In Situ and Laboratory Investigation of the Anti-Corrosion and Anti-Fouling Efficacy of an Innovative Biocide-Free Coating for Naval Steels
Abstract
1. Introduction
- Conductive polymer-mediated redox inhibition and passivation;
- Photocatalytic and barrier effects from TiO2;
- Magnetite-modified nanotubes for mechanical robustness and synergistic anti-biofouling activity.
2. Materials and Methods
2.1. Material
2.2. Synthesis of Antifouling Coating
2.2.1. Preparation of Polyaniline (PAni) Nanorods
2.2.2. Functionalization of PAni Nanorods with TiO2
2.2.3. Synthesis of MWCNTs/Fe3O4 Nanocomposites
2.2.4. Final Coating Preparation and Application
2.3. Laboratory Immersion Testing in Artificial Sea Water (ASW)
2.4. In Situ (Field) Seawater Immersion
3. Results
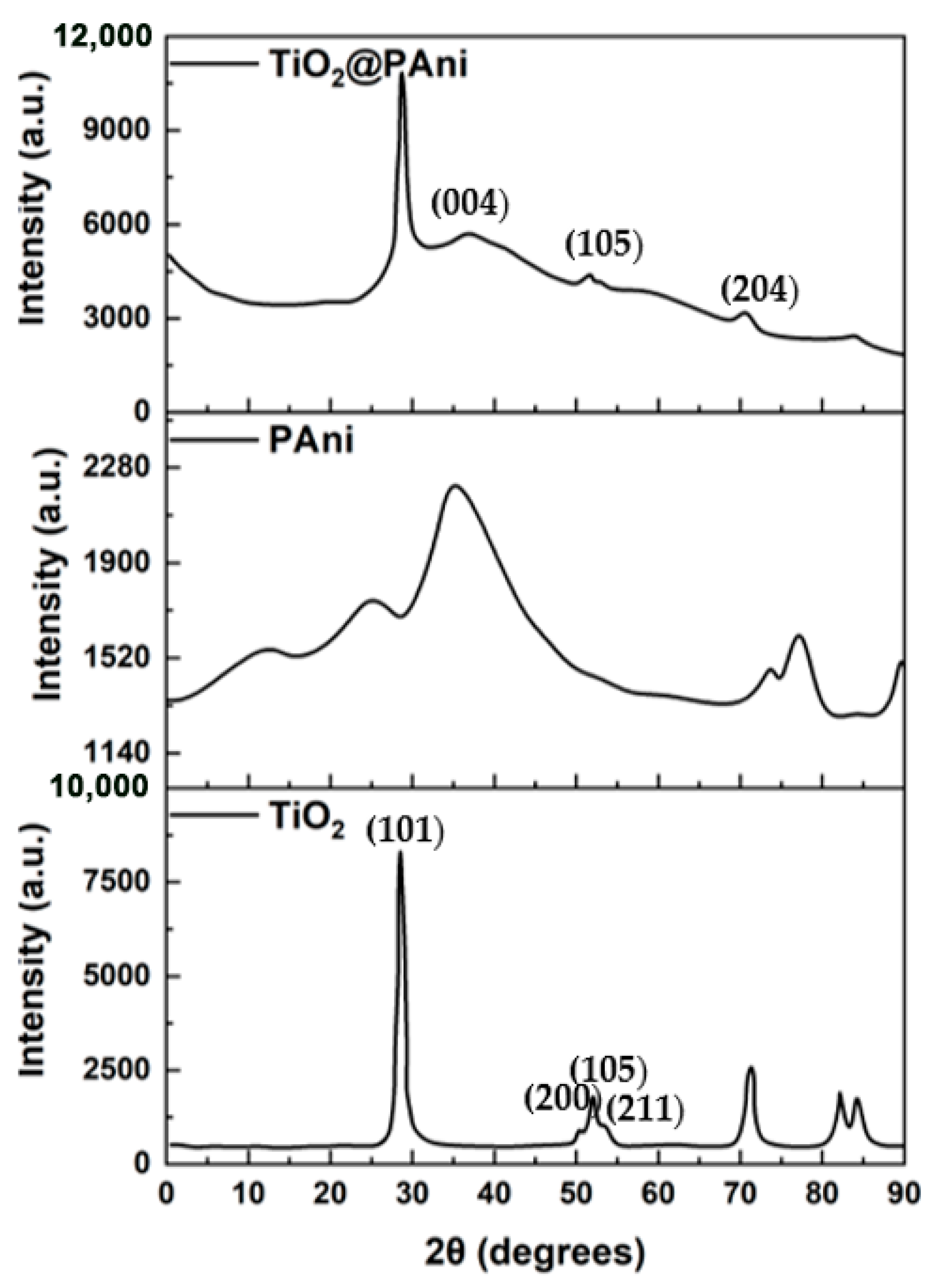
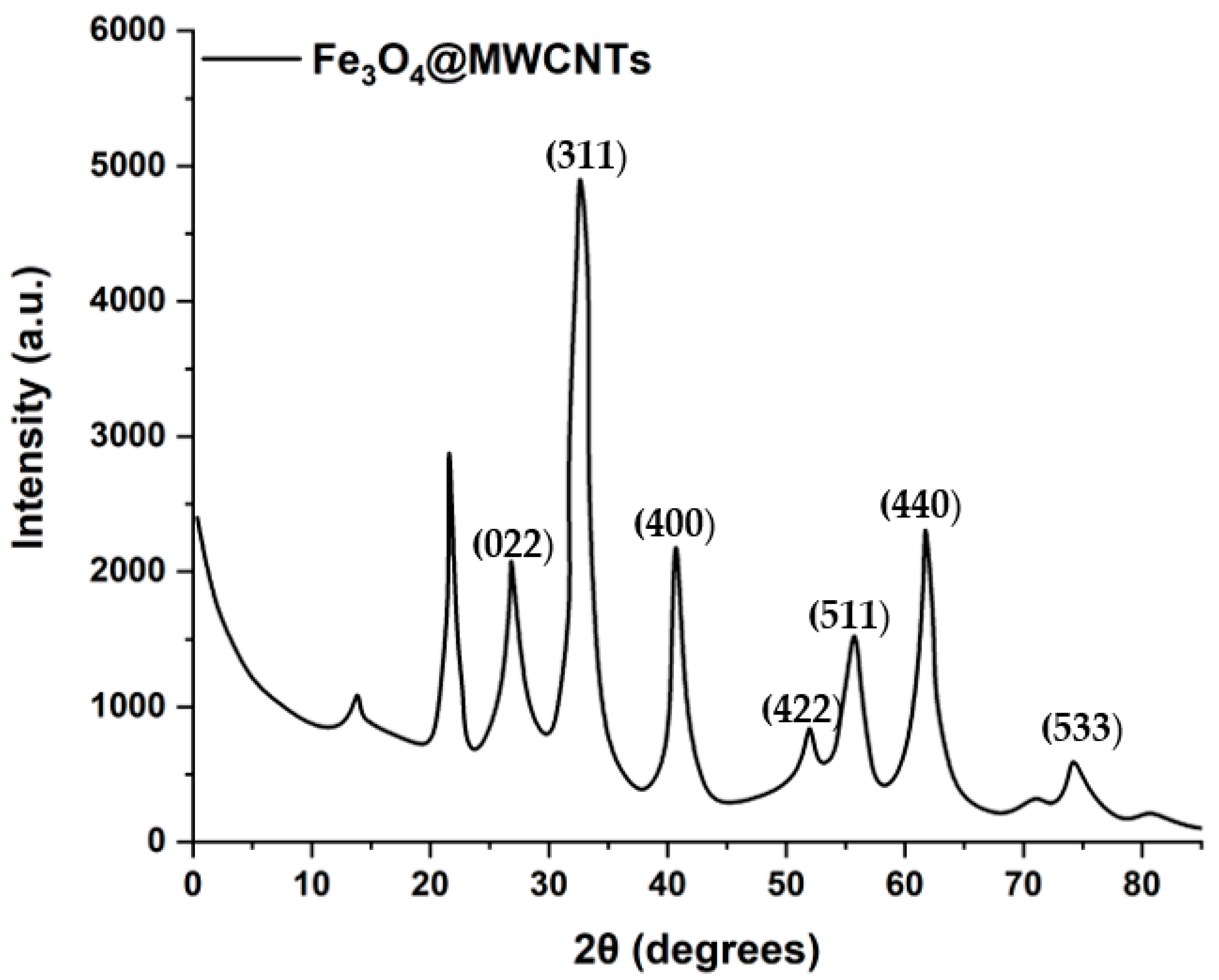
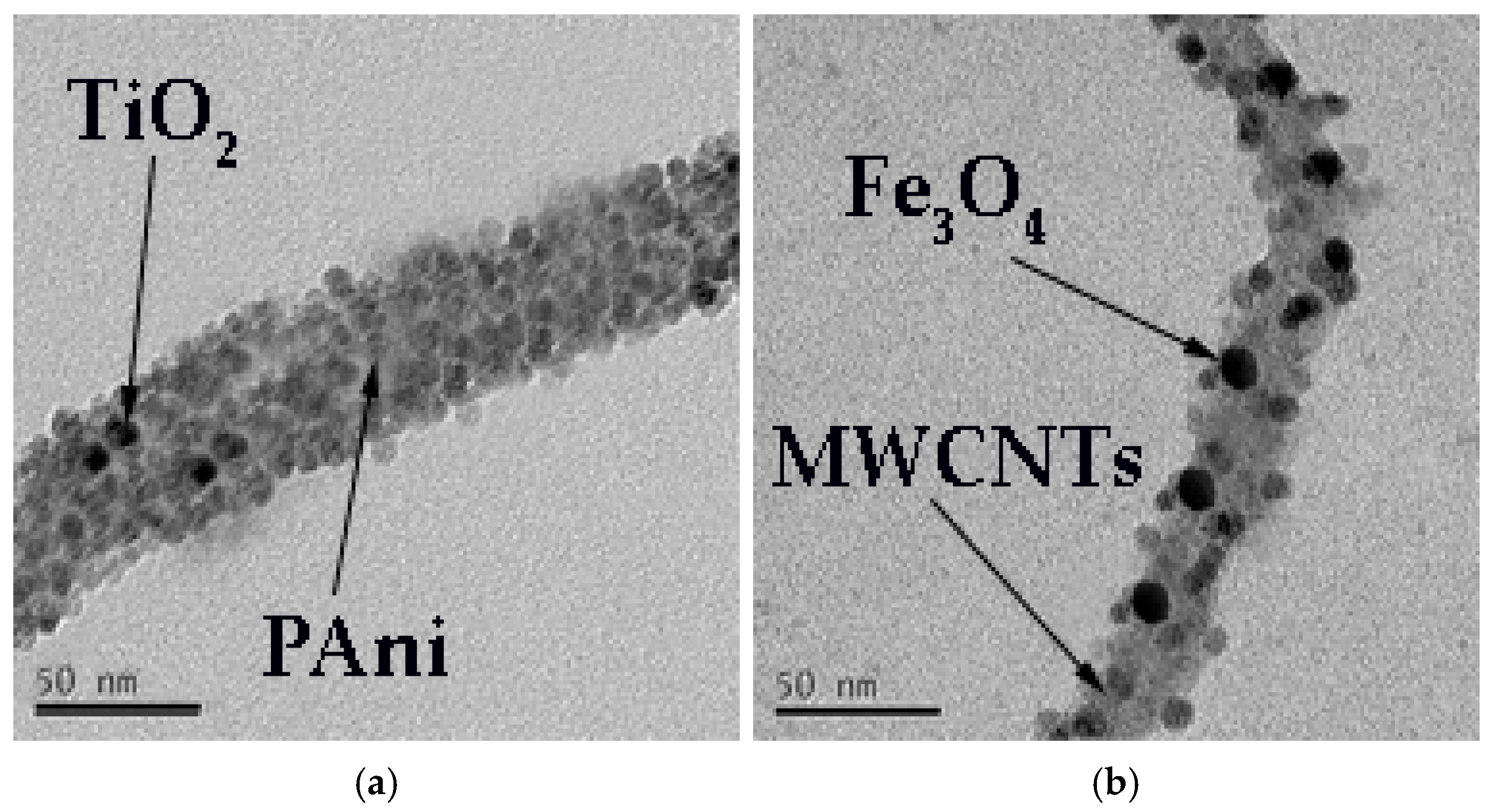
3.1. Characterization of Antifouling Coating
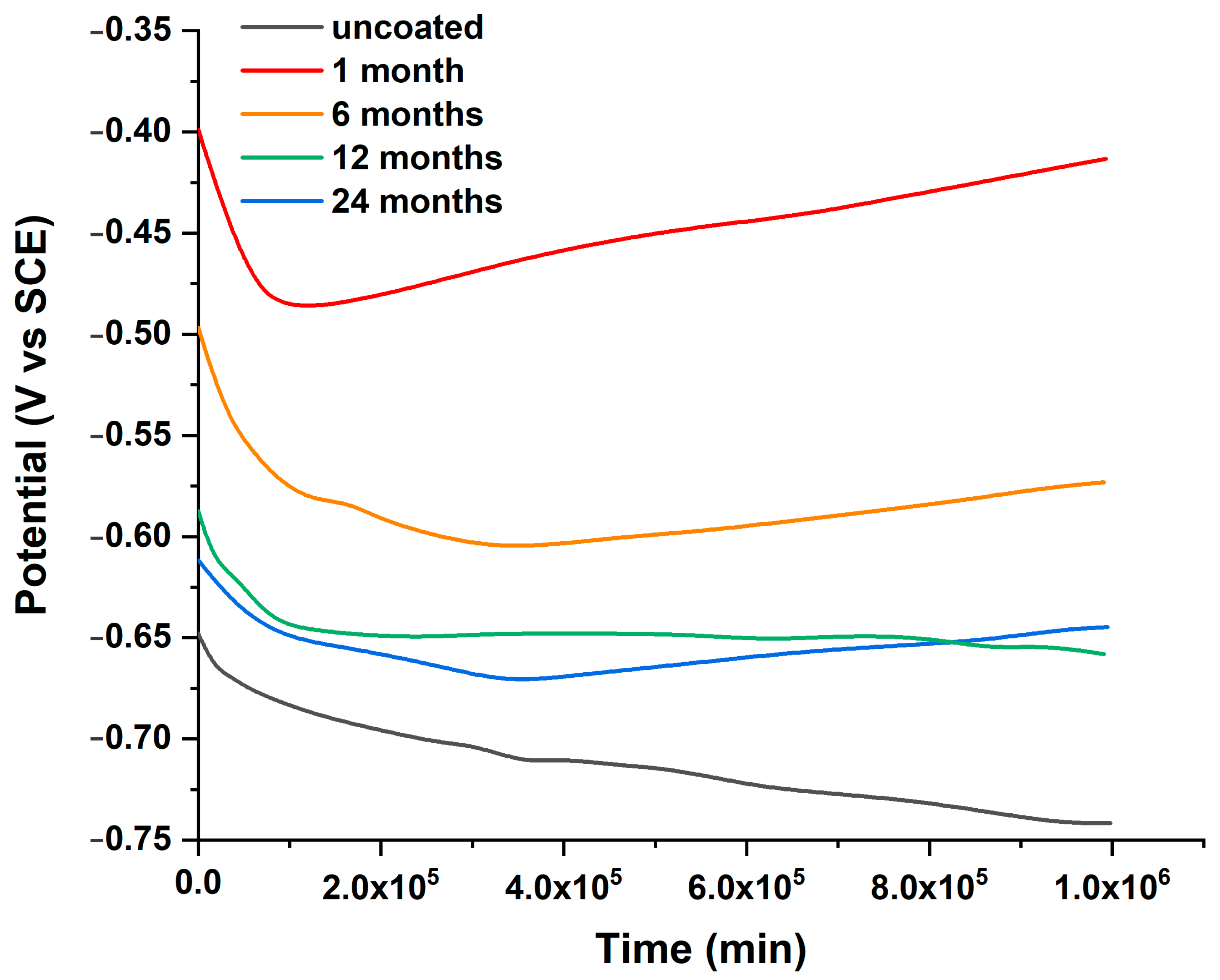
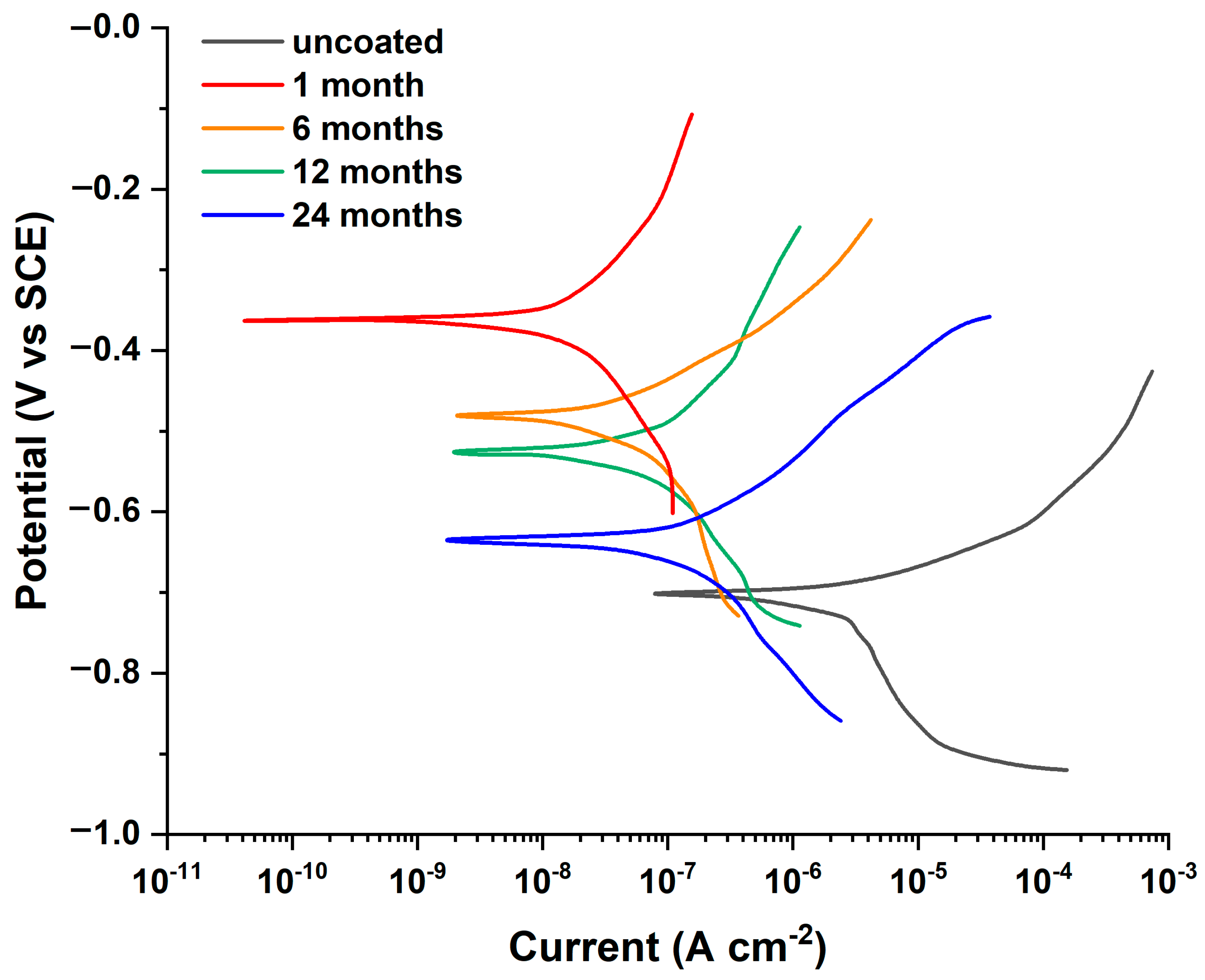
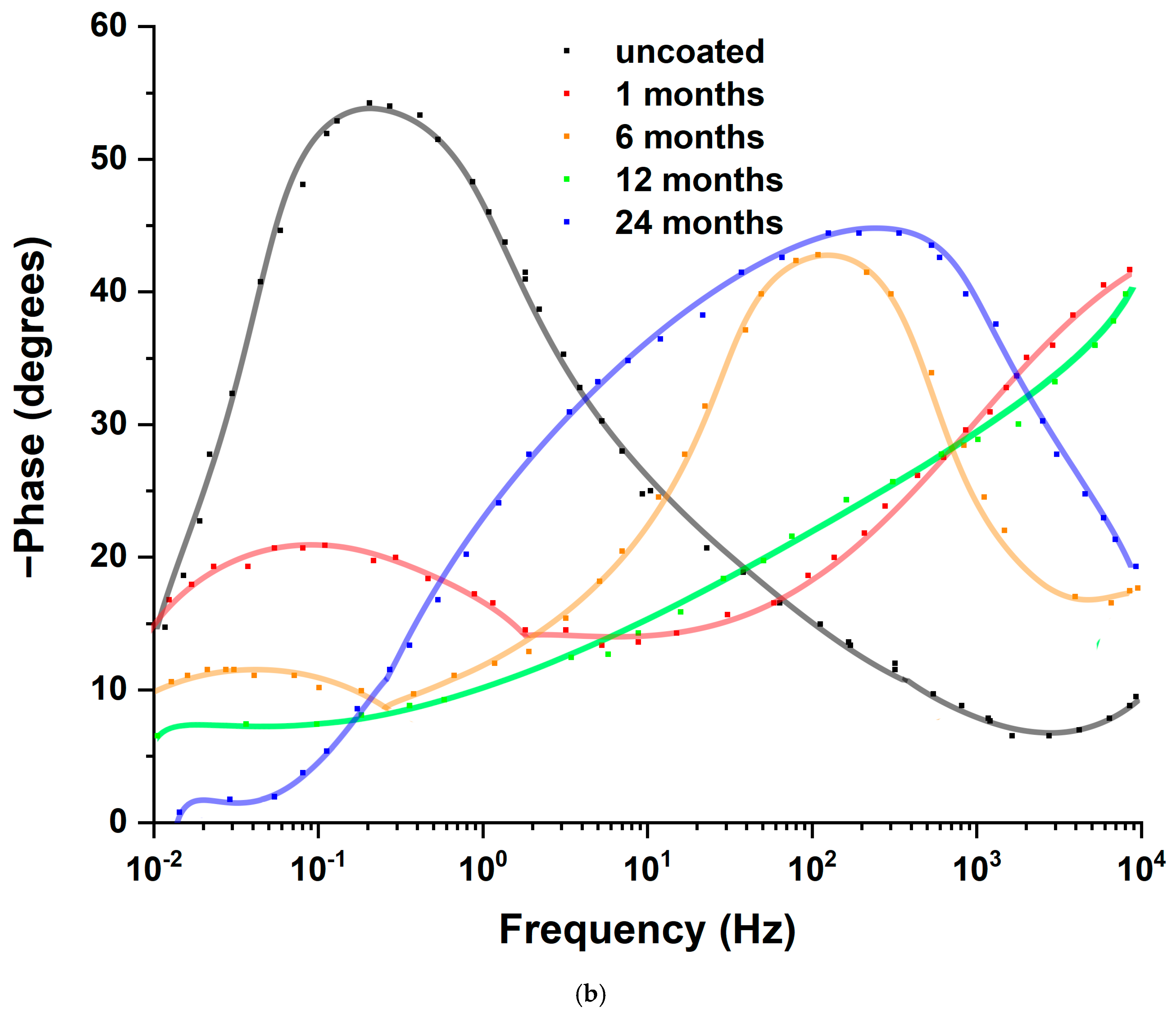
3.2. Laboratory Immersion Tests
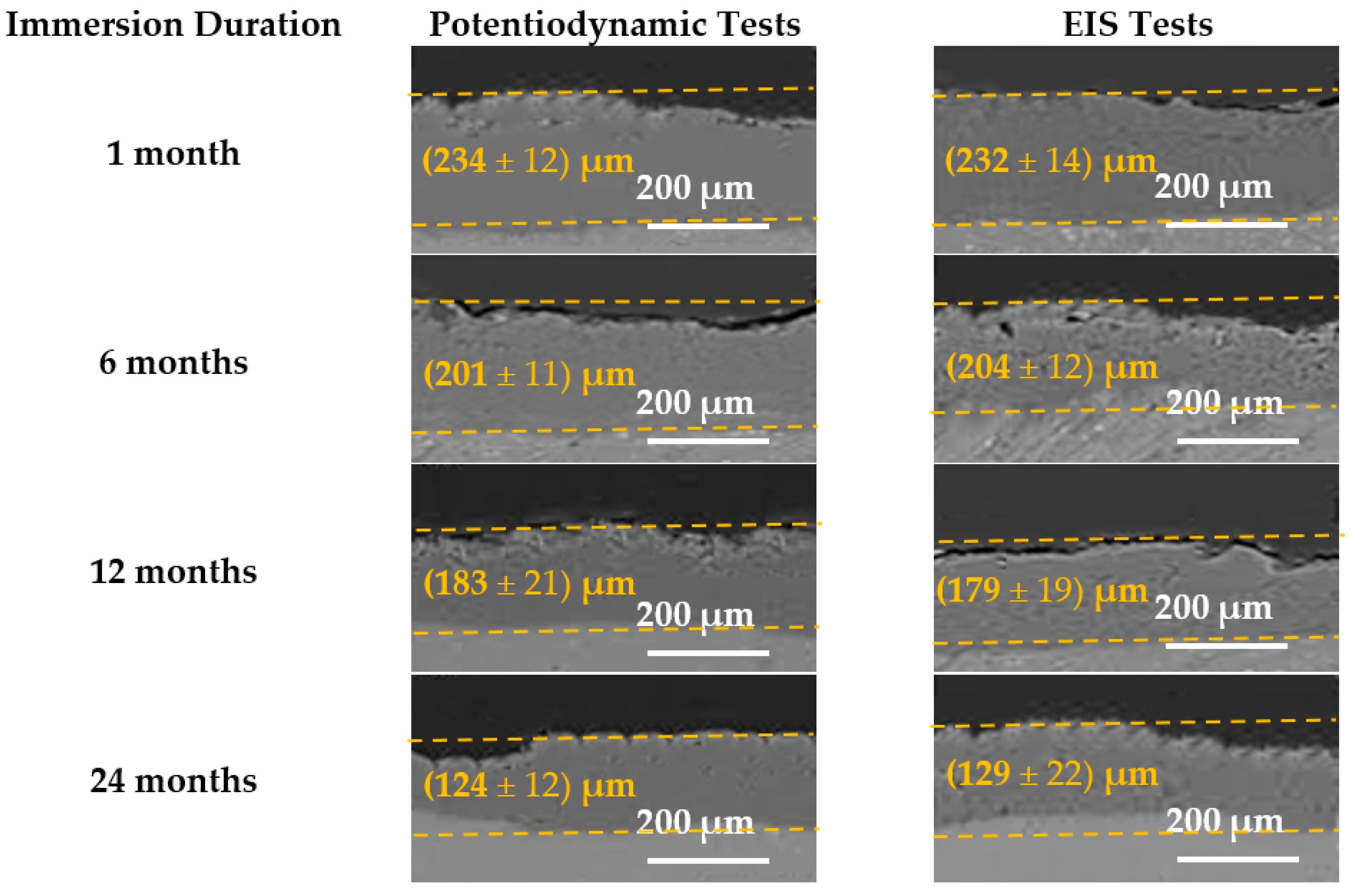
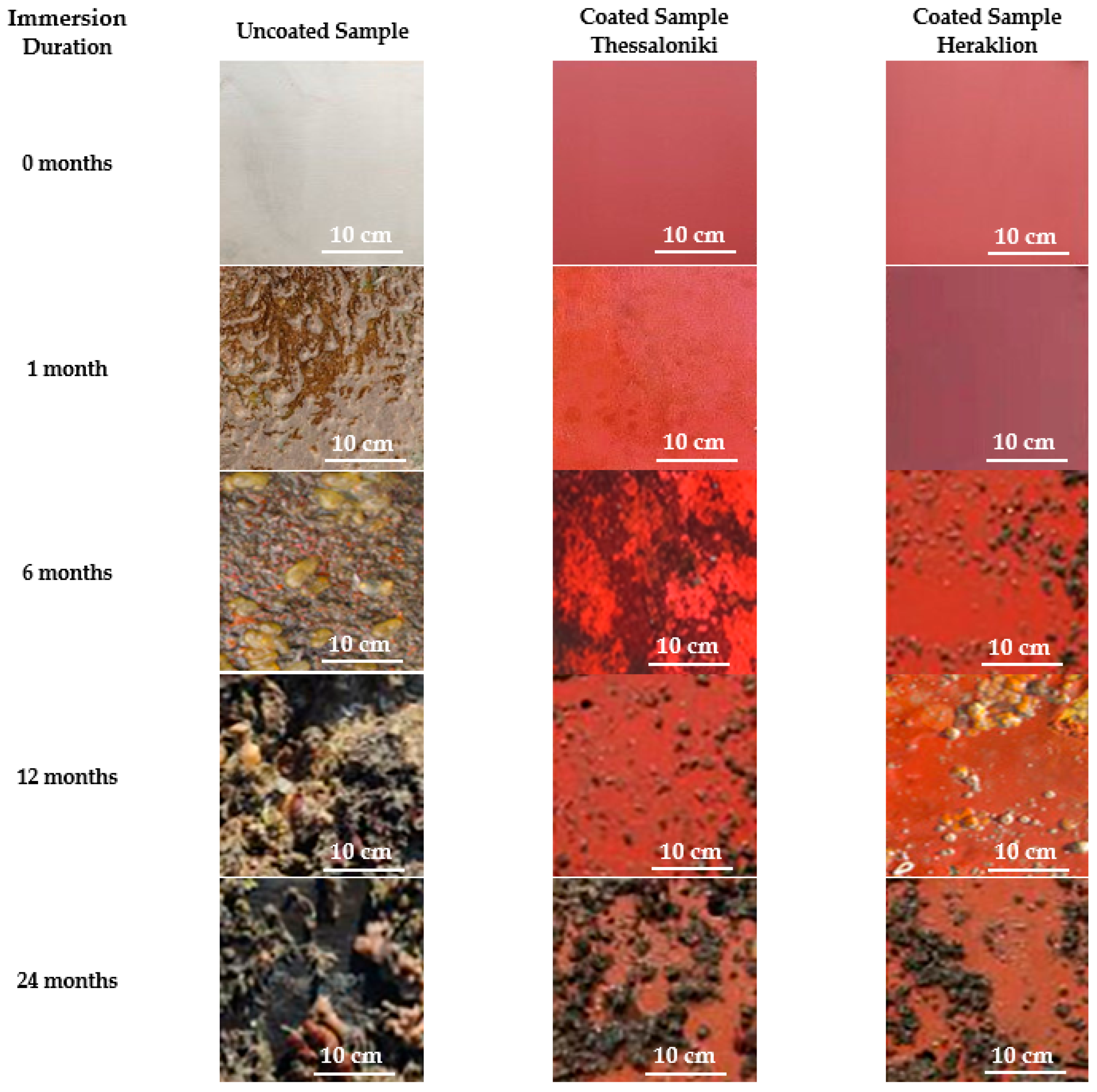
3.3. Field Immersion Tests
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APS | Ammonium peroxydisulfate |
| OCP | Open circuit potential |
| EIS | Electrochemical impedance spectroscopy |
| SCE | Saturated calomel electrode |
| Ag/AgCl | Silver/Silver chloride electrode |
| PAni | Polyaniline |
| TiO2 | Titanium dioxide |
| MWCNTs | Multiwalled carbon nanotubes |
| Fe3O4 | Magnetite |
| CR | Corrosion rate |
| Icorr | Corrosion current density |
| Ecorr | Corrosion potential |
| η (%) | Corrosion protection efficiency |
| CPE | Constant phase element |
| Rs | Solution resistance |
| Rc | Coating resistance |
| Rct | Charge transfer resistance |
| Rdiff | Diffusion resistance |
| n | Number of electrons exchanged |
| EW | Equivalent weight |
| TEM | Transmission electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| ASTM | American Society for Testing and Materials |
| IMO | International Maritime Organization |
| LCA | Life cycle assessment |
References
- Montemarano, T.W.; Sack, B.P.; Gudas, J.P.; Vassilaros, M.G.; Vanderveldt, H.H. High Strength Low Alloy Steels in Naval Construction. J. Ship Prod. 1986, 2, 145–162. [Google Scholar] [CrossRef]
- Abioye, O.P.; Loto, C.A.; Fayomi, O.S.I. Evaluation of Anti-Biofouling Progresses in Marine Application. J. Bio- Tribo-Corros. 2019, 5, 22. [Google Scholar] [CrossRef]
- Hopkins, G.; Davidson, I.; Georgiades, E.; Floerl, O.; Morrisey, D.; Cahill, P. Managing Biofouling on Submerged Static Artificial Structures in the Marine Environment—Assessment of Current and Emerging Approaches. Front. Mar. Sci. 2021, 8, 759194. [Google Scholar] [CrossRef]
- Liu, M.; Tan, Z.; Xu, S.; Zhao, Y.; Wang, H.; Zhang, S.; Ma, R.; Jiang, T.; Ma, Z.; Zhong, N.; et al. Synthesis and Characterization of Silane-Coupled Sodium Silicate Composite Coatings for Enhanced Anticorrosive Performance. Coatings 2025, 15, 428. [Google Scholar] [CrossRef]
- Yang, K.; Chen, J.; Zheng, L.; Zheng, B.; Chen, Y.; Chen, X.; Bai, W.; Jian, R.; Wei, F.; Xu, Y. Urushiol Titanium Polymer-Based Composites Coatings for Anti-corrosion and Antifouling in Marine Spray Splash Zones. J. Appl. Polym. Sci. 2021, 138, 50861. [Google Scholar] [CrossRef]
- Tesler, A.B.; Prado, L.H.; Thievessen, I.; Mazare, A.; Schmuki, P.; Virtanen, S.; Goldmann, W.H. Nontoxic Liquid-Infused Slippery Coating Prepared on Steel Substrates Inhibits Corrosion and Biofouling Adhesion. ACS Appl. Mater. Interfaces 2022, 14, 29386–29397. [Google Scholar] [CrossRef] [PubMed]
- Sfameni, S.; Rando, G.; Galletta, M.; Ielo, I.; Brucale, M.; De Leo, F.; Cardiano, P.; Cappello, S.; Visco, A.; Trovato, V.; et al. Design and Development of Fluorinated and Biocide-Free Sol–Gel Based Hybrid Functional Coatings for Anti-Biofouling/Foul-Release Activity. Gels 2022, 8, 538. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; De With, G. Non-Toxic, Non-Biocide-Release Antifouling Coatings Based on Molecular Structure Design for Marine Applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Cholkar, A.; Chatterjee, S.; Richards, C.; McCarthy, É.; Perumal, G.; Regan, F.; Kinahan, D.; Brabazon, D. Biofouling and Corrosion Protection of Aluminum Alloys Through Ultrafast Laser Surface Texturing for Marine Applications. Adv. Mater. Interfaces 2024, 11, 2300835. [Google Scholar] [CrossRef]
- Dobson, T.; Yunnie, A.; Kaloudis, D.; Larrosa, N.; Coules, H. Biofouling and Corrosion Rate of Welded Nickel Aluminium Bronze in Natural and Simulated Seawater. Biofouling 2024, 40, 193–208. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Lagerström, M.; Granhag, L.; Werner, S.; Larsson, A.I.; Ytreberg, E. A Novel Tool for Cost and Emission Reduction Related to Ship Underwater Hull Maintenance. J. Clean. Prod. 2022, 356, 131882. [Google Scholar] [CrossRef]
- Tamburri, M.N.; Davidson, I.C.; First, M.R.; Scianni, C.; Newcomer, K.; Inglis, G.J.; Georgiades, E.T.; Barnes, J.M.; Ruiz, G.M. In-Water Cleaning and Capture to Remove Ship Biofouling: An Initial Evaluation of Efficacy and Environmental Safety. Front. Mar. Sci. 2020, 7, 437. [Google Scholar] [CrossRef]
- Farkas, A.; Degiuli, N.; Martić, I. An Investigation into the Effect of Hard Fouling on the Ship Resistance Using CFD. Appl. Ocean Res. 2020, 100, 102205. [Google Scholar] [CrossRef]
- Scianni, C.; Georgiades, E. Vessel In-Water Cleaning or Treatment: Identification of Environmental Risks and Science Needs for Evidence-Based Decision Making. Front. Mar. Sci. 2019, 6, 467. [Google Scholar] [CrossRef]
- Weber, F.; Esmaeili, N. Marine Biofouling and the Role of Biocidal Coatings in Balancing Environmental Impacts. Biofouling 2023, 39, 661–681. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I.; Cahill, P.; Hinz, A.; Kluza, D.; Scianni, C.; Georgiades, E. A Review of Biofouling of Ships’ Internal Seawater Systems. Front. Mar. Sci. 2021, 8, 761531. [Google Scholar] [CrossRef]
- Compère, C.; Bellon-Fontaine, M.; Bertrand, P.; Costa, D.; Marcus, P.; Poleunis, C.; Pradier, C.; Rondot, B.; Walls, M.G. Kinetics of Conditioning Layer Formation on Stainless Steel Immersed in Seawater. Biofouling 2001, 17, 129–145. [Google Scholar] [CrossRef]
- Cao, Z.; Cao, P. Research Progress on Low-Surface-Energy Antifouling Coatings for Ship Hulls: A Review. Biomimetics 2023, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Khlyustova, A.; Park, K.; Stafslien, S.; Kang, G.; Chen, P.; Shindler, S.; Yang, R. Fluorine-Free Amphiphilic Copolymers for Broad-Spectrum Marine Biofouling Deterrence. Adv. Funct. Mater. 2025, 2502065. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.M.; Teplitski, M. Mini-Review: Inhibition of Biofouling by Marine Microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, W.H.; Lewandowski, Z. Manganese Biofouling and the Corrosion Behavior of Stainless Steel. Biofouling 1996, 10, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Venettacci, S.; Ponticelli, G.S.; Tagliaferri, F.; Guarino, S. Environmental and Economic Impact of an Innovative Biocide-Free Antifouling Coating for Naval Applications. Materials 2023, 16, 748. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Jamil, M.I.; Jiang, J.; Shoaib, M.; Amin, B.U.; Luo, S.; Zhan, X.; Chen, F.; Zhang, Q. An Overview of Controlled-Biocide-Release Coating Based on Polymer Resin for Marine Antifouling Applications. J. Polym. Res. 2020, 27, 85. [Google Scholar] [CrossRef]
- Papadatou, M.; Robson, S.C.; Dobretsov, S.; Watts, J.E.M.; Longyear, J.; Salta, M. Marine Biofilms on Different Fouling Control Coating Types Reveal Differences in Microbial Community Composition and Abundance. MicrobiologyOpen 2021, 10, e1231. [Google Scholar] [CrossRef]
- Miller, R.J.; Adeleye, A.S.; Page, H.M.; Kui, L.; Lenihan, H.S.; Keller, A.A. Nano and Traditional Copper and Zinc Antifouling Coatings: Metal Release and Impact on Marine Sessile Invertebrate Communities. J. Nanoparticle Res. 2020, 22, 129. [Google Scholar] [CrossRef]
- Fitridge, I.; Dempster, T.; Guenther, J.; De Nys, R. The Impact and Control of Biofouling in Marine Aquaculture: A Review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef]
- Qiu, H.; Hölken, I.; Gapeeva, A.; Filiz, V.; Adelung, R.; Baum, M. Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector. Materials 2018, 11, 2413. [Google Scholar] [CrossRef]
- Wu, S.; Wu, S.; Xing, S.; Wang, T.; Hou, J.; Zhao, Y.; Li, W. Research Progress of Marine Anti-Fouling Coatings. Coatings 2024, 14, 1227. [Google Scholar] [CrossRef]
- Liu, D.; Shu, H.; Zhou, J.; Bai, X.; Cao, P. Research Progress on New Environmentally Friendly Antifouling Coatings in Marine Settings: A Review. Biomimetics 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Truby, K.; Wood, C.; Stein, J.; Cella, J.; Carpenter, J.; Kavanagh, C.; Swain, G.; Wiebe, D.; Lapota, D.; Meyer, A.; et al. Evaluation of the Performance Enhancement of Silicone Biofouling-release Coatings by Oil Incorporation. Biofouling 2000, 15, 141–150. [Google Scholar] [CrossRef]
- Hu, P.; Xie, Q.; Ma, C.; Zhang, G. Silicone-Based Fouling-Release Coatings for Marine Antifouling. Langmuir 2020, 36, 2170–2183. [Google Scholar] [CrossRef]
- Pistone, A.; Scolaro, C.; Visco, A. Mechanical Properties of Protective Coatings against Marine Fouling: A Review. Polymers 2021, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Callow, M.E. Trends in the Development of Environmentally Friendly Fouling-Resistant Marine Coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.K.; Ober, C.K. Polymer-Based Marine Antifouling and Fouling Release Surfaces: Strategies for Synthesis and Modification. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Swain, G.W. Managing the Use of Copper-Based Antifouling Paints. Environ. Manag. 2007, 39, 423–441. [Google Scholar] [CrossRef]
- Lagerström, M.; Butschle, M.; Larsson, A.I.; Cachot, J.; Dam-Johansen, K.; Schackmann, M.; Le Bihanic, F. Investigation of Critical Copper Release Rates for Dose Optimization of Antifouling Coatings. Prog. Org. Coat. 2025, 198, 108928. [Google Scholar] [CrossRef]
- Pereira, D.; Almeida, J.R.; Cidade, H.; Correia-da-Silva, M. Proof of Concept of Natural and Synthetic Antifouling Agents in Coatings. Mar. Drugs 2024, 22, 291. [Google Scholar] [CrossRef]
- Xu, X.; Chen, R.; Zheng, Y.; Yu, J.; Liu, Q.; Liu, J.; Lin, C.; Duan, J.; Wang, J. Slippery-Liquid-Infused Electrostatic Flocking Surfaces for Marine Antifouling Application. Langmuir 2021, 37, 10020–10028. [Google Scholar] [CrossRef]
- Guo, R.; Tan, Y.; Fang, M.; Li, L.; Chen, Q.; Qin, W.; Liu, N.; Mo, Z. Multifunctional Superhydrophobic Coatings with Self-Cleaning and Anti-Fouling Properties for Corrosion Protection of Metals. Surf. Interfaces 2024, 50, 104476. [Google Scholar] [CrossRef]
- Guo, M.; Chen, Z.; Song, M.; Zhang, Y.; Wang, Z.; Feng, A.; Yang, D.; Wang, L.; Zhu, Z. Multifamily Nanozymes for Sustainable and Eco-Friendly Marine Antifouling. Nano Lett. 2025, 25, 4878–4886. [Google Scholar] [CrossRef]
- Kirschner, C.M.; Brennan, A.B. Bio-Inspired Antifouling Strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- Carrier, A.J.; Carve, M.; Shimeta, J.; Walker, T.R.; Zhang, X.; Oakes, K.D.; Jha, K.C.; Charlton, T.; Stenzel, M.H. Transitioning towards Environmentally Benign Marine Antifouling Coatings. Front. Mar. Sci. 2023, 10, 1175270. [Google Scholar] [CrossRef]
- Voulvoulis, N.; Scrimshaw, M.D.; Lester, J.N. Alternative Antifouling Biocides. Appl. Organomet. Chem. 1999, 13, 135–143. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Risks of Using Antifouling Biocides in Aquaculture. Int. J. Mol. Sci. 2012, 13, 1541–1560. [Google Scholar] [CrossRef]
- Romeu, M.J.; Mergulhão, F. Development of Antifouling Strategies for Marine Applications. Microorganisms 2023, 11, 1568. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, Z.; Liu, Y.; Dai, P.; Hai, G.; Liu, F.; Shang, Y.; Cao, Z.; Yang, W. Research Progress of Natural Products and Their Derivatives in Marine Antifouling. Materials 2023, 16, 6190. [Google Scholar] [CrossRef]
- Thomas, K.; Raymond, K.; Chadwick, J.; Waldock, M. The Effects of Short-term Changes in Environmental Parameters on the Release of Biocides from Antifouling Coatings: Cuprous Oxide and Tributyltin. Appl. Organometal. Chem. 1999, 13, 453–460. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Xu, M.; Zhou, P.; Ren, Y. Research Strategies to Develop Environmentally Friendly Marine Antifouling Coatings. Mar. Drugs 2020, 18, 371. [Google Scholar] [CrossRef]
- Yang, H.; Qin, L.; Zhao, W.; Mawignon, F.J.; Guo, H.; Wu, Y.; Zhang, Y.; Dong, G. Eco-Friendly Polysaccharide Coatings for Antifouling and Drag-Reduction and Potential Application for Marine Devices. Friction 2024, 12, 726–744. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.; Li, X.; Zheng, B.; Li, D.; Xu, D.; Wang, F. Nacre-Inspired Metal-Organic Framework Coatings Reinforced by Multiscale Hierarchical Cross-linking for Integrated Antifouling and Anti-Microbial Corrosion. Adv. Funct. Mater. 2023, 33, 2305995. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Yoshikawa, C.; Takagi, R.; Nakaji-Hirabayashi, T.; Ochi, T.; Kawamura, Y.; Thissen, H. Marine Antifouling Coatings Based on Durable Bottlebrush Polymers. ACS Appl. Mater. Interfaces 2022, 14, 32497–32509. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Macdonald, B.; Cho, T.H.; Repetto, T.; Sun, K.; Tuteja, A.; Dasgupta, N.P. Bioinspired Zwitterionic Nanowires with Simultaneous Biofouling Reduction and Release. Small 2024, 20, 2400784. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, X.; Jian, R.; Bai, W.; Zheng, G.; Xie, Z.; Lin, Q.; Lin, F.; Xu, Y. In Situ Reduction of Silver Nanoparticles/Urushiol-Based Polybenzoxazine Composite Coatings with Enhanced Antimicrobial and Antifouling Performances. Polymers 2024, 16, 1167. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Nanocoating Is a New Way for Biofouling Prevention. Front. Nanotechnol. 2021, 3, 771098. [Google Scholar] [CrossRef]
- Laftah, W.A.; Wan Abdul Rahman, W.A. Polymers for Anti-Fouling Applications: A Review. Environ. Sci. Adv. 2025, 4, 824–841. [Google Scholar] [CrossRef]
- Vourna, P.; Falara, P.P.; Hristoforou, E.V.; Papadopoulos, N.D. Corrosion and Antifouling Behavior of a New Biocide-Free Antifouling Paint for Ship Hulls Under Both Artificially Simulated and Natural Marine Environment. Materials 2025, 18, 3095. [Google Scholar] [CrossRef] [PubMed]
- Vourna, P.; Falara, P.P.; Papadopoulos, N.D. Investigation of Corrosion and Fouling in a Novel Biocide-Free Antifouling Coating on Steel. Micro 2025, 5, 34. [Google Scholar] [CrossRef]
- Saravanan, P.; Jayamoorthy, K.; Ananda Kumar, S. Design and Characterization of Non-Toxic Nano-Hybrid Coatings for Corrosion and Fouling Resistance. J. Sci. Adv. Mater. Devices 2016, 1, 367–378. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, X.; Wang, Y.; Sun, G.; Li, P.; Qu, L.; Sui, Y.; Dou, Y. Preparation and Corrosion Resistance of ETEO Modified Graphene Oxide/Epoxy Resin Coating. Coatings 2019, 9, 46. [Google Scholar] [CrossRef]
- Ying, L.; Wu, Y.; Nie, C.; Wu, C.; Wang, G. Improvement of the Tribological Properties and Corrosion Resistance of Epoxy–PTFE Composite Coating by Nanoparticle Modification. Coatings 2020, 11, 10. [Google Scholar] [CrossRef]
- Wang, S.; Tang, J.; Xiang, H.; Wei, C.; Huang, J.; Chen, Z.; Hu, K.; Han, C.; Zhu, S.; Ding, Y. Mechanism Insights in Anticorrosion Performance of Waterborne Epoxy Coatings Reinforced by PEI-Functionalized Boron Nitride Nanosheets. Langmuir 2024, 40, 10980–10991. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Zhao, H.; Wang, L. Synthesis of l-Histidine-Attached Graphene Nanomaterials and Their Application for Steel Protection. ACS Appl. Nano Mater. 2018, 1, 1385–1395. [Google Scholar] [CrossRef]
- Balgude, D.; Sabnis, A. Sol–Gel Derived Hybrid Coatings as an Environment Friendly Surface Treatment for Corrosion Protection of Metals and Their Alloys. J. Sol-Gel Sci. Technol. 2012, 64, 124–134. [Google Scholar] [CrossRef]
- Thomas, P.; Sahoo, B.N.; Thomas, P.J.; Greve, M.M. Recent Advances in Emerging Integrated Anticorrosion and Antifouling Nanomaterial-Based Coating Solutions. Environ. Sci. Pollut. Res. 2024, 31, 67550–67576. [Google Scholar] [CrossRef] [PubMed]
- Palippui, H. Integration of Technology and Regulations for Safe and Efficient Marine Logistics. Collab. Eng. Dly. Book Ser. 2024, 2, 1–7. [Google Scholar] [CrossRef]
- Ekmekçioğlu, A.; Çelebi, U.B.; Ünlügençoğlu, K.; Kuzu, S.L. Effect of the Low Sulphur Regulations of Maritime Fuels on Ambient Air Quality: A Case Study in the Bosphorus Strait. Int. J. Environ. Sci. Technol. 2025, 22, 4495–4502. [Google Scholar] [CrossRef]
- Bogatu, N.; Buruiana, D.L.; Muresan, A.C.; Ghisman, V.; Lupu, A.; Mardare, L.; Herbei, E.E.; Basliu, V.; Ceoromila, A.; Florescu, S. Assessment of the Effectiveness of Protective Coatings in Preventing Steel Corrosion in the Marine Environment. Polymers 2025, 17, 378. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, R.; Liu, K.; Zhang, Y.; Shi, X.; Sand, W.; Hou, B. Application of Nanomaterials in Antifouling: A Review. Nano Mater. Sci. 2024, 6, 672–700. [Google Scholar] [CrossRef]
- Baldissera, A.F.; Miranda, K.L.D.; Bressy, C.; Martin, C.; Margaillan, A.; Ferreira, C.A. Using Conducting Polymers as Active Agents for Marine Antifouling Paints. Mat. Res. 2015, 18, 1129–1139. [Google Scholar] [CrossRef]
- Sahoo, B.N.; Thomas, P.J.; Thomas, P.; Greve, M.M. Antibiofouling Coatings For Marine Sensors: Progress and Perspectives on Materials, Methods, Impacts, and Field Trial Studies. ACS Sens. 2025, 10, 1600–1619. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-Zinc Oxide Nanocomposite Coatings for the Prevention of Marine Biofouling. Chemosphere 2017, 168, 408–417. [Google Scholar] [CrossRef]
- Selim, M.S.; Azzam, A.M.; Higazy, S.A.; Shenashen, M.A.; Elmarakbi, A.; Ebara, M.; El-Safty, S.A. Hierarchical Biocide-Free Silicone/Graphene-Silicon Carbide Nanocomposite Coatings for Marine Antifouling and Superhydrophobicity of Ship Hulls. Chem. Eng. Sci. 2024, 291, 119929. [Google Scholar] [CrossRef]
- Vourna, P.; Falara, P.P.; Papadopoulos, N.D. The Investigation of a Biocide-Free Antifouling Coating on Naval Steels Under Both Simulated and Actual Seawater Conditions. Processes 2025, 13, 2448. [Google Scholar] [CrossRef]
- Committee D-19. D 1141-52 Standard Specifications for Substitute Ocean Water. In Manual on Industrial Water and Industrial Waste Water; ASTM International: West Conshohocken, PA, USA, 1960; pp. 398–399. ISBN 978-0-8031-6884-8. [Google Scholar]
- Hyder, P.; Simpson, J.H.; Christopoulos, S.; Krestenitis, Y. The Seasonal Cycles of Stratification and Circulation in the Thermaikos Gulf Region Of Freshwater Influence (ROFI), North-West Aegean. Cont. Shelf Res. 2002, 22, 2573–2597. [Google Scholar] [CrossRef]
- Falara, P.P.; Papadopoulos, N.D.; Vourna, P. Microstructure and Performance of Antibiofouling Coatings on High-Strength Steel Substrates Immersed in the Marine Environment. Micro 2022, 2, 277–294. [Google Scholar] [CrossRef]
- Chyrkin, A.; Pillai, R.; Galiullin, T.; Wessel, E.; Grüner, D.; Quadakkers, W.J. External α-Al2O3 Scale on Ni-Base Alloy 602 CA—Part I: Formation and Long-Term Stability. Corros. Sci. 2017, 124, 138–149. [Google Scholar] [CrossRef]



| Element | Chemical Composition Elemental % |
|---|---|
| Iron (Fe) | balanced |
| Carbon (C) | 0.170% |
| Silicon (Si) | 0.100% |
| Manganese (Mn) | 0.900% |
| Phosphorus (P) | 0.035% |
| Sulfur (S) | 0.035% |
| Aluminum (Al) | 0.015% |
| Titanium (Ti) | 0.020% |
| Copper (Cu) | 0.350% |
| Chromium (Cr) | 0.200% |
| Nickel (Ni) | 0.400% |
| Molybdenum (Mo) | 0.080% |
| Niobium (Nb) | 0.020% |
| Vanadium (V) | 0.050% |
| Parameter | Thessaloniki Seawater | Heraklion Seawater | Artificial Seawater (ASTM D1141) |
|---|---|---|---|
| Salinity (‰) | 37.0–39.0 | 38.5–39.5 | 35.0 |
| Temperature (°C) | 12–28 | 15–27 | Controlled to ~27 |
| pH | 7.9–8.3 | 8.0–8.4 | 8.1 |
| Major ions (mg/L) | |||
| Sodium (Na+) | 10,500–11,000 | 11,000–11,200 | 10,800 |
| Chloride (Cl−) | 19,000–20,000 | 19,500–20,500 | 19,350 |
| Sulfate (SO42−) | ~2500–2700 | ~2600–2800 | 2700 |
| Magnesium (Mg2+) | 1300–1400 | 1350–1450 | 1290 |
| Calcium (Ca2+) | 400–450 | 420–460 | 410 |
| Potassium (K+) | 380–400 | 390–410 | 380 |
| Trace metals (µg/L) | Elevated Cu, Zn, Pb due to pollution | Lower trace metal levels | Minimal to none |
| Nutrients | Higher nutrient (N, P) concentrations | Lower nutrient concentrations | Not typically present |
| Immersion Time (Months) | Ecorr (V) | Ιcorr (μA cm−2) | CR (mm/Year) | Mass Loss (Grams) | η (%) |
|---|---|---|---|---|---|
| uncoated | −0.697 | 2.428 | 1.41 × 10−2 | 9.20 × 10−4 (±0.32) | - |
| 1 | −0.360 | 0.017 | 9.89 × 10−5 | 6.47 × 10−5 (±0.42) | 99.30% |
| 6 | −0.481 | 0.073 | 4.25 × 10−4 | 1.67 × 10−4 (±0.31) | 96.99% |
| 12 | −0.512 | 0.077 | 4.48 × 10−4 | 3.52 × 10−4 (±0.30) | 96.83% |
| 24 | −0.637 | 0.141 | 8.20 × 10−4 | 7.07 × 10−4 (±0.31) | 94.19% |
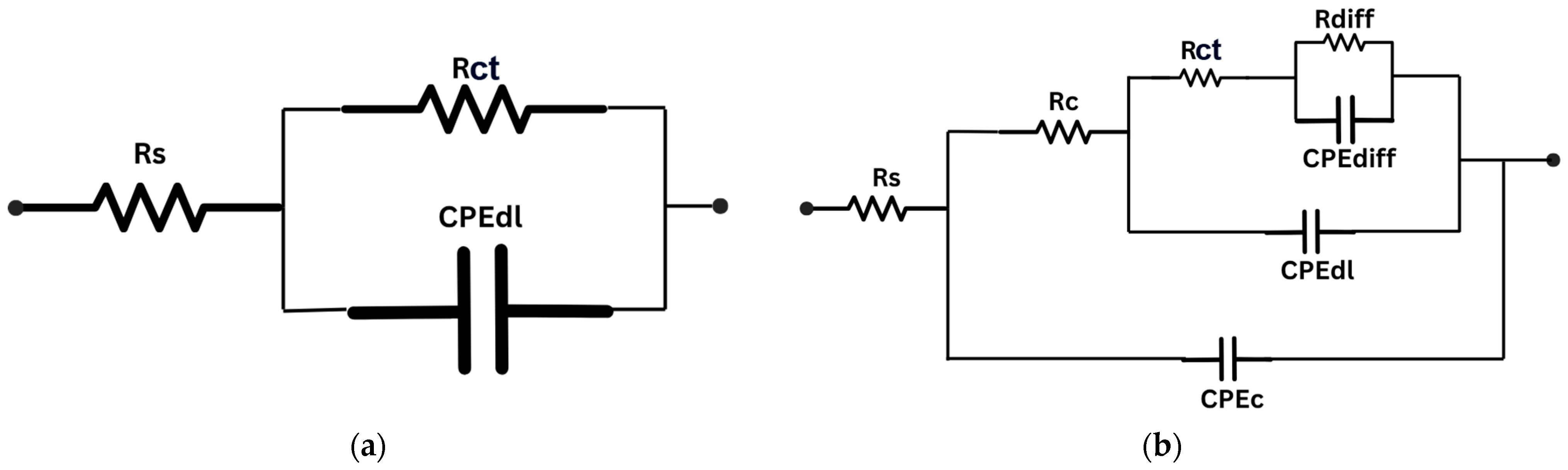
| Immersion Time (Months) | Rs (Ω) | CPEdl (F × cm−2) | ndl | Rct | CPEc (F × cm−2) | nc | Rc (Ω × cm2) | CPEdiff (F × cm−2) | ndiff | Rdiff (Ω × cm2) | η (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| uncoated | 50 | 1.70 × 10−3 | 0.80 | 1143 | |||||||
| 1 | 162 | 6.40 × 10−7 | 0.45 | 6.88 × 104 | 1.16 × 10−8 | 0.75 | 6140 | 8.80 × 10−6 | 0.45 | 3.80 × 105 | 98.34 |
| 6 | 200 | 2.40 × 10−7 | 0.85 | 4.58 × 104 | 9.70 × 10−7 | 0.43 | 5030 | 4.60 × 10−5 | 0.40 | 1.10 × 105 | 97.50 |
| 12 | 191 | 6.40 × 10−7 | 0.61 | 3.80 × 104 | 4.85 × 10−7 | 0.70 | 3710 | 2.00 × 10−6 | 0.80 | 5.70 × 104 | 96.99 |
| 24 | 157 | 6.61 × 10−6 | 0.80 | 1.78 × 104 | 6.70 × 10−9 | 0.80 | 3410 | 4.70 × 10−6 | 0.60 | 1.60 × 105 | 93.58 |
| Uncoated Sample | Coated Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locations | Fouling Organisms | 0 | 1 m | 6 m | 12 m | 24 m | 0 | 1 m | 6 m | 12 m | 24 m | |
| Thessaloniki | Micro-fouling | Biofilm | Χ | √ | √ | √ | √ | Χ | Χ | √ | √ | √ |
| Fungi and Protozoa | Χ | √ | √ | √ | √ | Χ | Χ | √ | √ | √ | ||
| Ultra spores | Χ | √ | √ | √ | √ | Χ | Χ | Χ | √ | √ | ||
| Brown algae | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Multicellular algae | Χ | Χ | √ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Tunicate | Χ | √ | √ | √ | √ | Χ | Χ | Χ | Χ | √ | ||
| Macro-fouling | Macroalgae | Χ | √ | √ | √ | √ | Χ | Χ | Χ | Χ | √ | |
| Bryozoans | Χ | √ | √ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Solitary anemones | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Hydrates | Χ | Χ | √ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Tubeworms | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Sedentary polychaetes | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Ascidians | Χ | Χ | √ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | ||
| Sponges | Χ | Χ | √ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Mussels | Χ | Χ | Χ | Χ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Oysters | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Barnacles | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Uncoated Sample | Coated Sample | |||||||||||
| Locations | Fouling Organisms | 0 | 1 m | 6 m | 12 m | 24 m | 0 | 1 m | 6 m | 12 m | 24 m | |
| Heraklion | Micro-fouling | Biofilm | Χ | √ | √ | √ | √ | Χ | Χ | √ | √ | √ |
| Fungi and Protozoa | Χ | √ | √ | √ | √ | Χ | Χ | √ | √ | √ | ||
| Ultra spores | Χ | √ | √ | √ | √ | Χ | Χ | Χ | √ | √ | ||
| Brown algae | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | √ | ||
| Multicellular algae | Χ | Χ | √ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Tunicate | Χ | √ | √ | √ | √ | Χ | Χ | Χ | Χ | √ | ||
| Macro-fouling | Macroalgae | Χ | √ | √ | √ | √ | Χ | Χ | Χ | Χ | √ | |
| Bryozoans | Χ | √ | √ | √ | √ | Χ | Χ | Χ | Χ | √ | ||
| Solitary anemones | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Hydrates | Χ | Χ | √ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Tubeworms | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Sedentary polychaetes | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Ascidians | Χ | Χ | √ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | ||
| Sponges | Χ | Χ | √ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Mussels | Χ | Χ | Χ | Χ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Oysters | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Barnacles | Χ | Χ | Χ | √ | √ | Χ | Χ | Χ | Χ | Χ | ||
| Coating Type | Antifouling Mechanism | Corrosion Protection | Environmental Profile | Durability | Typical η (%) (2 Years) | Ref. |
|---|---|---|---|---|---|---|
| Biocide-Based (Cu, Zn, organotin) | Toxic leaching and biocidal action | Moderate | Toxic; leaching restrictions | Moderate–short-lived | 70–85 | [22] |
| Silicone Foul-Release | Low adhesion/friction | Low–moderate | Non-toxic, but microplastics risk | Moderate | 80–88 | [19] |
| Biomimetic/Bio-derived | Surface topography, natural extracts | Low–moderate | Fully green, biodegradable | Low–moderate | 70–90 | [10] |
| Polymeric Nanocomposite | Barrier, redox, photocatalytic, etc. | High | Non-toxic, no leaching, robust | High | 93–99 | [6], (this study) |
| Coating/Trade Name | Main Biocidal Composition | Key Performance Metrics | Environmental Profile | Typical Longevity |
|---|---|---|---|---|
| International Intersmooth 7465HS SPC | Copper oxide + ZnO + triazine herbicide | A total of 70–90% fouling protection efficiency (12–18 mo); increases fuel efficiency by ~6% | Moderate to high toxicity (Cu, Zn leaching, organics) | 12–18 months |
| Jotun Sea Quantum Ultra | Copper oxide + zinc pyrithione | A total of 75–93% protection (1 yr, various routes); 7.5 g/m2/day Cu release | Cu leaching restricted in many ports | 24 months |
| Hempel Hempasil X3 (Foul-release) | Siloxane (biocide-free, control) | A total of 60–75% protection (macro-fouling); needs silicone topcoat | Biocide-free, microplastics risk | 18–24 months |
| Selektope™-containing paints (e.g., Chugoku SEA PREMIA) | Copper oxide + Selektope™ (Medetomidine) | A total of 70–90% (per barnacle settlement tests); up to 80% lower Cu leaching | Reduced copper load (but not zero) | 12–20 months |
| International Intercept 8500LPP | Copper oxide + rosin, self-polishing copolymer | A total of 60–80% biocidal efficiency (in tropical trials); Tin-free | Cu leaching, organics; lower than organotin | 12 months |
| Tributyltin (TBT)-based coatings (historic) | Tributyltin oxide, Cu2O | A total of >95% (up to 5 years, now banned); extremely effective | Extremely toxic; banned for global use | 60+ months (banned) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vourna, P.; Falara, P.P.; Papadopoulos, N.D. In Situ and Laboratory Investigation of the Anti-Corrosion and Anti-Fouling Efficacy of an Innovative Biocide-Free Coating for Naval Steels. Metals 2025, 15, 1000. https://doi.org/10.3390/met15091000
Vourna P, Falara PP, Papadopoulos ND. In Situ and Laboratory Investigation of the Anti-Corrosion and Anti-Fouling Efficacy of an Innovative Biocide-Free Coating for Naval Steels. Metals. 2025; 15(9):1000. https://doi.org/10.3390/met15091000
Chicago/Turabian StyleVourna, Polyxeni, Pinelopi P. Falara, and Nikolaos D. Papadopoulos. 2025. "In Situ and Laboratory Investigation of the Anti-Corrosion and Anti-Fouling Efficacy of an Innovative Biocide-Free Coating for Naval Steels" Metals 15, no. 9: 1000. https://doi.org/10.3390/met15091000
APA StyleVourna, P., Falara, P. P., & Papadopoulos, N. D. (2025). In Situ and Laboratory Investigation of the Anti-Corrosion and Anti-Fouling Efficacy of an Innovative Biocide-Free Coating for Naval Steels. Metals, 15(9), 1000. https://doi.org/10.3390/met15091000







