Abstract
Plasma electrolytic oxidation (PEO) is normally carried out under conditions with electrolyte cooling. However, the effect of the temperature of the electrolytes on the PEO behavior and properties of the resulting coatings is seldom investigated. In this study, PEO of pure Al was carried out in a dilute aluminate electrolyte with the electrolyte temperature being controlled under low (~10–30 °C), medium (~40–50 °C) and high (~70–80 °C) temperature ranges, respectively. The morphology, microstructure, composition and phase component of the coatings fabricated under the different temperature ranges were analyzed by scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS) and X-ray diffraction (XRD). The corrosion resistances of the coatings were evaluated by electrochemical methods. The hardness of the coatings and substrate following the PEO treatment in the different electrolyte temperature ranges were also tested. It was found that a higher electrolyte temperature resulted in a higher growth rate and rougher coatings. Moreover, the α-Al2O3 content was reduced as the electrolyte temperature increased. The highest corrosion resistance was registered for the coating obtained under the lowest temperature range, whereas the corrosion resistance of the coating obtained under the highest temperature range was the worst. The higher growth rate under high electrolyte temperatures was associated with the increased kinetics of the PEO reactions. However, the temperature of the electrolyte should be controlled under a suitable range to ensure reasonable coating properties.
1. Introduction
Plasma electrolytic oxidation (PEO), also known as micro arc oxidation, microplasma oxidation or spark anodizing, is an advanced surface treatment technology developed from conventional anodizing [,,]. This technology works under the breakdown voltages of the oxide film of the treated metals, with plasma discharges being observed during the PEO process. The plasma discharges generate very high temperatures (up to 4000–10,000 K) and pressures (~102 MPa) at the sites of discharge channels [], leading to the formation of ceramic coatings with various functions such as wear resistance [,,,], corrosion resistance [,], catalysis [,], biocompatibility [,], antifouling capability [] and antibacterial performance [] on so-called valve metals such as Al, Mg, Zr, Ti and their alloys. PEO technology has merits such as a high coating formation rate, an environmentally friendly electrolyte, and the ability to handle irregular workpieces. Furthermore, it can generate high-temperature ceramic coatings without causing thermal damage to the substrate metal, making it more favorable than other surface treatment methods such as thermal spraying.
Although PEO is a promising technology for obtaining high-quality coatings, it also has some shortcomings that constrain its development. One significant drawback is the high energy consumption, which is usually 20–50 times more energy-intensive than conventional anodizing []. Due to the high voltage and current densities of the PEO process, an enormous amount of heat is released during the PEO treatment. Therefore, the temperature of electrolyte is required to be controlled during the PEO process. A typical PEO treatment unit consists of an electrolyzer, a high-power electrical source and a cooling system []. The cooling system usually uses circulating water to control the temperature of the electrolyte during PEO.
The temperature of the electrolyte is controlled within a broad range in the literature. For example, in a recent study of the PEO of 2024 Al alloy by Gao et al., the electrolyte temperature was kept below 60 °C during the PEO process []. In the work of Liang et al. [], the electrolyte temperature was kept at 20 ± 2 °C by the cooling system during the PEO processes. Hakimizad et al. [] used a compressor chiller to control the PEO solution temperature in the range of 25 ± 2 °C. In the work of Michalska et al. [], the PEO of a Zr-Ti-Nb alloy was conducted at a lower temperature of 15 °C with intense mixing of the electrolyte by a magnetic stirrer. It should be pointed out that due to the huge energy input of the PEO process, it is difficult to control the electrolyte temperature at precise values. However, it will be useful to consider the effect of the electrolyte temperature on the PEO coating growth behavior and properties of the resulting coatings.
In anodizing practice, it is known that a lower electrolyte temperature helps to obtain higher-quality anodic coatings on aluminum. However, for PEO processing, this fact has not yet been established. Yerokhin [] studied the heat release during the PEO of aluminum in silicate (NaSiO3) solutions. In his work, the temperatures of the substrate (Ts) and electrolyte (Te) were measured. The Ts either did not rise or rose slowly to no more than 100–180 °C, while the electrolyte was kept below 60 °C. Yang et al. [] also measured the temperatures of the substrate, the electrolyte at 5 mm away from the sample surface and the bulk electrolyte (150 mm away) during the PEO of 7075 Al alloy in a silicate electrolyte. Similar results were found, in that the substrate temperatures were below 120 °C and the electrolyte temperature was below 38 °C depending on the distance from the sample surface. Although Yerokhin and Yang et al. studied the temperature conditions of the substrate and electrolyte during the PEO processes, the effect of the temperature of the electrolyte and substrate on the properties of the coatings has not been discussed yet. In another work of Vasil’eva et al. [], the effect of factors such as the current density, temperature and concentration of the electrolyte on the phase composition and electrical resistance of PEO coatings on titanium was studied in a borate electrolyte (Na2B4O7). It was found that the coating attained the highest rutile content in an electrolyte temperature of between 32–38 °C in 0.3 M Na2B4O7 at 0.1 A cm2. Therefore, the electrolyte temperature might have a certain influence on the properties of the resulting coatings. However, relevant studies on other valve metals and in other electrolytes are seldom reported.
In this work, the effects of the electrolyte temperature on the growth behavior and properties of PEO coatings on pure aluminum were studied. The hardness of the substrate following the PEO treatment in different electrolyte temperature ranges was also measured to make sure that the mechanical properties of the substrate were not seriously affected by the treatments. The results of this work are helpful in understanding the necessity of controlling the electrolyte temperature during the PEO process.
2. Experimental Methods
2.1. Preparation of Specimens
In this work, samples with dimensions of 20 mm × 10 mm × 5 mm were obtained by wire electrical discharge machining from a rolled pure aluminum (99.99 wt.%) plate. Each sample was connected with a copper wire and mounted with epoxy resin to expose a working area of 2 cm2. All samples were mechanically polished to 2000 grit SiC, and they were then rinsed with de-ionized water and finally dried in a cool air stream.
Figure 1 presents the schematic of the experimental setup. The setup consisted of a pulse power source, a 1 L jacketed glass cell and a water cooling system. PEO was carried out under the galvanostatic mode, with the positive and negative average current densities being fixed at 260 mA/cm2 and 160 mA/cm2, respectively. The frequency and duty cycle of the waveform were 1000 Hz and 20%, respectively. The electrolyte was 1 g L−1 KOH + 5 g L−1 NaAlO2, which was prepared by using high-purity chemicals and de-ionized water. During the PEO process, the electrolyte temperature was controlled under 3 different ranges with “low (~10–30 °C)”, “medium (~40–50 °C)” and “high (~70–80 °C)” temperatures, respectively. The temperature was controlled by adjusting the flow rate of the cooling water. A thermometer was inserted into the electrolyte to monitor the variation in the temperature of the electrolyte during the whole PEO processes.
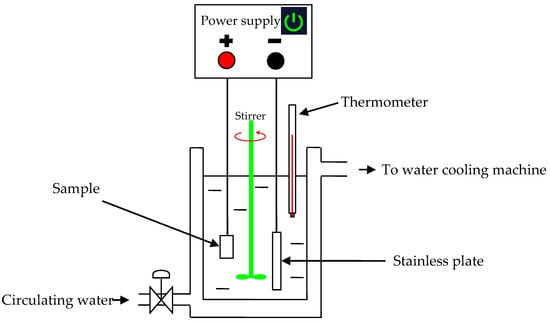
Figure 1.
Schematic diagram of the experimental setup.
2.2. Characterization
The thickness of the coatings was measured by a thickness gauge (TT260, Time Group, Beijing, China) based on the eddy current method. A total of 12 points on the sample surface were randomly measured to obtain the average and standard deviation. The surface and cross-sections of the resulting coatings were characterized using field emission scanning electron microscopy (SEM, Quanta FEG 250, FEI, Lausanne, Switzerland) assisted by energy dispersive spectroscopy (EDS, Oxford Instrument, Abingdon, UK). Platinum was sprayed on the surface of the PEO coatings before the SEM examinations. The phase structure of the coatings obtained under the different temperature ranges was characterized by X-ray diffraction (XRD, Rigaku D/max 2500 X-ray diffractometer, Cu Kα, 40 kV, 250 mA).
The corrosion properties of the coatings obtained under the different temperature ranges were tested by potentiodynamic polarization curves on an electrochemical workstation. The tests were conducted using a three-electrode system in a 3.5 wt.% NaCl solution. The specimens with an exposed area of 1 cm2 were used as the working electrode, whereas Pt foil and a saturated calomel electrode (SCE) were used as the counter electrode and reference electrode, respectively. The open-circuit potential (OCP) was first measured for an immersion time of 1800 s, and then the polarization curves were conducted by scanning from −500 mV (vs. OCP) to +1500 mV (vs. OCP) at a rate of 1 mV/s.
Microhardness measurements were carried out on the polished cross-sections of the coatings formed under the different electrolyte temperature ranges using an HX−1000 TM/LCD digital microhardness tester. Loads of 25 g and 10 g were used for the PEO coatings and the substrate under the coatings, respectively. The dwell time was 20 s. The microhardness tests for the substrate were carried out at locations just below the coatings and also away from the coating/substrate interface. More than 8 points and 15 points were tested on the coating and substrate, respectively, for each sample.
3. Results
3.1. V-t Responses and Growth Kinetics
Figure 2a,b show the positive cell voltage responses and the evolution of the electrolyte temperature, respectively, during the PEO of pure Al under the “low”, “medium” and “high” temperature ranges. For PEO under the “low” temperature range, the cell voltage rose rapidly with an initial surge to ~556 V at ~14 s, after which an inflexion point appeared on the curve, and the cell voltage rose with significantly lowered rates to a final voltage of ~695 V at 1800 s. The voltage at the inflexion point is normally called the “breakdown voltage” in the literature [,]. Before the breakdown voltage, the cell voltage rose linearly due to the thickening of the conventional anodic film. The cell voltage responses in the “medium” and “high” temperature ranges were similar to that in the “low” temperature range. However, the breakdown voltage was reduced to ~497 V and ~471 V in the cases of PEO in the “medium” and “high” temperature ranges, respectively. Although PEO in the “high” temperature range showed the lowest breakdown voltage, it exhibited the highest final voltage of ~708 V at 1800 s, exceeding the final voltage of ~695 V in the “low” temperature PEO and the final voltage of ~685 V for PEO in the “medium” temperature range.
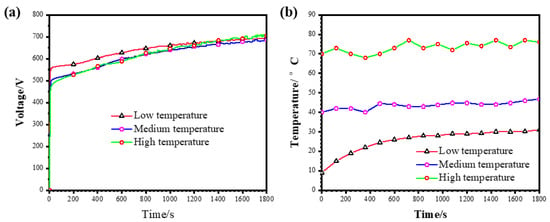
Figure 2.
(a) Voltage–time curves during PEO of pure Al in 1 g L−1 KOH + 5 g L−1 NaAlO2 under low, medium and high temperature ranges; (b) evolution of the electrolyte temperature during PEO of pure Al under the three different temperature ranges.
Figure 2b shows the variations in the electrolyte temperature recorded during PEO under the different conditions. For PEO under the “low” temperature range, the electrolyte temperature was ~10 °C at the initial time; however, the temperature approached ~30 °C at ~1800 s. The electrolyte temperature was kept between ~40–47 °C under the “medium” temperature PEO in this study. The electrolyte temperature fluctuated between ~70–80 °C during the “high”-temperature PEO process.
The kinetics of the coating growth under the different temperature ranges are shown in Figure 3. The thickness values shown in Figure 3 were measured by a thickness gauge using the eddy current method. It can be seen that increasing the electrolyte temperature led to an increased coating growth rate, especially for PEO at the later stage under the “high”-temperature conditions. At the early times of 300 and 600 s, the coating thicknesses were similar in the “medium” and “low” temperature ranges, but they were lower than those under the “high”-temperature conditions. The thicknesses of the coatings ranked in a decreasing order of 75 ± 6.3 μm, 71 ± 5.8 μm and 66 ± 4.3 μm at the final time of 1800 s from high to low temperatures.
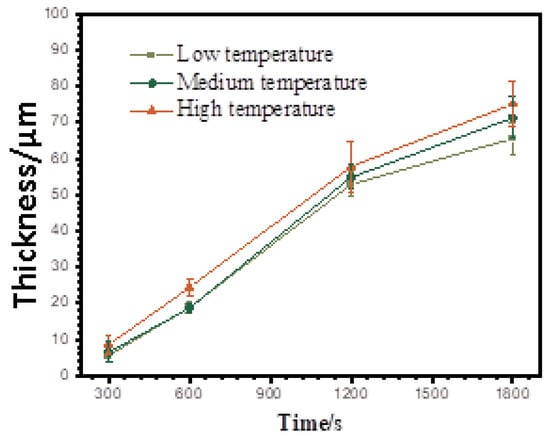
Figure 3.
Growth kinetics during PEO of pure Al under different temperature ranges.
3.2. Phase Structure
Figure 4 shows the phase structure of the coatings prepared under the different electrolyte temperatures. All the coatings consisted of α-Al2O3 and γ-Al2O3, with the former being the major phase. The peaks of aluminum came from the substrate, which was due to the fact that the coatings were penetrable by the X-rays. According to references [,], the relative content of α-Al2O3 to γ-Al2O3 in the coatings was proportional to the ratio of the integrated intensities of the (113) α-Al2O3 peak (Iα) and (400) γ-Al2O3 peak (Iγ), which were located at 43.3 degrees and 45.8 degrees (2θ) in the XRD patterns, respectively. The ratio of Iα/Iγ was calculated for the coatings shown in Figure 4 and is plotted in Figure 5. It can be seen in Figure 5 that the content of α-Al2O3 in the coating decreased with the increase in the electrolyte temperature. The ratios of Iα/Iγ were 10.5, 10.4 and 8.5 for the coatings prepared at the “low”, “medium” and “high” temperatures, respectively.
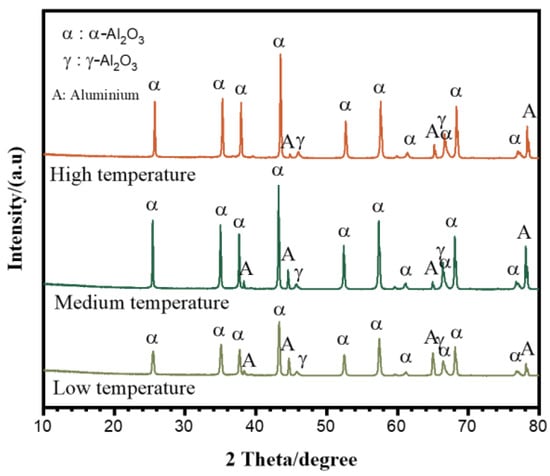
Figure 4.
XRD patterns of the PEO coatings on pure Al formed for 1800 s under low, medium and high temperature ranges.
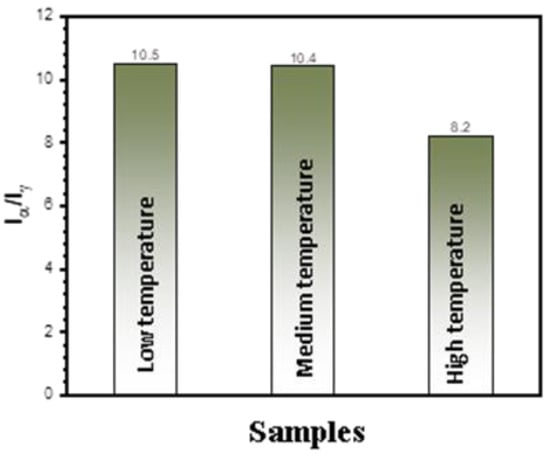
Figure 5.
The ratio of Iα/Iγ of the coatings formed for 1800 s on pure Al under different temperature ranges (Iα and Iγ represent the intensities of the XRD peaks of (113) α-Al2O3 and (400) γ-Al2O3 in Figure 4, respectively).
3.3. SEM Morphology
Figure 6 shows the surfaces and cross-sections of the coatings formed for 1800 s under the different temperature ranges. The surfaces of the coatings featured numerous pancake-like structures, which were the solidified discharge channels formed during the PEO process [,]. Micro-cracks can be observed on the surfaces of all the samples, which may have been caused by the thermal stress associated with the solidification of molten oxide during the PEO process. Besides the pancakes, some fine nodules were observed on the surfaces of the coatings prepared under the high temperature range. Fine nodules were also observed on the coatings at the low and medium temperatures, but there seemed to be fewer of them. The compositions of the different features on the surfaces were analyzed by EDS. Point A on the pancake of the coating formed under the low temperature showed a composition of O 35.94 wt.% and Al 64.06 wt.%. Point B of the pancake on the high-temperature coating had a composition of O 41.38 wt.% and Al 58.62 wt.%, whereas the fine nodules of the same coating (Point C) had a composition of O 43.89 wt.% and Al 56.11 wt.%. The results show that the different coatings formed under the different temperature ranges and the different features had similar compositions. There were no apparent differences in the surface morphologies among the different samples, indicating that the influence of the electrolyte temperature on the surface morphology was insignificant.
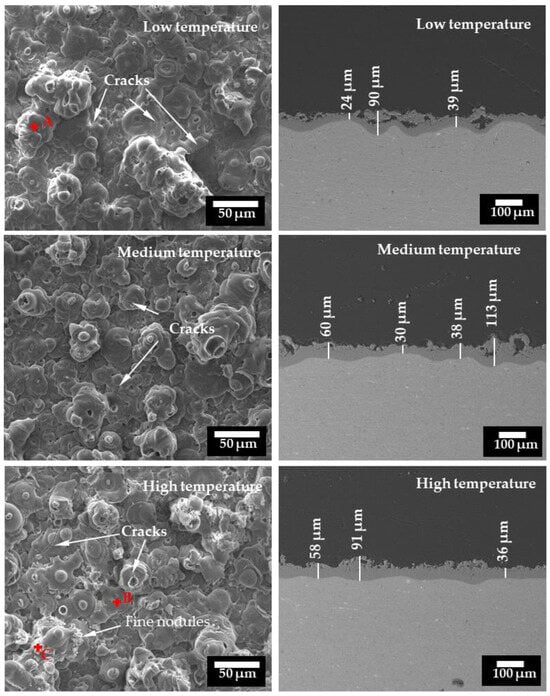
Figure 6.
SEM micrographs of the surfaces and cross-sections of the PEO coatings formed on pure Al for 1800 s under three temperature ranges.
The cross-sections of the coatings shown in Figure 6 revealed that the structure of the coatings consisted of a more porous outer part and a denser inner part. All the coatings showed an undulating interface between the substrate and the oxide coating. Big pores within the outer layer were observed on the cross-sections of the coatings formed under the low and medium temperature ranges. The thickness of the coatings was non-uniform at different locations. For the coatings formed under the low and medium temperature ranges, big variations were observed for the thickness values, showing the lowest value of 24 μm and the highest value of 113 μm. The coating formed under the high temperature range displayed a more uniform cross-section, with the thickness value varying between 36 and 91 μm. The average values calculated from 20 points on the SEM cross-sections shown in Figure 6 were 51 ± 21 μm, 60 ± 28 μm and 54 ± 19 μm for the coatings formed within the low, medium and high temperature ranges, respectively. It should be pointed out that the thickness values given in Figure 3 were measured by the eddy current method, and they show some differences compared to the values given by SEM.
3.4. Corrosion
The corrosion resistance of the coatings formed for 1800 s under the different temperature ranges was evaluated by electrochemical methods. Figure 7 shows the OCP during the immersion time of 1800 s. The coating formed under the low temperature showed an initial OCP of ~−0.70 V and a final OCP of ~−0.71 V. The coating formed under the medium temperature showed an initial OCP of ~−0.60 V, and then the OCP showed a decreasing trend but with some fluctuations to more positive values during the immersion time, reaching a final OCP of −0.75 V. The coating formed under the high temperature range showed a highest initial OCP of ~−0.59 V, but it decreased to ~−0.74 V at the final time of 1800 s.
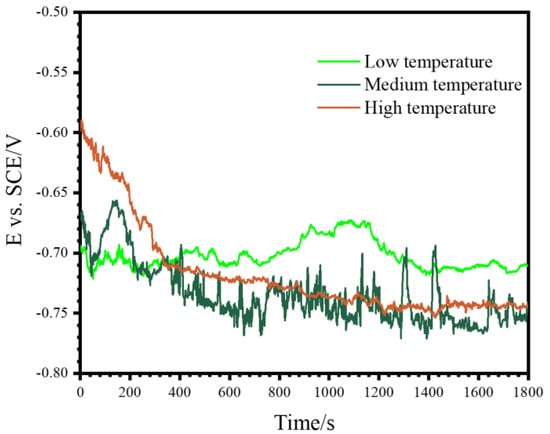
Figure 7.
Evolution of the OCP during immersion of the PEO coatings formed on pure Al for 1800 s under three different temperature ranges in 3.5 wt.% NaCl.
Figure 8 shows the polarization curves of the different coatings recorded in 3.5 wt.% NaCl. The parameters obtained from the polarization curves are listed in Table 1. The free corrosion current densities (icorr) were obtained by the Tafel extrapolation method. According to Table 1, the coating formed under the high temperature showed the most negative corrosion potential (Ecorr) of −0.758 V, whereas the Ecorr values of the coatings formed under the low and medium temperature ranges were nobler. A more negative Ecorr value normally indicates a proneness to corrosion. The cathodic Tafel slope (bc) of the coatings ranged between 380 and 613 mV/dec, whereas the anodic Tafel slope (ba) of the different coatings showed values of between 104 and 260 mV/dec. The free corrosion current density is a parameter that directly reflects the corrosion rate of a material. It can be seen that the coating obtained at the low temperature showed the lowest icorr value of 2.87 × 10−9 A cm−2, whereas the high-temperature coating showed the highest corrosion rate (5.38 × 10−7 A cm−2).
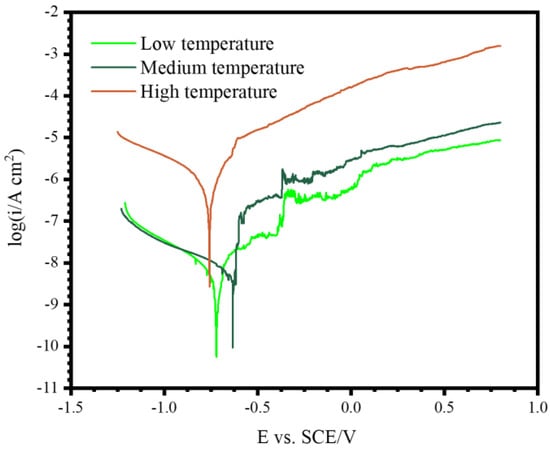
Figure 8.
Polarization curves of the PEO coatings formed on pure Al for 1800 s under three different temperature ranges in 3.5 wt.% NaCl.

Table 1.
Parameters obtained from the polarization curves in Figure 8.
3.5. Microhardness
Microhardness tests of the coatings and the substrate for PEO under the low, medium and high temperature ranges were also carried out. Figure 9 shows the indents left on the substrates and coatings after the microhardness tests. The indents on the substrate showed a similar size regardless of the PEO conditions and their distances from the coating/substrate interface. The indents on the coatings were much smaller and are shown in the insets. The average hardness values of the substrate and coatings for the different temperature ranges are listed in Table 2. The coatings formed under the low, medium and high temperature ranges showed hardness values of 1325 ± 142 HV, 1777 ± 496 HV and 1589 ± 260 HV, respectively. It seems that enhancing the electrolyte temperature increased the hardness of the PEO coatings. The hardness values of the substrate following the PEO treatment with different electrolytes were very close, showing values of between 58.2 HV and 64.2 HV. Therefore, the PEO treatment in the different temperature ranges did not affect the hardness value of the substrate.
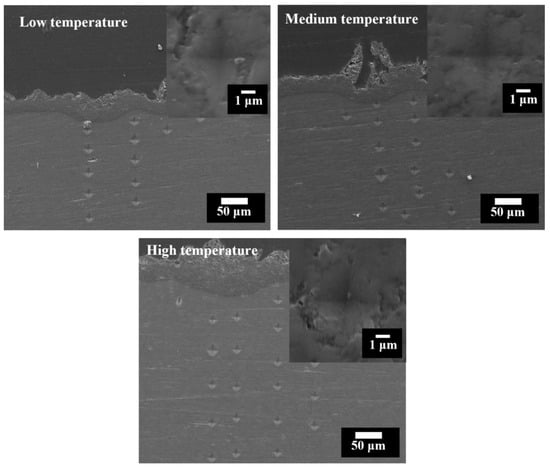
Figure 9.
SEM micrographs showing the microhardness indents on the substrates (load 10 g) and the PEO coatings (the insets, load 25 g) following 1800 s of PEO treatment under the low, medium and high temperature ranges.

Table 2.
Microhardness values of the coating and substrate of pure Al following 1800 s of PEO treatment under the low, medium and high temperature ranges.
4. Discussion
This study investigated the influence of the electrolyte temperature on the PEO behavior and coating morphology, phase composition and corrosion properties of the resulting coatings. The coatings’ characteristics, particularly their morphology, content and structure as well as their corrosion resistance, were expected to be identified with this study. The results showed that increasing the electrolyte temperature both had beneficial and disadvantageous effects on the properties of the coatings as well as on the PEO process itself.
The recording of the cell voltage responses showed that increasing the electrolyte temperature decreases the breakdown voltage of the PEO process. This phenomenon can be explained by the improved electrolyte conductivity at high temperatures.
According to Ikonopisov [], the relationship between the breakdown voltage (UB) and the electrical conductivity (κ) of the electrolyte is as follows:
where aB and bB are constants for a given metal and electrolyte composition.
When the temperature increases, due to reasons such as a decrease in the electrolyte viscosity, the enhancement of the mobility of the ions and the weakening of the ion hydration in the aqueous solution, the conductivity of the electrolyte increases []. Therefore, according to Equation (1), UB will be decreased.
Although the PEO process at the high temperature showed the lowest breakdown voltage, the final voltage surpassed that of the other two conditions in the low and medium temperature ranges. This phenomenon may be due to the fact that a coating with a higher thickness was formed in the high temperature range. The coating growth kinetics showed that increasing the electrolyte temperature is helpful for the formation of a coating with higher thickness. Although PEO is an electrochemical-based technique, complex physico-chemical processes are involved during the PEO process, including thermal and diffusion processes, new plasma chemical reactions and macro-particle transportation []. In this study, PEO was carried out under the galvanostatic mode, i.e., constant anodic and cathodic current densities were employed during the PEO processes in the different temperature ranges. However, it is known that non-Faradaic processes also contribute to PEO coating formation []. The non-Faradaic processes may include the plasma-assisted decomposition and deposition of the electrolyte species. It is well known that the temperature influences the reaction rate. In history, van’t Hoff summarized an approximate rule that for every 10 K increase in temperature, the reaction rate increases by 2 to 4 times. Therefore, it could be expected that a higher level of coating growth occurs with PEO under high electrolyte temperatures.
The phase composition of the present coatings was α-Al2O3 and γ-Al2O3, with the former being the major component. It is known that α-Al2O3, with its melting point of 2050 °C, is a stable phase of alumina, and γ-Al2O3 is one of the metastable phases among all the oxides of aluminum. γ-Al2O3 can be transformed into α-Al2O3 by heating it in the temperature range from 800 °C to 1200 °C. It is known that a higher cooling rate favors the formation of γ-Al2O3, and γ-Al2O3 is prone to distribute on the coating surface due to the high cooling rate caused by contact with the electrolyte []. Interestingly, the content of α-Al2O3 in this study was significantly higher than that of the coatings prepared by Xue et al., which were obtained in an electrolyte of 5 g/L NaOH []. The content of α-Al2O3 in the present coatings was also much higher than the coatings prepared by the PEO of Al alloy in a silicate electrolyte []. Therefore, the results presented in this study indicate that an aluminate electrolyte is more helpful for obtaining PEO coatings with a higher α-Al2O3 content.
It is interesting to note that the high-temperature electrolyte led to a slight decrease in the α-Al2O3 content in the PEO coatings in this study. This phenomenon seems to contradict the understanding that a higher cooling rate favors the formation of γ-Al2O3. However, the electrolyte temperature in this study was much lower than the temperature at the discharge channels, which can reach ~103–104 K [], and the phase transformation temperature of α-Al2O3 and γ-Al2O3. The effect of cooling may have been negligible whether the electrolyte temperature was low or high in this study. Another factor that may be considered is whether overheating of the substrate occurred at the high electrolyte temperatures in this study. However, the research of Yerokhin [] showed that the temperature of the substrate (aluminum) is ~160 °C when the temperature of the electrolyte is ~60 °C at the end of the PEO treatment. An even lower substrate temperature of ~120 °C was registered in the work of Yang et al. []. In this study, the highest electrolyte temperature was ~80 °C. Therefore, a similar substrate temperature would be expected. A substrate temperature below 200 °C is unlikely to cause the effect of overheating of the substrate and changes in its microstructure and properties. Therefore, the true reasons for the formation of less α-Al2O3 at high electrolyte temperatures still need to be researched in the future.
It was observed that the morphology of the coatings was not significantly changed as the electrolyte temperature increased. This result may imply that for Al, the electrolyte temperature is not a critical factor that should be precisely controlled during the PEO process. However, the corrosion properties of the coatings were seriously affected by the electrolyte temperature. The corrosion resistance of the coating obtained at the high electrolyte temperature was nearly two orders lower than that of the coatings prepared under the low and medium temperature ranges. Normally, coatings with more defects, such as micro-cracks and porosity, are less corrosion-resistant to a corrosive medium. Unfortunately, it was difficult to find evidence from the SEM morphology that the high-temperature coating had more defects. Conversely, the high-temperature coating seemed to be more homogeneous in its cross-section. Therefore, there should be other factors that may have influenced the corrosion resistance of the coatings.
One possible reason may be that the incorporated hydrogen species affected the corrosion resistance of the coatings prepared under the high temperature ranges. Gebarowski et al. [] found that PEO coatings on Al prepared with cathodic polarization (negative current) display a considerable drop in their charge transfer resistance after the occurrence of “soft sparking”. The authors postulate that hydrogen evolution during the cathodic cycle caused the barrier layer corrosion. Later, Rogov et al. [] proposed that an increase in the cathodic voltage leads to the incorporation of neutral hydrogen complexes ([H●]ox) within the coating, which accounts for the increase in the coating conductivity. Cheng et al. also found that the impedance of coatings prepared with high cathodic polarization was significantly lowered despite a compact cross-section []. In this study, negative current was also used in preparing the coatings (bipolar regime). It is possible that more hydrogen species were incorporated under the PEO process with a high electrolyte temperature, leading to the lowered corrosion resistance of the coatings.
The control of the electrolyte temperature under a suitable temperature range requires additional energy consumption. Therefore, it is unfavorable to cool the PEO system from the point of view of energy saving. However, the results presented in this study showed that for Al, the coatings prepared under the low and medium temperature ranges (~10–30 °C and ~40–50 °C) had similar morphologies in their surfaces and cross-sections and also similar corrosion resistance. This is a quite broad temperature range, which means that electrolyte cooling is not so critical for the PEO of Al. However, a much higher electrolyte temperature (~70–80 °C) was detrimental to the corrosion resistance of the PEO coatings.
The microhardness test conducted in the present study showed that the hardness of the substrate was not altered under PEO at the different electrolyte temperatures. These results support the point view that the temperature of the substrate did not exceed 200 °C during the PEO process even in the highest electrolyte temperature range of ~70–80 °C. The relatively short PEO treatment time (1800 s in this study) also helped to minimize the possible effects of substrate heating.
5. Conclusions
The PEO of pure Al was carried out in aluminate electrolyte under low (~10–30 °C), medium (~40–50 °C) and high (~70–80 °C) temperature ranges. The following conclusions can be drawn:
- The breakdown voltage of the PEO process decreased in the electrolyte with a high temperature.
- The coatings obtained in the high temperature range were more homogeneous than the coatings obtained in the medium and low temperature ranges and possessed higher thickness values according to the eddy current method.
- The coatings obtained in the different temperature ranges showed similar surfaces and cross-sectional morphologies.
- The phase composition of the coatings consisted of α-Al2O3 and γ-Al2O3, with α-Al2O3 being the dominant phase. PEO in the high temperature range led to a slight decrease in the α-Al2O3 content.
- The coatings formed under the low and medium temperature ranges showed excellent corrosion resistance, whereas the corrosion resistance of the coating formed under the high temperature range decreased by two orders according polarization tests.
- The coatings showed hardness values in the range from ~1325 to ~1777 HV. The hardness of the substrate was not affected by the PEO treatments at the different electrolyte temperatures.
Author Contributions
Conceptualization, Y.C.; Methodology, Y.C., X.S. and Y.L.; Validation, Y.L.; Formal analysis, Y.L.; Writing—original draft, Y.C. and X.Z.; Writing—review & editing, X.S. and X.Z.; Supervision, X.Z.; Funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 52371065) and the Hubei Provincial Natural Science Foundation of China (No. 2023AFB637).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Acknowledgments
The authors thank the technical support from the Analytical and Testing Centre in Huazhong University of Science and Technology (HUST) and the instrumental analysis and research centre of Shanghai Yanku (www.shuyanku.com, accessed on 12 February 2024) is also appreciated. All research data supporting this publication are directly available within this publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Xue, W.; Deng, Z.; Lai, Y.; Chen, R. Analysis of phase distribution for ceramic coatings formed by microarc oxidation on aluminum alloy. J. Am. Ceram. Soc. 1998, 81, 1365–1368. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Mao, M.K.; Cao, J.H.; Peng, Z.M. Plasma electrolytic oxidation of an Al-Cu-Li alloy in alkaline aluminate electrolytes: A competition between growth and dissolution for the initial ultra-thin films. Electrochim. Acta 2014, 138, 417–429. [Google Scholar] [CrossRef]
- Ji, R.N.; Wang, S.Q.; Zou, Y.C.; Chen, G.L.; Wang, Y.M.; Ouyang, J.H.; Jia, D.C.; Zhou, Y. One-step fabrication of amorphous/ITO-CNTs coating by plasma electrolytic oxidation with particle addition for excellent wear resistance. Appl. Surf. Sci. 2023, 640, 158274. [Google Scholar] [CrossRef]
- Molaei, M.; Fattah-alhosseini, A.; Nouri, M.; Kaseem, M. Assessing the wear properties of plasma electrolytic oxidation TiO2 coatings incorporated ZrO2 nanoparticles on Cp-Ti in simulated body fluid. Appl. Surf. Sci. 2024, 19, 100563. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, X.; Northwood, D.O. Investigation of Plasma Electrolytic Oxidation (PEO) coatings on a Zr–2.5Nb alloy using high temperature/pressure autoclave and tribological tests. Surf. Coat. Technol. 2010, 205, 1774–1782. [Google Scholar] [CrossRef]
- He, X.R.; Feng, T.; Cheng, Y.L.; Hu, P.F.; Le, Z.Z.; Liu, Z.H.; Cheng, Y.L. Fast formation of a black inner α-Al2O3 layer doped with CuO on Al–Cu–Li alloy by soft sparking PEO process. J. Am. Ceram Soc. 2023, 106, 7019–7042. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Tolnai, D.; Subroto, T.; Kainer, K.U.; Zhang, T.; Wang, F.; Zheludkevich, M.L. 3D reconstruction of plasma electrolytic oxidation coatings on Mg alloy via synchrotron radiation tomography. Corros. Sci. 2018, 139, 395–402. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, Y.P.; Lv, Y.; Dong, Z.H.; Hashimoto, T.; Zhou, X.R. Enhanced corrosion resistance of AZ31 Mg alloy by one-step formation of PEO/Mg-Al LDH composite coating. Corros. Commun. 2022, 6, 67–83. [Google Scholar] [CrossRef]
- Lukiyanchuk, I.V.; Rudnev, V.S.; Tyrina, L.M. Plasma electrolytic oxide layers as promising systems for catalysis. Surf. Coat. Technol. 2016, 307, 1183–1193. [Google Scholar] [CrossRef]
- Coto, M.; Troughton, S.C.; Knight, P.; Joshi, R.; Francis, R.; Kumar, R.V.; Clyne, T.W. Optimization of the microstructure of TiO2 photocatalytic surfaces created by Plasma Electrolytic Oxidation of titanium substrates. Surf. Coat. Technol. 2021, 411, 127000. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Ibrahim, M.; Etim, I.P.; Tan, L.; Yang, K. In vitro degradation and antibacterial property of a copper-containing micro-arc oxidation coating on Mg-2Zn-1Gd-0.5Zr alloy. Colloids Surf. B 2019, 179, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Lv, Y.; Fu, S.; Wu, Y.L.; Lu, X.Q.; Yang, L.; Liu, H.F.; Dong, Z.H. Synthesis, microstructure, anti-corrosion property and biological performances of Mn-incorporated Ca-P/TiO2 composite coating fabricated via micro-arc oxidation. Mater. Sci. Eng. C 2020, 117, 111321. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Cheng, Y.B.; Meng, X.Z.; Xu, J.; Dong, Z.H.; Zhang, X.X. Ultrasound-Auxiliary Preparation of Antifouling Cu-Enriched Titanium Oxide Ceramic Layer. Coatings 2023, 13, 1099. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, Y.; Lv, Y.; Zhang, X.X.; Dong, Z.H.; Yang, L.; Zhang, E.L. Ag distribution and corrosion behaviour of the plasma electrolytic oxidized antibacterial Mg-Ag alloy. Electrochim. Acta 2022, 411, 140089. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Matykina, E.; Arrabal, R.; Mohedano, M. Role of anodic precursor layer thickness on PEO coatings: Energy consumption and long-term corrosion performance. Surf. Coat. Technol. 2024, 476, 130186. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Xiao, S.; Li, T.J.; Wu, H.; Meng, X.Y.; Li, W.J.; Fan, S.Y.; Ye, Z.H.; Chen, G.H.; et al. Effects and mechanism of Zn on the structure and corrosion resistance of microarc oxidation coatings on aluminum alloy. Appl. Surf. Sci. 2024, 659, 159909. [Google Scholar] [CrossRef]
- Liang, J.; Peng, Z.J.; Cui, X.J.; Li, R.X.; Wang, B. Characterization of V-containing black plasma electrolytic oxide coatings on aluminium alloy: Impact of base electrolytes. J. Mater. Res. Technol. 2024, 30, 110–119. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Santamaria, M.; Asghari, M. Effects of pulse current mode on plasma electrolytic oxidation of 7075 Al in Na2WO4 containing solution: From unipolar to soft-sparking regime. Electrochim. Acta 2018, 284, 618–629. [Google Scholar] [CrossRef]
- Michalska, J.; Sowa, M.; Stolarczyk, A.; Warchoł, F.; Nikiforow, K.; Pisarek, M.; Dercz, G.; Pogorielov, M.; Mishchenko, O.; Simka, W. Plasma electrolytic oxidation of Zr-Ti-Nb alloy in phosphate-formate-EDTA electrolyte. Electrochim. Acta 2022, 419, 140375. [Google Scholar] [CrossRef]
- Yerokhin, A.L. Study of heat release during plasma-electrolytic oxidation of aluminum, In Electrophysical & Electrochemical Treatment of Materials; TulGU: Tula, Russia, 1996; pp. 30–36. (In Russian) [Google Scholar]
- Yang, X.; Chen, L.; Qu, Y.; Liu, R.; Wei, K.; Xue, W. Optical emission spectroscopy of plasma electrolytic oxidation process on 7075 aluminum alloy. Surf. Coat. Technol. 2017, 324, 18–25. [Google Scholar] [CrossRef]
- Vasil’eva, M.S.; Rudnev, V.S.; Tyrina, L.M.; Lukiyanchuk, I.V.; Kondrikov, N.B.; Gordienko, P.S. Phase composition of coatings formed on titanium in borate electrolyte by microarch oxidation. Russ. J. Appl. Chem. 2002, 75, 569–572. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.G.; Tian, J.; Liu, H.W.; Zhou, J.F.; Liu, W.M.; Xu, T. Effects of NaAlO2 on structure and corrosion resistance of microarc oxidation coatings formed on AM60B magnesium alloy in phosphate–KOH electrolyte. Surf. Coat. Technol. 2005, 199, 121–126. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Belca, I.; Petkovic, M.; Kasalica, B.; Nedic, Z.; Zekovic, L. Characterization of the plasma electrolytic oxidation of aluminium in sodium tungstate. Corros. Sci. 2010, 52, 3258–3265. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Discharge characterization in plasma electrolytic oxidation of aluminium. J. Phys. D Appl. Phys. 2003, 36, 2110–2120. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Raeissi, K.; Ashrafizadeh, F.; Hakimizad, A.; Santamaria, M. Incorporation mechanism of colloidal TiO2 nanoparticles and their effect on properties of coatings grown on 7075 Al alloy from silicate-based solution using plasma electrolytic oxidation. Trans. Nonferrous Met. Soc. 2021, 31, 3659–3676. [Google Scholar] [CrossRef]
- Hu, P.F.; Wei, B.J.; Cheng, Y.L.; Cheng, Y.L. Discharge channel structure revealed by plasma electrolytic oxidation of AZ31Mg alloy with magnetron sputtering Al layer and corrosion behaviors of treated alloy. Trans. Nonferrous Met. Soc. 2024, 34, 139–156. [Google Scholar] [CrossRef]
- Ikonopisov, S. Theory of electrical breakdown during formation of barrier anodic films. Electrochim. Acta 1977, 22, 1017–1082. [Google Scholar] [CrossRef]
- Fu, X.C.; Shen, W.X.; Yao, T.Y.; Hou, W.H. Physical Chemistry, 5th ed.; Higher Education Press: Beijing, China, 2006; Volume II, p. 3. [Google Scholar]
- Cheng, Y.L.; Cao, J.H.; Mao, M.K.; Peng, Z.M.; Skeldon, P.; Thompson, G.E. High growth rate, wear resistant coatings on an Al–Cu–Li alloy by plasma electrolytic oxidation in concentrated aluminate electrolytes. Surf. Coat. Technol. 2015, 269, 74–82. [Google Scholar] [CrossRef]
- Gębarowski, W.; Pietrzyk, S. “Growth characteristics of the oxide layer on aluminium in the process of plasma electrolytic oxidation. Arch. Metall. Mater. 2014, 59, 407–411. [Google Scholar] [CrossRef]
- Rogov, A.B.; Matthews, A.; Yerokhin, A. The role of cathodic current in plasma electrolytic oxidation of Aluminium: Phenomenological concepts of the ‘soft sparking’ mode. Langmuir 2017, 33, 11059–11069. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Feng, T.; Cheng, Y.L. A Systematic Study of the Role of Cathodic Polarization and New Findings on the Soft Sparking Phenomenon from Plasma Electrolytic Oxidation of an Al-Cu-Li Alloy. J. Electrochem. Soc. 2022, 169, 071505. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).