Abstract
This study presents the results of a two-stage autoclave processing of a copper–arsenic concentrate. Copper concentrate is an important raw material to produce copper and other metals. However, in some cases, the concentrate may contain increased amounts of arsenic, which makes further processing difficult. Therefore, the development of modern hydrometallurgical methods for processing copper concentrate with a high arsenic content is an urgent task, which could lead to the optimization of the raw material processing process and the improvement of the quality of the concentrate. It has been established that the optimal conditions for the sequential two-stage autoclave processing of copper–arsenic concentrate are: t = 220–225 °C, τoxidation = 20 min, τtot = 90 min, Po2 = 0.4 MPa, and L:S = 10:1, [H2SO4]initial = 40 g/dm3; in this case, 85% of zinc, 44% of iron, and 78% of arsenic, respectively, are extracted into the solution during both stages and the loss of copper was about 0.01%. This is explained by the fact that at the first stage (oxidation) of the autoclave processing of the copper–arsenic concentrate, copper, together with iron, leaches into the solution, and at the second stage (reduction), copper precipitates out of the solution in the form of chalcocite. Copper in the residue after autoclave leaching is in the form of Cu2S, iron is in the form of pyrite (FeS2), and lead is in the form of anglesite (PbSO4), respectively. The obtained micrographs and EDX mappings clearly show no iron arsenates. This confirms that at the oxidative stage of the developed process, arsenic, removed by 78%, remains in the solution. The remaining arsenic is associated with tennantite, indicating the effectiveness of the treatment process in removing arsenic from the copper–arsenic concentrate. A second important observation is the presence of pronounced areas of copper sulfides in the microphotos without iron and arsenic impurities. This confirms that copper is deposited as chalcocite during the reduction phase of the process, which is the desired result.
1. Introduction
Nowadays, due to the intensive development of deposits of non-ferrous metals, many reserves of rich and easy-to-dress ores have been largely exhausted, and there is a general trend towards a decrease in the metal content in mined ores. Hard-to-enrich, finely disseminated, low-quality, polymetallic, and technogenic raw materials are increasingly involved in the processing. The complex mineralogical composition of such materials inevitably leads to the need to use complex technologies that make it possible to isolate the main valuable components of raw materials and ensure processing profitability. An increased arsenic content in concentrates causes technological problems; in particular, the yield of production waste increases, the cyclic load rises, and copper extraction and productivity decrease, leading to increased operating and production costs.
Polymetallic ores, including copper ones with high arsenic content, have a complex and unique structure. This makes the process of extracting and processing using conventional technological methods more complex and costly.
The mineralogical composition of such polymetallic ores is extremely complex. It can be characterized by the presence of not only sphalerite, chalcopyrite, galena, arsenopyrite, and pyrite but, increasingly, tennantite and enargite [1,2]. The presence of arsenic makes processing these ores using conventional technological methods impractical.
In the era of industrial growth and development, enterprises involved in the processing of copper–arsenic raw materials are striving to modernize and optimize their technological processes. They make significant efforts to introduce more sophisticated and effective methods such as acid leaching [3,4,5,6], ammonia leaching [7], alkaline methods [8,9,10], bioleaching [11], and alternative methods [12,13,14,15]. However, all these innovations carry certain difficulties with them. Technological improvements inevitably entail increased energy and materials costs, which, in turn, increases the cost of the final product.
In addition, traditional pyrometallurgical technologies used in the processing of high-arsenic copper raw materials often lead to negative impacts on the environment [2,16,17,18,19,20,21,22,23,24,25]. The low environmental safety of such schemes is a serious obstacle to the environmentally sustainable development of enterprises. Even the introduction of environmental management systems is not always able to reduce pollution levels and often even leads to increased costs due to fines and sanctions imposed by environmental authorities.
In connection with the above, it seems very relevant to develop and implement new hydrometallurgical technologies for processing arsenic-containing copper raw materials, which are more promising from both environmental and economic viewpoints. One such technology could be autoclave leaching.
The use of autoclave processes for processing sulfide copper–arsenic raw materials will ensure high leaching intensity, selectivity, and completeness of extraction of valuable components into the solution [26,27,28,29,30,31,32,33,34,35,36]. High temperatures and pressure of the oxidizing agent (oxygen and air) in the autoclave, combined in some cases with the addition of oxidation catalysts or the ultrafine grinding of raw materials, make it possible to suppress the passivation of the chalcopyrite surface during leaching. Autoclave technologies are easily integrated with existing and widespread hydrometallurgical capacities for processing oxidized and secondary copper ores, including the SX–EW (solvent extraction and electrowinning) processing section. The sulfuric acid and iron (III) sulfate released during the oxidation process in the autoclave, as well as the heat obtained during the cooling process of the autoclave pulp, could be used for the atmospheric leaching of copper raw materials. In addition, the sulfate solution obtained after the oxidation stage can be used in the second stage (reduction) of the autoclave treatment to enrich the copper concentrate with low copper sulfides Cu1.8S, Cu1.94S, and Cu2S [37,38], suitable for processing using conventional pyrometallurgical methods.
In connection with the above, the purpose of this study was to determine the technological modes of oxidative autoclave leaching of copper concentrate with a high arsenic content. The selection of optimal conditions for the autoclave oxidation stage was carried out to maximize the tennantite dissolution, purification of concentrate from arsenic and zinc, and obtaining a conditioned, enriched copper concentrate suitable for processing using traditional pyrometallurgical methods.
2. Materials and Methods
2.1. Analysis
A chemical analysis of the copper–arsenic concentrate and the resulting solid conditioning products was carried out using an ARL Advant’X 4200 wavelength dispersive spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). The phase analysis was performed on an XRD 7000 Maxima diffractometer (Shimadzu Corp., Tokyo, Japan). The software package Match! v. 2.4.7 (Crystal Impact, Bonn, Germany) by Crystal Impact was used to analyze the obtained radiographs.
The chemical analysis of the resulting solutions was carried out using inductively coupled plasma mass spectrometry (ICP-MS) on an Elan 9000 instrument (PerkinElmer Inc., Waltham, MA, USA).
2.2. Materials and Reagents
A copper–arsenic concentrate was used as the main raw material. The particle size of this material was 95% class −0.074 μm; its chemical composition is presented in Table 1. The X-ray diffraction pattern of the copper concentrate used is presented in Figure 1. All other reagents used were of analytical grade.

Table 1.
Chemical composition of the copper concentrate.
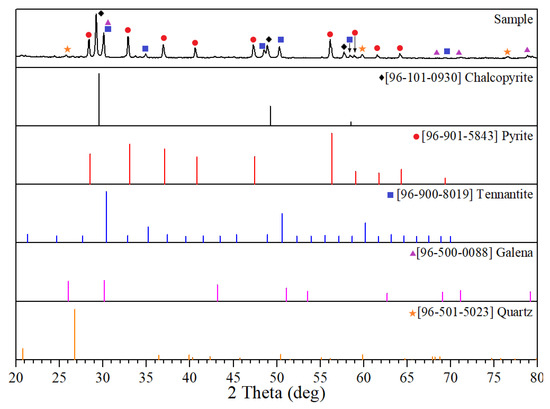
Figure 1.
X-ray diffraction pattern of the copper concentrate.
According to our X-ray phase and chemical analyses, the lead in the concentrate was in the form of galena (PbS)—2.34%, copper was chalcopyrite (CuFeS2)—29.36%, tennantite (Cu12As4S13)—14.11%, zinc was sphalerite (ZnS)—6.99%, and iron was pyrite (FeS2)—39.83%.
2.3. Experimental Procedure
Experiments were carried out in a 1-L Parr titanium autoclave (Parr Instrument Company, Davenport, IA, USA) with an adjustable stirrer speed. The autoclave was equipped with systems for electric heating, an oxygen supply from a standard cylinder, and sampling during experiments.
The rotation speed of the mixer was maintained at 1000 rpm, ensuring uniform pulp density. The resulting pulp was heated in an autoclave with constant stirring. A weighed sample of the concentrate and 600 cm3 of a solution of a given composition were loaded into the autoclave. Then, the stirrer drive was turned on, and the autoclave was heated to the required temperature. After the required temperature was reached, the oxygen supply and time countdown began. At the predetermined time intervals, a portion of the leaching pulp was taken with the help of excessive pressure in the reactor and cooled in a sealed vessel to atmospheric temperature. After the end of the calculated oxidation period, the stirrer was turned off, the oxygen was removed from the system, and the stage of hydrothermal copper deposition began.
After the end of the experiment, rapid cooling of the autoclave was performed with cold water down to 80–70 °C. The obtained pulp was filtered in a Buchner funnel (ECROSKHIM Co., Ltd., St. Petersburg, Russia); the solutions were sent for ICP-MS analysis. At the end of the experiment, the leaching cake was washed with distilled water, dried at 80 °C to a constant weight, weighed, and sent for XRF analysis. All the experiments were performed twice, and the mean values are presented.
3. Results and Discussion
3.1. Study of Two-Stage Autoclave Processing of Copper–Arsenic Concentrate
A sequential two-stage treatment, including high-temperature oxidative leaching of the concentrate in the first stage and hydrothermal copper deposition in the second stage, seems to be the most promising direction for the autoclave processing of copper concentrate.
The two-stage autoclave leaching was studied according to a second-order orthogonal experimental design obtained using Statgraphics software v. 18 (Statgraphics Technologies, Inc., The Plains, VA, USA) [39,40]. Second-order designs allow one to conduct an experiment to obtain a regression equation containing second powers of factors.
In all experiments, the duration of 30 min, the L:S ratio = 10:1, the concentration of sulfuric acid of 40 g/dm3, and the oxygen pressure of 0.4 MPa at the oxidative stage were constant. Independent variables were in a dimensional scale:
where A is temperature, °C, and B is the duration of the oxidative period.
190 < A< 220; 10 < B < 30,
When processing the experimental results in the Statgraphics program, the following regression models were obtained on a dimensional (1)–(3) scale:
As = 3793.89 − 36.82•A − 19.29•B + 0.09•A2 + 0.09•A•B + 0.07•B2
Zn = 1029.63 − 8.95•A − 8.21•B + 0.02•A2 + 0.05•A•B − 0.01•B2
Fe = 2693.21 − 24.83•A − 23.54•B + 0.06•A2 + 0.10•A•B + 0.11•B2
The multiple correlation coefficients for Equations (1)–(3) were greater than 0.96. The multiple correlation coefficients for Equations (1)–(3) were greater than 0.96. The results of the variance analysis are presented in Table 2.

Table 2.
The variance analysis results.
To assess the influence of autoclave processing parameters on the transition of arsenic, iron, and zinc into the solution from the concentrate, Pareto charts were plotted (Figure 2a–c).
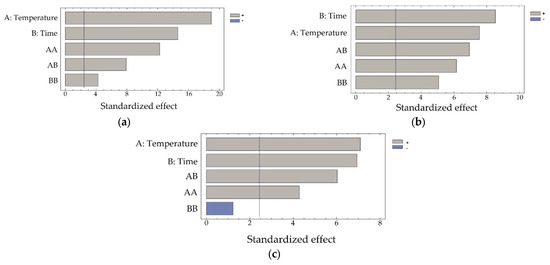
Figure 2.
Pareto charts for the recovery of arsenic (a), iron (b), and zinc (c) from the concentrate.
Considering the data presented in Table 2, it can be concluded that all variables have high statistical significance on the dissolution of iron, arsenic, and zinc. The results presented in Figure 2 confirm these data.
A diagram of the dependence of arsenic extraction into a solution on temperature and oxidation duration is presented in Figure 3.
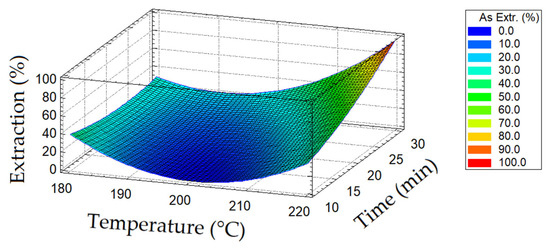
Figure 3.
Diagram of the dependence of arsenic recovery on temperature and oxidation duration.
Increasing the oxidation duration from 10 to 20 min had a positive effect on the extraction of arsenic; its transfer into a solution above 70–80% was achieved at temperatures of 210–220 °C.
A decrease in temperature from 220 to 200 °C led to a sharp decrease in the opening of tennantite; arsenic extraction did not exceed 40% over the entire range of duration of the oxidation period.
A diagram of the dependence of iron extraction on temperature and oxidation duration is presented in Figure 4.
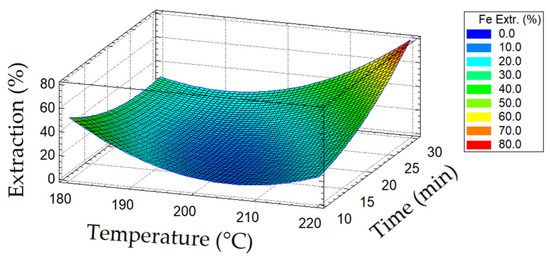
Figure 4.
Diagram of iron extraction depending on temperature and oxidation duration.
When the oxidation duration was increased from 20 to 30 min, the dissolution of pyrite increased, which indicates the almost complete opening of chalcopyrite since the proportion of iron in the concentrate associated with chalcopyrite was 32.5%. This effect negatively affects the subsequent hydrothermal copper precipitation, with chalcopyrite being the main precipitant. Copper precipitates through the following reactions:
CuFeS2 + CuSO4 = 2CuS + FeSO4
ZnS + CuSO4 = CuS + ZnSO4
PbS + CuSO4 = CuS + PbSO4
6CuS + 3CuSO4 + 4H2O = 5Cu1.8S + 4H2SO4
6MeS + 9CuSO4 + 4H2O = 5Cu1.8S + 6MeSO4 + 4H2SO4
where Me–Zn, Pb, Cu.
A decrease in temperature from 220 to 200 °C led to a sharp decrease in the extraction of iron into a solution by up to 30% over the entire duration range of the oxidation period.
A diagram of the dependence of zinc extraction into a solution on temperature and duration of oxidation is presented in Figure 5.
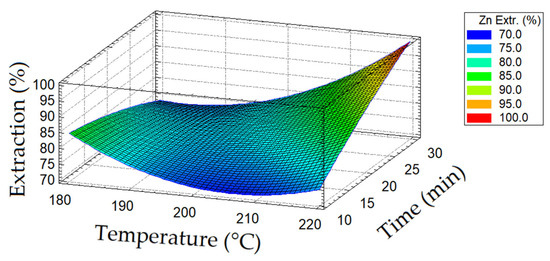
Figure 5.
Diagram of zinc extraction depending on temperature and oxidation duration.
Increasing the oxidation duration from 10 to 20 min had a positive effect on the extraction of zinc; its transfer into a solution above 75–85% was achieved at a temperature of 220 °C.
Reducing the temperature from 220 to 200 °C led to a decreased opening of sphalerite; the extraction of zinc did not exceed 70–75% over the entire duration range of the oxidation period.
3.2. Study of Mineral Dissolution of Copper Concentrate
The purpose of these studies was to assess the influence of the oxidative stage duration and the leaching temperature on the degree of opening of chalcopyrite and tennantite and the selection of process conditions to allow the maximum dissolution of arsenic, iron, and zinc while preventing copper transition into a solution.
In all experiments, the duration of 90 min, L:S ratio = 6:1, sulfuric acid concentration of 40 g/dm3, and oxygen pressure of 0.4 MPa at the oxidative stage were constant.
The dependence of the opening of chalcopyrite, tennantite, and sphalerite and the extraction of copper into a solution on the oxidative stage duration and leaching temperature is presented in Figure 6.
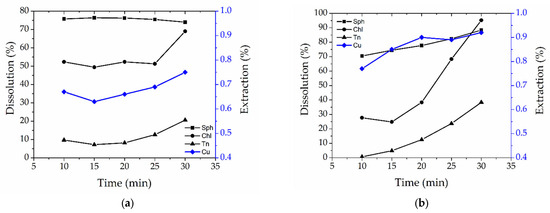
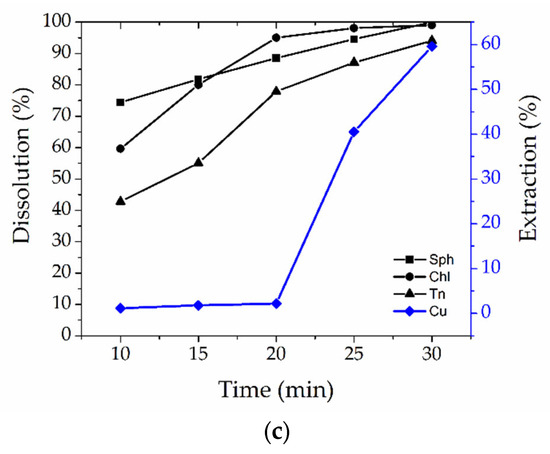
Figure 6.
Dependence of the dissolution of sphalerite (sph), chalcopyrite (chl), tennantite (tn), and the extraction of copper into a solution on the oxidative stage duration at temperatures of 190 °C (a), 205 °C (b), and 225 °C (c).
According to Figure 6a, the opening of sphalerite and chalcopyrite with an oxidation period of 10 min was 78.8% and 52.3%, respectively; a further increase in the oxidation duration to 25 min had virtually no effect on the dissolution of all minerals. Perhaps this effect is associated with the formation of elemental sulfur during autoclave leaching, according to the following reactions [41,42]:
ZnS + H2SO4 + 0.5O2 = ZnSO4 + S° + H2O
CuFeS2 + 2.5O2 + H2SO4 → CuSO4 + FeSO4 + S° + H2O
PbS + H2SO4 + 0.5O2 = PbSO4 + S° + H2O
At temperatures below 117 °C, sphalerite, galena, and chalcopyrite are oxidized to form solid elemental sulfur. In the 117–190 °C range, liquid sulfur is formed, which could wet sulfides. At temperatures higher than 160 °C, sulfur loses its liquid-flowing properties, and, in addition, the yield of So decreases significantly since the proportion of sulfur oxidized to sulfate increases, which also strongly depends on the acidity of the solution. As the temperature increases, the decomposition rate of sulfides and elemental sulfur rises; its passivating effect weakens upon reaching 200 °C, when elemental sulfur is quantitatively oxidized to sulfate ions.
The extraction of copper into a solution during both stages at 190 °C did not exceed 0.7%, which also indicates that chalcopyrite (the main precipitant of copper at the reduction stage) was not completely opened. Precipitation occurs according to Reaction 4 described above.
Tennantite is almost not leached at 190 °C, its degree of opening being 10–20%.
An increase in temperature from 190 to 205 °C led to a sharp increase in the degree of opening of minerals; with an increase in the duration of the oxidation period from 10 to 30 min, leaching of sphalerite and chalcopyrite was 88% and 92%, respectively (Figure 6b).
Sphalerite, chalcopyrite, and galena are decomposed during the oxidative period at a temperature of 205 °C through the following reactions:
ZnS + 2O2 = ZnSO4
2CuFeS2 + 4O2 = 2CuS + 2FeSO4
4CuS + 2O2 = CuSO4
During the reduction period, dissolved copper was deposited on the remaining sulfides, according to Reactions 4–8 described above.
The extraction of copper into a solution for both stages at 205 °C did not exceed 0.9%; the degree of chalcopyrite opening was 92%, which indicates that it is inappropriate to increase the duration of the oxidation period beyond 30 min since this would lead to an increased transition of copper into a solution after the two-stage treatment; the degree of tennantite leaching was 38%.
According to Figure 6c, the opening of sphalerite, chalcopyrite, and tennantite with an oxidation period of 25–30 min was 87–99%, while the extraction of copper into a solution for both stages was 40–60%, which indicates the complete opening of chalcopyrite during the oxidation period and the impossibility of complete copper deposition at the reduction stage.
The most optimal duration of the oxidation period is 20 min since up to 78% of tennantite is leached during the processing, while the extraction of copper into a solution after two stages is 2.2%, and the degree of opening of chalcopyrite reaches 95–97%.
Based on the results presented in Section 3.1 and Section 3.2, the optimal conditions for the autoclave two-stage processing of copper–arsenic concentrate are as follows: t = 225 °C, τoxidation = 20 min, τtot = 90 min, Po2 = 0.4 MPa, L:T = 6:1, and [H2SO4]initial = 40 g/dm3; in this case, 90% Zn, 2% Cu, 56% Fe, and 75–78% As are extracted into a solution during both stages.
The diffraction pattern of the cake after the autoclave treatment under optimal conditions is shown in Figure 7. Chemical analysis of the cake is presented in Table 3.
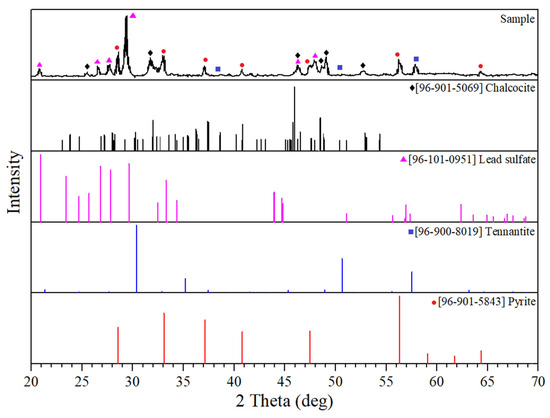
Figure 7.
X-ray diffraction pattern of the cake after the autoclave two-stage treatment (t = 225 °C, τoxidation = 20 min, τtot = 90 min, Po2 = 0.4 MPa, L:T = 6:1, and [H2SO4]initial = 40 g/dm3).

Table 3.
Chemical composition of the cake after autoclave treatment.
According to X-ray diffraction and chemical analysis data, pyrite, tennantite, chalcocite, and lead sulfate are the main compounds of the cake after the autoclave two-stage treatment. The presence of chalcocite in the cake confirms the proceeding of Reactions (4)–(8), which occur in the oxygen-free system at the reductive stage of autoclave processing [43].
3.3. Microphotographs
Microphotos and EDX mapping for the cake obtained using our autoclave two-stage processing of the copper–arsenic concentrate are t = 225 °C, τoxidation = 20 min, τtot = 90 min, Po2 = 0.4 MPa, L:T = 6:1, and [H2SO4]initial = 40 g/dm3, which are presented in Figure 8.
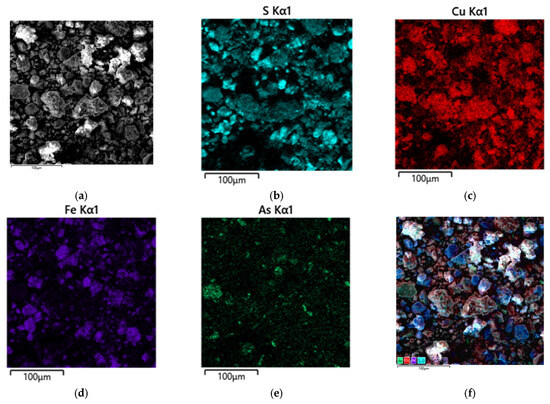
Figure 8.
SEM images of the solid residue after the autoclave two-stage treatment of copper–arsenic concentrate (a) and EDS mapping of the elemental composition of the particle surface (b–f).
The obtained micrographs and EDX mappings clearly show no iron arsenates. This confirms that at the oxidative stage of the developed process, arsenic, removed by 78%, remains in the solution. The remaining arsenic is associated with tennantite, indicating the effectiveness of the treatment process in removing arsenic from the copper–arsenic concentrate. A second important observation is the presence of pronounced areas of copper sulfides in the microphotos without iron and arsenic impurities. This confirms that copper is deposited as chalcocite during the reduction phase of the process, which is the desired result. No impurities indicate the efficiency of our copper deposition process.
4. Conclusions
The use of autoclave technologies for processing sulfide copper-containing raw materials ensures high leaching intensity, selectivity, and completeness of extraction of valuable components into a solution. High temperatures and pressure of the oxidizing agent (oxygen and air) in the autoclave, combined in some cases with the addition of oxidation catalysts or an ultrafine grinding of raw materials, make it possible to suppress the passivation of the chalcopyrite surface during leaching. Autoclave processing units are easily integrated with existing and widespread hydrometallurgical facilities for processing oxidized and secondary copper ores, including the SX–EW processing unit. Sulfuric acid and iron (III) sulfate released during the oxidation process in the autoclave, as well as the heat obtained during the cooling process of the autoclave pulp, could be used for the atmospheric leaching of copper raw materials.
Using methods of mathematical design of an experiment with a second-order orthogonal central compositional design, it was established that duration and temperature have the highest statistical significance for the oxidation process of copper–arsenic raw materials under autoclave conditions, which is confirmed via the calculated high coefficients of determination (more than 0.96).
The optimal parameters for the autoclave two-stage processing of copper–arsenic concentrate have been established: t = 220–225 °C, τox = 20 min, τtot = 90 min, PO2 = 0.4 MPa, L:S = 10:1, and [H2SO4]init = 40 g/dm3; in this case, 85% of zinc, 0.01% of copper, 44% of iron, and 78% of arsenic are extracted into the solution during both stages.
It is shown that after the second stage of autoclave treatment, copper is quantitatively concentrated in the leaching cake (Cu—29.9%, Fe—22.7%, and S—33.6%), which is suitable for further processing in traditional pyrometallurgical production.
Author Contributions
Conceptualization, K.K. and D.R.; methodology, O.D.; validation, M.T.; formal analysis, O.D.; investigation, K.K. and M.T.; resources, D.R.; data curation, K.K.; writing—original draft preparation, O.D.; writing—review and editing, K.K., D.R. and O.D.; visualization, M.T.; supervision, K.K.; project administration, D.R.; funding acquisition, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Russian Science Foundation Project No. 22-79-10290. Analytical studies were funded by State Assignment, grant No. 075-03-2021-051/5 (FEUZ-2021-0017).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vikentev, A. Selenium, tellurium and precious metal mineralogy in Uchalinsk copper-zinc-pyritic district, the Urals. IOP Conf. Ser. Mater. Sci. Eng. 2016, 123, 012027. [Google Scholar] [CrossRef]
- Lane, D.J.; Cook, N.J.; Grano, S.R.; Ehrig, K. Selective leaching of penalty elements from copper concentrates: A review. Miner. Eng. 2016, 98, 110–121. [Google Scholar] [CrossRef]
- Hernández, M.C.; Benavente, O.; Roca, A.; Melo, E.; Quezada, V. Selective Leaching of Arsenic from Copper Concentrates in Hypochlorite Medium. Minerals 2023, 13, 1372. [Google Scholar] [CrossRef]
- Jahromi, F.G.; Alvial-Hein, G.; Cowan, D.H.; Ghahreman, A. The kinetics of enargite dissolution in chloride media in the presence of activated carbon and AF 5 catalysts. Miner. Eng. 2019, 143, 106013. [Google Scholar] [CrossRef]
- Yang, W.; Qian, L.; Jin, B.; Feng, Q.; Li, L.; He, K.; Yang, J. Leaching behaviors of copper and arsenic from high-arsenic copper sulfide concentrates by oxygen-rich sulfuric acid leaching at atmospheric pressure. J. Environ. Chem. Eng. 2022, 10, 107358. [Google Scholar] [CrossRef]
- Lin, H.K. Electrochemical Behaviour of Tennantite in Chloride Solutions. J. Electrochem. Soc. 2006, 153, 74–79. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Álvarez, H.; Quezada, V.; García, A. The Enhancement of Enargite Dissolution by Sodium Hypochlorite in Ammoniacal Solutions. Materials 2021, 14, 4529. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, S.; Abdollahi, H.; Gharabaghi, M.; Mirmohammadi, M. Selective leaching of antimony from tetrahedrite rich concentrate using alkaline sulfide solution with experimental design: Optimization and kinetic studies. J. Taiwan Inst. Chem. Eng. 2021, 119, 298–312. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, Y.; He, X.; Lv, C.; Li, L.; Zhang, J.; Nan, J. Selective alkaline leaching of antimony from Low-grade refractory gold ores and process optimization. Miner. Eng. 2023, 201, 108198. [Google Scholar] [CrossRef]
- Cuevas, J.; Bruckard, W.J.; Pownceby, M.I.; Sparrow, G.J.; Torpy, A. Alkaline sulphide leaching of tennantite in copper flotation concentrates to selectively dissolve arsenic. Miner. Process. Extr. Metall. 2022, 131, 229–238. [Google Scholar] [CrossRef]
- Artykova, A.; Elkina, Y.; Nechaeva, A.; Melamud, V.; Boduen, A.; Bulaev, A. Options for Increasing the Rate of Bioleaching of Arsenic Containing Copper Concentrate. Microbiol. Res. 2022, 13, 466–479. [Google Scholar] [CrossRef]
- Anderson, C.G.; Twidwell, L.G. Hydrometallurgical processing of gold-bearing copper enargite concentrates. Can. Metall. Quart. 2008, 47, 337–346. [Google Scholar] [CrossRef]
- Rogozhnikov, D.A.; Shoppert, A.A.; Dizer, O.A.; Karimov, K.A.; Rusalev, R.E. Leaching Kinetics of Sulfides from Refractory Gold Concentrates by Nitric Acid. Metals 2019, 9, 465. [Google Scholar] [CrossRef]
- Karimov, K.A.; Rogozhnikov, D.A.; Kuzas, E.A.; Shoppert, A.A. Leaching Kinetics of Arsenic Sulfide-Containing Materials by Copper Sulfate Solution. Metals 2020, 10, 7. [Google Scholar] [CrossRef]
- Rogozhnikov, D.A.; Karelov, S.V.; Mamyachenkov, S.V.; Anisimova, O.S. Technology for the Hydrometallurgical Processing of a Complex Multicomponent Sulfide-Based Raw Material. Metallurgist 2013, 57, 247–250. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, G.; Zhang, L.; Zhou, C. Mineralogical and morphological factors affecting the separation of copper and arsenic in flash copper smelting slag flotation beneficiation process. J. Hazard. Mater. 2021, 401, 123293. [Google Scholar] [CrossRef]
- Kovyazin, A.; Timofeev, K.; Krauhin, S. Copper smelting fine dust autoclave leaching. Mater. Sci. Forume. 2019, 946, 615–620. [Google Scholar] [CrossRef]
- Jarošíková, A.; Ettler, V.; Mihaljevič, M.; Penížek, V.; Matoušek, T.; Culka, A.; Drahota, P. Transformation of arsenic-rich copper smelter flue dust in contrasting soils: A 2-year field experiment. Environ. Pollut. 2018, 237, 83–92. [Google Scholar] [CrossRef]
- Lv, X.-D.; Li, G.; Xin, Y.-T.; Yan, K.; Yi, Y. Selective Leaching of Arsenic from High-Arsenic Dust in the Alkaline System and its Prediction Model Using Artificial Neural Network. Min. Metall. Explor. 2021, 28, 2133–2144. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Yi, Y.; Shi, J.; Tian, Q.-H. Leaching behavior of metals from high-arsenic dust by NaOH-Na2S alkaline leaching. Trans. Nonferrous Met. Soc. China 2016, 26, 575–580. [Google Scholar] [CrossRef]
- Isabaev, S.M.; Kuzgibekova, K.M.; Zikanova, T.A.; Zhinova, E.V. Complex hydrometallurgical processing of lead arsenic-containing dust from copper production. Tsvetnye Met. 2017, 8, 33–38. [Google Scholar] [CrossRef]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of Arsenic Metallurgy: Treatment of Arsenical Minerals and the Immobilization of Arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Montenegro, V.; Sano, H.; Fujisawa, T. Recirculation of high arsenic content copper smelting dust to smelting and converting processes. Miner. Eng. 2013, 49, 184–189. [Google Scholar] [CrossRef]
- Schmidt, A.; Guy, B.; Montenegro, V.; Reuter, M.; Charitos, A.; Stelter, M.; Richter, A. Flue Dust Reactions and Sticking Mechanisms in a Copper Flash Smelting Furnace Waste Heat Boiler: A Sampling Study. J. Sustain. Metall. 2023, 9, 848–859. [Google Scholar] [CrossRef]
- Xue, J.; Long, D.; Zhong, H.; Wang, S.; Liu, L. Comprehensive recovery of arsenic and antimony from arsenic-rich copper smelter dust. J. Hazard. Mater. 2021, 413, 125365. [Google Scholar] [CrossRef]
- Mayhew, K.; Parhar, P.; Salomon-de-Frieberg, H. CESL process as applied to enargite-rich concentrates. Copper 2010, 5, 1983–1998. [Google Scholar]
- Karimov, K.; Rogozhnikov, D.; Kuzas, E.; Dizer, O.; Golovkin, D.; Tretiak, M. Deposition of Arsenic from Nitric Acid Leaching Solutions of Gold–Arsenic Sulphide Concentrates. Metals 2021, 11, 889. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, L.; Wu, S.; Jialin, Q.; Li, Q.; Cao, Z.; Wang, M.; Zhang, G.; Guan, W. Eco-friendly extraction of arsenic and tungsten from hazardous tungsten residue waste by pressure oxidation leaching in alkaline solutions: Mechanism and kinetic model. J. Environ. Manag. 2023, 325, 116586. [Google Scholar] [CrossRef]
- Karimov, K.; Shoppert, A.; Rogozhnikov, D.; Kuzas, E.; Zakhar’yan, S.; Naboichenko, S. Effect of Preliminary Alkali Desilication on Ammonia Pressure Leaching of Low-Grade Copper–Silver Concentrate. Metals 2020, 10, 812. [Google Scholar] [CrossRef]
- Han, B.; Altansukh, B.; Haga, K.; Takasaki, Y.; Shibayama, A. Leaching and Kinetic Study on Pressure Oxidation of Chalcopyrite in H2SO4 Solution and the Effect of Pyrite on Chalcopyrite Leaching. J. Sustain. Metall. 2017, 3, 528–542. [Google Scholar] [CrossRef]
- Fuentes, G.; Viñals, J.; Herreros, O. Hydrothermal Purification and Enrichment of Chilean Copper Concentrates: Part 1: The Behavior of Bornite, Covellite and Pyrite. Hydrometallurgy 2009, 95, 104–112. [Google Scholar] [CrossRef]
- Padilla, R.; Rivas, C.A.; Ruiz, M.C. Kinetics of Pressure Dissolution of Enargite in Sulfate-Oxygen Media. Met. Mater Trans B 2008, 39, 399–407. [Google Scholar] [CrossRef]
- Padilla, R.; Jerez, O.; Ruiz, M.C. Kinetics of the Pressure Leaching of Enargite in FeSO4–H2SO4–O2 Media. Hydrometallurgy 2015, 158, 49–55. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Vera, M.V.; Padilla, R. Mechanism of Enargite Pressure Leaching in the Presence of Pyrite. Hydrometallurgy 2011, 105, 290–295. [Google Scholar] [CrossRef]
- Padilla, R.; Rodríguez, G.; Ruiz, M.C. Copper and arsenic dissolution from chalcopyrite-enargite concentrate by sulfidation and pressure leaching in H2SO4-O2. Hydrometallurgy 2010, 100, 152–156. [Google Scholar] [CrossRef]
- Ji, G.; Liao, Y.; Xi, J.; Liu, Q.; Wu, Y.; Ma, H.; Li, J. Behavior and Kinetics of Copper During Oxygen Pressure Leaching of Complex Chalcopyrite Without Acid. J. Sustain. Metall. 2023, 9, 350–362. [Google Scholar] [CrossRef]
- Fuentes, G.; Viñals, J.; Herreros, O. Hydrothermal Purification and Enrichment of Chilean Copper Concentrates. Part 2: The Behavior of the Bulk Concentrates. Hydrometallurgy 2009, 95, 113–120. [Google Scholar] [CrossRef]
- Weidenbach, M.; Dunn, G.; Teo, Y.Y. Removal of impurities from copper sulfide mineral concentrates. In Proceedings of the ALTA Nickel-Cobalt-Copper Session, Perth, Australlia, 23–25 May 2016; pp. 335–351. [Google Scholar]
- Dobrosz-Gómez, I.; Ramos García, B.D.; GilPavas, E.; Gómez García, M.Á. Kinetic study on HCN volatilization in gold leaching tailing ponds. Miner. Eng. 2017, 110, 185–194. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; De Michelis, I.; Medici, F.; Vegliò, F. A hydrometallurgical process for the recovery of terbium from fluorescent lamps: Experimental design, optimization of acid leaching process and process analysis. J. Environ. Manag. 2016, 184, 552–559. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Kinetic Model for Direct Leaching of Zinc Sulfide Concentrates at High Slurry and Solute Concentration. Hydrometallurgy 2015, 153, 160–169. [Google Scholar] [CrossRef]
- Cháidez, J.; Parga, J.; Valenzuela, J.; Carrillo, R.; Almaguer, I. Leaching Chalcopyrite Concentrate with Oxygen and Sulfuric Acid Using a Low-Pressure Reactor. Metals 2019, 9, 189. [Google Scholar] [CrossRef]
- Kritskii, A.; Naboichenko, S. Hydrothermal Treatment of Arsenopyrite Particles with CuSO4 Solution. Materials 2021, 14, 7472. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).