cappitelli, F. Subaerial biofilms on outdoor stone monuments: Changing the perspective toward an ecological framework. Bioscience 2016, 66, 285–294. [Google Scholar] [CrossRef]
- Cozzolino, A.; Adamo, P.; Bonanomi, G.; Motti, R. The role of lichens, mosses, and vascular plants in the bi-odeterioration of historic buildings: A review. Plants 2022, 11, 3429. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Talluri, S.N.; Winter, R.M.; Salem, D.R. Conditioning film formation and its influence on the initial adhesion and biofilm formation by a cyanobacterium on photobioreactor materials. Biofouling 2020, 36, 183–199. [Google Scholar] [CrossRef]
- Jain, A.; Bhosle, N.B. Biochemical composition of the marine conditioning film: Implications for bacterial adhesion. Biofouling 2009, 25, 13–19. [Google Scholar] [CrossRef]
- Rummel, C.D.; Lechtenfeld, O.J.; Kallies, R.; Benke, A.; Herzsprung, P.; Rynek, R.; Wagner, S.; Potthoff, A.; Jahnling, N.; Geissler, R.; et al. Conditioning film and early biofilm succession on plastic surfaces. Environ. Sci. Technol. 2021, 55, 11006–11018. [Google Scholar] [CrossRef]
- Bhagwat, G.; O’connor, W.; Grainge, I.; Palanisami, T. Understanding the fundamental basis for biofilm formation on plastic surfaces: Role of conditioning films. Front. Microbiol. 2021, 12, 687118. [Google Scholar] [CrossRef]
- Ruibal, C.; Selbmann, L.; Avci, S.; Martin-Sanchez, P.M.; Gorbushina, A.A. Roof-inhabiting cousins of rock-inhabiting fungi: Novel melanized microcolonial fungal species from photocatalytically reactive subaerial surfaces. Life 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Viles, H.A.; Gorbushina, A.A. Soiling and microbial colonisation on urban roadside limestone: A three year study in Oxford, England. Build. Environ. 2003, 38, 1217–1224. [Google Scholar] [CrossRef]
- Ali, I.A.A.; Cheung, B.P.K.; Yau, J.Y.Y.; Matinlinna, J.P.; Lévesque, C.M.; Belibasakis, G.N.; Neelakantan, P. The influence of substrate surface conditioning and biofilm age on the composition of Enterococcus faecalis biofilms. Int. Endod. J. 2020, 53, 53–61. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Parrilli, E.; Tutino, M.L.; Marino, G. Biofilm as an adaptation strategy to extreme conditions. Rend. Lincei. Sci. Fis. E Nat. 2022, 33, 527–536. [Google Scholar]
- Tenore, A.; Wu, Y.; Jacob, J.; Bittermann, D.; Villa, F.; Buttaro, B.; Klapper, I. Water activity in subaerial microbial biofilms on stone monuments. Sci. Total Environ. 2023, 900, 165790. [Google Scholar] [CrossRef]
- Villa, F.; Cappitelli, F. The ecology of subaerial biofilms in dry and inhospitable terrestrial environments. Microorganisms 2019, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.T.; Chan, D.J.C. Physiology of microalgal biofilm: A review on prediction of adhesion on substrates. Bioengineered 2021, 12, 7577–7599. [Google Scholar] [CrossRef]
- Moreno Osorio, J.H.; Pollio, A.; Frunzo, L.; Lens, P.N.L.; Esposito, G. A review of microalgal biofilm technologies: Definition, applications, settings and analysis. Front. Chem. Eng. 2021, 3, 737710. [Google Scholar] [CrossRef]
- Oliva, R.L.; Khadka, U.B.; Camenzind, T.; Dyckmans, J.; Joergensen, R.G. Constituent of extracellular polymeric substances (EPS) produced by a range of soil bacteria and fungi. BMC Microbiol. 2025, 25, 298. [Google Scholar] [CrossRef]
- Kanematsu, H.; Barry, D.M. Formation and Control of Biofilm in Various Environments; Springer Nature: Dordrecht, The Netherlands, 2020. [Google Scholar]
- Fuentes, E.; Vázquez-Nion, D.; Prieto, B. Laboratory development of subaerial biofilms commonly found on buildings. A methodological review. Build. Environ. 2022, 223, 109451. [Google Scholar] [CrossRef]
- Vázquez-Nion, D.; Rodríguez-Castro, J.; López-Rodríguez, M.C.; Fernández-Silva, I.; Prieto, B. Subaerial bio-films on granitic historic buildings: Microbial diversity and development of phototrophic multi-species cultures. Biofouling 2016, 32, 657–669. [Google Scholar] [CrossRef]
- Morris, P.A.; Brigmon, R.L. Minerals and Microorganisms in Evaporite Environments. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2010; Volume 2010. [Google Scholar]
- Zanardini, E.; May, E.; Purdy, K.J.; Murrell, J.C. Nutrient cycling potential within microbial communities on culturally important stoneworks. Environ. Microbiol. Rep. 2019, 11, 147–154. [Google Scholar]
- Carr, E.C.; Harris, S.D.; Herr, J.R.; Riekhof, W.R. Lichens and biofilms: Common collective growth imparts similar developmental strategies. Algal Res. 2021, 54, 102217. [Google Scholar] [CrossRef]
- Berti, L.; Villa, F.; Toniolo, L.; Cappitelli, F.; Goidanich, S. Methodological challenges for the investigation of the dual role of biofilms on outdoor heritage. Sci. Total Environ. 2024, 954, 176450. [Google Scholar] [CrossRef]
- Roldán, M.; Hernández-Mariné, M. Exploring the secrets of the three-dimensional architecture of phototrophic biofilms in caves. Int. J. Speleol. 2009, 38, 5. [Google Scholar] [CrossRef]
- Udawattha, C.; Galkanda, H.; Ariyarathne, I.S.; Jayasinghe, G.Y.; Halwatura, R. Mold growth and moss growth on tropical walls. Build. Environ. 2018, 137, 268–279. [Google Scholar] [CrossRef]
- Komar, M.; Nowicka-Krawczyk, P.; Ruman, T.; Nizioł, J.; Konca, P.; Gutarowska, B. Metabolomic analysis of photosynthetic biofilms on building façades in temperate climate zones. Int. Biodeterior. Biodegrad. 2022, 169, 105374. [Google Scholar] [CrossRef]
- De La Rosa, J.P.M.; Warke, P.A.; Smith, B.J. Lichen-induced biomodification of calcareous surfaces: Bio-protection versus biodeterioration. Prog. Phys. Geogr. 2013, 37, 325–351. [Google Scholar] [CrossRef]
- Deng, Z.; Huang, D.; He, Q.; Chassagne, C. Review of the action of organic matter on mineral sediment flocculation. Front. Earth Sci. 2022, 10, 965919. [Google Scholar] [CrossRef]
- Dong, H.L.; Huang, L.Q.; Zhao, L.D.; Zeng, Q.; Liu, X.L.; Sheng, Y.; Shi, L.; Wu, G.; Jiang, H.C.; Li, F.R.; et al. A critical review of mineral–microbe interaction and co-evolution: Mechanisms and applications. Natl. Sci. Rev. 2022, 9, nwac128. [Google Scholar] [CrossRef]
- Mahrous, R.; Giancola, E.; Osman, A.; Asawa, T.; Mahmoud, H. Review of key factors that affect the implementation of bio-receptive façades in a hot arid climate: Case study north Egypt. Build. Environ. 2022, 214, 108920. [Google Scholar] [CrossRef]
- Green, T.G.A.; Lange, O.L. Photosynthesis in poikilohydric plants: A comparison of lichens and bryophytes. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 319–341. [Google Scholar]
- Calabria, L.M.; Petersen, K.S.; Bidwell, A.; Hamman, S.T. Moss-cyanobacteria associations as a novel source of biological N2-fixation in temperate grasslands. Plant Soil 2020, 456, 307–321. [Google Scholar] [CrossRef]
- Guo, S.; Clasen, L.A.; Rousk, K. Short-term fate of nitrogen fixed by moss-cyanobacteria associations under different rainfall regimes. Basic Appl. Ecol. 2024, 79, 9–16. [Google Scholar] [CrossRef]
- Ramakrishnan, D.K.; Wassermann, B.; Berg, C.; Abdelfattah, A.; Berg, G. Mosses as extraordinary reservoir of microbial diversity: A comparative analysing of co-occurring ‘plant-moss twins’ in natural alpine ecosystem. Environ. Microbiome 2025, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, D.O.; Rousk, K. Unraveling host–microbe interactions and ecosystem functions in moss–bacteria symbioses. J. Exp. Bot. 2022, 73, 4473–4486. [Google Scholar] [CrossRef]
- Holland-Moritz, H.; Stuart, J.E.M.; Lewis, L.R.; Miller, S.N.; Mack, M.C.; Ponciano, J.M.; McDaniel, S.F.; Fierer, N. The bacterial communities of Alaskan mosses and their contributions to N2-fixation. Microbiome 2021, 9, 53. [Google Scholar] [CrossRef]
- Marsaglia, V.; Guido, B.; Paoletti, I. Moss as a multifunctional material for technological greenery systems. Plan J. 2023, 8, 85–114. [Google Scholar] [CrossRef]
- Perini, K.; Castellari, P.; Gisotti, D.; Giachetta, A.; Turcato, C.; Roccotiello, E. MosSkin: A moss-based light-weight building system. Build. Environ. 2022, 221, 109283. [Google Scholar] [CrossRef]
- Julinova, P.; Beckovsky, D. Perspectives of moss species in urban ecosystems and vertical living-architecture: A review. In Advances in Engineering Materials, Structures and Systems: Innovations, Mechanics and Applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 2370–2375. [Google Scholar]
- Adamatzky, A. Towards intelligent living facades: On electrical activity of ordinary moss Brachythecium rutabulum. bioRxiv 2024. [Google Scholar] [CrossRef]
- Veeger, M.; Veenendaal, E.M.; Limpens, J.; Ottelé, M.; Jonkers, H.M. Moss species for bioreceptive concrete: A survey of epilithic urban moss communities and their dynamics. Ecol. Eng. 2025, 212, 107502. [Google Scholar] [CrossRef]
- Stohl, L.; Manninger, T.; von Werder, J.; Dehn, F.; Gorbushina, A.; Meng, B. Bioreceptivity of concrete: A review. J. Build. Eng. 2023, 76, 107201. [Google Scholar] [CrossRef]
- Jiang, L.; Pettitt, T.R.; Buenfeld, N.; Smith, S.R. A critical review of the physiological, ecological, physical and chemical factors influencing the microbial degradation of concrete by fungi. Build. Environ. 2022, 214, 108925. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Ortega-Morales, B.O. Biodeterioration and chemical corrosion of concrete in the marine environment: Too complex for prediction. Microorganisms 2023, 11, 2438. [Google Scholar] [CrossRef] [PubMed]
- Polo, A.; Gulotta, D.; Santo, N.; Di Benedetto, C.; Fascio, U.; Toniolo, L.; Villa, F.; Cappitelli, F. Importance of subaerial biofilms and airborne microflora in the deterioration of stonework: A molecular study. Biofouling 2012, 28, 1093–1106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jang, K.M. Moss on Rocks: Evaluating Biodeterioration and Bioprotection of Bryophytic Growth on Stone Masonry. Ph.D. Dissertation, University of Oxford, Oxford, UK, 2020. [Google Scholar]
- Nagy, B.; Kustermann, A. Rehabilitation of porous building components and masonry by MICP injection method. Buildings 2023, 13, 1273. [Google Scholar] [CrossRef]
- Soleimani, S.; Ormeci, B.; Isgor, O.B. Growth and characterization of Escherichia coli DH5α biofilm on concrete surfaces as a protective layer against microbiologically influenced concrete deterioration (MICD). Appl. Microbiol. Biotechnol. 2013, 97, 1093–1102. [Google Scholar] [CrossRef]
- Gianangeli, A. A Novel Failure Model to Predict Biofouling (Algae Growth) on Different Building Facades Under Different Environmental Conditions. 2019. Available online: https://iris.univpm.it/handle/11566/263614 (accessed on 26 July 2025).
- Piontek, M.; Lechów, H. Biodeterioration Due to Improper Exploitation of External Facades. Civ. Environ. Eng. Rep. 2018, 28, 145–154. [Google Scholar] [CrossRef]
- Johansson, S. Biological Growth on Rendered Façades. Doctoral Thesis, Lund University, Lund, Sweden, 2011. [Google Scholar]
- Cardellicchio, F.; Bufo, S.A.; Mang, S.M.; Camele, I.; Salvi, A.M.; Scrano, L. The bio-patina on a hypogeum wall of the materasassi rupestrian church “San Pietro Barisano” before and after Treatment with Glycoalkaloids. Molecules 2022, 28, 330. [Google Scholar] [CrossRef]
- Romani, M.; Warscheid, T.; Nicole, L.; Marcon, L.; Di Martino, P.; Suzuki, M.T.; Lebaron, P.; Lami, R. Current and future chemical treatments to fight biodeterioration of outdoor building materials and associated biofilms: Moving away from ecotoxic and towards efficient, sustainable solutions. Sci. Total Environ. 2022, 802, 149846. [Google Scholar] [CrossRef]
- Malaiškienė, J.; Jakubovskis, R. Influence of Pozzolanic Additives on the Structure and Properties of Ultra-High-Performance Concrete. Materials 2025, 18, 1304. [Google Scholar] [CrossRef]
- de Sousa Camposinhos, R. Stone Cladding Engineering; Springer Netherlands: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Wang, J.; Shi, D.; Song, Y. (Eds.) Advanced Materials in Smart Building Skins for Sustainability: From Nano to Macroscale; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Jakubovskis, R. Concrete for living Walls: Current status and a new design recommendation. Buildings 2023, 13, 3067. [Google Scholar] [CrossRef]
- Zhong, W.; Schröder, T.; Bekkering, J. Biophilic design in architecture and its contributions to health, well-being, and sustainability: A critical review. Front. Archit. Res. 2022, 11, 114–141. [Google Scholar] [CrossRef]
- Sayadi, S.; Hayati, A.; Salmanzadeh, M. Optimization of window-to-wall ratio for buildings located in different climates: An IDA-indoor climate and energy simulation study. Energies 2021, 14, 1974. [Google Scholar] [CrossRef]
- Goodrum, W.M.; Zhai, Z.J.; Robles, M. Impacts of architectural beauty to building energy performance. Archit. Struct. Constr. 2023, 3, 87–111. [Google Scholar] [CrossRef]
- Kozamernik, J.; Rakuša, M.; Nikšič, M. How green facades affect the perception of urban ambiences: Comparing Slovenia and the Netherlands. Urbani Izziv 2020, 31, 88–100. [Google Scholar] [CrossRef]
- Kellert, S.; Calabrese, E. The Practice of Biophilic Design; Terrapin Bright LLC: London, UK, 2015; Volume 3. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).