Abstract
A study has been carried out on Ta and Nb recovery by a liquid-liquid extraction process using 4-methylacetophenone (4-MAcPh) as the organic phase. The 4-MAcPh was compared to methyl isobutyl ketone (MIBK) with respect to extraction efficiencies (D values) at different concentrations of H2SO4 in the aqueous phase. The results showed a similar extraction of Nb for both solvents. However, for Ta, extraction efficiency is increased by a factor of 1.3 for 4-MAcPh. In addition, the MIBK solubilized completely after 6 mol∙L−1 of H2SO4 against only a loss of 0.14–4% for 4-MAcPh between 6 and 9 mol∙L−1 of H2SO4. The potential of 4-MAcPh has also been studied to selectively recover Ta from a model capacitor waste solution. The results showed a selectivity for Ta in the presence of impurities such as Ag, Fe, Ni and Mn. The 4-MAcPh also presents the advantage of having physicochemical properties adapted to its use in liquid-liquid extraction technologies such as mixer-settlers.
1. Introduction
Tantalum is a rare refractory metal with important and wide application in steel, electronic and other high-tech industries. The use of this metal being is in full swing in superalloys for aerospace, automotive or stationary turbines, as well as in components of carbide for cutting tools. It is mainly used in miniaturized capacitors (60% of current consumption) for mobile electronics (phone, laptops, tablets, etc.) [1,2].
These applications make Ta economically and strategically important to industrialized countries. From 2007–2015, the European consumption of tantalum (metal and oxide) quadrupled, from less than 100 t–380 t. Thus, the annual consumption of refined tantalum is around 2000 tons per year worldwide, with the supply exclusively resulting from mining, concentrated on three continents, Australia (40%) Brazil (17%) and the Great Lakes region in Africa (23%). On the other hand, with very similar chemical properties, tantalum (Ta) and niobium (Nb) occur naturally in the same ores, especially tantalite and columbite (Table 1).

Table 1.
Main economic minerals for Ta, data from [1].
Whereas less popular than tantalum, niobium is an essential metal for some industries. Thereby, it is involved in the manufacture of microalloyed steels for pipelines, stainless steels for the automotive industry, as well as in the production of alloys and superalloys for the aerospace industry, the nuclear industry, petrochemicals and some medical devices such as nuclear magnetic resonance equipment [3].
Since 1957, liquid-liquid extraction has replaced the Marignac process, a crystallization process less efficient, discontinuous and time-consuming for the production of Ta and Nb [4]. All commercial liquid-liquid extraction processes are carried out in the presence of fluorine and often with a second mineral acid such as sulfuric or hydrochloric acid.
Ta and Nb can reach several oxidation states such as +5, +4, +3, +2, +1, but only and are the most stable states in aqueous solutions [5]. reduction does not occur even in the presence of powerful reducers like Al, Zn, Cd. More reactive, is reducible to in acidic medium since it forms anionic complexes such as or in hydrochloric (HCl) and sulfuric () solution, respectively. However, the reduced is unstable and can rapidly be oxidized to in the presence of oxygen in the atmosphere [6].
In hydrofluoric medium, and form very stable monomeric complexes, each of them forming two types of complexes such as , and , [7]. The equilibrium between complexes is governed by the concentration of HF and elements (Ta or Nb), and it can be described as follows:
For the Ta: .
And for the Nb: .
The predominance domains of these complexes in the /—HF— system was described elsewhere [7], the formation of mixed fluoro-bisulfate, fluoro-chloride or fluoro-nitrate complexes is also observed with the presence of second mineral acid, , , [8].
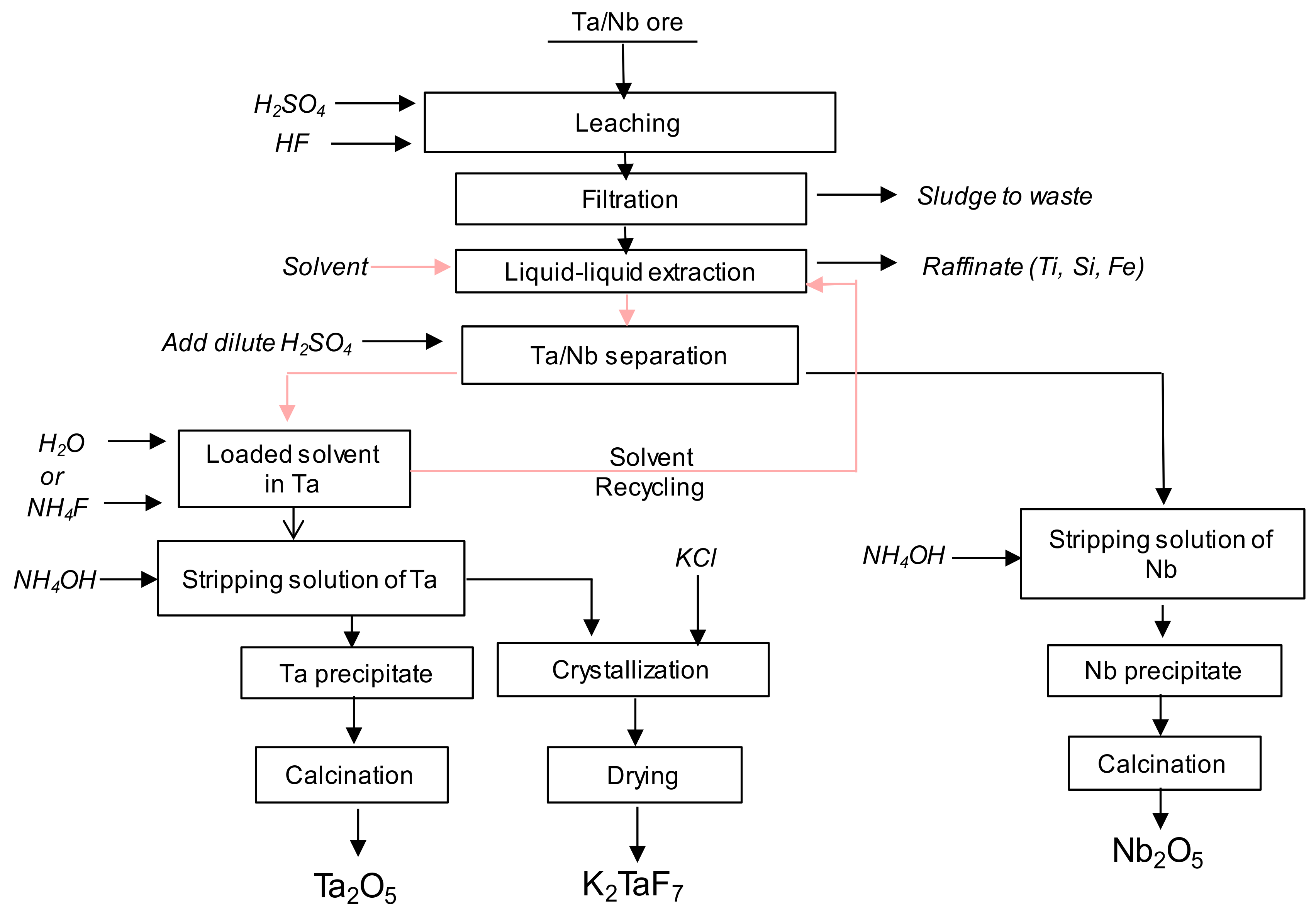
Figure 1 presents a process scheme for Ta and Nb production. The ore containing at least 25% of Ta and Nb is dissolved in the binary HF + H2SO4 [9]. After dissolution and residue removal by filtration, Ta () and Nb () are selectively extracted by contacting with a solvent. Here, two different routes are typically used [9]. One consists of a first Ta and Nb co-extraction from an aqueous phase at acidity greater than 9 mol∙L−1 followed by a selective separation of both elements at low acidity. The other method is to selectively extract firstly Ta and then Nb elements from aqueous solution by changing the acidity. In both cases (grouped or sequential extraction), Ta and Nb are back extracted using stripping solution such as ammonium fluoride or water.
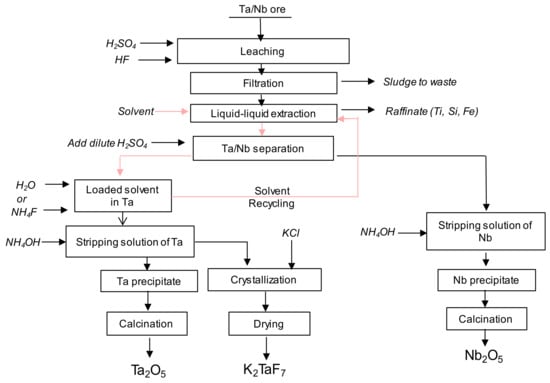
Figure 1.
Process scheme for Ta and Nb production (data from [4]).
In this process, several solvents were studied for the extraction of Ta and Nb, namely bis-(2-ethylhexyl) phosphoric acid (DEHP) [10], trioctyl-phosphine oxide (TOPO) [11], dibenzo-18-crown-6 (B18C6) [12], tri-n-butyl phosphate (TBP) [13], ketones such as diisopropyl ketone, diisopropyl butyl ketone, n-butyl ethyl ketone [14,15], methyl isobutyl ketone (MIBK) [16] and cyclohexanone [13], amides such as dimethyl heptyl acetamide [17] and bis-2-ethylhexylacetamide [18], secondary and tertiary amines [19,20] such as tri-n-octylamine [21], Alamine™ 336 and tri-n-decylamine, quaternary ammonium salts [10], such as tri-n-octylmethylammonium, tri-n-octylpropylammonium and ethyl benzyl dimethylammonium chlorides, tetrahexylammonium iodide, tri-n-octylmethylammonium hydroxide, bis-tri-sulfate n-octylmethylammonium, bistetrahexylammonium sulfate and tri-n-octylmethylammonium nitrate, as well as long chain alcohols, such as 1-octanol, 2-octanol [9,15] and isoamyl alcohol [5,17].
Among these solvents, only MIBK, TBP, cyclohexanone and 2-octanol are used industrially. However, despite their implementation in well-tried and effective processes for the production of Ta and Nb, some issues inherent in their use are observed.
Actually, MIBK, which is the most widely used in the world, has a relatively high solubility in water (of the order of 2 wt% weight percent) [5], a high volatility and a low flash point (14 °C). Therefore, MIBK displays safety problems since the mixers-settlers and the pumps that are used to recover Ta and Nb on an industrial scale must be able to operate in an explosive atmosphere.
TBP, which is more used in Russia and India, has a relatively low solubility in water (0.6 wt%) and a high flash point (193 °C) [5]. However, it is unstable in highly concentrated acidic solutions, especially in the presence of hydrofluoric acid and its hydrolysis products, such as phosphorus (V) acids, which has the consequence of decreasing the purity of Ta and Nb obtained [22].
Drawbacks are also related to other solvents; indeed cyclohexanone has a very high solubility in water (16 wt%), which justifies its weak use. Finally, 2-octanol has a very low solubility in water (<0.1 wt%) and a relatively high flash point (71 °C). This makes its use less hazardous than that of the MIBK. Nevertheless, it presents the double drawback of having a relatively high viscosity (8 MPa∙s) and a low extraction performance for Ta and Nb [5].
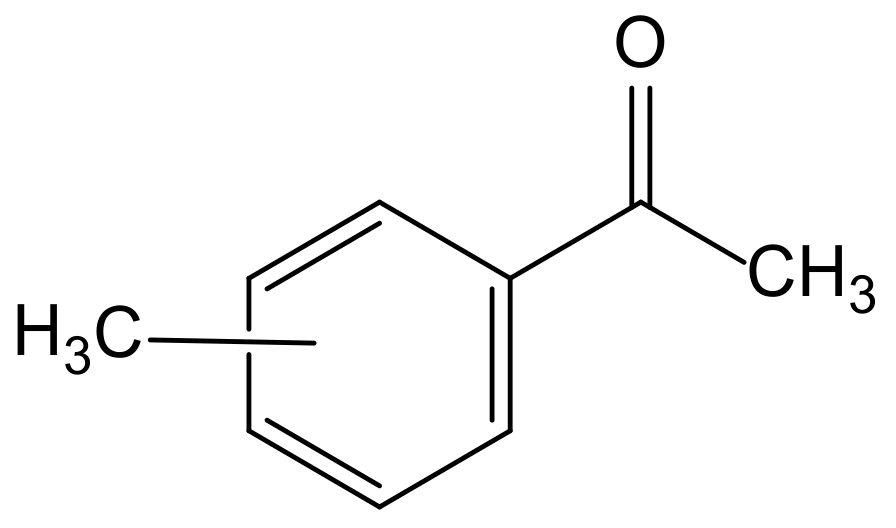
Considering the limitations associated with the physicochemical properties of the previous solvents, we explore the potential of methylacetophenone (MAcPh, Figure 2) as a solvent for the competitive extraction and purification of Ta and/or Nb. The para-substituted derivative 4-methyl-acetophenone (4-MAcPh) was selected, which highlights interesting physicochemical properties such as a very low solubility in water (<0.2 wt%), a relatively high flash point (85.1 ± 8.8 °C) and a moderate viscosity (1.88 MPa∙s).
Figure 2.
General structure of methylacetophenone molecules.
In a similar approach, Kasikova et al. [23,24] proposed a solvent mixture of acetophenone (30–50%) and triisooctylamine (20–40%) diluted in an inert diluent for Nb isolation from concentrated hydrochloric acid solutions (9 and 10 mol∙L−1) containing Ta and Ti as impurities.
In the same goal, and also in concentrated hydrochloric medium between 8 and 10 mol∙L−1, Gibala et al. [24] described a mixture of acetophenone and benzaldehyde. However, these methods do not allow the extraction of Ta, but rather of Nb, with precisely 4.4% of Ta extracted with acetophenone compared to 92.4% of Nb from a hydrochloric acid solution initially containing 0.43 g∙L−1 of Ta and 4.1 g∙L−1 of Nb. A drawback was also mentioned related to an insufficient density difference between pure acetophenone (1.028 g∙cm−3) and hydrochloric solutions concentrated in Nb, Ta and Ti for application in current mixers-settlers-type technologies.
A first step of our study was focused on the comparison of 4-MAcPh with MIBK in term of the selective extraction of Ta and Nb in the binary HF + H2SO4 system. The second part of the procedure was dedicated to the study of the ability of 4-MAcPh to selectively extract Ta from a model solution that mimics acidic leach solutions from capacitors’ waste.
2. Materials and Methods
To evaluate the selective properties of 4-MAcPh, experiments were performed under batch conditions. For this purpose, various ratios of aqueous phase (A) over organic (O) (A/O) were studied with 4-MAcPh as the organic phase and an aqueous acidic stock solution of cations.
Ta(V) and Nb(V) stock solutions (1–10 g∙L−1) were prepared at the desired acidity (H2SO4 1–9 M) from 10,000 mg∙L−1 Inductively Coupled Plasma ICP standard from SCP Science (aqueous solution of NH4TaF6 or NH4NbF6 with 1% fluorhydric acid). For the aqueous solution with competitive cations, Fe, Mn, Ag and Ni were introduced by dissolving their corresponding sulfate salts. The initial concentrations of metals were measured by inductively-coupled plasma/optical emission spectrometry ICP-OES SPECTRO ARCOS instrument (SPECTRO AMETEK, Kleve, Germany).
The two phases were mixed and stirred for 30 min at room temperature (22 ± 3 °C), and kinetic experiments confirmed that equilibrium was reached. After separation by centrifugation (3000 rpm for 5 min), the aqueous phases were collected and analyzed. Typically, volumes of 10–25 mL were used for the implementation of experiments.
From the results obtained by ICP-OES, the distribution coefficient (D) for each species was determined at equilibrium as follows (1):
where is the metal concentration in solvent and is the metal concentration in the aqueous phase.
The separation factor (SFM1/M2) is given by Equation (2):
where and are the distribution coefficients of the metal M1 and M2 ions, respectively.
The extraction percentage (E) is defined in relation to the distribution ratio D as given by Equation (3):
From the results obtained by total organic carbon (TOC) using a TOC-VCSH analyzer (Shimadzu, Kyoto, Japan) based on a 680 °C combustion catalytic oxidation/NDIR method, the loss by the solubility of the solvent (S) was evaluated as follows:
where refers to the solvent concentration in the organic phase and TOC refers to the amount of organic carbon in the aqueous phase after phase separation, considering that atoms other than carbon are negligible.
The interfacial tension measurements were carried out by the method of the hanging∙Liquid drop using a Drop Shape AnalyzerDSA30 (KRÜSS, Hamburg, Germany), and 8 mL of the solvent were previously loaded with metal for 30 min at room temperature. A 1-mL syringe was allowed to expel through a straight capillary of 1.494 mm in diameter a drop of an aqueous phase (20 µL) in the 8 mL of loaded solvent. The Drop Shape Analysis software (DSA1 v1.91, KRÜSS, Hamburg, Germany) was used to calculate the interfacial tension (IFT) (mN∙m−1) by the following relation:
where are the densities (g∙cm−3) of aqueous and loaded solvent, is the equatorial diameter and H an empirically-evaluated form factor. The density measurements were performed with a DSA 5000 thermo-regulated digital densimeter (Anton Paar, Graz, Austria).
All rheological measurements were performed using an AMVn automated microviscosimeter (Anton Paar, Graz, Austria). The apparatus measures viscosities using the rolling ball/falling ball principle, which consists of measuring ball rolling time in a diagonally-mounted glass capillary filled with the sample.
3. Results
3.1. Hydrodynamic Properties of 4-MAcPh
The interfacial tension, solubility, density and viscosity measurements of the 4-MAcPh were carried out according to the protocol described in the Materials and Methods and are presented in Table 2.

Table 2.
Physicochemical properties of 4-methylacetophenone (4-MAcPh).
The physical and chemical properties determined in this work were in close agreement with literature data when available. The solubility results highlight a low loss in the aqueous phase (0.197 wt%), which was much less than that of the commonly-used MIBK (about 2 wt%).
The density of pure 4-MAcPh (0.99997 g∙cm−3) did not cause settler difficulties during our tests. There was a significant difference between the two phases and, indeed, the density of the sulfuric aqueous phase loaded with Ta, which was about 1.32 g∙cm−3.
Pure 4-MAcPh (7.11 mol∙L−1) gave an interfacial tension (IFT) of 21.3 ± 0.4 mN∙m−1 in equilibrium with an aqueous phase composed of 0.4 mol∙L−1 of HF, 6 mol∙L−1 of H2SO4 and 6.6 g∙L−1 of Ta. For example, the IFT with TBP 30% in the PUREX process varied between 12 and 15 mN∙m−1 for about thirty seconds of phase separation time (PST) [25].
Liquid-liquid extraction is strongly influenced by hydrodynamic parameters such as viscosity. Indeed, the kinetics, the mass transfer and the phase separation time (PST) can be affected by the viscosity. The viscosity of pure 4-MAcPh increased slightly when loaded with Ta from 1.88 MPa∙s (without Ta) to 2.96 MPa∙s (when loaded with 0.97 g∙L−1 of Ta) and 4.54 MPa∙s (when loaded with 34.78 g∙L−1 of Ta). This increase had no significant influence on the PST. These values are of the same order of magnitude as those published for TBP and the N,N-dihexyloctanamide (DHOA); precisely, the viscosity of 1.1 mol∙L−1 of DHOA varied from 2.6 MPa∙s (without U) to 3.5 MPa∙s (at 30 g∙L−1 of U), and for 1.1 mol∙L−1 of TBP, it varied from 1.6 MPa∙s (without U) to 2.5 MPa∙s (at 30 g∙L−1 of U) [25]. This variation caused a small increase in the PST between 25 and 110 s.
3.2. Grouped and Sequential Extraction of Ta and Nb: Comparison between MAcPh and MIBK
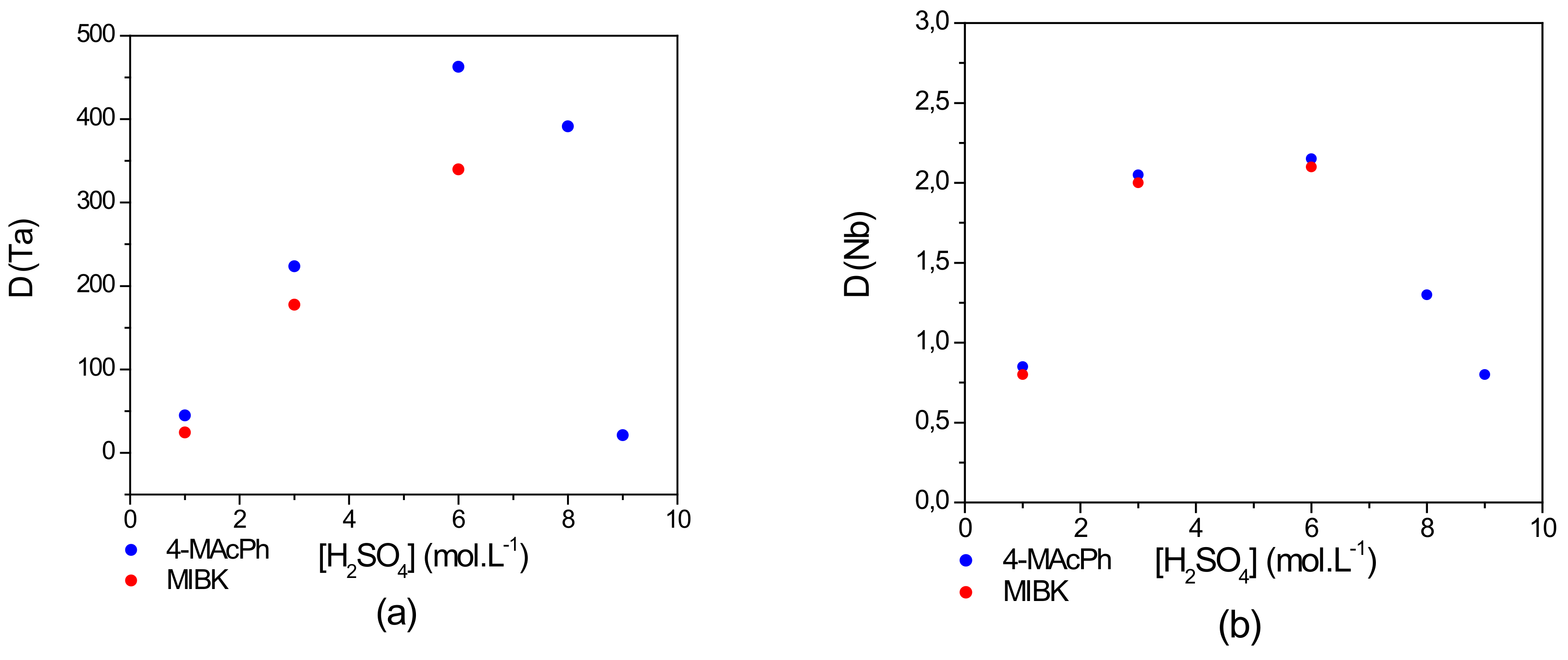
Extraction of Ta and Nb by 4-MAcPh and MIBK was evaluated on a synthetic aqueous solution comprised of Ta and Nb at 1 g∙L−1 dissolved in a mixture of 0.06 mol∙L−1 of HF and , the concentration of which varied from 1–9 mol∙L−1. The organics phases studied were pure 4-MAcPh and MIBK. Figure 3 shows the comparison between the 4-MAcPh and the MIBK with respect to the variation of the Ta and Nb D values as a function of the concentration of the (the data for D and SFTa/Nb values for Ta and Nb for both solvents are provided in Table S1; see the Supporting Information).
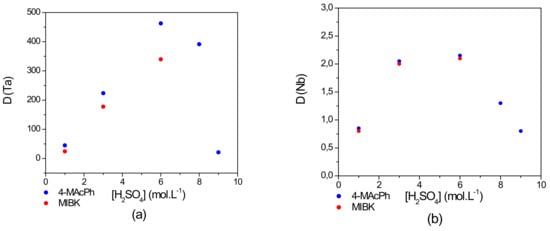
Figure 3.
Effect of concentration on the distribution coefficient D of (a) Ta and (b) Nb. Extraction conditions: O/A = 1; T = 22 °C. Extraction time: 30 min. Organic phase (O): 4-MAcPh (●) or methyl isobutyl ketone (MIBK) (●); Aqueous phase (A): [Ta] = [Nb] = 1 g∙L−1, [H2SO4] = 1–9 M, [HF] = 0.06 M.
With an increase in Ta and Nb extraction by 4-MAcPh from 1–6 mol∙L−1 of , the value of D increased from 45–463 for Ta and from 0.8–2 for Nb. Despite the increase of the extraction of Nb, the selectivity of Ta was well pronounced with a high separation factor SFTa/Nb of 56 and more than 220, respectively, for 1 and 6 mol∙L−1 of .
On the other hand, from 6 until 9 mol∙L−1 of , the D decreased to 21 for Ta corresponding to a decrease of 4% in the extraction of the metal cation (from 99.8–95.5%) and 0.8 for Nb corresponding to a decrease of 22% in the extraction (from 66.7–44.4%). This decrease could be due to the carbonyl protonation of the 4-MAcPh providing the enolization of the compound as demonstrated by Cox et al. [26], as well as significant increase in the solubility loss of 4-MAcPh. The total organic carbon (TOC) measurements in the equilibrium aqueous phase supported this hypothesis by reporting an increase in solubility loss of 4-MAcPh from 0.14–4 wt% between 6 and 9 mol∙L−1 of . This difference in their extraction performance could be used for the separation of the two metals. Niobium complexes in the solution are stronger Lewis acids than that of tantalum. Therefore, the extraction of niobium with 4-MAcPh requires stronger solution acidity than that of tantalum [9].
Thereby, these results highlight two possible routes to extract Ta and Nb. One possibility is to co-extract Ta and Nb from an aqueous phase of acidity between 3 and 6 mol∙L−1 of . Both elements could be then separated from an aqueous phase concentrated to less than 1 mol∙L−1 of since under these conditions, the Nb extraction performance is low. The second approach is based on a sequential extraction of the two elements. In this scheme, the Ta is selectively extracted in a first step at 1 mol∙L−1 of , followed by the Nb extraction between 3 mol∙L−1 and 6 mol∙L−1 of .
However, the result illustrated in Figure 3 shows a similar extraction tendency of Ta and Nb for the 4-MAcPh and the MIBK. The D values of Nb remained nearly similar from 1–6 mol∙L−1 of for these solvents. However, those of Ta were higher for the 4-MAcPh precisely by a factor of 1.3 at 6 mol∙L−1 of . In addition, MIBK was completely solubilized from 6 mol∙L−1 of , while only a loss of 0.14–4 wt% was observed with 4-MAcPh between 6 and 9 mol∙L−1 of . In terms of stability and efficiency, 4-MAcPh appeared to be more suitable for the extraction of Ta and Nb from a solution composed of 0.06 mol∙L−1 of HF and 1–9 mol∙L−1 of .
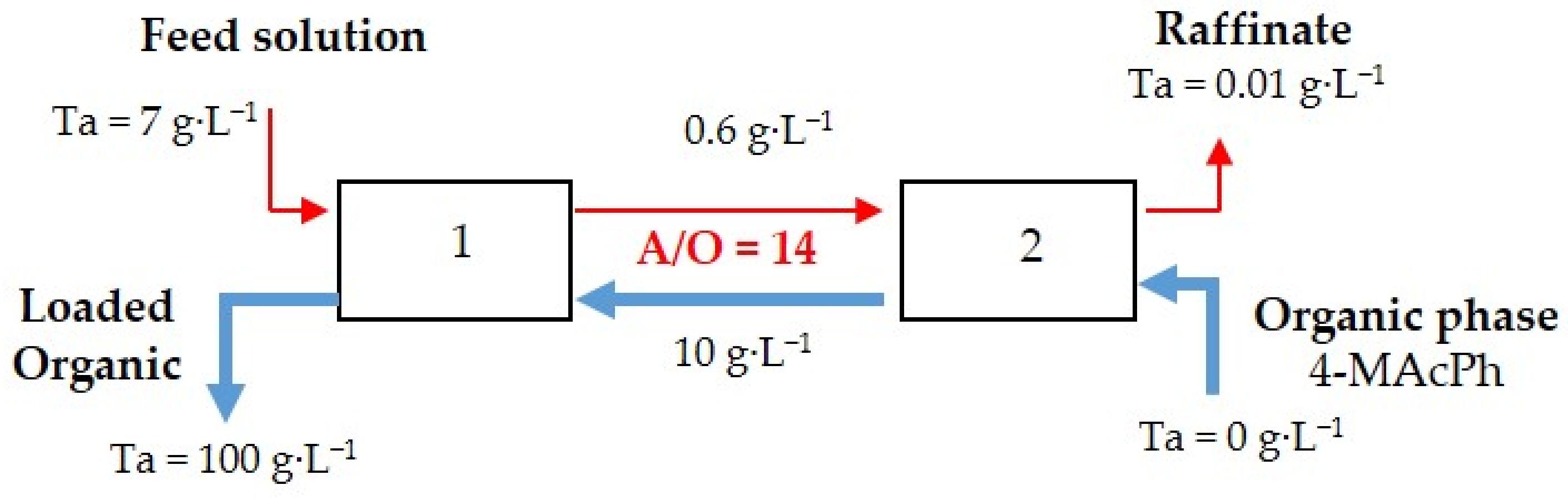
3.3. Extraction Isotherm and McCabe−Thiele Diagram Loading Capacity of 4-MAcPh for Ta
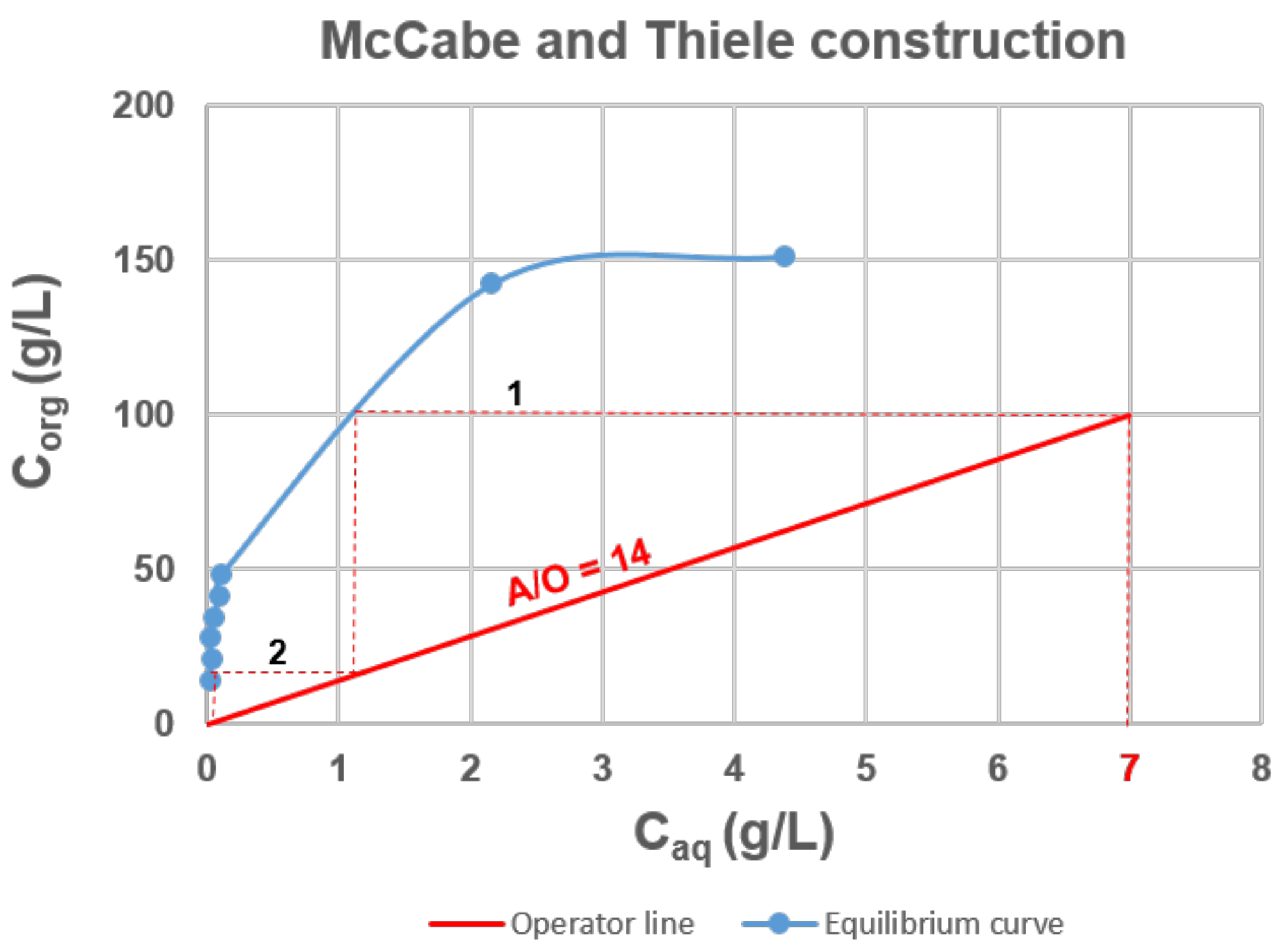
The Ta extraction distribution isotherm and the McCabe−Thiele diagram obtained with 4-MacPh are shown in Figure 4. The feed solution containing 7 g∙L−1 of Ta in acidic aqueous solution (0.4 mol∙L−1 of HF, 6 mol∙L−1 of ) was contacted with 4-MAcPh at different A/O phase ratios from 1–60 at ambient temperature. No third phase was observed, and the results presented in Figure 4 indicated a loading capacity higher than 150 g∙L−1 since the saturation seemed to be reached.
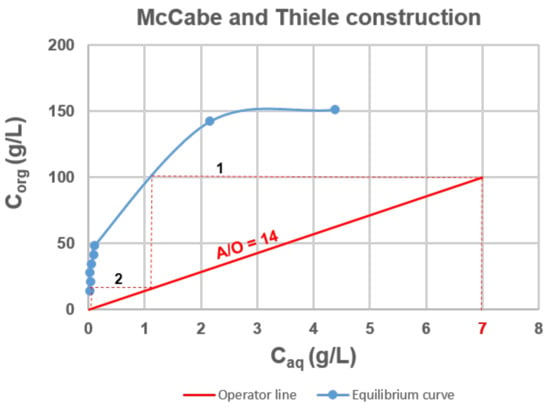
Figure 4.
McCabe and Thiele diagram for the 4-MAcPh-tantalum system. Extraction conditions: A/O = 1 to 60; T = 22 °C. Extraction time: 30 min. Organic phase (O): 4-MAcPh, Aqueous phase (A): [Ta] = 7 g∙L−1, [H2SO4] = 6 M, HF = 0.4 M.
The McCabe−Thiele diagram for Ta extraction highlight that for a flux composed of a feed containing 7 g∙L−1 of Ta and an organic phase leaving the process with 100 g∙L−1 of Ta, a flow ratio of 14 and two stages was required to have a process yield of 99.9%, i.e., a raffinate composed of 0.01 g∙L−1 of Ta.
The operator line equation is defined by the relation:
with the slope ; = 100 g∙L−1, i.e., Ta concentration in the solvent at the outlet of the process; = 7 g∙L−1, i.e., Ta concentration in the feed; = 0.01 g∙L−1, i.e., Ta concentration in the raffinate.
Table 3 shows the tantalum concentration in aqueous and organic phases and the distribution coefficient at equilibrium as a function of various phase ratios (A/O) from 1–60.

Table 3.
Tantalum concentration in aqueous and organic phases and the distribution coefficient (D) at equilibrium as a function of various phase ratios (A/O).
Figure 5 proposes a flow sheet for the recovery of Ta by 4-MAcPh from aqueous solution composed of 0.4 mol∙L−1 of HF, 6 mol∙L−1 of and 7 g∙L−1 of Ta. The phase ratio (A/O) was 14 for Extractions 1 and 2.
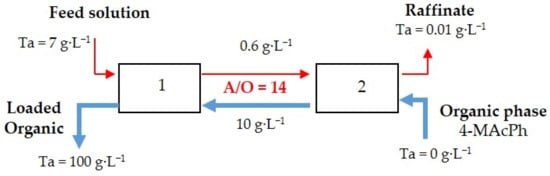
Figure 5.
Flow sheet for Ta recovery by 4-MAcPh with two stages at A/O = 14.
3.4. Stripping of Ta from Loaded 4-MAcPh
After the successful extraction of Ta from the feed aqueous solution (1 g∙L−1 of Ta, 0.06 mol∙L−1 of HF and 6 mol∙L−1 of with a phase ratio A/O = 1) by 4-MAcPh, another set of experiments was then carried out in order to recover the extracted targeted Ta from the organic phase. The 4-MAcPh already loaded with the extracted Ta was stripped then with ammonium oxalate (0.2 or 0.3 mol∙L−1) in an A/O ratio of one, allowing a quantitative recovery of Ta in the stripping phase without any precipitation phenomenon. In other cases, as with the use of water as the stripping agent, the formation of a third phase at the interface was observed.
3.5. Selective Recovery of Ta from a Model Solution of Capacitor Waste Containing Fe, Mn and Ni as Impurities
Recycling has the advantage of reducing the dependency on minerals. Actually, around 95% of electronic boards are recycled in copper smelters where Ta is dispersed in slags and cannot be recovered. Except the recycling of manufacturing chips, there is currently no technology that allows tantalum recycling.
Therefore, considering the Ta extraction performances by 4-MAcPh, we studied the efficiency and the selectivity of this system toward Ta contained in a simulated leaching solution of capacitor waste including Fe, Mn and Ni as impurities.
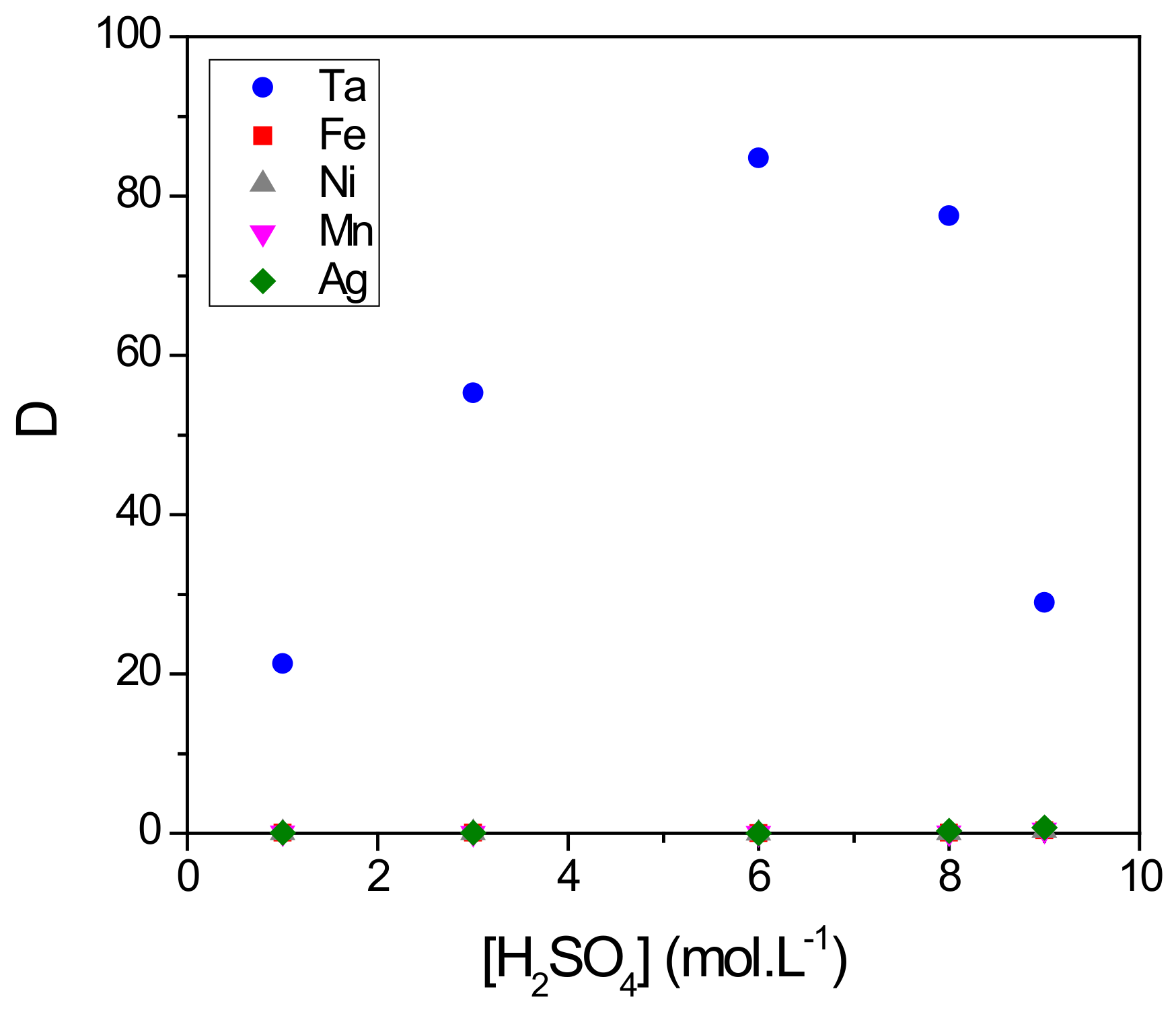
To be as representative as possible to the conditions encountered during the dissolution of capacitors of Ta [25,26], we studied the extraction performances from a synthetic aqueous solution containing 0.1 g∙L−1 of Ta, Fe, Mn and Ni. The whole was dissolved in a mixture of 0.06 mol∙L−1 of HF and , the concentration of which varied from 1–9 mol∙L−1. Figure 6 reports the D value for all elements as function of concentration (the data value for all elements as a function of concentration are provided in Table S2; see the Supporting Information).
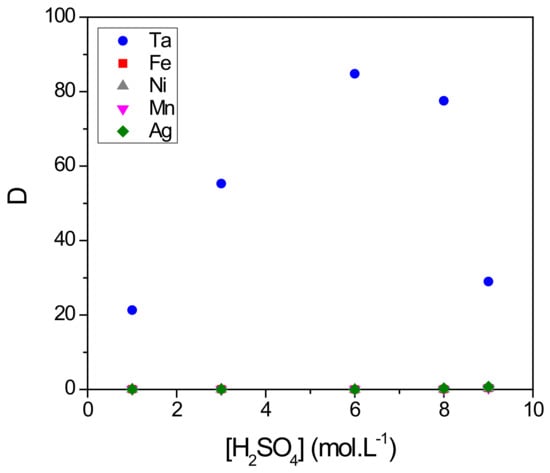
Figure 6.
Effect of concentration on the distribution coefficient D of Ta, Fe, Ni, Mn and Ag. Extraction conditions: A/O = 1; T = 22 °C. Extraction time: 30 min. Organic phase (O): 4-MAcPh; Aqueous phase (A): [Ta] = [Fe] = [Ni] = [Mn] = [Ag] = 0.1 g∙L−1, [] = 1–9 M, [HF] = 0.06 M.
As already observed, there was an increase of D values of Ta from 21–85 between 1 and 6 mol∙L−1 of . The decrease in the D values until 29 between 6 and 9 mol∙L−1 could be due to the competitive protonation of the 4-MAcPh accompanied by a loss per solubility from 0.14–4% as already mentioned. The D values for impurities (Fe, Mn, Ni, Ag) remained less than 0.7 between 1 and 9 mol∙L−1 of .
The present results demonstrate the potential of 4-MAcPh toward the selective extraction of Ta with respect to competitive ions such as Fe, Ni, Mn or Ag with a selectivity factor SFTa/M of about 120 at 6 mol∙L−1 of .
As indicated previously, the recovery of the Ta can then be considered using a stripping solution such as ammonium oxalate.
4. Discussion and Conclusions
The interest in 4-MAcPh for the extraction of Ta and Nb has been established. The following conclusions are highlighted:
- 4-MAcPh presents adapted intrinsic physicochemical properties for its use in the liquid-liquid extraction process. Indeed, its solubility highlights a low loss in the aqueous phase (0.2 wt%) which is much less than that of the commonly-used MIBK (about 2 wt%). The density of pure 4-MAcPh (0.99997 g∙cm−3) did not cause settler difficulties during our tests due to the significant difference with the sulfuric acid aqueous phase loaded with the metals. Pure 4-MAcPh gives an interfacial tension (IFT) of 21.3 ± 0.4 mN∙m−1 in equilibrium with an aqueous phase composed of 0.4 mol∙L−1 of HF. 6 mol∙L−1 of H2SO4 and 6.6 g∙L−1 of Ta. With these 21.3 mN∙m−1, we need more stirring energy than that for TBP 30% to create an emulsion. The viscosity of pure 4-MAcPh is of the same order of magnitude as those published for TBP and DHOA [27]. It increases slightly when loaded with Ta. This increase has no significant influence on the PST.
- From the results of the comparison between MIBK and MAcPh with respect to Ta and Nb extraction, it was concluded that there is a similar extraction tendency for both Ta and Nb. The D values of Nb remain nearly similar from 1–6 mol∙L−1 of but those of Ta are higher for the 4-MAcPh at 6 mol∙L−1 of . In addition, MIBK is completely solubilized from 6 mol∙L−1 of while only a loss of 0.14–4 wt% is observed with 4-MAcPh between 6 and 9 mol∙L−1 of . In terms of stability and efficiency, 4-MAcPh appears to be more suitable for the extraction of Ta and Nb from a solution composed of 0.06 mol∙L−1 of HF and 1–9 mol∙L−1 of . The decrease of the D value of Ta between 6 and 9 mol∙L−1 of in the case of MAcPh could be due to the carbonyl protonation providing the enolization of the compound as demonstrated by Cox et al. [28].
- From the results of the McCabe−Thiele diagram for Ta extraction, it can be concluded that for a flux composed of a feed containing 7 g∙L−1 of Ta and an organic phase leaving the process with 100 g∙L−1 of Ta, a flow ratio of 14 and two stages are required for a process yield of 99.9%, i.e., a raffinate composed of 0.01 g∙L−1 of Ta.
- From the results of the Ta stripping, it can be concluded that the ammonium oxalate (0.2 or 0.3 mol∙L−1) is adapted to recover Ta from loaded 4-MAcPh quantitatively.
- In regards to MIBK, the price of 4-MAcPh should be a drawback, but due to similar efficiencies without problems concerning safety issues (fire and explosion hazards and readily soluble in aqueous solutions), it appears that 4-MAcPh is a good alternative. Furthermore, the loss in the aqueous phase is low, and considering that the stripping step allows reusing the solvent, this reduces the impact of the price.
- From the results of the selective extraction of Ta from the simulated leaching solution of capacitor waste, it was concluded that the MAcPh is adapted with a separation factor of 120 for Ta with respect to Fe, Ni, Mn and Ag.
Supplementary Materials
The following are available online at http://www.mdpi.com/2075-4701/8/9/654/s1, Table S1: D and SFTa/Nb values for Ta and Nb for 4-MAcPh and MIBK. Table S2: D values for Ta, Ni, Mn, Fe and Ag as a function H2SO4 of concentration.
Author Contributions
M.T., G.A., J.D. and S.P.-R. conceived of and designed the experiments. M.T. performed the experiments. M.T., G.A., J.D. and S.P.-R. analyzed the data and wrote the paper.
Funding
This research was funded by Commissariat à l'Energie Atomique et aux énergies alternatives (CEA), Centre National de la Recherche Scientifique (CNRS) and University Montpellier.
Acknowledgments
The authors thank Véronique Dubois for her technical assistance. We gratefully acknowledge the financial support for this project by CEA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Monier, V.; Escalon, V.; Cassowitz, L.; Massari, F.; Deprouw, A. Etude du Potentiel de Recyclage de Certains Métaux Rares; ADEME: Angers, France, 2010. [Google Scholar]
- Polak, C. Métallurgie et Recyclage du Niobium et du Tantale; Techniques de l’ingénieur: Saint-Denis, France, 2012. [Google Scholar]
- Arrachart, G.; Duhamet, J.; Pellet-Rostaing, S.; Toure, M.; Turgis, R. Method for Extracting Tantalum and/or Niobium from an Acidic Aqueous Phase. WO Patent 2015193314A1, 23 December 2015. [Google Scholar]
- Singh, R.P. Processing of Ta2O5 Powders for electronic application. J. Electron. Mater. 2001, 30, 1584–1594. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, C.Y. Solvent extraction technology for the separation and purification of niobium and tantalum. A review. Hydrometallurgy 2011, 107, 1–12. [Google Scholar] [CrossRef]
- Gibalo, I.M. Analytical Chemistry of Niobium and Tantalum; Ann Arbor-Humphery Science Publishers: Ann Arbor, MI, USA, 1970; ISBN 9780250399246. [Google Scholar]
- Agulyansky, A. The Chemistry of Tantalum and Niobium Fluoride Compounds; Elsevier: New York, NY, USA, 2004; ISBN 0-444-51604-2. [Google Scholar]
- Varga, L.P.; Wakley, W.D.; Nicholson, L.S.; Madden, M.L.; Patterson, J. Solvent extraction studies of tantalum fluoride complexes with N-Benzoylphenylhydroxylamine tri-n-octylphosphine oxide and methyl isobutyl ketone using computer techniques. Anal. Chem. 1965, 37, 1003–1009. [Google Scholar] [CrossRef]
- Agulyansky, A.; Agulyansky, L.; Travkin, V.F. Liquid-liquid extraction of tantalum with 2-octanol. Chem. Eng. Process. 2004, 43, 1231–1237. [Google Scholar] [CrossRef]
- Niwa, K.; Ichikawa, I. Method of Purifying Tantalum. U.S. Patent 4673554A, 16 June 1987. [Google Scholar]
- Sanda, O.; Taiwo, E. Solvent extraction of tantalum(V) from aqueous sulphate/fluoride solution using trioctyl phosphine oxide in MIBK. Hydrometallurgy 2012, 127, 168–171. [Google Scholar] [CrossRef]
- Agrawal, Y.K. Liquid/liquid extraction separation recovery and transport of tantalum by crown-ether. Talanta 2002, 58, 875–882. [Google Scholar] [CrossRef]
- Gupta, C.K.; Suri, A.K. Niobium and Tantalum Separation Process. Extractive Metallurgy of Niobium; CRC Press Inc.: Boca Raton, FL, USA, 1994; ISBN 0-8493-6071-4. [Google Scholar]
- Werning, J.R.; Higbie, K.B.; Grace, J.T.; Speece, B.F.; Gilbert, H.L. Separation of Tantalum and Niobium by Liquid-Liquid Extraction. Ind. Eng. Chem. 1954, 46, 644–652. [Google Scholar] [CrossRef]
- Ellenburg, J.Y.; Leddicotte, G.W.; Moore, F.L. Separation of Tantalum and Niobium by Liquid-Liquid Extraction. Anal. Chem. 1953, 26, 1045–1047. [Google Scholar] [CrossRef]
- Yang, X.L.; Wang, X.H.; Wei, C.; Zheng, S.L.; Sun, Q.; Wang, D. Extraction kinetics of tantalum by MIBK from pulp using∙Lewis cell. Hydrometallurgy 2013, 131, 34–39. [Google Scholar] [CrossRef]
- Taili, Z.; Xiang, Z.; Rongjun, M.; Zhuoshu, H.; Ming, Q.; Zhonghua, Z. The amide type extractant A101 and its application to the separation of niobium and tantalum. And molybdenum and rhenium. Hydrometallurgy 1982, 8, 379–388. [Google Scholar] [CrossRef]
- Ohmori, H.; Shibata, J.; Nishimura, S.; Sano, M. Extraction of niobium and tantalum with bis-2-ethylhexyl acetamide. Solvent Extr. Ion Exch. 1987, 5, 227–243. [Google Scholar] [CrossRef]
- Bhattacharyya, S.N.; Ganguly, B. Solvent extraction separation of niobium and tantalum. Solvent Extr. Ion Exch. 1984, 2, 699–740. [Google Scholar] [CrossRef]
- Djordjevic, C.; Gorican, H.; Tan, S.L. Solvent extraction of niobium and tantalum: 3. Extraction mechanism in oxalic solutions with long chain tertiary amines. J. Less-Common Met. 1966, 11, 342–350. [Google Scholar] [CrossRef]
- Bludssus, W.; Reichert, K.; Bohmke, U. Process for Removing Antimony from Hydrofluoric Acid Solutions which Contain Ta/Nb. U.S. Patent 5908489A, 1 June 1999. [Google Scholar]
- Babkin, A.G.; Majorov, V.G.; Nikolaev, A.I.; Zolotov, Y.A. Solvent Extraction of Niobium, Tantalum and other Elements from Fluoride Solutions; AN SSSR, Apatity Nauka: Leningrad, Russia, 1988. [Google Scholar]
- Kassikova, N.I.; Kassikova, A.G.; Balabanov, Y.I.; Petrov, V.B.; Kalinnikov, V.T. Niobium, tantalum and titatnium extraction from natural and technogenic raw materials of the kola peninsula by liquid-liquid extraction methods. In Proceedings of the 3rd Balkan Metallurgical Conference (BMC), Ohrid, Republic of Macedonia, 24–27 September 2003; pp. 64–68. [Google Scholar]
- Gibala, I.M.; Albadri, J.S. Niobium and tantalum extraction from hydrochloric acid solution using benzaldehyde and acetophenone. Vestn. Mosk. Univ. Ser. II. Khim. 1969, 2, 98–101. [Google Scholar]
- Freeman, Y. Tantalum and Niobium-Based Capacitors; Springer: Manhattan, NY, USA, 2018; ISBN 978-3-319-67869-6. [Google Scholar]
- FIRADEC. Available online: http://banelec.online.fr/fab/firadec/cg2005.pdf (accessed on 10 July 2018).
- Pathak, P.N.; Kanekar, A.S.; Prabhu, D.R.; Manchanda, V.K. Comparison of Hydrometallurgical Parameters of N,N-Dialkylamides and of Tri-n-Butylphosphate. Solvent Extr. Ion Exch. 2009, 27, 683–694. [Google Scholar] [CrossRef]
- Cox, R.A.; Smith, C.R.; Yates, K. Excess acidity method—Basicities, and rates and mechanisms of enolization, of some acetophenones and acetone, in moderately concentrated sulfuric-acid. Can. J. Chem. 1979, 59, 2952–2958. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).