Selective Separation of Antimony and Preparation of Sodium Antimonate by Sodium Salt Leaching-Synergistic Oxidation from High Arsenic Antimony Residue
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
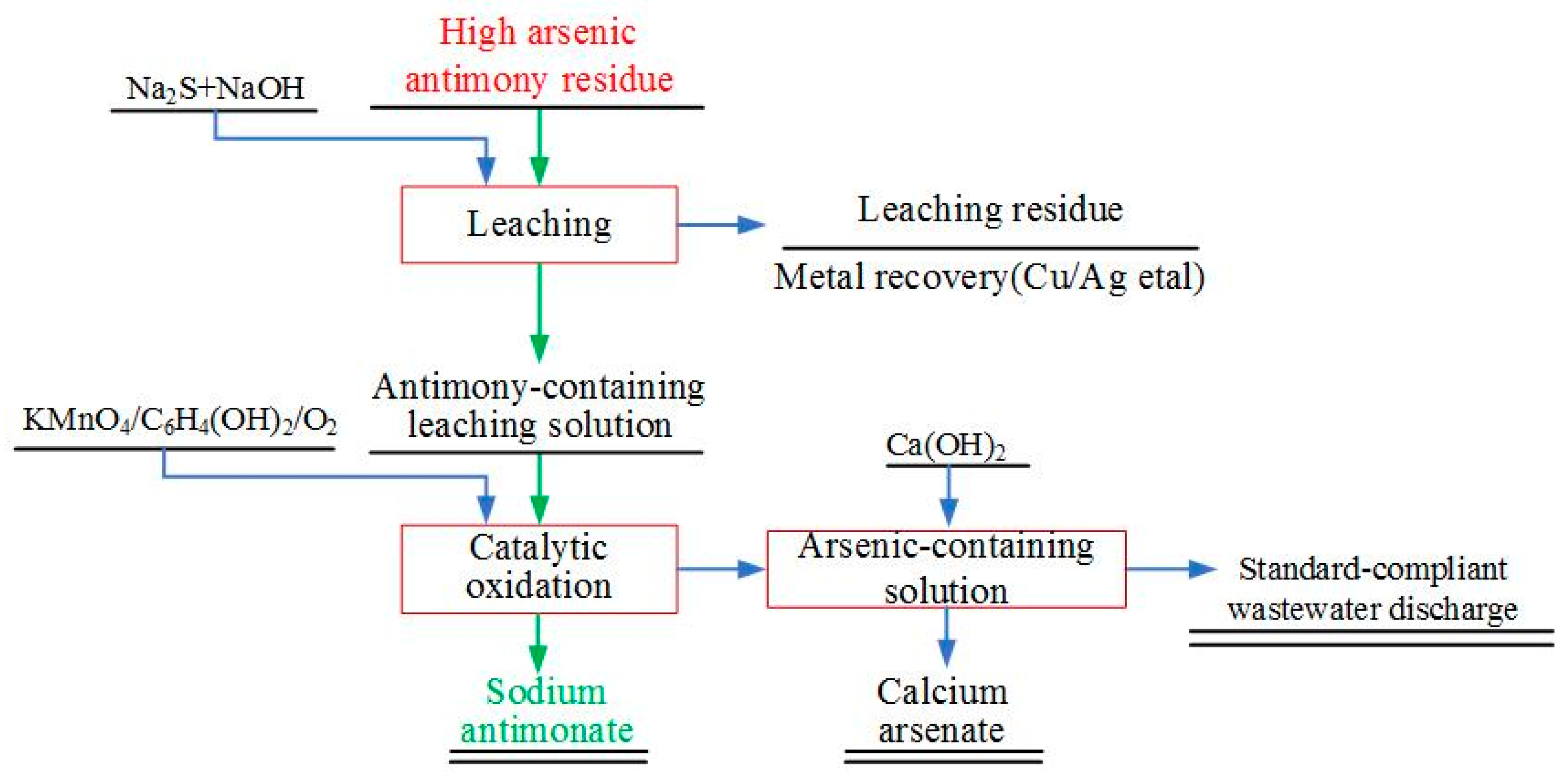
2.2. Experimental Principle
2.3. Experimental Method
2.3.1. First Compound Leaching
2.3.2. Synergistic Oxidation
2.3.3. Kinetics Analysis
2.4. Analytical Methods
2.4.1. Solid Material Analysis
2.4.2. Solution Analysis
3. Results and Discussion
3.1. Characterisation of High Arsenic Antimony Residue
3.2. Selective Separation of Antimony with Compound Leaching
3.3. Preparation of Sodium Antimonate by Catalytic Oxidation
3.3.1. Effect of Type of Oxidation
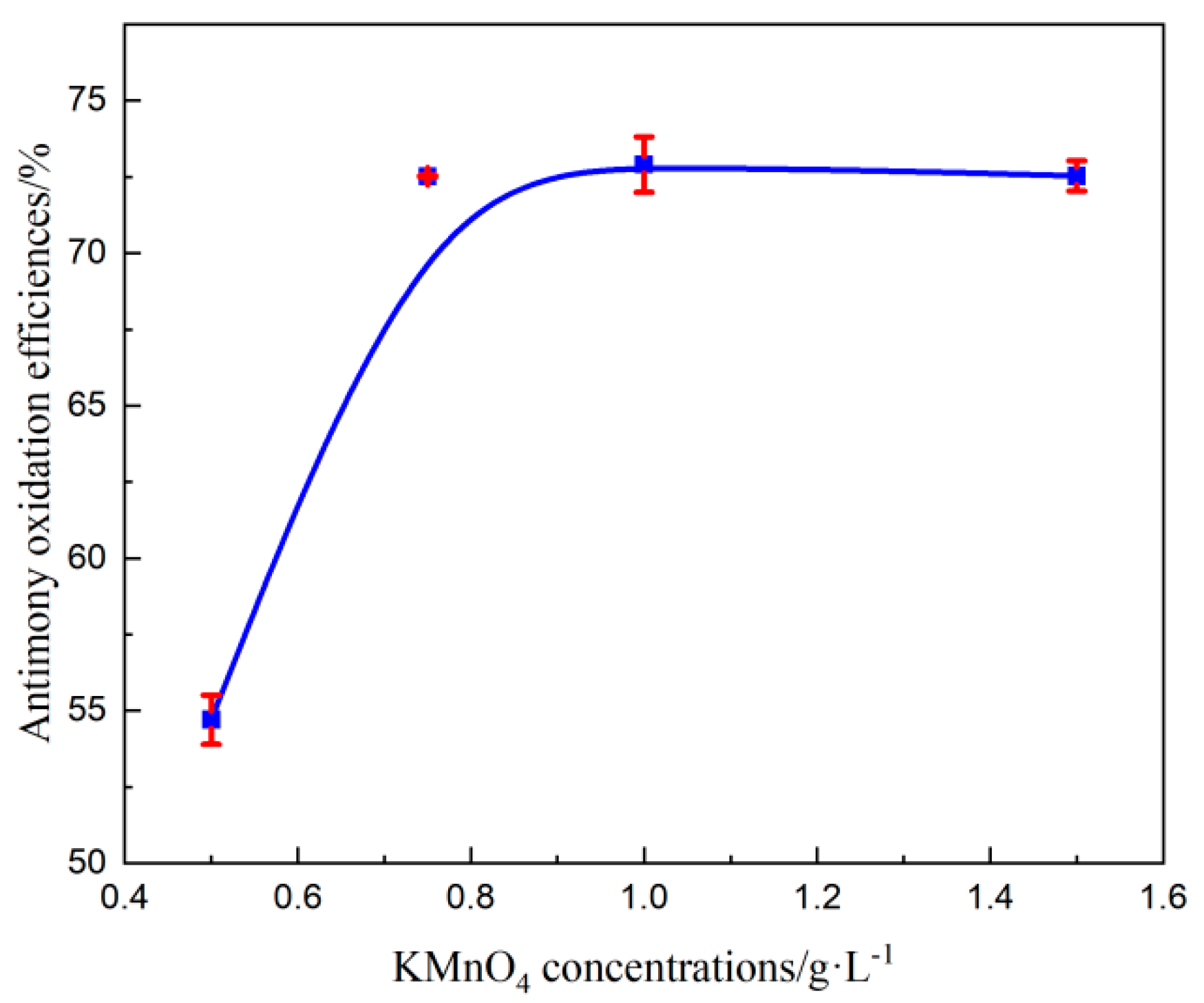
3.3.2. Effect of Catalysts Concentration
Effect of KMnO4 Concentration
Effect of Catechol Concentration
3.3.3. Effect of Air Flow Rate
3.3.4. Effect of Reaction Temperature
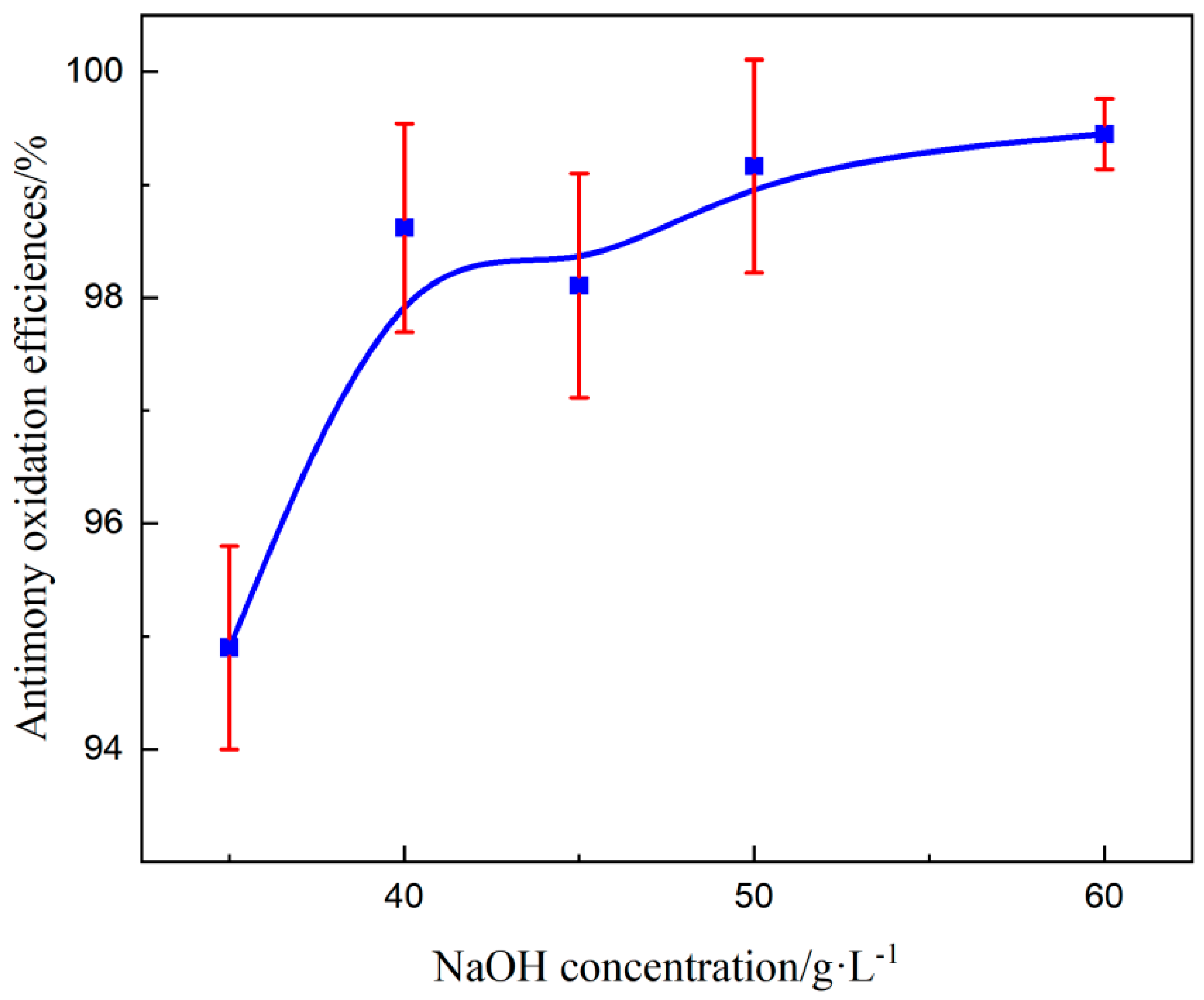
3.3.5. Effect of NaOH Concentration
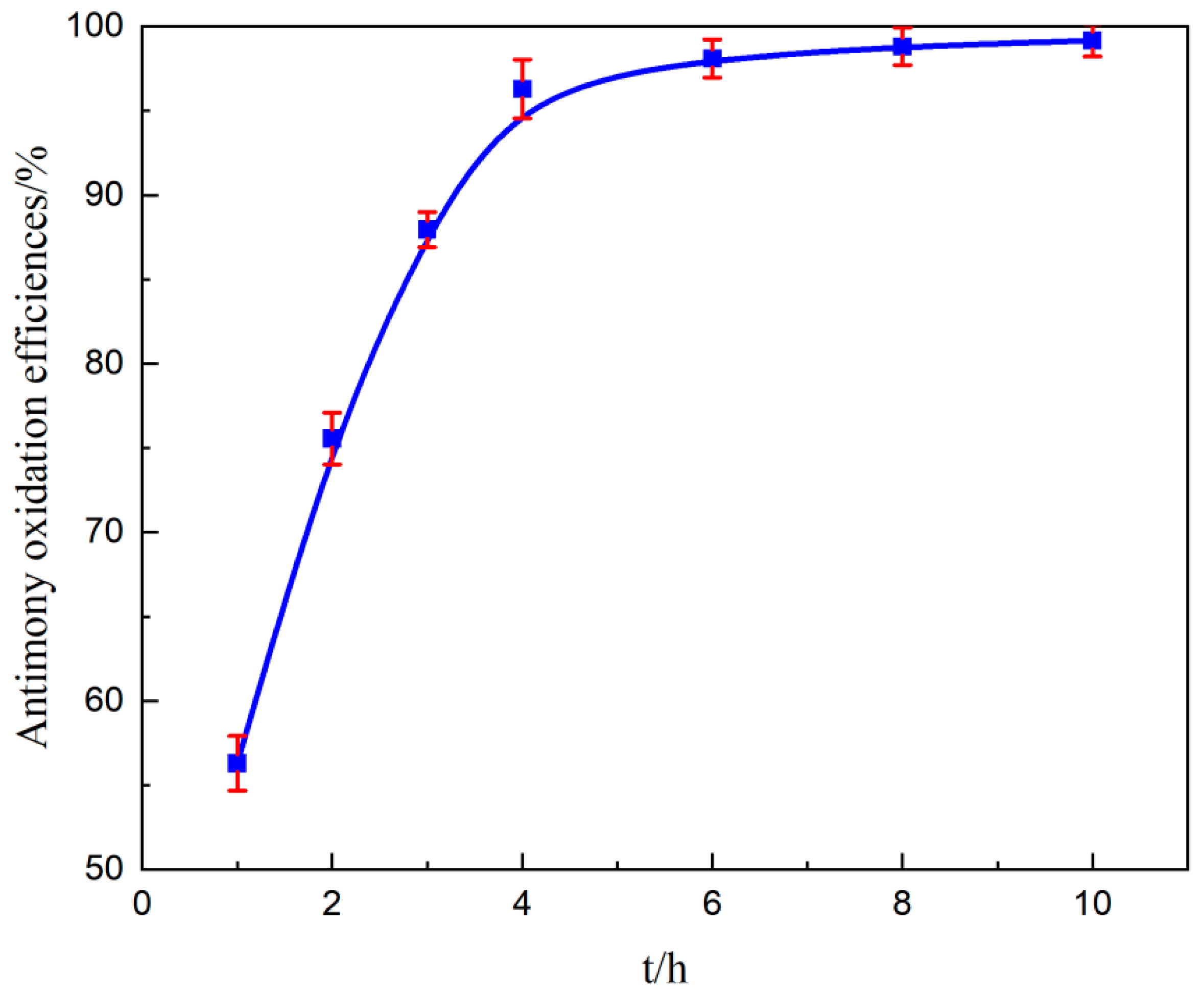
3.3.6. Effect of Reaction Time
3.4. Crude Sodium Antimonate Purification
3.5. Kinetic Analysis
- (1)
- The gaseous reactant, oxygen, diffuses from the gas phase to the gas–liquid phase interface.
- (2)
- Oxygen diffuses into the liquid phase from the gas–liquid phase interface and reacts in the liquid phase.
- (3)
- The reactants diffuse from the liquid phase to the gas–liquid reaction interface; and react with dissolved oxygen during the diffusion process.
- (4)
- The resulting product diffuses into the bulk of the liquid phase.
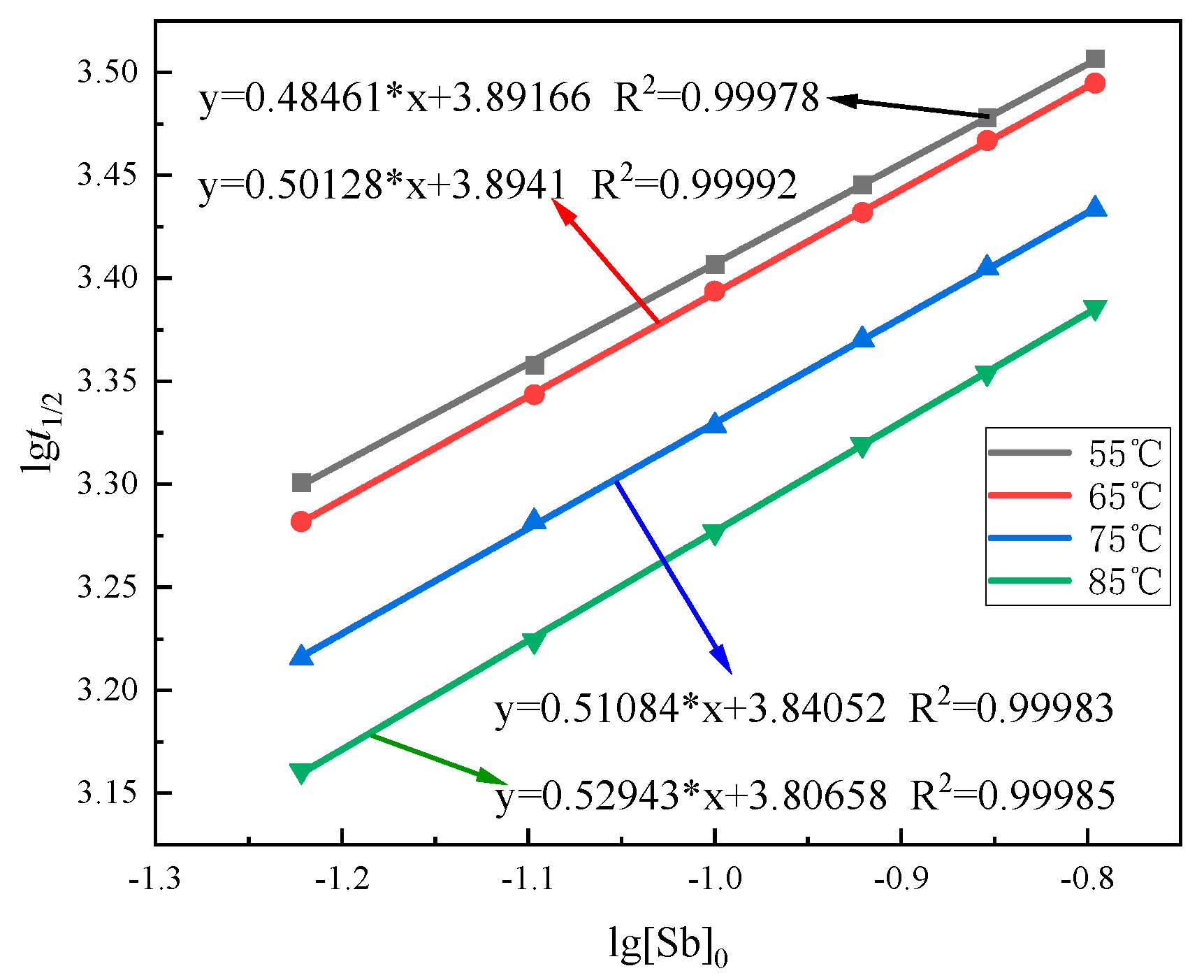
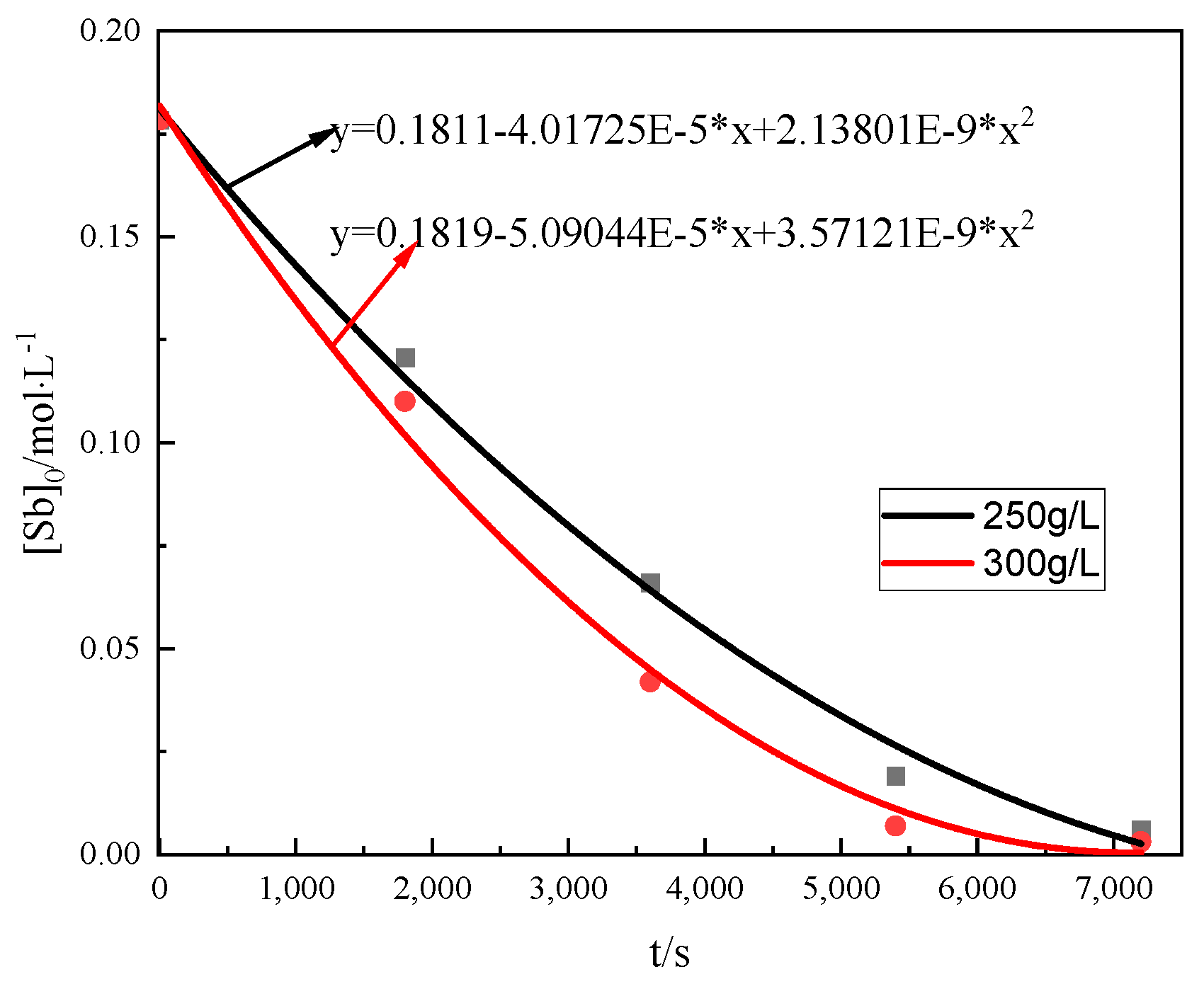
3.5.1. Half-Life Method
3.5.2. Isolation Method
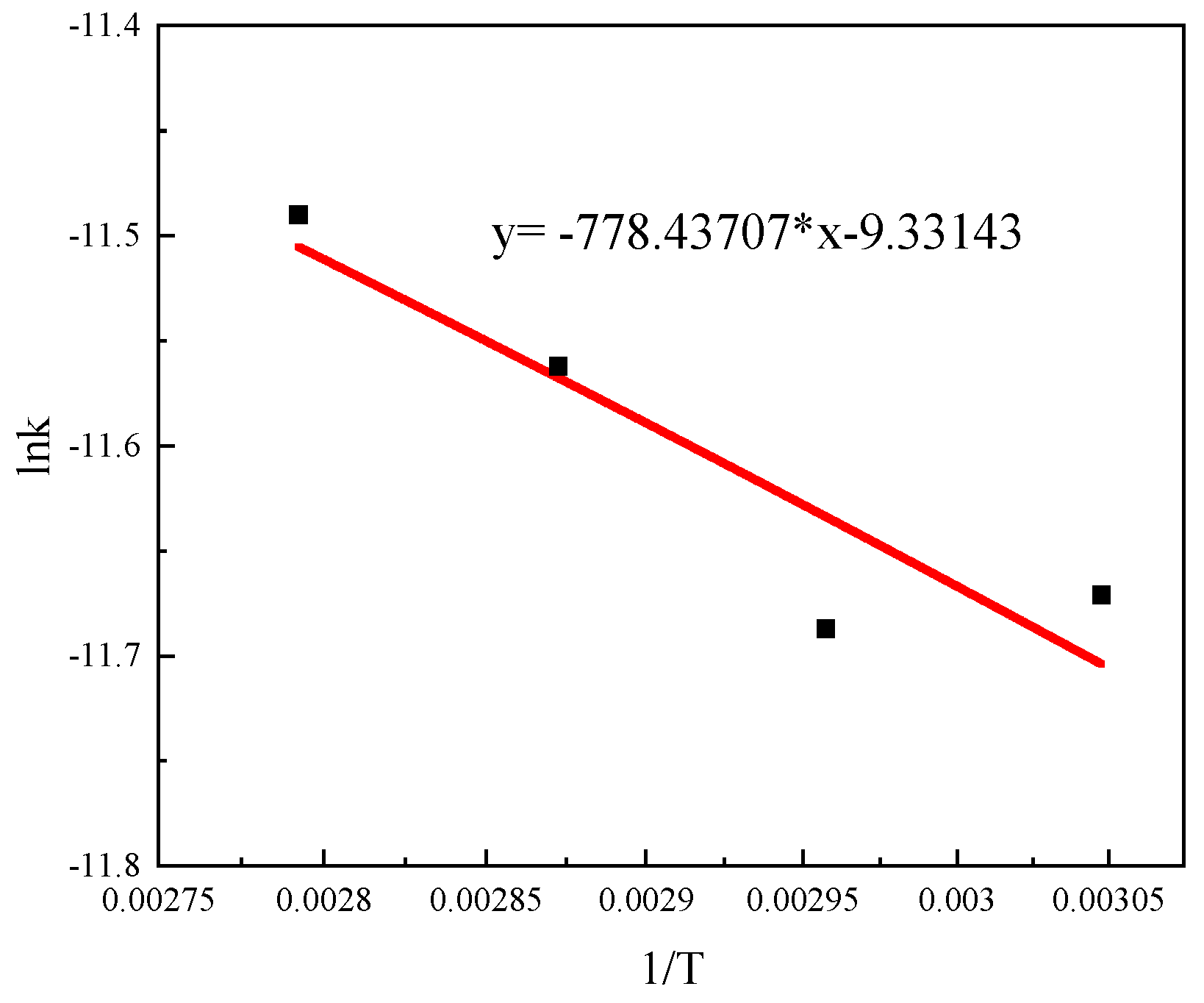
3.5.3. Reaction Activation Energy
4. Conclusions
- (1)
- In the first compound leaching stage, a leaching efficiency of 94% was achieved for antimony, while elements such as copper, lead, silver, and bismuth had leaching efficiencies of <3%. The results indicated that the enrichment of copper, lead, silver, and bismuth was approximately 290%, 290%, 280%, and 270%, respectively. The compound leaching slag can be returned to the copper anode slime smelting system for further recovery.
- (2)
- The oxidation rate of antimony reached more than 98% at the NaOH concentration of 50 g·L−1, KMnO4 concentration of 0.75 g·L−1, catechol concentration of 0.75 g·L−1 and under the air flow rates of 1.415 m3·min−1 at 75 °C for 8 h, and crude sodium antimonate products were formed.
- (3)
- After the crude sodium antimonate product was redissolved and adjusted to pH 12–14, refined sodium antimonate with uniform particle size distribution, good dispersibility, which met the second-class standard of the non-ferrous metal industry. The recovery rate of antimony was found to be >95.60%, and the liquid after neutralisation contained [As] < 0.10 g·L−1, [Sb] = 0.16-0.38 g·L−1, which can be reused in the composite leaching process.
- (4)
- The apparent activation energy (Ea) of the oxidation reaction was 6.47 kJ·mol−1, and the reaction process was diffusion controlled. The reaction rate equation was , and the reaction kinetics equation was .
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, Z.L.; Tang, X.C.; Xiao, Z.Y.; Liu, H.N. A novel approach to synthesizing sodium antimonate and recovering lead and zinc from arsenic—Bearing antimony white. Miner. Eng. 2023, 192, 108008. [Google Scholar] [CrossRef]
- Yang, T.; Tang, J.; Bing, W.; Liu, C. Review of the preparation of sodium pyroantimonate. Inorg. Chem. Ind. 1999, 31, 22–24. [Google Scholar]
- Yi, Y.; Shi, J.; Tian, Q.H.; Guo, X.Y. Novel technology for preparation of sodium pyroantimonate from alkali leaching residue of high arsenic dust. Chin. J. Nonferrous Met. 2015, 25, 241–249. [Google Scholar] [CrossRef]
- Liang, Y.; Zhong, N. Process for producing sodium antimonate by chlorination. Inorg. Chem. Inoustry 1991, 1, 14–18. [Google Scholar]
- Du, X. Research on the Production Technology of Sodium Pyroantimonite. Hunan Nonferrous Met. 2008, 24, 24–26. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Q.; Shen, W.; Min, X. Recovery of bismuth and antimony metals from pressure-leaching slag. Rare Met. 2012, 31, 102–106. [Google Scholar] [CrossRef]
- Cui, P.; Shen, Q.; Yu, X.; Yang, F.; Xie, G.; Jin, Y.; Jin, G. Experimental Study on Preparation of Sodium Antimonate from Tetrahedrite Alkaline System. Nonferrous Met. Engincering 2022, 12, 80–87+99. [Google Scholar] [CrossRef]
- Liu, W.; Jiao, A.; Liu, L.; Zhang, D.; Chen, L.; Yang, T. Clean technologies for producing sodium pyroantimonate by pressure oxidation method. Chin. J. Nonferrous Met. 2020, 30, 2379–2387. [Google Scholar] [CrossRef]
- Gu, K.; Liu, W.; Han, J.; Ou, Z.; Wu, D.; Qin, W. Arsenic and antimony extraction from high arsenic smelter ash with alkaline pressure oxidative leaching followed by Na2S leaching. Sep. Purif. Technol. 2019, 222, 53–59. [Google Scholar] [CrossRef]
- Zeng, O.; Zhao, R.; Guan, X. Study on Preparation of Sodium Antimonate by Oxidative Reflux Method. Inorg. Chem. Inoustry 1991, 1, 11–13. [Google Scholar]
- Feng, X. Production of sodium pyroantimonate by double decomposition. Guizhou Chem. Ind. 2000, 1, 22–23. [Google Scholar]
- Liu, W.; Zhang, K.; Zhang, D.; Chen, L.; Liu, L.; Yang, T. Preparation of Sodium Pyroantimonate from Antimony Trioxide by Pressure Oxidation in NaOH Solution. JOM 2019, 71, 3688–3695. [Google Scholar] [CrossRef]
- Liu, Q.; Shan, T.; Jin, C. Study of preparation of sodium antimonate from antimony oxidized ore with alkaline process. Hunan Nonferrous Met. 2014, 30, 31–33. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandstrom, A. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 15, 1227–1236. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, T.; Wang, W. Trial production of sodium pyroantimonate from antimony concentrate. Hunan Metall. 2005, 33, 17–20. [Google Scholar] [CrossRef]
- Xue, M.; Qian, F.; Zhang, Z.; Mo, Y.; Cao, J.; Pei, R. Method for Preparing Sodium Pyroantimonate Using Plant Polyphenols as Catalyst for Catalytic Air Oxidation. ZL20181038 1108.8, 2018.
- Zhang, L.; Guo, X.; Tian, Q.; Qin, H. Selective removal of arsenic from high arsenic dust in the NaOH-S system and leaching behavior of lead, antimony, zinc and tin. Hydrometallurgy 2021, 202, 105607. [Google Scholar] [CrossRef]
- Liao, C.; Zeng, Y.; Jin, J.; Jiang, P.; Lai, J. The Mechanism Research of Antimony Leaching from Copper Anode Slime Points Antimony Enrichment. Nonferrous Met. Eng. 2017, 7, 34–38. [Google Scholar] [CrossRef]
- Fu, B. Metallurgical Analysis of Modern Heavy Metals; Chemical Industry Press: Beijing, China, 2007; pp. 298–299. [Google Scholar]
- Chen, M.; Cong, D.Z.; Fang, T.N. Principles of Chemical Engineering, 3rd ed.; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Zhao, X. Chemical Reaction Kinetic Principles; Higher Education Press: Beijing, China, 1984; Volume 1, pp. 16–17. [Google Scholar]
- YS/T 22-2010; Sodium Antimonate. National Nonferrous Metals Standardization Technical Committee: Beijing, China, 2010.






| Element | Sb | Cu | Bi | As | Pb | Au | Ag | Cl |
|---|---|---|---|---|---|---|---|---|
| Content (%) | 34.7 | 8.99 | 0.703 | 7.47 | 1.61 | 0.006 | 0.36 | 9.38 |
| Element | Sb | Cu | As | Pb | Ag | Bi | Slag Rate |
|---|---|---|---|---|---|---|---|
| Raw materials (%) | 34.7 | 7.64 | 7.47 | 1.39 | 0.31 | 0.703 | |
| Compound leaching slage (%) | 5.39 | 22.73 | 1.04 | 4.09 | 0.87 | 1.907 | 31.24% |
| Type and Dosage of Oxidation (g·L−1) | Blank | KMnO4 0.5 | Phenol 0.5 | Catechol 0.5 | Catechol 0.5 + KMnO4 0.5 + Phenol 0.5 | Catechol 0.5 + KMnO4 0.5 |
|---|---|---|---|---|---|---|
| Antimony oxidation efficiency (%) | 17.50 | 29.61 | 32.24 | 33.47 | 53.92 | 54.71 |
| Composition (%) | Sb2O5 | Na2O≤ | Fe2O3≤ | CuO≤ | As2O3≤ | PbO≤ | Cr2O3≤ | V2O5≤ |
|---|---|---|---|---|---|---|---|---|
| Standard | 63.5–65.5 | 12–13.5 | 0.05 | 0.05 | 0.005 | 0.005 | 0.010 | 0.080 |
| Product | 62.39 | 12.57 | 0.023 | 0.015 | 0.013 | 0.006 | <0.005 | <0.005 |
| Composition (%) | Sb2O5 | Na2O≤ | Fe2O3≤ | CuO≤ | As2O3≤ | PbO≤ | Cr2O3≤ | V2O3≤ |
|---|---|---|---|---|---|---|---|---|
| Standard | 63.5–65.5 | 12–13.5 | 0.05 | 0.05 | 0.005 | 0.005 | 0.010 | 0.080 |
| Product | 64.9 | 12.57 | 0.023 | 0.003 | <0.005 | <0.005 | <0.005 | <0.005 |
| T (°C) | 50 | 60 | 70 | 80 | 90 |
| E × 10−3 (MPa) | 9.58 | 10.1 | 10.5 | 10.7 | 10.8 |
| T (°C) | 55 | 65 | 75 | 85 | Average Value |
|---|---|---|---|---|---|
| n | 0.515 | 0.487 | 0.489 | 0.471 | 0.491 |
| k | 7.56 × 10−5 | 7.44 × 10−5 | 8.43 × 10−5 | 9.06 × 10−5 | 8.12 × 10−5 |
| [Sb] (mol/L) | 0.16 | 0.14 | 0.12 | 0.10 | 0.08 | 0.06 | 0.04 | ||
|---|---|---|---|---|---|---|---|---|---|
| t/s | |||||||||
| [NaOH] (g/L) | |||||||||
| 250 | 540.8 | 1085.8 | 1669.2 | 2300.4 | 2993.6 | 3771.5 | 4676.0 | ||
| 300 | 444.0 | 877.1 | 1342.4 | 1848.6 | 2408.9 | 3045.3 | 3801.3 | ||
| [Sb] (mol/L) | 0.16 | 0.14 | 0.12 | 0.10 | 0.08 | 0.06 |
| V1 (mol/L/S) | 3.79 × 10−5 | 3.55 × 10−5 | 3.30 × 10−5 | 3.03 × 10−5 | 2.74 × 10−5 | 2.40 × 10−5 |
| V2 (mol/L/S) | 4.77 × 10−5 | 4.46 × 10−5 | 4.13 × 10−5 | 3.77 × 10−5 | 3.37 × 10−5 | 2.920 × 10−5 |
| 1.271 | 1.252 | 1.227 | 1.192 | 1.141 | 1.057 |
| T (°C) | 55 | 65 | 75 | 85 |
| k (mol·L−1)−1·s−1 | 8.54 × 10−6 | 8.40 × 10−6 | 9.52 E× 10−6 | 1.02 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Jin, J.; Liao, C.; Liu, F. Selective Separation of Antimony and Preparation of Sodium Antimonate by Sodium Salt Leaching-Synergistic Oxidation from High Arsenic Antimony Residue. Metals 2025, 15, 929. https://doi.org/10.3390/met15090929
Zeng Y, Jin J, Liao C, Liu F. Selective Separation of Antimony and Preparation of Sodium Antimonate by Sodium Salt Leaching-Synergistic Oxidation from High Arsenic Antimony Residue. Metals. 2025; 15(9):929. https://doi.org/10.3390/met15090929
Chicago/Turabian StyleZeng, Yanliang, Jun Jin, Chunfa Liao, and Fupeng Liu. 2025. "Selective Separation of Antimony and Preparation of Sodium Antimonate by Sodium Salt Leaching-Synergistic Oxidation from High Arsenic Antimony Residue" Metals 15, no. 9: 929. https://doi.org/10.3390/met15090929
APA StyleZeng, Y., Jin, J., Liao, C., & Liu, F. (2025). Selective Separation of Antimony and Preparation of Sodium Antimonate by Sodium Salt Leaching-Synergistic Oxidation from High Arsenic Antimony Residue. Metals, 15(9), 929. https://doi.org/10.3390/met15090929





