Abstract
Solvent extraction and separation of Pr, Nd and Dy from a synthetic leach solution of spent NdFeB magnet from wind turbines in the presence of aquo-complexing agent Ethylenediaminetetraacetic acid (EDTA) was studied using the nitrate form of Mextral® 336At ([336At][NO3]) as an extractant. The effect of different process parameters such as pH, extractant, nitrate, and EDTA concentrations on the extraction of Pr, Nd and Dy was studied. The extraction of these rare earths elements follows the order Pr > Nd > Dy, whereas EDTA forms stable complexes in the order Dy > Nd > Pr. The synergy of these two effects improved the selectivity among these elements as compared to when no aquo-complexing agent was used. The mechanism of extraction of rare earth elements was established by slope analysis method. The Fourier-Transform Infrared Spectroscopy (FTIR) spectra of [336At][NO3] and extracted Nd complex were recorded to understand the interaction of extractant with rare earth metal ions in the organic phase.
1. Introduction
Superior magnetic properties make NdFeB magnets suitable for various applications such as the hard drive of computers, amplifiers, MRI machines, and wind turbines. The NdFeB magnet industry is growing globally at a rate of 20% each year [1]. However, primary resources of rare earth elements are limited and they occur together in mineral deposits, which make their separation tedious and lead to over production of less demanding rare earth metals. For 1 MW of electricity generation, typically 250–650 kg of NdFeB magnets are required for the generators of wind turbines [2,3], and the life span of such magnets is only ~10 years. Furthermore, during shaping of the magnets, 20–30% of the magnet is wasted as sludge. Thus, there is huge generation of waste magnet which can serve as a potential source of Nd, Pr and Dy. Processing of spent magnets for the recovery of rare earth elements will reduce the burden on primary resources and their separation will be simpler, as these resources do not contain all rare earth elements.
A variety of approaches, such as liquid metal extraction, flux method, and chemical vapor transport method, have been attempted in the past for the recovery of rare earth elements from spent NdFeB magnets [4,5,6]. These processes are highly energy intensive and rare earth products obtained are in an impure form. The hydrometallurgical process is known as the most suitable method for obtaining metal of desired purity. Studies were carried out for selective recovery of rare earths from spent NdFeB magnets using a variety of mineral acids (H2SO4, HNO3, HCl etc.) to obtain rare earth (Nd3+, Pr3+ and Dy3+) in aqueous solution. However, individual separations of these metal ions are still a difficult task.
The solvent extraction process is the most suitable method for the separation of individual rare earths. Various studies have been carried out for the separation of rare earths using cation exchange extractants. Different cation exchange (acidic) extractants including Cyanex 272, D2EHPA, PC88A, and Cyanex 301 have been studied for the separation of La, Pr and Nd [7]. Cyanex 272 showed the best extraction efficiency among all extractants. The separation of heavy rare earths (Y, Dy, Tb and Gd) from crude concentrate using 2-ethylhexylphosphonic acid, mono-2-ethylhexyl ester (EHEHPA) as the extractant have also been studied [8]. Using two cycles of extraction, scrubbing and stripping, nuclear grade Dy2O3 was produced with more than 98% recovery. Acidic extractants release H+ ions in the aqueous phase, which lowers the extraction efficiency of extractants. Therefore, various studies have been carried out for the separation of rare earths using acidic extractants saponified by NaOH or NH3 solution [9,10]. The extraction of Nd(III) from chloride media have been studied using 40% saponified PC88A [11]. Similarly, extraction studies have been carried out for the separation of La, Ce, Pr and Nd using saponified PC-88A to obtain Nd2O3 oxide of purity >97% [12]. Although better separation can be achieved by using saponified extractants, the possibility of emulsification and third phase formation were found as major drawbacks during the extraction process. In addition, release of Na+ or NH4+ ions into the aqueous phase during extraction also affects the environment adversely [13]. To solve these problems, many separation studies were carried out by a mixture of acidic extractants with amine based extractants. Separation studies of Pr and Nd were carried out using a mixture of Cyanex 272 and different amine extractants such as Alamine 336, TOA, TEHA and Aliquat 336 [14,15]. The mixture of Cyanex 272 and Alamine 336 showed the maximum synergistic factors of 14.2 for Pr and 12.2 for Nd by using a 0.5 mole fraction of Cyanex 272 [14]. Attempts were also made by using neutral and anion exchange extractants for the separation of praseodymium, neodymium and dysprosium.
In the last decade, ionic liquid has emerged as a future solution for the separation of metal ions. Ionic liquid is the organic salt consisting of an organic cation and organic or inorganic anion, with a melting point below 100 °C. Various studies were carried out for the extraction of Nd, Pr and Dy using a variety of ionic liquids used as extractant or diluents. The extraction study of Pr, Nd and Dy derived from spent NdFeB magnet was carried out with TBP by using ionic liquid Aliquat nitrate ([A336][NO3] or R4N·NO3) as diluent [16]. A significant extraction of Nd(III) was obtained in the ionic liquid phase through repeated extraction. Another ionic liquid, trihexyl(tetradecyl)phosphonium nitrate was used for the separation of cobalt from rare earths (Nd, Dy) [17]. The detailed extraction study of Nd with Cyanex 923 was carried out in presence of ionic liquid containing the bis(trifluoromethylsulfonyl)imide anion with five different cations. It was found that ionic liquids with hydrophilic cation, [C4mim][Tf2N] and [N1444][Tf2N], extract Nd(III) efficiently in comparison with hydrophobic cations [18]. Other ionic liquids, such as [Hbet][Tf2N] and DODGAA, were also explored for the extraction of rare earths [19,20]. Some key extractant systems used in the literature for the separation of praseodymium, neodymium and dysprosium are summarized in Table 1.

Table 1.
Different solvent extractant systems related to the separation of praseodymium, neodymium and dysprosium.
For effective separation of chemically similar rare earth ions, new extraction systems were developed by adding water soluble complexing agent in the aqueous phase. The complexing agents form the thermodynamic stable chelate complex, which consequently enhances the selectivity of one metal ion by masking other ions in the aqueous phase. This kind of system could be very useful for the separation of adjacent rare earth ions such as praseodymium and neodymium. In Table 2, different extraction systems were summarized for separation of rare earths (mainly Pr, Nd and Dy) in the presence of water-soluble complexing agents. Several cation exchange extractants, such as such as D2EHPA, HEHEHPA, and P204 were studied in the presence of different complexing agents (lactic acid, citric acid, formic acid, acetic acid, EDTA, DTPA etc.) for the separation of rare earth ions [21,22,23]. The separation of La, Pr and Nd using Cyanex 272 and PC88A was studied by adding the complexing agent EDTA to the aqueous solution [24]. The separation studies were carried out at two pH conditions (2.8 and 4.9), with and without EDTA addition. It was found that the extraction of Pr and Nd was suppressed by the addition of EDTA, which enhances the possible separation between Pr/Nd and La. Similarly, the separation of Ce, La, Pr and Nd was studied with acidic extractant P2O4 in the presence of two complexing agents: Lactic acid and citric acid [25]. The experiments for the separation of two adjacent rare earths Pr and Nd in D2EHPA-HCl-LA (lactic acid) extraction system have also been carried out [26]. The maximum separation factor for Nd/Pr was obtained as 1.57 by using 0.6M lactic acid in aqueous solution at pH 3.5. An extraction and separation study of Ce (III) and Pr(III) was carried out with P2O4 by adding different ratios of citric acid and lactic acid in aqueous solution as the complexing agent [27]. It was found that with an increase in the ratio of citric acid:lactic acid, the separation factor increased and the maximum separation factor value was achieved as 5.78. Overall, it was observed that separation studies of rare earths were mostly carried by using acidic extractants in the presence of complexing agents.

Table 2.
Different extractant systems for separation of rare earths in presence of water-soluble complexing agents.
There is scant information available on the separation behavior of rare earths using ionic liquid extractants in the presence of complexing agents. Rare earth ions forms stable anionic species in the aqueous phase; therefore, quaternary ammonium salts (R4N+) may be effective for the separation of rare earth ions [28,29,30,31]. Therefore, in the present investigation, it is aimed to study the extraction of rare earth metal ions with an ionic liquid, trioctyl-methyl-ammonium nitrate, or R4N·NO3, in the presence of complexing/masking agent EDTA and to understand the influence of complexing agent on the separation of Pr, Nd and Dy. For this, the effect of pH, extractant concentration, and EDTA concentration were studied in detail. The extraction percentage and separation factor values were calculated for each experiment in order to determine the selectivity between Pr, Nd and Dy. The mechanism of extraction of metal ions with R4N·NO3 was also explored. FTIR (Fourier-Transform Infrared Spectroscopy) analysis was also carried out to comprehend the interaction of rare earth ions in the loaded organic phase.
2. Materials and Methods
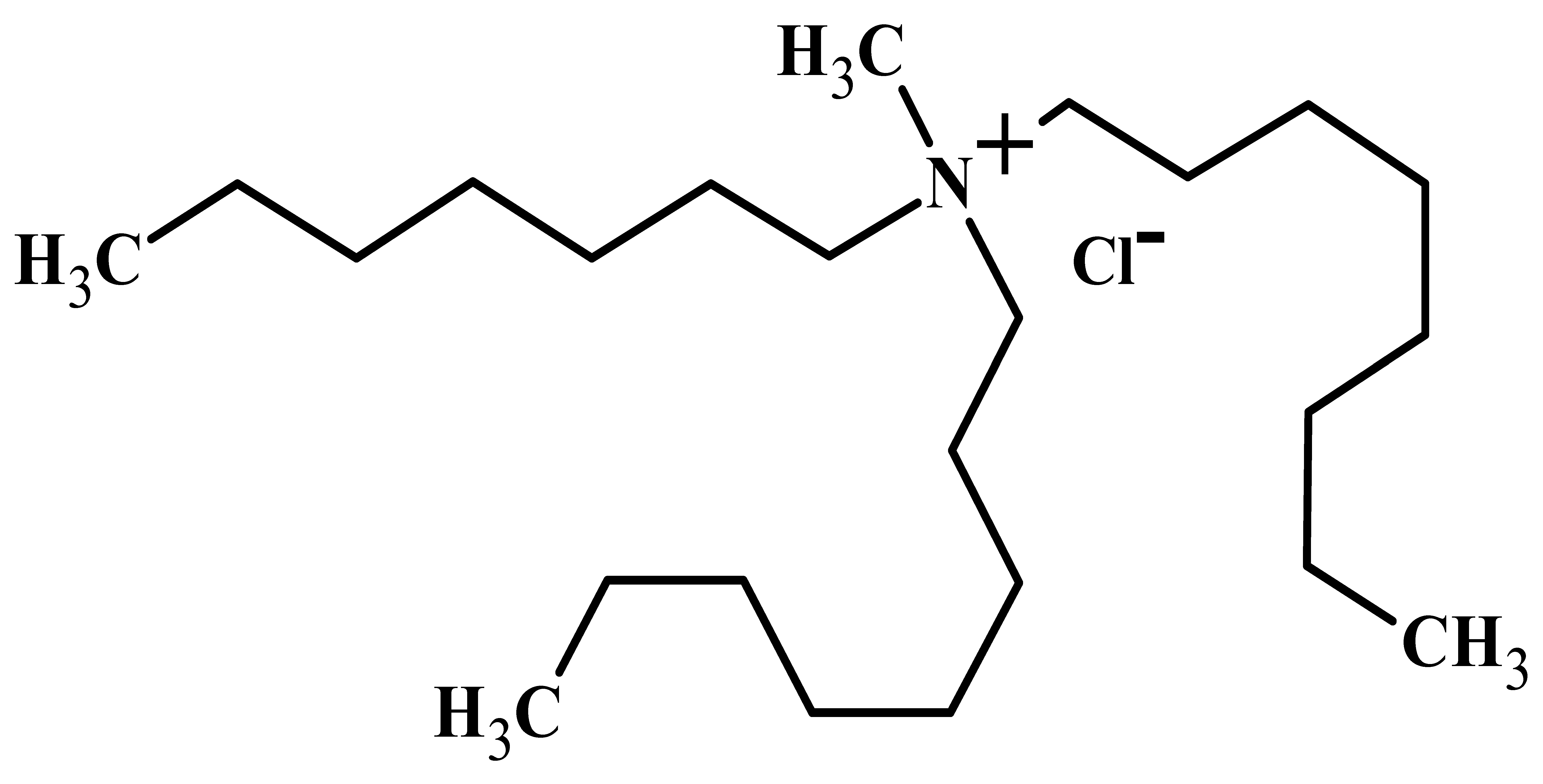
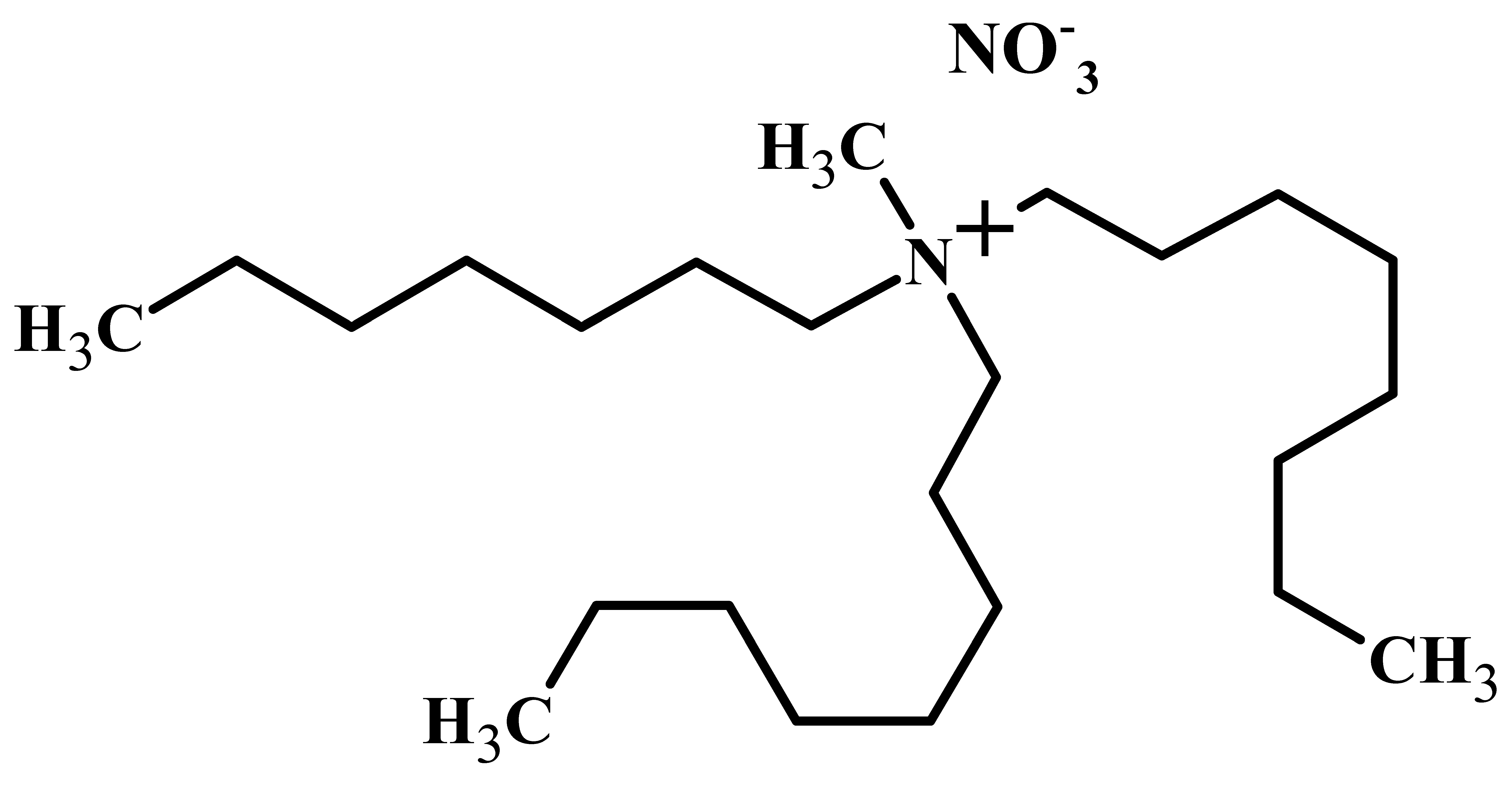
A stock solution containing 0.12 M Nd, 0.03 M Pr, and 0.002 M Dy was prepared by dissolving appropriate amounts of the respective rare earth chlorides in distilled water. For solvent extraction studies, the stock solution was diluted 10 times and nitrate concentration was maintained by the addition of an appropriate amount of sodium nitrate. Ethylenediaminetetraacetic acid (EDTA) was used as the complexing/ masking agent, and was also added in appropriate quantities while diluting the stock solution. The pH of the aqueous solution was maintained by the addition of HNO3 or NaOH solutions whenever required. Solvent extraction studies were carried out using the nitrate form of Mextral® 336At ([336At][NO3]) as extractant. Mextral® 336At in chloride form ([336At][Cl]) was obtained from Kopper Chem, Chongqing, China which is basically trioctyl-methyl-ammonium chloride (R4N·Cl). Prior to solvent extraction studies, trioctyl-methyl-ammonium nitrate (R4N·NO3) was prepared by converting trioctyl-methyl-ammonium chloride (Mextral® 336At) into nitrate form by mixing with 2.5 M NaNO3 solution three times in order to ensure complete exchange of chloride ions by nitrate ions [41]. The exchange was also confirmed by analysis of chloride ion in aqueous raffinate by Volhard’s test. After three contacts with NaNO3 solution, a negligible amount of chloride ions was detected in the raffinate. The properties of chloride (R4N·Cl or [336At][Cl]) and nitrate form (R4N·NO3 or [336At][NO3]) of Mextral® 336At are given in Table 3. Commercial grade kerosene and iso-decanol were used as diluent and phase modifier, respectively. All other chemicals used in the experiments were of analytical grade.

Table 3.
Properties of solvent extraction reagent.
Solvent extraction experiments were carried out by shaking 10 mL of each aqueous feed and organic phase solution for 15 min in a glass-stoppered vial with the help of a rotospin shaker (Revotek, Kolkata, India) at room temperature (298 ± 2K) and 30 rpm. After equilibration, the solution was allowed to settle for a few minutes to ensure complete phase separation. All the solvent extraction experiments were performed in duplicate. Eutech pH 510 was used to measure pH of aqueous solutions before and after the experiments. The concentration of metal ions in the raffinate was analyzed by ICP-OES (Thermo Scientific, Waltham, MA, USA). The metal ions extracted into the organic phase were determined by material balance. From these values, the extraction percentage (%E), distribution coefficients (D) and separation factors (β) were calculated using Equations (1)–(3):
where Mi and Mf represents the initial concentration of metal ions in the aqueous feed and final concentration of metal ions in the raffinate. The extraction percentage (%E) determines the efficiency of the extraction. The distribution coefficient (D) represents the ratio of metal ions transferred to organic phase to remaining metal ions in the raffinate solution. The separation factor (β) determines the selectivity of one lanthanide (Ln) ion over other, where D1 and D2 are the distribution coefficient of metal ions Ln1 and Ln2, respectively.
3. Results and Discussion
The spent NdFeB magnet of wind turbines received from Regen Power Tech. Ltd, Chennai, India. contained 23.2% Nd, 5.6% Pr, 0.42% Dy, 67.3% Fe, 1% B, and other traces of elements like Al and Co as minor impurities. As mentioned in our earlier publication [43], a process was developed to selectively recover rare earth elements by leaching with 0.5 M HCl at 368 K and 100 g/L pulp density for 300 min. The leach solution contained 0.12 M Nd, 0.03 M Pr and 0.002 M Dy along with only 0.0002 M Fe and other minor impurities. Iron and other impurities were removed by precipitation at pH 3.5. In the present investigation, solvent extraction and separation of rare earth elements from 10 times diluted synthetic leach solution containing 0.012 M Nd, 0.003 M Prand 0.0002 M Dy from nitrate medium with [336At][NO3] was studied.
Extraction of rare earth elements from nitrate solution with [336At][NO3] may be expressed as
where M3+ represents Nd3+, Pr3+ or Dy3+. The equilibrium constant K can be written as
The aqueous phase complexation of rare earth elements with EDTA is governed by the following equilibrium expression:
The stability constant, βEDTA can be expressed as
From Equations (5), (6) and (7), the distribution ratio D of metal ion can be written as
At very low concentrations of EDTA compared to metal ion concentration, particularly Nd and Pr concentration, formation of EDTA complex in the aqueous phase may be neglected and distribution ratio D can be rewritten as
By taking the logarithm of both sides as
3.1. Effect of pH on Solvent Extraction of Nd, Pr and Dy
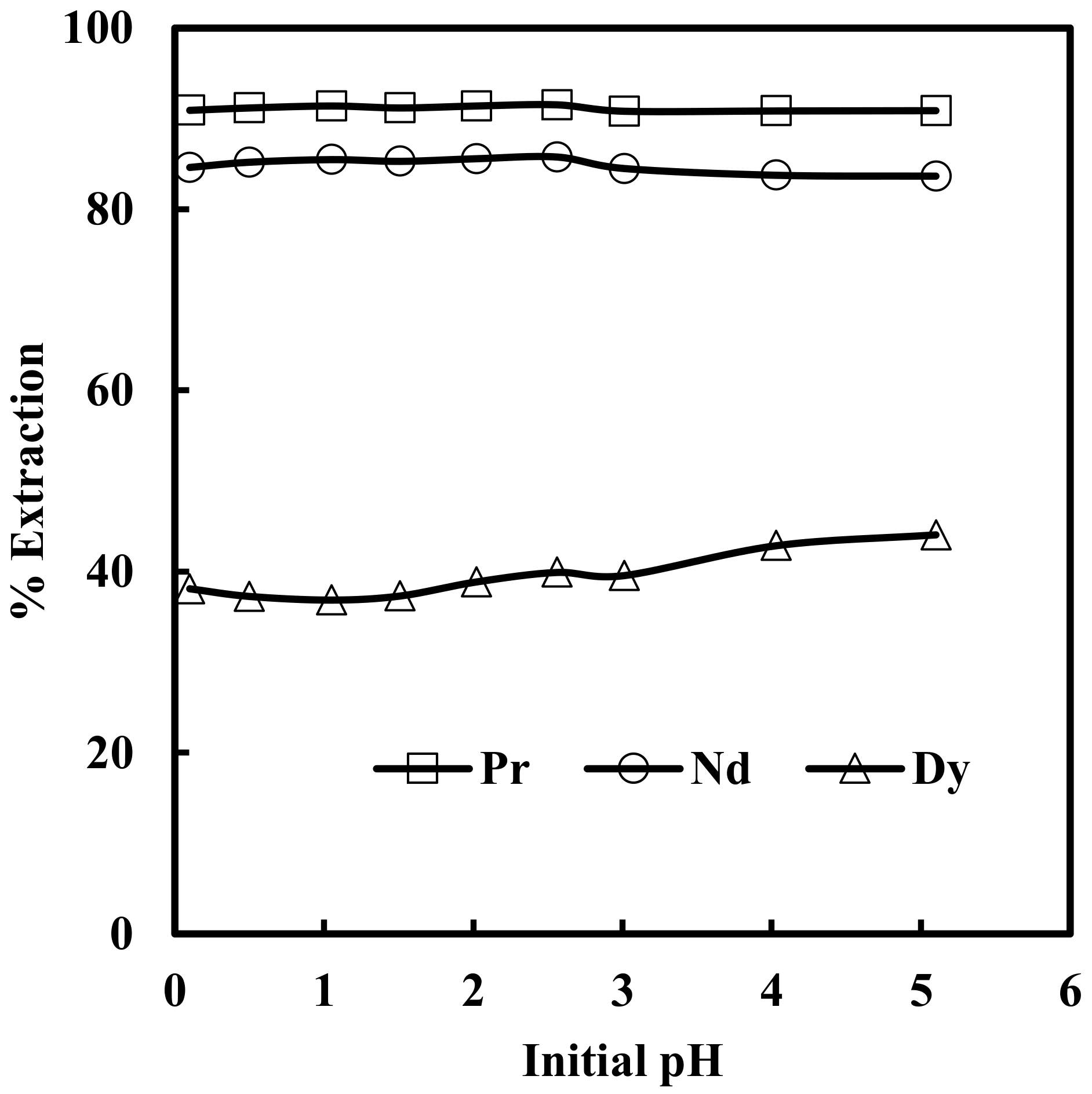
The effect of pH in the range of 0.1 to 5.0 on the solvent extraction of rare earth elements from 6 M NaNO3 solution containing 0.012 M Nd, 0.003 M Pr, 0.0002 M Dy, and 0.0001 M EDTA with 0.43 M nitrate form of Mextral® 336At (R4N·NO3) in kerosene was studied. It was found that aqueous feed pH had no significant effect on solvent extraction of rare earth elements, and the extraction of Pr, Nd and Dy remained almost constant at 91%, 85% and 42%, respectively, in the entire range of pH studied (Figure 1). It is clear from Figure 1 that the extraction of rare earth elements followed the order Pr > Nd > Dy and preferred to extract light rare earth elements first. This may be due to the fact that the stability of hydrated ions in the aqueous phase follows the order Pr < Nd < Dy. Therefore, the least soluble hydrated Pr3+ ion is easily dehydrated and converted to easily extractable species [28].
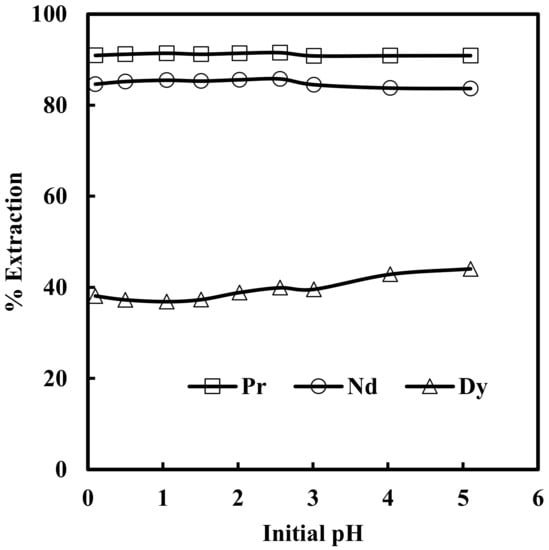
Figure 1.
Effect of initial pH of aqueous feed solution on extraction of Pr, Nd and Dy by [336At] [NO3]. Aqueous phase: [NO3−] = 6 M, [EDTA] = 0.0001 M. Organic phase: [336At][NO3] = 0.43 M + 10% iso-decanol in kerosene.
3.2. Effect of [336At][NO3] Concentration on Solvent Extraction of Nd, Pr and Dy
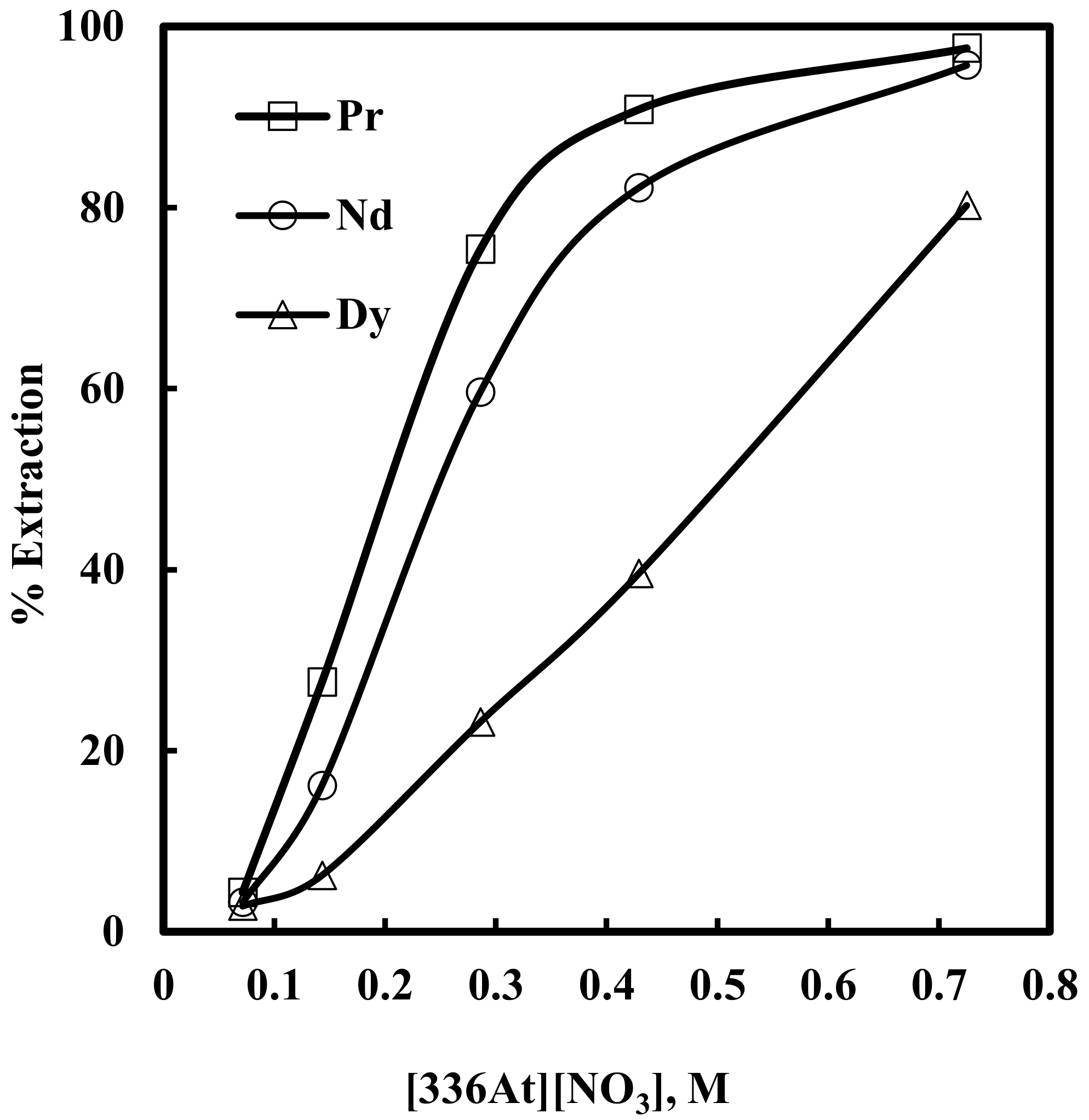
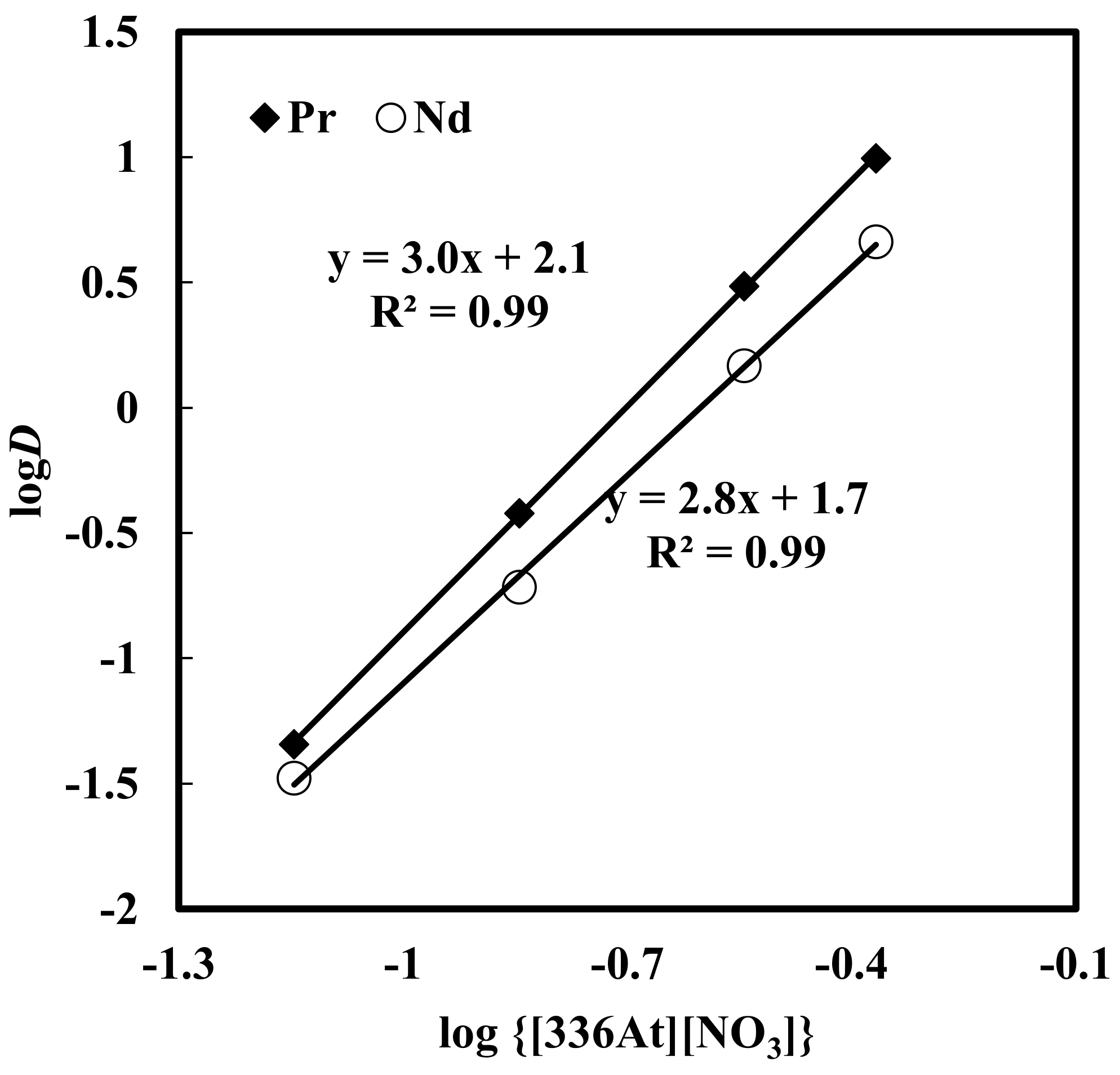
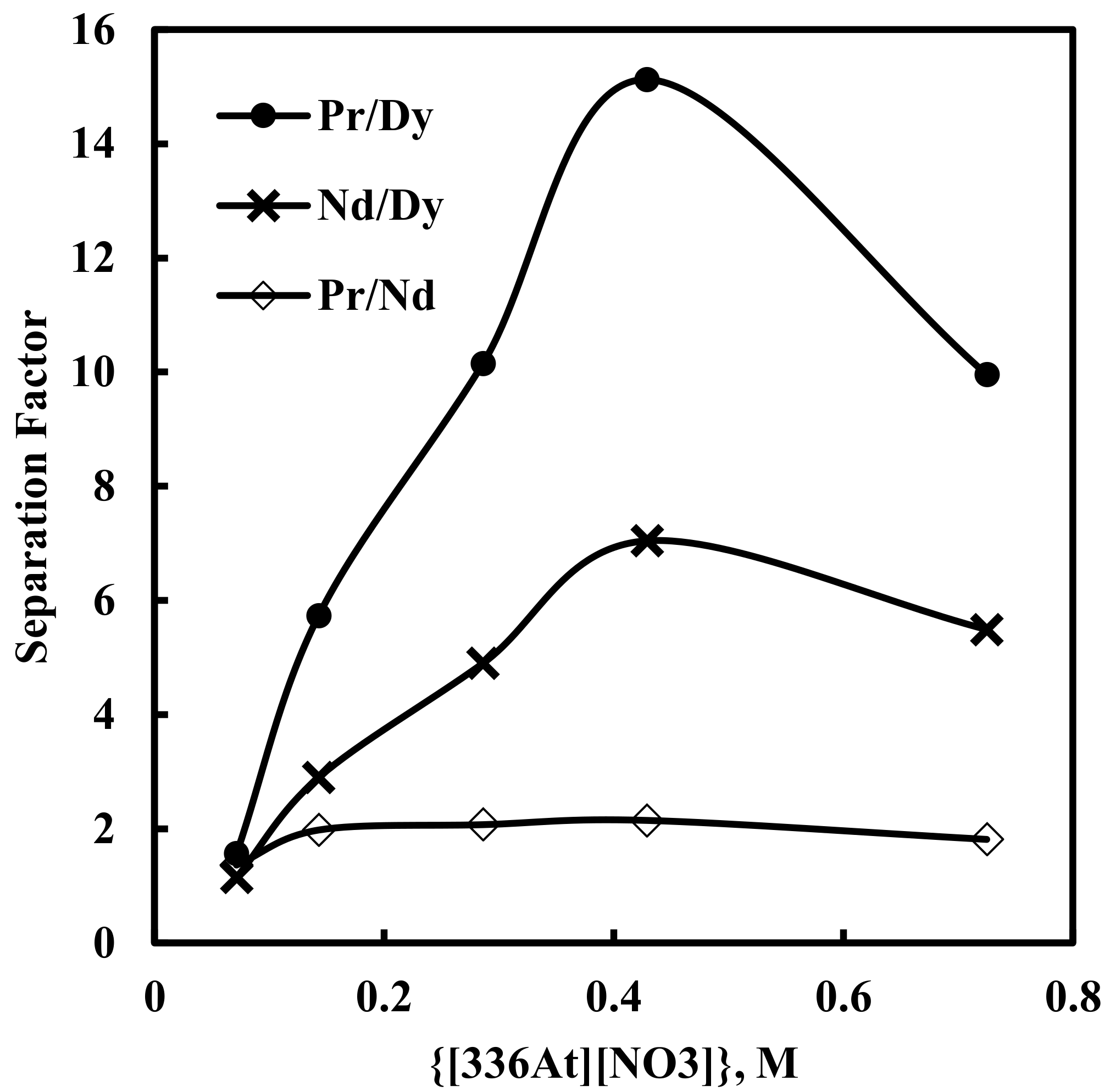
Here, solvent extraction of rare earth elements, from a 6 M NaNO3 solution containing 0.012 M Nd, 0.003 M Pr, 0.0002 M Dy, and 0.0001 M EDTA with varying concentrations of [336At][NO3] (in the range of 0.07–0.73 M) at constant aqueous feed pH of 3, was studied. Beyond 0.73 M [336At][NO3] concentration, the organic phase was found to be comparatively viscous and phase separation was difficult. The results are depicted in Figure 2 and Figure 3. It was found that extraction of Nd, Pr and Dy increased with an increase in [336At][NO3] concentration, and 90% Pr, 82% Nd and 39% Dy were extracted with 0.43 M [336At][NO3] (Figure 2). The extraction of Pr and Nd was found to be >95% along with 80% extraction of Dy by using 0.73 M [336At][NO3]. The plot of logD vs. log[336At][NO3] for Pr and Nd (Figure 3) had slopes of 3 and 2.8, respectively, with a correlation coefficient of 0.99, indicating association of 3 moles of the extractant for the extraction of 1 mole of metal ions into the organic phase (Equation 10). Separation factors between different pairs of rare earth elements (Pr/Dy, Nd/Dy and Pr/Nd) at varying concentrations of [336At][NO3] were calculated and are shown in Figure 4. It was found that the separation factors for each pair of rare earth elements increased with an increase in [336At][NO3] concentration up to 0.43 M. At 0.43 M [336At][NO3], the separation factor values were 15.1 for Pr/Dy, 7 for Nd/Dy and 2.1 for Pr/Nd.
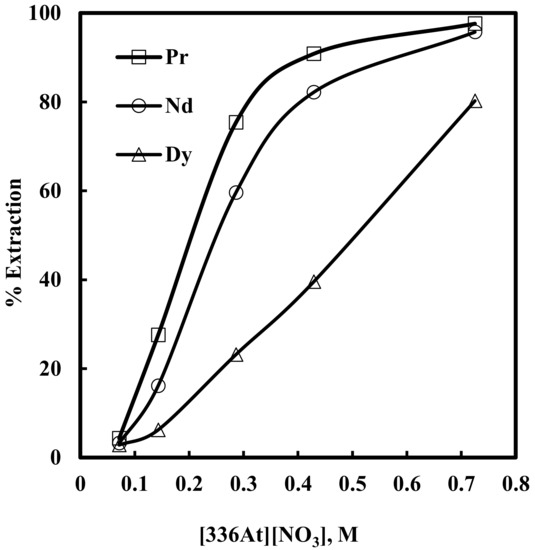
Figure 2.
Effect of concentration of [336At][NO3] on extraction of Pr, Nd and Dy in presence of EDTA. Aqueous phase: pH = 3, [NO3−] = 6 M, [EDTA] = 0.0001 M. Organic phase: [336At][NO3] = 0.07–0.73 M + 10% iso-decanol in kerosene.
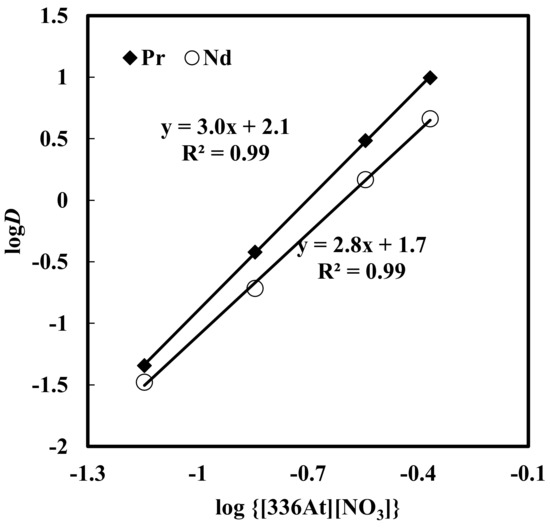
Figure 3.
Effect of concentration of [336At][NO3] on distribution ratio. Aqueous phase: pH = 3, [NO3−] = 6 M, [EDTA] = 0.0001M. Organic phase: [336At][NO3] = 0.07–0.43 M + 10% iso-decanol in kerosene.
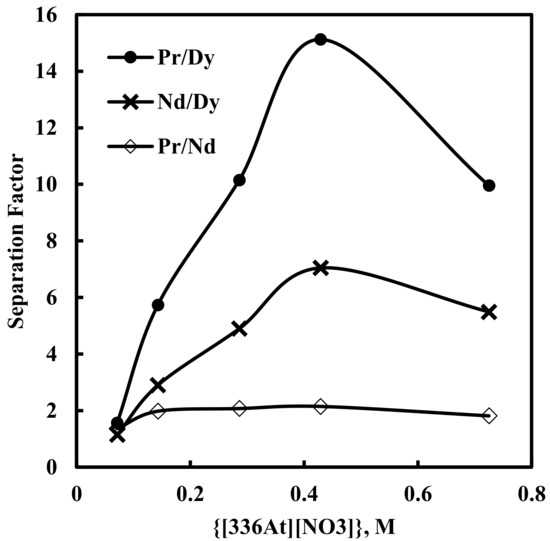
Figure 4.
Effect of concentration of [336At][NO3] on separation factors. Aqueous phase: pH = 3, [NO3−] = 6 M, [EDTA] = 0.0001 M. Organic phase: [336At][NO3] = (0.07–0.73 M)+ 10% iso-decanol in kerosene.
3.3. Effect of Nitrate Concentration on Solvent Extraction of Nd, Pr and Dy
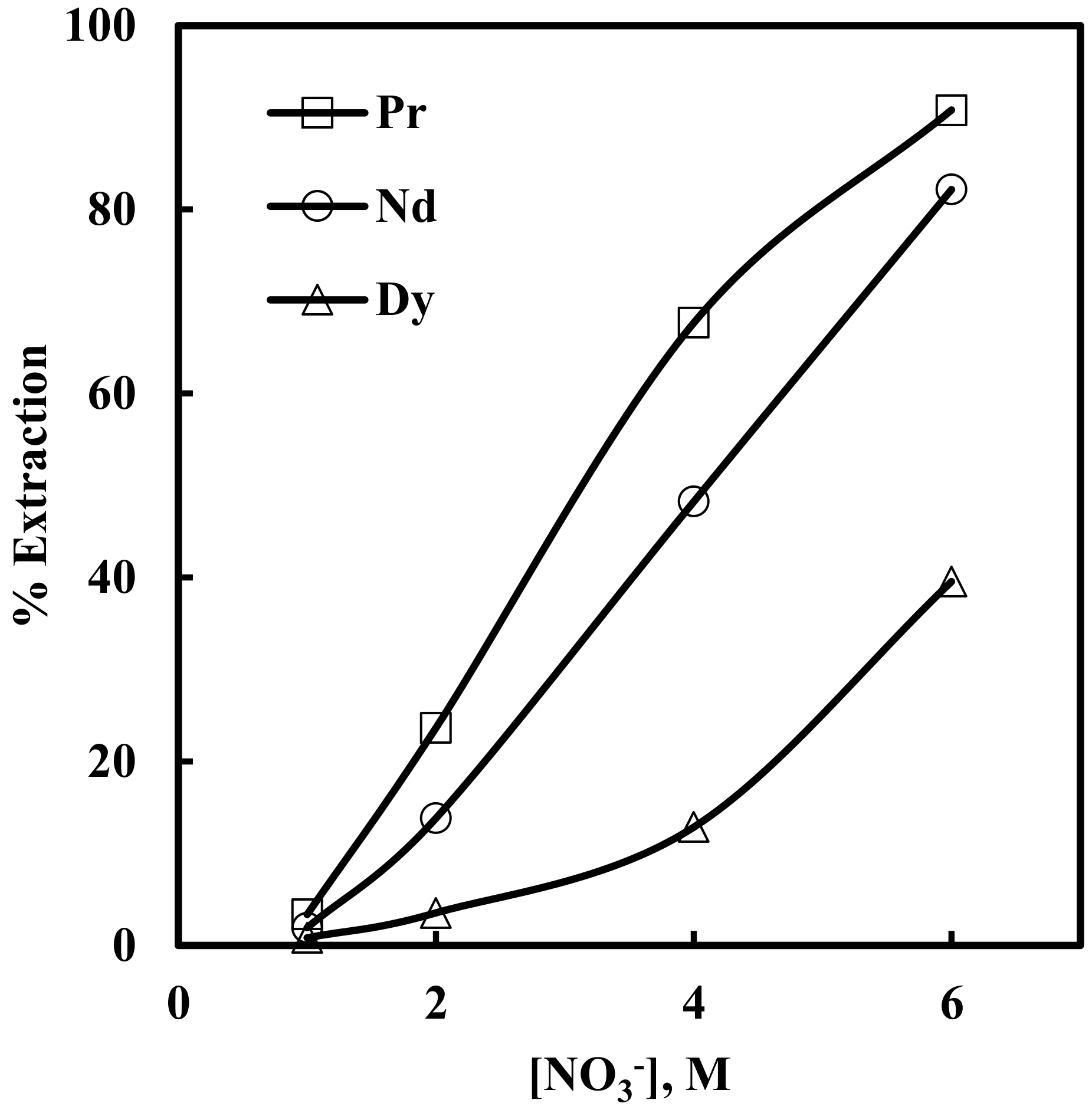
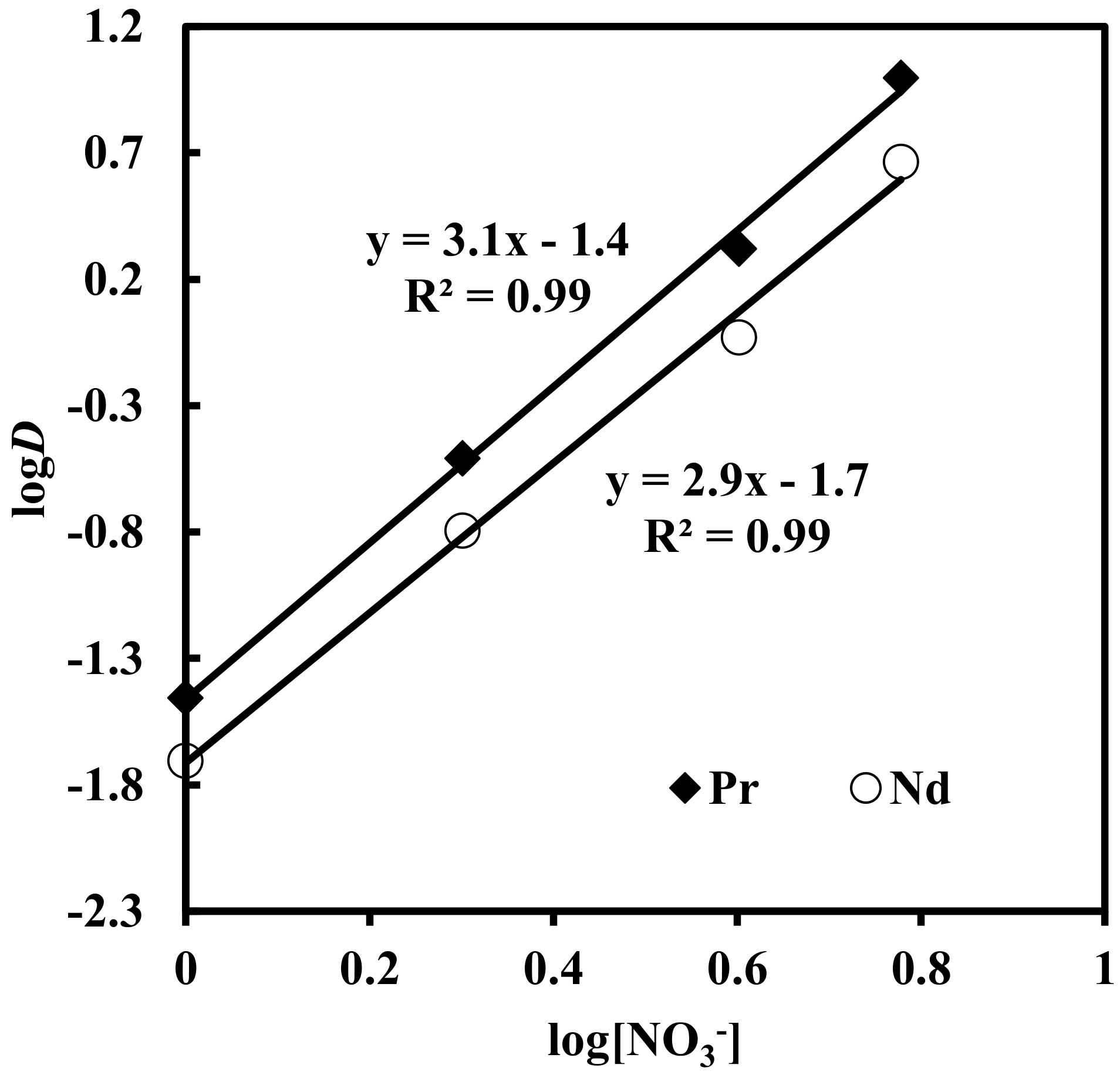
Solvent extraction of rare earth elements under varying concentrations (1–6 M) of NaNO3 in solutions containing 0.012 M Nd, 0.003 M Pr, 0.0002 M Dy, and 0.0001 M EDTA, with 0.43 M [336At][NO3], was studied. The results depicted in Figure 5 show that the extraction of Pr, Nd and Dy increased with an increase in nitrate concentration in the aqueous phase. When nitrate concentration increased from 1 to 6 M, rare earth extraction increased from 3% to 90% for Pr, 1.9% to 82% for Nd and 0.8% to 39% for Dy. The plot of logD vs. log[NO3−] for Pr and Nd (Figure 6) had slopes of 3.1 and 2.9, respectively. This indicates the involvement of 3 moles of nitrate ions for the extraction of one mole of metal ion into the organic phase. The overall reaction mechanism can be represented as
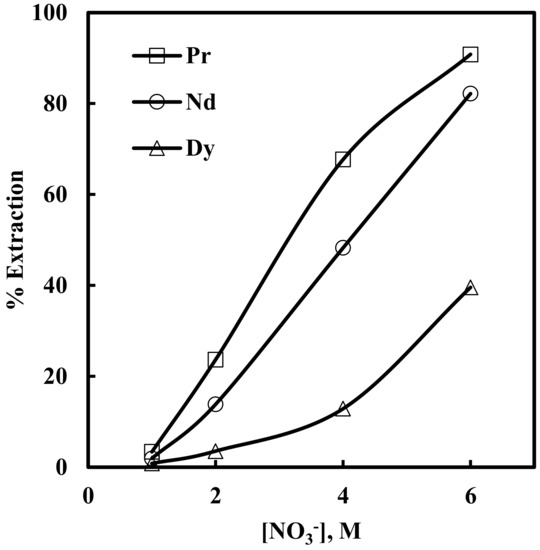
Figure 5.
Effect of nitrate ion concentration on the extraction of Pr, Nd and Dy in the presence of EDTA. Aqueous phase: pH = 3, [EDTA] = 0.0001 M. Organic phase: [336At][NO3] = 0.43 M + 10% iso-decanol in kerosene.
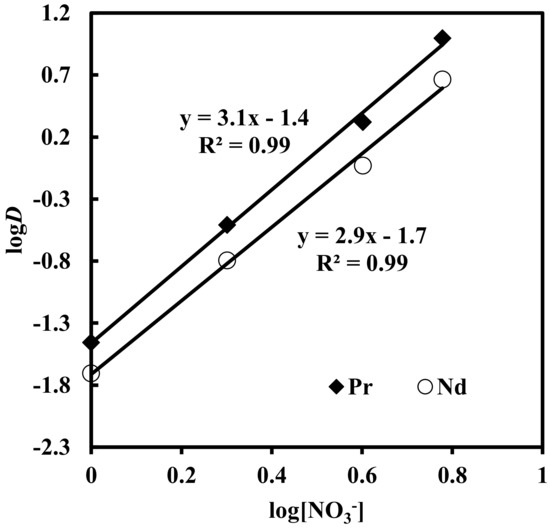
Figure 6.
Effect of concentration of nitrate ion on distribution ratio. Aqueous phase: pH = 3, [EDTA] = 0.0001 M. Organic phase: [336At][NO3] = 0.43 M + 10% iso-decanol in kerosene.
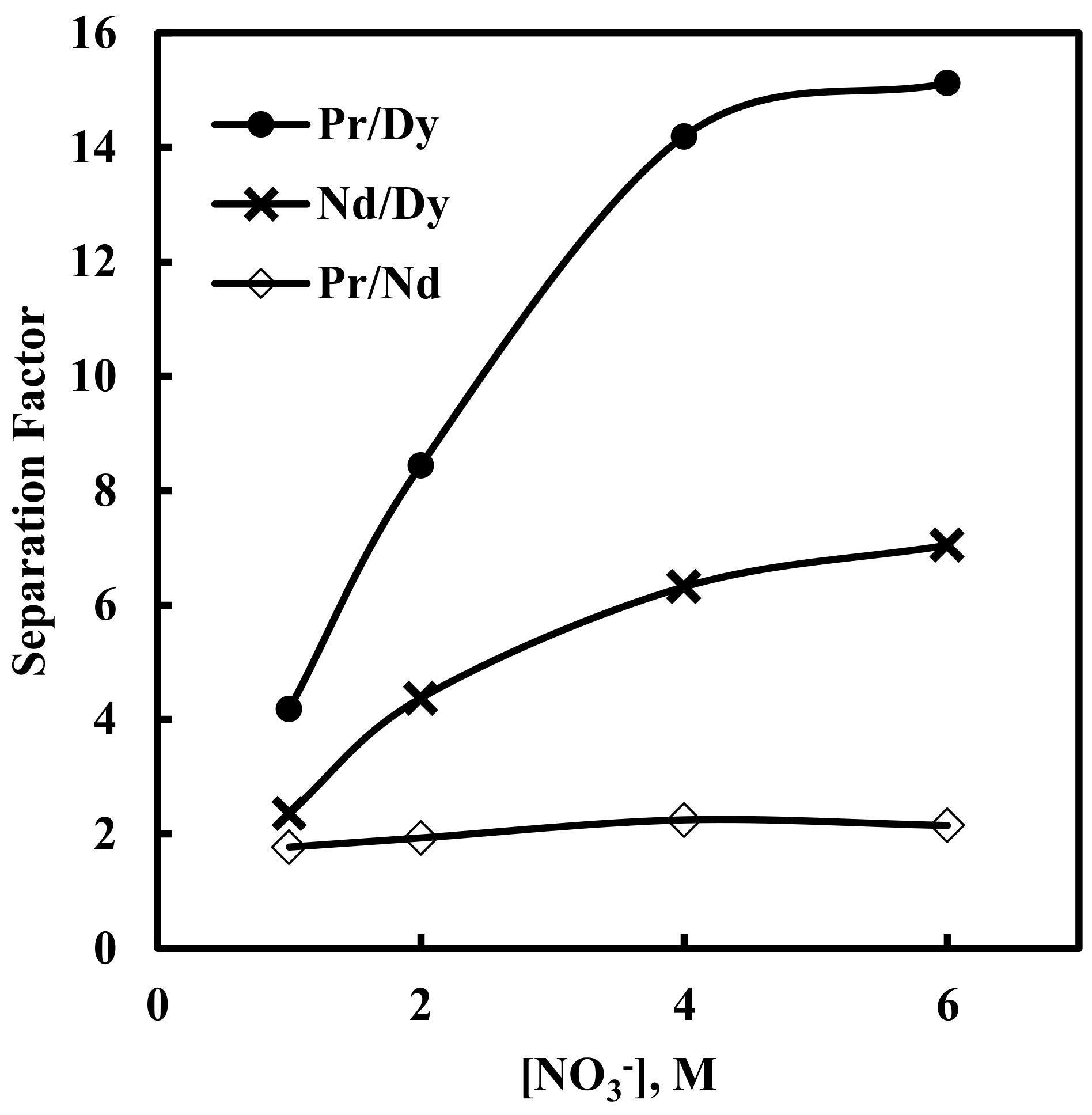
The separation factor values for different pairs of rare earth ions against various nitrate concentration are shown in Figure 7.
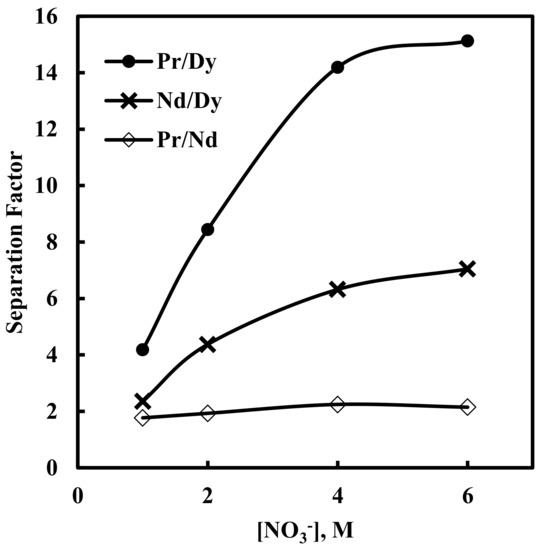
Figure 7.
Effect of nitrate ion concentration on separation factors. Aqueous phase: pH = 3, [EDTA] = 0.0001 M. Organic phase: [336At][NO3]= 0.43 M + 10% iso-decanol in kerosene.
3.4. Effect of EDTA on Solvent Extraction of Nd, Pr and Dy
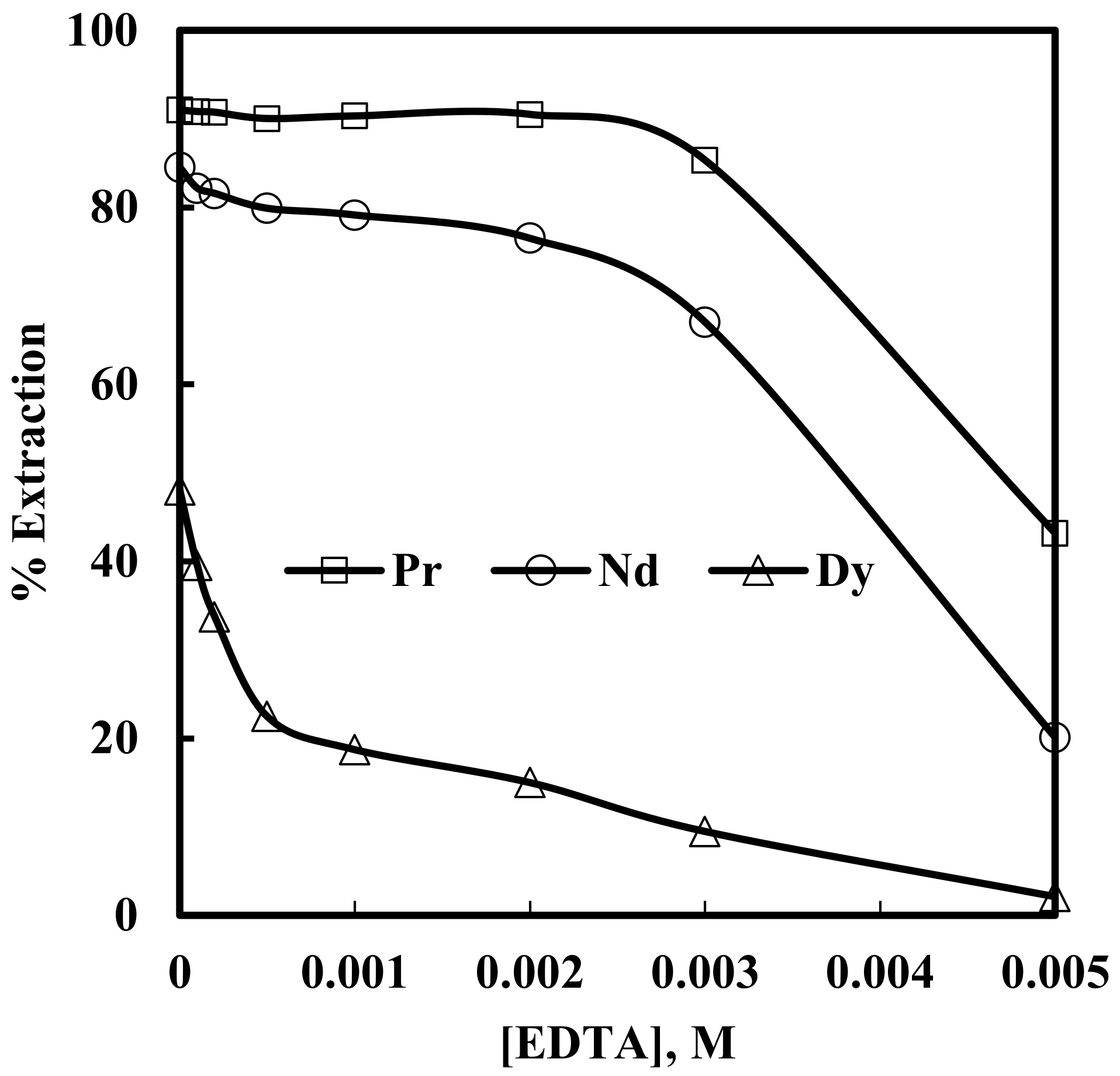
In order to investigate the effect of complexing agent EDTA, solvent extraction of rare earth elements were studied from 6 M NaNO3 solutions containing 0.012 M Nd, 0.003 M Pr, and 0.0002 M Dy, with 0.43 M [336At][NO3], and varying concentrations of EDTA in the range of 0.0001–0.005 M in the aqueous phase (assuming EDTA will make 1:1 complex with the rare earth ions). The results are depicted in Figure 8. It is clear from Figure 8 that the extraction of rare earth metal ions decreased with an increase in EDTA concentration. This is due to the fact that EDTA forms thermodynamically stable complexes with rare earth metal ions in the aqueous phase and suppresses their further extraction into the organic phase, as shown in Equation 6.
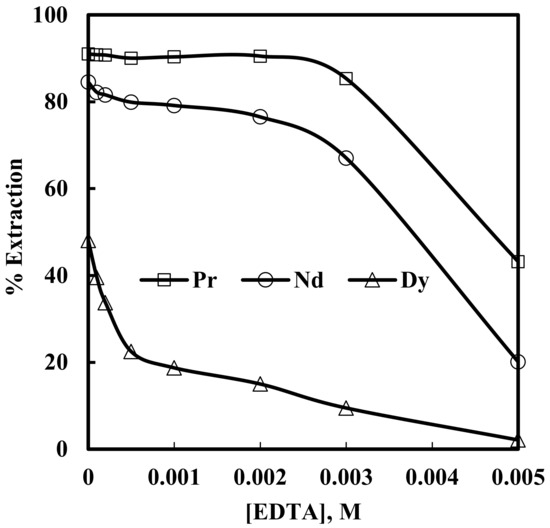
Figure 8.
Effect of EDTA concentration on the extraction of Pr, Nd and Dy. Aqueous phase: pH = 3, [NO3−] = 6 M. Organic phase: [336At][NO3] = 0.43 M + 10% iso-decanol in kerosene.
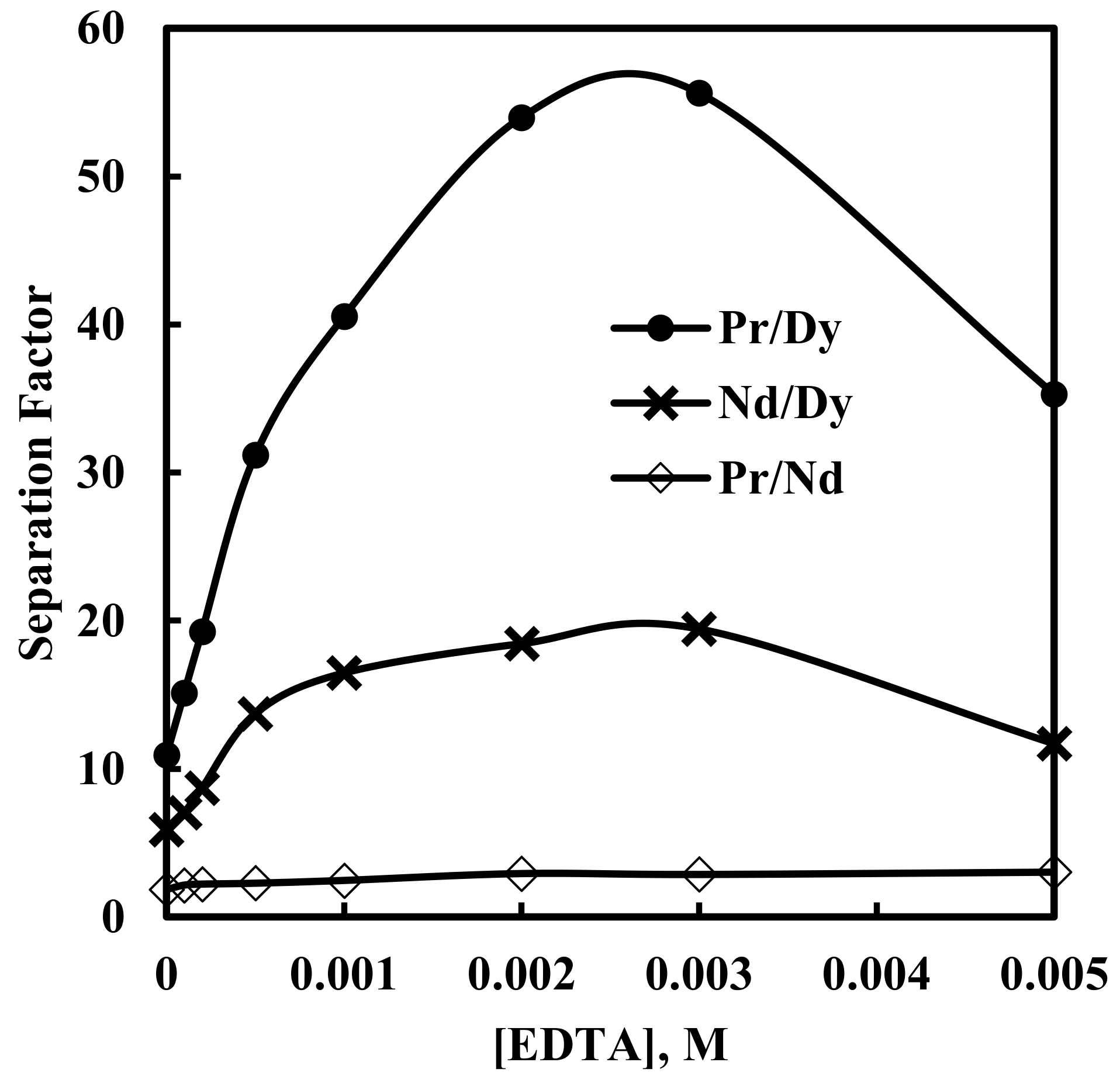
The plot of separation factor values against EDTA concentrations is shown in Figure 9. It is interesting to observe that separation factor values for Pr/Dy and Nd/Dy increased with an increase in EDTA concentration up to 0.003 M EDTA. Conversely, when EDTA was not used in the aqueous solution, the separation factor values for Pr/Dy, Nd/Dy and Pr/Nd were found to be 10.9, 5.9 and 1.8, respectively. However, when 0.003 M EDTA was used as the complexing agent, separation factor values increased to 55.6, 19.4 and 2.9, respectively (Table 4). Beyond 0.003 M EDTA the separation factor values decreased continuously. The stability constants (log βEDTA) of Pr, Nd and Dy ions with EDTA are 16.17, 16.48 and 18.01, respectively [44]. Dysprosium forms a stable complex with EDTA, in comparison with Pr and Nd. Therefore, in the present investigation, EDTA preferentially masked Dy ions present in the aqueous solution and increased the separation values for Pr/Dy and Nd/Dy up to 0.003 M EDTA. Beyond 0.003 M EDTA, Pr and Nd were also effectively masked by EDTA, thereby decreasing the separation factor values. At 0.003 M EDTA, separation factor values obtained for Pr/Dy and Nd/Dy were 55.6 and 19.4, respectively. The separation factor for Pr/Nd (2.9) was almost constant, as shown in Figure 9, due to a very small difference in their stability constant with EDTA. Comparisons of separation factor values for Pr/Nd obtained in the present investigation with the values reported in the literature are given in Table 5. It is clear from Table 5 that the use of EDTA as a complexing agent improved the separation factor for Pr/Nd.
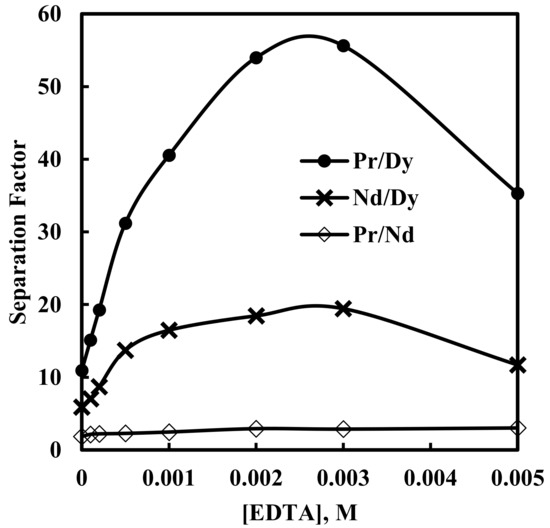
Figure 9.
Effect of EDTA concentration on separation factors. Aqueous phase: pH = 3, [NO3−] = 6 M. Organic phase: [336At][NO3] = 0.43 M + 10% iso-decanol in kerosene.

Table 4.
Extraction and separation behavior of Pr, Nd and Dy with and without EDTA, using [336At][NO3] as extractant. Aqueous phase: pH = 3, [NO3−] = 6 M. Organic phase: [336At][NO3] = 0.43 M + 10% iso-decanol in kerosene.

Table 5.
Comparison of separation factors obtained for Pr and Nd in different studies.
3.5. FTIR Spectra of [336At][NO3] and Nd Loaded [336At][NO3]
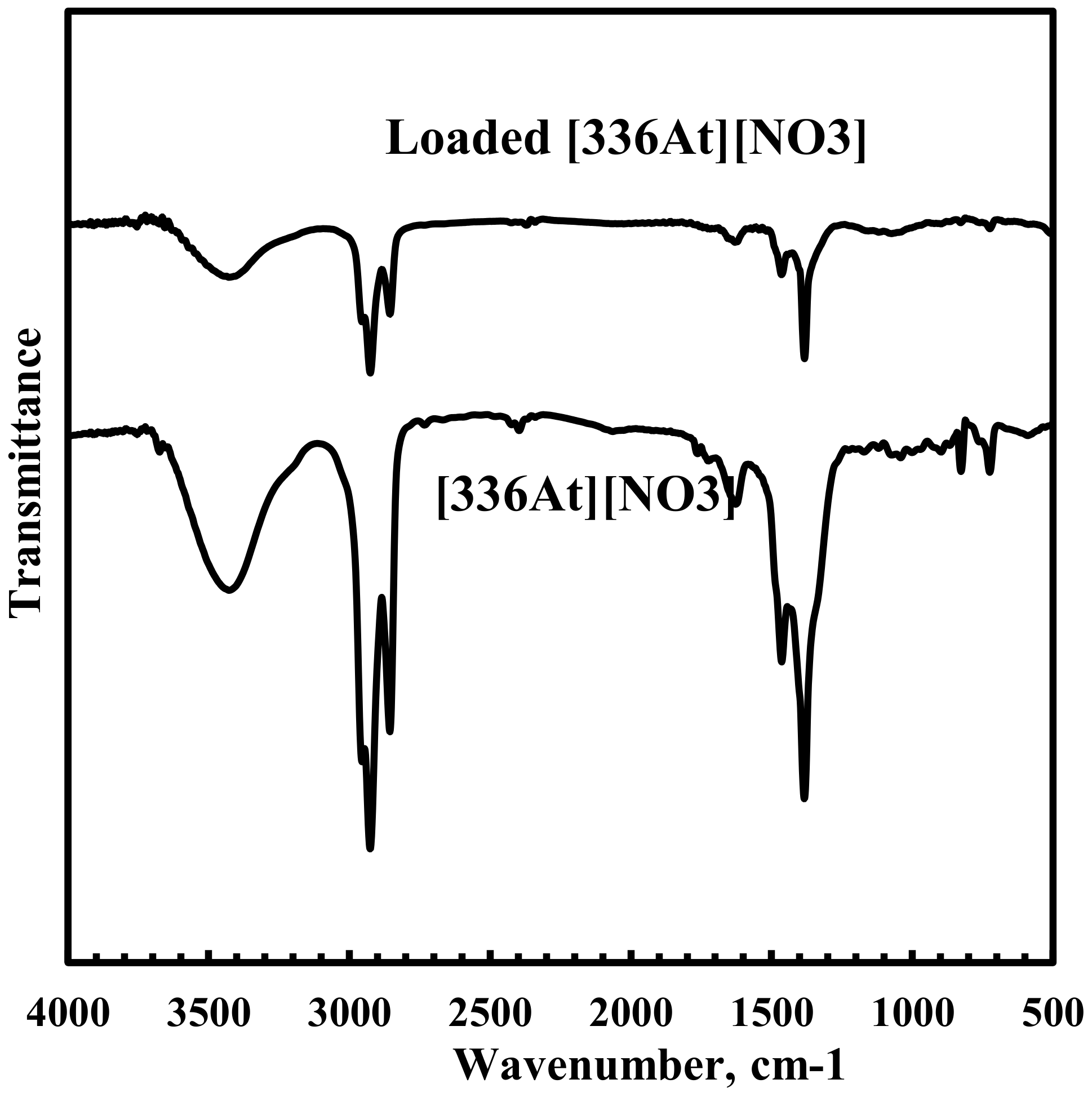
In order to understand the interaction between representative rare earth ion Nd3+ and [336At][NO3], FTIR absorption spectra of [336At][NO3] were recorded and are shown in Figure 10. Although both the spectra are very similar, it was found that the characteristic peak at 1462 cm−1 corresponding to N–CH2 symmetric vibration of [336At][NO3] shifted to 1450 cm−1 and became broader in the Nd loaded [336At][NO3]. This confirms the interaction of Nd(III) with nitrate ions of [336At][NO3], which led to a broadening of the peak due to N–CH2 symmetric stretching vibration. Furthermore, the characteristic peak at 1382 cm−1 due to N–O asymmetric stretching vibration in [336At][NO3] shifted to 1372 cm−1 in the Nd loaded [336At][NO3]. This indicates the interaction of nitrate groups with Nd3+ ion during extraction. This result supports the extraction mechanism as shown in Equation (4).
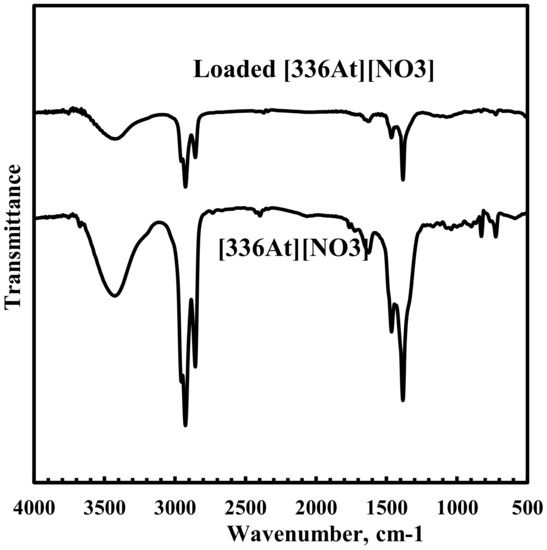
Figure 10.
FTIR spectra for [336At][NO3] and Nd loaded [336At][NO3].
4. Conclusions
The solvent extraction separation studies of praseodymium, neodymium and dysprosium obtained from NdFeB magnet leach liquor were performed by using ionic liquid, [336At][NO3] in the presence of complexing agent EDTA. It was found that the extraction of rare earths with [336At][NO3] in the organic phase follows the order Pr > Nd > Dy, whereas the complex formation stability of EDTA with rare earth ions in the aqueous solution follows the order Dy > Nd > Pr. This established the possibility of efficient separation of rare earths by using [336At][NO3] in the presence of complexing agent EDTA. With the addition of EDTA in the aqueous phase (upto 0.003 M), the extraction of rare earths decreased due to the masking effect, but the separation factor enhanced significantly. However, it is required to understand the interaction of rare earth metal ions and [336At][NO3] at the molecular level considering dielectric constant of diluents and viscosity of the extractant. Furthermore, the effect of other aquo-complexing agents, such as lactic acid and citric acid, may also be investigated.
Author Contributions
Desgining and performing the experiment, A.K.; Writing original draft, A.K.; Supervision, K.K.S. and S.K.S.; Writing review and editing, A.K., K.K.S. and S.K.S.
Funding
This research was funded by In-house Project Support Group (OLP - 0285) of CSIR-NML and CEFIPRA project (GAP-0271).
Acknowledgments
Authors are thankful to the Director of CSIR-National Metallurgical Laboratory (CSIR-NML), Jamshedpur, for giving permission to publish the paper. Financial support provided by In-house Project Support Group (OLP - 0285) of CSIR-NML and CEFIPRA project (GAP-0271) is gratefully acknowledged. Authors are also thankful to M/s Regen Powertech Pvt. Limited, Chennai, India for providing the spent NdFeB magnets of wind turbines and Kopper Chem, China for providing the reagent Mextral® 336At for research purposes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tao, X.; Huiqing, P. Formation cause, composition analysis and comprehensive utilization of rare earth solid wastes. J. Rare Earth 2009, 27, 1096–1102. [Google Scholar]
- Pavel, C.C.; Lacal-Arántegui, R.; Marmier, A.; Schüler, D.; Tzimas, E.; Buchert, M.; Blagoeva, D. Substitution strategies for reducing the use of rare earths in wind turbines. Resour. Policy 2017, 52, 349–357. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Guth, K.; Gauß, R.; Gutfleisch, O.; Binnemans, K. REE recovery from end-of-life NdFeB permanent magnet scrap: A critical review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Sun, M.; Hu, X.; Peng, L.; Fu, P.; Ding, W.; Peng, Y. On the production of Mg-Nd master alloy from NdFeB magnet scraps. J. Mater. Proc. 2015, 218, 57–61. [Google Scholar] [CrossRef]
- Abrahami, S.T.; Xiao, Y.; Yang, Y. Rare-earth elements recovery from postconsumer hard-disc drives. Miner. Proces. Extr. Met. 2015, 124, 106–115. [Google Scholar] [CrossRef]
- Itoh, M.; Miura, K.; Machida, K.I. Novel rare earth recovery process on Nd-Fe-B magnet scrap by selective chlorination using NH4Cl. J. Alloy. Compd. 2009, 477, 484–487. [Google Scholar] [CrossRef]
- Banda, R.; Seok, H.; Seung, M. Hydrometallurgy Solvent extraction separation of La from chloride solution containing Pr and Nd with Cyanex 272. Hydrometallurgy 2012, 121–124, 74–80. [Google Scholar] [CrossRef]
- Singh, D.K.; Kotekar, M.K.; Singh, H. Development of a solvent extraction process for production of nuclear grade dysprosium oxide from a crude concentrate. Desalination 2008, 232, 49–58. [Google Scholar] [CrossRef]
- Parhi, P.K.; Sethy, T.R.; Rout, P.C.; Sarangi, K. Separation and recovery of neodymium and praseodymium from permanent magnet scrap through the hydrometallurgical route. Sep. Sci. Technol. 2016, 51, 2232–2241. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, C.J.; Chung, K.W.; Kim, S.D.; Lee, J.Y.; Kumar, J.R. Solvent extraction, separation and recovery of dysprosium (Dy) and neodymium (Nd) from aqueous solutions: Waste recycling strategies for permanent magnet processing. Hydrometallurgy 2016, 165, 27–43. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.Y.; Kim, J.S.; Lee, G.S. Solvent extraction of neodymium ions from hydrochloric acid solution using PC88A and saponified PC88A. Sep. Puri. Tech. 2005, 46, 72–78. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Kumari, A.; Sinha, M.K.; Sahu, S.K.; Pandey, B.D. Solvent extraction and separation of trivalent lanthanides using cyphos IL 104, a novel phosphonium Ionic liquid as extractant. Solvent Extr. Ion Exc. 2016, 34, 469–484. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, H.S.; Lee, M.S. Solvent extraction of Pr and Nd from chloride solution by the mixtures of Cyanex 272 and amine extractants. Hydrometallurgy 2014, 150, 61–67. [Google Scholar] [CrossRef]
- Padhan, E.; Sarangi, K. Recovery of Nd and Pr from NdFeB magnet leachates with bi-functional ionic liquids based on Aliquat 336 and Cyanex 272. Hydrometallurgy 2017, 167, 134–140. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Matsumiya, M.; Kawakami, S. Extraction of rare earth ions from Nd–Fe–B magnet wastes with TBP in tri-capryl-methyl-ammonium nitrate. Solvent Extr. Res. Dev. 2014, 21, 137–145. [Google Scholar] [CrossRef]
- Riano, S.; Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015, 17, 2931–2942. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Influence of the ionic liquid cation on the solvent extraction of trivalent rare-earth ions by mixtures of Cyanex 923 and ionic liquids. Dalton Trans. 2015, 44, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Vander Hoogerstraete, T.; Onghena, B.; Binnemans, K. Homogeneous liquid-liquid extraction of rare earths with the betaine-betainium bis(trifluoromethylsulfonyl)imide ionic liquid system. Int. J. Mol. Sci. 2013, 14, 21353–21377. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Selective recovery of dysprosium and neodymium ions by a supported liquid membrane based on ionic liquids. Solvent Extr. Res. Dev. 2011, 18, 193–198. [Google Scholar] [CrossRef]
- Kashi, E.; Habibpour, R.; Gorzin, H.; Maleki, A. Solvent extraction and separation of light rare earth elements (La, Pr and Nd) in the presence of lactic acid as a complexing agent by Cyanex 272 in kerosene and the effect of citric acid, acetic acid and Titriplex III as auxiliary agents. J. Rare Earth 2018, 36, 317–323. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Li, D. Selective separation of yttrium by CA-100 in the presence of a complexing agent. J. Alloy Compd. 2006, 408–412, 999–1002. [Google Scholar] [CrossRef]
- Matsuyama, H.; Azis, A.; Fujita, M.; Teramoto, M. Enhancement in extraction rates by addition of organic acids to aqueous phase in solvent extraction of rare earth metals in presence of diethylenetriamine pentacetic acid. J. Chem. Eng. Japan 1995, 29, 126–133. [Google Scholar] [CrossRef]
- Banda, R.; Jeon, H.S.; Lee, M.S. Extraction behavior of REEs (La, Pr, and Nd) in the presence of EDTA from chloride solutions. Korean J. Met. Mater. 2013, 51, 421–427. [Google Scholar] [CrossRef]
- Yin, S.; Wu, W.; Bian, X.; Luo, Y.; Zhang, F. Solvent Extraction of La(III) from Chloride Medium in the Presence of Two Water Soluble Complexing Agents with di-(2-ethylhexyl) Phosphoric Acid. Ind. Eng. Chem. Res. 2013, 52, 8558–8564. [Google Scholar] [CrossRef]
- Yin, S.; Wu, W.; Zhang, B.; Zhang, F.; Luo, Y.; Li, S.; Bian, X. Study on separation technology of Pr and Nd in D2EHPA-HCl-LA coordination extraction system. J. Rare Earth 2010, 28, 111–115. [Google Scholar] [CrossRef]
- Yin, S.; Li, S.; Wu, W.; Bian, X.; Peng, J.; Zhang, Li. Extraction and separation of Ce(III) and Pr(III) in the system containing two complexing agents with di-(2-ethylhexyl) phosphoric acid. RSC Adv. 2014, 4, 59997–60001. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Separation of rare earths from transition metals by liquid-liquid extraction from a molten salt hydrate to an ionic liquid phase. Dalton Trans. 2014, 43, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Rout, A.; Binnemans, K. Solvent extraction of neodymium(III) by functionalized ionic liquid tri-octyl-methyl-ammonium dioctyl diglycolamate in fluorine-free ionic liquid diluent. Ind. Eng. Chem. Res. 2014, 53, 6500–6508. [Google Scholar] [CrossRef]
- Cerna, M.; Volaufova, E.; Rod, V. Extraction of light rare earth elements by amines at high inorganic nitrate concentration. Hydrometallurgy 1992, 28, 339–352. [Google Scholar] [CrossRef]
- Komasawa, I.; Hisada, K.; Miyamura, M. Extraction and separation of rare-earth elements by tri-octylmethylammonium nitrate. J. Chem. Eng. Japan 1990, 23, 308–315. [Google Scholar] [CrossRef]
- El-Kot, A.M. Solvent extraction of neodymium, europium and thulium by di-(2- ethylhexyl) phosphoric acid. J. Radioanal. Nucl. Chem. 1993, 170, 207–214. [Google Scholar] [CrossRef]
- Huang, Y.; Tanaka, M. Solvent extraction equilibrium of dysprosium(III) from nitric acid solutions with 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester. Trans. Nonferrous Met. Soc. China 2010, 20, 707–711. [Google Scholar] [CrossRef]
- Thakur, N.V.; Jayawant, D.V.; Iyer, N.S.; Koppiker, K.S. Separation of neodymium from lighter rare earths using alkyl phosphonic acid, PC 88A. Hydrometallurgy 1993, 34, 99–108. [Google Scholar] [CrossRef]
- Preston, J.S. The recovery of rare earth oxides from a phosphoric acid byproduct. Part 4. The preparation of magnet-grade neodymium oxide from the light rare earth fraction. Hydrometallurgy 1996, 42, 15–167. [Google Scholar] [CrossRef]
- Panda, N.; Devi, N.; Mishra, S. Solvent extraction of neodymium(III) from acidic nitrate medium using Cyanex 921 in kerosene. J. Rare Earth 2012, 30, 794–797. [Google Scholar] [CrossRef]
- Vander Hoogerstraete, T.; Wellens, S.; Verachtert, K.; Binnemans, K. Removal of transition metals from rare earths by solvent extraction with an undiluted phosphonium ionic liquid: Separations relevant to rare-earth magnet recycling. Green Chem. 2013, 15, 919. [Google Scholar] [CrossRef]
- Rout, A.; Kotlarska, J.; Dehaen, W.; Binnemans, K. Liquid-liquid extraction of neodymium(III) by dialkylphosphate ionic liquids from acidic medium: The importance of the ionic liquid cation. Phys. Chem. Chem. Phys. 2013, 15, 16533–16541. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Extractive separation of praseodymium and neodymium by di(2-ethylhexyl) phosphoric acid in the presence of water-soluble complexing agents using an electrostatic liquid-liquid contactor. Chem. Eng. Res. Des. 1997, 75, 447–452. [Google Scholar] [CrossRef]
- Lu, D.; Horng, J.S.; Hoh, Y.C. The separation of neodymium by quaternary amine from didymium nitrate solution. J. Alloy Compd. 1989, 149, 219–224. [Google Scholar] [CrossRef]
- Larson, K.; Binnemans, K. Separation of rare earths by solvent extraction with an undiluted nitrate ionic liquid. J. Sustain. Metall. 2017, 3, 73–78. [Google Scholar] [CrossRef]
- Riano, S.; Petranikova, M.; Onghena, B.; Hoogerstraete, T.V.; Banerjee, D.; Foreman, M.; Ekberg, C.; Binnemans, K. Separation of rare earths and other valuable metals from deep-eutectic solvents: A new alternative forthe recycling of used NdFeB magnets. Rsc. Adv. 2017, 7, 32100–32113. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, M.K.; Pramanik, S.; Sahu, S.K. Recovery of rare earths from spent NdFeB magnets of wind turbine: Leaching and kinetic aspects. Waste Manag. 2018, 75, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Wheelwright, E.J.; Spedding, F.H. The Use of Chelating Agents in the Separation of the Rare Earth Elements by Ion-Exchange Methods. Ames Laboratory ISC Technical Reports 1955. Available online: https://lib.dr.iastate.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=1098&context=ameslab_iscreports (accessed on 7 August 2018).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).