Abstract
This study reviews the current research status and future trends in the field of abrasive-resistant coating preparation, focusing on steel coating technology. The types of materials, preparation methods, and application status of the abrasive-resistant coatings are described in detail. First, the necessity for an abrasive-resistant coating is analyzed from the perspective of steel applications. Second, abrasive-resistant coating materials of different substrates, including metal-, ceramic-, composite-, and polymer-based materials, are systematically expounded, and the composition, property characteristics, and applications of the materials are systematically discussed. Next, the principles, advantages, and disadvantages of different preparation techniques are analyzed and compared, and the relevant research results are summarized, showing the urgent demand and application prospects of abrasive-resistant coatings. Finally, the performance of the coating is presented, and the development trend of abrasive-resistant coatings on steel surfaces is discussed, providing theoretical support and new ideas for innovation in this field.
1. Introduction
Steel is a core material used in modern industries. With the advancement of industrialization, the use of steel components has rapidly increased. Compared with other metal materials, steel has become an indispensable cornerstone of current and future society because of its stable performance, high strength, durability, malleability, versatility, low cost, and its ability to be recycled infinitely. It is the material of choice in infrastructure fields such as industrial engineering, including automobiles [1,2,3], ships [4], and structural buildings [5,6,7]. It is also included in daily necessities such as furniture and home appliances, such as refrigerators [8] and washing machines [9]. Steel can be classified based on various properties, including its chemical composition [10], quality, application, metallographic structure, smelting methods, and processing technology. Steels can also be divided based on their chemical composition into carbon and alloy steels. Carbon steels are further categorized into low-, medium-, and high-carbon steels, which are used in construction, mechanical parts, and tool manufacturing, respectively. Alloy steel is produced by adding one or more alloying elements (such as chromium, nickel, or molybdenum) to carbon steel to improve its performance [11]. Alloy steels are divided into low- [12], medium-, and high-alloy steels. Among them, stainless steel [13] is a type of high-alloy steel. Owing to its corrosion resistance, it has become the preferred material for kitchen equipment and medical devices and is one of the most widely used types of alloy steel.
Steel is widely used in machinery manufacturing, energy equipment [14], and civil engineering. For example, core components, such as gears [15,16], bearings, rolls, and excavation equipment, are mostly based on a steel matrix. The hardness and wear resistance of steel can be significantly improved through composition optimization and process control, and appropriate steel grades can be selected based on their specific working conditions (load, temperature, and medium); however, different degrees of wear inevitably occur on the steel surface. This issue is particularly prominent under special working conditions such as high loads, temperatures, and corrosion [17]. Wear can lead to a decrease in equipment precision and machine-production efficiency; in severe cases, it may cause component failure and scrapping and even result in safety issues. Previous studies have shown that approximately 80% of the damage to parts of mechanical equipment is caused by wear. The global industrial economic loss caused by wear exceeds USD 100 billion annually. In addition to the large financial loss, the frequent replacement of parts that fail owing to wear also requires extensive time, human resources, and material resources [18]. Based on this, a method for avoiding or reducing friction is considered during component design. By applying wear-resistant coatings to the steel surface, the surface hardness, wear resistance, and impact resistance of the steel can be significantly improved while maintaining the mechanical properties of the steel matrix. This extends the service lives of components, reduces the frequency of shutdown maintenance, and directly lowers the operating costs of industrial production [19]. The World Steel Association is committed to promoting sustainable steel development. It launched a series of plans and projects, including Sustainability Indicators and the Sustainability Charter, which are closely linked to the United Nations Sustainable Development Goals. Surface-strengthening technology has become a research focus to keep pace with the concept of sustainable green development. Common surface-strengthening measures are divided into three main categories: material surface-modification technology [20], surface alloying, and coating-strengthening processes [21]. Coating-strengthening technology refers to the formation of a covering layer with specific functions (such as strengthening and protection) on the surface of a metal or nonmetal matrix through physical, chemical, or other methods. This covering layer has properties that differ from those of the matrix material and it has a particular thickness. Many preparation methods are emerging owing to the increasing variety of coatings.
Part failure refers to a phenomenon in which a part loses its specific function during use. Wear is an important form of part failure and falls under the category of surface-loss failure [22]. Wear is defined as the phenomenon in which the surface material of an object is continuously lost (transferred) or undergoes residual deformation and fracture owing to mechanical friction, chemical actions (including thermochemical, electrochemical, and mechanochemical reactions), or thermal effects. This process leads to material loss, performance degradation, and the functional failure of an object. Wear occurs as a result of the interaction between the surface material and external environment (such as another contacting object or medium), which breaks the equilibrium state of the material surface. Thus, the surface material detaches from the main body in the form of tiny particles, fragments, or molecular layers or it undergoes morphological changes. This manifests as changes in the material properties and dimensions, as well as a shortened service life. Wear is a progressive dynamic process characterized by time-varying features that can be approximately classified into abrasive, fatigue, adhesive, erosive, corrosive, and fretting wear [23]. Based on the environmental medium, wear can be classified as dry, wet, or fluid wear.
The wear process of parts is approximately divided into three stages (as shown in Figure 1): slight, medium, and severe wear, which correspond to the running-in, steady wear, and serious wear stages, respectively.
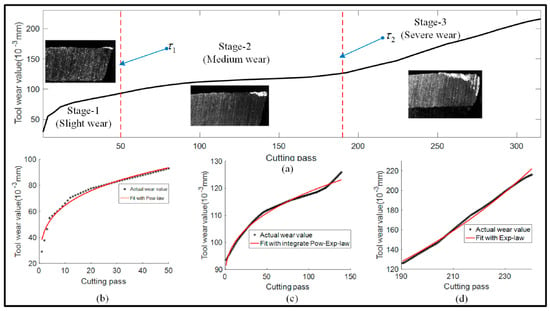
Figure 1.
Liu et al. Reprinted from Ref. [24] divided the typical tool degradation process into three stages based on the wear rate; (a) Typical degradation process of the tool; (b) Running-in stage; (c) Steady wear stage; (d) Severe wear stage.
Running-in stage: During initial operation, the contact area is small, and stress concentration occurs, leading to a relatively fast wear rate. As the operation continues, the protrusions on the surface are worn down, the contact area expands, and the wear rate gradually slows until it stabilizes.
Steady wear stage: After the running-in period, the surface condition of the part stabilizes. The wear rate is slow and uniform, and the wear amount has an approximately linear relationship with the working time, where the slope represents the wear rate. This stage is the main working phase of the part, and its duration directly determines its service life.
Severe wear stage: When a part wears to a certain extent, the surface precision and lubrication conditions deteriorate, causing the wear rate to accelerate sharply. As the wear increases, the wear loss increases, surface clearance expands, and surface quality deteriorates, leading to the rapid failure of the mechanical component. Continuing to use a part at this stage may cause malfunctions; therefore, the part must be replaced or repaired in a timely manner before entering this stage.
When parts operate under long-term wear conditions, their dimensional and shape accuracies, surface roughness, and other properties are affected. This leads to a decline in mechanical performance, impairs machine quality, shortens equipment service life, reduces machine efficiency, and results in higher energy consumption [25]. For example, after the piston rings of an engine wear out [26], the engine power decreases and fuel consumption increases. Severe wear can damage mechanical parts, thereby triggering equipment failure, reducing machine reliability, and creating safety hazards. For example, a worn journal may result in the vibration or even fracture of shafting components, material consumption, and the large-scale scrapping of mechanical materials. Measures such as improving the structural design, selecting appropriate manufacturing processes [27], implementing effective lubrication [28], and adopting appropriate surface-treatment methods are commonly used to reduce wear, and the application of wear-resistant coatings has emerged as a solution [29].
The latest market research shows that the compound annual growth rate of wear-resistant coatings has remained above 10% over the past five years, and this trend is expected to continue until 2025. Among other key figures, the global wear-resistant-coating market size already reached billions of US dollars in 2019 and is projected to reach tens of billions of US dollars by 2025. With the rapid development of the global manufacturing industry, the wear-resistant-coating market has exhibited a continuous expansion trend. This indicates a growing demand for wear-resistant coating materials across various fields, with particularly significant growth observed in high-performance and environmentally friendly wear-resistant coating segments. The main drivers for this increasing demand include the rising level of industrial automation and widespread application of wear-resistant coatings in key industries, such as machinery manufacturing, automotive manufacturing, and aerospace. In machinery manufacturing, wear-resistant coatings reduce wear and tear to enhance the durability of equipment parts, thereby extending their service lives. In automotive manufacturing, these coatings are used for the surface treatment of critical components such as engines and gearboxes to improve performance and reduce energy consumption. In the aerospace sector, wear-resistant coatings are widely applied to aircraft-engine and turbine blades to enhance their high-temperature and corrosion resistance.
The future development of the wear-resistant-coating market is influenced by multiple factors, including the global economic situation, technological innovation, policy guidance, and changes in consumer demand. Driven by rapid industrial development and the large-scale advancement of infrastructure construction, the demand for wear-resistant coatings will continue to increase and diversify. In the next few years, the wear-resistant-coating market is expected to maintain its growth momentum, especially in emerging-market countries such as China and India. High-performance wear-resistant coatings have become popular in the market owing to their excellent wear, corrosion, and high-temperature resistance. Particularly, in heavy-industry sectors such as iron, steel, and cement production, the application of wear-resistant coatings can significantly improve production efficiency and extend equipment service life. Additionally, increasingly stringent environmental regulations will drive the development of wear-resistant coatings toward high-performance, low-emission, and green production. For example, the continuous progress of emerging technologies such as nanocoatings has endowed wear-resistant coatings with outstanding performance while meeting the requirements of environmental protection and sustainable development. With global emphasis on industrial efficiency and safety, the role of wear-resistant coatings in enhancing equipment durability and reducing maintenance costs has become increasingly prominent.
Research on wear-resistant coatings for steel surfaces has significant value for resource conservation and environmental protection. Enhancing the wear resistance of steel components can reduce the total consumption of steel and lower reliance on mineral resources such as iron ore and coke, which aligns with the “reduction” concept of the circular economy. Moreover, coating technology enables the repair and reuse of waste steel components. For example, after re-cladding a wear-resistant coating on the surface of a worn roll, its service performance can be restored, thereby reducing material waste by more than 50% compared to replacing the roll with a new component. Additionally, under extreme working conditions (e.g., high-temperature or corrosive environments), wear-resistant coatings can further reduce the dust pollution generated by equipment wear and the consumption of lubricating oil, facilitating the transformation of industrial production toward low-carbon and green development.
This study focuses on new types of wear-resistant coating materials such as composite coatings, nanocoatings, and self-lubricating wear-resistant coatings. The preparation processes, performance characteristics, wear mechanisms, and application effects of these new materials on steel surfaces are analyzed, and the advantages of the new materials compared with traditional coating materials in terms of wear resistance, corrosion resistance, toughness, and other aspects are highlighted. The importance of innovation in the preparation process for improving the performance of wear coatings is emphasized. Moreover, this study introduces some of the latest preparation technologies, such as the improvement and optimization of gas-phase deposition, laser cladding, thermal spraying, and other technologies. We also discuss how these improved processes achieve precise control of the coating, improve the bonding strength of the coating and substrate, and improve the microstructure and performance of the coating to provide new ideas and methods for the preparation of wear-resistant coatings. In the analysis of wear-resistant coatings on steel surfaces, common problems such as the bonding strength of the coating, wear-resistance durability, and cost control are mentioned, as well as some new challenges that arise with the development of the industry, such as the performance stability of the coating under extreme conditions (high temperature, high pressure, and strong corrosion) and failure mechanism of the coating under complex working conditions. This provides a more reliable basis for selecting appropriate wear-resistant coatings for different application scenarios and a reference for subsequent research and practical applications.
In summary, research on wear-resistant coatings for steel surfaces is a technical means to solve industrial wear problems as well as an important approach to promote the progress of materials science, enhance industrial competitiveness, and achieve sustainable development, providing clear practical value and profound scientific significance.
2. Types of Wear-Resistant Coating Materials
2.1. Metal-Based Wear-Resistant Coating
Metal-matrix wear-resistant coatings use a metal or an alloy matrix. Typically, metals with sufficient toughness and bonding strength, such as cobalt-, nickel-, and iron-based alloys, are selected as the matrix to ensure firm bonding between the coating and substrate. Functional coatings are formed by the addition of other substances. For example, the incorporation of wear-resistant reinforcing phases (oxides, carbides, and nitrides) in the form of high-hardness particles endows the coating with excellent wear resistance. Alternatively, alloying elements can be added to improve the matrix performance: boron [30,31,32] or silicon reduces pores and inclusions in the coating [33] while improving the fluidity of the coating in its molten state, promoting bonding with the substrate, and enhancing the coating density; molybdenum and tungsten increase the high-temperature strength and hardness of the matrix, enhancing the deformation resistance and bonding strength of the coating under heavy loads and high-temperature conditions [34]. Other auxiliary elements are also involved: rare-earth elements [35] (e.g., La and Ce) refine the grain structure of the coating, reduce defects, improve the toughness and wear resistance of the coating, and simultaneously enhance its high-temperature oxidation resistance. Intermetallic compounds (e.g., Ni3Al and Co3Ti) form reinforcing phases in the matrix, which possess a certain degree of hardness and toughness, balancing the wear and impact resistances of the coating. Through the synergistic effect of these elements, the coating maintains the toughness of the matrix, resists wear via high-hardness reinforcing phases, and can adapt to different working conditions (e.g., high temperature, corrosion, and impact).
In wear-resistant materials with a metal matrix, metal or nonmetal elements are often added to improve the properties of the coating, such as wear resistance [36], hardness, density [37], and corrosion resistance. The specific types and functions are listed in Table 1.

Table 1.
Types of elements in the coating, functions and advantages.
These elements improve the performance of metal-based wear-resistant coatings in a targeted manner through methods such as solid solution strengthening, dispersion strengthening, the formation of hard phases, or protective films. Metals are typically used to enhance strength, toughness, and high-temperature stability, while nonmetals mainly strengthen wear resistance by forming high-hardness compounds or improve corrosion resistance through film-forming effects. In practical applications, these are often combined to balance the requirements of multiple properties.
The following sections provide a review of cobalt-, nickel-, and iron-based wear-resistant coatings, which cover the mainstream wear-resistant needs from general to extreme conditions and form a complementary and mature system in terms of “wear resistance, process adaptability, and cost controllability” corresponding to different strength requirements. Iron-based coatings have the lowest cost and are easy to process; thus, they are suitable for ordinary wear scenarios. Nickel-based coatings have both wear and corrosion resistance and are suitable for moderately harsh environments. Cobalt-based materials maintain high hardness under high temperature and high impact and are the first choice for extreme conditions (such as aircraft engines). They can all be perfectly matched with mainstream coating-preparation processes, such as plasma spraying, laser cladding, and submerged arc welding, with a high bonding force of the coating, ease of falling off, and mature industrial applications.
2.1.1. Cobalt-Based Wear-Resistant Coatings
Cobalt-based wear-resistant materials are widely used in the surface strengthening of components, such as pumps and valves, in the nuclear industry owing to their excellent wear resistance [38], high toughness, and corrosion resistance. The Stellite cobalt-chromium-tungsten alloy series, which has a long development history, is a high-temperature wear-resistant alloy based on cobalt, supplemented by elements such as chromium, tungsten, and molybdenum, and is a type of cobalt-based superalloy. Invented by Elwood Haynes in 1907, it was initially a cobalt-chromium binary alloy and later developed into a cobalt-chromium-tungsten ternary system. Originally developed for cutting tools, it is now widely applied in fields such as dentistry, surgery, energy, chemical engineering, and general engineering [39]. These coatings exhibit excellent high-temperature performance and maintain high strength and hardness in high-temperature environments. Owing to the addition of hard alloy elements, such as tungsten, they perform exceptionally well under sliding or impact wear conditions. Farnia et al. deposited a Stellite 6 coating on a low-carbon ferritic steel substrate using low-power pulsed-laser cladding technology, studied its microstructure characteristics and phase-transformation relationships, and observed a Greninger–Troiano orientation relationship between the coating and substrate [40]. With the development of modern science and technology, CoCrW alloys have entered a stage of challenging, extreme applications. In the aerospace field, a 0.3 mm thick CoCrW alloy coating on the turbine blades of Pratt & Whitney’s PW1100G engine can withstand the erosion of high-temperature gas at 1100 °C. The Cr2O3 oxide film on its surface reduces the oxidation rate to 0.01 mm/year, which is only 20% of that of nickel-based alloys. This material extends the engine overhaul cycle from 5000 h to 15,000 h, thereby significantly reducing the operating costs of airlines. In the medical field, CoCrW alloys have become the preferred materials for artificial joints because of their excellent wear resistance (friction coefficient of 0.1) and biocompatibility. Porous structures with porosities up to 60% can be prepared using 3D-printing technology to promote bone-tissue ingrowth. In the hydrocracking reactors of oil refineries, the stress-corrosion cracking resistance of CoCrW alloy valves in H2S + CO2 mixed media is ten times that of stainless steel. After adopting this alloy, the valve-replacement frequency decreased from four times per year to once every three years, greatly reducing maintenance costs. In addition to the wide applications of traditional Stellite-series alloys, composite materials formed by adding other elements also demonstrate excellent wear resistance. Li successfully prepared a Ti3SiC2-reinforced cobalt-based alloy self-lubricating coating on the surface of 35CrMo steel using laser cladding technology. A mixture of a cobalt-based alloy and Ti3SiC2 powder was used as the cladding material, and the effect of different Ti3SiC2 contents (with a focus on testing 10 wt%) on the coating performance was systematically studied. Microhardness and friction-wear test data verified the performance improvement of the coating; the hardness increased by 2.3 times, and the wear volume loss was significantly reduced by approximately 84% [41].
However, the release of cobalt ions can also lead to metallosis. Cobalt can generate radioactive isotopes (such as 60Co) in irradiated environments, which is not only costly but also significantly increases the risk of radiation exposure for staff. The European Union’s Batteries and Waste Batteries Regulation has set a target for the cobalt-recovery rate to reach 95% by 2030. China’s Interim Measures for the Administration of the Recycling and Utilization of Power Batteries for New Energy Vehicles also formulated strict standards for the recycling of cobalt-containing materials. Therefore, researchers have attempted to develop cobalt-free surfacing alloys [42,43] to replace traditional cobalt-based materials under the wear-resistant working conditions of nuclear-energy equipment.
2.1.2. Nickel-Based Wear-Resistant Coatings
Nickel-based alloys are widely used in the surface strengthening of high-performance steels that require wear and impact resistance owing to their excellent toughness, impact resistance, and oxidation resistance [44]. Jeyaprakash prepared cobalt-based (Co-Cr-W-C) and nickel-based (Ni-Cr-B-Si-C) laser claddings on 316 L stainless-steel substrates and compared them [45]. Microhardness tests showed that the cladding hardness was 2.75–2.90 times higher than that of the substrate. Among them, the nickel-based cladding exhibited the best wear resistance, with the surface roughness of the worn surface reduced by 1.31 times and 2.31 times compared to the cobalt-based cladding and substrate, respectively. This is attributed to the dendritic eutectic structure formed by the γ-Ni matrix and carbides/borides in the nickel-based coating, which constitutes a strengthening network. A wear-mechanism analysis indicated that the cobalt-based cladding and substrate mainly underwent plastic deformation and abrasive wear, while the outstanding performance of the nickel-based coating makes it a potential alternative to traditional cobalt-based coatings.
Nickel-based coatings contribute to improving coating compactness. Teng conducted a study in which two coating systems—a single Fe/WC coating and an Fe/WC-Ni60 composite coating—were prepared on the surface of 60Si2Mn spring steel using laser cladding technology [46]. Comparative analysis showed that the Ni60 transition layer significantly reduced the coating porosity and crack density but led to a decrease in the microhardness of the coating, resulting in a slight increase in the friction coefficient and wear loss.
2.1.3. Iron-Based Wear-Resistant Coatings
Compared with other wear-resistant coatings, iron-based alloy coatings have significant cost advantages because their raw materials are relatively inexpensive. When fused with iron-based substrates, these coatings form a high-strength metallurgical bonding layer with excellent bonding strength. In practical applications, they exhibit outstanding process performance, are suitable for various hot- and cold-working forming processes, and demonstrate incomparable advantages over traditional composite materials. When addressing wear conditions, iron-based coatings possess the dual advantages of toughness and wear resistance, which can extend the service lives of wear-resistant components [47]. This characteristic not only significantly reduces material costs but also greatly improves the comprehensive performance of the materials. Sekar employed empirical correlations to predict the porosity and microhardness of iron-based amorphous coatings, which yielded high-quality coatings under optimized process conditions. This parameter combination ensures the complete melting of the powder particles and results in a good spreading effect on the substrate surface. The experimental results showed that the coating prepared under the optimized parameters had excellent performance: the porosity was significantly reduced (0.32%) and the microhardness was significantly improved (1321 HV), as shown in Figure 2. The iron-based amorphous coating structure was dense and exhibited superior performance when the process parameters approached theoretically predicted values.
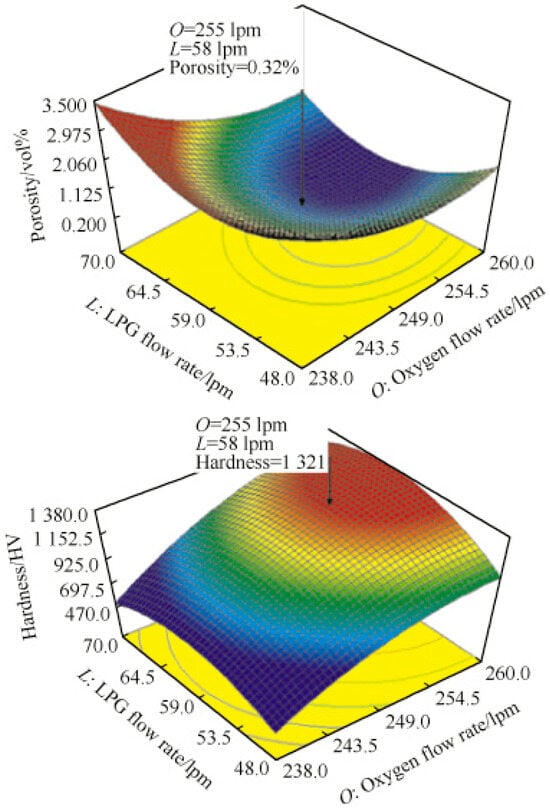
Figure 2.
Vignesh et al. Reprinted from Ref. [48] measured the response diagram of coating porosity and hardness.
During the coating-preparation process, boron can form high-hardness borides (e.g., FeB and Fe2B) with iron. These hard phases are uniformly distributed in the coating, which significantly improves its overall hardness, enhances its wear resistance, and extends its service life. Martins used 2205 duplex stainless steel and an iron alloy (Fe-B) as raw materials to prepare boron-modified steel powders using two methods: gas atomization and subsequent mechanical grinding. Boride-reinforced coatings were then prepared on the surface of low-carbon steel (AISI 1020) using selective laser melting technology [49]. Because boron can reduce the surface tension of the molten pool, promote the escape of bubbles, and reduce porosity, the coating structure becomes denser and the coating achieves metallurgical bonding with the substrate. Borides are uniformly distributed in the ferrite matrix. Consequently, the hardness of the coating reaches approximately 800 HV0.5, and the wear rate decreases by approximately two orders of magnitude.
2.2. Ceramic-Based Wear-Resistant Coatings
Ceramic-based wear-resistant coatings use ceramic materials as their main components and are applied to the surface of a substrate through specific processes. They are used to improve the wear, corrosion, and high-temperature resistance of substrates. These coatings can protect substrates from harsh environments, such as mechanical wear and chemical corrosion, thereby extending their service lives.
2.2.1. Oxide Ceramics
Oxide ceramics such as aluminum oxide (Al2O3), zirconium oxide (ZrO2), and chromium oxide (Cr2O3) are used as the main components. These ceramics are formed via processes such as spraying and sintering, they possess good oxidation and corrosion resistances and are suitable for medium-to-low temperature and mild wear environments, such as metal-surface protection.
The core advantage of using Al2O3 in coatings is that it can form a dense oxide layer with corrosion-protection properties. An Al2O3-30(Ni20Al) coating was deposited on an AISI 304 stainless-steel substrate. In terms of wear rate, the uncoated sample was 6.9 × 10−4 mm3/N·m, while the coated sample was reduced to 8.3 × 10−5 mm3/N·m [50]. Further research was conducted by preparing graphene oxide (GO)-reinforced Al2O3 ceramic coatings on medium-carbon-steel surfaces. After adding GO, the porosity of the coating decreased by 31%, the hardness increased by approximately 10%, and the bonding strength improved by 250%. The wear rates under 30 and 60 N loads were reduced by 81% and 84%, respectively [51].
ZrO2 can significantly enhance the oxidation resistance and load-bearing capacity under frictional loads [52]. A wear test was conducted on WC-Co cermet composites with zirconia as an additive. The addition of zirconia improved the oxidation resistance and load-bearing capacity of the composite. This is mainly because zirconia regulates the composition and surface morphology of the tribo-oxide layer, forms a dense protective oxide layer, and reduces the brittle-oxide content on the worn surface.
The Ni-20Cr2O3 coating significantly improves the erosion wear resistance of all materials. Ni-20Cr2O3 powder was deposited on SS 202, SS 304, and low-carbon steel, and the improvement in the low-carbon steel was the most significant [53]. The coating was denser on low-carbon steel (with the smallest thickness, approximately 250 μm) and had higher microhardness (715–760 HV). The Ni-20Cr2O3 coating enhanced the erosion resistance of low-carbon steel the most remarkably (by 4–6 times), followed by SS 304 and SS 202 (by 2–3 times). Its core advantages include high hardness, a dense structure, and a tough wear mechanism, and it performs particularly well under low-speed and high-concentration working conditions.
Different oxide types and contents have different effects on the coatings. For Y2O3-modified Mo-Si-B composite coatings, the content of Y2O3 has an impact on the coating properties [54]. The coating with 1 wt% Y2O3 exhibits the optimal comprehensive performance, with a microhardness of 931 HV0.5 (5.6 times that of the substrate) and stable oxidation resistance at 900 °C. Although the microhardness and oxidation resistance increase with increasing Y2O3 content, excess Y2O3 leads to increased crack sensitivity and decreased high-temperature friction and wear performance. Adding the appropriate content based on these requirements is particularly important. TiO2 often plays a lubricating role [55]. The NiCr-WS2-Ti composite material shows excellent tribological properties under high-temperature working conditions. Owing to the addition of Ti, it is preferentially oxidized to form TiO2 protrusions, forming a special surface-texture structure. A protective glaze layer is generated on the surface, which significantly reduces the friction coefficient and greatly improves the wear resistance.
2.2.2. Carbide Ceramics
Carbide ceramics use tungsten carbide (WC), chromium carbide (Cr3C2), and titanium carbide (TiC). They are typically compounded with metal binders (e.g., cobalt and nickel) and exhibit high hardness and extremely strong wear resistance. They are suitable for high-load and severe wear scenarios such as those on the surfaces of cutting tools and molds.
Shen conducted a test study on nickel-based coatings with different WC contents on carbon-steel substrates [56]. Three types of NiCrSiBC-WC composite coatings with different WC contents (40%, 50%, and 60%) were prepared on carbon-steel substrates via laser cladding technology, and the effect of the WC content on the coating properties was systematically investigated. The following conclusions were drawn: hardness increases with increasing WC content, residual stress increases with increasing WC content, and toughness decreases with increasing WC content.
You analyzed WC-Co-Cr composite coatings [57]. The WC particles underwent dissolution and regrowth during laser cladding. Under the optimal parameters, the microhardness of the composite coating increased by five-fold, and the wear mass loss was reduced by a factor of 25. However, coatings with a high WC content fail prematurely owing to local embrittlement [58].
Ortiz-Membrado implanted Ti, Cr, and N ions into a WC-Co matrix and evaluated the load-bearing capacity of the matrix through hardness tests. The treated matrices, especially those implanted with Ti and Cr ions, showed improved coating-adhesion strength. This effect is attributed to the introduction of residual stress in the matrix, which enhances its fracture toughness and load-bearing capacity [59]. Therefore, optimizing the coating performance by adding elements is applicable to ceramic-based materials.
Compared to oxide-ceramic coatings, carbide coatings have poorer high-temperature oxidation resistance [60]. Wu conducted a study on the microstructure, cyclic oxidation, and high-temperature wear properties of (Ti, W)C-reinforced stainless-steel coatings. Although the addition of (Ti, W)C particles reduced the oxidation resistance, it improved the high-temperature wear resistance of the coating and decreased the friction coefficient.
TiC optimizes the microstructure and interfacial bonding of the coating through recrystallization (releasing Ti) and interfacial reactions (forming a (Ti,W)C solid solution + reaction layer), ultimately achieving a comprehensive improvement in hardness, wear resistance, and friction performance [61]. Zhang prepared TiC/WC composite ceramic-reinforced nickel-based coatings on the surface of low-carbon steel using plasma-transferred arc technology. The addition of TiC refined the microstructure and improved the tribological properties of the coatings. When the TiC content was 20 wt%, the average microhardness of the coating reached 964 HV, which was 64% higher than that of the pure WC-reinforced Ni-based coating. It exhibited the lowest friction coefficient with the smallest fluctuation and minimum wear volume loss, and its wear resistance was seven times that of the substrate.
2.3. Polymer-Based Wear-Resistant Coatings
Polymer-based wear-resistant coatings are functional coatings formed using polymers (such as resins and rubbers) as the matrix material and wear-resistant fillers (such as hard particles and fibers). They are primarily used to improve the wear resistance of substrate surfaces. Fillers are functional components uniformly dispersed in the matrix material of the coating to form a composite coating structure. This method utilizes a combination of the properties of the fillers (such as high hardness, wear resistance, and lubricity) and the bonding force of the matrix material to enhance the overall performance of the coating [62]. Polymers, as the continuous phase, provide a coating with flexibility, adhesion, and formability, and can adapt to the surface characteristics of different substrates (such as metals and plastics). By adding hard fillers such as silicon carbide, aluminum oxide, and glass beads, or lubricating fillers such as graphite and molybdenum disulfide, the insufficient wear resistance of the polymers themselves is compensated for by the high-hardness or low-friction characteristics of the fillers. These coatings are often used on the surfaces of mechanical parts, pipelines, and equipment and are particularly suitable for friction environments with medium-low loads and non-extreme high temperatures, with both wear-resistant properties and certain anti-corrosion and shock-absorbing effects. Through the synergistic effect of polymers and fillers, such coatings provide wear-resistant protection of the substrate while ensuring convenient construction. Notably, the wear resistance of such wear-resistant coatings changes with the filler concentration. Tanaka used a pin-on-disk wear-test device to prove that the friction force and thickness of the transferred polymer layer have no clear relationship [63].
2.3.1. Thermosetting Resin Coatings
Thermosetting resins undergo chemical cross-linking reactions under heating, catalysis, and ultraviolet radiation, forming an insoluble and infusible 3D network structure. Once cured and shaped, they cannot be softened or melted via reheating. This characteristic endows the coatings with high hardness, chemical resistance, heat resistance, and mechanical strength, which can provide good protection and decoration for coated substrates (such as metals, wood, and plastics). These coatings are widely used in automobiles, furniture, and industrial equipment. Common thermosetting resins include epoxy, phenolic, and polyurethane resins.
Polysilazane is a thermosetting resin coating with ceramic conversion properties. The Si-N main chain forms a stable cross-linked structure after curing [64], combining the processability of organic coatings and the high-temperature resistance of inorganic ceramics. This characteristic makes it irreplaceable in high-end fields such as aerospace and electronic packaging. Wang evaluated the protective performances of three polymer-derived ceramic coating systems in high-temperature oxidation environments: SiON single-layer coatings, ZrSi2-reinforced SiOC composite coatings, and SiON/SiOC double-layer coating systems [65]. The experimental results showed that the double-layer system composed of SiON as the bonding layer and SiOC as the top layer exhibited the best protective effect on the metal substrates. Schuetz developed a new type of precursor-derived ceramic coating on steel substrates using polysilazane as the matrix and incorporated borosilicate-barium silicate glass-filler particles [66]. The coating had a nonporous and dense structure and formed a nickel-enriched transition layer (oxygen-diffusion barrier) with the substrate, demonstrating strong substrate adhesion, good wear resistance, and excellent high-temperature corrosion resistance.
2.3.2. Self-Lubricating Coatings
In addition to their superior wear resistance, solid lubricant coatings can reduce friction coefficients without additional lubrication, making them ideal for mechanical components that cannot be beaten regularly. Therefore, they are popular choices as coating materials. Solid-lubricant coatings are an application of solid lubricants. Compared to liquid lubricants, they cause leakage and environmental problems and can reduce maintenance costs and environmental impacts, making them a clean and pollution-free priority.
Polytetrafluoroethylene (PTFE) is a fluorine-containing high-molecular-weight polymer with excellent chemical stability, an extremely low friction coefficient [67,68], superior anti-sticking effects, good biocompatibility, and excellent temperature resistance. It is known as the “King of Plastics” and has good self-lubricating properties that can reduce friction and wear without the need for additional lubricants. Therefore, it is widely used as a self-lubricating coating. It is applied to the surfaces of metals, ceramics, and other substrates using methods such as spraying and dipping. Owing to its anti-sticking properties, corrosion resistance, and low friction, it is used in applications such as cookware (e.g., nonstick pans), pipelines, and bearings [69].
Sawyer tested the frictional properties of PTFE and aluminum-oxide composites on stainless-steel surfaces [70]. The wear resistance improved with an increase in the filler concentration, and a filler concentration that enhanced the wear resistance by up to 600 times was identified within the test range. Briscoe [71] and Blanchet [72] fabricated new nonabrasive PTFE systems with a wear resistance similar to that of filled PTFE composites using gamma radiation and electron irradiation. PTFE matrices are now compounded with ceramics, metal particles, and glass fibers. With further advancements in nanoparticle technology, such composites are expected to become noncorrosive solid lubricants/wear-resistant coatings with excellent wear resistance, providing new options for bearing materials.
Graphite is commonly used as an auxiliary material in self-lubricating coating-preparation systems. Graphite coatings are typically used as solid lubricants or inorganic nonmetallic coatings. In terms of composition, they are mainly made from natural or artificial graphite and are formed through methods such as coating and spraying. They exhibit good high-temperature resistance, corrosion resistance, and chemical stability. The layered crystal structure of graphite results in weak bonding forces between its layers, which can easily slide under external forces, leading to a low friction coefficient. They can achieve friction-reducing effects without requiring additional lubricants, thereby exhibiting self-lubricating properties. This characteristic enables wide application in scenarios requiring reduced friction and wear, such as in bearings, molds, and high-temperature mechanical components.
Bahadur conducted a test and analysis on graphite as a filler material and concluded that when graphite is used in combination with PTFE, a uniform and coherent filled polymer film is formed on the steel surface. The addition of graphite reduces the wear rate of PTFE by approximately 100 times while increasing the friction coefficient by approximately 30% [73]. Subsequently, Li introduced copper. They showed that the copper layer had good adhesion to the graphite surface, which prevented direct contact between the graphite and PTFE surfaces and improved the overall wear resistance [74].
In addition, the incorporation of graphite can serve as a method for reducing material costs and enhancing the load-bearing capacity of the coatings. Hang developed a new type of self-lubricating composite coating that was prepared on a stainless-steel substrate via a combined process of laser cladding and vacuum-pressure thermal-diffusion welding [75]. The coating consists of wear-resistant units (WU) and self-lubricating units (SU) fabricated using NiCrSiB alloy and copper-clad graphite composite powders, respectively. During the friction process, the SU can continuously release lubricating media to reduce the wear of the WU, whereas the WU plays a supporting and fixing role in delaying the excessive consumption of SU, thereby synergistically enhancing the lubrication effect. Fengmin prepared a Ni/B4C/graphite/Si3N4 composite coating on the surface of a Ti-6Al-4V alloy using laser surface-alloying technology. A self-lubricating metal matrix composite with excellent performance was obtained by optimizing the powder ratio (Ni:B4C:C:Si3N4 = 1:1:1.5:0.2 in molar ratio) [76]. The coating hardness reached 1385–1687 HV, and its wear resistance improved by more than 13 times compared with that of the substrate. Through the synergistic effect of high-hardness ceramic phases (B4C and Si3N4) and a solid lubricant (graphite), the composite coating achieves a good balance of hardness, lubricity, and toughness, thereby significantly improving the wear resistance of the titanium alloy.
2.4. Composite Wear-Resistant Coatings
Composite materials are new types of materials formed by combining two or more materials with different properties (such as metals, ceramics, and polymers) using physical or chemical methods [77]. Their compositions include matrix materials (continuous phase) and reinforcing materials (dispersed phase). Through a synergistic effect, they achieve a comprehensive performance superior to that of a single material. Their core feature is structural designability; their performance can be optimized by adjusting the proportion and distribution of components. With the development of industrial technology, mechanical parts face increasingly harsh working conditions, and traditional wear-resistant materials of a single type cannot meet the application requirements. Researchers have developed multiphase composite materials to improve the service lives of parts. By adding one or more reinforcing phases or alloy components, they realized the synergistic optimization of the material hardness and toughness [78]. Composite wear-resistant coatings are specifically designed for wear-prone working conditions. By combining high-hardness phases (such as ceramics and carbides) with tough matrices (such as metals and polymers), wear and impact resistance can be significantly enhanced.
In 2018, Zai prepared a zirconia coating on the surface of a CoCrMo alloy via the sol–gel method while introducing graphene sheets into the UHMWPE matrix, which significantly improved the comprehensive performance of the joint surface. Both the friction coefficient and wear volume were reduced by 50%. This synergistic modification strategy effectively mitigates the easy wear of polymers and the easy corrosion of metals in traditional metal-polymer joints. The lubricating effect of graphene enhanced the wear resistance of UHMWPE, whereas the dense ZrO2 coating significantly improved the corrosion resistance of CoCrMo. The optimal ratio (ZrO2-CoCrMo (T500) and UHMWPE-G (0.2%)) exhibited a wear resistance 19.5 times higher than that of the untreated materials. Observing the worn surfaces shown in Figure 3, the surfaces in Figure 3a,b are rougher than those in (c) and (d). This provides a new solution for the long-term service of artificial joints.
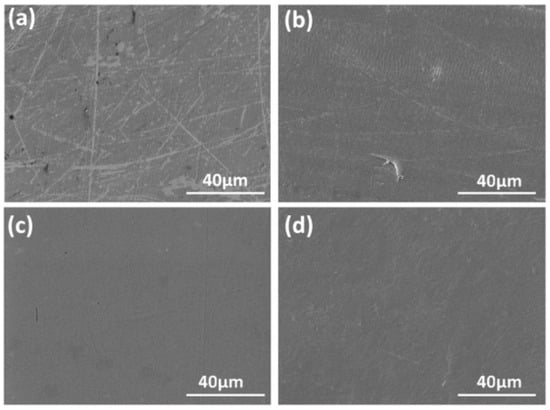
Figure 3.
Zai et al. Reprinted from Ref. [79] took the SEM micrographs of the worn surface of the UHMWPE-G composites: (a) CoCrMo, (b) UHMWPE, from wear test between CoCrMo and UHMWPE; and (c) CoCrMo-ZrO2 (T500), (d) UHMWPE-G (0.2%), from wear test between CoCrMo-ZrO2 (T500) and UHMWPE-G (0.2%).
In 2019, Ling developed a new type of PVDF/sulfonic-acid silica-gel porous composite membrane for vanadium redox flow batteries. After preparing the porous PVDF-based membrane using the phase-inversion method, sulfonic-acid silica gel was introduced to fill the pores via the sol–gel method [80]. Scanning electron microscopy (SEM) characterization showed that the composite membrane had a desirable porous structure, and its unique proton-transport channel design effectively inhibited vanadium-ion permeation while ensuring proton conduction. Tests conducted at a current density of 60 mA·cm−2 indicated that the single cell assembled with this composite membrane exhibited excellent performance, with a coulombic efficiency of 90.3% and an energy efficiency of 75.6%.
In 2020, Peng reported a new type of Ni-Fe LDHs composite coating [81]. It was prepared on the surface of carbon steel via a wet-dry cyclic corrosion method, and the loading of MoO42− significantly improved its corrosion resistance, achieving long-term and active anti-corrosion protection for Q235 steel.
2.5. Nano-Coatings
Nanowear-resistant coatings are materials prepared using nanotechnology. Nanoscale particles (such as nanoceramics, nanometals, and nanocarbon materials) are the core functional phase; they are uniformly dispersed in the matrix material and attached to the surface of objects through processes such as coating and spraying, which can significantly enhance the wear resistance of the substrate. Their core feature is that at least one phase in the coating has a size in the nanoscale range (1–100 nm), and extraordinary wear resistance is achieved by virtue of nanoeffects (such as the small-size and interface effects). Compared with traditional wear-resistant coatings, the small-size effect and high interface density of nanoparticles make the internal structure of the coating denser, and through the dispersion strengthening of nanoparticles, nanocoatings can improve toughness while maintaining high hardness, reducing the risk of cracking [82]. The thickness of nanocoatings can be controlled at the micrometer level without significantly increasing the weight of the substrate [83], making them suitable for scenarios with high lightweight requirements such as aerospace. In addition, the high activity of nanoparticles can improve the bonding force between the coating and substrate, thereby extending the service life. Nanocoatings are combined with 3D printing [84,85] and intelligent manufacturing to realize the precise and customized production of coatings, meeting the personalized wear-resistant needs of complex components.
In 2020, Kiryukhantsev-Korneev prepared Ti-Al-Ni-C-N nanocomposite coatings via direct-current magnetron sputtering and high-power impulse magnetron sputtering (HIPIMS) technologies in an argon atmosphere with different nitrogen concentrations (15% and 25%) [86]. The coatings were composed of a 3 nm FCC-TiCN phase and 15 nm Ni3Al phase, and their nanocrystalline structures were confirmed by X-ray diffraction and SEM analyses. The coatings exhibited optimal mechanical properties (hardness of 34 GPa) with a friction coefficient stably below 0.26 and wear rate lower than 5 × 10−6 mm3N−1m−1. HIPIMS technology significantly improved the bonding strength, tribological properties, and corrosion resistance of the coatings while reducing the friction coefficient and corrosion current density.
Movassagh-Alanagh deposited a multilayer nanocomposite coating on AISI 304 stainless steel using cathode arc evaporation technology [87]. The thicknesses of the coating layers were 0.71, 1.84, and 0.68 μm for the Ti, TiN, and TiSiN layers, respectively. The coating significantly reduced the friction coefficient to 0.1, increased the wear resistance by 18.7 times, and simultaneously improved the corrosion resistance.
Wang proposed a method for preparing nanotitanium carbide (TiC) functionally graded wear-resistant composite coatings on the surface of 40Cr gear steel using laser cladding technology, which significantly improved the wear resistance of the material under oil-starved lubrication conditions [88]. The microhardness of the graded coating increased from 612 to 1088 HV from the bottom to the top. Moreover, under heavy-load and oil-starved lubrication conditions, the friction coefficient was reduced by 50% and the wear loss decreased by 40%, significantly enhancing the wear resistance.
Dogus coated graphene oxide on the surface of chromium-coated steel rings. The corrosion resistance of the coating was evaluated, and the wear resistance of the coating was studied under lubricated conditions. The results showed that piston rings coated with graphene oxide exhibited excellent corrosion and wear resistance, with no wear marks on the surface [89]. Graphene is a 2D carbon nanomaterial composed of carbon atoms arranged in a hexagonal honeycomb lattice via sp2 hybridized orbitals. It is currently the thinnest known material (only one atomic layer thick) and appears to be a transparent and flexible film with a stable structure and extremely high mechanical strength. Graphene exhibits excellent mechanical properties, good thermal and electrical conductivity, and outstanding barrier-protection capabilities. These characteristics enable graphene to show great application potential in numerous fields, such as electronic devices [90,91], energy storage [92], composite materials [93], and coating protection [94].
3. Preparation Technologies for Wear-Resistant Coating Materials
3.1. Thermal Spraying Technology
Thermal spraying technology is a surface-treatment technique that uses a heat source to heat-spray materials (such as metals, alloys, ceramics, and plastics) to a molten or semimolten state, atomizes them with a high-velocity airflow, and sprays them onto the substrate surface to form a coating with specific functions [95]. Coatings prepared by thermal spraying are dense and have high surface roughness, which is conducive to secondary coating and enhances mechanical adhesion. In thermal spraying technology systems, flame spraying [96], arc spraying [97], and plasma spraying [98] are the main process methods, among which flame spraying and plasma spraying are widely used in the industry. This technology not only significantly improves the physical and chemical properties of coatings, such as wear and corrosion resistances [99], but also has important value in the field of mechanical part repair. Typical application scenarios include the repair of key components, such as aero-engines, oil-drilling equipment, and automotive power systems. From the perspective of functional expansion, thermal-spraying technology can also be used for industrial applications such as tool and mold manufacturing, surface hardness enhancement, and the anti-corrosion treatment of mechanical parts. Using customized material system design, this technology can be used to prepare composite coatings with conventional properties, such as wear, corrosion, and high-temperature resistance or special functions, such as electromagnetic shielding and heat insulation. This technology is highly compatible with different substrate types and geometric shapes. Its process characteristics are primarily reflected in its wide spraying range, high operational flexibility, and strong onsite adaptability. These advantages render it a highly versatile surface-strengthening solution. In the field of engineering practice, this technology has been successfully applied in key scenarios, such as steel-structure anti-corrosion, ship anti-corrosion, hot corrosion protection of water-cooled walls of power-plant boilers, and the repair of high-value equipment. It has shown significant technical advantages, especially in the treatment of large aluminum-alloy structural parts [100].
Each different thermal spraying process has its own advantages and disadvantages, Table 2 compares different spraying processes, which is convenient the selection of coating process to meet different needs.

Table 2.
Saroya et al. Reprinted from Ref. [101] compared different thermal spraying processes.
3.1.1. High-Velocity Oxygen Fuel Spraying
High-velocity oxygen fuel spraying (HVOF) is an important process in thermal-spraying technology and represents an upgrade from conventional flame spraying. This significantly improves the coating quality by increasing the flame-jet velocity [102]. The thickness and uniformity of the coating can be adjusted by modifying the number of spraying layers and spraying distance [103]. However, it incurs higher equipment costs and process complexities. Its working principle is as follows: fuel (such as kerosene and propane) is mixed with oxygen in a combustion chamber and ignited to generate a high-temperature, high-pressure flame (typically 2000–3000 °C). The high-velocity flame jet (with a speed exceeding 1000 m/s) heats the sprayed material to a molten or semimolten state while driving these particles to spray onto the substrate surface at a high speed, eventually forming a dense coating with high bonding strength (as shown in Figure 4). HVOF coatings have low porosity [104]; thus, they have wide applications in fields such as wear and corrosion resistance. Hai-Long prepared WC-Cr3C2-12Ni coatings on steel substrates using HVOF technology and investigated their coating properties under different oxygen flow rates; the coatings exhibited low porosity, uniform microstructure, and good mechanical properties [105]. Wu prepared Fe-Cr-Si-B-Mn coatings on the surface of 1Cr18Ni9Ti stainless steel via HVOF thermal spraying and tested their cavitation erosion resistance in freshwater environments. The experimental results indicated that HVOF thermal spraying is an effective method for preparing cavitation erosion-resistant coatings [106]. Samantha fabricated two types of coatings (with and without graphite layers) on tool steel using HVOF technology, and the calculated wear rate was reduced by an order of magnitude compared to similar coatings [107]. C. R C L conducted the first comparison between HVOF spraying and GTAW hot-wire surfacing technology [108]. This study examined the differences in the wear and corrosion properties of Stellite 6(R) coatings; HVOF-sprayed coatings performed better in abrasive wear, while GTAW hot-wire surfacing coatings showed higher resistance in erosive wear and corrosion tests.
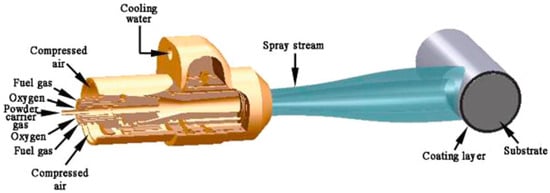
Figure 4.
Buytoz et al. Reprinted from Ref. [109] presented a schematic diagram of the HVOF system.
3.1.2. Arc Spraying
The core principle of arc spraying is to use the high temperature generated by an electric arc to melt metal wires, atomize the molten metal with a high-velocity airflow, and spray it onto the substrate surface to form a coating (Figure 5). The specific process is as follows. Two metal wires, serving as the spraying materials, are used as electrodes. When the power is turned on, the ends of the two wires come into contact, generating an electric arc. The high temperature of the arc (typically above 3000 °C) causes the ends of the metal wires to melt rapidly. A high-velocity airflow is formed by compressed air (or other gases) that blows directly toward the molten ends of the metal wires, atomizing the molten metal into fine droplets. Driven by the high-velocity airflow, the atomized metal droplets are sprayed at a very high speed onto a pretreated (such as a derusted and roughened) substrate surface. After impacting the substrate, the droplets cool and solidify rapidly, stack on top of each other, and form a dense coating. Compared to other thermal-spraying technologies, this process has a higher deposition rate.
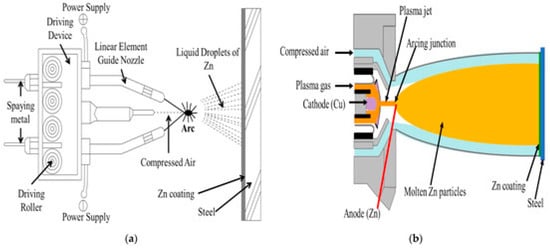
Figure 5.
Jitendra et al. Reprinted from Ref. [110] presented the schematic representation of (a) arc and (b) plasma arc thermal spraying processes.
Laima prepared FeMnCr/Cr3C2 coatings using high-velocity arc spraying and investigated their high-temperature erosion behavior under different impact angles. The erosion rate was the highest at an impact angle of 60°, and the erosion performance was superior to that of 20# steel [111].
Lei deposited zinc and aluminum coatings on the surface of Q235 carbon structural steel via arc spraying and used silicone resin for pore sealing to improve their corrosion resistance. The pore-sealed aluminum coating exhibited better corrosion resistance than the zinc coating in seawater environments containing sulfate-reducing bacteria [112]. In addition, the arc-spraying technology is used for other metal-composite coatings, such as aluminum-copper composite coatings, which possess excellent anticorrosion and antifouling properties [113].
Ndumia prepared FeCrMoCBWNb coatings on 65Mn and ASTM A238C steels via arc spraying and studied the thermal-shock behavior of the coatings on different substrates. The thermal-shock performance of the coating on ASTM A238C steel was superior to that on 65Mn, and the difference was mainly determined by the initial bonding strength [114]. Moreover, the performance mismatch between the coating and substrate affected the thermal-shock performance of the coating. As the number of thermal cycles increased, the iron compounds and oxide layers at the interface and splash boundaries increased, leading to internal cracks and spalling of the coating.
3.1.3. Plasma Spraying
Plasma spraying utilizes extremely high temperatures of plasma to melt materials and achieve high-velocity spraying. An electric arc is ignited between the cathode (tungsten electrode) and anode (copper nozzle) via a DC power supply (40–80 V). Inert gases such as argon and nitrogen are introduced and ionized by an intense arc to form the plasma. The powdered spray materials are then fed into a plasma-flame stream. The molten materials, driven by the high velocity of the plasma-flame stream (reaching several hundred meters per second), are sprayed onto the pretreated substrate surface. After rapid cooling and solidification, they accumulate to form a coating. A key advantage of plasma-spraying technology is its ability to process high-melting-point materials [115] because plasma releases thermal energy when recombined into a gaseous state during the spraying process. The instability of plasma causes ions in the flame stream to rapidly recombine and cool, with the temperature at the recombination point ranging from 6600 °C to 16,600 °C. Despite the extremely high temperature of the plasma-flame stream (which can exceed 10,000 °C), the surface temperature of the substrate is typically controlled below 150 °C. The coating properties of this process are influenced by multiple factors, including equipment parameters (spray gun type and power output), process parameters (gas type, flow rate, spray distance [116,117], and moving speed), material parameters (powder characteristics and feeder specifications), and environmental control (substrate-cooling conditions). Thus, it can be flexibly applied to scenarios with strict coating-quality requirements. Powder feeding can adopt internal [118] or external injection [119] methods. The powder particle size significantly affects the coating properties; fine particles move slowly but reach higher temperatures in the upper part of the molten pool, forming low-density coatings with high porosity between the lamellae.
3.2. Vapor-Deposition Technology
3.2.1. Physical Vapor Deposition
Physical-vapor deposition (PVD) is a technique that converts solid target materials (such as metals and ceramics) into vapor-phase particles through physical methods (e.g., sputtering, ion plating, and evaporation) in a vacuum environment, which are then deposited onto a substrate surface to form a coating. No chemical reactions occur during this process, and the film is formed solely by the migration and accumulation of atoms and molecules. This method enables the preparation of high-hardness, wear-resistant coatings.
Magnetron Sputtering
Magnetron sputtering (MS) is a PVD method based on the principle of glow discharge. In a vacuum environment, an electric field ionizes an inert gas (typically argon) to generate plasma. Positively charged Ar+ ions bombard the surface of the target material (serving as the cathode) at high speed under the action of an electric field, causing the target atoms to be “sputtered” out. Moreover, a magnetic field is applied near the target surface to confine electrons using the Lorentz force, extending their movement path in the plasma and increasing the probability of collisions with gas atoms, thereby improving the ionization efficiency and sputtering rate. The sputtered target atoms are eventually deposited on the substrate surface to form a thin-film coating (Figure 6). This technology has undergone three generations of development: traditional magnetron sputtering has a limited plasma confinement area (less than 60 mm from the target surface); unbalanced magnetron sputtering expands the plasma to a range of 100–150 mm from the target surface through an optimized magnetic-circuit design; and closed-field unbalanced magnetron sputtering forms high-density plasma through a symmetrical magnetic-field configuration, increasing the deposition rate. This technology can be categorized into cold-target and hot-target magnetron sputtering [120]. Key process parameters include the sputtering power, target-substrate distance, and deposition temperature, which synergistically regulate the structure and performance of the coating. Owing to the high energy of the sputtered atoms, they combine more tightly with the substrate, resulting in strong coating adhesion. This technology also provides good coverage on substrates with complex shapes, which can reduce the “shadow effect.” However, it requires complex magnetron and vacuum systems, leading to high initial investment and maintenance costs.
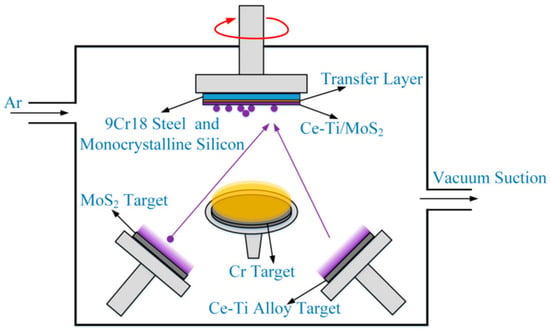
Figure 6.
Tian et al. Reprinted from Ref. [121] have drawn the principal diagram of the magnetron sputtering deposition process: The red circle indicates the rotation of the sample stage, while the purple dots represent the sputtered atoms or ions.
Researchers have combined plasma-spraying technology with PVD. Zhang proposed an improved PS-PVD method to prepare Al2O3-modified 7YSZ thermal-barrier coatings. This method effectively addresses the insufficient CMAS corrosion resistance and oxidation performance of traditional PS-PVD 7YSZ coatings, thereby providing a potential solution for advanced gas-turbine engines. Subsequently, through 350 water-quenching thermal cycles and particle erosion tests, the particle-erosion resistance and thermal-cycle performance of Al-modified coatings were significantly improved [122].
Ion-Plating Technology
Ion plating is an emerging technology. In a vacuum environment, coating materials are converted into vapor-phase particles through heating methods (such as evaporation and sputtering). Gas discharge (e.g., glow discharge) is utilized to ionize vapor-phase particles and inert gases (e.g., argon) to form plasma. A high-voltage electric field is applied between the substrate (serving as the cathode) and evaporation source (or anode). Positively charged ions bombard the substrate surface at high speed under the action of the electric field: this cleans the substrate (removing impurities and oxide layers) and endows the deposited particles with high energy. The particles then undergo strong physical and chemical interactions with the substrate surface, ultimately forming a thin film coating with strong adhesion (Figure 7). High-energy ion deposition can reduce the number of pores and defects in the film, resulting in a more uniform structure and higher density along with excellent wear and corrosion resistance. However, ion bombardment can generate heat. If the substrate is a heat-sensitive material (such as plastic), a high temperature may cause deformation or performance degradation.
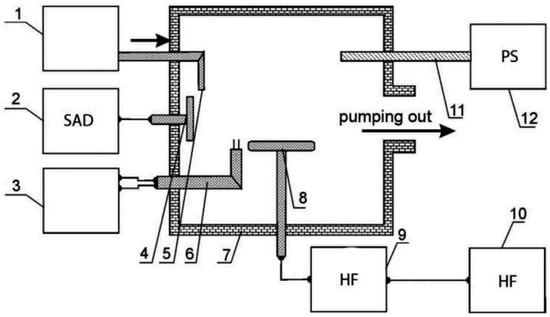
Figure 7.
Pogrebnjak et al. Reprinted from Ref. [123] have presented a flowchart of the vacuum arc discharge coating synthesis technique: 1—the gas-feeding device, 2—the sources for arc-discharge feeding, 3—the measuring probe, 4—the cathode, 5—the gas line for gas-feeding, 6—the double movable probe, 7—the vacuum chamber, 8—the substrate, 9—the device to match the HF-generator, 10—the HF-generator, 11—the quartz tube for dissociation of working gas molecules, 12—the power source.
Taran prepared nanostructured ZrN coatings on AISI 430 stainless steel at 150 °C using vacuum-arc ion-plating technology combined with radio-frequency discharge. The coatings exhibited a hardness of 26.6–31.5 GPa and showed excellent corrosion resistance in physiological saline. This technology addresses the issues of high temperatures and large particle defects associated with traditional arc plating, providing a new approach for low-temperature, high-performance protective coatings [124].
Wang prepared three types of coatings on 316 stainless-steel substrates using a multiarc ion-evaporation method [125]. The multilayer design significantly enhanced the corrosion resistance of the coatings through the triple synergistic effect of structural regulation (changing growth modes and plugging pinholes), defect suppression (reducing microcracks and porosities), and interface engineering (forming coherent interfaces and composite passivation films). The corrosion resistance of the TiAlN/CrN superlattice coating was more than three times higher than that of the single-layer coating.
Vacuum Evaporation Deposition Technology
Vacuum evaporation deposition is an early-developed technology in the field of PVD. In a high-vacuum environment, the material to be deposited is heated and evaporated into gaseous atoms or molecules. These gaseous particles move in the vacuum environment, are deposited onto the surface of the substrate to be coated and form a uniform thin-film coating through cooling and condensation. Compared with technologies such as sputtering, it has a higher evaporation rate (up to several micrometers per minute), making it suitable for mass production. The equipment has a relatively simple structure, which is convenient for maintenance and is inexpensive. However, this technology has limitations as preparing high-hardness coatings is difficult; thus, it is more suitable for manufacturing decorative and functional films (e.g., reflective and conductive films). Its applicability to high-performance coatings (e.g., wear- and high-temperature-resistant coatings) is relatively low.
Qiu deposited zinc coatings on interstitial-free steel sheets and other substrates using vacuum thermal-evaporation coating technology in a high-vacuum environment [126]. This technology avoids the oxidation and hydrogen-embrittlement problems of traditional hot-dip galvanization and has lower zinc consumption. Substrate temperature control is the key to achieving high-quality zinc coatings, providing a theoretical basis for industrial applications (such as anticorrosion of automotive steel sheets).
Surface-pretreatment methods can affect the performance of PVD coatings. Wolfgang was the first to investigate the effects of different surface-pretreatment methods on the surface integrity of WC-Co substrates and subsequent performance of PVD coatings [127]. Surface-pretreatment methods include heating, inert ion etching, metal-ion etching, and HIPIMS (Figure 8). This study showed that metal-ion etching and HIPIMS significantly increased the surface roughness, providing conditions for mechanical interlocking. Metal-ion etching released residual stress (by removing the Co phase) and substantially increased surface energy (58.6 mN/m), thereby enhancing wettability. Metal-ion etching is the preferred pretreatment for WC-Co substrates, improving the adhesion by 125%. Through the selective removal of the Co phase, it achieves the synergistic optimization of “exposed hard phase + high surface energy,” maximizing the adhesion of PVD coatings.
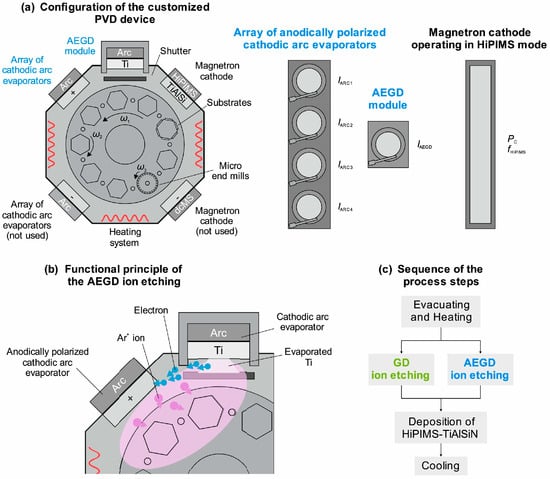
Figure 8.
Nelson et al. Reprinted from Ref. [128] Analyzed: (a) Configuration of the customized PVD device for AEGD ion etching and deposition of HiPIMS-TiAlSiN; (b) functional principle of the AEGD ion etching, and (c) sequence of the process steps.
3.2.2. Chemical Vapor Deposition
Chemical vapor deposition (CVD) is used to generate solid wear-resistant coatings through chemical reactions of gaseous chemical substances on the substrate surface (Figure 9). The core principle involves the introduction of gaseous precursors containing coating elements into the reaction chamber. Under particular temperature and pressure conditions, the precursors undergo reactions such as decomposition and combination on the substrate surface to form dense and uniform coatings while releasing byproduct gases. The wear-resistant coatings prepared by this method have a high bonding strength with the substrate and a uniform and dense structure, and the coating thickness and composition can be precisely controlled. It is suitable for preparing high-hardness and high-wear-resistance ceramic-based coatings (such as titanium nitride (TiN) and tungsten carbide (WC)) and is widely used in wear-resistant components such as cutting tools, molds, and bearings.
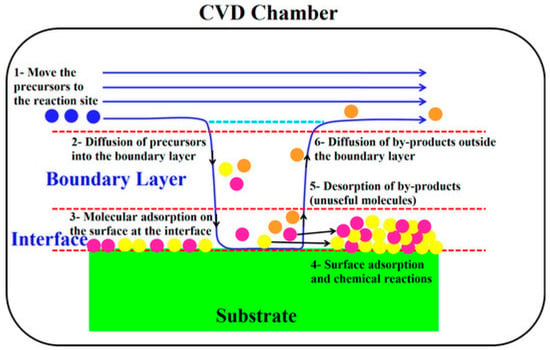
Figure 9.
Sabzi M et al. Reprinted from Ref. [129] presented a flow diagram of the main steps of the CVD process.
Kellermann deposited diamond films on C35 steel pretreated with a diffused chromium-carbide interlayer using the hot filament CVD (HFCVD) method [130]. They investigated the effects of different surface-roughness treatments on the tribological properties of steel and showed that the pretreatment significantly influenced the friction and wear characteristics of the coatings, providing a reference for the application of diamond coatings to steel components, such as mechanical seals.
Xiao proposed a new method for preparing diamond films with crack patterns on stainless-steel surfaces using W/W-N films as the interlayer and HFCVD technology [131]. The wear resistance of the films significantly improved, making them applicable to industrial stainless-steel scenarios requiring wear-resistant diamond coatings.
Muresan prepared hydrogenated diamond-like carbon double-layer protective coatings with a silicon-oxide interlayer on high-speed steel using plasma-enhanced CVD and confirmed that the coatings possessed high hardness and strong adhesion [132].
3.3. Surface-Modification Technology
3.3.1. Laser Cladding
Focusing on theoretical breakthroughs and principal discoveries, the development originated from Einstein’s proposal of stimulated emission theory in 1917, and Maiman developed the first ruby laser in 1960 to realize theoretical verification. Laser cladding technology has a development history of nearly 50 years. The AVCO Corporation led the application of this technology to repair vulnerable parts. The University of Sheffield designed a series of experiments to evaluate the performance of multiple repair positions in a single test [133]. Research has shown that laser cladding can be used as an on-site repair method to extend the service lives of railway rails. Subsequently, Rolls-Royce PLC applied this technology to clad a cobalt-based alloy protective layer on the surface of engine blades and verified the performance-improvement effect through hardness and wear-resistance tests. Because of its great potential and significant benefits, this technology has been widely used in many industrial fields. For example, the laser cladding of tungsten carbide coatings can reduce volume loss by up to 95% [134].
The core of laser cladding technology lies in utilizing the high energy density of lasers to achieve the rapid melting and solidification of materials. This process produces coatings with excellent properties (such as wear, corrosion, and high-temperature resistances) while minimizing the thermal impact on the substrate. It is classified as a green processing technology, with advantages such as low dilution rate, high bonding strength, high material-utilization rate, and low pollution. Specifically, it works by using a high-energy laser beam (e.g., CO2 laser and fiber laser) to irradiate the cladding material (which can be in powder, wire, or other forms) that is either pre-placed or fed synchronously. This irradiation causes both the cladding material and thin surface layer of the substrate to melt simultaneously, forming a molten pool. As the laser beam moves away, the molten pool rapidly solidifies, thereby creating a high-performance cladding layer on the substrate surface, which forms a metallurgical bond with the substrate. A stable and adjustable rotating magnetic-field device can be used to assist the laser cladding system (Figure 10).
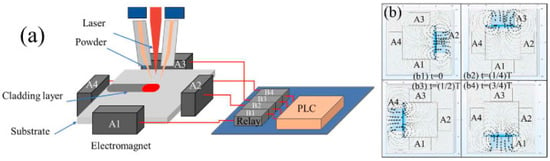
Figure 10.
Jiang et al. Reprinted from Ref. [135] presented the schematic diagram of the rotating magnetic field equipment-assisted laser cladding (a) and operation mode (b); The self-made rotating magnetic field system consisted of four electromagnets, four solid-state relays, a PLC, and a working power supply.
Compared with traditional surface plating and thermal-spraying processes, this technology can be used to prepare continuous large-area thick cladding layers, effectively meeting the needs of life extension and remanufacturing of engineering components. Specifically, laser cladding technology features a small heat-affected zone, and the rapid action of the laser minimizes the thermal deformation of the substrate, making it suitable for processing precision areas. It also offers excellent microstructural performance; the cladding layer has fine and uniform grains, along with high hardness, wear resistance, and corrosion resistance. Additionally, it boasts high process controllability [136]; the laser acts for a short time and the dilution rate is low, enabling precise control of the alloy composition. It exhibits broad material compatibility, supports a variety of powdered materials, and is particularly suitable for cladding high-melting-point alloys onto low-melting-point substrates. Furthermore, it has a high degree of automation with stable and reliable processes, allowing precise regulation of the composition and thickness of the cladding layer.
Owing to these advantages, this technology has been widely applied in fields such as metallurgy, energy, aerospace, and machinery manufacturing in recent years. Xing prepared Al-TiC-CeO2 composite coatings via laser cladding technology, investigated the microstructure and properties of the coatings under different laser powers, and showed that the coatings exhibited excellent metallurgical bonding, hardness, and corrosion resistance. At a laser power of 1.6 kW, the coating showed a high corrosion resistance [137]. However, the main factor hindering the industrialization of laser cladding technology in China is the insufficient quality stability of the cladding layer. Owing to extremely fast heating and cooling rates and differences in the thermophysical properties of materials, defects such as pores and cracks are prone to occur. In addition, online detection and automatic control technologies for the cladding processes remain immature. Moreover, the cladding layer is sensitive to cracking. Although studies on the crack-formation mechanism have been conducted, effective control methods remain underdeveloped, restricting its engineering applications.
Wang prepared Y2O3-added WC-reinforced Ni-based composite coatings on Ti-6Al-4V titanium-alloy substrates using laser cladding technology. The coatings exhibited remarkable microstructures and performance, achieving good metallurgical bonding with the substrate and enhancing the hardness and wear resistance of the coatings [138].
Chen showed that by adding different amounts of BiFeO3 to nickel-based WC coatings and using composite-field laser cladding technology, the hardness and wear resistance of the coatings are effectively improved. They showed that 5 wt% BiFeO3 was the optimal addition, and the best performance was achieved under a composite energy field of 40 mT [139].
Compared to conventional laser cladding, ultrahigh-speed laser cladding represents an innovation and improvement in the technology [140]. As a high-efficiency process developed from traditional laser cladding, ultrahigh-speed laser cladding achieves a cladding rate of 10–200 m/min. Through a specially designed optical system (e.g., a high-speed scanning galvanometer) and powder feeding device, the laser beam scans the substrate surface at an extremely high speed (usually several meters to dozens of meters per second) while precisely controlling the feeding amount and distribution of the cladding powder. The laser melts only the powder and an extremely thin layer (micrometer scale) on the substrate surface, forming a very thin molten pool with a faster solidification rate. Eventually, a cladding layer with a more uniform and thinner thickness (typically 50–500 μm) is formed on the substrate surface. In Yu’s experiment, Ni-based WC coatings were prepared using ultrahigh-speed laser cladding, with low-speed laser cladding used as a control [141]. The coatings prepared by ultra-high-speed laser cladding exhibited superior surface quality, lower heat input, and a faster cooling rate. Additionally, the coating-dilution rate was reduced, thermal damage was minimized, carbide precipitation and pore formation were inhibited, and the uniform distribution of WC in the coating was promoted. These improvements significantly reduced stress concentration and crack nucleation in the coating and decreased surface defects, such as coating porosity and cracks.
3.3.2. Electrospark Deposition
Currently, among the preparation technologies for wear-resistant coatings on steel surfaces, in addition to the commonly mentioned thermal spraying, PVD, and laser cladding methods, electrospark deposition (ESD) is the most widely used and enables rapid coating preparation. ESD is derived from electrical-discharge machining technology and is a surface-modification process based on the principle of pulsed discharge. The core mechanism is the directional transfer and deposition of materials through high-energy micro-arc discharge. The process consists of two main components: the material to be deposited (serving as the anode, connected to the positive pole of the pulsed power supply) and substrate workpiece (serving as the cathode, connected to the negative pole), which is conducted in a protective-gas or reactive-gas environment. The preparation of wear-resistant coatings via ESD involves two key steps: discharge and material deposition. During the discharge process, the electrode contacts the substrate at a specific frequency during the vibration and rotational movement. Instantaneous micro-arc discharge occurs at the moment of contact, causing the temperature in the discharge area to rise sharply and melt the electrode material into droplets. In the material-deposition process, droplets are ejected onto the substrate surface under the action of an electric field and gas dynamics to form a molten pool, which achieves metallurgical bonding with the surface layer of the substrate and solidifies rapidly to form deposition spots (Figure 11). By repeating this process, a completely deposited layer is formed.
Figure 11.
Claudia et al. Reprinted from Ref. [142] presented a flowchart of the electrospark deposition technique. (a) First stage: the electrode is moved until the tip locally contacts the substrate; (b) Second stage: a pulsed electrostatic discharge is generated between the electrode tip and the substrate, and the large amount of supplied energy causes fused droplets of electrode material and a small amount of substrate material to form; (c) Third stage: the molten droplet is rapidly driven into the melt pool, impinging on the substrate and solidifying; and (d) Fourth stage: the electrode and substrate contact again, initiating a new discharge cycle.; The yellow boxes represent the pulse signals, and the orange lines represent the sparks generated by discharge.
Korkmaz investigated the structural characteristics, mechanical properties, and corrosion resistance of chromium carbide coatings prepared on micro-alloyed steel using ESD technology. A hard and relatively thick coating was achieved by optimizing the electrical parameters [143]. The microhardness of the coating reached 1381 HV, which was significantly higher than that of the substrate (227 HV), and its corrosion resistance improved by approximately 30%.
Kudryashov prepared a barrier layer on an SPHN-60 white cast-iron substrate using ESD technology with Ni and Cr as the electrode materials. Subsequently, a double-layer protective coating was constructed by combining SHS-modified cemented carbide electrodes (e.g., TiC-NiAl and TiB2-NiAl). The coating-formation characteristics (phase composition, surface roughness, and mass-transfer kinetics) and adhesion performance with the substrate were systematically analyzed [144].
3.4. Other Preparation Technologies
3.4.1. Plasma Surfacing Welding
Plasma surfacing welding uses a plasma arc (a high-temperature, high-energy electric arc) as the heat source to melt the filler material (e.g., alloy powder and welding wire) while slightly melting the surface of the substrate. After the two fuse and cool, a surfacing layer with strong bonding to the substrate is formed. The high temperature of the plasma arc enables the rapid melting and solidification of the materials, and the coating composition can be adjusted flexibly. This technology is suitable for heavy-duty wear-resistant components (such as rolls and molds).
3.4.2. Submerged Arc Surfacing Welding (SASW)
In submerged arc surfacing welding (SASW), an electric arc burns beneath a layer of granular flux (a protective layer). The welding wire and substrate are heated and melted by the arc, and the flux melts to form a slag that covers the molten pool, isolating it from the air and participating in metallurgical reactions. After cooling, a surface coating is formed. This technology features a high welding current, large penetration depth, and thick coating (several to tens of millimeters), making it suitable for components requiring large-area coverage and high wear resistance (such as buckets and crusher jaw plates).
3.4.3. Electroplating
Electroplating utilizes electrolysis as follows: the substrate is used as the cathode, and the metal to be deposited (e.g., chromium and nickel) is used as the anode. Both are immersed in an electrolyte solution containing ions of the target metal. When direct current is applied, the metal ions are reduced and deposited on the surface of the cathode (substrate) to form a coating. For example, chromium plating has high hardness and wear resistance and is widely used in components such as shafts and molds.
3.4.4. Electroless Plating
Electroless plating does not require an external power supply. It relies on chemical reactions (redox reactions) to spontaneously reduce metal ions (e.g., Ni2+ and Co2+) in solution and deposit them on the substrate surface to form a coating. The reaction proceeds under the action of a catalyst (e.g., active sites on the substrate surface). The coating exhibits excellent uniformity and can cover surfaces with complex shapes. Electroless nickel-phosphorus alloy plating is commonly used to prepare wear-resistant coatings.
3.4.5. Sol–Gel Method
The sol–gel method involves dissolving precursors, such as metal alkoxides, in a solvent. A sol (colloidal solution) is formed via hydrolysis and polycondensation reactions. After the sol is coated onto the substrate surface, it undergoes drying and sintering (to remove solvents and organics and induce crystallization) to form oxide or composite oxide coatings (e.g., Al2O3 and ZrO2). This method can be used to prepare ceramic wear-resistant coatings with uniform compositions and high purity and is suitable for substrates that require low- or room-temperature processing (such as plastics and glass).
4. Evaluation of Wear-Resistant Coating Performance
4.1. Wear Resistance
The ability of the coating to resist material loss under specific friction conditions is the most critical evaluation index. It is typically determined through wear tests by the amount of wear (such as mass and volume loss) per unit time or distance; the lesser the wear, the better the abrasion resistance. A friction and wear test machine is commonly used to evaluate the abrasion resistance, and the instrument and its schematic are shown in Figure 12. The core principle of a friction wear test machine is to simulate friction under actual working conditions, allowing the coating sample to move relative to the counterpiece (such as a metal or grinding wheel) and apply a load. The abrasion resistance of the coating is quantitatively evaluated by detecting the physical changes during motion. The coating surface gradually produces wear (such as surface peeling material transfer) owing to mechanical action, and the key indicators are recorded or calculated in real time through sensors, including the friction coefficient (reflecting the size of the friction resistance) and amount of wear (measured by mass loss, volume loss, or depth of wear traces). Finally, these data are used to determine the abrasion resistance of the coating.
Figure 12.
Wei et al. Reprinted from Ref. [145] conducted friction and wear tests (a) Equipment (RTEC-MFT-5000, Rtec Instruments, San Jose, CA, USA) and drew (b) working diagram.
Currently, three main methods are used to evaluate wear: wear loss, wear resistance, and wear ratio.
The wear loss is divided into linear wear loss (Wl), volume wear loss (Wv), and weight wear loss (Ww).
Wear resistance refers to the ability of a material to withstand wear under certain working conditions. The most commonly used expression of wear resistance is the reciprocal of the volume wear loss. Material wear resistance can be classified into two types: relative and absolute. The relative wear resistance (ε) of a material is the ratio of the wear loss of two materials (A and B) under the same external conditions, where one of the materials (A) is a standard (or reference) sample. The formula is εA = WA/WB.
The wear ratio is used to measure wear during the erosion wear process and is calculated as wear ratio = erosion wear loss of the material/amount of abrasive used to cause the wear loss.
4.2. Bonding Strength
The coating bonding strength refers to the ability of the coating to resist separation from the substrate material or from other layers within the coating, essentially serving as an indicator of the degree of firm adhesion of the coating. If the bonding strength is insufficient, even if the coating itself has high hardness and good wear-resistance, it is prone to delamination and peeling under a friction load. Thus, the wear will intensify directly, leading to a significant decrease in wear resistance and its practical use value. Only when the bonding strength meets the standard can the wear-resistant characteristics of the coating be normally exerted, and the subsequent wear resistance data (such as the wear amount and friction coefficient) measured using the friction and wear tester have practical reference significance.
4.3. Hardness
Coating hardness refers to the ability of a coating to resist external mechanical penetration, scratching, or deformation and is a key indicator of the mechanical strength of the surface layer of a coating material, reflecting the ability of the coating to resist local plastic deformation.
The commonly used microhardness (such as Vickers hardness and nanohardness) is measured. Coatings with higher hardness values typically have stronger resistance to plastic deformation and wear; however, excessive hardness may lead to increased brittleness. Figure 13 schematically shows the Vickers microhardness test for HV-100 and its testing conditions. The Vickers hardness tester achieves testing through “ation diagonal length-conversion hardness value.” A diamond indenter in the shape of a regular square pyramid (with a top angle of 136°) is pressed vertically into the coating surface under a preset load (typically from a force of a few grams to tens of kilograms) and maintained for a certain period. After unloading, a square indentation remains on the surface of the coating. The indentation size is inversely proportional to the hardness of the coating (the higher the hardness, the smaller the indentation). The average lengths of the two diagonals of the indentation are measured using a microscope and substituted into the formula HV = 0.189 × F/d2 (where F is the load and d is the average length of the diagonal) to obtain the Vickers hardness value (HV).
Figure 13.
Schematic diagram of the HV-100 Vickers microhardness tester and its test sample situation.
4.4. Corrosion Resistance
Coating corrosion resistance refers to the ability to resist the erosion of environmental media (such as acids and alkalis, salt fog, and water vapor). If the coating has poor corrosion resistance, the substrate or coating itself will be damaged by corrosion, which indirectly affects the wear resistance and service life. It is commonly evaluated using methods such as cavitation erosion, salt spray, and immersion tests. Scratch tests can be performed before the corrosion tests (Figure 14) to accelerate the failure process. By scratching, the surface protective layer is destroyed, and “channels” for the penetration of corrosive medium are manufactured. Thus, the bonding strength between the substrate and coating and the overall corrosion resistance can be quick. However, the scratch method is not applicable to all types of coatings, such as flexible coatings that are prone to deformation with scratches and extremely thin coatings can be damaged by scratches that directly affect the substrate.
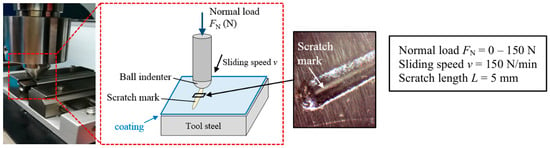
Figure 14.
Sulaiman et al. Reprinted from Ref. [146] captured the scratch pattern of the sample and drew the experimental schematic.
4.5. High-Temperature Resistance
High-temperature coating resistance refers to the ability of a material to maintain stable physical and chemical properties (such as hardness, bonding strength, and wear resistance) in a high-temperature environment. Specifically, this manifests as no oxidation, softening, decomposition, or harmful reactions with the substrate or environment within a particular temperature range, ensuring that the coating can still exert its protective effects under high-temperature working conditions. A common evaluation method is to place the coating in a predetermined high-temperature environment for a continuous period and then detect changes in its properties (e.g., hardness-retention rate and presence of peeling).
4.6. Other Key Properties
4.6.1. Coefficient of Friction
The coefficient of friction is a dimensionless parameter that describes the ratio of the frictional force to the normal force between two contacting surfaces and is divided into static and dynamic coefficients of friction. It reflects the magnitude of resistance during the relative motion of objects and is a key indicator for evaluating the wear resistance of materials or coatings. The changes in its value reflect the wear resistance of the coating. Studying the dynamic characteristics of the coefficient of friction helps control the loss behavior during friction and optimizes wear resistance. Generally, the coefficient of friction of a coating is significantly affected by the surface conditions (e.g., roughness and oxide film). The value of the coefficient of friction is directly related to the wear characteristics of the coating; a low coefficient of friction typically indicates a lower wear rate and longer service life. By analyzing the changes in the coefficient of friction, the coating-preparation processes can be optimized, such as by adjusting the surface roughness or adding lubricating components to reduce frictional loss.
4.6.2. Toughness
Toughness is the ability of a coating to resist fractures when subjected to stress or impact. Coatings with insufficient toughness are prone to cracking under impact loads or alternating stresses, which exacerbates wear. The crack resistance can be evaluated through impact or bending tests.
4.6.3. Impact Resistance
The impact resistance refers to the ability of a coating to resist deformation, cracking, or delamination when subjected to sudden impact forces or varying impact loads. In scenarios in which impacts are common (e.g., impact of mechanical components and scouring of materials), poor impact resistance leads to rapid failure of the coating. It is typically measured by impact tests (e.g., drop-weight impact tests) and is characterized by the impact energy or number of impacts when the coating is damaged.
4.6.4. Insulation Property
Insulation refers to the ability of a coating to prevent the flow of current, primarily for abrasion-resistant coatings that need to be functionally insulated (such as abrasion-resistant parts in electrical equipment). It is commonly measured by the volume resistivity or surface resistivity; the higher the resistivity, the better the insulation property. During evaluation, measurements must be conducted under specific temperature and humidity conditions to reflect the insulation performance in actual service environments.
4.6.5. Adhesion
Adhesion refers to the degree of firm bonding formed between the coating and the substrate (such as metal, plastic), or between different layers of multi-layer, through physical adsorption, chemical bonding and other actions, which is the foundation for the coating to stably exert its performance.
When the adhesion is used to evaluate the performance of the wear-resistant coating on the steel surface, the wear-resistant coating needs to withstand the shear force and impact force during the friction process. If the adhesion is insufficient, even if the coating itself has high hardness and good wear resistance, it will peel off and fall off after being stressed, resulting in the substrate being directly exposed and worn. Therefore, adhesion is the “lifeline” of the wear-resistant coating—the stronger the adhesion, the closer the coating is combined with the steel substrate, and it can more stably transfer and bear the wear stress, the higher the durability and reliability of the wear resistance; on the contrary, the wear resistance of the coating with poor adhesion cannot be effectively exerted, and even the wear resistance of the coating will accelerate the wear of the substrate.
4.6.6. Cohesion
Cohesion refers to the bonding force between molecules or particles within the coating material, enabling the coating itself to resist cracking, delaminating, or breaking, and it acts on the “interior of the coating.”
5. Conclusions and Prospects
5.1. Conclusions
This study systematically reviews and analyzes the types of and preparation technologies for wear-resistant coatings on steel surfaces.
Wear-resistant coatings on steel surfaces can be classified into metal-, ceramic-, and composite-based types, based on their core functional phases. Metal-based coatings (e.g., Ni-based and Fe-based alloy coatings) combine toughness with wear resistance, making them suitable for high-impact working conditions. Ceramic-based coatings (e.g., Al2O3 and WC coatings) have high hardness but significant brittleness, which must be improved through a composite design. Composite-based coatings (e.g., metal-ceramic composite coatings) achieve a “hardness–toughness” balance through the synergistic effect of high-hardness reinforcing phases (ceramic particles, nanophases) and tough matrices. These represent the mainstream development direction of current wear-resistant coatings, and their wear resistance is generally superior to that of single metal or ceramic coatings.
Among the existing preparation technologies, thermal-spraying technologies (e.g., HVOF and plasma spraying) feature flexible operation and a controllable coating thickness, making them suitable for the surface strengthening of large components. However, the coating and substrate are bonded mechanically, and pores are likely to develop. Vapor-deposition technologies (e.g., PVD and CVD) can produce dense, thin, and uniform coatings with high bonding strength. However, they have high equipment costs and low deposition efficiency and are suitable for small components such as precision cutting tools. Laser cladding technology uses a small heat-affected zone, and the coating forms a metallurgical bond with the substrate. This technology can prepare thick coatings (1–10 mm) and balance performance with applicability; however, it has relatively high energy consumption. Surfacing-welding technologies (e.g., plasma surfacing welding and submerged arc surfacing welding) enable a large coating thickness and high material utilization and are suitable for the repair of heavy-duty components. However, they cause significant thermal deformation, which requires subsequent processing to adjust precision.
Research on wear-resistant coatings for steel surfaces has always focused on improving wear resistance and service life, thereby advancing through the dual pathways of composite material design and preparation-technology optimization. Regarding the material front, the focus is on multiphase synergistic strengthening (e.g., nanoparticle-dispersion strengthening and gradient-structure design). On the technological front, emphasis is placed on reducing defects (pores and cracks), enhancing bonding strength, and improving process stability. Ultimately, this achieves a precise match between the coating performance and requirements of the service conditions.
5.2. Prospects
Although significant progress has been made in wear-resistant coatings for steel surfaces, the increasingly harsh service conditions in industrial fields necessitate in-depth exploration in the following directions. Currently, research on coating wear mechanisms is primarily focused on single-service conditions. The analysis of failure mechanisms under complex coupled service conditions must be strengthened (e.g., the synergistic effect of high-temperature wear corrosion) and correlation models must be established. Various service-life-prediction methods should be developed to provide theoretical support for the precise design and maintenance of coatings.
In the future, emphasis should be placed on developing “multifunctional synergistic” coatings, such as composite coatings with integrated properties including wear resistance, corrosion resistance, high-temperature resistance, and self-lubrication. The trade-off relationship between “hardness and toughness” can be further improved by regulating the coating composition and structure at the atomic level (e.g., nanolamellar structure and amorphous-nanocrystalline composite structure) or introducing new reinforcing phases (e.g., high-entropy ceramic particles). In response to the demand for lightweight materials, wear-resistant coatings with low densities and high strengths (e.g., aluminum-based composite coatings) should be developed to expand their applications in fields such as aerospace.
Existing technologies need to be improved toward “low energy consumption and greenization.” These include optimizing the energy-utilization efficiency of laser cladding and developing environmentally friendly thermally sprayed powders (to reduce harmful elements). However, technological innovation should be promoted. For example, combining PVD and CVD with laser cladding (depositing an ultrathin bonding layer first, then cladding a thick wear-resistant layer) to meet the requirements of both bonding strength and thickness, or introducing intelligent technologies, such as using artificial intelligence for process-parameter optimization and real-time online monitoring, to improve the quality stability of coatings and preparation efficiency.
In summary, research on wear-resistant coatings for steel surfaces must achieve interdisciplinary innovative integration to continuously enhance their coating performance and applicability, thereby providing key support for extending their service lives, saving energy, and improving the reliability of industrial equipment.
Author Contributions
Conceptualization, Y.W.; methodology, C.F. and J.Z.; software, T.L. and H.Y.; validation, Y.W., C.F. and J.Z.; formal analysis, R.Z. and J.Z.; investigation, Y.W., S.Y., J.H. and M.T.; resources, H.Y., S.Y., M.T. and G.W.; data curation, J.H. and G.W.; writing—original draft preparation, Y.W.; writing—review and editing, Y.W.; visualization, Y.W.; supervision, C.F., T.L., R.Z. and J.Z.; project administration, C.F., T.L. and J.Z.; funding acquisition, C.F. and R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Advanced Materials-National Science and Technology Major Project (grant number 2025ZD0611200), National Natural Science Foundation of China (grant number No. 52304343 and No. 52293392), Fundamental Research Funds for the Central Universities (grant number FRF-IDRY-23-013) and National Science and Technology Major Project (grant number 2024ZD1200404).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, J.; Xue, L. Laser Cladding of CPM Tool Steels on Hardened H13 Hot-Work Steel for Low-Cost High-Performance Automotive Tooling. JOM 2012, 64, 688–693. [Google Scholar] [CrossRef]
- Gullino, A.; Matteis, P.; D’Aiuto, F. Review of Aluminum-To-Steel Welding Technologies for Car-Body Applications. Metals 2019, 9, 315. [Google Scholar] [CrossRef]
- Jia, N.; Zhang, H.F.; Liu, X.F.; Cai, M.J. Research on Fire Resistance of Stainless Steel Car Body Floor Structure in Urban Rail Vehicles. In Proceedings of the ASME 2020 International Mechanical Engineering Congress and Exposition (IMECE2020), Virtual, 1 January 2020; Volume 6. [Google Scholar]
- Dolores, E.; Guillermo, G.; Christopher, J.U. Metal Shipwrecks in Patagonia, Argentina: Contributions to Their Research and Management. Int. J. Naut. Archaeol. 2020, 49, 303–317. [Google Scholar] [CrossRef]
- Smith, B.H.; Szyniszewski, S.; Hajjar, J.F.; Schafer, B.W.; Arwade, S.R. Steel Foam for Structures: A Review of Applications, Manufacturing and Material Properties. J. Constr. Steel Res. 2011, 71, 1–10. [Google Scholar] [CrossRef]
- Deng, E.F.; Zong, L.; Ding, Y.; Zhang, Z.; Zhang, J.F.; Shi, F.W.; Cai, L.M.; Gao, S.C. Seismic Performance of Mid-to-high Rise Modular Steel Construction—A Critical Review. Thin-Walled Struct. 2020, 155, 106924. [Google Scholar] [CrossRef]
- Gao, B.; Yang, C.; Zou, Y.X.; Wang, F.S.; Zhou, X.J.; Barbieri, D.M.; Wu, S.P. Compaction Procedures and Associated Environmental Impacts Analysis for Application of Steel Slag in Road Base Layer. Sustainability 2021, 13, 4396. [Google Scholar] [CrossRef]
- Yokoyama, K.; Oka, T.; Noto, K. Development of a Small-Size Superconducting Bulk Magnet System Using a 13 K Refrigerator. IEEE Trans. Appl. Supercond. 2010, 20, 973–976. [Google Scholar] [CrossRef]
- de Araujo Cardoso, R.F.; da Cunha, M.A.; Mendonça Brandão, L.P. Optimization of the Magnetic Losses of Electrical Steels Through Addition of Al and Si Using a Hot Dipping Process. J. Mater. Res. Technol. 2013, 2, 276–281. [Google Scholar] [CrossRef][Green Version]
- Lee, H.; Sohn, S.S.; Lee, S.; Kwak, J.; Lee, B. Thermodynamic analysis of the effect of C, Mn and Al on microstructural evolution of lightweight steels. Scr. Mater. 2013, 68, 339–342. [Google Scholar] [CrossRef]
- Sun, M.; Yang, X.; Du, C.; Liu, Z.; Li, Y.; Wu, Y.; San, H.; Su, X.; Li, X. Distinct Beneficial Effect of Sn on the Corrosion Resistance of Cr-Mo Low Alloy Steel. J. Mater. Sci. Technol. 2021, 81, 175–189. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, L.; Li, C.; Li, J.; Li, P. Evaluation of the Deformation Behaviors and Hot Workability of a High-Strength Low-Alloy Steel. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2021, 810, 141031. [Google Scholar] [CrossRef]
- Bai, W.; Chen, W.; Yang, C.; Hong, Y.; Wang, R. Fine Classification Method of Stainless Steel Based on LIBS Technology. Laser Optoelectron. Prog. 2022, 59, 2330001. [Google Scholar]
- Nagode, A.; Kosec, L.; Ule, B.; Kosec, G. Review of creep resistant alloys for power plant applications. Metalurgija 2011, 50, 45–48. [Google Scholar]
- Remigiusz, M.; Marek, K.; Anita, M.; Edyta, O.; Witold, P.; Andrzej, S.; Marian, S.; Waldemar, T.; Jan, W.; Andrzej, W. The Effect of a Gear Oil on Abrasion, Scuffing, and Pitting of the DLC-Coated 18Crnimo7-6 Steel. Coatings 2019, 9, 2. [Google Scholar]
- Waldemar, T.; Remigiusz, M.; Edyta, O.; Andrzej, S.; Marek, K.; Andrzej, N.W.; Jakub, N. Abrasive Wear, Scuffing and Rolling Contact Fatigue of DLC-Coated 18Crnimo7-6 Steel Lubricated by a Pure and Contaminated Gear Oil. Materials 2021, 14, 7086. [Google Scholar]
- Su, X.; Xu, G.; Zhu, M.; Zhang, Q.; Cai, F.; Liu, M. Investigation on the Interaction Between Corrosion and Wear of U68CuCr Rail Steel with Different Corrosion Periods. Wear 2023, 516, 204598. [Google Scholar] [CrossRef]
- Sun, J.; Xi, L.; Du, S.; Pan, E. Tool Maintenance Optimization for Multi-Station Machining Systems with Economic Consideration of Quality Loss and Obsolescence. Robot. Comput.-Integr. Manuf. 2009, 26, 145–155. [Google Scholar]
- Chaus, A.S.; Sitkevich, M.; Pokorny, P. Wear Resistance and Cutting Performance of High-Speed Steel Ball Nose End Mills Related to the Initial State of Tool Surface. Wear 2021, 472–473, 203711. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Hu, S.; Xi, Y.; Zhang, H.; Tian, K.; Fei, S.; Deng, H.; Shen, G. Effects of Surface Integrity on Wear Resistance of 42crmo Steel Subjected to Ultrasonic Shot Peening. J. Manuf. Process. 2025, 149, 679–696. [Google Scholar] [CrossRef]
- Grigoriev, S.N. Technologies of Coatings and Surface Hardening: Industrial Applications. Coatings 2023, 13, 511. [Google Scholar] [CrossRef]
- Julia, J.M.; Rodriguez-Tembleque, L. Wear and Subsurface Stress Evolution in a Half-Space under Cyclic Flat-Punch Indentation. Lubricants 2023, 11, 265. [Google Scholar] [CrossRef]
- Dwivedi, D.K. Surface Damage: Causes and Mechanisms. In Surface Engineering; Springer: New Delhi, India, 2018; pp. 17–43. [Google Scholar]
- Liu, W.C.; Yang, W.A.; You, Y.P. Three-Stage Wiener-Process-Based Model for Remaining Useful Life Prediction of a Cutting Tool in High-Speed Milling. Sensors 2022, 22, 4763. [Google Scholar] [CrossRef]
- Williams, J.A. Wear and wear particles—Some fundamentals. Tribol. Int. 2005, 38, 863–870. [Google Scholar] [CrossRef]
- Barai, V.; Dhanalkotwar, V.; Ramteke, S.M.; Jaju, S.B.; Untawale, S.; Sharma, A.; Chelladurai, H.; Amarnath, M. Intelligent Fault Diagnosis of Scuffed Piston Rings Using Vibration Signature Analysis. J. Vib. Eng. Technol. 2023, 12, 1019–1035. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Zhang, W.; Liao, W. Fracture Analysis of Stainless Steel Universal Joints in Power Grid Equipment: A Comparative Study of Cast and Forged Structures. Eng. Fail. Anal. 2023, 153, 107585. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Y.; Wan, Y.; Zhang, L.; Yang, D.; Gao, X.; Yu, Q.; Wang, D. Investigation on Anti-Wear and Corrosion-Resistance Behavior of Steel-Steel Friction Pair Enhanced by Ionic Liquid Additives under Conductive Conditions. Tribol. Int. 2023, 177, 108002. [Google Scholar] [CrossRef]
- Biswajit, S.; Subrat, B.; Rameswar, B.; Soumya, S.M.; Ajit, B. Wear: A Serious Problem in Industry. In Tribology in Materials and Manufacturing-Wear, Friction and Lubrication; Amazon: Seattle, WA, USA, 2021. [Google Scholar]
- Pashechko, M.; Dziedzic, K.; Jozwik, J. Analysis of Wear Resistance of Borided Steel C45. Materials 2020, 13, 5529. [Google Scholar] [CrossRef]
- Andrijana, M.; Vlatko, M.; Pejo, K.; Nikolina, B. Effect of Carbon Content and Boronizing Parameters on Growth Kinetics of Boride Layers Obtained on Carbon Steels. Materials 2022, 15, 1858. [Google Scholar] [CrossRef]
- Kayali, Y.; Kanca, E.; Gunen, A. Effect of Boronizing on Microstructure, High-Temperature Wear and Corrosion Behavior of Additive Manufactured Inconel 718. Mater. Charact. 2022, 191, 112155. [Google Scholar] [CrossRef]
- Michal, T.; Grzegorz, L.; Michal, K.; Ryszard, D.; Tadeusz, W. The Effect of Chemical Composition on the Microstructure and Properties of Multicomponent Nickel-Based Boride Layers Produced on C45 Steel by the Hybrid Method. Coatings 2024, 14, 197. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; Zhang, Y.; Lian, G.; Cao, Q.; Que, L. A Comparative Study on Microstructure and Tribological Characteristics of Mo2FeB2/Wc Self-Lubricating Composite Coatings with Addition of WS2, MoS2, and H-Bn. Mater. Des. 2023, 225, 111581. [Google Scholar] [CrossRef]
- Cui, G.J.; Liu, H.Q.; Li, S.; Gao, G.J.; Kou, Z.M. Design and High-Temperature Tribological Properties of CoCrW with Rare Earth Fluoride Composites. J. Mater. Res. Technol. 2020, 9, 2402–2411. [Google Scholar] [CrossRef]
- Yuan, J.; Yao, Y.; Zhuang, M.; Du, Y.; Wang, L.; Yu, Z. Effects of Cu and WS2 Addition on Microstructural Evolution and Tribological Properties of Self-Lubricating Anti-Wear Coatings Prepared by Laser Cladding. Tribol. Int. 2021, 157, 106872. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Zhou, J.; Wei, H.; Zeng, R.; Li, W. Effect of Cu Addition on the Microstructure and Tribological Performance of Ni60 Directional Structure Coating. Int. J. Miner. Metall. Mater. 2023, 30, 715–723. [Google Scholar] [CrossRef]
- Liu, X.; Bi, J.; Meng, Z.; Li, R.; Li, Y.; Zhang, T. Tribological Behaviors of High-Hardness Co-based Amorphous Coatings Fabricated by Laser Cladding. Tribol. Int. 2021, 162, 107142. [Google Scholar] [CrossRef]
- Riddihough, M. Stellite as a wear-resistant material. Tribology 1970, 3, 211–215. [Google Scholar] [CrossRef]
- Farnia, A.; Malek Ghaini, F.; Ocelík, V.; De Hosson, J.T.M. Microstructural Characterization of Co-Based Coating Deposited by Low Power Pulse Laser Cladding. J. Mater. Sci. 2012, 48, 2714–2723. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.H.; Zhang, S.; Wu, C.L.; Liu, Y.; Zhang, J.B.; Shahzad, M.B. Manufacturing of Ti3SiC2 Lubricated Co-based Alloy Coatings Using Laser Cladding Technology. Opt. Laser Technol. 2019, 114, 209–215. [Google Scholar] [CrossRef]
- Ocken, H. The galling wear resistance of new iron-base hardfacing alloys: A comparison with established cobalt- and nickel-base alloys. Surf. Coat. Technol. 1995, 76–77, 456–461. [Google Scholar] [CrossRef]
- Burdett, W.B. Development of cobalt free wear resistant alloys for nuclear applications. Surf Eng. 1992, 8, 131–135. [Google Scholar] [CrossRef]
- Fu, W.; Chen, Q.Y.; Yang, C.; Yi, D.L.; Yao, H.L.; Wang, H.T.; Ji, G.C.; Wang, F. Microstructure and Properties of High Velocity Oxygen Fuel Sprayed (Wc-Co)-ni Coatings. Ceram. Int. 2020, 46, 14940–14948. [Google Scholar] [CrossRef]
- Eyaprakash, N.; Yang, C.H.; Sivasankaran, S. Laser Cladding Process of Cobalt and Nickel Based Hard-Micron-layers on 316L-Stainless-steel-substrate. Mater. Manuf. Process. 2019, 35, 142–151. [Google Scholar] [CrossRef]
- Wu, T.; Shi, W.; Xie, L.; Gong, M.; Huang, J.; Xie, Y.; He, K. Study on the Effect of Ni60 Transition Coating on Microstructure and Mechanical Properties of Fe/WC Composite Coating by Laser Cladding. Opt. Laser Technol. 2023, 163, 109387. [Google Scholar] [CrossRef]
- Li, C.; Sun, R.; Li, Y.; Zhao, Z.; Qi, X.; Pei, M.; Li, F.; Li, J. Wear Mechanism of a Laser Cladded Fe-based Self-Lubricating Composite Coating for Protecting Counter-Abrasive Parts. Surf. Coat. Technol. 2023, 459, 129402. [Google Scholar] [CrossRef]
- Vignesh, S.; Shanmugam, K.; Balasubramanian, V.; Sridhar, K. Identifying the Optimal Hvof Spray Parameters to Attain Minimum Porosity and Maximum Hardness in Iron Based Amorphous Metallic Coatings. Def. Technol. 2017, 13, 101–110. [Google Scholar] [CrossRef]
- Martins Freitas, B.J.; De Oliveira, V.A.; Gargarella, P. Microstructural Characterization and Wear Resistance of Boride-Reinforced Steel Coatings Produced by Selective Laser Melting (SLM). Surf. Coat. Technol. 2021, 426, 127779. [Google Scholar] [CrossRef]
- Abu-Warda, N.; López, A.J.; López, M.D.; Utrilla, M.V. High Temperature Corrosion and Wear Behavior of HVOF-sprayed Coating of Al2O3-NiAl on AISI 304 Stainless Steel. Surf. Coat. Technol. 2018, 359, 35–46. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Deng, J.; He, J.; Qin, Y.; Xing, Y.; Yin, F. Fabrication of Graphene Oxide Reinforced Plasma Sprayed Al2O3 Coatings. Ceram. Int. 2023, 49, 1667–1677. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, H.; Liu, X.; Wang, H.; Li, D.; Liu, C.; Wang, M.; Song, X. Outstanding High-Temperature Oxidation-and Wear-Resistance of WC Based Cermets. J. Mater. Sci. Technol. 2023, 155, 33–46. [Google Scholar] [CrossRef]
- Anuj, B.; Deepak, K.G.; Prabhjot, S.; Anil, K.S.; Munish, K.G.; Niraj, B.; Jayant, K.; Gautam, S. Erosive Wear Behaviour of HVOF-sprayed Ni-20Cr2O3 Coating on Pipeline Materials. Int. J. Refract. Met. Hard Mater. 2020, 92, 105332. [Google Scholar]
- Ding, Z.; Fang, Z.; Wang, G.; Wu, C.; Yang, S. Microstructure and Properties of Mo-Si-B-Y2O3 Composite Coating on Nickel-Based Alloy by Laser Cladding. Opt. Laser Technol. 2023, 164, 109473. [Google Scholar] [CrossRef]
- Kong, X.; Sun, W.; Wang, Q.; Chen, M.; Zhang, T.; Wang, F. Improving High-Temperature Wear Resistance of NiCr Matrix Self-Lubricating Composites by Controlling Oxidation and Surface Texturing. J. Mater. Sci. Technol. 2022, 131, 253–263. [Google Scholar] [CrossRef]
- Shen, X.; He, X.; Gao, L.; Su, G.; Xu, C.; Xu, N. Study on Crack Behavior of Laser Cladding Ceramic-Metal Composite Coating with High Content of WC. Ceram. Int. 2022, 48, 17460–17470. [Google Scholar] [CrossRef]
- You, A.; Wang, N.; Chen, Y.; Jiang, C.; Zhang, Y.; Zhao, Q.; Shi, Y.; Li, Y.; Zhang, F.; Zhao, Y. Effect of Linear Energy Density on Microstructure and Wear Resistance of WC-Co-Cr Composite Coating by Laser Cladding. Surf. Coat. Technol. 2023, 454, 129185. [Google Scholar] [CrossRef]
- Marta, O.; Jon, I.A.; Aitzol, L.; Eneko, U. Study of the Flexural Behaviour and Bonding Strength of WC-Co Metal Matrix Composite Coatings Produced by Laser Directed Energy Deposition. Surf. Coat. Technol. 2023, 463, 129538. [Google Scholar]
- Ortiz-Membrado, L.; Garcia-Gonzalez, S.; Orrit-Prat, J. Improved Adhesion of Cathodic Arc Pvd Alcrsin Coating on Ion-Implanted Wc-Co Substrates. Int. J. Refract. Met. Hard Mater. 2023, 113, 106187. [Google Scholar] [CrossRef]
- Wu, Q.L.; Zheng, H.T.; Zhang, Z.H.; Hu, P.; Fan, C.H.; Zhong, N.; Liu, Y. High-temperature Wear and Cyclic Oxidation Behavior of (Ti, W)C Reinforced Stainless Steel Coating Deposited by PTA on a Plain Carbon Steel. Surf. Coat. Technol. 2021, 425, 127736. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Chi, J.; Wang, S.; Ren, L.; Fang, M.; Zhou, C. Microstructure Evolution, Recrystallization and Tribological Behavior of TiC/WC Composite Ceramics Coating. Vacuum 2019, 166, 64–71. [Google Scholar] [CrossRef]
- Bahadur, S.; Gong, D. The Action of Fillers in the Modification of the Tribological Behavior of Polymers. Wear 1992, 158, 41–59. [Google Scholar] [CrossRef]
- Tanaka, K. Transfer of Semicrystalline Polymers Sliding Against a Smooth Steel Surface. Wear 1982, 75, 183–199. [Google Scholar] [CrossRef]
- Ziegler, G.; Kleebe, H.J.; Motz, G.; Weibelzahl, W. Synthesis, Microstructure and Properties of Sicn Ceramics Prepared from Tailored Polymers. Mater. Chem. Phys. 1999, 61, 55–63. [Google Scholar] [CrossRef]
- Kaishi, W.; Martin, G.; Günter, M.; Rajendra, K.B. High performance environmental barrier coatings, Part II: Active filler loaded SiOC system for superalloys. J. Eur. Ceram. Soc. 2011, 31, 3011–3020. [Google Scholar] [CrossRef]
- Schuetz, A.; Guenthner, M.; Motz, G.; Greissl, O.; Glatzel, U. Characterisation of Novel Precursor-Derived Ceramic Coatings with Glass Filler Particles on Steel Substrates. Surf. Coat. Technol. 2012, 207, 319–327. [Google Scholar] [CrossRef]
- Biswas, S.K.; Vijayan, K. Friction and wear of PTFE-a review. Wear 1992, 158, 193–211. [Google Scholar] [CrossRef]
- Kameda, T.; Ohkuma, K.; Oka, S. Polytetrafluoroethylene (PTFE): A resin material for possible use in dental prostheses and devices. Dent. Mater. J. 2019, 38, 136–142. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Joshi, G.M. Performance properties and applications of polytetrafluoroethylene (PTFE)—A review. Adv. Compos. Hybrid Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Sawyer, W.G.; Freudenberg, K.D.; Bhimaraj, P.; Schadler, L.S. A Study on the Friction and Wear Behavior of Ptfe Filled with Alumina Nanoparticles. Wear 2003, 254, 573–580. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Ni, Z. The friction and wear of γ-irradiated polytetrafluoroethylene. Wear 1984, 100, 221–242. [Google Scholar] [CrossRef]
- Blanchet, T.A. Sliding wear mechanism of polytetrafluoroethylene (PTFE) and PTFE composites. Wear 1992, 153, 229–243. [Google Scholar] [CrossRef]
- Bahadur, S.; Tabor, D. The wear of filled polytetrafluoroethylene. Wear 1984, 98, 1–13. [Google Scholar] [CrossRef]
- Li, F.; Yan, F.Y.; Yu, L.G.; Liu, W.M. The Tribological Behaviors of Copper-Coated Graphite Filled Ptfe Composites. Wear 2000, 237, 33–38. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.F.; Xu, B.; Lu, Y.J.; Zhou, C.L.; Wu, X.Y.; Li, J.J. Fabrication and Tribological Properties of a Self-Lubricating Wear-Resistant Coating Based on Structural Coupling. Ceram. Int. 2018, 45, 3910–3920. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, H.; Sun, C.; Dai, J. Microstructure and Wear Properties of Multi Ceramics Reinforced Metal-Matrix Composite Coatings on Ti–6Al–4V Alloy Fabricated by Laser Surface Alloying. Surf. Eng. 2019, 35, 683–691. [Google Scholar] [CrossRef]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer composite materials: A comprehensive review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Z.; Du, K.; Zhang, Y.; Bi, Y.; Su, J.; Zhang, S. A Coordination-Driven Interface System for Improving the Performance of High-Filled Bamboo Fiber/poly (butylene Succinate-Co-butylene Adipate) (PBSA) Biocomposites. J. Clean. Prod. 2023, 418, 138198. [Google Scholar] [CrossRef]
- Zai, W.; Wong, M.H.; Man, H.C. Improving the Wear and Corrosion Resistance of CoCrMo-UHMWPE Articulating Surfaces in the Presence of an Electrolyte. Appl. Surf. Sci. 2018, 464, 404–411. [Google Scholar] [CrossRef]
- Ling, L.; Xiao, M.; Han, M.; Ren, S.; Wang, S.; Meng, Y.Z. Porous Composite Membrane of PVDF/Sulfonic Silica with High Ion Selectivity for Vanadium Redox Flow Battery. J. Membr. Sci. 2019, 585, 230–237. [Google Scholar] [CrossRef]
- Peng, G.; Qiao, Q.; Huang, K.; Wu, J.; Wang, Y.; Fu, X.; Zhang, Z.; Fang, T.; Zhang, B.; Huang, Y.; et al. Ni-Fe-MoO42− LDHs/epoxy Resin Varnish: A Composite Coating on Carbon Steel for Long-Time and Active Corrosion Protection. Prog. Org. Coat. 2020, 140, 105514. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, J.; Wang, M.; Wang, H.; Chen, Z.; Liu, X.; Li, S.; Zhao, W.; Yang, J. Enhanced Tribological Performance of NiAl-based Composite Coatings: Design of the Composition in Coatings by Addition of Mo and Nanodiamond. Tribol. Int. 2025, 203, 110405. [Google Scholar] [CrossRef]
- Beltrami, M.; Mavric, A.; Dal Zilio, S.; Fanetti, M.; Kapun, G.; Lazzarino, M. A Comparative Study of Nanolaminate CrN/Mo2N and CrN/W2N As Hard and Corrosion Resistant Coatings. Surf. Coat. Technol. 2023, 455, 129209. [Google Scholar] [CrossRef]
- Elder, B.; Neupane, R.; Tokita, E.; Ghosh, U.; Hales, S.; Kong, Y.L. Nanomaterial Patterning in 3D Printing. Adv. Mater. 2020, 32, e1907142. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Hou, Z.W.; Lin, L.; Li, F.; Zhao, Y.; Li, X.Z. 3D Nanoprinting of Semiconductor Quantum Dots by Photoexcitation-Induced Chemical Bonding. Science 2022, 377, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Vorotilo, S.A.; Levashov, E.A. Wear-resistant Ti–Al–Ni–C–N Coatings Produced by Magnetron Sputtering of SHS-targets in the DC and HIPIMS Modes. Ceram. Int. 2020, 46, 1775–1783. [Google Scholar] [CrossRef]
- Movassagh-Alanagh, F.; Mahdavi, M. Improving Wear and Corrosion Resistance of AISI 304 Stainless Steel by a Multilayered Nanocomposite Ti/TiN/TiSiN Coating. Surf. Interfaces 2020, 18, 100428. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Men, Y.; Li, X.; Liang, Y.; Ren, L. Fabrication of Nano-Tic Functional Gradient Wear-Resistant Composite Coating on 40cr Gear Steel Using Laser Cladding under Starved Lubrication Conditions. Opt. Laser Technol. 2020, 126, 106136. [Google Scholar] [CrossRef]
- Ozkan, D.; Erarslan, Y.; Kincal, C.; Gurlu, O.; Yagci, M.B. Wear and Corrosion Resistance Enhancement of Chromium Surfaces Through Graphene Oxide Coating. Surf. Coat. Technol. 2020, 391, 125595. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental Observation of the Quantum Hall Effect and Berry’S Phase in Graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, P.; Ma, J.; Wei, L.; Zhao, L.; Cheng, J.; Su, Y.; Gu, Y.; Lian, Y.; Peng, Y.; et al. Wax-Transferred Hydrophobic CVD Graphene Enables Water-Resistant and Dendrite-Free Lithium Anode Toward Long Cycle Li-Air Battery. Adv. Sci. 2021, 8, 2100488. [Google Scholar] [CrossRef]
- Wang, L.; Bai, X.; Wen, B.; Du, Z.; Lin, Y. Honeycomb-like Co/C Composites Derived from Hierarchically Nanoporous ZIF-67 As a Lightweight and Highly Efficient Microwave Absorber. Compos. Part B Eng. 2019, 166, 464–471. [Google Scholar] [CrossRef]
- Chang, H.; Luo, Z.Y.; Shi, X.R.; Cao, X.X.; Liang, S.Q. Stable and Reversible Zinc Metal Anode with Fluorinated Graphite Nanosheets Surface Coating. Trans. Nonferrous Met. Soc. China 2024, 34, 3358–3371. [Google Scholar] [CrossRef]
- Herman, H.; Sampath, S.; McCune, R. Thermal spray: Current status and future trends. MRS Bull. 2000, 25, 17–25. [Google Scholar] [CrossRef]
- Czupryński, A.; Adamiec, J.; Adamiak, M.; Żuk, M.; Kříž, A.; Mele, C.; Kciuk, M. High-Temperature Corrosion of Flame-Sprayed Power Boiler Components with Nickel Alloy Powders. Mater. Charact. 2023, 16, 1658. [Google Scholar] [CrossRef]
- Nyambura, S.M. A Review on the Wear, Corrosion and High-Temperature Resistant Properties of Wire Arc-Sprayed Fe-Based Coatings. Nanomaterials 2021, 11, 2527. [Google Scholar]
- Kwon, H.; Kim, J.; Lee, C. Surface Metallization and Ceramic Deposition on Thermoplastic-Polymer and Thermosetting-Polymer Composite Via Atmospheric Plasma Spraying. Met. Mater. Int. 2020, 27, 3293–3306. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, Z.; Zhou, Z.; Zhang, B.; Wang, Q.; Shen, B. Improved Corrosion and Corrosion-Wear Properties of Fe-based High-Entropy Amorphous Coatings by Modulating Heat Input of HVAF. Corros. Sci. 2024, 232, 112049. [Google Scholar] [CrossRef]
- Galedari, S.A.; Mahdavi, A.; Azarmi, F. A Comprehensive Review of Corrosion Resistance of Thermally-Sprayed and Thermally-Diffused Protective Coatings on Steel Structures. J. Therm. Spray Technol. 2019, 28, 645–677. [Google Scholar] [CrossRef]
- Talib, R.J.; Saad, S.; Toff, M.R.M. Thermal Spray Coating Technology—A Review. Int. J. Multidiscip. Res. 2023, 5, 1–8. [Google Scholar] [CrossRef]
- Picas, J.A.; Forn, A.; Rilla, R. HVOF thermal sprayed coatings on aluminium alloys and aluminium matrix composites. Surf. Coat. Technol. 2005, 200, 1178–1181. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, S.; Torres, B.; Lopez, A.J.; Otero, E.; Rams, J. Characterization and Mechanical Properties of Stainless Steel Coatings Deposited by HVOF on ZE41 Magnesium Alloy. Surf. Coat. Technol. 2019, 359, 73–84. [Google Scholar] [CrossRef]
- Ramesh, M.R.; Prakash, S.; Nath, S.K.; Sapra, P.K.; Venkataraman, B. Solid particle erosion of HVOF sprayed WC-Co/NiCrFeSiB coatings. Wear 2010, 269, 197–205. [Google Scholar] [CrossRef]
- Yao, H.L.; Yang, C.; Yi, D.L.; Zhang, M.X.; Wang, H.T.; Chen, Q.Y.; Bai, X.B.; Ji, G.C. Microstructure and Mechanical Property of High Velocity Oxy-Fuel Sprayed WC-Cr3C2-Ni Coatings. Surf. Coat. Technol. 2020, 397, 126010. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, P.; Chu, C.; Wang, Z.; Cao, M.; Hu, J. Cavitation Erosion Characteristics of a Fe–Cr–Si–B–Mn Coating Fabricated by High Velocity Oxy-Fuel (HVOF) Thermal Spray. Mater. Lett. 2007, 61, 1867–1872. [Google Scholar]
- Gateman, S.M.; Alidokht, S.A.; Mena-Morcillo, E.; Schulz, R.; Chromik, R.R.; Kietzig, A.-M.; Parkin, I.P.; Mauzeroll, J. Wear Resistant Solid Lubricating Coatings Via Compression Molding and Thermal Spraying Technologies. Surf. Coat. Technol. 2021, 426, 127790. [Google Scholar] [CrossRef]
- Lima, C.R.C.; Belem, M.J.X.; Fals, H.D.C.; Della Rovere, C.A. Wear and Corrosion Performance of Stellite 6(R) Coatings Applied by HVOF Spraying and GTAW Hotwire Cladding. J. Mater. Process. Technol. 2020, 284, 116734. [Google Scholar] [CrossRef]
- Buytoz, S.; Ulutan, M.; Islak, S.; Kurt, B.; Çelik, O.N. Microstructural and Wear Characteristics of High Velocity Oxygen Fuel (HVOF) Sprayed NiCrBSi-SiC Composite Coating on SAE 1030 Steel. Arab. J. Sci. Eng. 2013, 38, 1481–1491. [Google Scholar] [CrossRef]
- Jitendra, K.S.; Soumen, M.; Raihana, J.A.; Han-Seung, L.; Hyun-Min, Y. Role of Coating Processes on the Corrosion Kinetics and Mechanism of Zinc in Artificial Seawater. Materials 2021, 14, 7464. [Google Scholar] [CrossRef]
- Luo, L.; Liu, S.; Yu, J.; Zhao, Q.; Sun, N.; Li, J. High-temperature erosion behavior of high-velocity arc-spraying FeMnCr/Cr3C2 coatings. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2010, 39, 234–237. [Google Scholar]
- Qiao, L.; Duan, J.; Gao, W.; Hong, S. Corrosion Behavior of Arc-Sprayed Pore-Sealed Zn and Al Coatings in Seawater Containing Sulfate-Reducing Bacteria (SRB). J. Therm. Spray Technol. 2021, 30, 1557–1565. [Google Scholar] [CrossRef]
- Fang, L.; Huang, J.; Liu, Y.; Zhang, B.; Li, H. Cored-wire Arc Spray Fabrication of Novel Aluminium-Copper Coatings for Anti-Corrosion/fouling Hybrid Performances. Surf. Coat. Technol. 2019, 357, 794–801. [Google Scholar] [CrossRef]
- Ndumia, J.N.; Kang, M.; Gbenontin, B.V.; Lin, J.; Liu, J.; Nyambura, S.M. Evaluation of Thermal Shock Failure Mechanism of Arc-Sprayed Fe-based Coatings Deposited on Different Substrates. Surf. Coat. Technol. 2023, 474, 130081. [Google Scholar] [CrossRef]
- Jarligo, M.O.; Mack, D.E.; Mauer, G.; Vaßen, R.; Stöver, D. Atmospheric Plasma Spraying of High Melting Temperature Complex Perovskites for TBC Application. J. Therm. Spray Technol. 2010, 19, 303–310. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhou, K.S.; Deng, C.M.; Liu, M.; Deng, Z.Q.; Deng, C.G.; Song, J.B. Deposition and CMAS Corrosion Mechanism of 7YSZ Thermal Barrier Coatings Prepared by Plasma Spray-Physical Vapor Deposition. J. Inorg. Mater. 2015, 30, 287–293. [Google Scholar] [CrossRef]
- Deng, Z.Q.; Liu, M.; Mao, J.; Zhang, X.F.; Chen, W.L.; Chen, Z.K. Deposition Mechanism Based on Plasma Spray-Physical Vapor Deposition. J. Inorg. Mater. 2017, 32, 1285. [Google Scholar] [CrossRef]
- An, L.T.; Gao, Y.; Zhang, T. Effect of Powder Injection Location on Ceramic Coatings Properties When Using Plasma Spray. J. Therm. Spray Technol. 2007, 16, 967–973. [Google Scholar] [CrossRef]
- Srinivasan, V.; Friis, M.; Vaidya, A.; Streibl, T.; Sampath, S. Particle Injection in Direct Current Air Plasma Spray: Salient Observations and Optimization Strategies. Plasma Chem. Plasma Process. 2007, 27, 609–623. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Sidelev, D.; Rombaeva, M.; Syrtanov, M.S.; Bleykher, G.A. Chromium Coatings Deposited by Cooled and Hot Target Magnetron Sputtering for Accident Tolerant Nuclear Fuel Claddings. Surf. Coat. Technol. 2020, 389, 125618. [Google Scholar] [CrossRef]
- Tian, C.L.; Cai, H.C.; Xue, Y.J. Effect of Working Pressure on Tribological Properties of Ce-Ti/MoS2 Coatings Using Magnetron Sputter. Coatings 2022, 12, 1576. [Google Scholar] [CrossRef]
- Zhang, X.F.; Li, M.; Zhang, A.; Guo, S.Q.; Mao, J.; Deng, C.M. Al-modification for PS-PVD 7YSZ TBCs to Improve Particle Erosion and Thermal Cycle Performances. J. Adv. Ceram. 2022, 11, 1093–1103. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Shpak, A.P.; Beresnev, V.M.; Il'Yashenko, M.; Komarov, F.; Shypylenko, A.; Kaverin, M.; Zukovski, P.; Kunitskyi, Y.; Kolesnikov, D.; et al. Structure and Properties of Nano- and Microcomposite Coating Based on Ti-Si-N/WC-Co-Cr. Acta Phys. Pol. A Gen. Phys. Phys. Condens. Matter Opt. Quantum Electron. At. Mol. Phys. Appl. Phys. 2011, 120, 100–104. [Google Scholar] [CrossRef]
- Taran, A.; Garkusha, I.; Taran, V.; Muratov, R.; Starikov, V.; Baturin, A. Structure and Properties of Nanostructured ZrN Coatings Obtained by Vacuum-Arc Evaporation Using RF Discharge. Nanotechnol. Percept. 2018, 14, 167–177. [Google Scholar] [CrossRef]
- Wang, L.J.; Wang, M.C.; Chen, H. Corrosion Mechanism Investigation of TiAlN/CrN Superlattice Coating by Multi-Arc Ion Plating in 3.5 Wt% NaCl Solution. Surf. Coat. Technol. 2020, 391, 125660. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, X.; Jiang, S.; Jiang, G.; Zhang, Q. Growth Mechanism for Zinc Coatings Deposited by Vacuum Thermal Evaporation. J. Iron Steel Res. Int. 2021, 28, 1047–1053. [Google Scholar] [CrossRef]
- Tillmann, W.; Hagen, L.; Stangier, D.; Krabiell, M.; Schroeder, P.; Tiller, J. Influence of Etching-Pretreatment on Nano-Grained WC-Co Surfaces and Properties of PVD/HVOF Duplex Coatings. Surf. Coat. Technol. 2019, 374, 32–43. [Google Scholar] [CrossRef]
- Nelson, F.L.D.; Alexander, L.M.; Christoph, P.J.; Alexander, F.; Dirk, B.; Wolfgang, T. Arc-enhanced Glow Discharge Ion Etching of WC-Co Cemented Carbide for Improved PVD Thin Film Adhesion and Asymmetric Cutting Edge Preparation of Micro Milling Tools. Surf. Coat. Technol. 2024, 491, 131166. [Google Scholar]
- Sabzi, M.; Mousavi Anijdan, S.H.; Shamsodin, M.; Farzam, M.; Hojjati-Najafabadi, A.; Feng, P.; Park, N.; Lee, U. A Review on Sustainable Manufacturing of Ceramic-Based Thin Films by Chemical Vapor Deposition (CVD): Reactions Kinetics and the Deposition Mechanisms. Coatings 2023, 13, 188. [Google Scholar] [CrossRef]
- Kellermann, K.; Ehrhardt, S.; Fandrey, J.; Rosiwal, S.M.; Singer, R.F. Influence of Surface Roughness on the Tribological Properties of Hf-Cvd Diamond Coated Heat-Treatable Steel. Wear 2010, 269, 811–815. [Google Scholar] [CrossRef]
- Xiao, L.; Shan, Y.; Fan, X.; Chen, C.; Lu, S.; Hu, X. Preparation of Diamond Films with Cracked Textures on Stainless Steel Using W/W-N Film as an Interlayer. Coatings 2024, 14, 1494. [Google Scholar]
- Muresan, M.G.; Charvatova Campbell, A.; Ondracka, P.; Bursikova, V.; Perina, V.; Polcar, T. Protective Double-Layer Coatings Prepared by Plasma Enhanced Chemical Vapor Deposition on Tool Steel. Surf. Coat. Technol. 2015, 272, 229–238. [Google Scholar] [CrossRef]
- Tomlinson, K.; Fletcher, D.I.; Lewis, R. Evaluation of Laser Cladding as an In-Situ Repair Method on Rail Steel. Tribol. Int. 2023, 180, 108210. [Google Scholar] [CrossRef]
- Panziera, R.C.; Pereira, M.; Castro Rde, M.; Curi, E.I.M.; Neto, F.G. Effect of Tungsten Carbide Reinforcement Phase on the Abrasive Wear Performance of Metal Matrix Composites Deposited by Laser Cladding. J. Braz. Soc. Mech. Sci. Eng. 2023, 45, 596. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, P.; Lan, Q.; Meng, G.; Ren, Y.; Yang, Z. Recent research and development status of laser cladding: A review. Opt. Laser Technol. 2021, 138, 106915. [Google Scholar] [CrossRef]
- Brandau, B.; Hemschik, R.; Sousa, J.P.; Brueckner, F.; Kaplan, A. Enhancing Laser Cladding Stability: Defects and Schlieren-Based Analytics During L-DED. Addit. Manuf. 2025, 103, 104758. [Google Scholar] [CrossRef]
- Xing, H.; Kong, D.J.; Song, R.G. Microstructures and Properties of Laser Cladding Al-TiC-CeO2 Composite Coatings. Materials 2019, 48, 3634–3642. [Google Scholar]
- Wang, K.; Du, D.; Liu, G.; Chang, B.; Hong, Y. Microstructure and Properties of WC Reinforced Ni-based Composite Coatings with Y2O3 Addition on Titanium Alloy by Laser Cladding. Sci. Technol. Weld. Join. 2019, 24, 517–524. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Huang, X.; Qin, S.; Hu, X. Study on the Microstructure and Performance of the Multi-Field Composite-Assisted Laser Cladding of Nickel-Based Tungsten Carbide Coatings. Metals 2024, 14, 1188. [Google Scholar] [CrossRef]
- Yuan, W.; Li, R.; Chen, Z.; Gu, J.; Tian, Y. A Comparative Study on Microstructure and Properties of Traditional Laser Cladding and High-Speed Laser Cladding of Ni45 Alloy Coatings. Surf. Coat. Technol. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, L.Y.; Xu, Q.L.; Li, Y.; Li, C.J.; Li, C.X. Microstructure and Wear Resistance of Ni-Based WC Coating by Ultra-High Speed Laser Cladding. Acta Metall. Sin. 2020, 56, 1530–1540. [Google Scholar]
- Barile, C.; Casavola, C.; Pappalettera, G.; Renna, G. Advancements in Electrospark Deposition (ESD) Technique: A Short Review. Coatings 2022, 12, 1536. [Google Scholar] [CrossRef]
- Kemal, K. Investigation and Characterization of Electrospark Deposited Chromium Carbide-Based Coating on the Steel. Surf. Coat. Technol. 2015, 272, 1–7. [Google Scholar]
- Kudryashov, A.E.; Zamulaeva, E.I.; Levashov, E.A.; Manakova, O.S.; Petrzhik, M.I. Application of Electrospark Deposition Process and Modified SHS Electrode Materials to Improve the Endurance of Hot Mill Rolls. Part 1. Features of Coating Formation on SPHN-60 White Cast Iron Substrates. Surf. Eng. Appl. Electrochem. 2019, 55, 390–401. [Google Scholar] [CrossRef]
- Wei, S.S.; You, B.; Sun, S.L.; Li, Y.Y.; Lin, R.C.; Lin, Y.L. Study on Salt Spray Corrosion and Friction Wear Performance of CrN Coated Cemented Carbide Sealing Ring. Mater. Res. Express 2024, 11, 106511. [Google Scholar] [CrossRef]
- Sulaiman, M.H.; Sheng, A.O.C.; Farahana, R.N.; Bienk, K. Corrosion resistance of PVD hard coatings for tribological engineering applications. IOP Conf. Ser. Mater. Sci. Eng. 2019, 670, 012054. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).