Abstract
The paper presents the results and assessment of the properties and capabilities of new types of surface hydrophobic agents for the treatment of fresh and matured surfaces of concrete and other cement-bound layers. Hydrophobisation prevents the premature evaporation of water and thus plastic shrinkage, which inevitably leads to the formation of cracks in cement-based structures. The influence of the new type of hydrophobic agents, epoxy water-based (EWH) and acrylate (AH) containing solvent, on the physical and mechanical properties of the treated concrete samples was assessed, including the adhesion of hydrophobisation on the concrete surface layers. It was confirmed that surface hydrophobisation successfully prevents premature evaporation of water, and thus, plastic shrinkage (concrete treated with EWH_0.3 showed more than two times lower shrinkage than reference concrete). The concrete samples treated by hydrophobisation agents showed higher strength after 120 days (37.5 MPa) in comparison with untreated concrete (32.8 MPa). Different properties were recorded with different types of hydrophobisation agents, including compressive strength after 90 days (31.2 MPa with EWH_0.15, and 35.9 MPa with AH_0.15). Water absorption after 120 days was lowest with EWH_0.3 samples (3.77%), two times lower than AH_0.15 (6.98%). The layer of hydrophobisation agent EWH_0.3 was thicker than AH_0.15, leading to lower water absorption of treated concrete and higher resistance to defrosting chemicals—waste 8.5. g/m2 with EWH_0.3 in comparison to 35.7 g/m2 with AH_0.15. Furthermore, a difference in the hydrophobisation behaviour was shown with blocking of infiltration from the chemically aggressive environment into the concrete substrate. Deterioration of the surface concrete exposed to 10% HCl solution was worse with AH_0.15; but the concrete surface treated with AH_0.15 showed better resistance than the EWH treated surface, when exposed to 5% CH3COOH. Degradation of the hydrophobisation integrity and the loss of cohesion between the concrete and the surface treatment after exposure to acetic acid and hydrochloric acid was observed using scanning electron microscopy (SEM).
1. Introduction
If a concrete or other cement-based material is stressed due to plastic shrinkage as a result of drying or self-drying in the case of high-quality concretes, it may be damaged. This happens mainly due to non-compliance with measures intended to reduce the undesirable shrinkage of concrete. The pore size and gel characteristics of C-A-S-H have been considered as the main factors affecting the cement matrix shrinkage, and the formation of silica-gel hydration products, which are prone to shrinkage, partially explains the high drying shrinkage [1]. Products that eliminate plastic shrinkage, such as hydrophobisation agents, can provide a solution. However, the standard products are solvent-based; they contain about 70–90 wt.% of volatile solvents, which limits their effectiveness in the elimination of shrinkage. Their annual consumption in the Czech Republic is in the hundreds of tons, and a considerable amount of solvents evaporate into the air. Water-based hydrophobic agents without these solvents provide a more effective solution, but their development is still in the early stages. Such compositions are based on a different chemical base and contain more dry matter, which reduces the plastic shrinkage of the cement-bound layers much more effectively. It is preferable if they contain water instead of solvents, the evaporation of which does not produce any toxic emissions. When hydrophobising the concrete surface or other cement composites, a thin coating of liquid-repellent that is permeable to gases and water vapor is formed in the surface layer of the capillaries and pores [2]. The long-term effectiveness of surface hydrophobisation for un-cracked concrete depends mainly on the achieved penetration depth [3]. Surface treatment with hydrophobic agents is commonly used to improve the resistance of concrete against the penetration of fluids in aggressive environment, both in new and existing structures [4]. Water-repellent additive for concrete is effective in increasing strength, decreasing shrinkage, and increasing cracking resistance of concrete cured in dry environments [5]. For coastal areas with large annual rainfall, due to the very high relative humidity, the depth of penetration of the liquid solution into the concrete is significant, providing a medium for the transmission of corrosive ions into concrete [6]. Hydrophobisation, which creates a waterproof surface on the concrete, causes an increase in the so-called wetting angle for water. It is a well-established fact that hydrophobic treatment increases the water repellence of cement-based surfaces but does not fill the pores of the substrate, so it does not have the capability of inhibiting water penetration while under pressure [7]. The vast majority of hydrophobic impregnation formulations are made from a mixture of hydrophobising agents and auxiliary materials, and they are non-polar compounds containing carbon chains. Hydrophobic impregnations have been used in various forms in the construction industry to help prevent water and chloride ingress and their benefits are well documented [8,9,10]. The decrease in water penetration attributable to hydrophobic treatments decreases water loss of concrete and increases concrete resistance to freeze-thaw cycles [11].
Concretes with surface protection showed lower carbonation diffusion coefficients than non-protected concretes, due to the barrier effect introduced by the use of protective hydrophobic agents. The use of epoxy hydrophobisation agents showed better protection than the use of acrylic and siloxane resins [12]. Silanes belong to the pore liner category and are a group of silicones containing one silicon atom [13]. Alkoxy and alkyl silanes are routinely used for surface impregnations [14]. Of the hydrophobic agents, waterborne systems (microemulsions) have the combined advantages of being low-cost and environmentally friendly over 100% of active substances (such as silanes, siloxane oligomers or a mixture of these two components) and solvent-based hydrophobic agents. Nonetheless, the performance information of the waterborne hydrophobic agents is quite limited when compared with those of the solvent-based hydrophobic agents [15,16,17].
Hydrophobic impregnations are generally applied to reinforced concrete. Therefore, they must be resistant to the strong alkalinity (high pH) of the concrete, heat, UV radiation and low temperature [15,18]. The hydrophobic agents should sufficiently penetrate the impregnated cement composites deep enough to guarantee long-term durability [19]. The penetration depth of the waterborne hydrophobic agents results primarily from the micelle size, which plays an important role in determining the penetration depth of a waterborne hydrophobic agent [20]. Hydrophobic agents are also added directly in the concrete mixture in order to make both the surface and the whole concrete bulk hydrophobic [21,22]. The compatibility and interaction of hydrophobic treatment with concrete are evaluated by running a microstructure analysis, e.g., scanning electron microscopy (SEM) [23]. The improvement in durability of cement composites is attributed to the chemical reactions in the transition zone between cement paste and polymer hydrophobisation [24].
2. Materials and Methods
2.1. Hydrophobic Agents and Substrate
The tested materials were new types of polymer sprays that serve as hydrophobisation agents of silicate substrates, mainly concrete surfaces. The leading ability of the developed materials is that they can prevent the evaporation of water from concrete structures. This also prevents plastic shrinkage, which inevitably leads to the formation of cracks in cement-based materials and structures.
The tested hydrophobic agents are based on different polymer bases. There is a two-component water-based epoxy material (EWH) and a one-component material based on acrylate and solvents such as xylene (AH). The hydrophobic agents used in this research were newly developed and manufactured by IN-CHEMIE Technology Ltd. (Olomouc, Czech Republic), and are commercially available as from mid-2022. Both substances are intended for concrete floors, cement screeds and other concrete structures. The ratio of components A (epoxy resin based on Bisphenol A) and B (hardener based on the polyamine) of the water-soluble hydrophobisation was 1:1.08. For specific tests, the materials were delivered including a suitable pigment for better monitoring of the depth of impregnation into the substrate in connection with the given consumption of the material and some other properties. Both hydrophobisation agents were applied on the concrete surface by spraying on the surface. EWH hydrophobisation was applied in two ways, namely with a consumption of 0.15 kg/m2 (EWH_0.15) as hydrophobic impregnation which serves to reduce capillary water transport, and 0.30 kg/m2 (EWH_0.3) for ensuring long-term hydrophobisation of concrete. AH hydrophobisation was applied with a consumption of 0.15 kg/m2 (AH_0.15). The basic properties of the hydrophobic agents are reported in Table 1.

Table 1.
Basic properties of the hydrophobic agents.
Vibro-compacted concrete paving blocks were used as the substrate and the reference material (C_REF), having dimensions of 400 × 400 mm, and concrete class C25/30 that meets the parameters of the EN 1339 standard [25]. Concrete prisms with dimensions of 40 × 40 × 160 mm, prepared in laboratory conditions, were used as the substrate for strength tests, water absorption and shrinkage determination. The concrete mix for the prisms was based on 1350 g of standardised sand, 450 g of CEM II/B-M (S-LL) 32.5 R cement and 225 mL of water (water/cement ratio = 0.5). The prisms were cast in metal triple mould. The basic parameters of the substrates are reported in Table 2. The amounts of hydrophobic agents applied on the concrete surface in grams are presented in Table 3.

Table 2.
Basic properties of the substrate.

Table 3.
Weights of hydrophobic agents applied on concrete surfaces in [g].
2.2. Experimental Methods
2.2.1. Dynamic Viscosity
The EN ISO 255 standard [26] was used to determine the dynamic viscosity of the hydrophobic agents. An Anton Paar rotary viscometer, type ViscoQC 300 (Anton Paar GmbH, Graz, Austria), with a viscosity measurement range of 1 mPa·s to 6,000,000 mPa·s was used for the measurement. Viscosity was determined using the function of automatic adjustment of optimal spindle speed.
2.2.2. Thickness of Hydrophobic Agent
It is also important to monitor the bond of the hydrophobisation with the underlying concrete and the thickness of the resulting hydrophobisation layer for a given hydrophobisation coverage. The thickness of the hardened hydrophobisation layer on concrete pavements was determined using a digital optical microscope at appropriate magnification.
2.2.3. Adhesion on Concrete
The testing of adhesion of the hydrophobic agents on the concrete surface was performed in accordance with the standard EN ISO 4624 [27]. Before testing, the hydrophobic agents were applied on the concrete paving. After three days, metal targets with diameters of 20 mm were glued on the polymerised surfaces of the samples with hydrophobisation agents using a two-component epoxy adhesive. After the adhesive hardened, the targets were cut with a cutting tool down to the concrete substrate. Then, the targets were pulled off using the Elcometer 506 pull-off adhesion tester (Elcometer Instruments Ltd., Manchester, UK). The average value of hydrophobisation adhesion on the concrete was determined from three measurements.
2.2.4. Abrasion Resistance
To determine the resistance to wear according to Böhme in accordance with standard EN 13892-3 [28], vibro-compacted concrete pavement elements were used as the base material, on which tested hydrophobisation agents were applied to the required consumption. After seven days, the samples prepared in this way were cut to the required size, namely 71 × 71 mm. The test specimens were clamped in the N-1001 RT Böhm abrasion resistance tester (FORM + TEST Seidner & Co. GmbH, Riedlingen, Germany) on a grinding track. The samples were subjected to 16 test cycles, when a corundum was used as the abrasive. After each cycle (22 revolutions), the sample was rotated 90° and the abrasive was replaced with a new one. The test was carried out 28 days after the samples were made. Abrasion resistance by the Böhme method after 16 cycles was determined as a reduction in the volume of the test specimen. The abrasion resistance test was determined on 3 samples from each material, including the reference concrete.
2.2.5. Impact Resistance
To determine the impact resistance of the surface treatments in accordance with standard EN ISO 6272-2 [29] using the TQC impact test model SP1880 (TQC Sheen B.V., Capelle aan den Ijssel, Netherlands), the same pavement elements as in the previous tests were used as the base material. After 28 days from spraying the hydrophobic agent on the concrete surface, a free-falling weight was lowered and the resistance of the hydrophobising agent against cracking or peeling was monitored. By gradually increasing the height from which the weight was dropped, the value at which hydrophobisation damage occurred was determined. The result of the test refers to the drop height at which the specimen failed. The average value of hydrophobisation impact resistance was determined from five measurements.
2.2.6. Flexural and Compressive Strength
The flexural strength was determined using the three-point test according to the standard EN 12390-5 [30], and the compressive strength was determined in accordance with EN 12390-3 standard [31]. The testing equipment model RT 200/10-1 D servo (ratioTEC Prüfsysteme GmbH, Langenenslingen, Germany) was used for the compressive and flexural strength determination. As test samples, concrete prisms with dimensions of 40 × 40 × 160 mm were used. Hydrophobic agents were applied on the top side of the concrete surface, 24 h after unmoulding—a simulation of the application of hydrophobisation agents on real structures (e.g., a highway, where hydrophobisation agents are applied only on the top side exposed to the environment). The samples were tested after 28 days from the application of the hydrophobisation agents. The flexural strength was determined on 3 samples from each material, including the reference concrete. The average compressive strength was determined with the remains of the prisms after the determination of the flexural strength and was based on six values.
2.2.7. Shrinkage
The determination of the concrete shrinkage refers to the EN 12390-16 standard [32]. The aim of the test was the monitoring of concrete samples that are subject to changes in length under defined conditions of temperature and relative humidity. To determine the shrinkage, prisms of 40 × 40 × 160 mm size, with the same composition as the prisms used for strength determination, were first prepared and then demoulded after 24 h. The length of the samples was measured, a hydrophobic material was then applied on their entire surface in the given consumption, and the samples were placed in the cooled incubator Memmert ICP110 (MEMMERT GmbH, Schwabach, Germany) where the temperature was maintained at 20 °C and the relative humidity was 60%. Subsequently, the change in the length of the samples was monitored after 28, 90 and 120 days. The samples were measured at regular intervals, always in the same marked place, and the changes in length and shrinkage were monitored. The samples were also compared with the reference concrete, i.e., with samples without hydrophobic treatment. No surface cracking was observed in any of the monitored samples. The shrinkage was determined for three samples from each tested material (hydrophobised and reference concrete).
2.2.8. Water Absorption
The purpose of hydrophobising materials is to reduce the absorption of concrete structures. To determine the absorption of a concrete surface treated with hydrophobic agents, 40 × 40 × 160 mm prisms of the same composition as the samples used for the determination of strength were produced. Hydrophobisation agents were applied on their entire surface. Based on the EN 14617-1 [33] standard, the prisms prepared in this way were stored in an aquatic environment, and the maximum amount of water absorbed by the material during immersion in deionized water, at room temperature and pressure, was monitored by weighing the samples at intervals. The samples were also compared with reference concrete, i.e., with samples without hydrophobic coating. Water absorption was monitored over time: after 24 h, 28, 90 and 120 days of storage in water. The average water absorption was determined for three samples from each tested material (hydrophobised and reference concrete).
2.2.9. Resistance to Defrosting Chemicals
Resistance of the concrete samples to defrosting chemicals, treated by different hydrophobisation agents, was determined according to ČSN 73 1326 [34]. From the results of this test, the durability of the surfaces of concrete roads in the winter season when using chemical de-icing substances, was assessed. Samples with dimensions 140 × 140 mm were used for these tests, trimmed from the concrete pavement samples, on which hydrophobisation was also applied as for the other tests. The test was conducted after 28 days from the application of hydrophobisation agents on the concrete surface, while only the top surface mostly exposed to the 3% NaCl solution was treated by the hydrophobisation. The surface of the samples not treated with hydrophobisation was coated with epoxy resin. The samples were immersed in the solution so that the solution level reached a height of 5 mm above the tested surface. In total, 100 cycles of freezing and thawing were performed in the automatic device KD 20 (EKOFROST Ltd., Olomouc, Czech Republic) for testing the frost resistance and surface resistance of building materials against frost. Individual cycles consisted of cooling the tested surface to a temperature of −15 °C and heating it back to 20 °C, whereby the highest and lowest temperatures were maintained for at least 15 min. In total, one cycle lasted 2–2.5 h and the weight of the waste was determined after each 25 cycles. The resistance of the concrete surface against defrosting agents was determined by the weight of the waste per unit area in g/m2 after 100 cycles. The average resistance of the concrete to defrosting chemicals was determined for three samples from each tested material.
2.2.10. Chemical Resistance
After 28 days, inverted polyethylene funnels were attached to the surface of the concrete pavement samples, provided with the tested hydrophobisation, with silicone. A measured amount of the selected chemically aggressive media in the given concentrations was subsequently poured in the funnels, so that the level height was approximately 5 mm. The top ends of the funnels were sealed with plasticine to prevent the chemicals from evaporating into the environment. There was a total of five different chemically aggressive environments (5% H2SO4, 10% HNO3, 10% HCl, 5% CH3COOH and engine oil), while the effect of distilled water was also monitored. Aggressive substances and their concentration were chosen on the basis of aggressive conditions that act on many concrete structures in practical situations. Sulphate attack greatly reduces the service life of reinforced concrete structures, especially in partially filled concrete sewer pipes exposed not only to sulphates from waste water, but also to sulfuric acid produced during biogenic sulphate corrosion by the activity of bacteria. Therefore, the resistance of hydrophobised surfaces to sulfuric acid was also monitored. The environment in many cities is contaminated by the incomplete combustion of fuels and various industrial pollutants. These emissions produce nitrogen gas that reacts with water to produce a nitric acid compound that affects the durability of reinforced concrete structures [35]. Acetic acid is an organic acid produced from aerobic and anaerobic digestion that also corrodes the durability of concrete objects [36]. Hydrochloric acid is used in the metal industry as well as in the manufacture of fertilizers, dyes, and pigments, and the waste water from these industries can be very aggressive for concrete structures [37]. The increased permeability of concrete allows more hydrocarbons from engine oil into the interface of concrete repeatedly, and then hardened concrete deteriorates due to chemical attack [38]. Overall, the resistance of concrete treated with three different hydrophobisation agents to six types of liquid environments was monitored. The chemically aggressive environments acted on one selected spot of the hydrophobised concrete surface, while the stressed surface area considered was 2500 mm2 for all aggressive environments. The chemical resistance of the applied hydrophobisation was evaluated after 28 days of exposure to chemically aggressive environments. The assessment was conducted visually and also with the aid of a Keyence VHX950F digital optical microscope (Keyence Ltd., Osaka, Japan). A universal concrete saw was used to accurately cut the concrete paving blocks for the preparation of samples for the digital optical microscopy, intended to observe the influence of chemical aggressive media on treated concrete surfaces. The microstructure of most disrupted hydrophobised concrete due to chemical stress was examined using a scanning electron microscope (SEM) to assess the deterioration of hydrophobisation and the concrete surface in more detail. Samples for SEM were scraped off from the hydrophobised concrete surface exposed to chemical aggressive media, and then they were prepared by sputtering with gold in a high vacuum.
3. Results and Discussion
3.1. Dynamic Viscosity
The dynamic viscosity results of the tested hydrophobic agents are shown in Table 4. It can be seen that the water-soluble EWH hydrophobisation exhibited approximately 50× higher viscosity than the AH acrylate hydrophobisation. Not only the porosity of the concrete surface, but also the viscosity of the hydrophobisation affects the penetration of the surface treatment into the concrete surface.

Table 4.
Dynamic viscosity of tested hydrophobic agents.
3.2. Thickness of Hydrophobisation
The evaluation of the measurement of the thickness of the applied hydrophobisation agents and the depth of their penetration into the concrete surface after 28 days from their application can be seen in Figure 1. The average values of the thicknesses of individual hydrophobisation agents are shown in Table 5. The average value of the hydrophobisation thickness was determined from five measurements at different spots on the concrete cross-section, using a digital optical microscope. The highest average thickness was measured for hydrophobisation EWH_0.30. As can be seen in Figure 1, the layer on the surface of the concrete and also the depth of penetration of the hydrophobisation into the open structure of the concrete surface are considered to be the total thickness of the polymerised hydrophobisation. The thickness and penetration of hydrophobisation into the concrete surface were more uniform with surface treatment AH_0.15. The concrete substrate was the same for all hydrophobisations, while it can be seen that especially with the epoxy hydrophobisation EWH_0.3, not only the open pores of the concrete were covered but also a protective layer was formed on the surface of the concrete. Comparing acrylic and epoxy surface treatments with the same consumption, it can be seen that the AH_0.15 layer is approximately eight times thinner than EWH_0.15. The main reason may be the evaporation of xylene during the polymerization of acrylate hydrophobic agents.
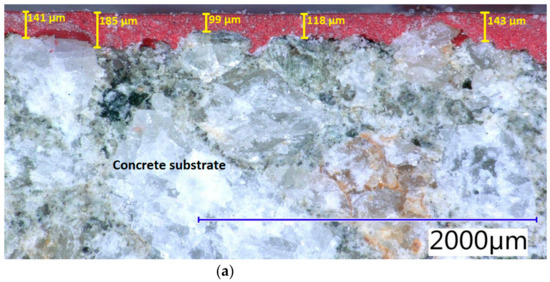

Figure 1.
Thicknesses of the hydrophobic agents including the depth of penetration in the concrete surface determined by the digital optical microscope at real magnification 32×: (a) EWH_0.15; (b) EWH_0.3; (c) AH_0.15.

Table 5.
Results of average thickness of the hydrophobic agents applied on the concrete.
3.3. Adhesion on Concrete
With water-based hydrophobisation (EWH_0.15 and EWH_0.30) cohesive failure occurred in the underlying concrete, and solvent-based hydrophobisation (AH_0.15) adhesive failure occurred in the adhesive—see Figure 2. Adhesion higher than 3.5 MPa was recorded for all tested hydrophobisations, which confirms excellent cohesion of the hydrophobisations with concrete. The adhesion of surface treatment to the concrete base ensures the efficiency of hydrophobisation [39]. From the results, it can be concluded that the minimum adhesion on concrete is the same for all tested hydrophobisations.
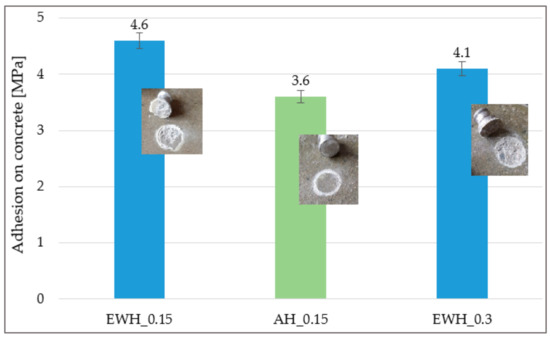
Figure 2.
Results of the adhesion of different hydrophobisation agents on the concrete.
3.4. Abrasion Resistance
Samples before and after the test are also shown in Figure 3, while the hydrophobisation from the concrete surface was almost completely removed after 16 cycles in the case of the AH_0.15 samples. Dang et al. [40] found that most polymer surface treatments could improve the abrasion resistance of concrete. Based on the results, it can be concluded that the applied hydrophobisation increases the abrasion resistance of the concrete. The highest abrasion resistance was shown for AH_0.15 samples, in which the smallest loss of material was observed after the abrasion resistance test—see Figure 3. However, it can also be concluded from the results that the water-soluble epoxy hydrophobisation layer showed higher abrasion resistance than the concrete itself.
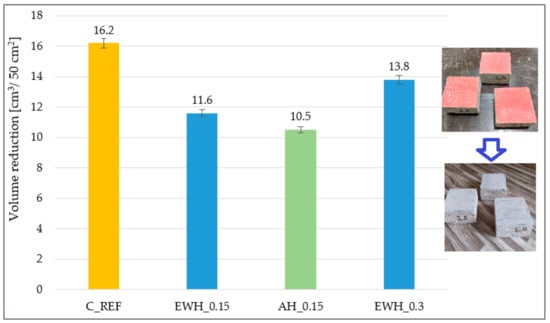
Figure 3.
Results of the abrasion resistance of the concrete samples treated by different hydrophobisation agents and reference sample.
3.5. Impact Resistance
In Figure 4 the results of impact resistance of tested hydrophobisation can be observed. There are also imprints from falling weights which, at the determined impact height, caused damage to the hydrophobisations—cracks, disruption of integrity, peeling, etc. The highest impact resistance (height of the falling weight) was recorded for hydrophobisation EWH_0.3. This fact is mainly related to the highest consumption, which created the strongest barrier to the falling weight. Gupta et al. [41] proved that credible correlation exists between the results of drop weight test, flexural strength of concrete and rebound test.
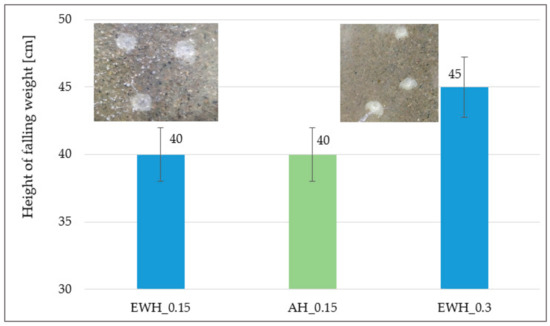
Figure 4.
Results of the impact resistance of the hydrophobic agents.
3.6. Flexural and Compressive Strength
Graphical evaluation of three-point flexural strength and compressive strength of concrete samples, on the top surface of which hydrophobisation agents were applied, and reference samples are shown in Figure 5 and Figure 6. It is evident from the results that hydrophobisation AH_0.15 showed the most positive effect on the concrete strength. The compressive strength after 90 days was the highest for samples provided with water-soluble hydrophobisation EWH_0.3. The application of hydrophobisation EWH_0.15 had almost no effect on concrete strength. The biggest difference in compressive strength was recorded after 28 days from the application of hydrophobisation. It is widely accepted that most surface treatments cannot directly improve the strength of concrete because they cannot improve the quality and porosity of the whole concrete object [42]. For a better comparison of strengths, it would be suitable to apply hydrophobisation agents on the entire surface of the concrete samples.
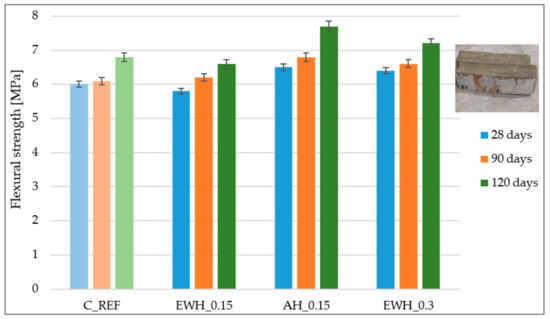
Figure 5.
Results of the three-point flexural strength of the concrete samples treated by different hydrophobisation agents and reference samples.

Figure 6.
Results of the compressive strength of the concrete samples treated by different hydrophobisation agents and reference samples.
3.7. Shrinkage
From Figure 7 it can be clearly seen that the most positive effect on concrete shrinkage was shown by hydrophobisation EWH_0.3, when after 120 days a shrinkage of less than 0.6mm/m was recorded. Compared to untreated concrete (C_REF), concrete treated with EWH_0.3 showed up to three times lower shrinkage. It can therefore be assumed that the application of water-soluble hydrophobisation can eliminate the occurrence of cracks arising mainly due to plastic shrinkage of concrete. Shi et al. [43] showed that the thicker the polymer surface treatment was and the earlier the resin was applied, the greater shrinkage reducing ratio is observed.
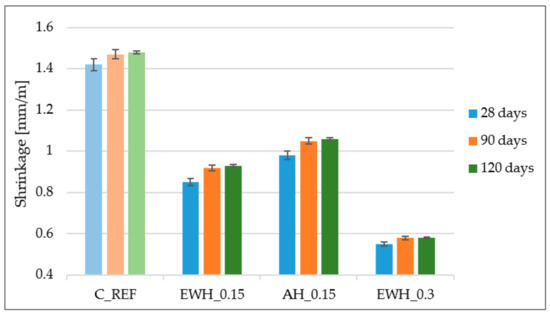
Figure 7.
Shrinkage of the concrete samples treated by different hydrophobisation agents and reference sample.
3.8. Water Absorption
One of the most important roles of hydrophobic agents is the reduction in concrete water absorption [44]. Water absorption of concrete samples treated with AH_0.15 hydrophobisation was approximately the same as that of the reference concrete: 7% after 120 days. Figure 8 demonstrates that the lowest water absorption was recorded in the samples treated by EWH_0.3 hydrophobisation, when after 120 days the water absorption was only 3.8%. From the point of view of water absorption results, water-soluble epoxy-based hydrophobisation showed a much more positive effect than acrylate hydrophobisation, even at the same consumption. Hydrophobic agents migrated into concrete and formed a hydrophobic barrier on the surface of concrete to improve waterproofing performance [45].
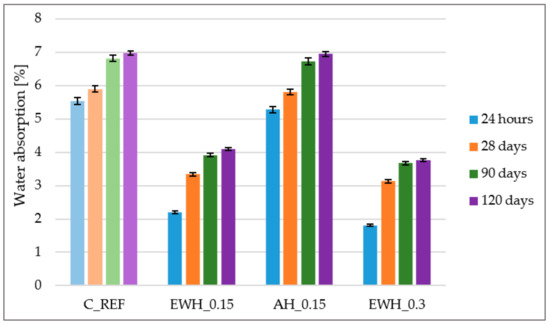
Figure 8.
Water absorption of the concrete samples treated by different hydrophobisation agents and reference sample.
3.9. Resistance to Defrosting Chemicals
The evaluation of the resistance to defrosting chemicals of concrete samples treated with hydrophobisation agents and reference concrete was based on the evaluation of the waste after 100 freezing and thawing cycles and is presented in Figure 9. The results demonstrate that the best resistance to defrosting agents was shown by samples provided with EWH_0.3 hydrophobisation. Resistance to de-frosting chemicals was improved by more than 10 times when compared to the reference concrete in this case. Water-soluble hydrophobisation at a consumption of 0.15 kg/m2 showed better resistance to defrosting agents than solvent-based hydrophobisation AH_0.15. From the results, it can be concluded that by applying EWH hydrophobisation, a significant improvement in resistance to defrosting agents can be achieved. This can be applied, for example, on concrete roads in winter. Mamaghani et al. [46] found out that the epoxy-based hydrophobic agents and the silane-based water repellent exhibit better performance in providing resistance to salt scaling, compared with other polymer surface treatments.

Figure 9.
Resistance to defrosting chemicals.
3.10. Chemical Resistance
Samples were exposed to different liquids in chemically aggressive environments for 28 days. The damaged samples were analysed using a digital optical microscope at optimal magnification to observe the resulting defects and are presented in Figure 10, Figure 11 and Figure 12. All tested hydrophobisations exposed to distilled water showed almost no change after 28 days and the treated concrete remained intact. The hydrophobisation AH_0.15 best resisted the acidic aggressive environment in the form of 5% H2SO4. Even though the hydrophobic layer was damaged, with deposits formed and colour change to light yellow, the hydrophobic agent prevented the penetration of sulfuric acid into the surface layers of concrete, more than in other cases—see Figure 10a,b. The water-soluble hydrophobisation of EWH, in both cases considered, changed its colour to orange after the action of 5% H2SO4 with surface etching and the formation of blisters evident (Figure 11a and Figure 12a). After drying, the hydrophobisation layer peeled off; however, intact concrete was visible under the formed residues—see Figure 11b and Figure 12b. Epoxy surface treatments provide a protective membrane on the concrete surface, which can be hardly penetrated by sulfates, thus mitigating capillary rise on the concrete [47]. The 10% HNO3 solution penetrated into the surface layers of concrete mostly in the case of the sample on which AH_0.15 hydrophobisation was applied (Figure 10c,d). Even though water-soluble EWH hydrophobic agents lost cohesion with the underlying concrete and formed microcracks in both applications, 10% HNO3 did not significantly affect the surface of the concrete after 28 days of exposure—see Figure 11c,d and Figure 12c,d. The aggressive environment in the form of 10% HCl caused the most significant degradation of the hydrophobisation and subsequently the surface layers of concrete. Disruption of the hydrophobisations and subsequent penetration into the concrete surface to a depth of approximately 3 mm is clearly visible in the cut sections as presented in Figure 10f, Figure 11f and Figure 12f. The average depth of concrete damage by a 10% HCl solution was the highest in the sample treated with hydrophobisation AH_0.15. This is related to the smallest thickness of this hydrophobisation, and therefore the weakest barrier to the penetration of aggressive substances into the concrete. Water-soluble epoxy hydrophobisations were significantly less resistant to acetic acid, with cracking in the entire layer visible after 28 days (Figure 11g and Figure 12g) and subsequent penetration into the concrete to a maximum depth of 300 µm (Figure 11h and Figure 12h). Immersion of epoxy-based coatings in acetic acid significantly affected the coating resistance [48]. The chemical structure of epoxy resin, hardener and additives affect diffusion of solutions into cured epoxy resin [49]. On the other hand, AH hydrophobisation containing xylene prevented concrete degradation to a considerable extent, in the case of acetic acid attack (Figure 10g,h). On exposure of fuel oil on the hydrophobised concrete surface, only a colour change was visible on the hydrophobised surface. No loss of cohesion between the hydrophobisation and the concrete was observed, and neither was the integrity of the hydrophobisation, which ensures the protection of the concrete affected—see Figure 11i,j. It has been already proven that in highly aggressive environments, surface treatment can significantly reduce the concrete degradation and steel corrosion which affect strength and stability of concrete structures [50].
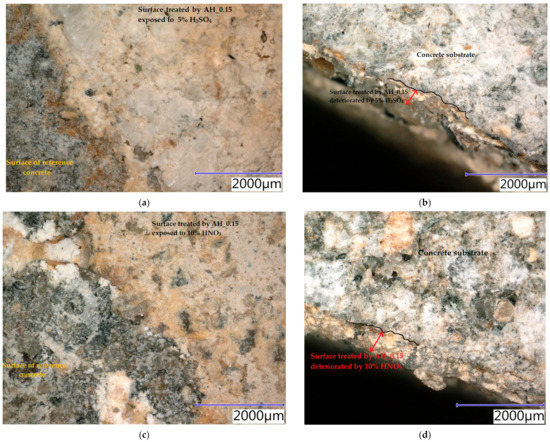
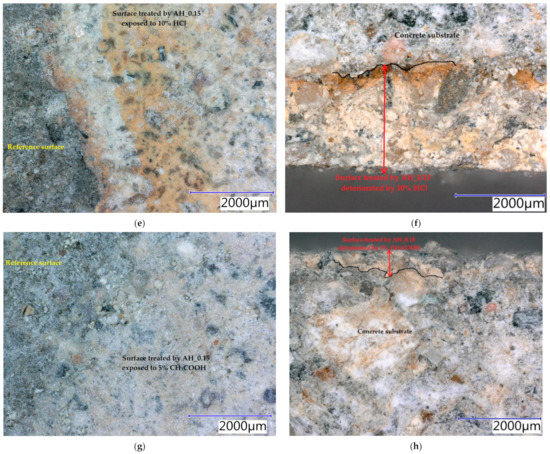
Figure 10.
Concrete samples treated by the hydrophobic agent AH_0.15 exposed to different liquid chemical aggressive environment after 28 days: (a) surface: 5% H2SO4; (b) cut edge: 5% H2SO4; (c) surface: 10% HNO3; (d) cut edge: 10% HNO3; (e) surface: 10% HCl; (f) cut edge: 10% HCl; (g) surface: 5% CH3COOH; (h) cut edge: 5% CH3COOH.
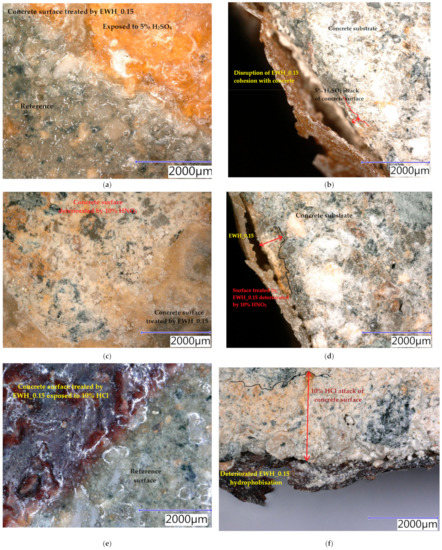
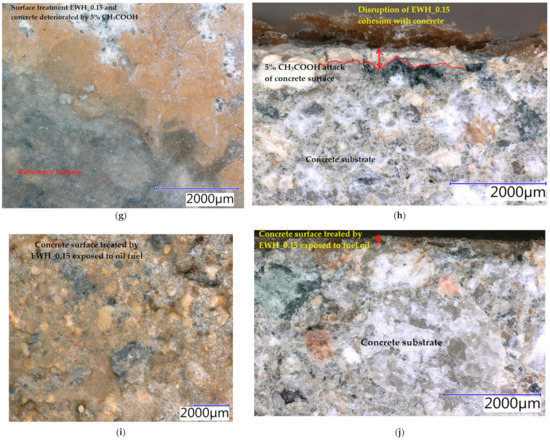
Figure 11.
Concrete samples treated by the hydrophobic agent EWH_0.15 exposed to different liquid chemical aggressive environment after 28 days: (a) surface: 5% H2SO4; (b) cut edge: 5% H2SO4; (c) surface: 10% HNO3; (d) cut edge: 10% HNO3; (e) surface: 10% HCl; (f) cut edge: 10% HCl; (g) surface: 5% CH3COOH; (h) cut edge: 5% CH3COOH; (i) surface: oil fuel; (j) cut edge: oil fuel.
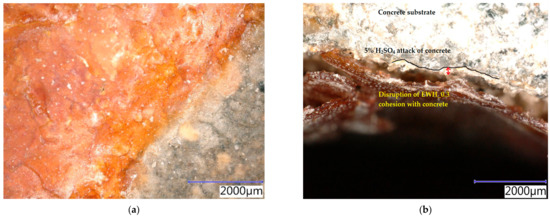
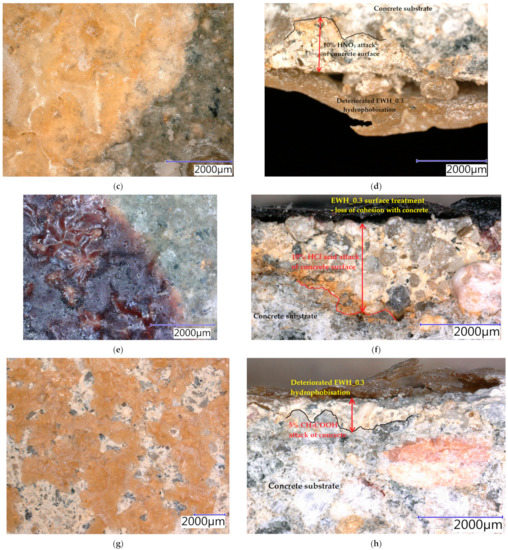
Figure 12.
Concrete samples treated by the hydrophobic agent EWH_0.3 exposed to different liquid chemical aggressive environment after 28 days: (a) surface—5% H2SO4; (b) cut edge: 5% H2SO4; (c) surface: 10% HNO3; (d) cut edge: 10% HNO3; (e) surface: 10% HCl; (f) cut edge: 10% HCl; (g) surface: 5% CH3COOH; (h) cut edge: 5% CH3COOH.
In Figure 13a, the disturbed hydrophobisation structure of AH_0.15 after the action of 10% HCl can be observed. This shows that the entire surface of the hydrophobisation was affected, and the surface of the concrete was thus exposed to the further action of a chemically aggressive environment. In Figure 13b, the cracked structure of hydrophobisation EWH_0.3 after the action of 10% HCl is visible. This sample was also extracted with pieces of surface layers of concrete, the particles of which are also visible in the image. The ‘defoliation’ of the hydrophobisation layer after exposure to 5% CH3COOH is evident in Figure 13c,d. It can be seen here that the integrity of both tested hydrophobisations was broken, and the cohesion between the concrete and the hydrophobisation layer is not visible either. There are also visible components of the concrete, which were also exposed to an aggressive environment after the functionality of the hydrophobisation was disrupted.

Figure 13.
SEM photomicrographs of the hydrophobic agents applied on the concrete samples exposed to different chemical aggressive environment for 28 days: (a) AH_0.15 exposed to 10% HCl; (b) EWH_0.3 exposed to 10% HCl; (c) AH_0.15 exposed to 5% CH3COOH; (d) EWH_0.3 exposed to 5% CH3COOH.
4. Conclusions
Based on the results achieved in the research, the following conclusions can be drawn, based on a comparison of the efficiency of water-soluble and solvent hydrophobic agents for concrete:
- Water-soluble EWH hydrophobisation showed 50× higher viscosity than the AH acrylate solvent hydrophobisation. The AH surface treatment was significantly thinner than water-soluble EWH, even in the same consumption;
- Adhesion of the surface treatments on concrete was higher than 3.5 MPa, and the minimum adhesion on concrete is the same for the hydrophobisations AH and EWH;
- Hydrophobisation blocks the absorption of water into the underlying concrete, which was also confirmed by the absorption test;
- Water-based hydrophobisations have been shown to prevent the penetration of chemicals into concrete almost as well as solvent-based hydrophobisation agents, thereby ensuring their longer durability;
- Hydrophobic agents successfully prevented the evaporation of water from the concrete, and thus, the plastic shrinkage that inevitably leads to the appearance of cracks in cement-based constructions;
- No significant difference was recorded in the strengths of concrete treated with hydrophobisations and concrete without surface treatment, while the highest flexural and compressive strengths were achieved for concrete treated with hydrophobisations AH_0.15 and EWH_0.30. For a better comparison of strengths, it would be necessary to apply hydrophobisations on the entire surface of the samples;
- Highest abrasion resistance was shown with concrete treated by AH_0.15 surface treatment; nevertheless, even water-soluble hydrophobisation significantly increased the abrasion resistance of concrete;
- Compared to untreated concrete (C_REF), concrete with EWH_0.3 hydrophobisation showed up to three times lower shrinkage;
- The lowest water absorption was recorded with the concrete treated by EWH_0.3 hydrophobisation—after 120 days the water absorption was only 3.8%;
- By applying EWH hydrophobisation, a significant improvement in resistance to defrosting agents was achieved in comparison with untreated concrete;
- Exposure of the water-soluble EWH hydrophobisation agents to acetic acid caused significant deterioration of the surface treatment and concrete surface. However, resistance to 5% H2SO4, 10% HNO3 and 10% HCl of the surface concrete treated by EWH was very similar with the concrete with AH surface treatment;
- Through SEM observations, the microstructure of AH and EWH hydrophobisations and its disruption and loss of cohesion with surface concrete after chemical aggressive environment attack was revealed;
- By evaluating the results achieved, it can be concluded that water-soluble epoxy-based hydrophobic agents can be used in practice for the same applications as solvent acrylate hydrophobic agents. Moreover, EWH surface treatment is more ecological, as no toxic solvents are released to the ambient environment after application.
Author Contributions
Conceptualization, J.H. (Jakub Hodul) and L.M.; methodology, J.H. (Jakub Hodul) and J.H. (Jana Hodná); formal analysis, J.H. (Jakub Hodul) and R.P.B.; investigation, J.H. (Jakub Hodul) and J.H. (Jana Hodná); resources, J.H. (Jakub Hodul), R.P.B. and J.H. (Jana Hodná); data curation, J.H. (Jakub Hodul) and L.M.; writing—original draft preparation, J.H. (Jakub Hodul); writing—review and editing, J.H. (Jakub Hodul) and R.P.B.; project administration, J.H. (Jakub Hodul). All authors have read and agreed to the published version of the manuscript.
Funding
This paper was created with the financial support of the Czech Science Foundation (GACR), Standard project No. 22-08888S “Increasing the durability of cement composites using water-based hydrophobization”.
Data Availability Statement
Not applicable.
Acknowledgments
The research was carried out through the collaboration between the Brno University of Technology, Faculty of Civil Engineering and the University of Malta, Faculty for the Built Environment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, J.; Hu, L.; Tang, L.; Zhang, E.Q.; Ren, J. Shrinkage behaviour, early hydration and hardened properties of sodium silicate activated slag incorporated with gypsum and cement. Constr. Build. Mater. 2020, 248, 118687. [Google Scholar] [CrossRef]
- Tittarelli, F. Oxygen diffusion through hydrophobic cement-based materials. Cem. Concr. Res. 2009, 39, 924–928. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Ghaffar, S.H.; Madyan, O.A.; Rahman, M.M. Development of low absorption and high-resistant sodium acetate concrete for severe environmental conditions. Constr. Build. Mater. 2020, 230, 117057. [Google Scholar] [CrossRef]
- Dai, J.-G.; Akira, Y.; Wittmann, F.H.; Yokota, H.; Zhang, P. Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks. Cem. Concr. Comp. 2010, 32, 101–109. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Ma, R.; Yang, L.; Hu, Y.; Zhang, J. Water-repellent additive that increases concrete cracking resistance in dry curing environments. Constr. Build. Mater. 2020, 249, 118704. [Google Scholar] [CrossRef]
- Jin, H.; Liu, J.; Jiang, Z.; Zhou, H.; Liu, J. Influence of the rainfall intensity on the chloride ion distribution in concrete with different levels of initial water saturation. Constr. Build. Mater. 2021, 281, 122561. [Google Scholar] [CrossRef]
- Matziaris, K.; Stefanidou, M.; Karagiannis, G. Impregnation and superhydrophobicity of coated porous low-fired clay building materials. Prog. Org. Coat. 2011, 72, 181–192. [Google Scholar] [CrossRef]
- Basheer, P.A.M.; Basheer, L.; Cleland, D.J.; Long, A.E. Surface treatments for concrete: Assessment methods and reported performance. Constr. Build. Mater. 1997, 11, 413–429. [Google Scholar] [CrossRef]
- Ibrahim, M.; Al-Gahtani, A.S.; Maslehuddin, M.; Almusallam, A.A. Effectiveness of concrete surface treatment materials in reducing chloride-induced reinforcement corrosion. Constr. Build. Mater. 1997, 11, 443–451. [Google Scholar] [CrossRef]
- Yang, C.C.; Wang, L.C.; Weng, T.L. Using charge passed and total chloride content to assess the effect of penetrating silane sealer on the transport properties of concrete. Mater. Chem. Phys. 2004, 85, 238–244. [Google Scholar] [CrossRef]
- Kong, X.; Liu, H.; Lu, Z.; Wang, D. The influence of silanes on hydration and strength development of cementitious systems. Cem. Concr. Res. 2015, 67, 168–178. [Google Scholar] [CrossRef]
- Aguiar, J.B.; Júnior, C. Carbonation of surface protected concrete. Constr. Build. Mater. 2013, 49, 478–483. [Google Scholar] [CrossRef]
- Concrete Society. Guide to Surface Treatments for Protection and Enhancement of Concrete. Surrey (UK); Technical Report 50; Concrete Society: London, UK, 1997; 87p. [Google Scholar]
- De Vries, R.B.; Polder, R.B. Hydrophobic treatment of concrete. Constr. Build. Mater. 1997, 11, 259–265. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, M.J.; Kim, H.; Ann, K. Effect of Hydrophobic Surface Treatment in Lowering Ionic Transport into Concrete. Adv. Mater. Sci. Eng. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Zhu, Y.; Kou, S.; Poon, C.; Dai, J.; Li, Q. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Almusallam, A.A.; Khan, F.M.; Dulaijan, S.U.; Al-Amoudi, O.S.B. Effectiveness of surface coatings in improving concrete durability. Cem. Concr. Compos. 2003, 25, 473–481. [Google Scholar] [CrossRef]
- Schueremans, L.; Gemert, D.V.; Giessler, S. Chloride penetration in RCstructures in marine environment-Long term assessment of a preventive hydrophobic treatment. Constr. Build. Mater. 2007, 21, 1238–1249. [Google Scholar] [CrossRef]
- Medeiros, M.; Helene, P. Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete. Mater. Struct. 2008, 41, 59–71. [Google Scholar] [CrossRef]
- Xue, X.; Li, Y.; Yang, Z.; He, Z.; Dai, J.G.; Xu, L.; Zhang, W. A systematic investigation of the waterproofing performance and chloride resistance of a self-developed waterborne silane-based hydrophobic agent for mortar and concrete. Constr. Build. Mater. 2017, 155, 939–946. [Google Scholar] [CrossRef]
- Mundo, R.D.; Labianca, C.; Carbone, G.; Notarnicola, M. Recent advances in hydrophobic and icephobic surface treatments of concrete. Coatings 2020, 10, 449. [Google Scholar] [CrossRef]
- Yan, B.; Ren, F.; Cai, M.; Qiao, C. Influence of new hydrophobic agent on the mechanical properties of modified cemented paste backfill. J. Mater. Res. Technol. 2019, 8, 5716–5727. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Rahman, M.M.; Chamberlain, D.A. Moisture evaluation of concrete pavement treated with hydrophobic surface impregnants. Int. J. Pavement Eng. 2020, 21, 1746–1754. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, S.; Wang, K.; Xie, T. Modification of bonding properties and microstructure of resin-cement interface by coupling agents. Compos. Interfaces 2018, 25, 27–37. [Google Scholar] [CrossRef]
- EN 1339; Concrete Paving Flags–Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2006.
- EN ISO 2555; Plastics—Resins in the Liquid State or as Emulsions or Dispersions—Determination of Apparent Viscosity Using a Single Cylinder Type Rotational Viscometer Method. European Committee for Standardization (CEN): Brussels, Belgium; Technical Committee ISO/TC61/SC5: Geneva, Switzerland, 2018.
- EN ISO 4624; Plastics—Paints and Varnishes—Pull-off Test for Adhesion. European Committee for Standardization (CEN): Brussels, Belgium; Technical Committee ISO/TC35/SC9: Geneva, Switzerland, 2016.
- EN 13892-3; Methods of Test for Screed Materials—Part 3: Determination of Wear Resistance—Böhme. European Committee for Standardization (CEN): Brussels, Belgium, 2014.
- EN ISO 6272-2; Paints and Varnishes—Rapid—Deformation (Impact Resistance) Tests—Part 2: Falling-Weight Test, Small-Area Indenter. European Committee for Standardization (CEN): Brussels, Belgium; Technical Committee ISO/TC35/SC9: Geneva, Switzerland, 2011.
- EN 12390-5; Testing Hardened Concrete–Part 5: Flexural Strength of Test Specimens. European Committee for Standardization (CEN): Brussels, Belgium, 2019.
- EN 12390-3; Testing Hardened Concrete–Part 3: Compressive Strength of Test Specimens. European Committee for Standardization (CEN): Brussels, Belgium, 2019.
- EN 12390-16; Testing Hardened Concrete–Part 16: Determination of the Shrinkage of Concrete. European Committee for Standardization (CEN): Brussels, Belgium, 2019.
- EN 14617-1; Agglomerated Stone–Test Methods–Part 1: Determination of Apparent Density and Water Absorption. European Committee for Standardization (CEN): Brussels, Belgium, 2013.
- ČSN 73 1326; Determination of the resistance of the surface of cement concrete against the action of water and chemical deicers. Czech Standardization Institute (CSI): Prague, Czech Republic, 1985.
- Chatveera, B.; Lertwattanaruk, P. Evaluation of nitric and acetic acid resistance of cement mortars containing high-volume black rice husk ash. J. Environ. Manag. 2014, 133, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, V. Corrosion of hardened cement paste by acetic and nitric acids Part III: Influence of water/cement ratio. Cem. Concr. Res. 1996, 26, 475–490. [Google Scholar] [CrossRef]
- Teymouri, M.; Behfarnia, K.; Shabani, A. Mix design effects on the durability of alkali-activated slag concrete in a hydrochloric acid environment. Sustainability 2021, 13, 8096. [Google Scholar] [CrossRef]
- Shill, S.K.; Al-Deen, S.; Ashraf, M. Concrete durability issues due to temperature effects and aviation oil spillage at military airbase—A comprehensive review. Constr. Build. Mater. 2018, 160, 240–251. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Smarzewski, P.; Suchorab, Z. Effect of hydrophobisation on durability related properties of ceramic brick. Constr. Build. Mater. 2016, 111, 275–285. [Google Scholar] [CrossRef]
- Dang, Y.; Xie, N.; Kessel, A.; McVey, E.; Pace, A.; Shi, X. Accelerated laboratory evaluation of surface treatments for protecting concrete bridge decks from salt scaling. Constr. Build. Mater. 2014, 55, 128–135. [Google Scholar] [CrossRef]
- Gupta, T.; Sharma, R.K.; Chaudhary, S. Impact resistance of concrete containing waste rubber fiber and silica fume. Int. J. Impact. Eng. 2015, 83, 76–87. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on surface treatment for concrete—Part 2: Performance. Constr. Build. Mater. 2017, 133, 81–90. [Google Scholar] [CrossRef]
- Shi, L.; Liu, J.; Liu, J. Effect of polymer coating on the properties of surface layer concrete. Procedia Eng. 2012, 27, 291–300. [Google Scholar] [CrossRef]
- Raupach, M.; Wolff, L. Long-term durability of hydrophobic treatment on concrete. Coat. Int. Part B Coat. Trans. 2005, 88, 127–133. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Tao, M.; Li, X. A cement paste–tail sealant interface modified with a silane coupling agent for enhancing waterproofing performance in a concrete lining system. RSC Adv. 2019, 9, 7165–7175. [Google Scholar] [CrossRef]
- Mamaghani, I.; Moretti, C.; Dockter, B.; Falken, L.; Tonnenson, J. Evaluation of penetrating sealers for reinforced concrete bridge decks. Transport. Res. Rec. J. Transport. Res. Board. 2009, 2108, 86–96. [Google Scholar] [CrossRef]
- Suleiman, A.R.; Soliman, A.M.; Nehdi, M.L. Effect of surface treatment on durability of concrete exposed to physical sulfate attack. Constr. Build. Mater. 2014, 73, 674–681. [Google Scholar] [CrossRef]
- Marotta, A.; Faggio, N.; Ambrogi, V.; Mija, A.; Gentile, G.; Cerruti, P. Biobased furan-based epoxy/TiO2 nanocomposites for the preparation of coatings with improved chemical resistance. Chem. Eng. J. 2021, 406, 127107. [Google Scholar] [CrossRef]
- Sembokuya, H.; Negishi, Y.; Kubouchi, M.; Tsuda, K. Corrosion behavior of epoxy resin cured with different amount of hardener in corrosive solutions. Mater. Sci. Res. Int. 2003, 9, 230–234. [Google Scholar] [CrossRef]
- Aguiar, J.B.; Camoes, A.; Moreira, P.M.; Kruger, D. Performance of concrete in aggressive environment. Concr. Struct. Materials. 2008, 2, 21–25. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).