Abstract
In this study, the morphology of high-temperature surface oxides in Ni-based superalloys was investigated as a function of Cr and Al content. First, the oxide layer morphologies of various alloys published in the literature were classified according to the basic model of Cr and Al content. Then, the high-temperature oxide layers of high-Cr/low-Al and low-Cr/high-Al alloys were investigated using SEM/EDX mapping. The high-temperature oxidation of Ni-based superalloys is affected by the amount of added elements such as Ti, Cr, and Al. The oxidation rate of each element is NiO > TiO2 > Cr2O3 > Al2O3, and the shape of the surface oxide layer is determined by the oxidation rate. The oxide layer is mainly classified according to the flat and fibrous shapes of Cr2O3 and Al2O3 on the outermost layer. In this study, IN738LC and IN792, as the high Cr-low Al alloys, were found to have flat Cr2O3 and fibrous type Al2O3 in the outermost layer after long-term exposure to high temperatures, while CM247LC and CMSX4 which are the low Cr-high Al alloys were found to have flat type Al2O3 in the outermost layer after long-term exposure to high temperatures, which is in good agreement with the results of various literature on oxide layers on Ni-based superalloys.
1. Introduction
Ni-based superalloys used in turbine blades under high temperature and pressure conditions are usually designed with an emphasis on creep strength and oxidation/corrosion resistance. Among the alloying elements in Ni-based superalloys, Ni, Cr, Al, and Ti are the main oxide-forming elements, and it has been reported that Co, W, Mo, Ta, and Si also affect the oxidation of the alloy [,]. Additionally, Hf, Re, and Fe are also added to enhance creep resistance in certain Ni-based alloys [,]. However, in order to simply and clearly understand the high-temperature oxidation behavior of Ni-based superalloys, it is necessary to analyze the effects of the main components of the alloy such as Ni, Cr, Al, and Ti.
Among the Ni-based superalloy components, the scale growth rates of Ni/Cr/Al and Ti/Co oxides are summarized in schematic figures presented in the literature [,]. The figures are schematic diagrams summarizing the oxide formation rates of each component depending on the temperature, and it has been confirmed that the oxidation rate of Ni/Cr/Al, as the main component of Ni-based superalloys, is “NiO > Cr2O3 > Al2O3”.
In Ni-Cr-Al ternary alloys, the shape of the high-temperature oxide layer changes depending on the content of each component, temperature, and holding time. Giggins and Pettit classified these oxide layer shapes into three groups as shown in Figure 1 []. These are: ① Group I (GI): NiO (outer layer)/(Al, Cr) oxide (inner layer), ② Group II (GII): Cr2O3 (outer layer)/Al2O3 (fibrous inner layer), and ③ Group III(GIII): Al2O3 (single layer). The change in the oxide layer shape of groups I, II, and III is interpreted as being due to the difference in the oxidation rate of NiO, Cr2O3, and Al2O3 depending on the amount of Ni, Cr, and Al components in the alloys.
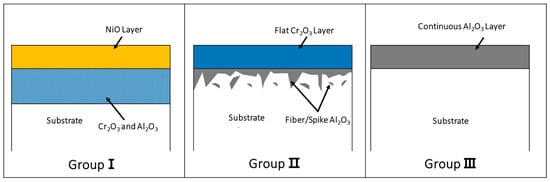
Figure 1.
Schematic diagram for the classification of oxide layers in Ni-Cr-Al ternary alloys: Group I, Group II, Group III [].
In this study, the high-temperature creep and surface oxidation phenomena in four kinds of Ni-based superalloys with different Cr, Al, and Ti contents were examined. The materials used in this study and the representative component contents affecting oxidation are IN738LC (16Cr-3Al-4Ti), IN792 (14Cr-4Al-5Ti), CMSX-4 (6Cr-6Al-1Ti), and CM247LC (8Cr-6Al-1Ti). Among these alloys, IN738LC and IN792 are the alloys with high-Cr and low-Al content, while CMSX-4 and CM247LC are characterized by low-Cr and high-Al contents. In this study, the oxide layer formed on the surface after high-temperature creep tests of these alloys was classified into variables such as creep temperature and time, and analyzed using SEM/EDS and mapping. Since the publication of the paper by Giggins and Pettit, most of the research on the high-temperature oxidation of nickel-based superalloys has been limited to individual alloys, and the high-temperature oxidation behaviors of various recently developed nickel-based superalloys have not been comprehensively discussed. Therefore, this study aims to summarize the high-temperature oxidation behaviors of various recently developed nickel-based superalloys according to their Cr/Al composition in order to identify and verify a common pattern. This is expected to contribute to the improvement of the high-temperature properties of nickel-based superalloys.
2. Experimental Method
In Ni-based superalloys, which are turbine blade materials, Ni, Ti, Cr, and Al affect high-temperature oxidation/corrosion. Among them, Ni and Ti have a high oxidation rate and low oxygen affinity, which leads to poor surface adhesion. Therefore, the morphology of high-temperature oxidation layers in superalloys tends to be determined by mainly Cr and Al contents. In Figure 1, Group II and Group III are classified into high-Cr/low-Al and low-Cr/high-Al alloy compositions, respectively. In this study, 26 high-temperature oxidation layers were classified by the contents of Cr and Al composition with reference to 17 literatures.
In order to verify whether the classification system of high-temperature oxide layer morphology for high-Cr/low-Al and low-Cr/high-Al alloys is appropriate, as shown in Table 1 and Table 2, four types of Ni-based superalloys were subjected to high-temperature oxidation under two different temperature/exposure time conditions for each alloy. The morphology of the oxide layers was analyzed using FE-SEM (field emission scanning electron microscopy, JEOL, Akishima, Tokyo, JP/JSM-7500F), and mapping analysis was performed using EDS (energy dispersive spectroscopy) attached to the FE-SEM to analyze the distribution of major elements. From the table, it can be seen that the alloys are “IN738LC and IN792” representing high-Cr/low-Al, and “CM247LC and CMSX-4” representing low-Cr/high-Al, respectively,

Table 1.
Four kinds of Ni-based superalloys with different Cr, Al, and Ti contents in wt%.

Table 2.
Two different temperature/exposure time conditions for each Ni-based superalloy.
3. Results
Results of studies on high-temperature oxidation of Ni-based superalloys are presented, including 13 GII (high Cr/low Al) and 13 GIII (low Cr/high Al) models cited in the literature [,,,,,,,,,,,,,,,,]. Results are also presented for the four alloys analyzed in this study (2 alloys of GII and 2 alloys of GIII). The oxide layers of all alloys analyzed in this study and the cited alloys were formed by high-temperature creep conditions. Each result in this study includes creep temperature and life, but does not include creep stress. That is, the effect of stress on the formation of high-temperature oxide layers is excluded and a stress-independent grip of the creep specimens is considered to be analyzed.
3.1. Classification of Group II and Group III Models
The oxidation conditions and oxide layer morphology of alloys presented as Group II (GII) in the literature surveyed are shown in Figure 2. The alloys in the GII category in the figure have high Cr (12Cr or more) and low Al (3Al or less). The surface oxide layer of the GII model in the figure has a relatively dense outermost layer of Cr2O3 and fibrous Al2O3 inside, and this oxide layer is designated as “Flat Cr2O3 + Fiber/spike Al2O3” [,,,,,,,,,].
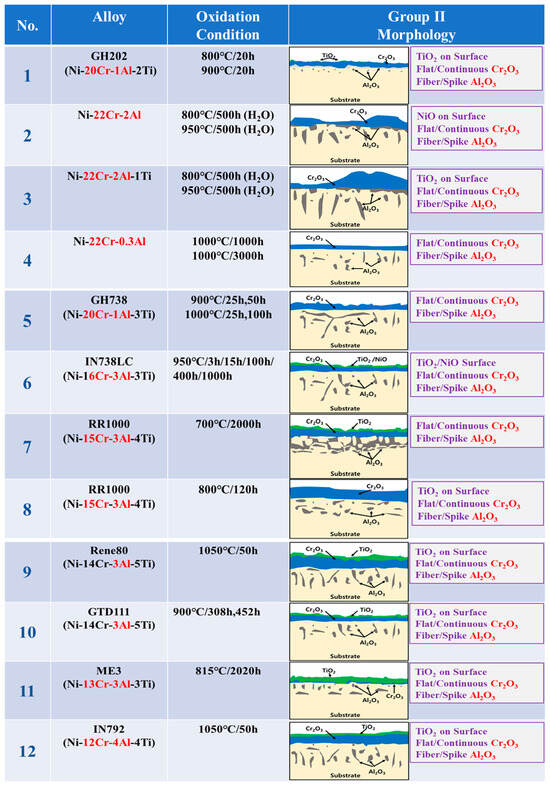
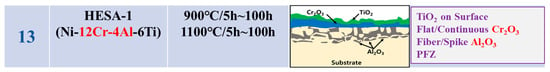
Figure 2.
Morphologies of high-temperature surface oxide layer in various Ni-based superalloys with high-Cr/low-Al composition corresponding to the GII model (1: [], 2: [], 3: [], 4: [], 5: [], 6: [], 7: [], 8: [], 9: [], 10: [], 11: [], 12: [], 13: []).
This means that in high Cr alloys, the oxidation rate of Cr is faster than that of Al; so, Cr is oxidized first, and a relatively dense flat Cr2O3 is formed on the outer surface layer. Then Al2O3 is formed under Cr2O3 by Al diffusion, which starts from a thin flat shape in Figure 2 and finally grows into fibrous Al2O3 as in the GII model. This is speculated to be because, in low-Al alloys, the first Al diffuses thinly to the surface with a low Al diffusion rate, which limits the subsequent Al content and forms fibrous oxides. We can see that all other high-Cr/low-Al alloys in the figure are generally composed of a “Flat Cr2O3 + Fiber/spike Al2O3” surface oxide layer.
The oxidation conditions and oxide layer morphology of the high-temperature surface oxide layer of alloys proposed as group III model (GIII) investigated in the literature are shown in Figure 3. The alloys corresponding to the GIII model are low-Cr (8Cr or less) and high-Al (5Al or more) [,,,,,,]. The surface oxide layer of the GIII model is composed of relatively dense and continuously formed Al2O3 inside after the initial oxidized NiO/Cr2O3 is removed. The oxide layer is designated as “Continuous Al2O3” except for NiO/Cr2O3 which may be formed in the outermost layer. This means that most of the alloys shown in the figure consist of the continuous Al2O3 in the outer layer, in rare cases the NiO/Cr2O3 remains on the outer surface.
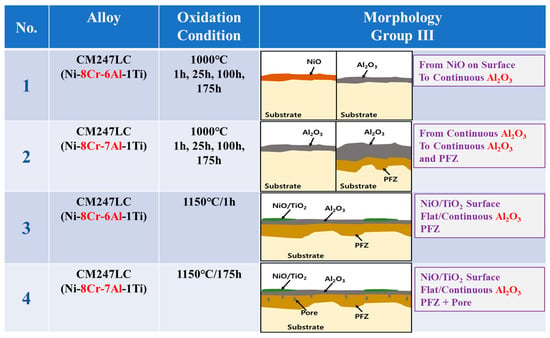
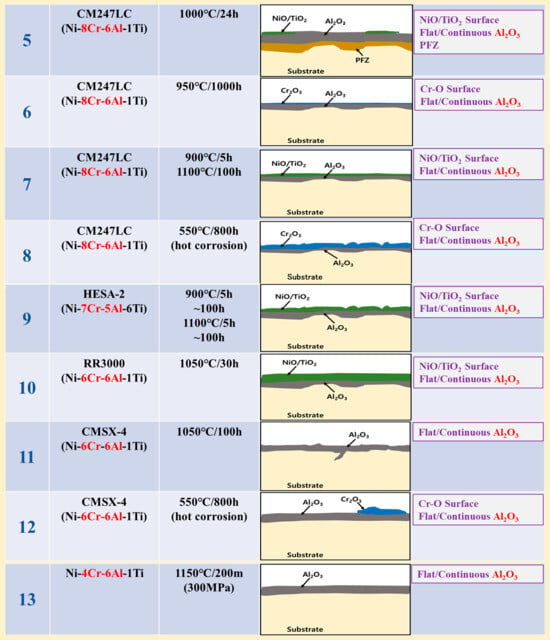
Figure 3.
Morphologies of high-temperature surface oxide layer in various Ni-based superalloys with low-Cr/high-Al composition corresponding to the GIII model (1: [], 2: [], 3: [], 4: [], 5: [], 6: [], 7: [], 8: [], 9: [], 10: [], 11: [], 12: [], 13: []).
This is because the low-Cr content alloys have a limited oxidation rate, making it difficult to form a sufficient, stable, and dense Cr2O3 oxide layer on the surface. As the high-temperature oxidation time progresses, not only the NiO but also the Cr2O3 on the surface is lost. This is evidenced by the fact that NiO/Cr2O3 remains in some alloys of the figure. On the other hand, in high-Al content alloys, the oxidation rate of Al is slow but the content is sufficient so that stable and dense Al2O3 of the GIII model is formed on the surface instead of Cr and Ni oxides during high-temperature oxidation. As a result, It can be seen that all alloys containing low-Cr and high-Al components have a common “Continuous Al2O3” as a surface oxide layer.
3.2. IN738LC and IN792 for Group II Model
In this study, we analyzed the surface oxidation layers of high-Cr/low-Al alloys such as IN738LC (16Cr-3Al) and IN792 (14Cr-4Al) after high-temperature creep tests. Figure 4 shows the surface oxidation layers of IN738LC exposed to 950 °C during 5 h to 157 h. In Figure 4a, unlike the GII model, the outermost layer is Cr2O3 and the inner part is smooth Al2O3. Whereas in Figure 4b, after long-term exposure during 157 h, the outermost layer is Cr2O3 and the inner part is fibrous Al2O3, which is consistent with the GII model of Figure 1. That is, the high-temperature oxidation of high-Cr/low-Al alloys progresses from the initial flat/smooth Al2O3 to the later fibrous Al2O3 inside flat Cr2O3 as in the GII model. It is also evident that the initially oxidized TiO2 remains on the outside of the flat Cr2O3.
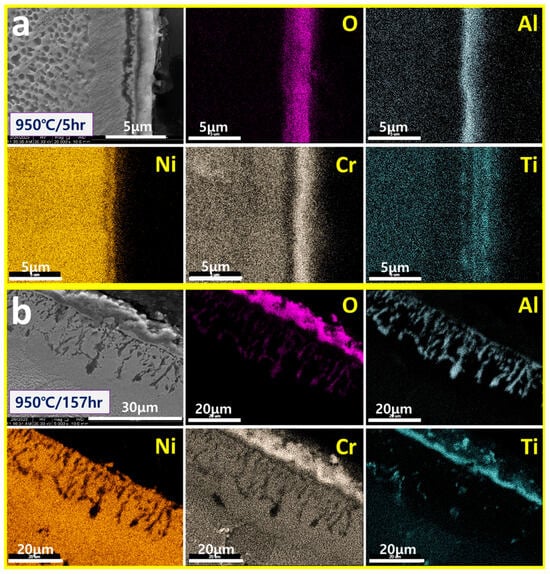
Figure 4.
SEM/Mapping of surface oxidation layers exposed to (a) 950 °C/5 h and (b) 950 °C/157 h of IN738LC.
As shown in the figure, it was confirmed that the high-temperature oxidation of IN738LC (16Cr-3Al), a high Cr/low Al alloy, progresses in the following order according to the degree of high-temperature exposure time: (i) surface Cr/Ti oxide layer + inner flat/smooth Al2O3, (ii) surface Cr/Ti oxide layer and inner fiber/spike Al2O3. The final high-temperature oxidation morphology of IN738LC was confirmed to be consistent with the GII model.
Figure 5 shows the surface oxide layer of IN792 after 5 h and 328 h of exposure at 950 °C. It can be seen that this is “flat Cr2O3 + fiber/spike Al2O3” which is consistent with the GII model. It also shows the TiO2 remaining on the outside of the Cr2O3 before oxidation, similar to the IN738LC oxide layer as shown in Figure 4a.
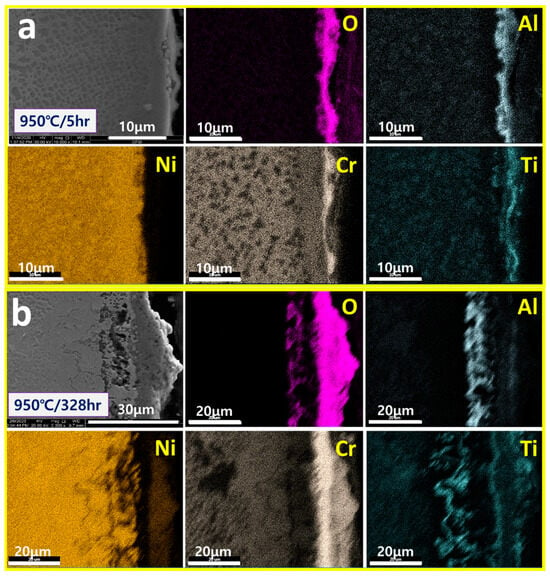
Figure 5.
SEM/Mapping of surface oxidation layers exposed to (a) 950 °C/5 h and (b) 950 °C/328 h of IN792.
3.3. CM247LC and CMSX4 of Group III Model
In this study, the surface oxide layers of low Cr/high Al alloys CM247LC (8Cr-6Al) and CMSX4 (6Cr-6Al) after high-temperature creep tests were analyzed. Figure 6 shows the surface oxide layers of CM247LC exposed to high temperatures (880 °C and 982 °C) for 166 to 209 h. Figure 6a shows the surface oxide layer after high-temperature exposure at 880 °C for 166 h. The oxide layer is mainly composed of Al2O3, which is similar to the GIII model classified above, but the Al2O3 in the figure has irregularities. Also, some Cr/Ti oxide remains on the surface. Figure 6b shows the surface oxide layer after exposure to 982 °C during 209 h, which is higher and longer than the 880 °C/166 h in Figure 6a. The oxide layer is composed of continuous Al2O3, which is consistent with the GIII model. From this, the high-temperature oxidation of CM247LC is interpreted as the initial irregular Al2O3 developing into a continuous Al2O3 stage with sufficient Al diffusion, while there is almost no Cr/Ti oxide on the surface. In Figure 6, it is observed that the Cr2O3 oxide layer remains on the outside of the Al2O3 layer. In Figure 6a, it is observed that at a high-temperature exposure of 880 °C/166 h, a Cr-depleted layer is formed due to the surface diffusion of Cr just below the surface oxide layer and the formation of Cr2O3. However, in Figure 6b, it is confirmed that a Cr-accumulated layer is formed due to the surface diffusion of Cr just below the inner layer.
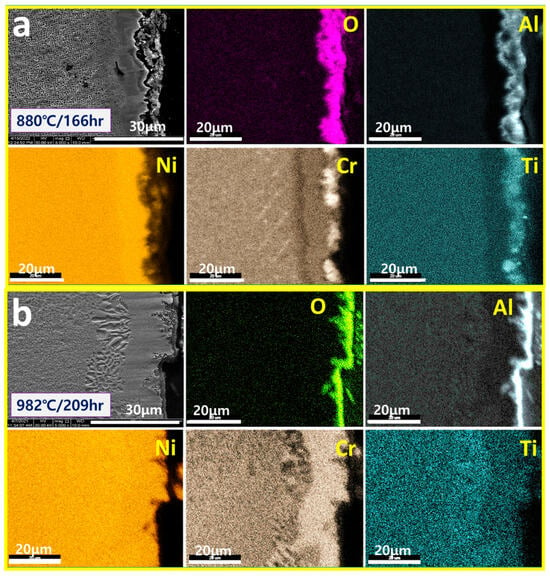
Figure 6.
SEM/mapping of surface oxidation layers exposed to (a) 880 °C/166 h and (b) 982 °C/209 h of CM247LC.
As shown in Figure 6, the high-temperature oxidation of the low Cr/high Al alloy CM247LC (8Cr-6Al) was confirmed to progress in the following order depending on the duration of high-temperature exposure: (i) surface Cr/Ti oxide layer + inner irregular Al2O3, (ii) inner continuous Al2O3 with remained some surface Cr/Ti oxide layer. The final high-temperature oxidation morphology is consistent with the GIII model. That is, the initial irregular surface Cr/Ti oxide layer of CM247LC is removed by high-temperature exposure, and only inner continuous Al2O3 remains. Then, the initial inner irregular/unstable Al2O3 is stabilized to continuous Al2O3 by high-temperature exposure. In addition, the Cr content is depleted initially due to the formation of Cr2O3, as shown in Figure 6a, and Cr inside the alloy is diffused and accumulated in the inner layer by high-temperature exposure, as shown in Figure 6b.
Figure 7 shows the surface oxide layers formed at creep conditions of 930 °C/60 h and 982 °C/38 h in CMSX4. Figure 7a shows the surface oxide layers formed at high temperatures of 930 °C/60 h. The oxide layers at relatively low temperatures in the figure are mainly composed of Al2O3, which is similar to the fibrous/spiked Al2O3 of the GII model classified above. This indicates that in the low-Cr/high-Al alloys, the formation of “fibrous/spiked Al2O3” may be induced due to the limited diffusion of Al, even with long exposure, at relatively low temperatures.
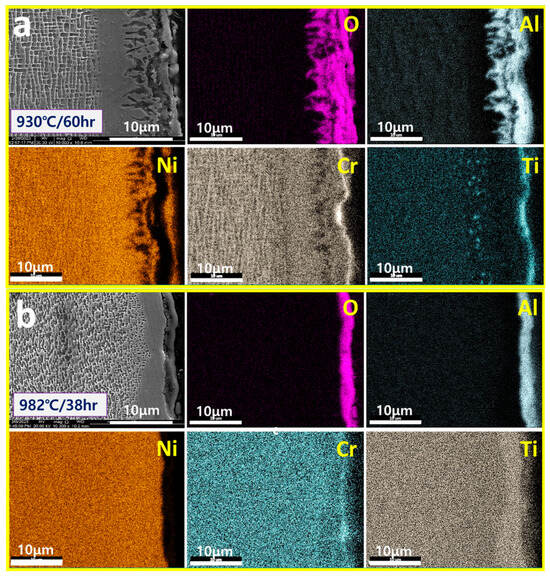
Figure 7.
SEM/mapping of surface oxidation layers exposed to (a) 930 °C/60 h and (b) 982 °C/38 h of CMSX4.
Figure 7b shows the surface oxidation layer after high-temperature exposure of 982 °C/38 h. As the alloy is exposed at relatively high temperatures in the figure, the oxidation layer becomes the “continuous Al2O3” of the GIII model previously classified. This indicates that in the low-Cr/high-Al alloys, the formation of “continuous Al2O3” can be induced due to the high content Al diffusion, even with short time exposure, at relatively high temperatures. From this, the high-temperature oxidation of CMSX-4 is interpreted as the initial irregular “fiber/spike Al2O3” evolving into the continuous Al2O3 stage with sufficient Al diffusion. As shown in the figure, outside the main Al2O3, Cr2O3, and TiO2 remaining due to the preferential oxidation of Cr/Ti are observed. In addition, it is observed that a Cr/Ti depleted layer is formed just below some of the surface oxide layers due to the surface diffusion of Cr/Ti and the formation of Cr2O3/TiO2.
It was confirmed that the high-temperature oxidation of CMSX4 (6Cr-6Al) as a low-Cr/high-Al alloy, proceeds in the following order depending on the degree (temperature and time) of high-temperature exposure: (i) surface Cr/Ti oxide layer + inner fibrous/spike and irregular Al2O3, (ii) inner continuous Al2O3 with remained some surface Cr/Ti oxide layer. Its final high-temperature oxidation morphology is consistent with the GIII model. That is, the initial irregular surface Cr/Ti oxide layer of the alloys is removed by high-temperature exposure, and only inner continuous Al2O3 remains. And the initial inner fibrous/spike and irregular/unstable Al2O3 are stabilized to continuous Al2O3 by high-temperature exposure. Also, the Cr content is depleted internally due to the formation of Cr2O3, as shown in Figure 7a. Also, as continuous Al2O3 forms, only Al is selectively oxidized, and Cr is no longer oxidized. As a result, due to the concentration difference of Cr in the inner layer and high-temperature exposure, Cr diffuses and accumulates, as shown in Figure 7b.
4. Discussion
This study analyzed the high-temperature oxidation behavior of Ni-based superalloys based on the classification of high-Cr/low-Al (GII) and low-Cr/high-Al (GIII) models. A total of 13 GII and 13GIII models from the literature were reviewed, along with experimental results from four Ni-based superalloys (IN738LC, IN792, CM247LC, and CMSX4) tested under high-temperature creep conditions.
- (1)
- Influence of Cr and Al on oxide layer formation
GII (high Cr/low Al) alloys exhibited a dense Cr2O3 outer layer and fibrous Al2O3 inner layer, confirming that Cr oxidizes preferentially, forming a protective Cr2O3 scale, while Al2O3 develops underneath due to limited Al diffusion. GIII (low Cr/high Al) alloys predominantly formed a continuous Al2O3 layer, replacing the initially formed NiO/Cr2O3, as Cr2O3 was unstable and depleted over time. The classification of high Cr and high Al is generally based on the threshold values of approximately 12% Cr and 5% Al, distinguishing oxidation behavior in Ni-based superalloys.
These findings indicate that Cr dictates the initial oxidation kinetics, while Al determines long-term oxidation stability.
- (2)
- Experimental validation of the GII/GIII models
IN738LC and IN792 (GII alloys) initially showed smooth Al2O3 under Cr2O3 but transitioned to fibrous Al2O3 after prolonged exposure, consistent with the GII oxidation mechanism. CM247LC and CMSX4 (GIII alloys) transitioned from irregular Al2O3 with residual Cr2O3 to fully continuous Al2O3, validating the GIII oxidation model.
- (3)
- Cr diffusion behavior of high-temperature oxide layer in IN738LC as high-Cr/low-Al alloy
Cr diffusion induced in the surface oxidation layer of high-Cr/low-Al alloy changes according to the degree of high-temperature exposure time. As shown in Figure 8a, in the early high-temperature oxidation, a Cr oxide layer is formed on the surface due to the rapid oxidation of Cr, and a Cr-depleted layer due to Cr diffusion can be seen beneath the oxide layer, and it is confirmed that smooth Al2O3 is formed inside. As shown in Figure 8b, in the middle high-temperature oxidation, as Cr oxidation continues, the formation of the Cr oxide layer on the surface increases to a thickness of about 10 μm, and a Cr-depleted layer due to Cr diffusion can still be seen inside, and the Al2O3 inside changes to an irregular shape. As shown in Figure 8c, in the subsequent high-temperature oxidation, the increase of the Cr oxide layer on the surface stops due to limited oxidation of Cr, and as a result, the Cr-depleted layer inside disappears. And then, the Al2O3 inside changes to a fibrous/spiked shape due to the low-Al content, and finally forms an oxide layer of the GII model.
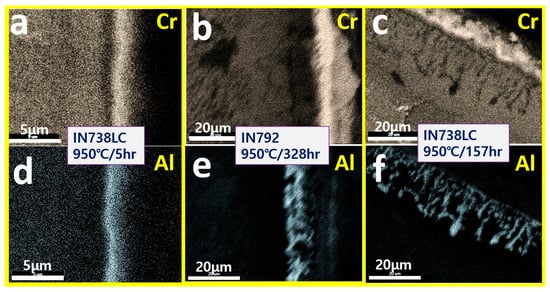
Figure 8.
SEM/mapping of surface oxidation layers exposed to (a,d) 950 °C/5 h of IN738LC, (b,e) 950 °C/328 h of IN792, and (c,f) 950 °C/157 h of IN738LC.
During high-temperature exposure, the IN738LC (16Cr-3Al) and IN792 (14Cr-4Al) as high Cr/low Al alloys show behaviors of Cr accumulation and depletion by inner diffusion in addition to surface oxidation. Figure 9 shows the analysis of the blade surface of each material after use in a high-temperature turbine, referencing Han’s previous paper []. The figure confirms that Cr accumulation is observed in all surface-coated blades, and that all alloys form high Cr content TCP phases.

Figure 9.
SEM image of the cross-section of the oxide layer on the surface of the used blades (a) IN738LC, (b) IN792, and (c) Cr mapping of (b).
- (4)
- Cr diffusion behavior of high-temperature oxidation layer in CM247LC as a low-Cr/high-Al alloy
When low-Cr/high Al alloy CM247LC (8Cr-6Al) is exposed to high temperatures, Cr shows behaviors of accumulation and depletion by inner diffusion along with surface oxidation. Figure 10 shows the surface oxidation of CM247LC exposed to 982 °C for 209 h and includes extracted Cr and Al results from the mapping analysis, referencing Choe’s previous paper []. In the figure, it can be seen that the surface oxidation layer is composed of a single layer of Al2O3, and the Cr/Ti oxide layer is spalled off. It can be seen that a Cr accumulation layer of about 20 μm thickness is formed beneath the oxide layer, accompanied by a depletion of Cr underneath. In the figure, the substrate has a typical γ/γ′ structure, but the Cr-accumulated layer and the Cr-depleted layer are pure γ and coarse γ′ structures, respectively. In the Cr-accumulated layer, γ′ precipitation is suppressed and pure γ is formed, whereas in the Cr-depleted layer, γ′ precipitation is promoted and coarse γ′ is formed.

Figure 10.
SEM/mapping of surface oxidation layers exposed to 982 °C/209 h (a) SEM, (b) Cr mapping, and (c) Al mapping.
5. Conclusions
The high-temperature oxide layer morphology of these Ni-Cr-Al superalloys is classified according to the Cr and Al content as follows:
- (1)
- The high-temperature oxide layer of Ni-based superalloys is divided into fibrous Al oxide of the Group II model and flat-shaped Al oxide of the Group III model according to the Cr and Al content. The high-Cr/low-Al alloys such as IN738LC and IN792 are classified into the Group II model, and the low-Cr/high-Al alloys such as CM247LC and CMSX4 are classified into the Group III model.
- (2)
- According to the classification and analysis of this study, the standards for the Cr and Al content are estimated to be approximately 12% and 5%, respectively.
- (3)
- Based on this standard, it is confirmed that for high Cr/low Al alloys such as IN738LC (16Cr-3Al) and IN792 (14Cr-4Al), the high Cr surface Cr oxide layer is formed flat, thick, and stable, and the inner Al oxide layer is group II due to the low-Al content, which is composed of fibrous shape.
- (4)
- For low Cr/high Al alloys such as CM247LC (8Cr-6Al) and CMSX4 (6Cr-6Al), it is confirmed that Group III is made by forming a thin or peeled Cr oxide layer due to the low Cr content, and forming a flat, thick, and stable inner Al oxide layer due to the high Al content.
- (5)
- In high-Cr/low-Al alloys such as IN738LC and IN792, Cr diffuses to the surface, but its content is sufficient to form only an accumulated layer without a depletion layer inside. And due to the high Cr content of the Cr accumulated layer, there is a tendency for the high-Cr TCP σ phase to precipitate upon exposure to high temperatures.
- (6)
- In low Cr/high Al alloys such as CM247LC and CMSX-4, Cr diffused to the surface to form Cr2O3, but its content is not sufficient to form a Cr accumulated layer and a Cr depletion layer exists in the inner layer. As a result, a pure γ microstructure is formed in the Cr accumulated layer and a coarse γ’ microstructure is formed in the Cr depletion layer.
Author Contributions
K.C.: Writing—original draft, conceptualization, formal analysis, investigation, validation, visualization, B.C.: writing—review and editing, visualization, methodology, investigation, S.H.: writing—review and editing, visualization, methodology, investigation, D.K.: writing—review and editing, visualization, methodology, investigation, S.-M.S.: writing—review and editing, visualization, methodology, investigation, D.W.Y.: writing—review and editing, visualization, methodology, investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “Power Generation & Electricity Delivery (No. 20193310100090)” of the Korea Institute of Energy Technology Evaluation and Planning from the Ministry of Trade, Industry and Energy, Republic of Korea. This research was supported by “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2022RIS-005).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pettit, F.S.; Meier, G.H. Oxidation and Hot Corrosion of Superalloys; The Minerals, Metals & Materials Society: Pittsburgh, UK, 1984; pp. 651–687. [Google Scholar]
- Smialek, J.; Meier, G.M. High Temperature Oxidation in Superalloy, Superalloy Ⅱ; A Wiley-Interscience publication: Hoboken, NJ, USA, 1987; pp. 293–326. [Google Scholar]
- Saunders, N. Phasediagram Calculationsforni-Uased Superalloys; The Minerals, Metals & Materials Society: Pittsburgh, UK, 1996; pp. 101–110. [Google Scholar]
- Yadav, P.; Abro, M.A.; Lee, D.B.; Yoon, J.H. High-temperature corrosion of pure Ni3Al and its alloyed (2.99 wt.%Ti) inAr-0.2%SO2 gas environment, JMR&T 17. J. Mater. Res. Technol. 2022, 17, 3055–3065. [Google Scholar] [CrossRef]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al Alloys Between 1000° and 1200 °C. J. Electrochem. Soc. 1971, 118, 1782–1790. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.; Hua, Y.; Chen, R.; Li, Z.; Ye, Y. Microstructure and hot corrosion behavior of the Ni-based superalloy GH202 treated by laser shock processing. Mater. Charact. 2017, 125, 67–75. [Google Scholar] [CrossRef]
- Pillai, R.; Romedenne, M.; Haynes, J.A.; Pint, B.A. Oxidation behavior of candidate NiCr-alloys for engine exhaust valves—Part I: Effect of minor alloying elements. Oxid. Met. 2021, 95, 157–187. [Google Scholar] [CrossRef]
- Duan, R.; Jalowicka, A.; Unocic, K.A.; Pint, B.A.; Huczkowski, P.; Chyrkin, A.; Grüner, D.; Pillai, R.; Quadakkers, W.J. Predicting Oxidation-Limited Lifetime of Thin Walled Components of NiCrW alloy 230. Oxid. Met. 2017, 87, 11–38. [Google Scholar] [CrossRef]
- Wang, J.; Xue, H.; Wang, Y. Oxidation behavior of Ni-based superalloy GH738 in static air between 800 and 1000 °C. Rare Met. 2020, 40, 616–625. [Google Scholar] [CrossRef]
- Mallikarjuna, H.T.; Caley, W.F.; Richard, N.L. Oxidation Kinetics and Oxide Scale Characterization of Nickel-Based Superalloy IN738LC at 900 °C. J. Mater. Eng. Perform. 2017, 26, 4838–4846. [Google Scholar] [CrossRef]
- Cruchley, S.; Evans, H.; Taylor, M. An overview of the oxidation of Ni-based superalloys for turbine disc applications: Surface condition, applied load and mechanical performance. Mater. High Temp. 2016, 33, 465–475. [Google Scholar] [CrossRef]
- Foss, B.J.; Hardy, M.C.; Child, D.J.; McPhail, D.S.; Shollock, B.A. Oxidation of a Commercial Nickel-Based Superalloy under Static Loading. JOM 2014, 66, 2516–2524. [Google Scholar] [CrossRef]
- Nowak, W.J.; Wierzba, B.; Sieniawski, J. Effect of Ti and Ta on Oxidation Kinetics of Chromia Forming Ni-Base Superalloys in Ar-O2-Based Atmosphere. High Temp. Mater. Process. 2018, 37, 9–10. [Google Scholar] [CrossRef]
- Brenneman, J.; Wei, J.; Sun, Z.; Liu, L.; Zou, G.; Zhou, Y. Oxidation behavior of GTD111 Ni-based superalloy at 900 °C in air. Corros. Sci. 2015, 100, 267–274. [Google Scholar] [CrossRef]
- Tsao, T.K.; Yeh, A.C.; Kuo, C.M.; Murakami, H. High Temperature Oxidation and Corrosion Properties of High Entropy Superalloys. Entropy 2016, 18, 62. [Google Scholar] [CrossRef]
- Chiou, M.S.; Yeh, A.C.; Jian, S.R.; Kuo, C.M. High Temperature Oxidation Behavior of CM-247LC Nickel Base Superalloy. Adv. Mater. Res. 2014, 922, 61–66. [Google Scholar] [CrossRef]
- Chiou, M.S.; Jian, S.R.; Yeh, A.C.; Kuo, C.M. Effects of Al Addition on the High Temperature Oxidation Behavior of CM-247 LC Ni-Based Superalloy; The Minerals, Metals & Materials Society: Pittsburgh, UK, 2013; pp. 521–527. [Google Scholar] [CrossRef]
- Ghoussoub, J.N.; Klupś, P.; Dick-Cleland, W.J.B.; Rankin, K.E.; Utada, S.; Bagot, P.A.J.; McCartney, D.G.; Tang, Y.T.; Reed, R.C. A New Class of Alumina-Forming Superalloy for 3D Printing. Addit. Manuf. 2022, 52, 102608. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Parlikar, C.; Alam, M.Z.; Das, D.K. Type-I Hot Corrosion of Ni-Base Superalloy CM247LC in Presence of Molten Na2SO4 Film. Met. Mater. Trans. A 2020, 52, 378–393. [Google Scholar] [CrossRef]
- Chapman, N.; Gray, S.; Sumner, J.; Nicholls, J. Surface Roughness Evolution to Identify. Incubation Time for Hot Corrosion of Nickel-Base Superalloys: CMSX-4, CM247LC DS and IN6203DS at 550 °C. Oxid. Met. 2020, 94, 447–463. [Google Scholar] [CrossRef]
- Younes, C.M.; Allen, G.C.; Nicholson, J.A. High Temperature Oxidation Behaviour of Single Crystal Superalloys RR3000 and CMSX-4. Corros. Eng. Sci. Technol. 2007, 42, 80–88. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, W.; Wang, J.; Zhang, Y.; Zhang, Z. Initial Oxidation Behavior of a Single Crystal Superalloy During Stress at 1150 °C. Sci. Rep. 2020, 10, 3089. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Choe, B.H.; Kim, D.H.; Kim, J.H.; Choi, K.S.; Kim, Y.S. The effect of TCP-σ precipitates on surface pitting and cracking in a Ni-based superalloy turbine blade. Eng. Fail. Anal. 2024, 158, 107989. [Google Scholar] [CrossRef]
- Choe, B.H.; Choi, K.S.; Han, S.H.; Kim, D.H.; Ahn, J.K.; Kang, D.S.; Seo, S.M. γ′-Precipitation Free Zone and γ′ Rafting Related to Surface Oxidation in Creep Condition of Directionally Solidified CM247LC Superalloy. Korean J. Mater. Res. 2023, 33, 10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).