Abstract
In this study, a certain amount of Cu was added into tentative steel to introduce novel Cu-rich nanoprecipitates, thus enhancing strength yet without sacrificing toughness. This type of precipitates was quite different from previous ε-Cu, and was a novel type of Cu-rich nanoprecipitates, which contained more than 50% Cu. The microstructure, mechanical properties and precipitates of the steels aged at 550 °C for different holding times and were carefully examined. The microstructure of the tested steel was mainly bainite and gradually evolved into equilibrium state after aging. Mechanical properties results showed that after being aged at 550 °C for 10 min, the steel can have an excellent mechanical property combination of strength and toughness. In addition, a large amount of tiny precipitates was uniformly distributed in the matrix of the aging steels, and their size kept at nanoscale. In particular, when the steel was aged at 550 °C for 10 min, it produced the largest number of tiny precipitates of this type. This type of Cu-rich nanoprecipitates emerging from the steel aged at 550 °C for 10 min also brought about a remarkable antibacterial property. It revealed that novel Cu-rich precipitates not only have positive effects on strength and toughness, but also played an important role in antibacterial properties.
1. Introduction
High strength steels are increasingly used in many areas, such as engineering machinery, marine platforms, naval vessels, pipelines, storage tanks, and bridges []. Traditionally, in terms of elements put into steels, the strengthening of steels mainly relies on adding more carbon and more alloying elements, which commonly are Mn, Mo, Cr, Ni, Nb, V, Ti, etc. [,,,]. In regards to the strengthening mechanism, it often involves solid solution strengthening, dislocation strengthening, precipitation strengthening, subcrystal strengthening and so on []. Unfortunately, more carbon and more alloying elements can easily have harmful effects on the weldability. Moreover, to some extent, large size precipitations containing Nb, Ti, and V not only weaken precipitation strengthening and the dislocation strengthening effect, but also are unfavorable for weldability and toughness. In other words, as the strength of the steel in demand is becoming higher and higher, only adopting these methods mentioned above makes it very difficult to enhance the strength level of steels without a reduction of toughness [].
Recently, the novel Cu -rich nanoprecipitates in high strength steel have grabbed wide attention and become one important turning point for the development of high strength steels [,,,,,,,,]. As we know, the effect of precipitation strengthening highly depends on its type, number density, size and distribution conditions []. Recent research has shown that, through suitable composition and heat treatment, novel Cu-rich nanoprecipitates can offer an excellent performance combination of high strength and toughness, yet the carbon content of these high strength steel can be very low [,,].
Microbiologically influenced corrosion is a normal phenomenon for steels in soil and sea, and has attracted considerable attention due to the great loss it can cause [,]. Various methods have been used to prevent microbiologically influenced corrosion, such as biocides, coating, cathodic protection and so on []. However, these methods have some restrictions in many aspects: expensive cost, harmful effects on environment and its inefficiency. If steels themselves have antibacterial performance, the harmfulness can be greatly reduced. This would be a new effective and environmentally friendly strategy for microbial corrosion control. In this paper, the novel Cu-rich nanoprecipitates in the tentative steel under different techniques were studied, as well as its effects on the mechanical and antibacterial properties of the steel.
2. Materials and Methods
The experimental steel investigated in this study was produced by a 25 kg vacuum induction melting furnace. The chemical composition of the steel is listed in Table 1. The steel ingot was forged into a block of 120 mm × 120 mm × 240 mm and then austenitized at 1200 °C for 2 h. Afterwards, it was rolled to 70 mm at 860 °C with 3 passes and then was rolled to 20 mm at 800 °C with several passes, and a fast cooling rate (20~25 °C/s) after rolling by water spraying. An aging process was applied to control the precipitation of nanoscale Cu-rich precipitates to strengthen the steel. Some of the as-rolled plates were aged at 550 °C for 5 min, 10 min, 30 min, 60 min respectively.

Table 1.
Chemical composition of experimental steels (wt.%).
Optical microscope (OM, Leica DMI5000M, Leica, Germany) and transmission electron microscope (TEM, JEM2100F, JEOL, Tokyo, Japan) were used to observe the microstructures of these steels. For the Optical microscope observation, samples were ground to #2000 with sand papers, and then mechanically polished, and then were etched with 4 vol.% nital solution. The transmission electron microscope (TEM) was used to survey the characteristics of nanoscale Cu-rich precipitates. 0.3 μm thin slices were manually thinned to 50 μm thick and were examined by TEM.
The tensile specimens were 5 mm in diameter and 25.4 in gauge length and were machined from the tentative plates perpendicular to the rolling direction. The tensile tests were conducted at room temperature. To enhance the reliability of the tensile results, two parallel samples were tested to get the average value.
The antibacterial property was examined according to agar plate method (GB4789.2-94), and the tested bacteria were Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). The tested bacterial strain was obtained from slant culture at logarithmic phase, and the tentative steel and the control specimen were individually incubated both in 105 CFU/mL Escherichia coli (E. coli) and 106 CFU/mL Staphylococcus aureus (S. aureus) at 37 °C for 24 h. Then these bacterial fluids were diluted to 103 CFU/mL and were incubated by agar for 24 h at the same temperature and humidity. The number of bacterial colonies in culture dishes can be calculated. The calculation of antibacterial ratio was as follows:
R = (C − A)/C × 100%,
In this equation, R is antibacterial ratio, C is the viable bacterial count of the control specimen, A is the viable bacterial count of the tentative steel [,].
3. Results
3.1. Microstructure
Figure 1 shows the optical microstructures of the TMCP and aged specimens. On the whole, the microstructure of the tested steel was mainly bainite, yet there were slight differences under processes involving different techniques. Before the aging process, most of the microstructure of the TMCP steel was tiny granular bainite and polygonal ferrite, with a small amount of lath bainite (Figure 1a). After aging at 550 °C for 5 min, there were several not quite obvious changes (Figure 1b). When the aging holding time was prolonged to 10 min, the proportion of polygonal ferrite began to increase. As the holding time was further extended from 30 min to 60 min, the main microstructure became polygonal ferrite, and the proportion of granular bainite significantly decreased (Figure 1c–e). In regards to all types of bainite, polygonal ferrite is the most stable microstructure, and granular bainite is inferior to it, with the most unstable one being lath bainite. The terminal point of microstructure evolution is equilibrium microstructure—polygonal ferrite []. From these microstructure evolution photos, it was evident that as the holding time became longer, the microstructure gradually evolved into an equilibrium state.
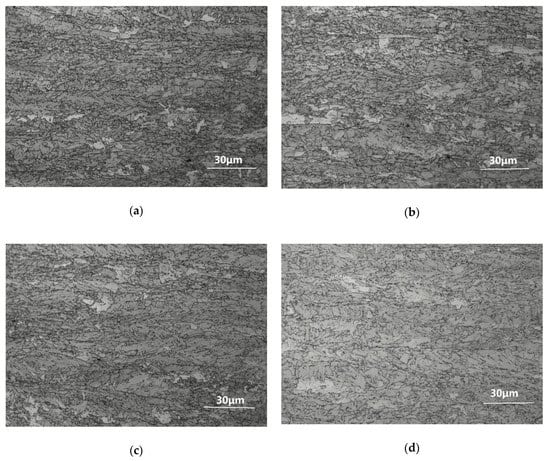
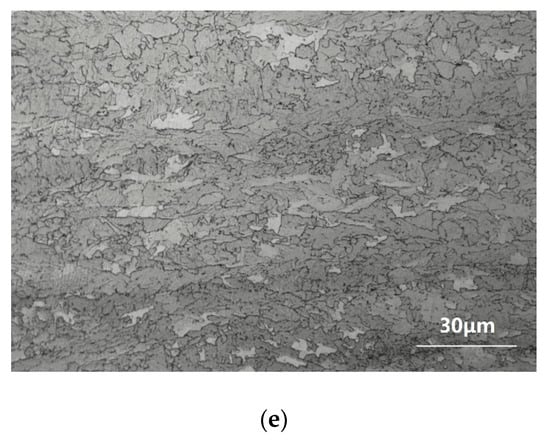
Figure 1.
Optical microstructures of tested steel: (a) TMCP (Thermo Mechanical Control Process); (b) TMCP + aged at 550 °C for 5 min; (c) TMCP + aged at 550 °C for 10 min; (d) TMCP + aged at 550 °C for 30 min; (e) TMCP + aged at 550 °C for 60 min.
3.2. Tensile Properties and Microhardness
The strength curve of the specimens under different processing techniques was shown in Figure 2a. From Figure 2a, it can be found that the aging holding time had obvious effects on the mechanical properties of the steel. After TMCP, the yield strength and the tensile strength were 616 MPa and 744 MPa respectively, and the elongation rate was 24%. After aging at 550 °C for 5 min, there is a small increase in both the yield strength and the tensile strength by about 13 MPa, with 22% of the elongation rate. When the steel was aged at the same temperature for 10 min, the yield strength and the tensile strength rose dramatically to 660 MPa and 782 MPa respectively, yet the elongation rate decreased slightly to 21%. As the aging holding time was prolonged to 30 min, the yield strength grew to 692 MPa and the tensile strength remained in the previous level, with the elongation rate declining to 18%. When it was aged for 60 min, its yield strength and the tensile strength reached their highest point at 756 MPa and 806 MPa, and there is no significant change in the elongation rate. Generally, there was a relatively stable increase in both the yield strength and the tensile strength. By contrast, the elongation rate saw an opposite trend.
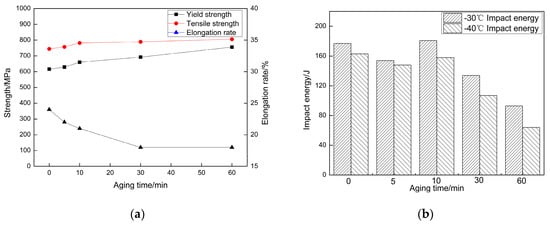
Figure 2.
Mechanical property: (a) Tensile property; (b) Impact energy.
The chart (Figure 2b) illustrated the low temperature impact property of the steels under different processing techniques. With the elongation of the holding time, the impact absorbing energy slightly fell first under 5 min holding time, and then rose to its peak point at 10 min aging time. After that, the impact absorbing energy began to drop again, and the longer the holding time, the lower it became. Overall, after aging at 550 °C for 10 min, the steel can have excellent mechanical properties.
3.3. Precipitation
The distribution condition of Cu-rich nanoprecipitates in the rolled steel sample and in the steel samples aged at 550 °C for different times is shown in Figure 3. For the rolled sample, numerous entangled dislocations can be clearly seen, and no nanoprecipitates were observed (Figure 3a). After the steel was aged at 550 °C for 5 min, a large amount of tiny precipitates uniformly distributed in the matrix, and their size kept at nanoscale (Figure 3b). These precipitates were analyzed by energy spectrum (see Figure 4a, Table 2). The results revealed that these tiny precipitates were Cu-rich phase. After holding for 10 min at the same temperature, the number of these tiny precipitates continued to rise and the size did not apparently grow larger (Figure 3c). As the holding time was further prolonged, precipitates notably become larger, in particular when the sample was aged for 60 min, the size reached above 20 nm (Figure 3d,e). The precipitation process of microalloy elements usually undergoes four steps: segregation of microalloy elements and nucleation, growth, and coarsening of precipitates. From the results, it can be concluded that with the prolonged holding time, Cu element constantly segregated and resulted in the continuous nucleation, growth, and coarsening of Cu-rich precipitates.
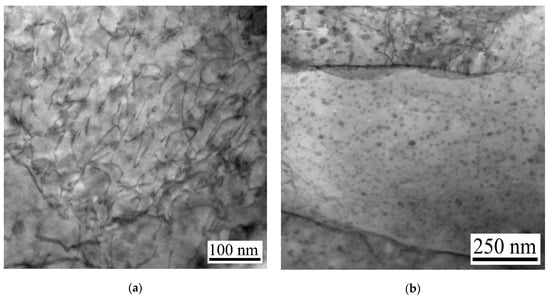
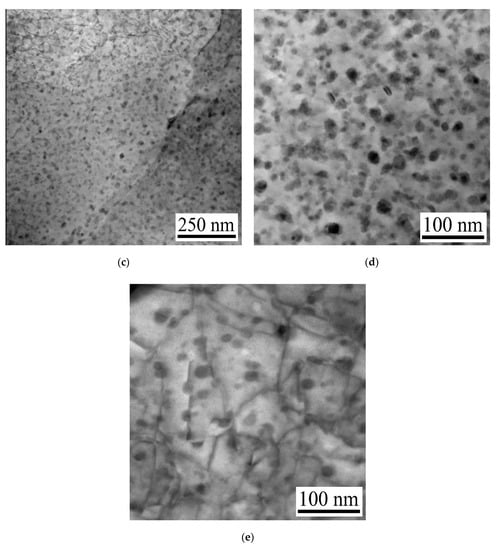
Figure 3.
The distribution condition of Cu-rich nanoprecipitates: (a) As-rolled; (b) As-aged at 550 °C for 5 min; (c) As-aged at 550 °C for 10 min; (d) As-aged at 550 °C for 30 min; (e) As-aged at 550 °C for 60 min.
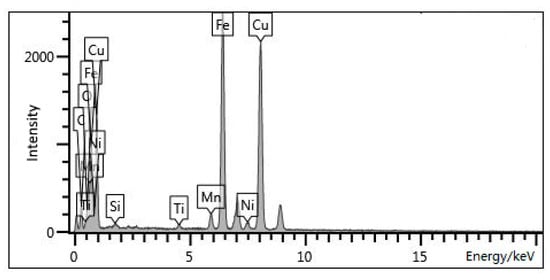
Figure 4.
The photos of energy spectrum.

Table 2.
Chemical composition.
3.4. The Antibacterial Performance
To further study the antibacterial property of the tested steel, two control experiments: as-rolled and aged at 550 °C for 10 min, were chosen to conduct an antibacterial test. Figure 5 shows antibacterial rates against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) of the two specimens. It conveyed that the antibacterial property of the as-rolled sample was obviously poor, and its antibacterial rates against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were 63% and 70%, respectively. Compared with the as-rolled sample, the control sample (as-aged at 550 °C for 10 min) had excellent antibacterial performance, of which antibacterial rates against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) reached 100% and 99.9%.
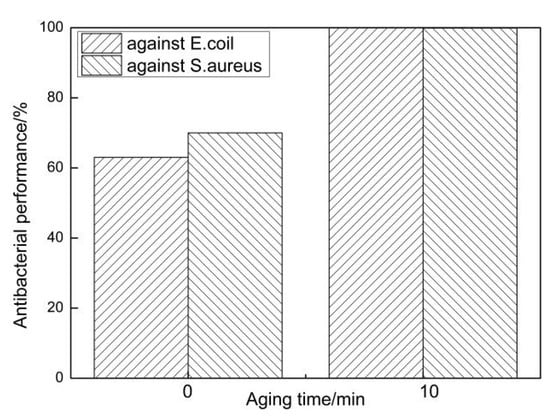
Figure 5.
Antibacterial rates against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) of steels under rolled and aged conditions respectively.
The photos of antibacterial performance against E. coil and S. aureus on petridishes cultured with two control samples were shown in Figure 6 and Figure 7. As it was illustrated, after the as-rolled sample was cultured with bacterial liquid, the number of bacterial colonies was greater and the sample exhibited poor antibacterial performance (Figure 6a, Figure 7a). By contrast, when the sample aged at 550 °C for 10 min and bacterial liquid were cultured together, there was no bacterial colony and the sample had an excellent antibacterial performance (Figure 6b, Figure 7b).
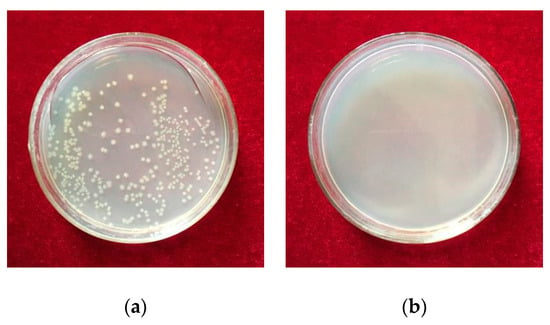
Figure 6.
Representative photos of antibacterial performance against E. coil on petridishes cultured with steels: (a) As-rolled; (b) Aged at 550 °C for 10 min.

Figure 7.
Representative photos of antibacterial performance against S. aureus on petridishes cultured with steels: (a) As-rolled; (b) Aged at 550 °C for 10 min.
4. Discussion
As shown in experimental results, the strength of the steel continued to grow with the prolonged holding time, and the low temperature impact property reached the best condition when it was aged for 10 min. However, from the optical microstructures evolution, the holding time can have slight softening effects on microstructure. This means that the strength range of the steel after aging was not caused by the transformation of the microstructure, but resulted from aging-induced Cu-rich nanoprecipitates. Precipitation strengthening is the most important strengthening method for Cu-rich high-strength steel. Because the early observed strengthening effect mainly depends on the precipitation of ε-Cu on the matrix, the precipitates phase is still a controversial issue. But with the introduction of more advanced detection methods into the research of the Cu-containing precipitates phase, researchers began to develop different opinions on this problem. Goodman [] discovered that the strength rise at aging peak was not from the ε-Cu phase as people used to think, but was from the uniformly distributed precipitates which contained 50% Cu. It was also found that the precipitation strengthening effect of ε-Cu was far worse than this novel type of precipitates. Actually, the characteristics of Cu-rich precipitates in aspect of size, number density, shape, etc. that determine final mechanical properties greatly depend on the compositional and resultant structural evolution during the aging process [,,,,,,].
With the beginning of the aging process, the Cu element can gain energy to avoid an ambient potential barrier and then segregate in nearby positions. The longer holding time, the more precipitates develop, which also gradually grow larger. In this experiment, when the samples were holding for both 5 min and 10 min, Cu-rich precipitates were all nanoscale. During this process, the precipitates were small and dislocations cut through them. The amount and the volume fraction of precipitates are so large that dislocation had to cut them continuously, thus constantly enhancing the precipitation effect and resulting in the rising strength. However, as holding time was prolonged to 60 min, the size of precipitates gradually grew larger. Although the strength rose in the process, the increasing size of precipitates would produce negative effects on the toughness. If the holding time was extended further, it could have resulted in an over-aging phenomenon. This cannot be observed in the optical microstructure because of the quite small nanoprecipitates.
In this experiment, the nanoscale and the rising volume fraction of these precipitates made it possible for the uniformly distributed ones to inhibit the dislocation motion, thus gaining higher strength. In fact, tiny novel Cu-rich phase was precipitated from supersaturated matrix during the aging process, and the interaction effect of these precipitates and movable dislocations is the essence of the resulting precipitation strengthening [].
As for antibacterial properties, the as-aged at 550 °C for 10 min sample obviously had better performance than the as-rolled sample. Shi and Wang etc. also revealed that the precipitation of Cu-rich phase is the precondition for ensuring having antibacterial properties, and Cu-rich phase precipitating in the aging process can significantly promote antibacterial properties [,]. Therefore, novel Cu-rich precipitates not only have positive effects on strength and toughness, but also was a decisive factor for antibacterial properties.
Firstly, the observed antibacterial activity of Cu nanoparticles is highly dependent on their morphology, especially their large ratio of surface to volume, which enables them to directly interact with the outer microbial membrane of each bacterium []. Yin also discovered that the size and the number per unit area of the precipitates were important factors for excellent antibacterial properties []. The aging tested plates produced a large amount of uniformly distributed Cu-rich nanoprecipitates, and the number reached the peak when it was aged at 550 °C for 10 min. That means under this technique processing, their surface value was the largest possible and the volume was relatively smaller, thus having largest ratio of surface to volume among these specimens. This was the reason for the steel having remarkable antibacterial property.
However, the underlying mechanism of the antibacterial activity of Cu-rich nanoprecipitates is still controversial. One possible explanation is that cell membranes of bacteria containing nano-sized pores can be overcome by the nanoparticles []. The appearance of nanoparticles in suspension can continuously produce ions into the nutrient media. Cu ions from the nanoprecipitates attach to the bacterial cell membrane and rupture it, thus resulting in protein denaturation and cell death. The attachment of both nanoparticles and copper ions to the cell membrane leads to the accumulation of envelope protein precursors and it could cause dissipation of the proton motive force. Cu-rich nanoprecipitates also display destabilization of the outer membrane and rupture of the plasma membrane, thereby causing depletion of intracellular ATP [,]. Cu-rich nanoprecipitates and released ions that produce hydroxyl radicals damage or disturb the working of essential proteins and DNA, leading to the cell death [,].
5. Conclusions
The microstructure of the tested steel was mainly bainite, yet there were slight differences under different techniques process. Before the aging process, most of the microstructure of the TMCP steel was tiny granular bainite and polygonal ferrite, with a small amount of lath bainite. After aging at 550 °C, with the prolonged holding time, the microstructure gradually evolved into an equilibrium state. As the holding time was prolonged, the strength of the tested steel constantly increased and the elongation rate gradually decreased. The impact of absorbing energy reached its peak at 10 min of aging time. Overall, after aging at 550 °C for 10 min, the steel can have excellent mechanical properties. By using transmission electron microscope, numerous entangled dislocations were observed in the rolled sample, while no nanoprecipitates were observed. After aging, a large amount of tiny precipitates was uniformly distributed in the matrix, and their size was kept at nanoscale. But as the holding time was extended, the size of precipitates obviously grew. Compared with the as-rolled sample, the sample aged at 550 °C for 10 min had excellent both mechanical properties and antibacterial performance. It revealed that novel Cu-rich precipitates not only have positive effects on strength and toughness, but also was a decisive factor for antibacterial properties.
Author Contributions
Y.F. analyzed the data and wrote the whole manuscript; C.M. provided paper revision; S.L. designed the experiments; H.Z. performed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Argon, A.S. Strengthening Mechanisms in Crystal Plasticity; Oxford University Press: Oxford, UK, 2008; pp. 100–110. [Google Scholar]
- Lee, W.B.; Hong, S.G.; Park, C.G.; Kim, K.H.; Park, S.H. Influence of Mo on precipitation hardening in hot rolled HSLA steels containing Nb. Scr. Mater. 2000, 43, 319–324. [Google Scholar] [CrossRef]
- Takahashi, A.; Iino, M. Microstructural refinement by Cu addition and its effect on strengthening and toughening of sour service line pipe steels. ISIJ Int. 1996, 36, 241–245. [Google Scholar] [CrossRef]
- Kong, J.H.; Zhen, L.; Guo, B. Influence of Mo content on microstructure and mechanical properties of high strength pipeline steel. Mater. Des. 2004, 25, 723–728. [Google Scholar]
- Koh, S.U.; Lee, J.M.; Yang, B.Y.; Kim, K.Y. Effect of molybdenum and chromium addition on the susceptibility to sulfide stress cracking of high-strength, low-alloy steels. Corrosion 2007, 63, 220–230. [Google Scholar] [CrossRef]
- Wang, Y.M.; Li, M.Y.; Wei, G. Controlled Rolling and Controlled Cooling, 1st ed.; Metallurgical Industry Press: Beijing, China, 2007; pp. 14–19. [Google Scholar]
- Zhang, Z.W. Research development of high strength low alloy (HSLA) steels. Mater. China 2016, 35, 141–150. [Google Scholar]
- Czyryca, E.J.; Vassilaros, M.G. Advances in Low Carbon, High Strength Ferrous Alloys. Key Eng. Mater. 1993, 34, 85–91. [Google Scholar]
- Asfahani, R.; Tither, G. International symposium on low-carbon steels for the 90’s. Mater. Soc. 1993, 1, 511–516. [Google Scholar]
- Vaynman, S.; Fine, M.; Ghosh, G.; Bhat, S. Materials for the new millennium. In Proceedings of the Fourth Materials Engineering Conference, Washington, DC, USA, 10–14 November 1996. [Google Scholar]
- Hattestrand, M.; Andren, H.O. Influence of strain on precipitation reactions during creep of an advanced 9% chromium steel. Acta Mater. 2001, 49, 2123–2128. [Google Scholar] [CrossRef]
- Vaynman, S.; Isheim, D.; Kolli, R.P.; Bhat, S.P.; Seidman, D.N.; Fine, M.E. High-strength low-carbon ferritic steel containing Cu-Fe-Ni-Al-Mn precipitates. Metall. Mater. Trans. A 2008, 39, 363–373. [Google Scholar] [CrossRef]
- Mulholland, M.D.; Seidman, D.N. Nanoscale co-precipitation and mechanical properties of a high-strength low-carbon steel. Acta Mater. 2011, 59, 1881–1897. [Google Scholar] [CrossRef]
- Xu, W.; Rivera-Diaz-del-Castillo, P.E.J.; Yan, W.; Yang, K.; Martin, D.S.; Kestens, L.A.I.; Van Der Zwaag, S. A new ultrahigh-strength stainless steel strengthened by various coexisting nanoprecipitates. Acta Mater. 2010, 58, 4067–4075. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Liu, C.T.; Wang, X.L.; Ma, D.; Chen, G.; Williams, J.R.; Chin, B.A. Effects of proton irradiation on nanocluster precipitation in ferritic steel containing fcc alloying additions. Acta Mater. 2012, 60, 3034–3046. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Zhang, Z.W.; Miller, M.K.; Ma, W.B.; Liu, C.T. Synergistic effects of Cu and Ni on nanoscale precipitation and mechanical properties of high-strength steels. Acta Mater. 2013, 61, 5996–6005. [Google Scholar] [CrossRef]
- Yu, X.H.; Caron, J.L.; Babu, S.S.; Lippold, J.C.; Isheim, D.; Seidman, D.N. Characterization of microstructural strengthening in the heat-affected zone of a blast-resistant naval steel. Acta Mater. 2010, 58, 5596–5609. [Google Scholar] [CrossRef]
- Liu, H.W.; Xu, D.K.; Wu, Y.N.; Yang, K.; Liu, H.F. Research progress in corrosion of steels induced by sulfate reducing bacteria. Corros. Sci. Prot. Technol 2015, 27, 409–418. [Google Scholar]
- Videla, H.A. Manual of Biocorrosion, 1st ed.; CRC-Press: Boca Raton, FL, USA, 1996; pp. 24–26.3. [Google Scholar]
- Shi, X.B.; Xu, D.K.; Yan, M.C.; Yan, W.; Shan, Y.Y.; Yang, K. Study on Microbiologically influenced corrosion behavior of novel Cu-bearing pipeline steels. Acta Metall. Sin. 2017, 53, 153–162. [Google Scholar]
- Xia, J.; Xu, D.K.; Nan, L. Study on mechanisms of microbiologically influenced corrision of metal from the perspective of bio-electrochemistry and bio-energetics. Chin. J. Mater. Res. 2016, 30, 161–170. [Google Scholar]
- Kang, Y.L.; Chen, Q.J.; Wang, K.L.; Sun, H.; Yu, H. Heattreatment techniques research of 700 MPa low carbon bainite steel. Tran. Mater. Heat Treat. 2005, 26, 96–99. [Google Scholar]
- Goodman, S.R.; Brenner, S.S.; Low, J.R. An FIM-atom probe study of the precipitation of copper from lron-1.4 at. pct copper. Part II: Atom probe analyses. Metall. Trans. 1973, 4, 2371–2378. [Google Scholar] [CrossRef]
- Isheim, D.; Kolli, R.P.; Fine, M.E.; Seidman, D.N. An atom-probe tomographic study of the temporal evolution of the nanostructure of Fe-Cu based high-strength low-carbon steels. Scr. Mater. 2006, 55, 35–40. [Google Scholar] [CrossRef]
- Kolli, R.P.; Seidman, D.N. Comparison of compositional and morphological atom-probe tomography analyses for a multicomponent Fe-Cu steel. Microsc. Microanal. 2007, 13, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Kolli, R.P.; Mao, Z.; Seidman, D.N.; Keane, D.T. Identification of a Ni0.5(Al0.5−xMnx) B2 phase at the heterophase interfaces of Cu-rich precipitates in an α-Fe matrix. Appl. Phys. Lett. 2007, 91, 1903–1908. [Google Scholar] [CrossRef]
- Kolli, R.P.; Seidman, D.N. The temporal evolution of the decomposition of a concentrated multicomponent Fe-Cu-based steel. Acta Mater. 2008, 56, 2073–2088. [Google Scholar] [CrossRef]
- Mulholland, M.D.; Seidman, D.N. Multiple dispersed phases in a high-strength low-carbon steel: An atom-probe tomographic and synchrotron X-ray diffraction study. Scr. Mater. 2009, 60, 992–995. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Liu, C.T.; Miller, M.K.; Wang, X.; Wen, Y.R.; Fujita, T.; Hirata, A.; Chen, M.W.; Chen, G. A nanoscale co-precipitation approach for property enhancement of Fe-base alloys. Sci. Rep. 2013, 3, 1327–1334. [Google Scholar] [CrossRef]
- Kolli, R.P.; Seidman, D.N. Co-Precipitated and Collocated Carbides and Cu-Rich Precipitates in a Fe–Cu Steel Characterized by Atom-Probe Tomography. Microsc. Microanal. 2014, 20, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Studies on Aging Behavior of Cu-Enriched Nano Cluster Strengthened HSLA Steels. Master’s Thesis, Harbin Engineering University, Harbin, China, 2015. [Google Scholar]
- Wang, S.; Yang, C.G.; Xu, D. Effect of heat treatment on antibacterial performance of 3Cr13MoCu martensitic stainless steel. Acta Metall. 2014, 50, 1453–1460. [Google Scholar]
- Khalid, H.; Shamaila, S.; Zafar, N.; Sharif, R.; Nazir, J.; Rafique, M.; Ghani, S.; Saba, H. Antibacterial behavior of laser-ablated copper nanoparticles. Acta Metall. 2016, 29, 748–754. [Google Scholar] [CrossRef]
- Yin, H.X. Copper Precipitation Mechanism and Performance Control of Ferritic Antibacterial Stainless Steel. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Jeffrey, C.P. Alcamo’s Fundamentals of Microbiology, 9th ed.; Jones and Bartlett Publishers: Burlington, MA, USA, 2011; pp. 57–85. [Google Scholar]
- Pinto, T.D.J.A.; Okamoto, R.T.; Traple, M.A.L.; Lourenco, F.R. Development and validation of microbiological assay for ceftriaxone and its application in photo-stability study. Current Pharmaceutical Analysis. Curr. Pharm. Anal. 2013, 9, 573–580. [Google Scholar]
- Malathi, S.; Ramya, V.; Ezhilarasu, T.; Abiraman, T.; Balasubramanian, S.J. Green Synthesis of Novel Jasmine Bud-Shaped Copper Nanoparticles. Int. J. Nanotechnol. 2014, 1, 34–40. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).